Abstract
Background
Triple negative Breast tumor (TNBC) is an aggressive tumor with sparse data worldwide.
Methods
We analyzed non-metastatic TNBC from 2013 to 2019 for demographics, practice patterns, and survival by the Kaplan Meir method. Prognostic factors for OS and DFS were evaluated using Cox Proportional Hazard model estimator for univariate and multivariable analysis after checking for collinearity among the variables.
Results
There were 1297 patients with median age of 38 years; 41 (33.3%) among 123 tested were BRCA-positives. Among these 593 (45.7%) had stage III disease, 1279 (98.6%) were grade III, 165 (13.0%) had peri-nodal extension (PNE), 212 (16.0%) lympho-vascular invasion (LVI), and 21 (1.6%) were metaplastic; 1256 (96.8%) received chemotherapy including 820 (63.2%) neoadjuvant with 306 (40.0%) pCR. Grade ≥3 toxicities occurred in 155 (12.4%) including two deaths and 3 s-primaries. 1234 (95.2%) underwent surgery [722 (55.7%) breast conservations] and 1034 (79.7%) received radiotherapy.
At a median follow-up of 54 months, median disease-free (DFS) was 92.2 months and overall survival (OS) was not reached. 5-year estimated DFS and OS was 65.9% and 80.3%. There were 259 (20.0%) failures; predominantly distant (204, 15.7%) - lung (51%), liver (31.8%).
In multivariate analysis presence of LVI (HR-2.00, p-0.003), PNE (HR-2.09 p-0.003), older age (HR-1.03, p-0.002) and stage III disease (HR-4.89, p-0.027), were associated with poor OS.
Conclusion
Relatively large contemporary data of non-metastatic TNBC confirms aggressive biology and predominant advanced stage presentation which adversely affects outcomes. The data strongly indicate the unmet need for early detection to optimize care.
Keywords: Triple negative breast cancer (TNBC), Non-metastatic, Low-middle income countries (LMIC)
Highlights
-
•
TNBC affects young women and majority are locally advanced at presentation.
-
•
Multimodal management achieves favorable survival with limited resources.
-
•
Most relapses are at distant visceral sites, outcomes dismal after relapse.
-
•
Challenges in implementing resource intensive interventions.
1. Introduction
Triple negative breast cancers (TNBC) constitute 12–17% of total breast cancers, however, the incidence is up to 30% in low and middle-income countries (LMIC) [[1], [2], [3]]. TNBC patients are younger and are known to have aggressive biology and a propensity for early visceral metastases leading to poor survival outcomes [4,5].
Chemotherapy has been the cornerstone for the management of TNBC patients. Recent studies show that immunotherapy and PARP inhibitors have some efficacy, but their prohibitive cost limits its utility among patients from LMICs [6]. Treating a relatively larger proportion of TNBC patients with resource constraints pose unique challenges in LMICs which are underreported in literature. Majority of the patients hail from socio-economic underprivileged background and lack prompt access to tertiary health care. Financial hardships, limited insurance coverage compounded with lack of awareness leads to delayed presentation, often in advanced stages with resultant compromised outcomes. Compliance to the intended treatment is also an important issue due to several challenges and treatment abandonment is prevalent specially in advanced stages. This is in contrast to high income countries where insurance penetration is wide and patients access healthcare in earlier stages of the disease and comply with the intended treatment. [7,8] Thus, our study aimed to report the demographics, practice patterns, and survival outcomes for TNBC patients from LMICs in a real-life setting.
2. Methods
The study included consecutive, non-metastatic TNBC patients registered between January 1, 2013, and December 31, 2019, and received at least partial treatment at our institute. The data was captured from the electronic medical records (EMR) and patient files; follow-up was updated telephonically when required. Tumors were staged using the AJCC 7th edition and treatment decisions were made by a multidisciplinary team (MDT). A prospective trial of NACT in TNBC was actively recruiting patients during the study period and 254 patients of this trial were included in this study.
Pathological complete response (pCR) was defined as an absence of residual invasive cancer in the resected breast and regional nodes. We also describe unique subgroups among TNBCs of young breast cancer (YBC) aged <40 years and breast cancer diagnosed during pregnancy (PrBC) or in the first postpartum year (PPBC) [9,10].
3. Subpart of methods
Descriptive statistics were used for reporting patient, tumor, and treatment-related characteristics. Limits of normal blood parameters were defined as per the institutional cut-offs and are as follows; hemoglobin (12-15) grams/dl, serum albumin (3.5–5.2) grams/dl, and elevated serum alkaline phosphatase >120U/L.
Survival was analyzed using the Kaplan Meir method and compared with the log-rank test. The disease-free survival (DFS) was appraised from the date of diagnosis to the date of relapse of breast cancer (local or distant), or date of diagnosis of contralateral breast cancer, second primary cancer, or death due to any cause. Similarly, the overall survival (OS) was defined as the duration from the date of diagnosis to the date of death from any cause, and patients were censored at their last follow-up [11]. Every effort was made to establish contact with patients not coming to the hospital for their follow-up. Data cut-off time for analysis of this study was Jan 2022. Patients who couldn't be contacted and were not seen in the last one and half year were deemed as lost to follow-up. Collinearity was checked for all the variables with OS and DFS before analysis. Prognostic factors for OS and DFS were evaluated using Cox proportional hazard model estimator both for univariate analysis and multivariate analysis. If the variables had a significant hazard ratio for OS or DFS, they were checked for proportional hazard assumption based on Schoenfeld residuals (ph-test), and only if the test reported not-significance i.e. the variable has met the assumption, they were included in the analysis. The variables which had a significant hazard ratio in the univariate analysis were only included for the multivariate analysis (MVA). Statistical analysis was accomplished by operating SPSS (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp) and two-sided p-value of ≤.05 was taken as significant.
