Graphical abstract
Keywords: β-Glucan, Mushroom, Food ingredient, Safety, Toxicity
Highlights
-
•
β-Glucan from Hericium erinaceus was investigated for subchronic toxicity and mutagenicity.
-
•
In the subchronic study, The No-Observed-Adverse-Effect Level (NOAEL) for Hericium erinaceus β-glucan was 2000 mg/kg bw/day.
-
•
In mutagenicity studies, Hericium erinaceus β-glucan did not reveal genotoxic effects.
Abstract
The medicinal effects of Hericium erinaceus have been long documented in scientific studies of Eastern traditional medicine. It is widely consumed, because of its nutritional qualities and perceived health benefits. Also, it is rich in β-glucans, which has been shown to have immunomodulating and antitumor effects. The objective of the present study was to investigate adverse effects, if any, of β-glucan extract preparation from H. erinaceus in subchronic toxicity and genotoxicity studies. The conduct of these studies was in compliance with Good Laboratory Practice (GLP) and test guidelines established by the Organization for Economic Cooperation and Development (OECD). In the subchronic toxicity study, Sprague Dawley rats (12/sex/group) were administered (gavage) H. erinaceus β-glucan extract preparation at dose levels of 0, 500, 1000 and 2000 mg/kg body weight (bw)/day for 90 days. Treatment with H. erinaceus β-glucan extract preparation did not result in any toxicologically significant treatment-related changes in clinical observations, ophthalmic examinations, body weights, body weight gains, feed consumption, and organ weights. Clinical pathology including hematology, serum chemistry, urinalysisand terminal necropsy (gross or histopathology findings) did not reveal any treatment-related adverse effects. The results of genotoxicity studies as evaluated by gene mutations in Salmonella typhimurium, in vitro chromosome aberrations and in vivo micronucleus test in mice did not reveal any genotoxicity of H. erinaceus β-glucan extract preparation. Based on the subchronic study, the no observed-adverse-effect level (NOAEL) for H. erinaceus β-glucan extract preparation was determined as 2000 mg/kg bw/day, the highest dose tested.
1. Introduction
Mushrooms have been consumed as food for millennia for its delicious and nutritive value. It has also been evaluated for use in Traditional Asian Medicine (Valverde et al., 2015). Many mushrooms not only become the protagonists of a healthy diet, but also turn out to be an important component of many dietary supplements, health foods, antioxidant, anticancer and immunomodulatory products (Chen, 2018, Patel and Goyal, 2012, Su et al., 2016, Li et al., 2014). In recent years, many studies have shown that a variety of special components in mushrooms, especially beta-glucans, can effectively improve both the physiology and immune function (Meena et al., 2013).
Mushrooms are regarded as excellent functional foods because of their biologically active compounds, which can have various beneficial effects on human health. The most important active ingredient is β-glucan (Ren et al., 2012). β-glucan mainly exist in fungi, yeast and grains (Driscoll, 2009). The primary chemical structure of β-glucan polymers differs from source to source, but mainly consists of a linear glucose polymer with β (1,3), β (1,4) or β (1,6) linkages. Mushroom β-glucans have shown very different activities from β-glucan derived from oats and barley. Mushroom β-glucans have shown effectiveness as an anti-tumor defense and as an immune system booster while that from oats and barley help in lowering cholesterol and blood sugar (Zhao and Cheung, 2011). Also, β-glucans from mushrooms have a highly branched main chain with mixed glycosidic 1,3 and 1,6 β-linkages. Kim et al. (Kim, 2011) reported that β-glucans obtained from mushrooms contained 514 g/kg of (1,3)-β-glucans with (1,6)-β-linked side chains. Zhang et al. (Zhang et al., 2007) reported that the most common chemical structure of β-glucans from mushrooms is a β-1,3 backbone with different degrees of β-1,6 and/or β-1,4 branching. The immunomodulatory function of β-glucans would be affected by their structural variability. With regard to the differences in immunomodulatory activity ofmushroom β-glucan, when compared with that from oats/barley, is considered more effective as it has a certain degree of branching (1 → 3) -β-glucan units.
Among the different types of mushrooms that are rich in beta-glucans, Hericium erinaceus, also known as Lion's mane mushroom, is rich in beta 1–3, 1–6 glucan (Wang, 2001), in addition to erinacines (Kawagishi, 1992, Kawagishi et al., 1994, Nagai et al., 2006); hericenones (Mori et al., 2009); dilinoleoyl-phosphatidylethanolamine, DLPE (Kawagishi et al., 2006), terpenes compounds (Kenmoku et al., 2001), lectins (Kawagishi et al., 1994), proteins, vitamins (Wu et al., 2012), amino acids (Li et al., 2014), trace elements (Fu et al., 2002), and lipids. H. erinaceus is both a food in Chinese cuisine, and it is also an important component of Asian Medicine. The potential medicinal aspects have been investigated and H. erinaceus is reported to increase longevity in animal models, possibly due to reduced tumorigenesis and oxidation. (Hetland et al., 2020, Li et al., 2019). Anti-inflammatory properties have also been attributed to H. erinaceus (Ren et al., 2012). Due to its health benefits, it is thought to be valuable for its therapeutic use in neurasthenia, gastritis, dyspepsia, and gastric and duodenal ulcers (Zhang et al., 2015, Ren et al., 2018). Recent studies have indicated that H. erinaceus has potential in protecting and repairing the nervous system, thrombolysis, anti-cancer and improving immune response (Wang, 2001).
H. erinaceus are mainly distributed in temperate climatic zones of the northern hemisphere in Asia, America and Europe. It is mostly parasitic on the dead parts or wounds of walnut, oak, beech or pine trees. The growth temperature range is 10–34 °C and is a low temperature and light-favored fungus which can grow in humid and acidic environments (He et al., 2017, Thongbai et al., 2015).
Global regulatory agencies including the US Food and Drug Administration (FDA, 2007) and the European Food Safety Authority (EFSA, 2011) have permitted the health claim on a food label for the maintenance of normal blood cholesterol concentrations for soluble cereal fibers, particularly β-glucans from oat and barley. β-Glucan-enriched extracts from different sources have also been incorporated into a variety of food products such as baked foods, dairy and confectionery products (Kim, 2011, Lazaridou and Biliaderis, 2007).
Extracting β-glucan from mushrooms for food applications has become the trend of healthy and functional foods, among which H. erinaceus β-glucan is the focus of much attention. Hence, many artificially cultivated H. erinaceus have been extracted to produce functional foods that are popular with consumers (Thongbai et al., 2015) with the benefits of β-glucan. Although toxicological studies on new sources of β-glucans have been conducted to assess their efficacy and safety (Chen, 2018, Chen, 2011), a comprehensive safety profile of β-glucan extract preparation from H. erinaceus has not yet been established. Therefore, the objective of the present study was to investigate adverse effects, if any, of a standardized H. erinaceus β-glucan extract preparation in mutagenicity studies as evaluated by the Ames test, in vitro chromosome aberration assay, and in vivo micronucleus test in mice, and in a repeat-dose subchronic toxicity study in rats. The effects of H. erinaceus β-glucan extract preparation was investigated in a dose–response manner.
2. Materials and methods
2.1. Test material
High-purity standardized β-glucan extracted from H. erinaceus used in the genotoxicity and subchronic toxicity studies was produced and provided by Super Beta Glucan Inc. (Irvine, California). The H. erinaceus (BCRC No. 35669) used in the production of β-glucan was identified by Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan. H. erinaceus strain was subcultured and maintained in Yeast Mold (YM) agar medium (BD Difco Yeast Mold Agar, BD-271210) under conditions of 28℃ and relative humidity at 70–80% with an average illumination of 300 lx, 12 h per day for 14 days. The manufacturing process was initiated by preparing the culture medium, containing 35 g glucose, 10 g mannose, and 15 g yeast extract per liter. Following autoclaving procedure at 121℃ for 15 min, the mycelia of H. erinaceus were introduced into the sterile medium, and cultured at 25–28 °C for 5 to 6 weeks using a batch fermentation tank to allow full growth of the mushroom culture. Subsequently, H. erinaceus β-glucan was extracted from the mycelium using a high-speed homogenizer (12500 rpm/ 8 min) and ultrasonic vibration (30 kHz/ 25 min). The resulting solution was then filtered and separated using a ceramic membrane to strip most of the residual small carbohydrate molecules (Molecular Weight < 3 kDa). The concentrated H. erinaceus β-glucans were then pooled, dried and ground into powder form. The physical and chemical specifications of the product have been fully developed (Table 1). It is a water-soluble and fine cream powder without any characteristic odor. The product contains ∼ 93% carbohydrate of which 68% is β-glucan(Megazyme, Mushroom and Yeast Beta-Glucan Assay, K-YBGL, Ireland). In addition to the carbohydrate, the product contains small amount of fat (0.6%), protein (0.9%) and moisture (2.9%). The ash content of the product is reported as 2.3%. The material provided by the supplier met these specifications.
Table 1.
