Abstract
Small trials have suggested that heterologous vaccination with first-dose ChAdOx1 and second-dose BNT162b2 may generate a better immune response than homologous vaccination with two doses of ChAdOx1. In this cohort analysis, we use linked data from Catalonia (Spain), where those aged <60 who received a first dose of ChAdOx1 could choose between ChAdOx1 and BNT162b2 for their second dose. Comparable cohorts were obtained after exact-matching 14,325/17,849 (80.3%) people receiving heterologous vaccination to 14,325/149,386 (9.6%) receiving homologous vaccination by age, sex, region, and date of second dose. Of these, 464 (3.2%) in the heterologous and 694 (4.8%) in the homologous groups developed COVID-19 between 1st June 2021 and 5th December 2021. The resulting hazard ratio (95% confidence interval) is 0.66 [0.59–0.74], favouring heterologous vaccination. The two groups had similar testing rates and safety outcomes. Sensitivity and negative control outcome analyses confirm these findings. In conclusion, we demonstrate that a heterologous vaccination schedule with ChAdOx1 followed by BNT162b2 was more efficacious than and similarly safe to homologous vaccination with two doses of ChAdOx1. Most of the infections in our study occurred when Delta was the predominant SARS-CoV-2 variant in Spain. These data agree with previous phase 2 randomised trials.
Subject terms: Epidemiology, Health policy, Vaccines, SARS-CoV-2
Different homologous and heterologous vaccination regimens have been used for COVID-19. Here the authors show in a cohort analysis from Catalonia that heterologous vaccination with ChAdOx1 followed by BNT162b2 has better vaccine effectiveness than two doses of ChAdOx1.
Introduction
The rapid development of vaccines to prevent Coronavirus Disease 2019 (COVID-19) has allowed remarkable progress in the global fight against the SARS-CoV-2 pandemic. As of 27 October 2021, around half of the world’s population had received at least one dose of a COVID-19 vaccine. While 15 vaccines have been approved for use by at least one authority, most countries have received more than four approved vaccines1.
The Chimpanzee Adenovirus-vectored Oxford (ChAdOx1) and BioNTech/Pfizer mRNA (BNT162b2) vaccines were among the first approved by the European Medicines Agency for emergency use in the European Union. Both vaccines were tested in large phase 3 randomised controlled trials and found to be highly effective against symptomatic SARS-CoV-2 infection when given as two doses2–5. Follow-up studies have demonstrated their clinical effectiveness against severe disease, including preventing hospitalisations and mortality, overall6,7 and in previously under-researched populations8.
Spanish guidelines initially recommended the use of ChAdOx1 for people aged younger than 60 years due to the under-representation of elderly people in the initial pivotal trials2. Key workers were targeted in this initial stage to maximise the impact of vaccination on community transmission9. Despite their efficacy, reports of thrombotic events after the first dose of adenovirus-based COVID-19 vaccines led to recommendations for heterologous vaccination for those vaccinated with a first dose of ChAdOx1, i.e. many European authorities recommended the use of BNT162b2 for second doses to avoid further exposure to ChAdOx1. A small randomised controlled trial was rapidly conducted that demonstrated better immunogenicity from heterologous vaccination in Spain10, but larger studies on clinical effectiveness and safety are urgently needed11.
The Spanish authorities allowed citizens previously vaccinated with a first dose of ChAdOx1 to choose between ChAdOx1 and BNT162b2 for their second dose. The majority chose homologous vaccination with two doses of ChAdOx112. In the absence of phase 3/4 randomised controlled trials, this created a natural experiment for studying the comparative safety and effectiveness of these two vaccination schedules. We leveraged routinely collected health data, including electronic medical records linked to vaccination data and laboratory tests, to study the comparative effectiveness and safety of homologous (two-dose ChAdOx1) and heterologous (ChadOx1 followed by BNT162b2) vaccination.