4. Results
We studied 1297 consecutive patients with a median age of 38 years [interquartile range (IQR) 33–42 years]. Table 1a showing baseline characteristics which indicates that a large majority of our patients belonged to the YBC (n = 781, 60%) and among them, a few had PrBC or PPBC (n = 16, 1.2%). Despite the young age (n = 781) and family history of cancer (n = 182, 14%), only 123 patients (9.1%) underwent germline BRCA 1/2 testing. Germline pathogenic variants were detected in 42/123 (34%) patients. Nearly half of the study patients (45.7%) had stage III disease. Staging information was missing for 4.5% of the patients. Androgen receptor testing by immunohistochemistry is not standard institutional practice for all TNBCs, thus it was available for only 655 (50.5%) patients.
Table 1a.
Baseline characteristics.
| Variable | Frequency n = 1297 (%) |
|---|---|
| Median age (IQR) | 38 years (33–42 years) |
| Young breast cancer (<40 years) | 781 (60%) (95%CI, 57.5–62.9) |
| Pregnancy associated breast cancer | 16 (1.2%) (95%CI, 0.7–2.0) |
| Family history of cancer | 182 (14%) (95%CI, 12.2–16.0) |
| Known hereditary syndromes | 41 (3.2%) (95%CI, 2.3–4.3) |
| BRCA positive | 40 (3.1%) (95%CI, 2.2–4.2) |
| Other mutations | 1 (<1%) |
| AJCC Stage (7th edition) | |
|
39 (3.0%) |
|
606 (46.7%) |
|
593 (45.7%) |
|
59 (4.5%) |
| Staging | |
|
570 (44.0%) |
|
668 (51.5%) |
|
59 (4.5%) |
| Pathological details | |
| Grade of Tumor | |
|
1 (<1%) |
|
17 (1.3%) |
|
1279 (98.6%) |
| Histology | |
|
1275 (98.3%) |
|
21 (1.6%) |
|
1 (<1%) |
| Androgen receptor IHC | |
|
7 (<1%) |
|
648 (50%) |
|
642 (49.5%) |
| Laboratory parameters | |
|
11.8 g/dL (IQR 11.0–12.6) |
|
669 (51.6%) |
|
122 (9.4%) |
|
229 (17.7%) |
| Post surgical pathological details | |
|
231 (17.8%) (95%CI, 15.8–19.8) |
|
522 (40.2%) (95%CI, 37.5–42.9) |
|
191 (14.7) (95%CI, 12.8–16.6) |
|
416 (32.1%) (95%CI, 29.5–34.7) |
|
275 (21.2%) (95%CI, 19.0–23.5) |
|
82 (6.3%) (95%CI, 5.1–7.8) |
|
59 (4.5%) (95%CI, 3.5–5.8) |
|
212 (16.0%) (95%CI, 14.0–18.1) |
|
165 (13.0%) (95%CI, 11.2–15.0) |
|
306 (40%a) |
|
63 (4.8%) |
IQR – Inter-quartile range, CI- Confidence interval, AJCC -American Joint Committee on Cancer, LVI- Lympho-vascular space invasion, PNE-Perinodal extension, pCR-pathological complete response.
Out of patients who underwent surgery after neoadjuvant chemotherapy (n = 766).
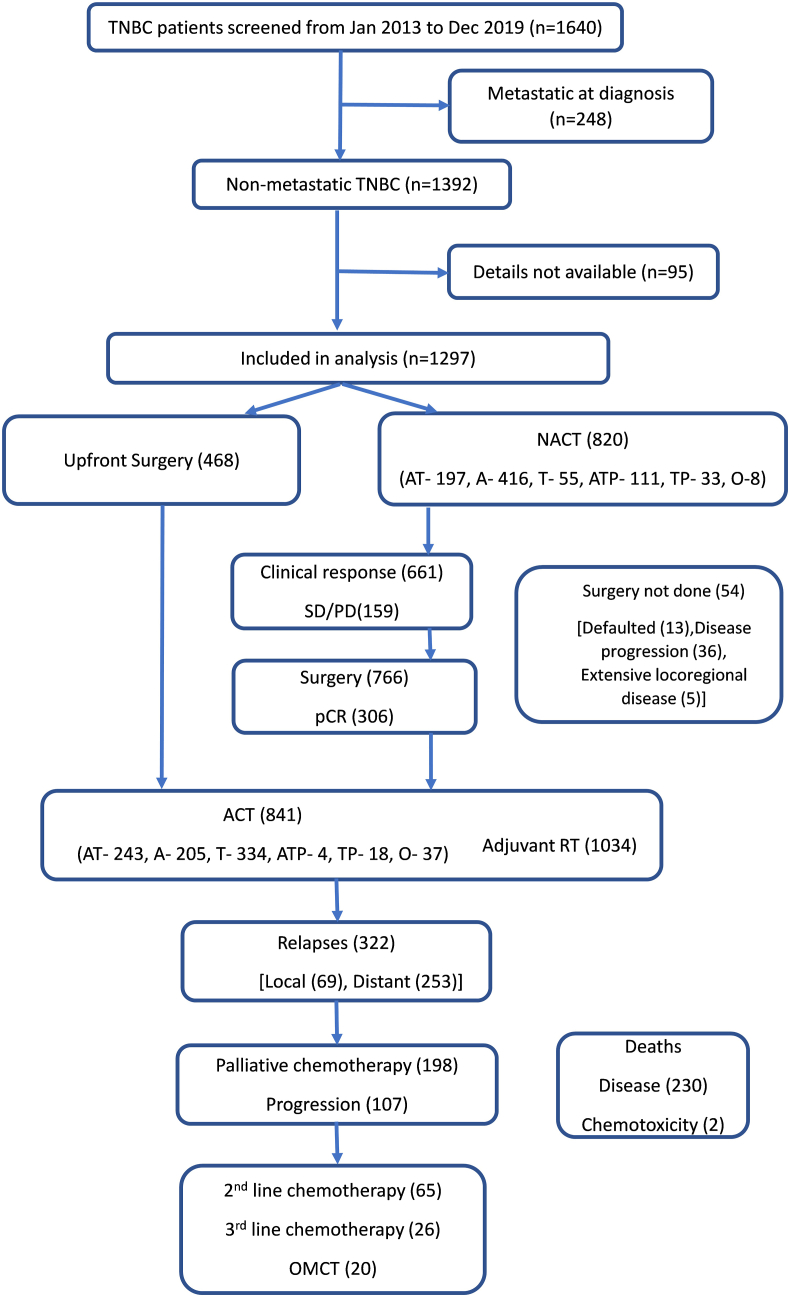
Treatment Characteristics:Fig. 1 show details of patient selection and their treatments within our study group. Key treatment characteristics are summarized in Table 1b. 1256 (96.8% of all) patients received chemotherapy where the entire chemotherapy was delivered as neoadjuvant (NACT) in 415 patients, and as adjuvant (ACT) in 436 patients whereas surgery was sandwiched between NACT and ACT for 405 patients. Of the 820 (65.3%) patients that received NACT, 766 underwent surgery and 306 (40%) achieved a pCR. Higher proportion of patients (excluding NACT trial patients) received NACT overall (59.2% vs 51.4%; p value - 0.013) and for stage II tumors (43.1% vs 31.9%; p value - 0.010) during the study period 2017 to 2019 vs 2013 to 2016 (Table 1c). The details of various chemotherapy regimens used in NACT and ACT are summarized in Table 1b. Anthracyclines (A) or Taxanes (T) were used as chemotherapy in 1176 (93.6%) and 995 (79.2%) patients respectively. Platinum agents were added to chemotherapy in 179 (14.2%) patients, predominantly as a part of an ongoing trial. Chemotherapy was well tolerated and grade ≥3 chemotherapy toxicities were observed in 155 (12.4%) patients (Table 2a). There were two chemotherapy-related deaths (0.15%) due to septic shock secondary to febrile neutropenia during adjuvant anthracycline-based therapy. Data for long term toxicity like cardiac toxicity was not available for analysis.
