Abstract
Purpose
Outbreaks of Campylobacter infection are common, but studies exploring the clinical features of acute illness in the outbreak setting are scarce in existing literature. The main purpose of the present study was to investigate the clinical features of self-reported acute illness in gastroenteritis cases during a large waterborne Campylobacter outbreak in Askøy municipality, Norway, in 2019.
Methods
A web-based self-administered questionnaire, and invitation to participate was sent by the municipality of Askøy as text message to mobile phones using the municipality’s warning system to the inhabitants during the ongoing outbreak.
Results
Out of 3624 participants, 749 (20.7%) were defined as cases, of which 177 (23.6%) reported severe gastroenteritis. The most common symptoms were loose stools (90.7%), abdominal pain (89.3%) and diarrhea (88.9%), whereas 63.8% reported fever, 50.2% joint pain and 14.2% bloody stools. Tiredness, a symptom non-specific to gastroenteritis, was the overall most common symptom (91.2%).
Conclusion
About one in four of the cases reported symptoms consistent with severe gastroenteritis. We found more joint pain and less bloody stools than reported in published studies of laboratory confirmed campylobacteriosis cases. Tiredness was common in the current study, although rarely described in previous literature of acute illness in the outbreak setting.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-021-01652-3.
Keywords: Campylobacter infections, Disease outbreaks, Gastroenteritis, Waterborne diseases
Introduction
Campylobacter spp. is considered the most common bacterial cause of gastroenteritis worldwide, as well as in Europe and Norway [1–3]. Approximately 3000 cases are reported annually in Norway, of which more than 50% are acquired abroad. The domestically infected cases are either sporadic or associated with smaller outbreaks, most commonly waterborne [4, 5].
Common symptoms of gastroenteritis caused by Campylobacter include loose stools, diarrhea (≥ 3 loose stools in 24 h), nausea, vomiting, abdominal pain, bloody stools and fever, and the severity varies from mild and self-limiting symptoms (most common) to lethal disease [6–14]. Bloody stools and fever are considered markers of more severe infections [6, 13, 15, 16]. Several studies report symptoms and clinical features of campylobacteriosis, but these studies were predominantly published in the period from late 1970s to 2000, and based mainly on surveillance data or sporadic cases of laboratory confirmed infection [7–12, 15, 16]. Such cases represent a selected group that may differ from the total symptomatic population in the community [17, 18]. During large outbreaks of gastrointestinal infections, many cases are not tested and thus not registered. Most epidemiological studies on campylobacteriosis outbreaks aim to identify the source of the outbreak and rarely include detailed descriptions of clinical features.
In some cases, campylobacteriosis is complicated with joint symptoms, or post-infectious neuropathy or irritable bowel syndrome (IBS) [8, 19–23]. Antibiotics are usually not needed in treatment of campylobacteriosis but may be useful in patients with severe disease [6, 15, 16, 24–27]. The burden of symptoms during an outbreak, including the extent of more severe disease is difficult to investigate since research cannot be planned in advance. Hence, comprehensive baseline data from outbreaks are relatively rare.
In June 2019, there was a large community-wide waterborne Campylobacter outbreak in the island municipality Askøy (population 29,500) in Norway. The outbreak was detected on 6 June 2019, and the outbreak investigation team consisting of the municipality of Askøy and the National Institute of Public Health’s (NIPH) later concluded that the drinking water had been contaminated by Campylobacter jejuni sometime in late May 2019 [28]. The outbreak investigation findings were published in a report stating that more than 1500 inhabitants were ill during the outbreak [28]. Further, 67 patients were admitted to hospital and 2 deaths were related to the outbreak [28, 29]. Our group has long experience with research on clinical manifestations and complications of gastroenteritis in an outbreak setting [30, 31] and established a large cohort study within days after the outbreak was acknowledged. The primary aim of this study was to describe the clinical features of self-reported acute gastroenteritis in a Campylobacter outbreak setting. Secondary aims were to investigate factors associated with severe gastroenteritis.
Methods
The current paper is based on data from the baseline survey out of totally four surveys in the Askøy Campylobacter Outbreak Study (ASCOS), a longitudinal cohort study following the outbreak. Households in Askøy received an invitation to participate in the study on 20 June 2019. Invitations and a webpage link to the survey were sent by the municipality of Askøy by one text message (SMS) to approximately 16,000 mobile phones using the municipality’s warning system, encouraging as many household members as possible to answer a questionnaire. Information was also presented in public meetings arranged by the municipality, on the municipality’s web site, and on posters in municipality and GP offices during the study period. Participants of all ages were included in the study. Consent from parents was needed for participants under the age of 16, and parents were asked to answer the questionnaire on behalf of younger children. Inclusion was closed on 1 July 2019.
Participants were asked if they were ill during the outbreak. Participants responding ‘yes’ were further asked about the acute disease (symptoms, duration of disease and perceived severity), management (use of health care services and medication) and consequences of the disease (absence from work or school). Participants answering ‘no’ or ‘uncertain’ about acute illness did not receive these follow-up questions. Furthermore, all participants were asked if they had stayed continuously outside Askøy from 31 May up to the time for answering. The study population consists of all participants who answered the question whether they were ill during the outbreak, excluding those reporting that they had not been in Askøy during the outbreak (Fig. 1).
Fig. 1.
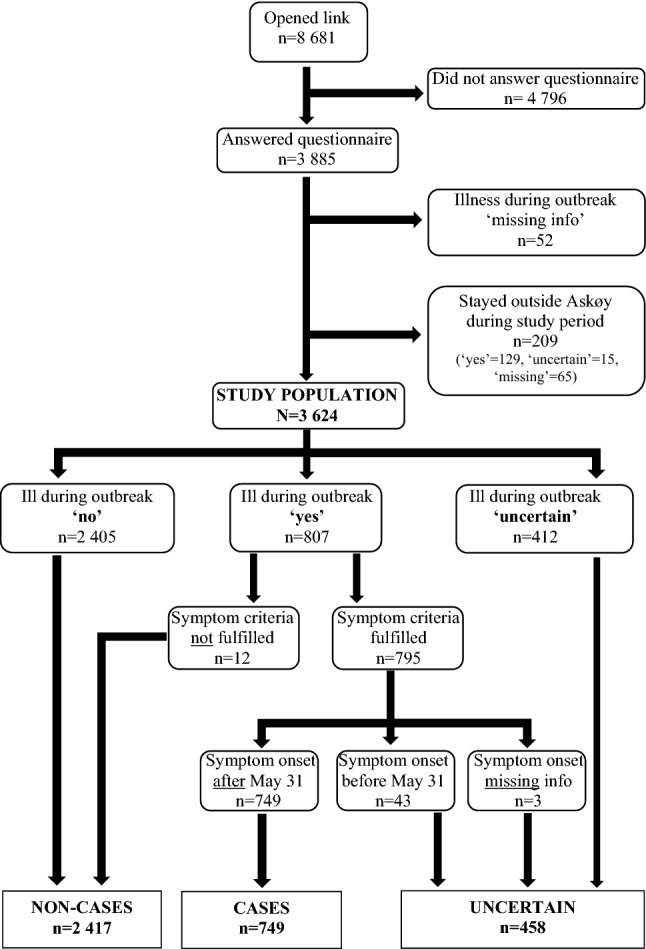
Flow chart of inclusion and exclusion of the study population, and of the cases, non-cases and the uncertain group, during the Campylobacter outbreak in Askøy
We defined a ‘case’ as a participant who reported being ill with gastrointestinal symptoms during the outbreak, with symptom onset in the study period, and who experienced at least one of the following symptoms: loose stools, diarrhea, bloody stools, abdominal pain, vomiting and nausea. A ‘non-case’ was a participant reporting not being ill during the outbreak or who reported being ill but did not fulfill the symptom criteria. Participants reporting that they were uncertain whether being ill during the outbreak or reported being ill and fulfilling the symptom criteria but with symptom onset either before the study period or missing, were assigned to the ‘uncertain’ group (Fig. 1). The participants were not asked whether they had submitted stool samples for laboratory verification of Campylobacter infection, as the aim of the study was to study self-reported gastroenteritis in the population during the Campylobacter outbreak.
