Abstract
Due in part to overall improvements in health, the population of elderly individuals is increasing rapidly. Similarly, obstructive sleep apnoea (OSA) is both gaining increased recognition and also increasing due to the worldwide obesity epidemic. The overlap of OSA and aging is large, but there is strong plausibility for causation in both directions: OSA is associated with pathological processes that may accelerate aging and aging related processes; aging may cause physical and neurological changes that predispose to obstructive (and central) apnoea. In addition, the common symptoms (e.g. excessive daytime somnolence, defects in memory and cognition), possible physiological consequences of OSA (e.g. accelerated cardiovascular and cerebrovascular atherosclerosis), and changes in metabolic and inflammatory markers overlap with the symptoms and associated conditions seen in aging. There is also the possibility of synergy in the effects of these symptoms and conditions on quality of life, as well as a need to separate treatable consequences of OSA from age-related complaints. Taken together, the above make it essential to review the interaction of OSA and aging, both proven and suspected. The present review examines some aspects of what is known and points to the need for further investigation of the relationships, given the large number of potentially affected subjects.
Keywords: OSA, Obstructive sleep apnoea, Sleep disordered breathing, SDB, cardiovascular, neurocognitive. CPAP, continuous positive airway disease, elderly, Alzheimer’s disease.
1. Introduction:
International trends show a world population that is becoming older(1) but also healthier with a postponement of disabilities(2). A potentially important source of unrecognized morbidity in this group is obstructive sleep apnoea (OSA). OSA is associated with multiple health consequences, including neurological dysfunction (sleepiness and decreased cognitive function)(3), vascular disease (hypertension, cardiac and stroke)(4), metabolic dysfunction (type 2 diabetes)(5), depression(6), and cancer(7, 8). Many of these putative consequences of OSA overlap with changes seen in aging(9). This has motivated speculation on the bidirectional causal relationships that could exist between these overlapping conditions. Further complicating the science is that symptomatic OSA is clinically more prominent, although not less common, in middle age than in the elderly. It may also cause progressive damage over time that manifests only later, while OSA itself may change character and become less evident with aging(10).
OSA is very common in late life, especially in the mild-moderate range of severity, where its health consequences have not been conclusively demonstrated. However, if one accepts widely used criteria such as having an apnoea-hypopnoea index (AHI) defined by >5 events/hour, OSA is found in upwards of 20-60% of adults(11). In our own series, these criteria are met in at least 50% of the elderly population(12, 13). Using definitions of OSA including hypopnea’s definition (AHI defined by arousals or 3% desaturation), it may affect up to 75% of individuals >65 years(14, 15). Sleepiness also seems less prominent among the elderly, suggesting the possibility of differential susceptibility and/or disorders, despite similar event indices(16). Moreover, there is conflicting information on the health consequences of OSA in the very old (>80 years) and the impact (or lack thereof) of treatment. In this manuscript, we review available information on the pathophysiology, epidemiology, diagnostic, and treatment aspects of OSA focusing on studies that included elderly individuals, and pointing out future challenges for this emerging field.
2. Pathophysiological aspects:
OSA is characterized by recurrent obstructions of the upper airway (UA) during sleep. While it was originally thought that the obstructions were entirely the result of abnormal anatomy interacting with the loss of muscle tone that occurs during sleep(17), it is now appreciated that there can be multiple other pathophysiological contributors to the phenomenon. In one approach first proposed by Wellman et al(18), these factors are divided into contributing components: i. collapsibility of the passive airway (Pcrit or closing pressure); ii. responsiveness of UA muscles to respiratory stimuli; iii. arousability; and, iv. ‘loop gain’ (i.e. response of the respiratory controller to a transient disturbance). As a result, there would be different endotypes of OSA, each characterized by combinations/predominance of the different factors. This approach has shown promise in describing populations of OSA patients and predicting responses to therapy(19), but it remains to be confirmed as a tool to describe the pathophysiology(20). In a complimentary approach to describing the spectrum of OSA, clinical symptoms or consequences can also play a role in defining phenotypes(21). Over the past 30 years, definitions of the OSA syndrome have required a clinical consequence such as excessive daytime sleepiness (EDS), but this is not uniformly accepted(22). Clearly, inclusion of asymptomatic individuals, in contrast to requiring both an elevated index of obstructions and sleepiness or downstream organ dysfunction, will impact the epidemiology of OSA(23).
Aging effects on the individual elements implicated in the pathophysiology have been invoked to account for the clinical observations that: a. OSA is more common in the elderly(10); b. the risk factors for OSA (e.g. obesity) are different in the elderly phenotype(24-27); and, c. symptoms and natural history may be different than in the middle-age patient(28, 29). Aging has potential effects on all of these pathophysiological factors. The ability of pharyngeal dilator muscles to respond to chemical and mechanical stimuli during sleep declines with age due to: an increased deposition of para-pharyngeal fat(30); changes in muscle activity(31); changes in lung volume(32); changes in the UA dimensions(30); increased UA resistance and collapsibility(33); and sleep fragmentation(34). Table 1 shows some possible factors associated to greater airway collapsibility in elderly. From these age-related physiological changes, two studies, one performed by Eikermann et al in 21 healthy individuals (age 18-75 years)(33), the second by Edwards et al in 10 young (20-40 years) and old (>60 years) patients with OSA(32), suggest that UA anatomy/collapsibility plays a greater pathogenic role in late-life. This effect, however, would be mitigated by a reduced ventilatory demand and feedback control sensitivity that are typical in late-life(35, 36); in contrast to the OSA phenotype of younger adults, in which the airway is less collapsible, but where higher feedback control and ventilatory demand are dominant traits(32). Similarly, there is some evidence that the elderly become less sleepy as a result of sleep disruption(37-39), and it has been suggested that some consequences of OSA (e.g. EDS), are less prominent in the elderly(40). Finally, the association of OSA with mortality seems to be stronger in the young than in the elderly, but this remains to be conclusively established.
Table 1.
Pharyngeal collapsibility in elderly
| Airway resistance during sleep(33, 172) | Increased |
| Deposition of parapharyngeal fat(173) | Increased or Decreased |
| Pharyngeal musculature activity(30) | Decreased |
| Lung elastic recoil (affects lung volume and tracheal traction)(32) | Decreased |
| Upper airway dimensions (fat deposition, structure changes, loss of teeth, etc.)(173) | Decreased |
| Sleep fragmentation(34) | Increased |
| Alterations of the pharynx muscular dilatory reflexes(174) | Increased |
| Respiratory instability during sleep (central events)(175) | Increased |
3. Prevalence and epidemiology projection:
By 2050, 20% of world population will be older than 60 (2019 Revision of World Population Prospects)(1). As demonstrated by several community-based studies, the prevalence of OSA in people older than 60 ranges from 27 to 80%(11) depending on the definition and on the population studied. In sleep clinic-based series, the prevalence can be even higher(41). Furthermore, as the prevalence of OSA continues to increase due to the obesity epidemic(42), this can be expected to eventually be reflected in the elderly population(10). A recent retrospective cross-sectional study performed in Spain (projected to become the world’s oldest country in 2050)(1) using 51,229 medical records, demonstrated that almost 25% of current sleep-clinic studies were performed in individuals 65 and older, 71.5% of which showed an AHI ≥10 events/hour, with a mean AHI of 38.3±7.4events/hour (41). Table 2 below shows the prevalence of OSA observed in several seminal epidemiological studies that only included older individuals.
Table 2.
Prevalence of obstructive sleep apnoea in studies exclusively of the elderly.
| Author, year | n | Age, yrs | Females % | BMI Kg/m2 | Population | AHI≥5 % | AHI≥10/15 % |
|---|---|---|---|---|---|---|---|
| Ancoli-israel(176) | 233 | 65-101 | 65 | 30.6 ± 6 | Nursing home | 70 | -- |
| Ancoli-Israel(177) | 385 | 65-99 | 62.6 | 21.1 ± 4 | Community | 81 | 62 |
| Bixler(48) | 75 | 65-100 | 0 | 29.4 | Community | 31 | 24 |
| Young(45) | 3448 | 60-99 | -- | -- | Community | 54 | 20 |
| Carskadon(178) | 40 | 62-86 | 55 | -- | Community | 36 | -- |
| McGinty(179) | 26 | 61-81 | 0 | -- | Community | 62 | |
| Hoch(180) | 105 | 60-91 | 52 | -- | Community | 26 | 13 |
| Martinez-Garcia(41) | 12,468 | +65 | -- | -- | Clinical series | 71.5 | -- |
BMI: Body mass index; AHI: Apnoea-hypopnoea index;
Snoring frequency increases with age up to 50 to 60 years of age and then decreases in both men and women(43, 44). In contrast, the prevalence of OSA increases throughout the life span, becoming in late middle age at least double than that seen in younger adults. There is a plateau in OSA frequency in people 60 and above(45, 46). The difference in prevalence observed between the different studies shown in Table 2 has been attributed to the different populations studied (e.g. community based vs. sleep clinic vs. nursing home), methodologies (e.g. limited channel vs. polysomnography), and hypopnoea definitions (e.g. 4% desaturation vs. 3% desaturation and arousal). There is a critical need for these data to be reconciled, and for large scale population-based studies to be performed with agreed criteria involving a broad range of elderly patients who represent real-world patients(47). However, it is important to highlight that clinically relevant nocturnal hypoxemia or AHI cutoffs (e.g. based on an AHI of 5 events/hour or greater) that can distinguish normal from abnormal sleep breathing are still not defined or agreed upon, especially in the elderly(12). Furthermore, even though the number of apnoeas/hypopnoeas increases with age(48), their impact on EDS, quality of life (QoL), cognition, depression, cardiovascular disease, or increased mortality is still a matter of much debate(47).
4. Why sleep apnoea in elderly is a special entity?
4.1. Clinical presentation and common symptoms.
OSA is largely under-recognized and under-diagnosed in older adults(49, 50). Those that are symptomatic or are referred to sleep clinics for OSA evaluation, however, seem to have a similar presentation to that of middle-age, except that elderly patients underreport snoring as a chief complaint, tend to be less obese, are less likely to be men, and are less objectively sleepy (see Table 3). Moreover, the incidence of EDS(37), metabolic syndrome(51), hypertension(52) and mortality associated with OSA decline with age(53). While this may reflect underreporting of OSA or its sequellae in this population, survivor or collider biases (e.g. stroke may preclude participation in epidemiological studies), it also suggests that older people with OSA may not suffer the same health consequences as their younger counterparts.
Table 3.