Results
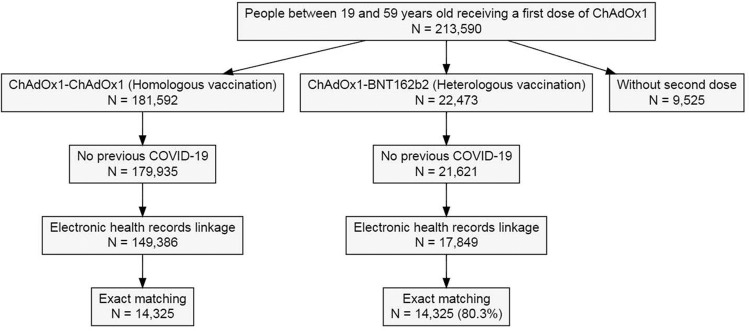
Of 167,235 eligible people, 17,849 (10.7%) chose heterologous vaccination and 149,386 (89.3%) chose homologous vaccination. Figure 1 shows the inclusion/exclusion steps used to identify the study participants. For primary analyses, 14,325/17,849 (80.3%) people in the heterologous group were matched to 14,325/149,386 (9.6%) in the homologous group vaccinated with their second dose on the same date (±2 days). The resulting cohorts were comparable in terms of all observed demographics, comorbidity, medicine use, area of residence and socio-economic status (Supplementary Fig. 1). Exact matching ensured the same average (SD) age (42.2 (9.6) years) and proportion of female participants (62.5%) in the two groups. A comparable proportion lived in the most socio-economically deprived (15.8% heterologous vs 15.9% homologous) and rural (19.4% heterologous vs 19.5% homologous) areas of the country (Table 1).
Fig. 1.
Population flowchart.
Table 1.
Baseline characteristics of study participants according to vaccination schedule.
| Variable | Heterologous | Homologous |
|---|---|---|
| N | 14,325 | 14,325 |
| Socio-demographic and socio-economic | ||
| Mean (SD) age, years | 42.20 (9.60) | 42.21 (9.57) |
| Female sex | 8959 (62.5%) | 8959 (62.5%) |
| Socio-economic status: first quartile (least deprived) | 2725 (19.02%) | 2745 (19.16%) |
| Socio-economic status: second quartile | 4403 (30.74%) | 4363 (30.46%) |
| Socio-economic status: third quartile | 2162 (15.09%) | 2145 (14.97%) |
| Socio-economic status: fourth quartile (most deprived) | 2260 (15.78%) | 2276 (15.89%) |
| Residence in a rural area | 2775 (19.37%) | 2796 (19.52%) |
| Medicines use | ||
| Analgesics | 654 (4.57%) | 533 (3.72%) |
| Sedatives/hypnotics | 1049 (7.32%) | 948 (6.62%) |
| Anticoagulants | 201 (1.40%) | 116 (0.81%) |
| Antidepressants | 1228 (8.57%) | 1077 (7.52%) |
| Antiepileptics | 449 (3.13%) | 353 (2.46%) |
| Antipsychotics | 281 (1.96%) | 157 (1.10%) |
| Antacids | 618 (4.31%) | 533 (3.72%) |
| Systemic corticosteroids | 101 (0.71%) | 82 (0.57%) |
| Oral antidiabetics | 187 (1.31%) | 150 (1.05%) |
| Insulin | 105 (0.73%) | 73 (0.51%) |
| Lipid modifying agents | 453 (3.16%) | 410 (2.86%) |
| Alpha blockers | 5 (0.03%) | 2 (0.01%) |
| Other antihypertensives | 4 (0.03%) | 1 (0.01%) |
| Beta blockers | 202 (1.41%) | 193 (1.35%) |
| Calcium channel blockers | 135 (0.94%) | 101 (0.71%) |
| Combination antihypertensives | 209 (1.46%) | 176 (1.23%) |
| Diuretics | 103 (0.72%) | 108 (0.75%) |
| ACE inhibitors/ARBs | 423 (2.95%) | 424 (2.96%) |
| Chronic obstructive pulmonary disease/asthma inhalers | 579 (4.04%) | 528 (3.69%) |
| Comorbidities | ||
| Atrial fibrillation | 23 (0.16%) | 19 (0.13%) |
| Osteoarthritis | 513 (3.58%) | 533 (3.72%) |
| Asthma | 935 (6.53%) | 910 (6.35%) |