Participants were asked to report ‘perceived severity’ at the worst time point during the acute illness, using a scale from 1 (well) to 9 (life-threatening illness). In addition, we defined the outcome ‘severe gastroenteritis’ as cases reporting diarrhea for ≥ 5 days and at least one of either fever for ≥ 2 days or bloody stools. Cases not fulfilling these criteria were defined as having ‘non-severe gastroenteritis’.
Duration of each of the following symptoms were specified by the predefined categories 1–2, 3–4 or 5–7 days, or 1–2 or > 2 weeks: loose stools, diarrhea, bloody stools, nausea, vomiting, abdominal pain, fever, joint pain, tiredness and other symptoms, in addition to total duration of illness (number of days). Questions whether still being ill and whether each symptom was still present at the time of answering the survey were also included. The study questionnaire furthermore included questions about age (number of years), sex, educational level (elementary school, high school, and college/university), employment situation (student/pupil, worker, self-employed, unemployed, on welfare, and pensioner), marital status (single, married, divorced/separated, and widow/widower), household total income in Norwegian kroner (< 250,000, 250,000–499,999, 500,000–749,999, 750,000–1,000,000, and > 1,000,000), self-reported previous diseases (diabetes, ulcerative colitis, Crohn’s disease, esophagitis, irritable bowel syndrome (IBS), celiac disease, peptic ulcer, anxiety, depression, and rheumatic disease), intake of glasses with tap water during the week prior to outbreak (0, 1–2, 3–5, > 5), intake of alcohol units during a normal week (number of units) and tobacco use (daily, sporadic, former daily smoker, and never smoked). Participants registered by their e-mail address, and were asked to voluntarily state their name, national identity number, telephone number and postal address for the purpose of follow-up studies and possibility/opportunity to be invited into adjacent research studies. All personally identifiable information were deidentified prior to analyses.
Two different categorical variables were made for age, with three (0–24, 25–54 and ≥ 55 years) and 10 categories (0–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84 and ≥ 85 years), respectively. Duration of illness was categorized into 0–3, 4–7, 8–14 and ≥ 15 days. Alcohol units were categorized into the following six categories: 0, 1–2, 3–5, 6–9, 10–14 and ≥ 15 units per week. Tobacco use was dichotomized. Analyses of alcohol and tobacco use were restricted to participants ≥ 16 years. Analyses of the variables educational level, employment situation and marital status, were restricted to participants ≥ 18 years. There was a high proportion of missing data for the variables age and sex in the baseline survey (29% and 26%, respectively), but we were able to add data from the follow-up surveys for 580 and 507, respectively, giving a final of 13% missing age and 12% missing sex information in the study population.
The data were collected online using SuveyXact by Rambøll. All data have been stored, processed, and analyzed on the University of Bergen’s solution for secure processing of sensitive personal data in research (SAFE). The software R, StataSE 16.1 and Microsoft Excel for Windows 365 MSO have been used for processing and analyzing the data.
Descriptive statistics and Pearson’s x2-tests for associations were used to examine the distribution of different characteristics by two outcomes: (1) cases, non-cases and the uncertain group, and (2) cases with severe gastroenteritis vs. non-severe gastroenteritis. For the outcome severe gastroenteritis vs non-severe gastroenteritis, we further explored the associations by estimating relative risks (RRs) with 95% confidence intervals using a modified Poisson regression model [32], adjusting for sex and age. Distribution of symptoms, illness duration, management, and short-term consequences of the acute disease by sex and age were explored using descriptive statistics and Pearson’s x2-tests for associations. Level of statistical significance was set at p < 0.05.
Results
During the study period, 8681 individuals accessed the web site, of which 3885 answered the questionnaire (Fig. 1). Of these, 261 were excluded because information about whether they had been ill was missing (n = 52) or because they had stayed outside Askøy (n = 209). In the study population of 3624 participants, 749 (20.7%) were cases, 2417 (66.7%) non-cases and 458 (12.6%) were uncertain. Tables 1 and 2 show the distribution of characteristics by cases, non-cases, and the uncertain group. The proportion of missing data for most variables, including sex and age, was highest in the uncertain group, followed by non-cases.
Table 1.