Physical, chemical and microbiological specifications of Hericium erinaceus β-Glucan.
| Parameter | Specifications | Assay method |
|---|---|---|
| Physical parameters | ||
| Appearance | Fine powder | Visual |
| Odor | Odorless | Olfactory |
| Taste | Tasteless | Taste |
| Chemical parameters | ||
| Total Carbohydrate (%) | ∼93 | By Difference (Calculation) |
| β-Glucan (%) | 68 | Internal Methods |
| Fat (%) | 0.6 | AOAC |
| Protein (%) | 0.9 | AOAC |
| Moisture (%) | 2.9 | AOAC |
| Ash (%) | 2.3 | AOAC |
| Heavy metals | ||
| Lead | <0.5 ppm | ICP-MS |
| Arsenic | <0.5 ppm | Cold Vapor |
| Cadmium | <0.5 ppm | ICP-MS |
| Mercury | <0.05 ppm | ICP-OES |
| Microbiological parameters | ||
| Aerobic Plate Count (CFU/g) | <100 | FDA BAM |
| Yeast and Mold (CFU/g) | <10 | FDA BAM |
| Total Coliforms | Negative | FDA IV USDA/3M PETRIFILM |
| E. coli | Negative | FDA IV USDA/3M PETRIFILM |
| Salmonella sp. | Negative | FDA BAM |
2.2. Genotoxicity studies
2.2.1. Ames test
This test was carried out to investigate the potential of H. erinaceus β-glucan extract preparation to induce gene mutations (Ames test). Salmonella typhimurium strains TA98, TA100, TA102, TA1535, TA1537 were used, and the plate incorporation method in the presence or absence of a S9 metabolic activation system was applied. The initial dose range finding study at doses of 5.0, 2.5, 1.25, 0.625 and 0.313 mg of H. erinaceus β-glucan extract preparation/plate revealed significant colony growth that interfered with the counting of revertant colony. Hence, the following concentrations of the β-glucan extract preparations were tested: 0.039, 0.078, 0.157, 0.313 and 0.625 mg/plate. Chemicals used as positive control for assays without or with metabolic activation included 2-nitrofluorene, sodium azide, mitomycin C, 9-aminoacridine, 2-aminoanthracene, and benzo(a)pyrene. S9 fraction (Aroclor 1254-induced; Moltox, Boone, USA) with cofactor was used to mimic the metabolic activation system. The plate incorporation method was employed, and the following components were added sequentially: A: phosphate buffer; B: each testing concentration of H. erinaceus β-glucan, negative or positive control solution; C: overnight culture of the Salmonella typhimurium strains (containing approximately 1–2 × 109 cells/ml); D: molten top agar with histidine/biotin. The contents were mixed and poured onto the surface of minimal glucose agar plates. When the top agar was solidified, the plates were inverted and placed in an incubator for 48 to 72 h at 37 ± 1 °C. Following which the colonies were counted. All experiments were carried out in triplicate.
A cytotoxic effect was concluded when a decrease in revertant colonies over the negative control was lower than 0.5-fold, if loss of bacterial lawn was observed, or if a pin colony appeared. An increase in revertant over the negative control (two-fold for TA98, TA100, and TA102 and three-fold for TA1535 and TA1537) was considered as potentially mutagenic. If the H. erinaceus β-glucan extract preparation was considered a potential mutagen, the data would be further analyzed by ANOVA to evaluate the difference between the negative control group and the treatment groups. If the data were statistically significant, the dose-related response was evaluated in response to the number of revertant colonies on the treatment groups when compared to the negative controls.
2.2.2. In vitro chromosomal aberration assay
The potential of H. erinaceus β-glucan extract preparation to induce structural and numerical chromosome aberrations was evaluated in Chinese hamster ovary cells (CHO-K1). The test was performed following GLP guidelines and in accordance with the OECD guideline for testing of chemicals #473- In vitro Mammalian Chromosome Aberration Test (1997). Five doses (0.313, 0.625, 1.25, 2.50, and 5.00 mg/ml) of H. erinaceus β-glucan extract preparation were tested for cytotoxicity using the MTT assay. CHO-K1 cells with epithelial-like morphology and modal chromosome number 20 ± 2 were used. The culture medium employed was HAM’s F12 supplemented with 10% heat-inactivated fetal bovine serum, 2.0 mM L-glutamine, 100 U/ml penicillin and streptomycin. The cells were cultured in a humidified atmosphere and exposed to the S9 mixture consisted of S9 fraction (Arcola 1254-induced) and cofactor. The growing cells were seeded in a 6-well culture plate. Seeded cells were cultured in the culture medium for 18–24 h before treatment.
The culture medium was used as the negative control. The positive controls in the different treatments included mitomycin C (3 and 18 h), benzo(a)pyrene (3 h). Each test was performed in duplicate and incubated for 3 or 18 h. Cytotoxicity was examined by the MTT assay to determine cell viability, and by microscopic examination to determine cell morphology. Cell viability was calculated according to the following formula:
The non-cytotoxic dosages of 1.25, 2.5, and 5 mg/ml of H. erinaceus β-glucan extract preparation with or without S9 in short-term (3 h) and without S9 in long term (18 h) were selected for the chromosome aberration test. Cell viability of over 50% meant that the H. erinaceus β-glucan extract preparation was non-cytotoxic in CHO-K1 cells. Any changes in general morphology, vacuolization, detachment, lysis, and membrane integrity were assessed and recorded. At least 200 well-spread metaphase cells with a number of centromeres equal to the modal number (20 ± 2) were scored for every concentration and control group. Structural chromosome aberrations, including chromosome breakage and exchange, chromatid breakage and exchange, and other abnormalities were scored and image-recorded. The Poisson distribution was used for statistical analysis (P < 0.05). The positive control group was significantly increased (P < 0.05) when compared with the negative control group. If more than two significant doses exist, H. erinaceus β-glucan extract preparation resulted in chromosome aberration in CHO-K1 cells. If only one significant dose existed, the Cochran-Armitage trend test (C-A test) was used for dose-dependent analysis. H. erinaceus β-glucan extract preparation would have been considered to display genotoxicity had the dose-dependent analysis been positive.
2.2.3. In vivo mammalian erythrocyte micronucleus test
This study was performed in accordance with the OECD guideline for the testing of chemicals #474 (1997): mammalian erythrocyte micronucleus test. For this dose–response study, CD-1® (ICR) mice (SPF grade, about 7 weeks old) were quarantined and acclimated for seven days before dosing. H. erinaceus β-glucan extract preparation was administered orally to randomly selected mice (5/sex/group; the control group 6/sex). H. erinaceus β-glucan extract preparation was suspended in sterile water and administered to mice at dose level of 80, 500, 1000 and 2000 mg/kg bw. Animals in control group received sterile water and served as the negative control. Cyclophosphamide (80 mg/kg bw) was chosen as the positive control and administered intraperitoneally. The peripheral blood samples (2 µl) from the tail vein were collected at 24 ± 2, 48 ± 2, and 72 ± 2 h after dosing. It was then smeared on acridine orange-coated slides and the staining was performed at room temperature for 2–3 h. The positive control group was only sampled at 48 ± 2 h after dosing.
For each time-point and each animal, over 1000 erythrocytes were counted the polychromatic erythrocytes (PCE) and the percentage of PCE in erythrocytes (PCE%) were calculated. At least 2000 PCE per animal were scored for the incidence of PCE with micronucleus (MN 0/00 PCE). Examination of PCE% and micronucleus frequency (MN 0/00 PCE) was conducted by fluorescent microscope. The fluorescent microscope (Zeiss AXIO Imager.AI) with 488 nm exiting and 515 nm long pass filter was used for PCE and micronucleus identification and counting. The PCE % was calculated. At least 1000 PCEs were observed and the micronucleus frequency (MN 0/00 PCE) was calculated. Data were presented as mean ± standard deviation. The micronucleus frequency was analyzed by the model of Poisson distribution. The p value of<0.05 (p < 0.05) was considered statistically significant. If testing group presents significant result, the Cochran Armitage trend test (C-A test) was used for identifying the dose dependence.
2.3. Subchronic study
2.3.1. Study design
This study was performed according to a well-designed protocol based on OECD Guidelines for Testing Chemicals, Health Effects Test Guidelines, for Repeated Dose 90-Day Oral Toxicity Study in Rodents, Section 408. The study was conducted in compliance with the technical requirements of the protocol, and all applicable guidance and regulations that included Good Laboratory Practices for Non-Clinical Laboratory Studies (FDA, 21 CFR, Part 58), and OECD principles on GLP. The Animal Study Protocol, along with the detailed description of the proposed use of animals was reviewed and approved by the IACUC review committee.
2.3.2. Animals
In this study, CD® (SD) IGS strain rats from BioLASCO Taiwan Company Ltd. (Taipei, Taiwan) were used. The animals were allowed to acclimatize for a minimum of six days before the initiation of experiments. A total of 96 rats (∼6 weeks old) were selected for the study. Two animals of the same sex and treatment were housed together in polycarbonate cages with paddy husk bedding. The room temperature and relative humidity were maintained at 21 ± 2 °C and 55 ± 20%, respectively, with a 12 h light/dark cycle. Throughout the study periods all animals received Rodent feed 5010, LabDiet, PMI® Nutrition International (Brentwood, MO) and drinking water ad libitum.