Fig. 1.
CONSORT Diagram Showing the patient inclusion, treatment selection and outcomes in our contemporary cohort NACT- Neoadjuvant chemotherapy, SD-stable disease, PD- Progressive disease, pCR-pathological complete response, RT- Radiotherapy, ACT- Adjuvant chemotherapy, OMCT- Oral metronomic chemotherapy, AT- Anthracyclines and taxanes, A- Anthracycline alone, T- Taxane alone, ATP- Anthracycline, taxane and platinum,TP- Taxane and platinum, O- Other.
Table 1b.
Treatment characteristics.
| Treatment attribute | N = 1297 (%) |
|---|---|
| Surgery | |
|
468 (36.1%) |
|
766 (59.1%) |
|
63 (4.8%) |
| Type of surgery | |
|
722 (55.7%) |
|
512 (39.5%) |
|
63 (4.8%) |
| Radiotherapy | |
|
1034 (79.7%) |
|
263 (20.3%) |
| Chemotherapy | |
|
415 (32.0%) |
|
436 (33.6%) |
|
405 (31.2%) |
|
41 (3.2%) |
| Chemotherapy regimen | |
| NACT | N = 820 (100%) |
|
197 (24.0%) |
|
111 (13.5%) |
|
416 (50.7%) |
|
94 (11.5%) |
|
2 (0.2%) |
| ACT | N=841 (100%) |
|
243 (28.9%) |
|
4 (0.47%) |
|
205 (24.4%) |
|
359 (42.7%) |
|
30 (3.6%) |
NACT-Neoadjuvant chemotherapy, BCS-Breast conservation surgery, MRM-Modified radical mastectomy, RT-Radiotherapy.
Table 1c.
Usage of NACT in routine practicea.
| Group | Year 2013–2016 (%) | Year 2017–2019 (%) | P value |
|---|---|---|---|
| Overall (n = 1043) | 284/553 (51.4%) | 290/490 (59.2%) | 0.013 |
| Stage I (n = 37) | 2/19 (10.5%) | 7/18 (38.9%) | 0.062 |
| Stage II (n = 523) | 95/298 (31.9%) | 97/225 (43.1%) | 0.010 |
| Stage III (n = 426) | 183/221 (82.8%) | 176/205 (85.9%) | 0.426 |
| Stage unknown (n = 57) | 4/15 (26.7%) | 10/42 (23.8%) | 1.000 |
254 patients of NACT trial excluded.
Table 2a.
Chemotherapy related grade 3 or higher adverse events.
| Adverse events | Frequency n = 1256 (%) |
|---|---|
| Hematological | |
| Anemia | 18 (1.4%) |
| Neutropenia | 101 (8.0%) |
| Thrombocytopenia | 10 (<1%) |
| Non – hematological | |
| Febrile Neutropenia | 49 (3.9%) |
| Infection | |
| Pneumonia | 4 (<1%) |
| Varicella | 2 (<1%) |
| Otitis media | 1 (<1%) |
| Paclitaxel hypersensitivity | 1 (<1%) |
| Peripheral neuropathy | 8 (<1%) |
| AST/ALT elevation | 2 (<1%) |
| Diarrhea | 4 (<1%) |
| Mucositis | 4 (<1%) |
| Acute kidney injury | 2 (<1%) |
| Reduced ejection fraction | 2 (<1%) |
AST- Aspartate aminotransferase, ALT- Alanine aminotransferase.
Patients underwent surgery as appropriate for stage, where 468 (36.1%) were operated upfront and 766 (59.1%) after NACT. Breast conservation (BCS) was possible in 55.7% (n = 722) patients and only 4 of them had microscopic margin positivity needing a mastectomy (n = 1) or a margin revision (n = 3). In total, 820 patients (63.2%) received a median of 4 (1-8) cycles of NACT and 80.6% (n = 661) of those experienced clinical complete (n = 287, 35%) or partial response (n = 374, 45.6%) whereas 15% (n = 123) had stable disease. Among 766 patients who had undergone surgery after NACT, 306 (40.0%) were found to have pCR. Only 79.7% (n = 1034) patients received loco-regional radiation therapy (40–60Gy). Overall, 65 (5%) patients could not complete the planned treatment due to non-compliance (n = 42), intolerance (n = 16) and disease progression (n = 7).