| Ischaemic heart disease | 48 (0.34%) | 38 (0.27%) |
| Diabetes mellitus | 266 (1.86%) | 209 (1.46%) |
| Liver disease | 289 (2.02%) | 278 (1.94%) |
| Hypertension | 826 (5.77%) | 814 (5.68%) |
| Heart failure | 5 (0.03%) | 2 (0.01%) |
| Cerebrovascular disease | 41 (0.29%) | 26 (0.18%) |
| Chronic obstructive pulmonary disease | 46 (0.32%) | 37 (0.26%) |
| Chronic kidney disease | 44 (0.31%) | 54 (0.38%) |
| Cancer (all except non-melanoma skin cancer) | 344 (2.40%) | 325 (2.27%) |
| Obesity | 1539 (10.74%) | 1391 (9.71%) |
| Valvular disease | 73 (0.51%) | 64 (0.45%) |
| Hepatitis B | 20 (0.14%) | 15 (0.10%) |
| Hepatitis C | 49 (0.34%) | 34 (0.24%) |
| HIV infection | 49 (0.34%) | 47 (0.33%) |
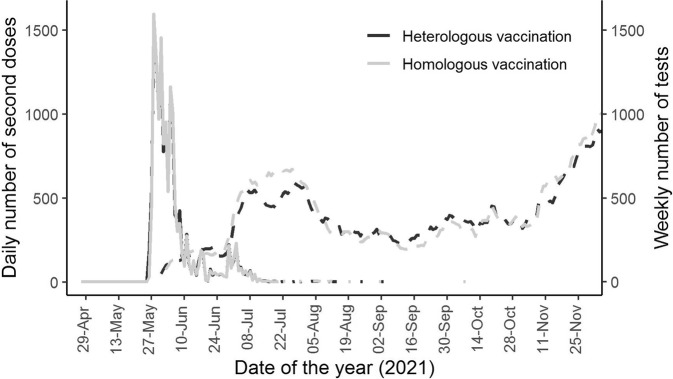
Study participants received their second doses between 27 April 2021 and 8 October 2021. Second doses occurred on the same date for 8742 (61.0%) matched pairs, 1 day apart (before/after) for 4302 (30.0%) pairs and 2 days apart for the remaining 1281 (9.0%) pairs. Test rates were similar in the matched cohorts, with 4874 (34.0%) people on the heterologous schedule and 3268 (35.8%) on the homologous schedule tested at least once during the study period. The average (SD) number of tests for heterologous and homologous groups was 0.82 (1.65) vs 0.87 (1.68) overall and 2.42 (2.04) vs 2.44 (2.03) among those tested at least once during follow-up, respectively. Table 2 shows number, type of test (lateral flow test (LFT) vs polymerase chain reaction (PCR) test) and test incidence rates according to vaccination regimen. The incidence rate ratio [95% confidence interval] of testing for heterologous versus homologous vaccination was 1.01 [0.96–1.06]. Figure 2 shows that the matched cohorts had similar timings for vaccination and testing over time.
Table 2.
Number and incidence rate (per 1000 person-years) of tests and SARS-CoV-2 infection (positive test) following second-dose vaccination according to vaccination schedule.
| Heterologous | Homologous | HR/IRR | |||
|---|---|---|---|---|---|
| N / Mean | IR / SD | N / Mean | IR / SD | [95% CI] | |
| Tested | 4874 | 2.21/1000 py | 5134 | 2.35/1000 py | n/a |
| Number of tests (overall) | 0.82 | 1.65 | 0.87 | 1.68 | 1.01 [0.96–1.06] |
| N of tests among the tested | 2.42 | 2.04 | 2.44 | 2.03 | n/a |
| N of PCR tests (overall) | 0.37 | 1.15 | 0.38 | 1.20 | 1.02 [0.96–1.08] |
| N of PCR tests among the tested | 1.86 | 1.98 | 1.87 | 2.08 | n/a |
| N of LFT tests overall | 0.45 | 1.03 | 0.50 | 1.05 | 1.00 [0.96–1.04] |
| N of LFT tests among the tested | 2.18 | 1.17 | 2.17 | 1.07 | n/a |
| SARS-CoV-2 infection | 464 | 0.18/1000 py | 694 | 0.27/1000 py | 0.66 [0.59–0.74] |
CI confidence interval, HR hazard ratio, IR incidence rate, IRR incidence rate ratio, N number, py person-years of follow-up, SD standard deviation.
Fig. 2.