2.3.3. Treatment
Rats were divided into four groups (12/sex/group) based on stratified randomization by using body weights taken before the initiation of treatment. Rats were treated orally (gavage) once daily with H. erinaceus β-glucan extract preparation at dose levels of 0 (Group I- control), 500 (Group II- low-dose), 1000 (Group III- mid-dose), or 2000 (Group IV- high-dose) mg/kg bw (dosing volume 10 mL/kg) for 90 consecutive days. The dosing solutions were prepared fresh daily with sterile water and stirred until the proper dose was achieved. The control animals received sterile water.
2.3.4. Parameters studied
2.3.4.1. Clinical signs, body weights and feed consumption
During the course of study, all animals were observed twice daily for mortality or morbidity. Clinical observations were performed daily after dosing and any abnormalities were recorded and documented. Ophthalmologic examinations were performed on all animals at the grouping day and before euthanasia. Body weights were recorded before the first dosing, weekly thereafter, prior to the termination of the study, and on the day of necropsy. Mean body weight and mean body weight gains were recorded. Feed consumption was measured at weekly intervals.
2.3.4.2. Clinical pathology
After 90 days of treatment, hematology (including coagulation), serum chemistry, and urinalysis measurements were performed on all surviving rats. Blood samples were drawn through the abdominal aorta and collected in three different sampling tubes: 1. Containing K2-EDTA (hematology); 2. Containing sodium citrate (coagulation); and 3. No anticoagulant (serum chemistry). Urine samples were collected approximately 12–16 h using metabolism cages prior to euthanasia. All surviving animals were anesthetized with ketamine/xylazine mixture, followed by blood collection, exsanguinations, and necropsy. Hematology parameters analyzed included: Red blood cell counts (RBC), White blood cell counts (WBC), Platelet counts (PLT), Hemoglobin (HGB), Hematocrit (HCT), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), WBC differential (neutrophils- NEUT; eosinophils- EOSIN; basophils- BASO; monocytes- MONO; lymphocytes- LYMPH), Activated partial thromboplastin time (APTT) and Prothrombin time (PT). Clinical biochemistry parameters analyzed included: Amylase (AMY), Albumin (ALB), Alkaline phosphatase (ALP), Total bilirubin (T-BIL), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Gamma-glutamyl transferase (γ-GT), Total protein (TP), Creatinine (CRE), Blood Urea nitrogen (BUN), Cholesterol (CHO), Triglycerides (EFSA, 2011), Creatine kinase (CK), Chloride (Cl), Sodium (Na), Potassium (K), Glucose (GLU), Calcium (Ca), and Phosphorus (P).
2.3.4.3. Necropsy, organ weight and histopathology
At termination, gross necropsy was performed and recorded for each animal, including examinations of the external surface of the body, thoracic and abdominal cavities, intestines, and visceral organs. Following gross necropsy, sampled tissue/organs, including adrenals, brain, epididymitis, heart, kidneys, liver, ovaries, pituitary, spleen, testis, thymus, uterus, prostates and seminal vesicles were weighed. The sampled tissue/organ was fixed and preserved in 10% neutral buffered formalin or other appropriate fixative for subsequent histopathology examination. The selected tissues (over 40) in the control and high-dose groups were trimmed, embedded, sectioned, and H&E stained, followed by microscopy examination.
2.3.5. Statistical analysis
All measured parameters were calculated and expressed as mean ± standard deviation (12 rats/group). Comparisons of data collected from treated and control groups were performed by one-way ANOVA, followed by Dunnett’s method (SPSS ver. 12.0). All analyses and comparisons were evaluated at the 95% level of confidence (P < 0.05).
3. Results
3.1. Genotoxicity studies
3.1.1. Bacterial reverse mutation test
In the Bacterial reverse mutation test, the analysis results of the five different strains showed that: No matter whether S9 was present or not, the number of revertant colonies did not increase significantly. The number of revertant colonies in the negative control groups of each strain was within the range of historic control data. The revertant colonies in the positive control group were more than two times (TA98, TA100, and TA102) and three times (TA1535 and TA1537) the negative control groups. These results show no significant increase in the number of revertant colonies at all concentrations of test article in any of the strains whether with or without S9 mixture. Thus, the results of the study indicated that the H. erinaceus β-glucan extract preparation was non-mutagenic.
3.1.2. In vitro chromosomal aberration assay
In the three-hour (short-term) treatment group, cell viability in the absence of S9 metabolic activation at the H. erinaceus β-glucan concentrations 5, 2.5, 1.25, 0.625, and 0.313 mg/ml were 98.23 ± 2.15, 98.14 ± 1.21, 97.88 ± 2.21, 96.37 ± 1.92, 94.87 ± 1.31% respectively. In the short-term treatment in the presence of S9 metabolic mixture, the cell viabilities at 5, 2.5, 1.25, 0.625, and 0.313 mg/ml were 117.24 ± 5.85, 114.58 ± 6.46, 109.44 ± 5.14, 129.57 ± 6.47, 131.02 ± 10.52% respectively. In the 18-hour (long-term) treatment in the absence of S9 metabolic mixture, the cell viabilities were 93.72 ± 1.24, 94.25 ± 1.04, 95.52 ± 1.48, 96.21 ± 0.76, 98.45 ± 6.53%, respectively. As the cell viability was greater than 50%, the H. erinaceus β-glucan extract preparation was not considered as cytotoxic. The results of this test suggested that H. erinaceus β-glucan extract preparation do not cause significant structural and numerical aberrations under the experimental conditions described.
3.1.3. In vivo mammalian erythrocyte micronucleus test
During this study, no abnormal clinical symptoms were observed in any group of animals, and there were no mortalities either. No significant difference in mean body weights were noted between the groups. The PCE% of the negative control, positive control and treatment groups are summarized in Table 2. The PCE% of the positive control group at 48 h after dosing in females was 1.51 ± 0.52% and males was 1.63 ± 0.45% respectively. A decrease in the PCE% of the positive control group, at after 48 h, indicated inhibition of erythropoiesis by cyclophosphamide. However, the PCE% in all the treatment groups showed no significant decrease as compared to the negative control group indicating H. erinaceus β-glucan extract preparation did not inhibit erythropoiesis.
Table 2.
Dose related changes in rat polychromatic erythrocytes and micronucleus frequency following administration of H. erinaceus β-glucan preparation to rats.
|
PCE% |
MN 0/00PCE |
||||
|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | ||
| Females | |||||
| Negative control | Sterile water | 3.44 ± 0.64 | 3.58 ± 0.58 | 0.18 ± 0.26 | 0.18 ± 0.66 |
| Positive control | Cyclophosphamide 80 mg/kg/day |
1.51 ± 0.52 | – | 21.00 ± 5.55* | – |
| H. erinaceus β-glucan | 500 mg/kg/day | 3.62 ± 0.55 | 3.72 ± 0.42 | 0.20 ± 0.27 | 0.40 ± 0.27 |
| 1000 mg/kg/day | 3.75 ± 0.72 | 3.82 ± 0.55 | 0.30 ± 0.45 | 0.30 ± 0.45 | |
| 2000 mg/kg/day | 3.45 ± 0.65 | 3.84 ± 0.66 | 0.30 ± 0.45 | 0.60 ± 0.45 | |
| Males | |||||
| Negative control | Sterile water | 3.62 ± 0.52 | 3.61 ± 0.36 | 0.64 ± 0.52 | 0.60 ± 0.45 |
| Positive control | Cyclophosphamide 80 mg/kg/day | 1.63 ± 0.45 | – | 21.50 ± 6.50* | – |
| H. erinaceus β-glucan | 500 mg/kg/day | 3.65 ± 0.75 | 3.72 ± 0.42 | 0.20 ± 0.27 | 0.30 ± 0.22 |
| 1000 mg/kg/day | 3.72 ± 0.60 | 3.45 ± 0.45 | 0.30 ± 0.45 | 0.20 ± 0.27 | |
| 2000 mg/kg/day | 3.65 ± 0.65 | 3.48 ± 0.33 | 0.20 ± 0.27 | 0.10 ± 0.22 | |
Values are mean ± SD for 12 (n = 12) rats in each group unless indicated.
* Poisson distribution, p < 0/05 indicated significant difference.
The micronucleus frequency (MN 0/00 PCE) in thousand PCEs was examined with a fluorescent microscope and is summarized in Table 2. The micronucleus frequency in thousand PCEs of the negative control group at 48 h after dosing was 0.18 ± 0.26 0/00 PCE, and 72 h was 0.18 ± 0.66 0/00 PCE in females, and 0.64 ± 0.52, and 0.60 ± 0.45 0/00 PCE in males respectively. The micronucleus frequency in thousand PCEs of the positive control group at 48 h after dosing was 21.00 ± 5.550/00 PCE in females, and 21.50 ± 6.50 0/00 PCE in males. After Poisson distribution analysis, there was no significant difference between the three treatment groups and the negative control group in both males and females, which indicated that H. erinaceus β-glucan extract preparation exhibited no genotoxicity in the testing system applied in the study.