Familial and hereditary cancers: Out of the 133 patients referred to the cancer genetics clinic, 123 (92.4%) agreed to genetic testing after genetic counselling. A germline pathogenic variant was found in 41 patients (33.3% of all tested and 3% of the total study population) mostly in BRCA1 (n = 37) and BRCA2 (n = 3). The uptake of prophylactic contralateral mastectomy (n = 7, 17.0%) and risk-reducing bilateral salpingo-oophorectomy (n = 6, 14.6%) was low. Most of them opted for personalized intensive surveillance during follow-up. Among BRCA positive patients, 12 patients underwent surgery after NACT and 66.7% (n = 8) achieved pCR.
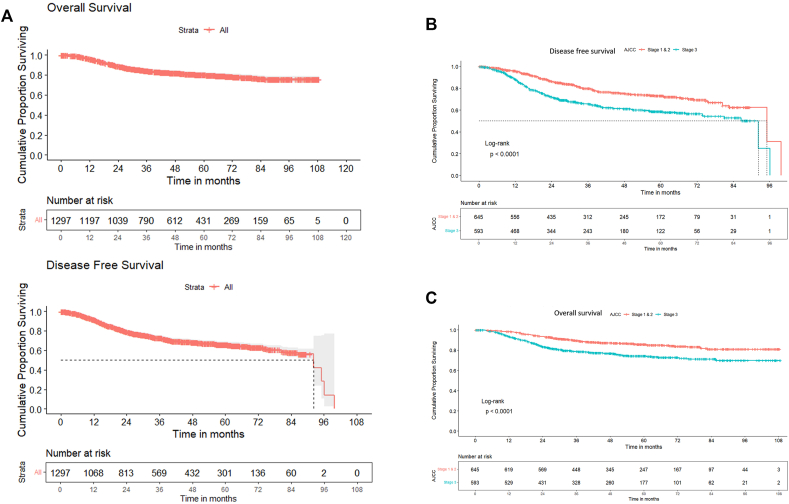
Survival outcomes: At 54 (95% CI 51.6–56.4) months of median follow up, 211 (16.3%) patients were lost to follow up. The median DFS was 92.2 (95%CI 82.5–102) months and 3 and 5-year DFS were 72.7% (95% CI 70.0%–75.3%) and 65.9% (95% CI 62.7%–68.9%) respectively. The median OS was not reached and the 3 and 5-year OS were 84.3% (95% CI 82%–86.2%) and 80.3% (95% CI 77.7%–82.6%) respectively as shown in Fig. 2a. Table 2b shows the stage-wise DFS and OS. Among the patients who had undergone surgery after NACT (n = 766), the 5-year DFS and OS for patient not achieving pCR (50.4%; 95%CI 44.7%–56.1% and 70.4%; 95%CI 65.5%–75.3%) was significantly worse than those achieving pCR (81.2%; 95%CI 76.3%–86.1%; p < .0001 and 90.1%; 95%CI 86.6%–93.6%; p < .0001) respectively. BRCA positive patients had significantly longer 5 years OS [(95.0%, 95%CI - 88.3 -100) vs (79.8%, 95% CI - 77.3-82.3) p value = .022] as compared to BRCA negative patients.
Fig. 2.
A: Overall Cohort (N = 1297): Disease-free and Overall Survival Curves Kaplan-Meier survival curves demonstrating flattening of survival curves after 3 years suggesting the early relapse pattern in TNBCs, DFS- Disease free survival, OS – Overall survival Fig. 2B Overall cohort (n = 1254): Stage wise Disease-free Survival Curves Kaplan-Meier survival curves demonstrating that outcomes are superior in early stages in comparison to higher stage (III); p < .0001 Fig. 2C Overall cohort (n = 1254): stage wise Overall Survival curves. Kaplan-Meier survival curves demonstrating that outcomes are superior in early stages in comparison to higher stage (III); p < .0001.
Table 2b.
Stage wise disease free survival and overall survival at 5 years.
| Stage (n) | 5-year DFS | 95% CI for DFS | 5-year OS | 95% CI for OS |
|---|---|---|---|---|
| I and II (645) | 73.0% | 69.8%–76.2% | 85.9% | 82.9%–88.8% |
| III (593) | 58.6% | 53.9%–63.3% | 70.4% | 73.9%–78.2% |
DFS – Disease free survival, OS – Overall survival, CI – Confidence interval.
Patterns of relapse: During the follow-up 259 patients (20.0%) had disease recurrence; the recurrence was local in 55 (21.2%), distant in 143 (55.2%) and both (local and distant) in 61 (23.5%) patients. The distant failures were predominantly in lung 104 (51%), liver 65 (31.8%), bone 62 (30.4%), brain 50 (24.5%) and other sites 72 (35.3%). Multi-organ metastases were present in 91 (44.6%) patients. Three patients developed second primary cancers (ovarian cancer, ALK-positive NSCLC, and esophageal cancer) during follow-up.
Prognostic markers: Factors found significant for DFS and OS in univariate analysis are shown in supplementary table 1. Older age, stage III disease, presence of LVI, and PNE were among the independent prognostic factors indicating poor DFS and OS in multivariate analysis (Table 3).
Table 3.
Multivariable analysis for variables affecting disease free survival and overall survival.
| Variable | HR (95% CI) for DFS | P value | HR (95% CI) OS | P value |
|---|---|---|---|---|
| Age (continuous) | – | – | 1.02 (1.00–1.03) | 0.016 |
| Stage III vs Stage I & II | 1.64 (1.32–2.05) | <0.001 | 1.89 (1.38–2.40) | <0.001 |
| LVI present | 2.08 (1.56–2.76) | <0.001 | 2.13 (1.50–3.01) | <0.001 |
| PNE present | 2.21 (1.65–2.97) | <0.001 | 2.29 (1.59–3.29) | <0.001 |
HR – Hazards ratio, CI = Confidence interval, DFS- Disease free survival, OS – Overall survival, LVI- Lymphovascular space invasion, PNE – Perinodal extension, NACT- Neoadjuvant chemotherapy.