Demographic characteristics of the study population, by cases with self-reported gastroenteritis, non-cases and the uncertain group, during the Campylobacter outbreak in Askøy
| All | Cases | Non-cases | Uncertain | x2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | pa | ||
| Total | 3624 | 100 | 749 | 20.7* | 2417 | 66.7* | 458 | 12.6* | ||
| Sex | 0.174 | |||||||||
| Male | 1243 | 34.3 | 291 | 38.9 | 822 | 34.0 | 130 | 28.4 | ||
| Female | 1957 | 54.0 | 405 | 54.1 | 1291 | 53.4 | 261 | 57.0 | ||
| Missing | 424 | 11.7 | 53 | 7.1 | 304 | 12.6 | 67 | 14.6 | ||
| Age range (years) | 1–91 | 1–82 | 1–91 | 1–81 | ||||||
| Age | < 0.01 | |||||||||
| 0–4 | 22 | 0.6 | 6 | 0.8 | 12 | 0.5 | 4 | 0.9 | ||
| 5–14 | 30 | 0.8 | 8 | 1.1 | 14 | 0.6 | 8 | 1.7 | ||
| 15–24 | 236 | 6.5 | 72 | 9.6 | 136 | 5.6 | 28 | 6.1 | ||
| 25–34 | 483 | 13.3 | 112 | 15.0 | 296 | 12.2 | 75 | 16.4 | ||
| 35–44 | 701 | 19.3 | 159 | 21.2 | 460 | 19.0 | 82 | 17.9 | ||
| 45–54 | 691 | 19.1 | 171 | 22.8 | 427 | 17.7 | 93 | 20.3 | ||
| 55–64 | 477 | 13.2 | 101 | 13.5 | 328 | 13.6 | 48 | 10.5 | ||
| 65–74 | 445 | 12.3 | 52 | 6.9 | 349 | 14.4 | 44 | 9.6 | ||
| 75–84 | 82 | 2.3 | 10 | 1.3 | 67 | 2.8 | 5 | 1.1 | ||
| ≥ 85 | 2 | 0.1 | 0 | 0.0 | 2 | 0.1 | 0 | 0.0 | ||
| Missing | 455 | 12.6 | 58 | 7.7 | 326 | 13.5 | 71 | 15.5 | ||
| Education level** | < 0.01 | |||||||||
| Elementary school | 175 | 4.9 | 47 | 6.4 | 102 | 4.3 | 26 | 5.9 | ||
| High school | 1192 | 33.6 | 299 | 40.9 | 748 | 31.5 | 145 | 32.7 | ||
| University/college | 1431 | 40.4 | 290 | 39.7 | 981 | 41.4 | 160 | 36.0 | ||
| Missing | 748 | 21.1 | 95 | 13.0 | 540 | 22.8 | 113 | 25.5 | ||
| Employment** | < 0.01 | |||||||||
| Student/pupil | 146 | 4.1 | 34 | 4.7 | 95 | 4.0 | 17 | 3.8 | ||
| Worker | 1879 | 53.0 | 459 | 62.8 | 1193 | 50.3 | 227 | 51.1 | ||
| Self-employed | 94 | 2.7 | 20 | 2.7 | 62 | 2.6 | 12 | 2.7 | ||
| Unemployed | 89 | 2.5 | 22 | 3.0 | 52 | 2.2 | 15 | 3.4 | ||
| On welfare | 177 | 5.0 | 55 | 7.5 | 95 | 4.0 | 27 | 6.1 | ||
| Pensioner | 423 | 11.9 | 50 | 6.8 | 336 | 14.2 | 37 | 8.3 | ||
| Missing | 738 | 20.8 | 91 | 12.4 | 538 | 22.7 | 109 | 24.5 | ||
| Houshold income | 0.28 | |||||||||
| < 250,000 | 86 | 2.4 | 23 | 3.1 | 51 | 2.1 | 12 | 2.6 | ||
| 250,000–499,999 | 393 | 10.8 | 89 | 11.9 | 254 | 10.5 | 50 | 10.9 | ||
| 500,000–749,999 | 609 | 16.8 | 130 | 17.4 | 388 | 16.1 | 91 | 19.9 | ||
| 750,000–1,000,000 | 683 | 18.8 | 171 | 22.8 | 437 | 18.1 | 75 | 16.4 | ||
| > 1,000,000 | 900 | 24.8 | 191 | 25.5 | 621 | 25.7 | 88 | 19.2 | ||
| missing | 953 | 26.3 | 145 | 19.4 | 666 | 27.6 | 142 | 31.0 | ||
| Marital status** | < 0.01 | |||||||||
| Single | 440 | 12.4 | 131 | 17.9 | 251 | 10.6 | 58 | 13.1 | ||
| Married/cohabitant | 2152 | 60.7 | 459 | 62.8 | 1446 | 61.0 | 247 | 55.6 | ||
| Divorced/separated | 153 | 4.3 | 40 | 5.5 | 93 | 3.9 | 20 | 4.5 | ||
| Widow/widower | 61 | 1.7 | 9 | 1.2 | 45 | 1.9 | 7 | 1.6 | ||
| Missing | 740 | 20.9 | 92 | 12.6 | 536 | 22.6 | 112 | 25.2 | ||
Distribution within characteristics is given by column unless stated by *
*Distribution by row, i.e., within study population
**Analyses restricted to participants ≥ 18 years
aP values from Pearson’s x2-test of association calculated from cross tables not including missing values and ‘uncertain group’
Table 2.
Characteristics of the study population, by cases with self-reported gastroenteritis, non-cases, and the uncertain group, during the Campylobacter outbreak in Askøy
| All | Cases | Non-cases | Uncertain | x2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | pa | ||
| Total | 3624 | 100 | 749 | 20.7* | 2417 | 66.7* | 458 | 12.6* | ||
| Tap water (glasses/day)b | < 0.01 | |||||||||
| 0 | 225 | 6.2 | 21 | 2.8 | 183 | 7.6 | 21 | 4.6 | ||
| 1–2 | 963 | 26.6 | 158 | 21.1 | 664 | 27.5 | 141 | 30.8 | ||
| 3–5 | 1450 | 40.0 | 319 | 42.6 | 952 | 39.4 | 179 | 39.1 | ||
| > 5 | 976 | 26.9 | 247 | 33.0 | 612 | 25.3 | 117 | 25.5 | ||
| missing | 10 | 0.3 | 4 | 0.5 | 6 | 0.2 | 0 | 0.0 | ||
| Alcohol (units/week)**c | < 0.01 | |||||||||
| 0 | 1396 | 39.1 | 328 | 44.6 | 894 | 37.5 | 174 | 39.0 | ||
| 1–2 | 999 | 28.0 | 191 | 26.0 | 682 | 28.6 | 126 | 28.3 | ||
| 3–5 | 539 | 15.1 | 100 | 13.6 | 375 | 15.7 | 64 | 14.3 | ||
| 6–9 | 191 | 5.4 | 45 | 6.1 | 126 | 5.3 | 20 | 4.5 | ||
| 10–14 | 81 | 2.3 | 15 | 2.0 | 57 | 2.4 | 9 | 2.0 | ||
| ≥ 15 | 26 | 0.7 | 4 | 0.5 | 18 | 0.8 | 4 | 0.9 | ||
| missing | 336 | 9.4 | 52 | 7.1 | 235 | 9.8 | 49 | 11.0 | ||
| Tobacco** | 0.75 | |||||||||
| Yes | 1602 | 44.9 | 322 | 43.8 | 1076 | 45.1 | 204 | 45.7 | ||
| No | 1895 | 53.1 | 401 | 54.6 | 1260 | 52.8 | 234 | 52.5 | ||
| Missing | 71 | 2.0 | 12 | 1.6 | 51 | 2.1 | 8 | 1.8 | ||
| Previous diseases | ||||||||||
| None | 1860 | 51.3 | 332 | 44.3 | 1329 | 55.0 | 199 | 43.4 | < 0.01 | |
| Diabetes | 142 | 3.9 | 22 | 2.9 | 105 | 4.3 | 15 | 3.3 | 0.09 | |