Characteristics of older adults with obstructive sleep apnoea (OSA)
| Typical presentations (i.e. overweight male with loud snoring, nocturnal choking, nocturia, excessive daytime sleepiness, and cardiovascular consequences) do exist. |
| They are less likely to be men (particularly after menopause in women). |
| The association with obesity and neck circumference is present but less prominent, and snoring as a chief complaint is underreported. |
| There are decreased reports of excessive daytime sleepiness and fatigue. |
| Health consequences are rated differently for the same level of OSA (i.e. less debilitating), while cardiovascular consequences of OSA are less evident. |
| Atypical presentations like secondary enuresis and nocturia, cognitive impairment, ophthalmic conditions and repeated falls are common. |
OSA could present distinctly in older populations owing to differences in the underlying risk factors for OSA (e.g. UA collapsibility and reduced ventilatory control); the characteristics of breathing disturbances (e.g. central vs. obstructive); to differences in physiological responses to UA occlusion (e.g. increased chemoresponsiveness, diminished inspiratory effort, or milder cardiovascular response to arousals); or to perceptions and expectations about symptoms such as EDS. Sympathetic tone is widely found to be increased during aging(54), while the sensitivity of α/β adrenergic receptors in the heart and blood vessels is reduced(55). Similarly, heart rate (HR) variability decreases with age(56) and HR changes in response to muscarinic receptor blocking agents are blunted(57). All of which would impair the ability to react to internal stimuli such as intermittent hypoxia or arousals with changes in autonomic function(54). Lastly, fatigue, nocturia, unintentional napping, and cognitive dysfunction may be ascribed to the aging process or to other disorders(58); while retirement or living alone may allow more opportunities to compensate for the consequences of sleep disruption(59).
4.2. Choosing the best diagnostic procedure
OSA is traditionally diagnosed in the laboratory with a full polysomnogram (PSG) that captures not only the respiratory phenomenon but also the electroencephalogram (EEG) and related sleep measures. Over the last several decades it has become clear that home testing, usually with more limited monitoring and particularly without the direct measures of sleep (i.e. EEG, electrooculogram and electromyogram) can capture moderate and severe OSA(60), and is more cost-effective with a lower per-patient cost(61). The trend to rely on limited testing has been accelerated by the current COVID-19 pandemic, as laboratory testing was felt to be associated with unjustified risks for both patients and technical staff(62). There remains, however, much debate about whether limited testing is sufficient to ‘rule-out’ OSA(63). Probably the clinical pre-test probability of suffering from OSA is especially important even in elderly. The position of the American Academy of Sleep Medicine (AASM) is that a negative home test in the presence of a strong clinical suspicion should still prompt a full laboratory PSG(63). This is, however, debated, and the recommendation is not followed uniformly in many settings and countries other than the USA(64). The strongest arguments for performing laboratory PSGs are when there is a substantial differential diagnosis other than OSA, when patients, especially the infirm or elderly, are not able to self-apply testing sensors in the home, when subjects have difficulty with sleep or obtaining a suitably long test, or when there is potential respiratory muscle weakness due to neuromuscular condition, awake hypoventilation or suspicion of sleep related hypoventilation(63). These situations are particularly common in the elderly: living alone, caregiving, dementia, history of stroke, limited mobility and dexterity, and coexisting conditions like heart failure that increase the likelihood of central sleep apnoea and other causes of sleep disruption not easily identified on typical home sleep tests(65).
A growing trend has been the use of non-EEG sensors that capture surrogates of recurrent arousals or the sympathetic surges that accompany obstructive apnoea. Thus, peripheral arterial tonometry (e.g. WatchPAT(66)) has been increasingly used to diagnose OSA without a laboratory study and show good results for moderate and severe disease. Other devices that capture surrogates of arousal (i.e. body movements, pulse changes, etc.) are being explored, including wrist based, mattress or even non-contact sensors, but these have had only limited validation to date, and suffer many of the limitations of actigraphy, which is not generally considered sufficient to diagnose OSA(67). It seems likely that the fully attended laboratory PSG will continue to have utility in most cases where the diagnosis is in question.
5. Sleep apnoea impact in elderly
5.1. Symptoms and Quality of life
OSA in symptomatic patients causes daytime and sleep-related symptoms, health consequences, and decreased quality of life (QoL)(68-71). The main daytime symptom of OSA is EDS. Its impact can be measured by both objective (e.g. Psychomotor Vigilance Test or Multiple Sleep Latency Test) and subjective methods (e.g. ESS or some QoL questionnaires)(72). QoL is commonly measured by subjective, non-specific (e.g. Short-term-36 Health Survey [SF-36](73, 74)), and specific (e.g. Functional Outcomes of Sleep Questionnaire [FOSQ], Quebec Sleep Questionnaire [QSQ], Sleep apnoea quality of life index [SAQLI](70-74)) questionnaires. As with other aspects of OSA research, the vast majority of validation studies for QoL instruments have been performed in middle-aged individuals(75-77), and their findings may not extrapolate to the elderly. This is because the perception of sleep-related symptoms and QoL is subjective(78), and could be impacted by age in a similar manner to the gender related differences seen in sleep-related QoL across all age ranges(78). One of the most important limitations in the measurement of QoL in elderly with OSA is the presence of comorbidities such as sleep fragmentation, poly-pharmacy, cognitive impairment, different social environments, lifestyle factors (e.g. retirement), and decreased physical activity, all of which could act as confounders due to their impact on both QoL and EDS(12, 79). Moreover, there is no diagnostic tool of OSA specifically validated for the elderly nor are there established definitions of “clinically relevant” OSA(12, 79).
Several studies have analyzed whether the impact on symptoms and QoL in OSA patients is the same in advanced aged compared with middle age. Appleton et al., in a study of 837 men participants 40 years and older, observed that, overall, AHI was associated with an increase in EDS -measured with the Epworth Sleepiness Scale- (ESS) and a decrease in QoL, measured by SF-36. However, this association was not observed in patients that were older than 70(80). In a similar study that Martinez-Garcia et al performed in 212 OSA patients that were divided into those over and under 65 years, both the presence of OSA and ESS>11 were associated with an important deterioration of QoL compared with reference values adjusted by sex and age in the younger group(81). In contrast, in those patients over 65, the presence of OSA or ESS>11 had only a slight impact on QoL when compared with the reference values. Variables that were better predictors of poor QoL in this age group were advanced age, medical comorbidities, oxygen desaturation, and use of psychotropic medication(81).
5.2. Cardiovascular system and prognosis
Intermittent hypoxemia (IH) and sleep fragmentation can produce alterations related to a greater cardiovascular risk such as higher inflammation status, endothelial dysfunction, arterial stiffness or an increase in oxidative stress in elderly with OSA(82). However, some studies show that the impact of these cardiovascular consequences is less severe than the one seen in younger patients. Li et al(83) observed that OSA had less impact on cerebrovascular reactivity in elderly individuals, suggesting the existence of an age-related reduction in cerebrovascular susceptibility to OSA. Similarly, Castro-Grattoni et al(84) using a murine model for OSA, concluded that cardiovascular pathological remodeling induced by severe IH was moderated by the age at which the onset of that hypoxia occurred, suggesting that deleterious cardiovascular effects may be more pronounced in younger populations reflecting age-related change in cardiovascular structural integrity(84). This finding could explain the disparity found in OSA longitudinal studies on the incidence of cardiovascular events and their prognosis in late life. At the same time, it is worth mentioning that the vast majority of longitudinal studies, both on clinical and population-based samples, demonstrate that the presence of untreated severe OSA (AHI>30 events/hour) can increase cardiovascular morbidity and mortality in the elderly even in advanced ages Table 4)(85-90). However, it seems that this increase in the incidence of fatal and non-fatal cardiovascular events is more pronounced in the cerebral (mainly stroke), than in the coronary circulation (e.g. angina and myocardial infarction) where there is no strong evidence of a greater risk(91-93). Figure 1 shows the increase in the incidence of all-cause and cardiovascular mortality due to the increased cerebrovascular and heart failure mortality but not due to ischemic heart disease mortality in elderly with severe non-treated OSA compared with mild-to moderate OSA or OSA treated with CPAP in a total of 969 elderly patients sent to a sleep units with suspected OSA(87).
Table 4.
Main characteristics and results on the impact of OSA and the effects of CPAP on different risk factors and cardiovascular events, both in observational studies and clinical trials that only included elderly
| Observational studies | ||||||
|---|---|---|---|---|---|---|
| Study | Methodology | Design | Mean age ± SD | Main outcome | Follow-up | Cardiovascular results |
| Martinez-Garcia et al(87) | Multicenter Prospective (≥65 yo) | AHI<15 (n=155) AHI 15-29 (n=108) AHI≥30 without CPAP (n=173) OSA with CPAP (n=503) |
70.8 ± 4.6 | Cardiovascular death | Median 69 (IQR, 48-87 mo) | AHI≥30 without CPAP risk; OR 2.53 (95%CI, 1.28-4.99) respect to control group OSA with CPAP risk; OR 1.07 (95%CI, 0.54-2.01) respect to control group |
| Catalan-Serra et al(1)(85) | Multicenter Prospective (≥65 yo) | AHI <15 (n=141) AHI 15-29 (n=99) AHI>30 without CPAP (n=149) OSA with CPAP (n=470) |
71.1 ± 4.9 | Nonfatal stroke | Median 72 (IQR, 50-88.5) months | AHI≥30 without CPAP risk; OR 3.42 (95%CI, 1.37-8.52) respect to control group OSA with CPAP risk; OR 1.02 (95%CI, 0.41-2.56) respect to control group |
| Catalan-Serra et al(2)(85) | Multicenter Prospective (≥65 yo) | AHI <15 (n=138) AHI 15-29 (n=84) AHI≥30 without CPAP (n=146) OSA with CPAP (n=426) |
70.9 ± 5.1 | Non-fatal coronary disease | Median 71 (IQR, 51.1-89) months | AHI≥30 without CPAP risk; OR 2.05 (95%CI, 0.65-6.47) respect to control group OSA with CPAP risk; 1.07 (95%CI, 0.34-3.30) respect to control group (IAH<15) |
| Lopez-Padilla et al(86) | Single-center (≥80 yo) | CPAP (n=79) vs no CPAP (n=76) | 81.5 ± 1.7 | All-cause mortality (28% cardiovascular) | Median 53 (IQR 41-77 mo) | Decrease all-cause mortality adjusted by other CV risk factors, age, gender, and BMI. Treated OSA, HR 0.46 (95%CI, 0.27-0.78) |
| Ou et al(90) | Single-center (≥60 yo) | CPAP (n=36) vs no CPAP (n=88) | 72.8 ± 6.2 | Fatal and non-fatal death from CVE | Median 5 (2.5) (range 1-8 yrs) | Reduce mortality in moderate-severe OSA no treated Survival rate CPAP vs no CPAP; 5.6 vs 21.6%; p=0.035 |
| Nishihata et al(89) | Single center (65-86 yo) Retrospective AH≥15 Prior hospitalization due to CVD | CPAP (n=64) vs no CPAP (n=66) | 65-86 yo | All cause death or admission for relapse of CVE | Mean 32.9 ± 23.8 months | HR 5.13; 95%CI, 1.01-42 in moderate-to-severe OSA not treated with CPAP CPAP normalice the risk |
| Muñoz et al(88) | Single center 70-100 yrs old Population-based | 394 non-institutionalized CPAP-free patients | 70-100 yo | Incident of first-ever stroke | 6 years | AHI≥30 vs control HR, 2.52; 95%CI:1.04-6.01 |
| Randomized Clinical Trials | ||||||
| McMillan et al(140) | Multicenter (≥65 yo) | 140 CPAP vs 138 no CPAP | 71.1 ± 3.7 | ESS and cost-effectiveness | 12 months | Reduced total and LDL colesterol at 3 months (p=0.048) in the CPAP group (not at 12 months) Improvement in SBP (−3.7; 0.2-7.2 mmHg; p=0.04) at 12 months No changes in incident CVE |
| Martinez-Garcia et al(139) | Multicenter (≥70 yo) Severe OSA | 115 CPAP vs 109 no CPAP | 75.5 ± 3.9 | QoL (QSQ) | 3 months | No changes in SBP and DBP levels |
| Ponce et al(141) | Multicenter (≥70 yo) Moderate OSA | 73 CPAP vs 72 no CPAP | 74.9 ± 4.6 | ESS | 3 months | No changes in SBP and DBP levels |
AHI: Apnoea hypopnoea index; CPAP: Continuous positive airway pressure; QoL: Quality of Life; QSQ: Quebec Sleep Questionnaire; OSA: Obstructive sleep apnnoea; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; CE: cardiovascular events: LDL: Low-density lipoprotein; HR: Hazard ratio; CVD: Cardiovascular disease; ESS: Epworth Sleepiness Scale
Figure 1.