5. Discussion
To the best of our knowledge, this is the largest study of non-metastatic TNBCs from a single tertiary care centre in India and the third largest in the world [[12], [13], [14], [15], [16], [17], [18], [19]]. These outcomes will establish a benchmark against which future studies from India and other low-middle income countries can be planned.
Consistent with the published literature from India, our study patients were younger (median age 38 years) with predominantly high grade (98.6%), and stage III disease (45.7%) [14,15] possibly due to limited access to cancer care [16,17]. Contrary to our findings, a larger TNBC series by Li et al. (n = 2649) showed a comparatively smaller proportion of stage-III patients or grade-III disease (26.6% and 34.4% respectively) [18]. Few patients in our study had uncommon histology (n = 22) mostly metaplastic cancer. Other studies have also shown predominant TNBC subtype among uncommon histology [20,21].
The higher BCS rates (n = 722, 55.7%) in our contemporary cohort compared to the other Indian studies [14,22,23] may be a reflection of systematic training of surgeons, improved access to radiotherapy because of increased capacity, and from using moderate hypofractionation [24,25]. However, the BCS rates may vary across centres due to differences in surgical expertise, RT services, and patients’ preferences.
Interestingly, we observed an increase in the proportion of patients undergoing NACT as a routine practice during the later part of the study (2017–2019) than in the first half (2013–2016). This is possibly due to lowered threshold to start NACT among biologically aggressive TNBC subtypes. Notably, NACT helps in gauging in vivo sensitivity and allows less radical surgery [26]. We observed grade-3 or higher chemotherapy induced neutropenia, febrile neutropenia, and peripheral neuropathy of 8%, 3.9% and 1% which were comparable to 12%, 5%, and 7% respectively in the CALGB trial 9741 with similar regimens [27]. The lower rates of toxicities observed in our study compared to others may be partly due to suboptimal documentation and underreporting observed in busy clinics [28,29]. Additionally, there are significant concerns regarding quality-of-life issues including concerns regarding chemo induced alopecia especially in these young women [10,28]. Nevertheless, the grade-5 or life-threatening toxicities are always documented and were found to be low, perhaps due to a relatively younger population.
Though a few patients in our study received platinum based NACT as part of on-going trial, this was not routine practice. Incorporating platinum based chemotherapy in NACT leads to higher pCR rates in TNBC which translates into survival advantage [29,30]. Besides, there have been emerging data of adjuvant capecitabine in TNBC patients with residual invasive cancer post NACT. The treatment intensification provides survival advantage in this unfavourable subset of patient albeit at cost of increased toxicity [31,32]. However, patients in LMIC are often nutritionally challenged and higher toxicity with addition of platinum or capecitabine may compromise the intensity of therapy and overall compliance with treatment. Thus, this approach requires careful patient selection to improve the outcome [33].
Recent years have witnessed advent of immunotherapy in early TNBC, it has shown better pCR rates and improvement in DFS [34,35]. Similarly early data has shown DFS benefit of adjuvant PARP inhibitor in germline BRCA mutated breast cancer [36]. Despite the benefit, incorporation of these interventions remains a challenge in LMIC. As majority of the patients are from underprivileged background and outside insurance ambit, adoption of immunotherapy and PARP inhibitors requires critical cost benefit analysis [7]. There are other potential solutions which might be useful like an abbreviated course of immunotherapy which has shown benefit in a large data set from resource constrained setting in India [37]. Besides, subsided pricing of these therapies through health policy intervention may improve their access in LMICs.
Due to resource constraints, TNBCs patients were offered genetic testing only when they had a strong family history or YBC. As expected the higher pCR rate (66.7%) among patients with germline BRCA pathogenic variants translated into better OS (5-year OS 95.0% vs 79.8%) as has been observed in other studies as well [38,39].
The 5-year DFS (65.9%) and OS (80.3%) in our cohort were comparable to that reported in the literature [4,12,40,41]. However, in a similar study by Suhani et al. the lower 5-year DFS and OS of 55.6% and 72.9% respectively, may be because only 45% of their patients received taxanes [14]. Contrarily, Doval et al. have reported a higher 5 year DFS and OS among TNBC patients (n = 769) of 85.5% and 92.7% respectively [22]. However, their cohort predominantly had patients with early stages (stage I & II – 78.6%), higher grade 2 and higher socioeconomic strata patients, making a direct comparison with our cohort inappropriate [22]. In a study from New Zealand, the survival statistics were somewhat superior albeit with 60% postmenopausal women and relatively less tumor burden as well as shorter follow-up [42].
In a Turkish study also the survival was slightly higher (the 5-year OS and DFS rates were 84.6% and 71.6%, respectively) [43]. There are conflicting reports about the effect of race on survival of TNBC patients. Data supporting the role of ethnic influence on survival comes from studies that have documented a higher prevalence and higher mortality of TNBC among African American women when compared with white women [44,45].
As expected, in our study stage III disease showed poorer survival compared to early stages. A similar study by Agarwal et al. also showed a statistically higher mean OS for stage I and II (102.6 ± 7.2 months) vs stage III (47.4 ± 5.3 months) among TNBC patients [3,22,46,47]. Thus, the major emphasis should be on early detection and our data underscore this vital need [48]. On MVA, the presence of LVI and PNE were associated with poorer DFS and OS as has been reported by other studies as well [[49], [50], [51], [52]]. As expected, older age was a poor prognostic factor for OS; possibly because of reduced life expectancy from several competing morbidities or poor tolerance of systemic treatment [53].