| Ulcerative colitis | 50 | 1.4 | 6 | 0.8 | 34 | 1.4 | 10 | 2.2 | 0.20 | |
| Crohn’s disease | 16 | 0.4 | 4 | 0.5 | 7 | 0.3 | 5 | 1.1 | 0.32 | |
| Oesophagitis | 150 | 4.1 | 41 | 5.5 | 88 | 3.6 | 21 | 4.6 | 0.03 | |
| Irritable bowel syndrome | 318 | 8.8 | 76 | 10.1 | 187 | 7.7 | 55 | 12.0 | 0.04 | |
| Celiac disease | 35 | 1.0 | 12 | 1.6 | 17 | 0.7 | 6 | 1.3 | 0.02 | |
| Peptic ulcer | 93 | 2.6 | 24 | 3.2 | 51 | 2.1 | 18 | 3.9 | 0.09 | |
| Anxiety | 325 | 9.0 | 83 | 11.1 | 187 | 7.7 | 55 | 12.0 | < 0.01 | |
| Depression | 344 | 9.5 | 89 | 11.9 | 207 | 8.6 | 48 | 10.5 | < 0.01 | |
| Rheumatic/inflammatory | 207 | 5.7 | 54 | 7.2 | 121 | 5.0 | 32 | 7.0 | 0.02 | |
Distribution within characteristics is given by column unless stated by *
*Distribution by row, i.e., within study population
**Analyses restricted to participants ≥ 16 years old
ap values from Pearson’s x2-test of association calculated from cross tables not including missing values and ‘uncertain group’
bAverage daily number of tap water glasses during week before outbreak
cUnits of alcohol during a normal week
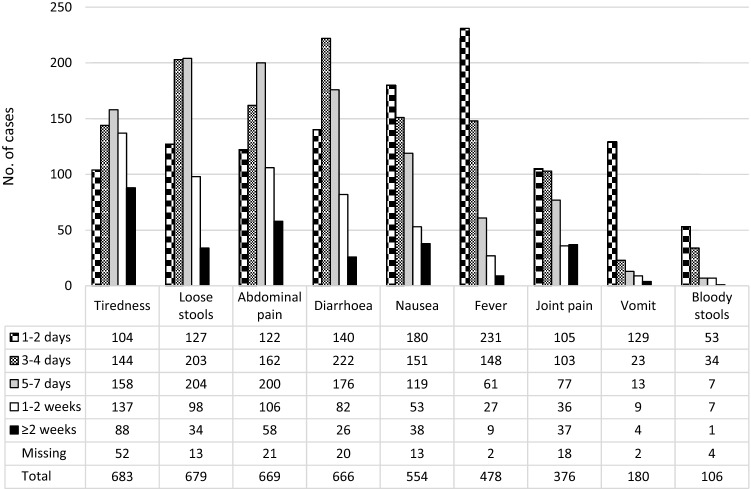
The most common symptoms reported by the 749 cases were tiredness (91.2%), loose stools (90.7%), abdominal pain (89.3%) and diarrhea (88.9%) (Table 3 and Fig. 2). Bloody stools (14.2%) and vomiting (24.0%) were the least frequently reported of the listed symptoms. Nausea and joint pain were reported by 74.0% and 50.2%, respectively, whereas 63.8% of the cases reported fever. More women than men reported nausea (79.3% in females vs. 66.7% in males, p < 0.01), abdominal pain (91.4% in females vs. 87.3% in males, p = 0.02) and joint pain (54.1% in females vs. 45.4 in males, p = 0.02). Vomiting, fever, and tiredness were more commonly reported among the youngest and the oldest, compared to those aged 25–54 years, whereas diarrhea was most common in age category 25–54 years. Bloody stools were most frequently reported by cases in the age category 0–24 years (25.6%); however, none of these was under the age of 15 years. In age category 25–54 years, bloody stools were reported in 14.7%, and in 8.6% of those 55 years or older.
Table 3.
Symptoms, management, and consequences of illness during the Campylobacter outbreak in Askøy, by age
| Age category (years) | All | 0–24 | 25–54 | ≥ 55 | Missing | x2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | p** | ||
| Total | 749 | 100 | 86 | 11.5* | 442 | 59.0* | 163 | 21.8* | 58 | 7.7* | ||
| Symptoms | ||||||||||||
| Loose stools | 679 | 90.7 | 77 | 89.5 | 408 | 92.3 | 144 | 88.3 | 50 | 86.2 | 0.73 | |
| Diarrhea | 666 | 88.9 | 70 | 81.4 | 408 | 92.3 | 138 | 84.7 | 50 | 86.2 | 0.04 | |
| Bloody stools | 106 | 14.2 | 22 | 25.6 | 65 | 14.7 | 14 | 8.6 | 5 | 8.6 | < 0.01 | |
| Nausea | 554 | 74.0 | 71 | 82.6 | 321 | 72.6 | 120 | 73.6 | 42 | 72.4 | 0.12 | |
| Vomit | 180 | 24.0 | 31 | 36.0 | 93 | 21.0 | 40 | 24.5 | 16 | 27.6 | 0.01 | |
| Abdominal pain | 669 | 89.3 | 78 | 90.7 | 400 | 90.5 | 142 | 87.1 | 49 | 84.5 | 0.94 | |
| Fever | 478 | 63.8 | 69 | 80.2 | 271 | 61.3 | 107 | 65.6 | 31 | 53.4 | < 0.01 | |
| Joint pain | 376 | 50.2 | 40 | 46.5 | 225 | 50.9 | 84 | 51.5 | 27 | 46.6 | 0.22 | |
| Tiredness | 683 | 91.2 | 82 | 95.3 | 396 | 89.6 | 151 | 92.6 | 54 | 93.1 | < 0.01 | |
| Illness duration (days) | 0.43 | |||||||||||
| 0–3 | 120 | 16.0 | 13 | 15.1 | 75 | 17.0 | 16 | 9.8 | 16 | 27.6 | ||
| 4–7 | 316 | 42.2 | 40 | 46.5 | 196 | 44.3 | 53 | 32.5 | 27 | 46.6 | ||
| 8–14 | 171 | 22.8 | 22 | 25.6 | 108 | 24.4 | 33 | 20.2 | 8 | 13.8 | ||
| ≥ 15 | 33 | 4.4 | 6 | 7.0 | 16 | 3.6 | 10 | 6.1 | 1 | 1.7 | ||
| Missing | 109 | 14.6 | 5 | 5.8 | 47 | 10.6 | 51 | 31.3 | 6 | 10.3 | ||
| Consulted doctor | 203 | 27.1 | 31 | 36.0 | 109 | 24.7 | 53 | 32.5 | 10 | 17.2 | 0.01 | |
| Hospitalized | 33 | 4.4 | 3 | 3.5 | 16 | 3.6 | 12 | 7.4 | 2 | 3.4 | 0.12 | |
| Absence school/work | 414 | 55.3 | 59 | 68.6 | 281 | 63.6 | 46 | 28.2 | 28 | 48.3 | < 0.01 | |
| Medication | ||||||||||||
| None | 184 | 24.6 | 21 | 24.4 | 109 | 24.7 | 42 | 25.8 | 12 | 20.7 | 0.03 | |
| Antibiotics | 21 | 2.8 | 2 | 2.3 | 10 | 2.3 | 8 | 4.9 | 1 | 1.7 | 0.12 | |
| Loperamide | 93 | 12.4 | 7 | 8.1 | 49 | 11.1 | 35 | 21.5 | 2 | 3.4 | < 0.01 | |
| Tramadol | 13 | 1.7 | 1 | 1.2 | 5 | 1.1 | 6 | 3.7 | 1 | 1.7 | 0.10 | |
| Codeine + paracetamol | 41 | 5.5 | 3 | 3.5 | 24 | 5.4 | 8 | 4.9 | 6 | 10.3 | 0.39 | |
| Paracetamol | 470 | 62.8 | 59 | 68.6 | 281 | 63.6 | 92 | 56.4 | 38 | 65.5 | 0.58 | |
| NSAIDs | 238 | 31.8 | 41 | 47.7 | 156 | 35.3 | 21 | 12.9 | 20 | 34.5 | < 0.01 | |
| Probiotics | 167 | 22.3 | 17 | 19.8 | 101 | 22.9 | 35 | 21.5 | 14 | 24.1 | 0.02 | |
Distribution within characteristics is given by column unless stated by *
*Distribution by row
**p value from Pearson’s x2-test of association calculated from cross tables not including missing values
Fig. 2.