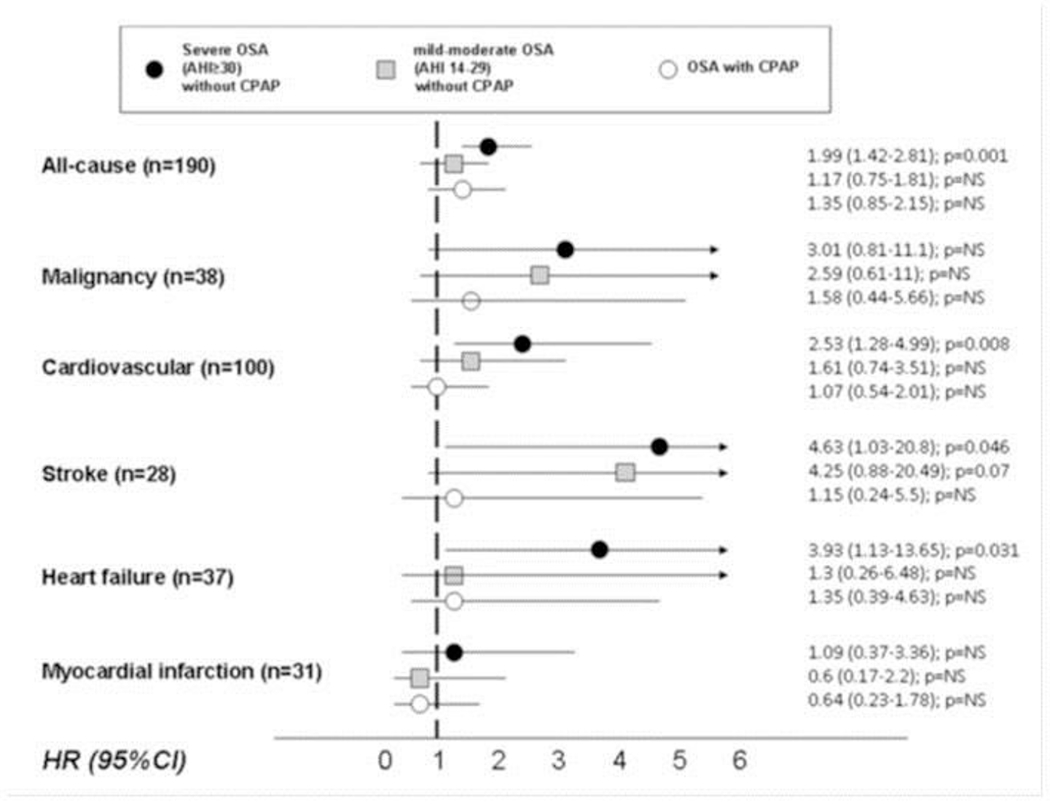
Risk of cardiovascular mortality due to different causes in 939 elderly patients with severe OSA without CPAP (black circle), mild-moderate OSA without CPAP (grey square), and OSA with (good adherence to) CPAP (white circle). Martínez-García MA, et al. American Journal of Respiratory and Critical Care Medicine 2012; 186(9): 909-16. With permission.
OSA: Obstructive sleep apnoea, CPAP: Continuous positive airway pressure; AHI: Apnoea-hypopnoea index; NS: non-significant; HR: Hazard ratio; CI: Confidence interval
To explain this phenomena, a few years ago the ‘preconditioning hypoxia hypothesis’ promulgated by Lavie et al gained notoriety, after they observed that the presence of OSA did not produce excess mortality in the elderly as opposed to younger individuals(94, 95). This was attributed to the fact that a low-grade, continuous IH produced coronary vascular collaterals through the activation of some key molecules such as hypoxia-inducible factor-1α and vascular endothelial growth factor (VEGF). The majority of studies on preconditioning hypoxia have been carried out in coronary circulation and the formation of this neovascularization has indeed been observed(96). However, in cerebral circulation the vascular regulation may be different. Thus, according to Wegener et al(97), cerebrovascular control seems to be governed more by neuro-hormonal factors than by neovascularization, which may at least partially explain the different action of IH in both types of circulations and thereby explain the different effects seen in clinical studies(97). On the other hand, some authors point out that with age the effect of IH on circulation may be affected(98). Along these lines, Dalmases et al(99) suggest that brain oxidative stress in aged rats is lower than in young rats in response to recurrent apnoeas mimicking OSA. This could be due to the different partial pressure of oxygen (PtO2) response observed between age groups and the increased antioxidant expression in aged rats(99). Table 4 shows the most significant characteristics and the most relevant results of the impact of OSA on the cardiovascular system in studies that exclusively included elderly patients.
5.3. Cognitive function:
OSA is believed to cause cognitive impairment by EDS or drowsiness from sleep fragmentation(100-102), neurological damage due to intermittent hypoxia(100, 103, 104), as well as from contributing effects of obesity, hypertension, diabetes, anxiety, or depression(100, 105), among others. For the most part, there is substantial evidence suggesting weak to strong associations between OSA and cognitive performance in middle age on measures of attention(106-109), memory(110-115), reaction time(115-117), processing speed(109), and executive function(106, 107, 109, 110, 113-115). Studies that restricted their populations to older adults (i.e., >60 years) generally show weaker, if any, links to impaired cognition(118-122). An important meta-analysis of several of these studies performed by Cross et al showed a small association between OSA severity and combined measures of cognition, processing speed and memory(123). However, this effect appeared to be driven by selection bias, with small case-control studies from sleep clinics observing the greatest associations, while larger cohort studies from community samples demonstrating no effects(123). An interpretation by the same authors is that the link between OSA severity and impaired cognition may be most pronounced in those seeking specialist assessments while absent in asymptomatic older adults or those with unrecognized symptoms. In this latter group, a recent study from Sforza et al in a randomly selected, population-based study of 812 healthy older adults, found that sex and depression score -with a weak contribution of obesity-, but not AHI severity, were the only significant factors affecting the presence of EDS(124). This finding underlines the link between the perception of sleepiness and reduced mood and QoL in OSA patients, effectively showing mood disorders impacting cognition more than typical OSA-related symptoms in some cases. However, although a number of studies have been carried out on the link between OSA and depression(125), the results in the elderly have so far been inconclusive with most studies reporting no associations(38, 126).
Longitudinal studies from Blackwell et al in the Osteoporotic Fractures in Men (MrOS)(127), Martin et al in the PROgnostic indicator OF cardiovascular and cerebrovascular events (PROOF)(128), and Lutsey et al in the Atherosclerosis Risk in Communities (ARIC)(129) cohorts have also shown null(129) to modest associations with global measures of cognition(127) and attention(128), which in some cases were only evident in patients with severe OSA. In the ARIC cohort, some differences between subjects examined at follow-up and those excluded after the first cognitive and/or polygraphic studies were found (i.e. AHI of the lost to follow-up subjects was higher, and they differed in the rates of obesity and hypertension), which suggests potential selection biases in those studies that used non-random samples of healthy elderly with normal cognition. In contrast, studies examining the association between OSA and dementia outcomes (i.e. decline to mild cognitive impairment [MCI] and dementia or Alzheimer’s disease [AD]) have been notoriously more consistent in their findings. Yaffe et al(130) in her seminal prospective study of OSA and cognition among women participating in the osteoporotic fractures (SOF) cohort, found that older adult women with OSA had an 85% higher risk of developing MCI/Dementia at follow-up. The expected high prevalence of Alzheimer disease (AD) in this age group (mean age 82 at inclusion) and absence of AD biomarker assessments do not preclude the possibility of reverse causation (i.e. preclinical or prodromal AD increasing the incidence of OSA). These findings however, have been replicated in subsequent studies among older male veterans(130, 131), severe OSA patients from the ARIC cohort(132), and several large national health insurance studies(133-135). We note here that several of these studies used medical records or administrative claims to make the diagnosis of OSA, which in most cases incorporates abnormal sleep breathing events alongside associated symptoms (e.g. EDS, stroke) or cardiovascular consequences that prompted these subjects to seek medical treatment. Thus, the link between OSA and cognitive decline to MCI or AD in these cases might also be driven by those seeking specialist assessments(136) and potentially absent in asymptomatic patients. For a systematic review of the above studies and findings see(136).