Our patterns of relapse are consistent with those in literature [54,55]. A fourth of our patients with distant relapse had brain metastasis which can be a site for active research on effective systemic agents as well as on the role of prophylactic cranial irradiation [56,57]. Palliative chemotherapy was planned and given to 75.6% (n = 196) of the patients (n = 259) who relapsed; however, the outcomes after metastasis remained dismal and studies to improve their outcomes are required.
The strength of our study is the large numbers and the contemporary nature of the cohort who were uniformly treated at a single tertiary care centre from India. Since we receive patients from all the parts of our country including those who are underprivileged, our results may be considered representative for the rest of the country and similar LMIC. Even though this is a retrospective study, the prospective nature of data capture helped us to derive meaningful inferences. While our real-world observational study was simple it did have limitations that stem from its single-centre, nonrandomized design. Further, there were ∼16% lost to follow-ups. Although, the lost to follow-up rate is comparable to other studies (19% Dovel et al., 18% at Roswell Park Cancer Institute) and alarm towards this important issue outside clinical trials [22,58]. Also lost to follow up is higher in first year after completion of treatment (48% of 211 patients lost to follow up in present study) [22,59]. Furthermore, post relapse lost to follow-up is also common in LMIC as patient went back to native places and often even opt for alternative treatments. The median follow-up (54 months) was relatively short, and therefore we were unable to study the long term toxicities and outcomes among long-term survivors.
6. Conclusion
Relatively large contemporary data of TNBC patients from a single tertiary care centre suggest that the TNBC subtype is characterized by young age at presentation, a higher grade of tumor, and frequent visceral relapses. Germline BRCA1/2 alteration is seen with a better response to NACT and favorable survival among this subset of patients. The patients who develop relapse have limited treatment options and carry a dismal prognosis. Stage-wise outcomes for adequately treated patients were comparable to other published studies and strongly indicate increasing awareness and to develop strategies to ensure adequate follow-up of the patients in India and other LMIC to enhance early detection and timely optimal treatment.
Declaration
This manuscript has been read and approved by all the authors, the requirements for authorship have been met. We believe that the manuscript represents honest work and this information is not provided in another form.
Institute Review Board approval
Investigator initiated study and approval obtained.
Funding source
None.
Authors contribution statement
Jyoti Bajpai (JB): Conception, design, patient recruitment, management, follow-up, analysis, administration, supervision, first and final manuscript. Lakhan Kashyap (LK): patient recruitment, data entry, treatment, follow-up, analysis, first and final manuscript. Dilip Harindran Vallathol (DV), Anbarasan Sekar (AS), Ankita Das (AD): Maneesh Singh (MS): Ravindra Nandhana (RN), Rahul Ravind (RR): Asha Reddy (AR): patient recruitment, data entry, treatment, follow-up, final manuscript. Reema Pathak (RP), Sushmita Rath, Shalaka Joshi (SJ): Tabassum Wadasadawala (TW): patient recruitment, treatment, follow-up, first and final manuscript. Subham Mohanta (SM): Statistical analysis. Vani Parmar (VP), Nita Nair (NN), Jaya Ghosh, Seema Gulia: management follow-up, analysis, final manuscript. Sangeeta Desai (SD), Tanuja Shet (TS): Meenakshi Thakur (MT), Asawari Patil (AP): lab reports, final manuscript. Sudeep Gupta (SG), Rajendra Badwe (RB), Rajiv Sarin (RS): management, statistical analysis, administration, supervision, final manuscript.
Declaration of competing interest
Nil relevant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu G.S., Erqou S., Patterson H., Mathew A. Prevalence of triple-negative breast cancer in India: systematic review and meta-analysis. J Glob Oncol. 2016 Dec;2(6):412–421. doi: 10.1200/JGO.2016.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair N., Shet T., Parmar V., Havaldar R., Gupta S., Budrukkar A., et al. Breast cancer in a tertiary cancer center in India - an audit, with outcome analysis. Indian J Cancer. 2018 Mar;55(1):16–22. doi: 10.4103/ijc.IJC_484_17. [DOI] [PubMed] [Google Scholar]
- 4.Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2007 Aug 1;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Trivers K.F., Lund M.J., Porter P.L., Liff J.M., Flagg E.W., Coates R.J., et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control CCC. 2009 Sep;20(7):1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyawali B., Sullivan R. Economics of cancer medicines: for whose benefit? New Bioeth Multidiscip J Biotechnol Body. 2017 Apr;23(1):95–104. doi: 10.1080/20502877.2017.1314885. [DOI] [PubMed] [Google Scholar]
- 7.Philip C., Mathew A., John M. vol. 1. Cancer Research Statistics and Treatment; 2018. pp. 58–62. (Cancer care: challenges in the developing world). [Google Scholar]