Duration of symptoms reported by cases with self-reported gastroenteritis, during the Campylobacter outbreak in Askøy. N = 749
Illness duration ranged from 0 to 24 days, with a median at 6 days (interquartile range: 4–9 days) (Table 3). Nine percent (n = 68) reported still being ill at the time of answering the survey (data not shown). Slightly more men than women reported illness duration of 0–3 days (17.9% vs. 13.3%, p = 0.02), 4–7 days (42.3% vs. 41.2%, p = 0.02), and ≥ 15 days (5.2% vs. 4.2%, p = 0.02), while an illness duration of 8–14 days was more common in women than men (27.7% vs. 17.5%, p = 0.02). No significant differences across age were observed for the illness duration distribution (Table 3).
Each symptom’s duration turned out to fit into one of to two main patterns (Fig. 2): symptoms with (1) an approximate bell-shaped distribution where most cases reported either 3–4 days or 4–7 days duration (tiredness, loose stools, abdominal pain and diarrhea), and (2) symptoms most frequently reported to last for either 1–2 days or 3–4 days with a subsequent decrease (nausea, fever, joint pain, vomiting and bloody stools). At the time of answering the survey, the proportions of cases reporting each symptom being ‘still present’ were: tiredness 15.9%, abdominal pain 9.0%, joint pain 8.5%, nausea 6.1%, diarrhea 6.0%, loose stools 5.9%, fever 1.3%, bloody stools 1.2%, and vomiting 0.5% (data not shown).
Paracetamol (62.8%) was the medication most frequently reported for treatment of the acute illness, followed by non-steroidal anti-inflammatory drugs (NSAIDs) (31.8%) (Table 3). Antibiotics use was reported by 2.8% (n = 21) of the cases, of which none were under the age of 15 years. Among the hospitalized cases, 30.3% (n = 10) reported antibiotics use, compared to 1.5% (n = 11) of those not hospitalized. Loperamide was reported by 12.4% (n = 93) of the cases, most commonly used by those aged ≥ 55 years (21.5%) and least common in age category 0–24 years (8.1%). No cases under the age of 15 years reported use of loperamide. No significant differences between the sexes were observed for consulting a doctor or hospitalization (data not shown), whereas for medication more women than men reported use of paracetamol (65.4% vs. 58.1%, p = 0.04).
Twenty-seven percent (n = 203) of the cases reported to have consulted a primary care doctor, which was more common among the youngest and oldest compared to the middle-aged cases (Table 3). Four percent had been admitted to hospital, most commonly in the age category ≥ 55 years (Table 3). No cases below 15 years of age reported being admitted to hospital.
Twenty-four percent of the cases (n = 177) fulfilled the definition of ‘severe gastroenteritis’, and Supplementary tables 1 and 2 show the distribution and characteristics of these patients. There were significant positive associations between the outcome ‘severe gastroenteritis’, and the reported perceived severity at the worst time point of the illness (Table 4). Furthermore, cases with severe gastroenteritis more often had been in contact with a primary care doctor or were hospitalized (Table 4).
Table 4.
Health care seeking and perceived severity of illness during the Campylobacter outbreak in Askøy, by cases with severe or non-severe gastroenteritis
| All | Non-severe | Severe | x2 | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | pa | ||
| Total | 749 | 100 | 572 | 76.4* | 177 | 23.6* | ||
| Contacted doctor | < 0.01 | |||||||
| Yes | 203 | 27.1 | 117 | 20.5 | 86 | 48.6 | ||
| No | 539 | 72.0 | 450 | 78.7 | 89 | 50.3 | ||
| Uncertain | 5 | 0.7 | 3 | 0.5 | 2 | 1.1 | ||
| Missing | 2 | 0.3 | 2 | 0.3 | 0 | 0.0 | ||
| Hospitalized | 0.01 | |||||||
| Yes | 33 | 4.4 | 16 | 2.8 | 17 | 9.6 | ||
| No | 715 | 95.5 | 555 | 97.0 | 160 | 90.4 | ||
| Missing | 1 | 0.1 | 1 | 0.2 | 0 | 0.0 | ||
| Perceived severityb | < 0.01 | |||||||
| 1 well | 8 | 1.1 | 6 | 1.0 | 2 | 1.1 | ||
| 2 | 39 | 5.2 | 37 | 6.5 | 2 | 1.1 | ||
| 3 | 125 | 16.7 | 118 | 20.6 | 7 | 4.0 | ||
| 4 | 152 | 20.3 | 133 | 23.3 | 19 | 10.7 | ||
| 5 | 157 | 21.0 | 133 | 23.3 | 24 | 13.6 | ||
| 6 | 160 | 21.4 | 99 | 17.3 | 61 | 34.5 | ||
| 7 | 83 | 11.1 | 37 | 6.5 | 46 | 26.0 | ||
| 8 | 21 | 2.8 | 8 | 1.4 | 13 | 7.3 | ||
| 9 life threatening | 4 | 0.5 | 1 | 0.2 | 3 | 1.7 | ||
Distribution within characteristics is given by column unless stated by *
*Distribution by row
ap values from Pearson’s x2-test of association calculated from cross tables between cases with non-severe and severe gastroenteritis
bSelf-reported perceived severity at worst time point of illness. Scale from 1 (well) to 9 (life threatening)
Compared to the others, cases with severe gastroenteritis more often reported drinking > 5 glasses of tap water (41.2% vs 30.4%, p = 0.02), previous peptic ulcer (13.6% vs 2.3%, p = 0.01) and previous depression (16.9% vs 10.3%, p = 0.02) (Supplementary table 2). In the adjusted regression analyses, previous depression (RR: 1.62, 95% CI 1.17–2.26) and previous peptic ulcer (RR: 1.73, 95% CI 1.00–3.00) remained significant (Table 5). Further, age 55–64 years (RR: 0.63, 95% CI 0.41–0.94) and 35–44 (RR: 0.53, 95% CI 0.36–0.78), were associated with a lowered risk of severe gastroenteritis as compared to the reference age category 45–54 years, although the RR for age 55–64 years was not significant in the unadjusted regression model.