Vaccine uptake and testing rates according to vaccination schedule.
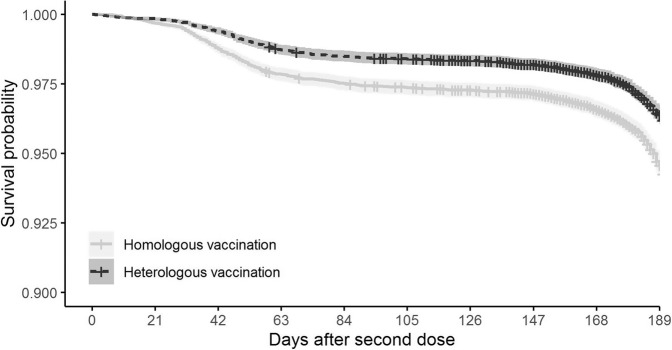
Between 1 June and 5 December 2021, SARS-CoV-2 infections were recorded for 464 (3.2%) people in the heterologous group, equivalent to an incidence rate of 0.18/1000 person-years, and 694 (4.8%) people in the homologous group, equivalent to an incidence rate of 0.27/1000 person-years. These rates are equivalent to a hazard ratio of 0.66 [0.59–0.74], favouring heterologous vaccination (Fig. 3 and Table 2). This was equivalent to an absolute risk reduction (ARR) of 0.016 [0.012–0.021] in the study period. Only 2 (0.01%) hospital admissions with COVID-19 were identified in the heterologous group, compared with 5 (0.03%) in the homologous. No deaths were seen in either group. A total of 489 (3.4%) and 318 (2.2%) of the participants in the heterologous and homologous cohorts received a third dose of an mRNA vaccine during follow-up, respectively, and were censored on that date.
Fig. 3.
Kaplan–Meier plot of COVID-19 infection (primary outcome) after second-dose vaccination according to vaccination schedule.
Regarding safety, primary analyses of 14,325 people per group found only one venous thromboembolism event (0.007%) and one venous thromboembolism with thrombocytopenia event (0.007%), both in the heterologous group. No myopericarditis events were seen in either group (Table 3).
Table 3.
Number (%) of safety events in the 21 days following second dose, according to vaccination schedule.
| Heterologous | Homologous | |||||
|---|---|---|---|---|---|---|
| 1:1 matching | 1:2 matching | 1:5 matching | 1:1 matching | 1:2 matching | 1:5 matching | |
| N participants | 14,325 | 12,512 | 8569 | 14,325 | 25,024 | 42,845 |
| N (%) of venous thromboembolism events | 1 | 1 | 1 | 0 | 0 | 0 |
| N (%) of venous thromboembolism with thrombocytopenia | 1 | 1 | 1 | 0 | 0 | 0 |
| N (%) of myopericarditis | 0 | 0 | 0 | 0 | 0 | 1 |
Back pain episodes were used as a negative control outcome. They were recorded at similar frequencies in the two groups (Supplementary Fig. 2).
Sensitivity analyses were conducted using 1:2 and 1:5 exact matching, resulting in 12,512 people with heterologous vaccination matched to 25,024 people with homologous vaccination and 8569 matched to 42,845, respectively. The hazard ratios for SARS-CoV-2 infection were comparable to those in the primary analysis: 0.65 [0.58–0.73] in the 1:2 and 0.65 [0.57–0.73] in the 1:5 matched cohorts. No safety concerns were identified in these larger cohorts, with only one additional safety event of myopericarditis identified in the homologous vaccination group for 1:5 matching (Table 3).
Finally, a post-hoc analysis was conducted using propensity scores and vaccine date matching instead of the matching described above (see Methods). Comparable cohorts were obtained after matching 17,173/17,849 (96.0%) of those vaccinated with heterologous regimen to 17,173/149,386 (11.5%) in the homologous (Supplementary Fig. 3). A total of 551/17,173 (3.2%) and 809/17,173 (4.7%) COVID-19 cases were identified amongst those in the heterologous and homologous vaccination cohorts, respectively, equivalent to incidence rates of 0.18/1000 and 0.27/1000 person-years. The resulting HR was similar to that seen in the main analysis: HR 0.67 [0.60–0.75].