6. Effect of CPAP treatment of Sleep apnoea in elderly
6.1. Symptoms and Quality of life:
There is a clear evidence of CPAP efficacy in improving QoL in OSA patients particularly in those that are most symptomatic. However, the number of studies that focus on elderly adults is still small. Most studies report positive effects in both EDS (measured by EES) and QoL measured by different tools (SF-36, SAQLI, Fatigue Severity Scale [FSS], Patient-Health Questionnaire-9 [PHQ-9] and FOSQ)(137, 138). However, CPAP improvement is less apparent in elderly than in younger OSA patients(80), probably because the clinical impact of OSA on QoL is less pronounced(81).
With regards to randomized controlled trials (RCTs), only four studies have analyzed the effect of CPAP on EDS or QoL (as a primary(139–141) or as a secondary outcome(142)) in elderly OSA patients. In the PREDICT trial(140), 278 elderly patients (>65 years) were randomly allocated in parallel groups to receive either CPAP or best supportive care. EDS reduction at 3 and 12 months was greater in those patients with higher CPAP usage and those with higher baseline EES. However, objective sleepiness (measured by the Osler test) was reduced at 3 months but not at 12 months. Similarly, two methodological-mirrored studies from Martinez-Garcia and Ponce et al(139, 141) (n=224 and 145, respectively) performed in elderly OSA patients (≥70 years) concluded that treatment with CPAP reduced EES by 3.4 and 2.6 points, respectively. These two studies also observed a significant reduction in snoring, witnessed apnoeas, choking, and nocturia. In contrast, Dalmases et al did not find differences in EES in their small RCT aimed to randomly comparing 33 patients(142).
Findings from the same four RCTs with regards to QoL change are more controversial. In the PREDICT trial from McMillan et al(140), no significant change in the SF36 at 3 and 12 months was found except for the energy/vitality domain, whereas a clear improvement was observed using the sleep-specific SAQLI at both 3 and 6 months. Similarly, the two Spanish RCTs from Martinez-Garcia and Ponce(139, 141), using the sleep-specific QSQ, observed a significant improvement in all 5 domains (hypersomnolence, diurnal symptoms, nocturnal symptoms, emotions and social interaction) in severe OSA patients, but only in the nocturnal symptoms and emotions in the moderate group. Improvements in QSQ’s nocturnal symptoms and social interaction were also observed in the Dalmases et al trial(142). To conclude, a recent meta-analysis(143) of RCTs on CPAP efficacy for improvement in sleepiness and QoL (which included the four RCT previously discussed) concluded that CPAP treatment was associated with a decrease in 2.62 (95%CI, 1.93 to 3.30) points in EES and an improvement in all the QSQ domains although the quality of evidence was considered low using the GRADE system. Therefore, larger and longer follow-up studies are needed.
6.2. Cardiovascular system and prognosis:
The number of clinical studies that focus on the cardiovascular system among elderly OSA patients is also very small(85–90, 140, 141). Of these, only three are clinical trials(87, 140, 141), and none of them included a cardiovascular measure as the primary outcome. Table 4 shows the main characteristics and results of CPAP treatment on different risk factors and cardiovascular events, both in observational studies and clinical trials that included exclusively elderly adults. Regarding the clinical trials, neither of the two RCTs performed by Martinez-Garcia(87) and Ponce et al(141), in OSA patients 70 and older, observed changes in systolic (SBP) or diastolic blood pressure (DBP) levels after three months of CPAP treatment. In contrast, the study performed by McMillan et al(140), in OSA patients 65 and older, observed that CPAP achieved a significant reduction in total and low-density lypoproteins (LDL) cholesterol levels at 3 months of treatment (which were not maintained at 12 months of follow-up), as well as in SBP of 3.7 mmHg at 12 months. However, these findings did not translate into a clinically relevant reduction in the incidence of cardiovascular events at the one year of follow-up (most of which were episodes of atrial fibrillation)(140).
Among the observational studies, the largest to date has been published by Martinez-Garcia et al(87) (Figure 1) in a multicenter cohort of 969 elderly individuals who attended a sleep clinic for suspected OSA. After dividing the subjects into 4 groups: those without OSA (AHI<15 events/hour), those with mild-moderate disease (AHI ≥15 and <30 events), severe disease (AHI ≥30 events) treated and non-treated effectively with CPAP; they observed that the group with good adherence to CPAP showed a decrease in the risk of total cardiovascular death, stroke and heart failure to values similar to that seen in the control group without OSA. However, no effect was observed on fatal coronary events. In an attempt to delve into the analysis on the differences found between the effect of CPAP on the incidence of cerebrovascular and coronary events, Catalan-Serra et al(85), in a secondary analysis using the same series of patients, divided the sample into those who had and had not suffered a previous coronary and cerebrovascular event, respectively. Both series were followed, dividing the patients into the 4 groups already described according to the severity of OSA and CPAP treatment. Again, the results were similar, and while CPAP seemed to protect against new non-fatal cerebrovascular events in the elderly, it had no effect on coronary events.
A meta-analysis published by Yan et al(144) (9), which included 7 studies, both observational and RCT, observed that those elderly patients with severe OSA undergoing CPAP treatment had a lower cardiovascular risk (HR, 0.49; 95%CI, 0.36 to 0.66), although they only included 4 studies in this analysis. On the other hand, these subjects also presented lower mortality HR, 2.22 (95%CI, 1.64 to 3.01), although only 3 studies were included in this analysis. In both analyzes, due to its size, the study by Martinez-Garcia et al with 969 included individuals had a great influence on the results(87).
6.3. Cognitive function
The number of RCTs studies that focus exclusively on cognition as a primary or secondary outcome among elderly OSA patients is also very small (Table 5). Nonetheless, a similar picture to that of observational studies on the relationship between OSA and cognition described in section 5.3 emerges: with single-center small-sample trials from Aloia and Dalmases et al (n=12 and n=33)(142, 145) showing the greatest improvements in attention, psychomotor speed, memory and executive function; in clear contrast to the larger, multicenter clinical cohorts from Ponce, Martinez-Garcia and McMillan et al (n=145, 224 and 278 respectively) where CPAP showed significant improvement only in attention/working memory(139) or null effects(140, 141).
Table 5.
Neurocognitive impairment results in randomized clinical trial on the effect of CPAP in exclusively elderly series
| Study (RCT) | n | Age | CPAP vs no-CPAP | |||||
|---|---|---|---|---|---|---|---|---|
| Digit Span | Digit Symbol test | HADS depression | HADS anxiety | TMT-A | TMT-B | |||
| Aloia et al(145) | 12 | 64.8 ± 4.7 | - | 5.74 (C95%, 1.69 - 9.79), p<0.001 | - | - | - | 1.8–48.0 (6.8 <0.05 |
| Dalmases et al(142) | 33 | 71.3 ± 5.5 | 0.79 (CI95%,−0.30, 1.88), N.S | 5.74 (CI95%,1.69, 9.79), p<0.01 | −0.42 (CI95%:−1.84, 0.99), N.S | −0.43 (CI95%:−1.0, −1.88), N.S | −2.68 (CI95%:−16, 10.6), N.S | −47.64 (CI95%:−81.8, −13.4), p<0.01 |
| Mc Millan et al(140) | 278 | 71.1 | - | 1.1 (CI95%,−0.6, 2.7) N.S. | −0.4 (CI95%: −1.0, 0.3); N.S | −0.2 (CI95%: −0.9, 0.5); N.S | - | 6.2 (CI95% −3.4, 15.8); N.S |
| Martinez-Garcia et al(139) | 224 | 75.5 ± 3.9 | −0.08 (CI95%, −0.55, 0.4; N.S | −1.43 (CI95%, −2.84, −0.02); p<0.05. | 1.98 (CI95%: 1.18-2.7), p<0.001 | 1.19 (CI95% 0.22-2.16); p=0.016 | 10 (−4–34) versus 0 (−16–11); p=0.029) | −9.4 (95% CI −24–5.3) N.S |
| Ponce et al(141) | 145 | 74.9 ± 4.6 | −0.2 (CI95%, −0.6, 0.9); NS | −0.9 (CI95%, −2.5, −4.3) N.S | 0.2 (CI95%, −0.9, −0.5), N.S | 0.8 (CI95%, 0.2–1.7), N.S | −5.6 (CI95%, −14.3–3), N.S | −9.4 (95% CI −24–5.3). p<0.05 |
| Richards et al(146) | 54 | 70.1 ± 8.3 | - | 1.68 (CI95%:0.73-2.62), p<0.05 | - | - | - | - |
HADS: Hospital Anxiety and Depression Scale; TMT: Trail making Test; CPAP: Continuous positive airway pressure, CI: Confidence interval; RCT: Randomized controlled trail.
The low adherence and short CPAP usage in the McMillan et al RCT(140), as well as the presence of comorbidities that affected cognition might have diluted the treatment effect in this trial. In addition, OSA participants were starting from a very high cognitive baseline indicating potential ceiling effects (i.e. cognitive scores were often within the age-adjusted normative range). Compliance, however, was good (>70%) with at least 4 h-day in both Ponce’s and Martinez-Garcia et al trials(139, 141), showing levels of CPAP use similar or higher to those observed in middle-aged patient trials. It is noteworthy that Martinez-Garcia et al trial only included severe OSA patients, suggesting that cognitive impairment is more closely associated with severe OSA than with moderate OSA in late-life, and so a cognitive improvement with CPAP would be more likely only in the former. Of particular interest from the same trial are the findings that CPAP treatment was associated with marked improvements in anxiety and depression as measured by the Hospital Anxiety and Depression Scale (HADS). With regards to these changes in mood, the same meta-analysis(143) described on section 6.1, which pooled four of the RCT previously discussed, found that CPAP treatment was associated with a decrease of 0.94 points in the HADS-depression domain, with subgroup analyses showing better results in studies including populations ≥70 years old. Whether some of the observed improvements in cognition were mediated by improvements in mood in the elderly was not reported in any of the trials.
To conclude, a recent quasi-experimental study from Richards et al(146) with two comparison groups (pooled mean age of 70.1±7.9 years): 1) among prodromal AD patients: an MCI, OSA, and CPAP-adherent group (MCI + CPAP ≥4 h mean CPAP use per night for 1 year, n=29); and, 2) an MCI, OSA, CPAP-non-adherent group (MCI −CPAP <4 h mean CPAP use per night for 1 year, n=25), demonstrated significant improvements in psychomotor/cognitive processing speed using the Digits Symbol in the MCI + CPAP group vs the MCI −CPAP group after adjustment for age, race, and marital status(146).