Table 5.
Severe gastroenteritis by characteristics, during the Campylobacter outbreak in Askøy. Unadjusted and adjusted relative risks (RR) with 95% confidence intervals (CIs)
| Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|
| RR | CI | RR | CI | ||
| Sex | |||||
| Male | 1.20 | 0.93—1.6 | 1.33 | 1.03—1.72 | |
| Female | Reference | Reference | |||
| Age group (years) | |||||
| 0–4 | 1.02 | 0.32—3.22 | 1.23 | 0.34—4.41 | |
| 5–14 | 0.38 | 0.06—2.42 | 0.35 | 0.05—2.23 | |
| 15–24 | 0.89 | 0.59—1.36 | 0.77 | 0.50—1.19 | |
| 25–34 | 0.85 | 0.59—1.22 | 0.77 | 0.53—1.12 | |
| 35–44 | 0.54 | 0.36—0.80 | 0.53 | 0.36—0.78 | |
| 45–54 | Reference | Reference | |||
| 55–64 | 0.67 | 0.43—1.02 | 0.63 | 0.41—0.96 | |
| 65–74 | 0.65 | 0.37—1.14 | 0.63 | 0.36—1.09 | |
| 75–84 | 0.92 | 0.35—2.42 | 0.84 | 0.30—2.37 | |
| ≥ 85 | NA | NA | |||
| Tap water (glasses/day)b | |||||
| 0 | 0.83 | 0.34—2.06 | 0.75 | 0.31—1.78 | |
| 1–2 | 0.72 | 0.48—1.08 | 0.72 | 0.48—1.08 | |
| 3–5 | Reference | Reference | |||
| > 5 | 1.29 | 0.98—1.71 | 1.29 | 0.97—1.70 | |
| Diseases | |||||
| None | 0.84 | 0.65—1.10 | |||
| Diabetes | 1.56 | 0.89—2.76 | |||
| Ulcerative colitis | 2.14 | 0.95—4.81 | |||
| Crohn’s disease | 1.06 | 0.19—5.81 | |||
| Oesophagitis | 1.37 | 0.86—2.19 | |||
| Irritable bowel syndrome | 1.26 | 0.86—1.84 | |||
| Celiac disease | 0.35 | 0.05—2.29 | |||
| Peptic ulcer | 2.00 | 1.27—3.16 | 1.73 | 1.00—3.00 | |
| Anxiety | 1.32 | 0.92—1.89 | |||
| Depression | 1.51 | 1.09—2.09 | 1.62 | 1.17—2.26 | |
| Rheumatic/inflammatory | 0.94 | 0.56—1.57 | |||
aAdjusted for sex, age, intake of tap water, peptic ulcer and depression
bAverage daily number of tap water glasses during week before outbreak
NA not applicable
Discussion
The typical gastroenteritis cases during the Campylobacter outbreak in our study were adults experiencing illness lasting for 4–7 days, with diarrhea, abdominal pain, and tiredness as the most common symptoms. About one in two of the cases reported fever or joint pain, whereas bloody stools and vomiting were less common. One in four of the cases fulfilled our definition of severe gastroenteritis. Risk factors associated with severe gastroenteritis were having depression or peptic ulcer prior to the outbreak, in addition to high consumption of tap water. Approximately 1 in 4 had consulted a doctor, and 4% had been hospitalized.
A main strength of this study was that data were collected during the acute phase of a large outbreak, which increases statistical power and reduces recall bias considerably. This also constitutes a solid basis for follow-up studies of post-infectious complaints after the outbreak.
Invitations to participate in the study were sent by the municipality of Askøy as text message to mobile phones using the municipality’s warning system, an approach that had recently been used by the municipality and the Norwegian Institute of Public Health as part of their outbreak investigation [28]. Askøy municipality has 29,500 inhabitants, and not all could be reached by this approach. Information about age and sex were extracted from the national identity number, for those who had stated this. Using this procedure secured precise information for those who responded to this request, but resulted in missing data for those who did not want to give this information.
As many as 458 participants, 12.6% of the study population, were uncertain whether they had been ill with acute gastrointestinal infection during the outbreak. Many persons in this group were likely having incident diffuse symptoms of other causes, but the size of the groups suggests that a fraction represents the less severe end of the spectrum of Campylobacter infection. As this group was not asked questions about symptoms, we could not categorize them as neither cases or non-cases based on such information. The uncertain group had the greatest proportion of missing data for most variables, representing a group with more uncertain answers overall. Since the cases were not laboratory confirmed nor did we have variables to verify exposure to Campylobacter, such as detailed information on the drinking water supply, we could not investigate potential risk factors for developing campylobacteriosis during the outbreak.
Cases were not verified by a clinician’s diagnosis or by laboratory information. Using this population-based approach, we were able to investigate a broad spectrum of symptoms during the outbreak. However, this also introduces some limitations. By use of a self-administered online questionnaire, we defined a ‘case’ based on the participants’ self-reported information about their geographical presence, onset of illness and symptoms related to the outbreak. There exist no common, symptom-based definitions of campylobacteriosis or gastroenteritis that are widely used for research purposes. Thus, our case definition was a modification of case definitions used in previous studies [33–36].
Our definition of ‘severe gastroenteritis’ was based on existing literature [6, 13, 15, 16], as well as clinical experiences and expertise among members in the research group, and aimed to capture a set of symptoms which indicated a greater extent of both local inflammation in the bowels (diarrhea for ≥ 5 days or bloody stools) and more generalized disease (fever > 2 days). We observed an association between ‘severe gastroenteritis’, and perceived severity at the worst time point during the illness, and health care use, which to some extent suggests validity to the definition.
Population-based cohort studies describing the clinical features of Campylobacter infection during an ongoing outbreak, are scarce in existing literature. The proportion of children with acute gastroenteritis was lower in our study compared to previous studies of Campylobacter infections [9, 10]. Selection bias may have led to underrepresentation of the elderly and children. The latter is suggested by our previous finding of 17 patients under the age of 16 years in the study that characterized hospitalized patients during the same outbreak [29], whereas the present study found no hospitalized cases under the age of 15 years.