3.2. Subchronic study
3.2.1. Survival, clinical observations, body weights and feed consumption
There was no mortalities or treatment-related abnormal clinical signs in any of the groups during the study period. Some clinical signs were observed due to housing behavior (wounds: male- 4/12 in Group II and; females- 3/12 in Group II and 8/12 in Group IV) or individual animal difference (hair loss: male-4/12 and 1/12 in Group II and III, respectively; female 3/12 and 8/12 in Group I and III, respectively). The severities of these clinical signs were slight (wounds and hair loss). The ophthalmological examinations did not reveal any abnormalities in any group before dosing and necropsy.
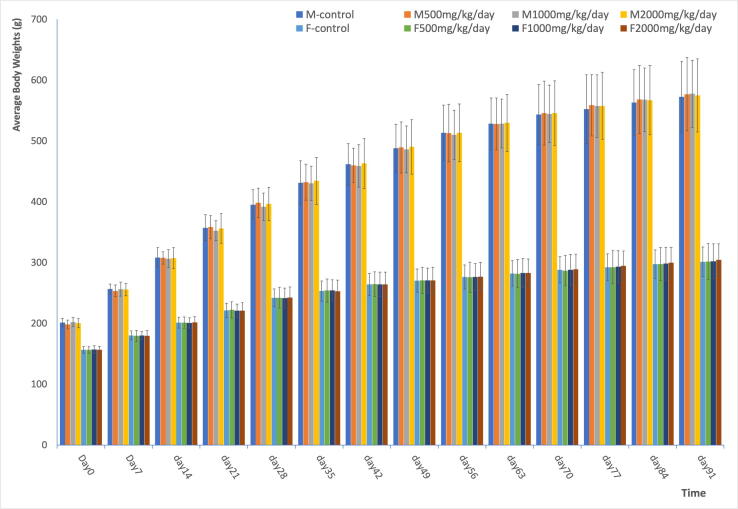
The mean body weights (Fig. 1) and body weight gains of treatment groups were comparable to control group animals throughout the treatment period and no statistically significant (p < 0.05) difference was noted. Thus, compared to the control group, no treatment-related biologically significant effects of the β-glucan extract preparation were noted on body weight or body weight gain at dose levels up to 2000 mg/kg bw/day (Fig. 1). In addition, there were no biologically significant differences in feed consumption in males and females in the vehicle control and treatment groups during the course of study (data not shown). At week 9, male rats in the high-dose group showed statistically significant reduction in food intake. At week 7, all female rats in treatment group, showed significantly higher feed intake. These significant increases cannot be confirmed as related to the administration of H. erinaceus β-glucan extract preparations.
Fig. 1.
3.2.2. Clinical pathology
3.2.2.1. Urinalysis
The results of urinalysis parameters in male and female rats following administration of H. erinaceus β-glucan extract preparation at dose levels of 0, 500, 1000, and 2000 mg/kg bw/day for 90 consecutive days are summarized in (Table 3). There were no significant differences or physiological abnormalities noted in males and females in the control and treatment groups. The urine analysis parameters such as volume, pH, specific gravity, and urobilinogen did not show any significant difference from the respective control groups.
Table 3.
Effect of H. erinaceus β-glucan preparation on urinalysis parameters in male and female rats.
|
Dose (mg/kg/day) |
|||||
|---|---|---|---|---|---|
| Parameters | 0a | 500 | 1000 | 2000 | |
| Males | |||||
| Volume (mL) | 22.28 ± 7.02 | 19.85 ± 8.55 | 21.12 ± 9.09 | 20.63 ± 6.62 | |
| Specific gravity | 1.02 ± 0.0044 | 1.02 ± 0.0045 | 1.02 ± 0.0045 | 1.02 ± 0.0048 | |
| pH | 7.23 ± 0.55 | 7.31 ± 0.21 | 7.28 ± 0.33 | 7.26 ± 0.40 | |
| Urobilinogen (EU/dL) | 0.39 ± 0.32 | 0.45 ± 0.35 | 0.46 ± 0.38 | 0.48 ± 0.37 | |
| Females | |||||
| Volume (mL) | 16.773 ± 8.39 | 13.52 ± 9.28 | 13.255 ± 9.62 | 14.52 ± 7.65 | |
| Specific gravity | 1.02 ± 0.0045 | 1.02 ± 0.0048 | 1.02 ± 0.0045 | 1.02 ± 0.0050 | |
| pH | 6.85 ± 0.44 | 6.92 ± 0.49 | 6.95 ± 0.18 | 6.73 ± 0.38 | |
| Urobilinogen (EU/dL) | 0.34 ± 0.31 | 0.42 ± 0.31 | 0.37± 0.26 | 0.40 ± 0.36 | |
aVehicle control: Sterile water for injection (WFI).
Values are mean ± SD for 12 rats in each group unless indicated.
*p < 0.05.
3.2.2.2. Hematology
Treatment with H. erinaceus β-glucan extract preparation did not result in any biologically significant adverse effects in hematology parameters in male and female rats (Table 4, Table 5). However, some statistically significant differences were noted when the control and treatment groups were compared. In male rats, a statistically significant (p < 0.05) increase in blood levels of eosinophil were noted in the mid-dose treated group (1000 mg/kg bw/day; Group III). The above noted significant change following administration of the β-glucan extract preparation in the 90 day study was not observed in both sexes, lacked correlating changes in other red cell parameters, was of small magnitude, and/or was not noted in a dose-related manner hence this change was considered as incidental variation and not treatment-related adverse effect. There were no other statistically significant differences when the respective control and treatment groups were compared.
Table 4.
Effect of H. erinaceus β-glucan preparation on hematological parameters in male rats.
| Parameter | Units |
Dose (mg/kg/day) |
|||
|---|---|---|---|---|---|
| 0a | 500 | 1000 | 2000 | ||
| RBC | 106/μL | 9.532 ± 0.385 | 9.425 ± 0.384 | 9.505 ± 0.482 | 9.468 ± 0.338 |
| WBC | 103/μL | 8.715 ± 3.725 | 8.782 ± 3.246 | 8.425 ± 3.662 | 8.682 ± 3.017 |
| Neutrophil | % | 24.82 ± 7.15 | 26.15 ± 4.73 | 25.93 ± 6.75 | 26.22 ± 8.36 |
| Lymphocyte | % | 70.84 ± 7.80 | 69.12 ± 8.40 | 71.47 ± 9.38 | 70.15 ± 7.58 |
| Monocyte | % | 4.30 ± 1.25 | 4.15 ± 1.50 | 4.00 ± 1.45 | 4.24 ± 1.25 |
| Eosinophil | % | 0.28 ± 0.15 | 0.31 ± 0.10 | 0.42 ± 0.22* | 0.31 ± 0.17 |
| Basophil | % | 0.03 ± 0.04 | 0.03 ± 0.05 | 0.03 ± 0.02 | 0.0 3 ± 0.04 |
| MCV | fL | 47.27 ± 1.82 | 46.85 ± 1.67 | 47.23 ± 1.78 | 48.11 ± 1.58 |
| MCH | pg | 17.44 ± 0.60 | 17.50 ± 0.48 | 17.44 ± 0.56 | 17. 51 ± 0.48 |
| MCHC | g/dL | 38.88 ± 0.45 | 36.75 ± 0.78 | 38.56 ± 0.54 | 38.43 ± 0.68 |
| Platelet | 103/μL | 1285.3 ± 170.8 | 1301.5 ± 162.7 | 1294.8 ± 145.6 | 1283.9 ± 166.3 |
| Hemoglobin | g/dL | 16.34 ± 0.45 | 15.92 ± 0.58 | 16.36 ± 0.33 | 16.52 ± 0.86 |
| Hematocrit | % | 44.83 ± 1.84 | 46.75 ± 1.72 | 45.26 ± 2.27 | 45.13 ± 1.94 |
| APTT | Sec | 17.31 ± 0.99 | 17.19 ± 1.14 | 16.95 ± 0.88 | 16.92 ± 1.65 |
| Prothrombin time | Sec | 11.32 ± 1.82 | 11.51 ± 1.72 | 11.44 ± 1.66 | 11.38 ± 1.92 |
aVehicle control: Sterile water for injection (WFI).
Values are mean ± SD for 12 rats in each group.
*p < 0.05; in females there were no statically significant differences between the vehicle control and the test article treatment groups.
RBC = red blood cells; WBC = white blood cells; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; APTT = activated partial thromboplastin time.
Table 5.