- 8.de Souza J.A., Hunt B., Asirwa F.C., Adebamowo C., Lopes G. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol Off J Am Soc Clin Oncol. 2016 Jan 1;34(1):6–13. doi: 10.1200/JCO.2015.62.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajpai J., Simha V., Shylasree T.S., Sarin R., Pathak R., Popat P., et al. Pregnancy associated breast cancer (PABC): report from a gestational cancer registry from a tertiary cancer care centre, India. Breast. 2021 Apr 1;56:88–95. doi: 10.1016/j.breast.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajpai J., Ventrapati P., Joshi S., Wadasadawala T., Rath S., Pathak R., et al. Unique challenges and outcomes of young women with breast cancers from a tertiary care cancer centre in India. Breast Edinb Scotl. 2021 Dec;60:177–184. doi: 10.1016/j.breast.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudis C.A., Barlow W.E., Costantino J.P., Gray R.J., Pritchard K.I., Chapman J.-A.W., et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol Off J Am Soc Clin Oncol. 2007 May 20;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 12.Sarin R., Khandrika L., Hanitha R., Avula A., Batra M., Kaul S., et al. Epidemiological and survival analysis of triple-negative breast cancer cases in a retrospective multicenter study. Indian J Cancer. 2016 Sep;53(3):353–359. doi: 10.4103/0019-509X.200682. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni A., Kelkar D.A., Parikh N., Shashidhara L.S., Koppiker C.B., Kulkarni M. Meta-analysis of prevalence of triple-negative breast cancer and its clinical features at incidence in Indian patients with breast cancer. JCO Glob Oncol. 2020 Jul;6:1052–1062. doi: 10.1200/GO.20.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suhani Parshad R., Kazi M., Seenu V., Mathur S., Dattagupta S., et al. Triple-negative breast cancers: are they always different from nontriple-negative breast cancers? An experience from a tertiary center in India. Indian J Cancer. 2017 Dec;54(4):658–663. doi: 10.4103/ijc.IJC_348_17. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmaiah K.C., Das U., Suresh T.M., Lokanatha D., Babu G.K., Jacob L.A., et al. A study of triple negative breast cancer at a tertiary cancer care center in southern India. Ann Med Health Sci Res. 2014 Nov;4(6):933–937. doi: 10.4103/2141-9248.144917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sathwara J.A., Balasubramaniam G., Bobdey S.C., Jain A., Saoba S. Sociodemographic factors and late-stage diagnosis of breast cancer in India: a hospital-based study. Indian J Med Paediatr Oncol Off J Indian Soc Med Paediatr Oncol. 2017;38(3):277–281. doi: 10.4103/ijmpo.ijmpo_15_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur P., Sathishkumar K., Chaturvedi M., Das P., Sudarshan K.L., Santhappan S., et al. Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol. 2020 Jul;6:1063–1075. doi: 10.1200/GO.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C.-Y., Zhang S., Zhang X.-B., Wang P., Hou G.-F., Zhang J. Clinicopathological and prognostic characteristics of triple- negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev APJCP. 2013;14(6):3779–3784. doi: 10.7314/apjcp.2013.14.6.3779. [DOI] [PubMed] [Google Scholar]
- 19.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.-N., et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills M.N., Yang G.Q., Oliver D.E., Liveringhouse C.L., Ahmed K.A., Orman A.G., et al. Histologic heterogeneity of triple negative breast cancer: a National Cancer Centre Database analysis. Eur J Cancer Oxf Engl. 2018 Jul;98:48–58. doi: 10.1016/j.ejca.2018.04.011. 1990. [DOI] [PubMed] [Google Scholar]
- 21.Balkenhol M.C.A., Vreuls W., Wauters C.A.P., Mol S.J.J., van der Laak J.A.W.M., Bult P. Histological subtypes in triple negative breast cancer are associated with specific information on survival. Ann Diagn Pathol. 2020 Jun;46:151490. doi: 10.1016/j.anndiagpath.2020.151490. [DOI] [PubMed] [Google Scholar]
- 22.Doval D.C., Radhakrishna S., Tripathi R., Kashinath R.I., Talwar V., Batra U., et al. A multi-institutional real world data study from India of 3453 non-metastatic breast cancer patients undergoing upfront surgery. Sci Rep. 2020 Apr 3;10(1):5886. doi: 10.1038/s41598-020-62618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogia A., Deo S.V., Shukla N.K., Mathur S., Sharma D.N., Tiwari A. Clinicopathological profile of breast cancer: an institutional experience. Indian J Cancer. 2018 Sep;55(3):210–213. doi: 10.4103/ijc.IJC_73_18. [DOI] [PubMed] [Google Scholar]
- 24.Hassan Ali S., Somashekhar S.P., Arun Kumar N. Rate of breast-conserving surgery vs mastectomy in breast cancer: a tertiary care centre experience from south India. Indian J Surg Oncol. 2019 Mar;10(1):72–76. doi: 10.1007/s13193-018-0818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budach W., Bölke E., Matuschek C. Hypofractionated radiotherapy as adjuvant treatment in early breast cancer. A review and meta-analysis of randomized controlled trials. Breast Care Basel Switz. 2015 Aug;10(4):240–245. doi: 10.1159/000439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedel F., Hoffmann A.S., Moderow M., Heublein S., Deutsch T.M., Golatta M., et al. Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer. 2020 Dec 1;147(11):3049–3058. doi: 10.1002/ijc.33122. [DOI] [PubMed] [Google Scholar]
- 27.Citron M.L., Berry D.A., Cirrincione C., Hudis C., Winer E.P., Gradishar W.J., et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol Off J Am Soc Clin Oncol. 2003 Apr 15;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 28.Bajpai J., Kagwade S., Chandrasekharan A., Dandekar S., Kanan S., Kembhavi Y., et al. Randomised controlled trial of scalp cooling for the prevention of chemotherapy induced alopecia. Breast Edinb Scotl. 2020 Feb;49:187–193. doi: 10.1016/j.breast.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poggio F., Bruzzone M., Ceppi M., Pondé N.F., La Valle G., Del Mastro L., et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol Off J Eur Soc Med Oncol. 2018 Jul 1;29(7):1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 30.Geyer C.E., Sikov W.M., Huober J., Rugo H.S., Wolmark N., O'Shaughnessy J., et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol Off J Eur Soc Med Oncol. 2022 Jan 31 doi: 10.1016/j.annonc.2022.01.009. S0923-7534(22)00018-7. [DOI] [PubMed] [Google Scholar]