Our finding of diarrhea and abdominal pain as the most common, and bloody stools and vomiting as the least common symptoms of acute gastroenteritis in the outbreak setting, is in line with previous literature on Campylobacter infection [8–10, 14, 34, 37]. The proportion of cases reporting bloody stools were 14% in the present study, which is higher compared to the findings in two previous outbreak investigation studies: a population-based study of an outbreak in Røros, Norway (2%) [34], and in a study of cases included among patients seeking health care services (of which 16% were laboratory confirmed) during an outbreak in Finland (4%) [37]. The two previously published studies of the Askøy outbreak; NIPH’s population-based outbreak investigation study (6%) [34] and the study of hospitalized cases (9%), also found lower proportions of bloody stools than the present study [28, 29]. Higher proportions of bloody stools, ranging from 30 to 58%, are reported in a study of laboratory confirmed cases in general practice in the Netherlands [9], of sporadic notified cases in Norway [10], of laboratory confirmed cases aged 0–14 years in an outbreak in Greece [38], and notified cases in Australia, Canada and the United states [14]. The latter study also reported association between age and bloody stools, in line with our finding, although their proportions of bloody diarrhea among the youngest (59% in age < 5 years, 49% in 5–24 years) were higher than in our study (25.6% aged < 25 years, but none < 15 years). The reason for lower proportions of bloody stools reported in outbreak studies, including the present study, may be that they capture a broader scope of clinical features than represented by the laboratory confirmed cases. The corresponding low proportion of bloody stools observed in the study of hospitalized patients during the Askøy outbreak, can be explained by a possible lowered threshold for referral due to fatal outcome in the initial phase of the outbreak, thus leading to hospitalization of less severe cases [29]. However, virulence factors associated with bloody stools of the particular strain of Campylobacter jejuni cannot be ruled out [29].
Joint pain was more common in our study compared to a Norwegian study of sporadic campylobacteriosis from 1992 (50% vs. 27%) [10], but otherwise seems to be scarcely described in existing literature as a symptom during the acute phase of Campylobacter infection. Tiredness was not a case-defining symptom, but still the most frequently reported in our study. We could not find descriptions of tiredness in published studies, probably because it is unspecific to gastroenteritis or Campylobacter infection. However, documenting the baseline level of the symptom at the time of the outbreak is useful to follow-up studies of post-infectious complaints, and should perhaps be investigated further in future outbreaks. Our findings of more common joint pain and less bloody stools in these cases with self-reported gastroenteritis than previously reported in studies of laboratory confirmed Campylobacter cases, may reflect that the population-based approach may capture a broader spectrum of clinical features of acute gastroenteritis during in the Campylobacter outbreak setting.
Median duration of illness observed in our study (6 days) is in line with what is commonly reported in previous studies (5–6 days) [14, 34, 37], except for the Norwegian study of sporadic cases from 1992 reporting median 11 days duration [10].
A total of 3%, none under the age of 15 years, and 30% of the hospitalized cases in the current study received antibiotic treatment. The study of hospitalized patients during the same outbreak found that one in two of children and one in ten of adults received antibiotics [29]. Kapperud et al. reported in 1992 that 16% of 135 sporadic laboratory confirmed cases in Norway were treated with antibiotics [10], and White et al. 2019 reported an antibiotic treatment proportion of 35% in culture confirmed cases in Australia, Canada and the United States [14], although neither discriminated between hospitalized and non-hospitalized treatment proportions. Our finding of low antibiotic treatment proportion is concordant with the recommendations, and with a generally cautious policy regarding use of antibiotics in Norway [6, 24, 25, 39].
Risk factors associated with severe gastroenteritis were high consumption of tap water, having depression or peptic ulcer prior to the outbreak, whereas being in the age category 35–44 seemed to be protective. As the outbreak was waterborne, the association between high consumption of tap water and severe gastroenteritis probably indicates a dose–response relationship. Psychological comorbidity has previously been shown to increase susceptibility to develop infectious gastroenteritis [23], but bias may lead to reporting of more severe symptoms in cases with depression, as the symptom pressure can be perceived as more burdensome in this patient group. However, this effect should have been reduced because the outcome ‘severe gastroenteritis’ in these analyses was defined by reported symptoms rather than the cases’ own assessment of perceived illness severity. Peptic ulcer as a risk factor for severe illness is reasonable, as a gastrointestinal disease, and not least presumably often treated with anti-acidic medication which may cause vulnerability to a more severe illness.
Conclusions
We present clinical features of self-reported acute gastroenteritis in a population during a large waterborne outbreak of Campylobacter infection. The most common symptoms were loose stools, abdominal pain, and diarrhea. About one in four of the cases reported symptoms consistent with severe gastroenteritis. Although not a gastroenteritis specific symptom, tiredness was the overall most common symptom, but is rarely described in previous studies of acute campylobacteriosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Parts of the work were carried out at the Biostatistics and Data analysis core facility (BIOS) and were thus supported by the Faculty of Medicine at the University of Bergen and its partners. Thanks to the Municipality of Askøy and the Norwegian Institute of Public Health for collaboration in the project.
Financial support
Open access funding provided by University of Bergen (incl Haukeland University Hospital). This study was supported by Faculty of Medicine at the University of Bergen, Western Norway Regional Health Authority (Helse Vest RHF), NORCE Norwegian Research Centre, and Municipality of Askøy.
Data availability
The data underlying this article cannot be shared publicly due to limitations given by the ethical approval by the Regional Committee for Medical and Health Research Ethics.
Declarations
Conflict of interest
None.
Ethical approval
Regional Committee for Medical and Health Research Ethics, REC West (project number 2019/1086).
Footnotes
The original online version of this article was revised: Modifications have been made to the Section Results and also to Table 1, Table 5 and Supplementary file 1. Full information regarding the corrections made can be found in the correction for this article.
Change history
1/20/2022
A Correction to this paper has been published: 10.1007/s15010-021-01746-y
References
- 1.European Centre for Disease Prevention and Control (ECDC). Campylobacteriosis. In: Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available from: https://www.ecdc.europa.eu/en/publications-data/campylobacteriosis-annualepidemiological-report-2017. Accessed 10 Apr 2021.
- 2.World Health Organization (WHO). Campylobacter. Key facts. Geneva: WHO. Available from: https://www.who.int/en/news-room/fact-sheets/detail/campylobacter. Accessed 10 April 2021.
- 3.Norwegian Veterinary Institute. The Norwegian Zoonoses Report 2019. Oslo: Norwegian Veterinary Institute; 2020. Available from: https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2020/the-norwegian-zoonoses-report-2019. Accessed 10 April 2021.
- 4.Sandberg M, Nygard K, Meldal H, Valle PS, Kruse H, Skjerve E. Incidence trend and risk factors for campylobacter infections in humans in Norway. BMC Public Health. 2006;6:179. doi: 10.1186/1471-2458-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald E, White R, Mexia R, Bruun T, Kapperud G, Lange H, et al. Risk factors for sporadic domestically acquired campylobacter infections in Norway 2010–2011: a National Prospective case-control study. PLoS ONE. 2015;10:e0139636. doi: 10.1371/journal.pone.0139636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allos B, Calderwood SB, Bloom A. Clinical manifestations, diagnosis, and treatment of Campylobacter infection. Up-to-date. (2021). https://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-treatment-of-campylobacter-infection?search=campylobacter&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed 24 April 2021.