Effect of H. erinaceu β-glucan preparation on hematological parameters in female rats.
| Parameter | Units |
Dose (mg/kg/day) |
|||
|---|---|---|---|---|---|
| 0a | 500 | 1000 | 2000 | ||
| RBC | 106/μL | 8.332 ± 0.354 | 8.427 ± 0.385 | 8.382 ± 0.328 | 8.395 ± 0.354 |
| WBC | 103/μL | 7.452 ± 2.862 | 7.345 ± 3.285 | 7.128 ± 2.873 | 7.622 ± 2.179 |
| Neutrophil | % | 18.85 ± 6.24 | 17.83 ± 7.28 | 18.15 ± 4.65 | 17.13 ± 8.06 |
| Lymphocyte | % | 80.38 ± 5.74 | 78.48 ± 8.71 | 77.44 ± 6.48 | 78.36 ± 7.85 |
| Monocyte | % | 4.04 ± 0.77 | 3.65 ± 0.87 | 3.92 ± 0.68 | 3.74 ± 0.77 |
| Eosinophil | % | 0.28 ± 0.14 | 0.26 ± 0.17 | 0.25 ± 0.18 | 0.26 ± 0.18 |
| Basophil | % | 0.03 ± 0.09 | 0.02 ± 0.04 | 0.01 ± 0.03 | 0.02 ± 0.04 |
| MCV | fL | 55.22 ± 1.11 | 54.77 ± 2.56 | 53.67 ± 2.83 | 54.84 ± 1.44 |
| MCH | pg | 18.60 ± 0.38 | 18.25 ± 0.49 | 18.35 ± 0.78 | 18.45 ± 0.35 |
| MCHC | g/dL | 34.64 ± 0.58 | 35.27 ± 0.68 | 35.79 ± 0.42 | 35.86 ± 0.75 |
| Platelet | 103/μL | 1090.3 ± 130.2 | 1092.2 ± 153.8 | 1084.6 ± 135.1 | 1102.2 ± 151.6 |
| Hemoglobin | g/dL | 15.48 ± 0.46 | 15.32 ± 0.51 | 15.42 ± 0.64 | 15.57 ± 0.68 |
| Hematocrit | % | 42.78 ± 1.56 | 43.12 ± 1.45 | 43.36 ± 1.64 | 43.75 ± 1.82 |
| APPT | Sec | 14.75 ± 1.27 | 14.67 ± 0.92 | 14.33 ± 0.90 | 14.65 ± 1.06 |
| Prothrombin time | Sec | 9.10 ± 0.24 | 9.14 ± 0.22 | 9.16 ± 0.25 | 9.08 ± 0.20 |
aVehicle control: Sterile water for injection (WFI).
n = 12.
Values are mean ± SD for 12 rats in each group unless indicated. There were no statically significant differences between the vehicle control and the test article treatment groups.
RBC = red blood cells; WBC = white blood cells; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; APTT = activated partial thromboplastin time.
3.2.2.3. Serum chemistry
There were no treatment-related biologically significant adverse effects of the H. erinaceus β-glucan extract preparation on serum chemistry parameters in male and female rats (Table 6, Table 7). However, some statistically significant differences were noted when the respective control and treatment groups were compared. In male rats, as compared to control group, a significant (p < 0.05) increase in serum sodium levels in mid- (Group III; 1000 mg/kg bw/day) and the high-dose (Group IV; 2000 mg/kg bw/day) groups; serum albumin in the high-dose group (Group IV; 2000 mg/kg bw/day); and serum chloride in mid-dose (Group III; 1000 mg/kg bw/day) group was noted. Similarly, in female rats, compared to the control group, a statistically significant decrease in serum levels of total protein in the mid-dose group (Group III; 1000 mg/kg bw/day); increase in alkaline phosphatase in the low-dose (Group II; 500 mg/kg bw/day) and high-dose group (Group IV; 2000 mg/kg bw/day); and increase in serum sodium levels in the low- and mid-dose groups (Group II & III; 500 and 1000 mg/kg bw/day) was noted. There were no other statistically significant differences when the respective control and/or treatment groups were compared. The all described changes in serum sodium, alkaline phosphatase and proteins were well within the normal laboratory control range and hence considered as incidental changes or biological variations and not as H. erinaceus β-glucan treatment-related effects.
Table 6.
Effect of H. erinaceus β-glucan preparation on serum chemistry parameters in male rats.
| Parameter | Units |
Dose (mg/kg/day) |
Historical control data1 |
|||
|---|---|---|---|---|---|---|
| 0a | 500 | 1000 | 2000 | |||
| Glucose | mg/dL | 170.35 ± 25.64 | 165.42 ± 24.38 | 163.25 ± 28.56 | 170.21 ± 24.74 | – |
| Cholesterol | mg/dL | 58.42 ± 15.20 | 60.03 ± 16.34 | 55.78 ± 14.02 | 59.62 ± 12.46 | – |
| Triglyceride | mg/dL | 28.46 ± 19.47 | 24.59 ± 14.32 | 26.13 ± 9.81 | 25.62 ± 11.37 | – |
| Creatinine | mg/dL | 0.44 ± 0.09 | 0.48 ± 0.05 | 0.44 ± 0.05 | 0.456 ± 0.08 | – |
| AST | U/L | 122.75 ± 44.29 | 114.70 ± 20.85 | 128.44 ± 20.28 | 130.87 ± 33.65 | – |
| ALT | U/L | 29.40 ± 2.53 | 28.57 ± 3.06 | 28.49 ± 4.02 | 31.42 ± 5.10 | – |
| Total protein | g/dL | 6.60 ± 0.16 | 6.55 ± 0.22 | 6.64 ± 0.39 | 6.77 ± 0.30 | – |
| Total bilirubin | mg/dL | 0.053 ± 0.005 | 0.060 ± 0.005 | 0.060 ± 0.006 | 0.056 ± 0.017 | – |
| gamma-glutamyl transferase | U/L | NA | NA | NA | NA | – |
| Alkaline phosphatase | U/L | 227.42 ± 35.25 | 230.24 ± 36.72 | 234.88 ± 35.36 | 238.07 ± 39.62 | – |
| Amylase | U/L | 1348.5 ± 175.4 | 1393.8 ± 165.6 | 1432.8 ± 225.3 | 1417.1 ± 200.3 | – |
| Albumin | g/dL | 4.03 ± 0.16 | 4.15 ± 0.22 | 4.09 ± 0.2 | 432 ± 0.152* | 3.46 ∼ 4.49 |
| Creatine kinase | U/L | 715.52 ± 310.12 | 661.43 ± 249.18 | 695.89 ± 270.63 | 704.75 ± 300.55 | – |
| Sodium | mmol/L | 141.56 ± 1.05 | 144.50 ± 1.52 | 148.12 ± 1.76* | 148.25 ± 1.47* | 140.95 ∼ 148.99 |
| Potassium | mmol/L | 4.492 ± 0.245 | 4.483 ± 0.249 | 4.534 ± 0.167 | 4.510 ± 0.274 | – |
| Phosphorus | mg/dL | 6.84 ± 0.73 | 6.68 ± 0.86 | 6.75 ± 0.85 | 6.92 ± 0.52 | – |
| Calcium | mg/dL | 10.05 ± 0.35 | 10.12 ± 0.42 | 10.09 ± 0.67 | 10.17 ± 0.29 | – |
| Chloride | mmol/L | 104.32 ± 2.93 | 103.14 ± 2.17 | 110.62 ± 1.72* | 102.12 ± 1.62 | 101.15 ∼ 114.43 |
| BUN | mg/dL | 14.81 ± 1.37 | 15.27 ± 1.52 | 14.49 ± 2.37 | 14.96 ± 1.56 | – |
aVehicle control: Sterile water for injection (WFI).
NA = No data available (Due to below detection limit).
1Historical control data: Level Biotechnology Inc., Preclinical Testing Center.
Values are mean ± SD for 12 rats.
*p < 0.05.
AST = aspartate aminotransferase; ALT = alanine aminotransferase; BUN = blood urea nitrogen.
Table 7.
Effect of H. erinaceus β-glucan preparation on serum chemistry parameters in female rats.