- 31.Masuda N., Lee S.-J., Ohtani S., Im Y.-H., Lee E.-S., Yokota I., et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017 Jun 1;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 32.Joensuu H., Kellokumpu-Lehtinen P.-L., Huovinen R., Jukkola A., Tanner M., Ahlgren J., et al. Adjuvant capecitabine for early breast cancer: 15-year overall survival results from a randomized trial. J Clin Oncol Off J Am Soc Clin Oncol. 2022 Jan 12:JCO2102054. doi: 10.1200/JCO.21.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dotan E., Tew W.P., Mohile S.G., Ma H., Kim H., Sun C.-L., et al. Associations between nutritional factors and chemotherapy toxicity in older adults with solid tumors. Cancer. 2020 Apr 15;126(8):1708–1716. doi: 10.1002/cncr.32718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid P., Cortes J., Dent R., Pusztai L., McArthur H., Kümmel S., et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022 Feb 10;386(6):556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 35.Mittendorf E.A., Zhang H., Barrios C.H., Saji S., Jung K.H., Hegg R., et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet Lond Engl. 2020 Oct 10;396(10257):1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 36.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021 Jun 24;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham G., Noronha V., Rajappa S., Agarwal A., Batra U., Somani N., et al. The clinical utility and safety of short-course immune checkpoint inhibitors in multiple tumours-A real-world multicentric study from India. Int J Cancer. 2022 Mar 15;150(6):1045–1052. doi: 10.1002/ijc.33868. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Zhang J., Wang Y., Ouyang T., Li J., Wang T., et al. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2015 Mar;26(3):523–528. doi: 10.1093/annonc/mdu559. [DOI] [PubMed] [Google Scholar]
- 39.De Talhouet S., Peron J., Vuilleumier A., Friedlaender A., Viassolo V., Ayme A., et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020 Apr 27;10(1):7073. doi: 10.1038/s41598-020-63759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandra D., Suresh P., Sinha R., Azam S., Batra U., Talwar V., et al. Eight year survival analysis of patients with triple negative breast cancer in India. Asian Pac J Cancer Prev APJCP. 2016;17(6):2995–2999. [PubMed] [Google Scholar]
- 41.Kumar S., Furrukh M., Al-Baimani K., Al-Ajmi A., Burney I.A., Al-Moundhri M.S. Outcomes of women with non-metastatic triple-negative breast cancer in Oman: a single-centre experience. Sultan Qaboos Univ Med J SQUMJ. 2019 Nov 5;19(3):e209–216. doi: 10.18295/squmj.2019.19.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James M., Dixit A., Robinson B., Frampton C., Davey V. Outcomes for patients with non-metastatic triple-negative breast cancer in New Zealand. Clin Oncol R Coll Radiol G B. 2019 Jan;31(1):17–24. doi: 10.1016/j.clon.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Eralp Y., Kılıç L., Alço G., Başaran G., Doğan M., Dinçol D., et al. The outcome of patients with triple negative breast cancer: the Turkish oncology group experience. J Breast Health. 2014 Oct;10(4):209–215. doi: 10.5152/tjbh.2014.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey L.A., Perou C.M., Livasy C.A., Dressler L.G., Cowan D., Conway K., et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006 Jun 7;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 45.Chlebowski R.T., Chen Z., Anderson G.L., Rohan T., Aragaki A., Lane D., et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005 Mar 16;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal G., Nanda G., Lal P., Mishra A., Agarwal A., Agrawal V., et al. Outcomes of triple-negative breast cancers (TNBC) compared with non-TNBC: does the survival vary for all stages? World J Surg. 2016 Jun;40(6):1362–1372. doi: 10.1007/s00268-016-3422-4. [DOI] [PubMed] [Google Scholar]
- 47.Viral P., Pavithran K., Beena K., Shaji A., Vijaykumar D.K. Ten-year survival outcome of breast cancer patients in India. J Carcinog. 2021;20:1. doi: 10.4103/jcar.JCar_26_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birnbaum J.K., Duggan C., Anderson B.O., Etzioni R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Global Health. 2018 Aug 1;6(8):e885–e893. doi: 10.1016/S2214-109X(18)30257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neri A., Marrelli D., Roviello F., De Stefano A., Guarnieri A., Pallucca E., et al. Prognostic value of extracapsular extension of axillary lymph node metastases in T1 to T3 breast cancer. Ann Surg Oncol. 2005 Mar;12(3):246–253. doi: 10.1245/ASO.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 50.Rakha E.A., Martin S., Lee A.H.S., Morgan D., Pharoah P.D.P., Hodi Z., et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012 Aug 1;118(15):3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 51.Maksimovic S., Gojkovic Z., Opric M. Positive axillary lymph node metastases in T1–T3 breast cancer: prognostic value of extracapsular extension. Breast Cancer Res. 2007 Jun 19;9(1):SP10. [Google Scholar]
- 52.Kanyılmaz G., Fındık S., Yavuz B.B., Aktan M. The significance of extent of extracapsular extension in patients with T1-2 and N1 breast cancer. Eur J Breast Health. 2018 Oct;14(4):218–224. doi: 10.5152/ejbh.2018.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wedding U., Honecker F., Bokemeyer C., Pientka L., Höffken K. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control J Moffitt Cancer Cent. 2007 Jan;14(1):44–56. doi: 10.1177/107327480701400106. [DOI] [PubMed] [Google Scholar]
- 54.Tseng L.M., Hsu N.C., Chen S.C., Lu Y.S., Lin C.H., Chang D.Y., et al. Distant metastasis in triple-negative breast cancer. Neoplasma. 2013;60(3):290–294. doi: 10.4149/neo_2013_038. [DOI] [PubMed] [Google Scholar]
- 55.Yao Y., Chu Y., Xu B., Hu Q., Song Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. 2019 Jun 28;39(6) doi: 10.1042/BSR20190288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin J., Gao Y., Zhang J., Wang L., Wang B., Cao J., et al. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer. 2018 Apr 19;18(1):446. doi: 10.1186/s12885-018-4371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brosnan E.M., Anders C.K. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. 2018 May;6(9):163. doi: 10.21037/atm.2018.04.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kukar M., Watroba N., Miller A., Kumar S., Edge S.B. Fostering coordinated survivorship care in breast cancer: who is lost to follow-up? J Cancer Surviv Res Pract. 2014 Jun;8(2):199–204. doi: 10.1007/s11764-013-0323-5. [DOI] [PubMed] [Google Scholar]
- 59.Swaminathan R., Rama R., Shanta V. Lack of active follow-up of cancer patients in Chennai, India: implications for population-based survival estimates. Bull World Health Organ. 2008 Jul;86(7):509–515. doi: 10.2471/BLT.07.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.