6.4. CPAP adherence in the elderly
One of the key factors for a positive effect of CPAP on the cardiovascular prognosis is good adherence to the treatment, even in elderly patients(147–149). Although there is some debate regarding whether adherence to CPAP is different in young and elderly people, it is certain that the elderly can have characteristics that could be related to reduce adherence: living on their own, chronic pain, cognitive impairment, dental problems, etc(150, 151). A study undertaken exclusively on OSA patients aged over 65 demonstrated that the mean adherence to CPAP treatment fell below acceptable limits – i.e., less than 3 hours per day (2.9 ± 1.7 hours) –, particularly in patients aged 80 and older. In fact, only 23% of the patients used CPAP for at least 4 hours a day, on average, making it difficult to evaluate any positive effect of CPAP in the oldest old(152).
7. Central sleep apnoea in the elderly.
Thus far, most of the discussion has centered around OSA. The occurrence of events that transiently reduce breathing, but are not due to obstruction, i.e. central sleep apnoea (CSA), is a confound that affects very few of the younger patients coming to clinical attention in sleep clinics, with the exception of obvious neurological conditions such as genetic syndromes and disruptions of the neuro-respiratory apparatus(153–156). However, in the elderly, congestive heart failure is common, and upwards of 50% of patients with a reduced ejection fraction show the pattern of central apnoea called Hunter Cheyne Stokes (a waxing and waning respiration, with minimal obstruction and a somewhat longer period than OSA)(157). CSA may overlap with OSA and can be difficult to extricate(158, 159). The importance of distinguishing the disorders is twofold. CSA has not been shown to have the same clinical associations as OSA, and may be an incidental manifestation of the cardiac dysfunction that is not implicated in the clearly observed increased mortality(160). In addition, CSA does not respond to the same therapy as OSA: Hunter Cheyne Stokes in heart failure has been resistant to positive airway pressure therapy, and even shown an increased mortality on some variations of PAP therapy (ASV)(161). The most effective therapy for Hunter Cheyne Stokes respiration has consistently been shown to be treatment of the cardiac dysfunction(162). The other major form of Hunter Cheyne Stokes and central apnoea is that associated with neurological dysfunction, such as strokes, which are clearly common in the elderly. While there are data supporting that untreated OSA leads to recurrent stroke(163, 164), the data for treating CSA associated with stroke is much less clear(165, 166). Finally, while few studies have exclusively addressed Hunter Cheyne Stokes or central apnoea in the elderly, these are the subjects with the greatest incidence of the underlying heart failure.
8. Other therapeutical options.
The most used therapy for OSA is the application of continuous positive airway pressure(167). This therapy, originally proposed by Colin Sullivan in 1981(168), is remarkably effective, as it appears to “splint” the upper airway and eliminates the obstructive events. However, there is increasing data showing that, even in ideal and symptomatic populations, it is used much less than would be anticipated from its efficacy; most clinical trials using CPAP average less than 4-5 hours of therapy, much less than the desirable 7-8 hours of good sleep recommended(169). However real-world studies using big data have shown better CPAP compliance(170). In the elderly, there is conflicting evidence, but CPAP usage may be higher than(137), or as poor as(140), in younger subjects with OSA in clinical populations(137). In contrast, usage may be much lower in epidemiological studies where asymptomatic elderly are being recruited for therapy, or to test an effect on dementia(171). Thus CPAP is rarely successfully implemented in clinical elderly populations with minimal symptoms where the rationale for therapy is only the associated (presumed) consequences of OSA discussed above (hypertension, memory loss, perceived risk of or early evidence of AD). While it makes sense to try other therapies for OSA, such as mandibular advancement devices, positional therapy and other modalities, most of these are less predictable in their efficacy than CPAP and only recently are being subjected to monitoring of use and long term studies in the elderly. Many clinicians balance the difficulty and discomfort of OSA therapy against the lack of proven benefit in asymptomatic populations (including the elderly), and chose to treat only willing patients and leave the others untreated for anything other than severe OSA. Clinical trial showing clear benefit on cardiovascular and neurological long-term outcomes and higher levels of acceptance of therapy will be needed to change this.
9. Conclusions and Future challenges
The epidemiology of OSA and its apparent consequences overlap heavily with the complaints of aging populations and common chronic conditions seen in the elderly. However, the number of elderly patients in sleep clinics referred due to OSA suspicion is gradually rising, and it is predicted to continue rising in the next future. There is strong biological plausibility for causal associations between these common conditions, in both directions: OSA, perhaps though intermittent hypoxia or through sleep disturbance, could well cause accumulated neurological damage and/or accelerate AD pathology; conversely, the neurological deterioration that underlies aging and aging-related conditions like AD could easily affect the regulation of breathing and some of the mechanisms causing OSA. Alternatively, having OSA related consequences and symptoms could act on quality of life and exacerbate many of the most troublesome complaints of aging and its chronic conditions, independently of impacting their progression. However, at the present time there are insufficient data to prove causality of the associations between OSA and aging pathology; to do so would require a successful intervention for either condition as well as a long term follow-up. The implication is that when confronted with an elderly subject with OSA, one should treat severe symptomatic OSA as in all other groups. The implications for moderate, mild and asymptomatic disease are less clear. A rational approach is to offer therapeutic trials of CPAP and possibly mandibular advancement devices when disease is mild-moderate or minimally symptomatic, but not be too insistent on continuing therapy if the patient is not aware of improvements. The definitive approach will depend on yet-to-be performed clinical trials and improvements in obtaining long-term adherence to current or novel treatments. A better definition of the OSA phenotype in the elderly, with improved clinical criteria as well as novel treatment outcomes (e.g. novel AD blood biomarkers of neurodegeneration) are also important future directions. Table 6 shows some of most important future challenges that need to be urgently tackled in order to increase our understanding of OSA in these patients. Meanwhile, some important take home-messages can be stated on the management of OSA in the elderly (Table 7).
Table 6.
Future challenges in obstructive sleep apnoea (OSA) in the elderly
| To develop better polysomnographic parameter (beyond AHI) for evaluating OSA Validation of specific questionnaires (neurocognitive, diagnostic prediction, hypersomnia) |
| Validation of simplified diagnostic sleep test and development of new devices specifically for the elderly |
| Analysis of CPAP effectiveness (phenotypes of responders) in very old patients (more than 80 years old) and evaluation of the consequences of the withdrawal of CPAP in extreme old age |
| Educational programs aimed specifically at elderly people |
| To explore other therapeutic possibilities beyond CPAP |
| To analyze (especially by randomized controlled trials) the impact of OSA and the effect of CPAP on cardiovascular outcomes and mortality as well as Alheimer’s disease risk |
| Impact of comorbidities in the management of OSA in elderly |
Table 7.
Some messages with clinical implications about obstructive sleep apnoea (OSA) and CPAP treatment in the elderly
| OSA prevalence increases with age |
| Since it is not known what is the cut off in the apnoea-hypopnoea index and Epworth sleepiness scale that should be considered as pathological, it should be used as in younger individuals |
| A clinical history that includes findings specific to elderly people must be always undertaken |
| Sleepiness must not be considered a physiological situation in elderly. However it is not be assumed that it must be associated to OSA |
| The clinical picture of OSA in elderly is different than that seen in younger patients. Neurocognitive symptoms are especially prevalent |
| Full polysomnography is the gold standard in the diagnosis of OSA in elderly. However simplified tests can be used in patients without important comorbidities. |
| Randomized clinical trials demonstrated that CPAP is effective in symptomatic moderate to severe OSA patients |
| It is not known what happens in extreme old age (over 80 years) when the compliance to CPAP is very low, therefore a less aggressive approach may be appropriate when recommending treatment with CPAP |
Figure 2.
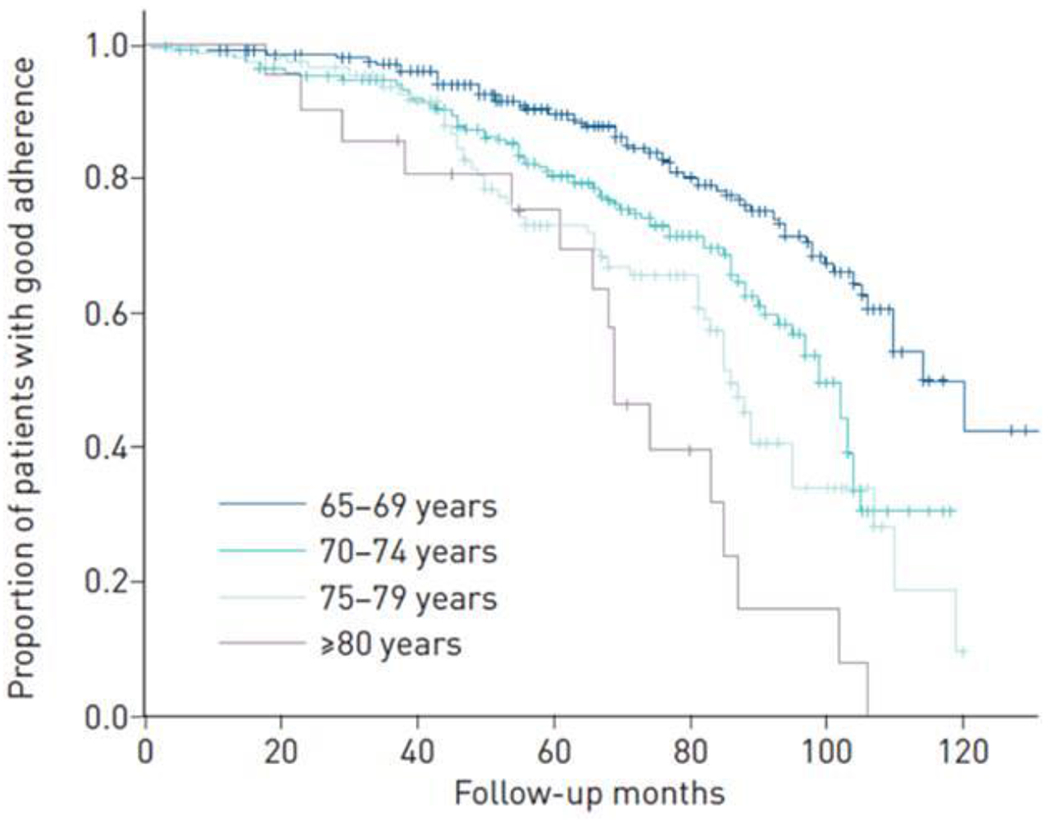
Kaplan-Meier curve with the proportion of elderly patients with good adherence (at least 4 hours.day) depending on the age (between 65-69 yo, between 70-74 yo, between 75-79 yo, at least 80 yo). Martinez-Garcia et al. ERJ Open Res. 2019 Mar 4;5(1). pii: 00178-2018, with permission.
Take Home Message:
Current evidence supporting obstructive sleep apnoea (OSA) in the elderly as a different clinical entity is still insufficient. In the meantime, older patients should be diagnosed and treated in the same manners as their younger counterparts with a particular focus on cognition and multi-morbidity.