| Parameter | Units |
Dose (mg/kg/day) |
Historical control data1 |
|||
|---|---|---|---|---|---|---|
| 0a | 500 | 1000 | 2000 | |||
| Glucose | mg/dL | 156.00 ± 19.19 | 148.33 ± 26.93 | 144.48 ± 17.43 | 142.16 ± 22.08 | – |
| Cholesterol | mg/dL | 74.55 ± 19.71 | 69.45 ± 12.70 | 69.98 ± 9.87 | 68.88 ± 15.24 | – |
| Triglyceride | mg/dL | 23.82 ± 9.56 | 26.79 ± 9.68 | 24.49 ± 5.12 | 29.91 ± 19.65 | – |
| Creatinine | mg/dL | 0.53 ± 0.09 | 0.55 ± 0.06 | 0.51 ± 0.08 | 0.53 ± 0.09 | – |
| AST | U/L | 95.72 ± 14.46 | 89.34 ± 17.68 | 91.03 ± 15.30 | 98.37 ± 19.25 | – |
| ALT | U/L | 24.12 ± 14.29 | 25.35 ± 8.45 | 26.83 ± 10.40 | 27.34 ± 8.88 | – |
| Total protein | g/dL | 6.52 ± 0.42 | 6.45 ± 0.33 | 7.13 ± 0.26* | 6.61 ± 0.18 | 5.72 ∼ 7.98 |
| Total bilirubin | mg/dL | 0.063 ± 0.015 | 0.063 ± 0.005 | 0.067 ± 0.006 | 0.076 ± 0.027 | – |
| gamma-glutamyl transferase | U/L | NA | NA | NA | NA | – |
| Alkaline phosphatase | U/L | 114.80 ± 34.33 | 125.16 ± 30.6* | 118.77 ± 25.35 | 132.35 ± 38.18* | 58.16 ∼ 176.63 |
| Amylase | U/L | 997.30 ± 156.20 | 1022.50 ± 178.40 | 1062.50 ± 160.30 | 1005.30 ± 170.50 | – |
| Albumin | g/dL | 4.65 ± 0.27 | 4.82 ± 0.37 | 4.92 ± 0.56 | 4.58 ± 0.37 | – |
| Creatine kinase | U/L | 389.50 ± 143.71 | 354.16 ± 128.63 | 351.93 ± 98.37 | 393.69 ± 163.03 | – |
| Sodium | mmol/L | 140.25 ± 1.65 | 148.15 ± 1.20* | 147.20 ± 2.26* | 142.18 ± 1.66 | 132.51 ∼ 151.47 |
| Potassium | mmol/L | 3.982 ± 0.318 | 3.945 ± 0.250 | 4.025 ± 0.332 | 4.106 ± 0.217 | – |
| Phosphorus | mg/dL | 5.84 ± 0.69 | 6.02 ± 0.77 | 5.82 ± 0.93 | 5.96 ± 0.65 | – |
| Calcium | mg/dL | 10.40 ± 0.35 | 10.45 ± 0.75 | 10.05 ± 0.64 | 10.36 ± 0.35 | – |
| Chloride | mmol/L | 105.66 ± 1.68 | 104.75 ± 1.33 | 105.22 ± 2.70 | 105.20 ± 1.80 | – |
| BUN | mg/dL | 15.90 ± 2.06 | 16.10 ± 1.63 | 16.60 ± 1.86 | 16.72 ± 1.70 | – |
aVehicle control: Sterile water for injection (WFI).
NA = No data available (Due to below detection limit).
1Historical control data: Level Biotechnology Inc., Preclinical Testing Center.
Values are mean ± SD for 12 rats in each group unless mentioned.
*p < 0.05.
AST = aspartate aminotransferase; ALT = alanine aminotransferase; BUN = blood urea nitrogen.
3.2.2.4. Organ weights
The changes in organ weights following treatment with H. erinaceus β-glucan extract preparation administration are summarized in Table 8. There were no statistically significant differences when the respective control and treatment groups were compared.
Table 8.
Effect of H. erinaceus β-glucan preparation on absolute organ weights (g) in male and female rats.
| Organs | Sex |
Dose (mg/kg/day) |
|||
|---|---|---|---|---|---|
| 0a | 500 | 1000 | 2000 | ||
| Liver | M | 14.851 ± 1.625 | 14.632 ± 1.792 | 15.023 ± 2.147 | 14.783 ± 1.928 |
| Kidneys | M | 3.652 ± 0.372 | 3.580 ± 0.263 | 3.577 ± 0.314 | 3.558 ± 0.246 |
| Heart | M | 1.580 ± 0.107 | 1.552 ± 0.126 | 1.567 ± 0.213 | 1.565 ± 0.167 |
| Adrenals | M | 0.05435 ± 0.00814 | 0.05447 ± 0.00713 | 0.05522 ± 0.00805 | 0.05616 ± 0.00665 |
| Pituitary | M | 0.01266 ± 0.00105 | 0.01318 ± 0.00157 | 0.01321 ± 0.00122 | 0.01254 ± 0.00132 |
| Prostates & Seminal vesiclesb | M | 3.651 ± 0.335 | 3.5620 ± 0.317 | 3.702 ± 0.420 | 3.712 ± 0.205 |
| Testes | M | 3.245 ± 0.327 | 3.312 ± 0.267 | 3.332 ± 0.212 | 3.370 ± 0.258 |
| Brain | M | 2.168 ± 0.066 | 2.143 ± 0.050 | 2.148 ± 0.025 | 2.159 ± 0.034 |
| Thymus | M | 0.419 ± 0.135 | 0.428 ± 0.076 | 0.448 ± 0.068 | 0.435 ± 0.87 |
| Spleen | M | 0.925 ± 0.135 | 0.916 ± 0.069 | 0.924 ± 0.073 | 0.920 ± 0.76 |
| Epididymides | M | 1.411 ± 0.153 | 1.392 ± 0.091 | 1.448 ± 0.106 | 1.432 ± 0.152 |
| Liver | F | 7.678 ± 0.768 | 7.802 ± 0.548 | 7.741 ± 0.857 | 7.759 ± 0.891 |
| Kidneys | F | 1.954 ± 0.211 | 2.003 ± 0.185 | 1.973 ± 0.208 | 2.005 ± 0.298 |
| Heart | F | 0.951 ± 0.068 | 0.960 ± 0.065 | 0.957 ± 0.080 | 0.965 ± 0.073 |
| Adrenals | F | 0.06500 ± 0.01054 | 0.06752 ± 0.01484 | 0.06535 ± 0.01025 | 0.06705 ± 0.00838 |
| Pituitary | F | 0.01625 ± 0.00263 | 0.01606 ± 0.00315 | 0.01582 ± 0.00842 | 0.01642 ± 0.00230 |
| Uterus with cervix | F | 0.751 ± 0.208 | 0.794 ± 0.468 | 0.788 ± 0.242 | 0.753 ± 0.313 |
| Brain | F | 1.972 ± 0.054 | 2.002 ± 0.046 | 1.985 ± 0.068 | 2.005 ± 0.082 |
| Thymus | F | 0.292 ± 0.045 | 0.288 ± 0.072 | 0.302 ± 0.083 | 0.303 ± 0.085 |
| Spleen | F | 0.533 ± 0.105 | 0.558 ± 0.094 | 0.546 ± 0.076 | 0.551 ± 0.057 |
| Ovariesc | F | 0.13452 ± 0.01885 | 0.13315 ± 0.02100 | 0.13582 ± 0.02156 | 0.13395 ± 0.01980 |
aVehicle control: Sterile water for injection (WFI).
bwith coagulating glands.
cwith oviducts.
Values are mean ± SD for 12 rats in each group.
* p < 0.05.
3.2.2.5. Macroscopic and microscopic examinations
No treatment-related macroscopic findings were noted in any of the groups at the scheduled necropsy following administration of the β-glucan extract preparation to rats. At terminal euthanasia, only one female from low-dose group showed focal mass in subcutaneous tissue (mammary gland). According to severity and incidence based on histopathological evaluation of this lesion (fibroadenoma), the finding was considered as spontaneous abnormality and not related to test article.
There were no treatment-related histopathological findings. The incidence and severity of lesions are summarized in Table 9. The histopathological observations in the high-dose group were considered to be spontaneous due to incidence, significance, and severity. These changes were observed across all groups and no dose-related response was noted. It was inferred that there were no pathological changes in the organs that could be attributed to H. erinaceus β-glucan extract preparation treatment. All findings observed were consistent with normal background lesions in clinically normal rats of the age and strains used in this study, and were considered spontaneous and/or incidental in nature and unrelated to the treatment.
Table 9.
Incidence and severity of histopathological findings following H. erinaceus β-glucan preparation treatment to rats.
|
Vehicle control |
High-dose (2000 mg/kg/day) |
||||
|---|---|---|---|---|---|
| Gender | M | F | M | F | |
| Histopathological findings | S2 | (N/N)1 | |||
| Thyroid | |||||
| Cyst, ultimobronchial, focal | 1 | 1/12 | 1/12 | 0/12 | 0/12 |
| Necrosis, focal | 1 | 1/12 | 0/12 | 0/12 | 0/12 |
| Lung | |||||
| Alveolar histocytosis, focal | 1 | 1/12 | 1/12 | 1/12 | 1/12 |
| Haemorrhage, acute, focal | 2 | 0/12 | 2/12 | 0/12 | 0/122 |
| Adrenals | |||||
| Vacuolar degeneration, cortex, foci | 1 | 1/12 | 0/12 | 0/12 | 0/12 |
| Heart (Aorta) | |||||
| Focal round cell collection | 2 | 0/12 | 1/12 | 0/12 | 0/12 |
| Harderian glands | |||||
| FRCC, stromal | 1 | 0/12 | 1/12 | 0/121 | 0/12 |
| Lymph nodes, mandibular | |||||
| Focal round cell collection | 1 | 0/12 | 1/12 | 0/12 | 1/12 |
| Thymus | |||||
| Congestion, focal | 1–2 | 1/12 | 1/12 | 0/12 | 0/12 |
| Kidneys | |||||
| Cyst, medulla, focal | 1–2 | 2/12 | 0/12 | 0/12 | 0/12 |
| Focal round cell collection, cortex | 1–2 | 1/12 | 0/12 | 1/12 | 0/12 |
| Congestion, foci, cortex | 1 | 0/12 | 0/12 | 1/12 | 0/12 |
| Mineralization, tubular lumen, foci | 1–2 | 1/12 | 2/12 | 0/12 | 1/12 |
| Liver | |||||
| Clear cell, portal zone, focal | 1–2 | 3/12 | 3/12 | 1/12 | 0/12 |
| Necrosis, portal zone, focal | 1 | 2/12 | 1/12 | 0/12 | 0/12 |
| Focal round cell collection, portal zone | 1 | 2/12 | 1/12 | 1/12 | 0/12 |
| Prostate | |||||
| FiRCC, stromal | 1–2 | 1/12 | – | 1/12 | – |
| Pancreas | |||||
| Focal round cell collection | 1 | 1/12 | 1/12 | 0/12 | 0/12 |
| Hyperplasia, acinar cell, foci | 1 | 1/12 | 0/12 | 0/12 | 0/12 |
| Spleen | |||||
| Hemangioma, focal | 1–2 | 1/12 | 2/12 | 0/12 | 0/12 |
FRCC – Focal round cells collection; FiRCC – Foci round cells collection.