- 7.Blaser MJ, Berkowitz ID, LaForce FM, Cravens J, Reller LB, Wang WL. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979;91:179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 8.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 9.de Wit MA, Koopmans MP, Kortbeek LM, van Leeuwen NJ, Bartelds AI, van Duynhoven YT. Gastroenteritis in sentinel general practices, The Netherlands. Emerg Infect Dis. 2001;7:82–91. doi: 10.3201/eid0701.700082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapperud G, Lassen J, Ostroff SM, Aasen S. Clinical features of sporadic campylobacter infections in Norway. Scand J Infect Dis. 1992;24:741–749. doi: 10.3109/00365549209062459. [DOI] [PubMed] [Google Scholar]
- 11.Kendall EJ, Tanner EI. Campylobacter enteritis in general practice. J Hyg (Lond) 1982;88:155–163. doi: 10.1017/s0022172400070030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKendrick MW, Geddes AM, Gearty J. Campylobacter enteritis: a study of clinical features and rectal mucosal changes. Scand J Infect Dis. 1982;14:35–38. doi: 10.3109/inf.1982.14.issue-1.07. [DOI] [PubMed] [Google Scholar]
- 13.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, et al. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White AE, Ciampa N, Chen Y, Kirk M, Nesbitt A, Bruce BB, et al. Characteristics of campylobacter and salmonella infections and acute gastroenteritis in older adults in Australia, Canada, and the United States. Clin Infect Dis. 2019;69:1545–1552. doi: 10.1093/cid/ciy1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dryden MS, Gabb RJ, Wright SK. Empirical treatment of severe acute community-acquired gastroenteritis with ciprofloxacin. Clin Infect Dis. 1996;22:1019–1025. doi: 10.1093/clinids/22.6.1019. [DOI] [PubMed] [Google Scholar]
- 16.Thielman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. New Engl J Med. 2004;350:38–47. doi: 10.1056/NEJMcp031534. [DOI] [PubMed] [Google Scholar]
- 17.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. Br Med J. 1999;318:1046–50. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaser MJ. Epidemiologic and clinical features of campylobacter jejuni infections. J Infect Dis. 1997;176:S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–50. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 21.Scallan Walter EJ, Crim SM, Bruce BB, Griffin PM. Postinfectious Irritable Bowel Syndrome After Campylobacter Infection. Am J Gastroenterol. 2019;114:1649–56. 10.14309/ajg.0000000000000408. [DOI] [PubMed]
- 22.Uotila T, Korpela M, Vuento R, Laine J, Lumio J, Kuusi M, et al. Joint symptoms after a faecal culture positive Campylobacter infection associated with a waterborne gastroenteritis outbreak: a questionnaire study. Scand J Rheumatol. 2014;43:524–526. doi: 10.3109/03009742.2014.920916. [DOI] [PubMed] [Google Scholar]
- 23.Wouters MM, Van Wanrooy S, Nguyen A, Dooley J, Aguilera-Lizarraga J, Van Brabant W, et al. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65:1279–1288. doi: 10.1136/gutjnl-2015-309460. [DOI] [PubMed] [Google Scholar]
- 24.Norwegian Directorate of Health. Norwegian guidelines for the use of antibiotics in primary care. Available from: https://www.helsedirektoratet.no/retningslinjer/antibiotikabruk-i-primaerhelsetjenesten. Accessed 27 Apr 2021.
- 25.Norwegian Directorate of Health. Norwegian guidelines for the use of antibiotics in hospitals. Available from: https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus/. Accessed: 14 May 2021.
- 26.Ruiz-Palacios GM. The Health Burden of Campylobacter Infection and the Impact of Antimicrobial Resistance: Playing Chicken. Clin Infect Dis. 2007;44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 27.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis. 2007;44:696–700. doi: 10.1086/509924. [DOI] [PubMed] [Google Scholar]
- 28.Hyllestad S, Iversen A, MacDonald E, Amato E, Borge BÅS, Bøe A, et al. Large waterborne Campylobacter outbreak: use of multiple approaches to investigate contamination of the drinking water supply system, Norway, June 2019. Eurosurveillance. 2020;25:1–10. doi: 10.2807/1560-7917.ES.2020.25.35.2000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortensen N, Jonasson SA, Lavesson IV, Emberland KE, Litleskare S, Wensaas KA, et al. Characteristics of hospitalized patients during a large waterborne outbreak of Campylobacter jejuni in Norway. PLoS ONE. 2021;16:e0248464. doi: 10.1371/journal.pone.0248464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;61:214–219. doi: 10.1136/gutjnl-2011-300220. [DOI] [PubMed] [Google Scholar]
- 31.Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland N, Wensaas KA. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after giardia infection. Clin Gastroenterol Hepatol. 2018;16:1064–72.e4. doi: 10.1016/j.cgh.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 32.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 33.Bartholomew N, Brunton C, Mitchell P, Williamson J, Gilpin B. A waterborne outbreak of campylobacteriosis in the South Island of New Zealand due to a failure to implement a multi-barrier approach. J Water Health. 2014;12:555–563. doi: 10.2166/wh.2014.155. [DOI] [PubMed] [Google Scholar]
- 34.Jakopanec I, Borgen K, Vold L, Lund H, Forseth T, Hannula R, et al. A large waterborne outbreak of campylobacteriosis in Norway: The need to focus on distribution system safety. BMC Infect Dis. 2008;8:128. doi: 10.1186/1471-2334-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhn KG, Falkenhorst G, Emborg HD, Ceper T, Torpdahl M, Krogfelt KA, et al. Epidemiological and serological investigation of a waterborne Campylobacter jejuni outbreak in a Danish town. Epidemiol Infect. 2017;145:701–709. doi: 10.1017/S0950268816002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majowicz SE, Hall G, Scallan E, Adak GK, Gauci C, Jones TF, et al. A common, symptom-based case definition for gastroenteritis. Epidemiol Infect. 2008;136:886–894. doi: 10.1017/S0950268807009375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuusi M, Klemets P, Miettinen I, Laaksonen I, Sarkkinen H, Hanninen ML, et al. An outbreak of gastroenteritis from a non-chlorinated community water supply. J Epidemiol Community Health. 2004;58:273–277. doi: 10.1136/jech.2003.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karagiannis I, Sideroglou T, Gkolfinopoulou K, Tsouri A, Lampousaki D, Velonakis EN, et al. A waterborne Campylobacter jejuni outbreak on a Greek island. Epidemiol Infect. 2010;138:1726–1734. doi: 10.1017/S0950268810002116. [DOI] [PubMed] [Google Scholar]
- 39.NORM/NORM-VET 2018. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo: NORM/NORM-VET: 2019. Available from: https://www.vetinst.no/overvaking/antibiotikaresistens-norm-vet. Accessed 27 April 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to limitations given by the ethical approval by the Regional Committee for Medical and Health Research Ethics.