Short sentence:
From clinical, prognostic and therapeutic points of view, obstructive sleep apnoea (OSA) in the elderly is both similar to, but also shows differences from, the syndrome in younger individuals and may contribute to unique disorders of the elderly. There are many gaps of knowledge of OSA in the elderly; the large number of aging people all over the world and the frequency of OSA make the overlap of these conditions an important area for future research
Funding:
Dr. Osorio is supported by R01AG056531, R01AG056031 and P30AG066512.
Footnotes
Previous presentations:
These data have not been presented in any scientific congress or meeting.
Conflict of interest:
For complete conflict of interest, see the ICJME documents
Publisher's Disclaimer: This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online.
REFERENCES
- 1.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects; 2019. [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosselin N, Baril AA, Osorio RS, Kaminska M, Carrier J. Obstructive Sleep Apnea and the Risk of Cognitive Decline in Older Adults. American journal of respiratory and critical care medicine. 2019; 199(2):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet (London, England). 2009;373(9657):82–93. [DOI] [PubMed] [Google Scholar]
- 5.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep medicine reviews. 2018;42:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2015; 11(2):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-García M, Campos-Rodriguez F, Barbé F. Cancer and OSA: Current Evidence From Human Studies. Chest. 2016;150(2):451–63. [DOI] [PubMed] [Google Scholar]
- 8.Almendros I, Martinez-Garcia MA, Farré R, Gozal D. Obesity, sleep apnea, and cancer. Int J Obes (Lond). 2020;44(8):1653–67. [DOI] [PubMed] [Google Scholar]
- 9.Jaul E, Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Frontiers in public health. 2017;5:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan A, Morrell MJ. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheffield, England). 2016;12(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep medicine reviews. 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-García MA, Durán-Cantolla J, Montserrat JM. [Sleep apnea-hypopnea syndrome in the elderly]. Archivos de bronconeumologia. 2010;46(9):479–88. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. American journal of respiratory and critical care medicine. 2018;197(7):933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Menezes Duarte RL, Soares De Oliveira E Sá T, Magalhães da Silveira FJ. Aging and its association with increased prevalence of obstructive sleep apnoea: A six-year experience in a Brazilian sleep center. 2016;48(suppl 60):PA337. [Google Scholar]
- 15.Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences – Results of SHIP-Trend. 2019;28(5):e12770. [DOI] [PubMed] [Google Scholar]
- 16.Iannella G, Vicini C, Colizza A, Meccariello G, Polimeni A, Greco A, et al. Aging effect on sleepiness and apneas severity in patients with obstructive sleep apnea syndrome: a meta-analysis study. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019;276(12):3549–56. [DOI] [PubMed] [Google Scholar]
- 17.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. Journal of applied physiology: respiratory, environmental and exercise physiology. 1978;44(6):931–8. [DOI] [PubMed] [Google Scholar]
- 18.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. Journal of applied physiology (Bethesda, Md : 1985). 2011;110(6):1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - New pathways for targeted therapy. Sleep medicine reviews. 2018;37:45–59. [DOI] [PubMed] [Google Scholar]
- 20.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep medicine reviews. 2017;35:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom Subtypes of Obstructive Sleep Apnea Predict Incidence of Cardiovascular Outcomes. American journal of respiratory and critical care medicine. 2019;200(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altintas N, Riha RL. Non-sleepy obstructive sleep apnoea: to treat or not to treat? European respiratory review : an official journal of the European Respiratory Society. 2019;28(154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams R, Appleton S, Taylor A, McEvoy D, Wittert G. Are the ICSD-3 criteria for sleep apnoea syndrome too inclusive? The Lancet Respiratory medicine. 2016;4(5):e19–20. [DOI] [PubMed] [Google Scholar]
- 24.Lee YG, Lee YJ, Jeong DU. Differential Effects of Obesity on Obstructive Sleep Apnea Syndrome according to Age. Psychiatry investigation. 2017;14(5):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmelli D, Swan GE, Bliwise DL. Relationship of 30-year changes in obesity to sleep-disordered breathing in the Western Collaborative Group Study. Obesity research. 2000;8(9):632–7. [DOI] [PubMed] [Google Scholar]
- 26.Degache F, Sforza E, Dauphinot V, Celle S, Garcin A, Collet P, et al. Relation of central fat mass to obstructive sleep apnea in the elderly. Sleep. 2013;36(4):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Archives of internal medicine. 2005;165(20):2408–13. [DOI] [PubMed] [Google Scholar]
- 28.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS medicine. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T, Lee CS, Lee SD, Kang SH, Han JW, Malhotra A, et al. Impacts of comorbidities on the association between arterial stiffness and obstructive sleep apnea in the elderly. Respiration; international review of thoracic diseases. 2015;89(4):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. The American journal of medicine. 2006;119(1):72.e9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veldi M, Vasar V, Hion T, Kull M, Vain A. Ageing, soft-palate tone and sleep-related breathing disorders. Clinical physiology (Oxford, England). 2001;21(3):358–64. [DOI] [PubMed] [Google Scholar]
- 32.Edwards BA, O’Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Seminars in respiratory and critical care medicine. 2010;31(5):618–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131(6):1702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Archives of internal medicine. 2004;164(4):406–18. [DOI] [PubMed] [Google Scholar]
- 35.Browne HA, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. The European respiratory journal. 2003;21(3):523–9. [DOI] [PubMed] [Google Scholar]
- 36.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. The Journal of physiology. 2007;581(Pt 1):291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. The European respiratory journal. 2012;40(2):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips BA, Berry DT, Lipke-Molby TC. Sleep-disordered breathing in healthy, aged persons. Fifth and final year follow-up. Chest. 1996;110(3):654–8. [DOI] [PubMed] [Google Scholar]
- 39.Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalai D, Bingeliene A, Shapiro C. Sleepiness in the Elderly. Sleep medicine clinics. 2017;12(3):429–41. [DOI] [PubMed] [Google Scholar]
- 41.Martínez-García M, Amilibia J, Chiner E, Queipo C, Díaz de Atauri MJ, Carmona-Bernal C, et al. [Sleep apnoea in patients of elderly: care activity in Spain (2002-2008)]. Archivos de bronconeumologia. 2010;46(10):502–7. [DOI] [PubMed] [Google Scholar]
- 42.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 43.Lindberg E, Taube A, Janson C, Gislason T, Svärdsudd K, Boman G. A 10-year follow-up of snoring in men. Chest. 1998;114(4):1048–55. [DOI] [PubMed] [Google Scholar]
- 44.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129(4):933–41. [DOI] [PubMed] [Google Scholar]
- 45.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. American journal of respiratory and critical care medicine. 2002;165(9):1217–39. [DOI] [PubMed] [Google Scholar]
- 46.Sforza E, Hupin D, Pichot V, Barthílímy JC, Roche F. A 7-year follow-up study of obstructive sleep apnoea in healthy elderly: The PROOF cohort study. Respirology (Carlton, Vic). 2017;22(5):1007–14. [DOI] [PubMed] [Google Scholar]
- 47.Kitakata H, Kohno T, Fukuda K. Sleep-disordered breathing in the elderly: is it distinct from that in the younger or middle-aged populations? Journal of thoracic disease. 2018;10(Suppl 9):S1102–s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. American journal of respiratory and critical care medicine. 1998;157(1):144–8. [DOI] [PubMed] [Google Scholar]
- 49.Braley TJ, Dunietz GL, Chervin RD, Lisabeth LD, Skolarus LE, Burke JF. Recognition and Diagnosis of Obstructive Sleep Apnea in Older Americans. Journal of the American Geriatrics Society. 2018;66(7):1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep & breathing = Schlaf & Atmung. 2002;6(2):49–54. [DOI] [PubMed] [Google Scholar]
- 51.Assoumou HG, Gaspoz JM, Sforza E, Pichot V, Celle S, Maudoux D, et al. Obstructive sleep apnea and the metabolic syndrome in an elderly healthy population: the SYNAPSE cohort. Sleep & breathing = Schlaf & Atmung. 2012;16(3):895–902. [DOI] [PubMed] [Google Scholar]
- 52.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–21. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbí F. Obstructive sleep apnoea and cardiovascular disease. The Lancet Respiratory medicine. 2013;1(1):61–72. [DOI] [PubMed] [Google Scholar]
- 54.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatrics & gerontology international. 2010;10 Suppl 1:S127–36. [DOI] [PubMed] [Google Scholar]
- 55.Lakatta EG. Deficient neuroendocrine regulation of the cardiovascular system with advancing age in healthy humans. Circulation. 1993;87(2):631–6. [DOI] [PubMed] [Google Scholar]
- 56.Korkushko OV, Shatilo VB, Plachinda Yu I, Shatilo TV. Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. Journal of the autonomic nervous system. 1991;32(3):191–8. [DOI] [PubMed] [Google Scholar]
- 57.Poller U, Nedelka G, Radke J, Pönicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. Journal of the American College of Cardiology. 1997;29(1):187–93. [DOI] [PubMed] [Google Scholar]
- 58.Norman D, Loredo JS. Obstructive sleep apnea in older adults. Clinics in geriatric medicine. 2008;24(1):151–65, ix. [DOI] [PubMed] [Google Scholar]
- 59.van den Berg JF, Tulen JH, Neven AK, Hofman A, Miedema HM, Witteman JC, et al. Sleep duration and hypertension are not associated in the elderly. Hypertension (Dallas, Tex : 1979). 2007;50(3):585–9. [DOI] [PubMed] [Google Scholar]
- 60.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31(10):1423–31. [PMC free article] [PubMed] [Google Scholar]
- 61.Corral J, Sánchez-Quiroga M, Carmona-Bernal C, Sánchez-Armengol Á, de la Torre AS, Durán-Cantolla J, et al. Conventional Polysomnography Is Not Necessary for the Management of Most Patients with Suspected Obstructive Sleep Apnea. Noninferiority, Randomized Controlled Trial. American journal of respiratory and critical care medicine. 2017;196(9):1181–90. [DOI] [PubMed] [Google Scholar]