Incidence rate – animal numbers of histopathologic findings / animal numbers of histopathologic examinations (N/N).
The severity grading scheme: 1. minimal (<10%), 2. mild (10–39%), 3. moderate (40–79%), 4. marked (80–100%).
4. Discussion
The results of present subchronic dose–response study in rats did not reveal adverse effects of H. erinaceus β-glucan extract preparation treatment, at dose levels up to 2000 mg/kg bw/day, as evaluated by general condition, appearance of the animals, growth, feed consumption, ophthalmoscopy, clinical pathology, organ weights and histopathology. There were a few statistically significant changes between rats treated with the H. erinaceus β-glucan extract preparation and controls, although these changes were not considered as adverse effects of the test article as discussed below. In this study, statistically significant variations were noted in some of the clinical pathology parameters in H. erinaceus β-glucan treated groups. For example, treatment with H. erinaceus β-glucan extract preparation revealed a significant increase in blood levels of eosinophil in the mid-dose male group (1000 mg/kg bw/day). Additionally, serum chemistry parameters revealed a significant increase in serum sodium levels in male rats receiving 1000 and 2000 mg/kg bw/day; serum albumin in male rats receiving 2000 mg/kg bw/day; and serum chloride in male rats treated with 1000 mg/kg bw/day. In female rats statistically significant decrease in serum levels of total protein in rats receiving 1000 mg/kg bw/day; increase in alkaline phosphatase in rats receiving 500 and 2000 mg/kg bw/day; and increase in serum sodium levels in rats receiving 500 and 1000 mg/kg bw/day was noted. These statistically significant changes observed in clinical pathology parameters following administration of H. erinaceus β-glucan extract preparation were considered as incidental and not related to the treatment, as they were either limited to one sex, lacked dose dependent response, were within the normal laboratory ranges, and/or were not supported by any other changes in related clinical parameters or histopathological observations.
Traditionally, edible mushrooms have been used in medical and health foods (Chen, 2012). Also, more recent studies have shown that β-glucans in different mushrooms have been provento be highly active and safe (Chen, 2018, Chen, 2011). Over the past decade, H. erinaceus has been made its way into many medical and health care products demonstrating beneficial effects to alleviate epigastric pain caused by chronic superficial gastritis, gastric ulcer, or atrophic gastritis (Ren et al., 2018). In addition to exhibiting antitumor and immunomodulatory activities (Wang, 2001); H. erinaceus extracts have shown to contain bioactive ingredients with potential to protect neuronal cells (He et al., 2017). Studies on the ingestion of whole mushroom or extracts of H. erinaceus have confirmed their exceptional safety, but there is still a lack of in-depth research on the H. erinaceus β-glucan (Lakshmanan et al., 2016). Many different mushroom β-glucans have been evaluated for their safety, including Ganoderma lucidum and Antrodia cinnamomea. In a 90-day study, conducted as per OECD guidelines, Chen et al. (Chen, 2018) investigated the safety of A. cinnamomea β-glucan. In that study, Sprague-Dawley rats were administered A. cinnamomea β-glucan via oral gavage at doses of 0, 500.1000 and 2000 mg/kg bw/day. The results did not reveal genotoxicity, mortality or adverse changes in clinical signs, body weight and ophthalmological examinations. Similarly, urinalysis, hematology, serum biochemistry parameters, necropsy and histopathological observations did not show any treatment related changes between the treatment and control groups. The investigators identified the no-observed-adverse-effect level (NOAEL) of A. cinnamomea β-glucan to be greater than 2000 mg/kg bw/day. Although Chen et al. (Chen, 2018) used A. cinnamomea β-glucan, the findings are consistent with the results of the present study with H. erinaceus β-glucan extract preparation.
Several regulatory agencies and authoritative bodies around the world have reviewed the safety of β-glucans from different sources and permitted its use as dietary supplement and food additive, (FDA, 2007, EFSA, 2011, FDA, 2012, Chen, 2011). The safety and efficacy of β-glucans derived from barley, yeast, mushroom, etc., and from different sources has been investigated in several animal experimental studies, as well as in human clinical trials. These studies do not raise any new safety concerns (Chen, 2012, Chen, 2011, Jonker et al., 2010, Jonker et al., 2010, Babicek, 2007, Delaney et al., 2003). Chen et al. (Chen, 2011) investigated the subchronic toxicity and potential genotoxic effects of Ganoderma lucidum β-glucans These studies revealed that G. lucidum β-glucan was not genotoxic and the results of the subchronic toxicity study in rats identified a NOAEL of 2000 mg/kg bw/day, the highest dose tested. The results of present study in which oral gavage administration of H. erinaceus β-glucan extract preparation to rats at dose levels of up to 2000 mg/kg bw/day did not cause any significant treatment-related adverse effects further support the previous observation on the safety of β-glucan from different sources. The intake level of β-glucan from the present rat study is approximately 40-fold higher than that recommended for lowering blood cholesterol (at least 3 g/person/day, corresponding to 50 mg/kg bw/day for a person weighing 60 kg) (FDA, 2005). The findings from the present study suggest that intake of H. erinaceus β-glucan extract preparation by humans for health benefits are unlikely to cause adverse effects.
In retrospect, although this study confirmed the safety of the use of β-glucan extract preparation, in the future, there is availability of complex in vitro platforms and in silico models that could be used to support an evaluation of repeat system toxicity. The European Partnership for Alternative Approaches to Animal Testing (EPAA) recently organized a workshop whose goals were to frame strategic research essentials and follow up actions for pioneering innovative approaches for repeated-dose systemic toxicity (Mahony et al., 2020). This we believe is a beginning and would allow for the use of newer testing methods for food ingredients to support safety assessment from similar natural sources.
In summary, the results of present subchronic toxicity study suggest that oral administration of the H. erinaceus β-glucan extract preparation at levels up to 2000 mg/kg bw/day does not cause adverse effects in male and female rats. Based on the results of this study, the no-observed effect level (NOAEL) of the H. erinaceus β-glucan extract preparation was found to be 2000 mg/kg bw/day, the highest dose tested. The findings from present investigations also suggest that H. erinaceus β-glucan extract preparation is unlikely to cause any genotoxic effects.
CRediT authorship contribution statement
S.N. Chen: Conceptualization, Methodology, Investigation, Validation, Supervision, Writing – review & editing. C.S. Chang: Investigation, Data curation, Resources, Writing – original draft, Project administration. M.F. Yang: Investigation, Data curation, Software, Formal analysis. S. Chen: Conceptualization, Methodology, Writing – review & editing, Visualization, Funding acquisition. M. Soni: Conceptualization, Methodology, Writing – review & editing, Visualization. B. Mahadevan: Writing – review & editing, Visualization.
Declaration of Competing Interest
S. N. Chen, C.S. Chang, and M.F. Yang work as independent Consulting Biologists for the studies. S. Chen is currently employed by Glytheron Inc. (Irvine, CA, USA) that funded these studies. M. G. Soni & B. Mahadevan work as independent Consulting Toxicologists and assisted in the preparation of this manuscript.
Acknowledgement
Super Beta Glucan Inc. (Irvine, CA, USA) for supplying standardized Lion’s Mane (Hericium erinaceus) mushroom β-glucan extract preparation for use in these studies.
References
- Babicek K., et al. Toxicological assessment of a particulate yeast (1,3/1,6)-beta-D-glucan in rats. Food Chem. Toxicol. 2007;45(9):1719–1730. doi: 10.1016/j.fct.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Chen S.N., et al. Safety assessment of mushroom beta-glucan: subchronic toxicity in rodents and mutagenicity studies. Food Chem. Toxicol. 2011;49(11):2890–2898. doi: 10.1016/j.fct.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., et al. Ethanol extracts of fruiting bodies of Hericium erinaceu suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid. Based Complement Alternat. Med. 2012;2012 doi: 10.1155/2012/378415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.N., et al. Subchronic toxicity and genotoxicity studies of Antrodia mushroom beta-glucan preparation. Regul. Toxicol. Pharm. 2018;92:429–438. doi: 10.1016/j.yrtph.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Delaney B., Carlson T., Frazer S., Zheng T., Hess R., Ostergren K., Kierzek K., Haworth J., Knutson N., Junker K., Jonker D. Evaluation of the toxicity of concentrated barley β-glucan in a 28-day feeding study in Wistar rats. Food Chem. Toxicol. 2003;41(4):477–487. doi: 10.1016/s0278-6915(02)00298-3. [DOI] [PubMed] [Google Scholar]
- Driscoll M., et al. Therapeutic potential of various beta-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol. Ther. 2009;8(3):218–225. doi: 10.4161/cbt.8.3.7337. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on dietetic products, nutrition and allergies (NDA); scientific opinion on the safety of “yeast beta-glucans” as a novel food ingredient. EFSA J. 2011;9(5):2137–2159. [Google Scholar]
- FDA, Department of Health and Human Services, Food labeling: health claims; oats and coronary health disease. Fed Reg, 1997. 62: p. 3584-3601.