- 62.Cilea R, Guaraldi P, Barletta G, Lucchi M, Baschieri F, Baldelli L, et al. Performing sleep studies after the COVID-19 outbreak: practical suggestions from Bologna’s sleep unit. Sleep medicine. 2021;77:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2017;13(3):479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas JA, Chai-Coetzer CL, McEvoy D, Naughton MT, Neill AM, Rochford P, et al. Guidelines for sleep studies in adults - a position statement of the Australasian Sleep Association. Sleep medicine. 2017;36 Suppl 1:S2–s22. [DOI] [PubMed] [Google Scholar]
- 65.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3(7):737–47. [PMC free article] [PubMed] [Google Scholar]
- 66.Penzel T, Kesper K, Ploch T, Becker HF, Vogelmeier C. Ambulatory recording of sleep apnea using peripheral arterial tonometry. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2004;2004:3856–9. [DOI] [PubMed] [Google Scholar]
- 67.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018;14(7):1209–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batool-Anwar S, Goodwin JL, Kushida CA, Walsh JA, Simon RD, Nichols DA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). Journal of sleep research. 2016;25(6):731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopes C, Esteves AM, Bittencourt LR, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2008;41(10):908–13. [DOI] [PubMed] [Google Scholar]
- 70.Lacasse Y, Godbout C, Sériès F. Health-related quality of life in obstructive sleep apnoea. The European respiratory journal. 2002;19(3):499–503. [DOI] [PubMed] [Google Scholar]
- 71.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep medicine reviews. 2003;7(4):335–49. [DOI] [PubMed] [Google Scholar]
- 72.Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring Subjective Sleep Quality: A Review. International journal of environmental research and public health. 2021;18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 74.McHorney CA, Ware JE Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31(3):247–63. [DOI] [PubMed] [Google Scholar]
- 75.Lacasse Y, Bureau MP, Sériès F. A new standardised and self-administered quality of life questionnaire specific to obstructive sleep apnoea. Thorax. 2004;59(6):494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–43. [PubMed] [Google Scholar]
- 77.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. American journal of respiratory and critical care medicine. 1998;158(2):494–503. [DOI] [PubMed] [Google Scholar]
- 78.Silva GE, Goodwin JL, Vana KD, Quan SF. Obstructive Sleep Apnea and Quality of Life: Comparison of the SAQLI, FOSQ, and SF-36 Questionnaires. Southwest journal of pulmonary & critical care. 2016;13(3):137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Launois SH, Pépin JL, Lévy P. Sleep apnea in the elderly: a specific entity? Sleep medicine reviews. 2007;11(2):87–97. [DOI] [PubMed] [Google Scholar]
- 80.Appleton SL, Vakulin A, McEvoy RD, Vincent A, Martin SA, Grant JF, et al. Undiagnosed obstructive sleep apnea is independently associated with reductions in quality of life in middle-aged, but not elderly men of a population cohort. Sleep & breathing = Schlaf & Atmung. 2015;19(4):1309–16. [DOI] [PubMed] [Google Scholar]
- 81.Martínez-García MA, Soler-Cataluña JJ, Román-Sánchez P, González V, Amorós C, Montserrat JM. Obstructive sleep apnea has little impact on quality of life in the elderly. Sleep medicine. 2009;10(1):104–11. [DOI] [PubMed] [Google Scholar]
- 82.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung. 2009;13(1):11–7. [DOI] [PubMed] [Google Scholar]
- 83.Li N, Liu Y, Zhao Y, Wu X, Tong J, Hua Y. Cerebrovascular reactivity in young and old patients with obstructive sleep apnea. Sleep medicine. 2020;73:125–9. [DOI] [PubMed] [Google Scholar]
- 84.Castro-Grattoni AL, Suarez-Giron M, Benitez I, Torres M, Almendros I, Farre R, et al. Effect of age on the cardiovascular remodelling induced by chronic intermittent hypoxia as a murine model of sleep apnoea. Respirology (Carlton, Vic). 2020;25(3):312–20. [DOI] [PubMed] [Google Scholar]
- 85.Catalan-Serra P, Campos-Rodriguez F, Reyes-Nuñez N, Selma-Ferrer MJ, Navarro-Soriano C, Ballester-Canelles M, et al. Increased Incidence of Stroke, but Not Coronary Heart Disease, in Elderly Patients With Sleep Apnea. Stroke. 2019;50(2):491–4. [DOI] [PubMed] [Google Scholar]
- 86.López-Padilla D, Alonso-Moralejo R, Martínez-García M, De la Torre Carazo S, Díaz de Atauri MJ. Continuous positive airway pressure and survival of very elderly persons with moderate to severe obstructive sleep apnea. Sleep medicine. 2016;19:23–9. [DOI] [PubMed] [Google Scholar]
- 87.Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. American journal of respiratory and critical care medicine. 2012;186(9):909–16. [DOI] [PubMed] [Google Scholar]
- 88.Munoz R, Duran-Cantolla J, Martínez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–21. [DOI] [PubMed] [Google Scholar]
- 89.Nishihata Y, Takata Y, Usui Y, Kato K, Yamaguchi T, Shiina K, et al. Continuous positive airway pressure treatment improves cardiovascular outcomes in elderly patients with cardiovascular disease and obstructive sleep apnea. Heart and vessels. 2015;30(1):61–9. [DOI] [PubMed] [Google Scholar]
- 90.Ou Q, Chen YC, Zhuo SQ, Tian XT, He CH, Lu XL, et al. Continuous Positive Airway Pressure Treatment Reduces Mortality in Elderly Patients with Moderate to Severe Obstructive Severe Sleep Apnea: A Cohort Study. PloS one. 2015;10(6):e0127775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. American journal of respiratory and critical care medicine. 2016;194(5):613–20. [DOI] [PubMed] [Google Scholar]
- 92.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. The New England journal of medicine. 2016;375(10):919–31. [DOI] [PubMed] [Google Scholar]
- 93.Javaheri S, Martinez-Garcia MA, Campos-Rodriguez F, Muriel A, Peker Y. Continuous Positive Airway Pressure Adherence for Prevention of Major Adverse Cerebrovascular and Cardiovascular Events in Obstructive Sleep Apnea. American journal of respiratory and critical care medicine. 2020;201(5):607–10. [DOI] [PubMed] [Google Scholar]
- 94.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. Journal of sleep research. 2009;18(4):397–403. [DOI] [PubMed] [Google Scholar]
- 95.Lavie L Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep medicine reviews. 2015;20:27–45. [DOI] [PubMed] [Google Scholar]
- 96.Steiner S, Schueller PO, Schulze V, Strauer BE. Occurrence of coronary collateral vessels in patients with sleep apnea and total coronary occlusion. Chest. 2010;137(3):516–20. [DOI] [PubMed] [Google Scholar]
- 97.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616–21. [DOI] [PubMed] [Google Scholar]
- 98.Della-Morte D, Cacciatore F, Salsano E, Pirozzi G, Del Genio MT, D’Antonio I, et al. Age-related reduction of cerebral ischemic preconditioning: myth or reality? Clinical interventions in aging. 2013;8:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalmases M, Torres M, Márquez-Kisinousky L, Almendros I, Planas AM, Embid C, et al. Brain tissue hypoxia and oxidative stress induced by obstructive apneas is different in young and aged rats. Sleep. 2014;37(7):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Archives of internal medicine. 1992;152(3):538–41. [PubMed] [Google Scholar]
- 101.Telakivi T, Kajaste S, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Cognitive function in middle-aged snorers and controls: Role of excessive daytime somnolence and sleep-related hypoxic events. Sleep. 1988; 11(5) :454–62. [PubMed] [Google Scholar]
- 102.Valencia-flores M, Bliwise DL, Guilleminault C, Cilveti R, Clerk A. Cognitive Function in Patients with Sleep Apnea after Acute Nocturnal Nasal Continuous Positive Airway Pressure (CPAP) Treatment: Sleepiness and Hypoxemia Effects. Journal of Clinical and Experimental Neuropsychology. 1996;18(2):197–210. [DOI] [PubMed] [Google Scholar]
- 103.Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90(5):686–90. [DOI] [PubMed] [Google Scholar]
- 104.Greenberg GD, Watson RK, Deptula D. Neuropsychological Dysfunction in Sleep Apnea. Sleep. 1987; 10(3):254–62. [DOI] [PubMed] [Google Scholar]
- 105.Hobzova M, Prasko J, Vanek J, Ociskova M, Genzor S, Holubova M, et al. Depression and obstructive sleep apnea. Neuro endocrinology letters. 2017;38(5):343–52. [PubMed] [Google Scholar]
- 106.Bawden FC, Oliveira CA, Caramelli P. Impact of obstructive sleep apnea on cognitive performance. Arquivos de neuro-psiquiatria. 2011;69(4):585–9. [DOI] [PubMed] [Google Scholar]
- 107.Mathieu A, Mazza S, Décary A, Massicotte-Marquez J, Petit D, Gosselin N, et al. Effects of obstructive sleep apnea on cognitive function: A comparison between younger and older OSAS patients. Sleep medicine. 2008;9(2):112–20. [DOI] [PubMed] [Google Scholar]
- 108.Naëgelé B, Thouvard V, Pépin JL, Lévy P, Bonnet C, Perret JE, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18(1):43–52. [PubMed] [Google Scholar]
- 109.Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, et al. Obstructive sleep apnea–hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep medicine. 2006;7(6):498–507. [DOI] [PubMed] [Google Scholar]
- 110.Hrubos-Strøm H, Nordhus IH, Einvik G, Randby A, Omland T, Sundet K, et al. Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep and Breathing. 2012;16(1):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kloepfer C, Riemann D, Nofzinger Eric A, Feige B, Unterrainer J, O’Hara R, et al. Memory Before and After Sleep in Patients with Moderate Obstructive Sleep Apnea. Journal of Clinical Sleep Medicine. 2009;05(06):540–8. [PMC free article] [PubMed] [Google Scholar]
- 112.Naëgelé B, Launois SH, Mazza S, Feuerstein C, Pépin J-L, Lévy P. Which Memory Processes are Affected in Patients With Obstructive Sleep Apnea? An Evaluation of 3 Types of Memory. Sleep. 2006;29(4):533–44. [DOI] [PubMed] [Google Scholar]
- 113.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of Sleep Disordered Breathing and Cognitive Deficit in APOE ε4 Carriers. Sleep. 2013;36(6):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24(1):93–100. [DOI] [PubMed] [Google Scholar]
- 115.Sharma H, Sharma SK, Kadhiravan T, Mehta M, Sreenivas V, Gulati V, et al. Pattern & correlates of neurocognitive dysfunction in Asian Indian adults with severe obstructive sleep apnoea. The Indian journal of medical research. 2010;132:409–14. [PubMed] [Google Scholar]
- 116.Alchanatis M, Zias N, Deligiorgis N, Liappas I, Chroneou A, Soldatos C, et al. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep and Breathing. 2008;12(1):17–24. [DOI] [PubMed] [Google Scholar]
- 117.Kim H, Dinges DF, Young T. Sleep-Disordered Breathing and Psychomotor Vigilance in a Community-Based Sample. Sleep. 2007;30(10):1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berry DTR, Phillips BA, Cook YR, Schmitt FA, Honeycutt NA, Arita AA, et al. Geriatric Sleep Apnea Syndrome: A Preliminary Description. Journal of Gerontology. 1990;45(5):M169–M74. [DOI] [PubMed] [Google Scholar]
- 119.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34(10):1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ju G, Yoon I-Y, Lee SD, Kim TH, Choe JY, Kim KW. Effects of Sleep Apnea Syndrome on Delayed Memory and Executive Function in Elderly Adults. Journal of the American Geriatrics Society. 2012;60(6):1099–103. [DOI] [PubMed] [Google Scholar]
- 121.Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, et al. Sleep-Disordered Breathing and Cognition in Older Women. Journal of the American Geriatrics Society. 2008;56(1):45–50. [DOI] [PubMed] [Google Scholar]
- 122.Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Preliminary Communication: Intellectual Deficit and Sleep-Related Respiratory Disturbance in the Elderly. Sleep. 1985;8(1):30–3. [DOI] [PubMed] [Google Scholar]
- 123.Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is Obstructive Sleep Apnoea Related to Neuropsychological Function in Healthy Older Adults? A Systematic Review and Meta-Analysis. Neuropsychology Review. 2017;27(4):389–402. [DOI] [PubMed] [Google Scholar]
- 124.Sforza E, Pichot V, Martin MS, Barthélémy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep medicine. 2015;16(8):981–6. [DOI] [PubMed] [Google Scholar]
- 125.Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Bonanni E, Magnavita N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behavioral sleep medicine. 2020;18(1):35–57. [DOI] [PubMed] [Google Scholar]
- 126.Hongyo K, Ito N, Yamamoto K, Yasunobe Y, Takeda M, Oguro R, et al. Factors associated with the severity of obstructive sleep apnea in older adults. Geriatrics & gerontology international. 2017;17(4):614–21. [DOI] [PubMed] [Google Scholar]
- 127.Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-lsrael S, Ensrud KE, et al. Associations Between Sleep-Disordered Breathing, Nocturnal Hypoxemia, and Subsequent Cognitive Decline in Older Community-Dwelling Men: The Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society. 2015;63(3):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort. Sleep. 2015;38(2):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lutsey PL, Bengtson LGS, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive Sleep Apnea and 15-Year Cognitive Decline: The Atherosclerosis Risk in Communities (ARIC) Study. Sleep. 2016;39(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep Quality and Risk of Dementia Among Older Male Veterans. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2015;23(6):651–4. [DOI] [PubMed] [Google Scholar]
- 131.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–11. [DOI] [PubMed] [Google Scholar]
- 132.Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: The Atherosclerosis Risk in Communities Study. Alzheimer’s & Dementia. 2018;14(2):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PloS one. 2013;8(10):e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee JE, Yang SW, Ju YJ, Ki SK, Chun KH. Sleep-disordered breathing and Alzheimer’s disease: A nationwide cohort study. Psychiatry Research. 2019;273:624–30. [DOI] [PubMed] [Google Scholar]
- 135.Tsai M-S, Li H-Y, Huang C-G, Wang RYL, Chuang L-P, Chen N-H, et al. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Patients With or Without Treatment: Real-World Evidence. The Laryngoscope. 2020;130(9):2292–8. [DOI] [PubMed] [Google Scholar]
- 136.Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep medicine reviews. 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serrano Merino J, Pérula de Torres L, Bardwell WA, Muñoz Gómez R, Roldán Villalobos A, Feu Collado N, et al. Impact of Positive Pressure Treatment of the Airway on Health-Related Quality of Life in Elderly Patients With Obstructive Sleep Apnea. Biological research for nursing. 2018;20(4):452–61. [DOI] [PubMed] [Google Scholar]
- 138.Pallansch J, Li Y, Bena J, Wang L, Foldvary-Schaefer N. Patient-Reported Outcomes in Older Adults With Obstructive Sleep Apnea Treated With Continuous Positive Airway Pressure Therapy. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2018;14(2):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martínez-García MÁ, Chiner E, Hernández L, Cortes JP, Catalán P, Ponce S, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. 2015;46(1):142–51. [DOI] [PubMed] [Google Scholar]
- 140.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. The Lancet Respiratory medicine. 2014;2(10):804–12. [DOI] [PubMed] [Google Scholar]
- 141.Ponce S, Pastor E, Orosa B, Oscullo G, Catalán P, Martinez A, et al. The role of CPAP treatment in elderly patients with moderate obstructive sleep apnoea: a multicentre randomised controlled trial. The European respiratory journal. 2019;54(2). [DOI] [PubMed] [Google Scholar]
- 142.Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia M, et al. Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study. Chest. 2015;148(5):1214–23. [DOI] [PubMed] [Google Scholar]
- 143.Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for Improvements in Sleepiness, Cognition, Mood, and Quality of Life in Elderly Patients With OSA: Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest. 2020;158(2):751–64. [DOI] [PubMed] [Google Scholar]
- 144.Yan B, Jin Y, Hu Y, Li S. Effects of continuous positive airway pressure on elderly patients with obstructive sleep apnea: a meta-analysis. Medecine sciences : M/S. 2018;34 Focus issue F1:66–73. [DOI] [PubMed] [Google Scholar]
- 145.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. Journal of psychosomatic research. 2003;54(1):71–6. [DOI] [PubMed] [Google Scholar]
- 146.Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, et al. CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea. Journal of the American Geriatrics Society. 2019;67(3):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wickwire EM, Bailey MD, Somers VK, Srivastava MC, Scharf SM, Johnson AM, et al. CPAP adherence is associated with reduced risk for stroke among older adult Medicare beneficiaries with obstructive sleep apnea. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wickwire EM, Bailey MD, Somers VK, Srivastava MC, Scharf SM, Johnson AM, et al. CPAP adherence reduces cardiovascular risk among older adults with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2020. [DOI] [PubMed] [Google Scholar]
- 149.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep medicine reviews. 2007;11(2):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kostikas K, Browne HA, Ghiassi R, Adams L, Simonds AK, Morrell MJ. The determinants of therapeutic levels of continuous positive airway pressure in elderly sleep apnea patients. Respiratory medicine. 2006;100(7):1216–25. [DOI] [PubMed] [Google Scholar]
- 151.Netzer NCC, Ancoli-Israel SC-C, Bliwise DL, Fulda S, Roffe C, Almeida F, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. The European respiratory journal. 2016;48(4):992–1018. [DOI] [PubMed] [Google Scholar]
- 152.Martinez-Garcia MA, Valero-Sánchez I, Reyes-Nuñez N, Oscullo G, Garcia-Ortega A, Gómez-Olivas JD, et al. Continuous positive airway pressure adherence declines with age in elderly obstructive sleep apnoea patients. ERJ open research. 2019;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2010;6(2):131–7. [PMC free article] [PubMed] [Google Scholar]
- 154.Siccoli MM, Valko PO, Hermann DM, Bassetti CL. Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. Journal of neurology. 2008;255(11):1687–92. [DOI] [PubMed] [Google Scholar]
- 155.Berger KI, Fagondes SC, Giugliani R, Hardy KA, Lee KS, McArdle C, et al. Respiratory and sleep disorders in mucopolysaccharidosis. Journal of inherited metabolic disease. 2013;36(2):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hilz MJ, Moeller S, Buechner S, Czarkowska H, Ayappa I, Axelrod FB, et al. Obstructive Sleep-Disordered Breathing Is More Common than Central in Mild Familial Dysautonomia. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2016;12(12):1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. Journal of the American College of Cardiology. 2017;69(7):841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Redeker NS, Muench U, Zucker MJ, Walsleben J, Gilbert M, Freudenberger R, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30(12):1756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mehra R Sleep apnea and the heart. Cleveland Clinic journal of medicine. 2019;86(9 Suppl 1):10–8. [DOI] [PubMed] [Google Scholar]
- 161.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. The New England journal of medicine. 2015;373(12):1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Selim BJ, Ramar K. Management of Sleep Apnea Syndromes in Heart Failure. Sleep medicine clinics. 2017;12(1):107–21. [DOI] [PubMed] [Google Scholar]
- 163.McDermott M, Brown DL. Sleep apnea and stroke. Current opinion in neurology. 2020;33(1):4–9. [DOI] [PubMed] [Google Scholar]
- 164.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. The New England journal of medicine. 2005;353(19):2034–41. [DOI] [PubMed] [Google Scholar]
- 165.Mohsenin V Is sleep apnea a risk factor for stroke? A critical analysis. Minerva medica. 2004;95(4):291–305. [PubMed] [Google Scholar]
- 166.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Archives of internal medicine. 2008;168(3):297–301. [DOI] [PubMed] [Google Scholar]
- 167.Epstein LJ, Kristo D, Strollo PJ Jr., Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 168.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–5. [DOI] [PubMed] [Google Scholar]
- 169.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep health. 2015;1(4):233–43. [DOI] [PubMed] [Google Scholar]
- 170.Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep medicine. 2019;59:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(4):305–9. [PMC free article] [PubMed] [Google Scholar]
- 172.Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. Journal of applied physiology (Bethesda, Md : 1985). 1993;74(5):2198–204. [DOI] [PubMed] [Google Scholar]
- 173.Gao F, Li YR, Xu W, An YS, Wang HJ, Xian JF, et al. Upper airway morphological changes in obstructive sleep apnoea: effect of age on pharyngeal anatomy. The Journal of laryngology and otology. 2020;134(4):354–61. [DOI] [PubMed] [Google Scholar]
- 174.Shaker R, Lang IM. Effect of aging on the deglutitive oral, pharyngeal, and esophageal motor function. Dysphagia. 1994;9(4):221–8. [DOI] [PubMed] [Google Scholar]
- 175.Chowdhuri S, Pranathiageswaran S, Loomis-King H, Salloum A, Badr MS. Aging is associated with increased propensity for central apnea during NREM sleep. Journal of applied physiology (Bethesda, Md : 1985). 2018;124(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M. Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest. 1989;96(5):1054–8. [DOI] [PubMed] [Google Scholar]
- 177.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carskadon MA, Dement WC. Respiration during sleep in the aged human. J Gerontol. 1981;36(4):420–3. [DOI] [PubMed] [Google Scholar]
- 179.McGinty D, Littner M, Beahm E, Ruiz-Primo E, Young E, Sowers J. Sleep related breathing disorders in older men: a search for underlying mechanisms. Neurobiol Aging. 1982;3(4):337–50. [DOI] [PubMed] [Google Scholar]
- 180.Hoch CC, Reynolds CF 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry. 1986;47(10):499–503. [PubMed] [Google Scholar]