- FDA, GRN 413. Beta Glucans Derived from Ganoderma Lucidum Mycelium. GRAS Notification by Super Beta Glucan Inc. 2012.
- 10.FDA, Food labeling: Soluble dietary fibre from certain foods and coronary heart disease. Federal Register, 2005. 70(246): p. 76150-76162.
- Fu S.G., Yoon Y., Bazemore R. Aroma-active components in fermented bamboo shoots. J. Agric. Food Chem. 2002;50(3):549–554. doi: 10.1021/jf010883t. [DOI] [PubMed] [Google Scholar]
- He X., Wang X., Fang J., Chang Y.u., Ning N., Guo H., Huang L., Huang X., Zhao Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion's Mane) mushroom: A review. Int. J. Biol. Macromol. 2017;97:228–237. doi: 10.1016/j.ijbiomac.2017.01.040. [DOI] [PubMed] [Google Scholar]
- Hetland G., Tangen J.M., Mahmood F., Mirlashari M.R., Nissen-Meyer L.S.H., Nentwich I., Therkelsen S.P., Tjønnfjord G.E., Johnson E. Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies. Nutrients. 2020;12(5):1339. doi: 10.3390/nu12051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker D., Hasselwander O., Tervilä-Wilo A., Tenning P.P. 28-Day oral toxicity study in rats with high purity barley beta-glucan (Glucagel) Food Chem. Toxicol. 2010;48(1):422–428. doi: 10.1016/j.fct.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Jonker D., Kuper C.F., Maquet V., Nollevaux G., Gautier S. Subchronic (13-week) oral toxicity study in rats with fungal chitin-glucan from Aspergillus niger. Food Chem. Toxicol. 2010;48(10):2695–2701. doi: 10.1016/j.fct.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Kawagishi H., et al. Erinapyrone-a and Erinapyrone-B from the Cultured Mycelia of Hericium-Erinaceum. Chem. Lett. 1992;12:2475–2476. [Google Scholar]
- Kawagishi H., Mori H., Uno A., Kimura A., Chiba S. A sialic acid-binding lectin from the mushroom Hericium erinaceum. FEBS Lett. 1994;340(1-2):56–58. doi: 10.1016/0014-5793(94)80172-x. [DOI] [PubMed] [Google Scholar]
- Kawagishi H., Murakami H., Sakai S., Inoue S. Carnitine-esters from the mushroom Suillus laricinus. Phytochemistry. 2006;67(24):2676–2680. doi: 10.1016/j.phytochem.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Kenmoku H., Oozone T., Sugai T., Sassa T. Mass production of pure gibberellin A1 by Phaeosphaeria sp. L487 and the fungal preparation of [U-13C]gibberellin A1. Biosci. Biotechnol. Biochem. 2001;65(9):2095–2097. doi: 10.1271/bbb.65.2095. [DOI] [PubMed] [Google Scholar]
- Kim J., et al. (1–3)(1–6)-beta-glucan-enriched materials from Lentinus edodes mushroom as a high-fibre and low-calorie flour substitute for baked foods. J. Sci. Food Agric. 2011;91(10):1915–1919. doi: 10.1002/jsfa.4409. [DOI] [PubMed] [Google Scholar]
- Lakshmanan H., Raman J., David P., Wong K.H., Naidu M., Sabaratnam V. Haematological, biochemical and histopathological aspects of Hericium erinaceus ingestion in a rodent model: A sub-chronic toxicological assessment. J. Ethnopharmacol. 2016;194:1051–1059. doi: 10.1016/j.jep.2016.10.084. [DOI] [PubMed] [Google Scholar]
- Lazaridou A., Biliaderis C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007;46(2):101–118. [Google Scholar]
- Li I.C., Lee L.Y., Chen Y.J., Chou M.Y., Wang M.F., Chen W.P., Chen Y.P., Chen C.C. Erinacine A-enriched Hericium erinaceus mycelia promotes longevity in Drosophila melanogaster and aged mice. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217226. e0217226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yu K., Li F., Xu K., Li J., He S., Cao S., Tan G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014;153(2):521–530. doi: 10.1016/j.jep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Mahony C., Ashton R.S., Birk B., Boobis A.R., Cull T., Daston G.P., Ewart L., Knudsen T.B., Manou I., Maurer-Stroh S., Margiotta-Casaluci L., Müller B.P., Nordlund P., Roberts R.A., Steger-Hartmann T., Vandenbossche E., Viant M.R., Vinken M., Whelan M., Zvonimir Z., Cronin M.T.D. New ideas for non-animal approaches to predict repeated-dose systemic toxicity: Report from an EPAA Blue Sky Workshop. Regul. Toxicol. Pharm. 2020;114:104668. doi: 10.1016/j.yrtph.2020.104668. [DOI] [PubMed] [Google Scholar]
- Meena D.K., Das P., Kumar S., Mandal S.C., Prusty A.K., Singh S.K., Akhtar M.S., Behera B.K., Kumar K., Pal A.K., Mukherjee S.C. Beta-glucan: an ideal immunostimulant in aquaculture (a review) Fish Physiol. Biochem. 2013;39(3):431–457. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- Mori K., Inatomi S., Ouchi K., Azumi Y., Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother. Res. 2009;23(3):367–372. doi: 10.1002/ptr.2634. [DOI] [PubMed] [Google Scholar]
- Nagai K., Chiba A., Nishino T., Kubota T., Kawagishi H. Dilinoleoyl-phosphatidylethanolamine from Hericium erinaceum protects against ER stress-dependent Neuro2a cell death via protein kinase C pathway. J. Nutr. Biochem. 2006;17(8):525–530. doi: 10.1016/j.jnutbio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Patel S., Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3. Biotech. 2012;2(1):1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Geng Y., Du Y., Wang L., Lu Z.-M., Xu H.-Y., Xu G.-H., Shi J.-S., Xu Z.-H. Polysaccharide of Hericium Erinaceus Attenuates Colitis in C57BL/6 Mice via Regulation of Oxidative Stress, Inflammation-Related Signaling Pathways and Modulating the Composition of the Gut Microbiota. J. Nutr. Biochem. 2018;57:67–76. doi: 10.1016/j.jnutbio.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Ren L., Perera C., Hemar Y. Antitumor activity of mushroom polysaccharides: a review. Food Funct. 2012;3(11):1118–1130. doi: 10.1039/c2fo10279j. [DOI] [PubMed] [Google Scholar]
- Su C.-H., Lai M.-N., Lin C.-C., Ng L.-T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl. Microbiol. Biotechnol. 2016;100(10):4385–4393. doi: 10.1007/s00253-015-7260-3. [DOI] [PubMed] [Google Scholar]
- Thongbai B., Rapior S., Hyde K.D., Wittstein K., Stadler M. Hericium erinaceus, an amazing medicinal mushroom. Mycological Progress. 2015;14(10) [Google Scholar]
- Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: Improving human health and promoting quality life. Inter. J. Microbiol. 2015;2015:1–14. doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C., et al. Antitumor and immunoenhancing activities of polysaccharide from culture broth of Hericium spp. Kaohsiung J. Med. Sci. 2001;17(9):461–467. [PubMed] [Google Scholar]
- Wu S.-H., Kirk P.M., Redhead S.A., Stalpers J.A., Dai Y.-C., Norvell L.L., Yang Z.-L., Ryvarden L., Su C.-H., Li Y.u., Zhuang W.-Y., Yao Y.-J., Chen C.-J., Chen L.-C., Yu Z.-H., Wang X.-C. Resolution of the nomenclature for niu-chang-chih (Taiwanofungus camphoratus), an important medicinal polypore. Taxon. 2012;61(6):1305–1310. [Google Scholar]
- Zhang M., Cui S.W., Cheung P.C.K., Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007;18(1):4–19. [Google Scholar]
- Zhang C.-C., Yin X., Cao C.-Y., Wei J., Zhang Q., Gao J.-M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015;25(22):5078–5082. doi: 10.1016/j.bmcl.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Zhao J., Cheung P.C. Fermentation of beta-glucans derived from different sources by bifidobacteria: evaluation of their bifidogenic effect. J. Agric. Food Chem. 2011;59(11):5986–5992. doi: 10.1021/jf200621y. [DOI] [PubMed] [Google Scholar]