Discussion
To the best of our knowledge, this is the first report to date comparing the safety and effectiveness of homologous vaccination against COVID-19 with two-dose ChAdOx1 and heterologous vaccination with first-dose ChAdOx1 and second-dose BNT162b2. Our primary analysis included over 28,000 people, over 14,000 per group, exactly matched on age, sex, region and date of second-dose vaccination. In this rich linked cohort, we found a 34% relative risk reduction of SARS-CoV-2 infection (primary outcome) among those on the heterologous vaccination schedule compared with those on the homologous vaccination schedule, despite similar testing rates in the two groups.
No safety concerns were identified, with only one event (<0.01%) of venous thromboembolism and one event of venous thromboembolism with thrombocytopenia in the heterologous group in the main analysis. One additional event of myopericarditis was observed in 42,845 people receiving homologous vaccination in the 1:5 matched sensitivity analysis.
Sensitivity analyses using 1:2 and 1:5 matching increased the sample size to >37,000 and >50,000 participants, respectively, and confirmed the safety and effectiveness findings. Similarly, a post-hoc analysis using propensity score matching methods yielded consistent findings.
The null association between vaccination schedule and our chosen negative control outcome of back pain supported the robustness of our findings, ruling out residual confounding.
Our findings that heterologous vaccination was more effective than homologous vaccination against COVID-19 agrees with emerging efficacy evidence based on immunological endpoints. Two small, randomised trials have reported higher immunogenicity, characterised by humoral and cellular responses, from ChAdOx1/BNT162b2 than ChAdOx1/ChAdOx110,13. These results were also corroborated by several cohort studies14–16. Although people vaccinated with heterologous ChAdOx1/mRNA vaccine (e.g. BNT162b2) were reported to have a 68% lower risk of symptomatic COVID-19 infection than unvaccinated people17, little was known about the comparative effectiveness of the heterologous and homologous vaccination schedules against clinical endpoints. Our study showed that heterologous ChAdOx1/BNT162b2 vaccination conferred 34% more protection against SARS-CoV-2 infection than homologous two-dose ChAdOx1 vaccination, and corroborate the potential of immunological surrogate endpoints of COVID-19 vaccines being predictive for clinical protection18,19.
Data on the post-marketing safety of heterologous vaccination schedules remain sparse, particularly for rare safety events, with most evidence from evaluations of reactogenicity10,13–15,20. Although reactogenicity endpoints are informative for assessing potential vaccine side effects, these trials are underpowered to study rare safety outcomes. Adverse events related to the ChAdOx1 vaccine include the rare (<1/1000 to ≥1/10,000) outcome venous thrombosis and the very rare (<1/10,000) outcome vaccine-induced immune thrombosis with thrombocytopenia syndrome21,22. Similarly, myocarditis and pericarditis outcomes possibly associated with BNT162b2 are expected to affect around 10–24 people per 10 million fully vaccinated people aged ≥30 years23. In this study with >28,000 participants, we identified one venous thromboembolism event and one venous thromboembolism with thrombocytopenia event. The number of events did not increase much in sensitivity analyses including up to >50,000 participants, suggesting that larger studies are needed to investigate these safety signals for heterologous vaccination schedules.
Our study has several limitations. The main limitation is the observational nature of our data. However, exact matching on age, region, and date led to a good balance in all observed confounders, including socio-demographics, comorbidity and medicines use. Propensity score matching was used in a post-hoc analysis to confirm the robustness of our exact-matching approach. Additionally, our analysis of a negative control outcome (back pain) suggested comparability of the matched cohorts, including unobserved covariates.
As most of our participants were middle-aged adults aged less than 60 years old, our risk-benefit assessment may not be valid for younger or elderly people. In addition, we could not include the cohort of people receiving 2 doses of BNT162b2 in our analyses, as they were vaccinated during a different calendar period, as depicted in Supplementary Fig. 4. Failing to match on vaccination date would lead to biased results due to changes in community transmission during the study period. Finally, our sample size was insufficient for studying severe COVID-19 outcomes, including hospitalisation and mortality, or rare safety outcomes.
This study also has strengths. The rich, representative linked dataset used allowed a robust analysis of vaccine exposure and outcomes at speed to inform ongoing international vaccination campaigns. Catalonia has a universal healthcare system and uses a centralised, secure data ecosystem with a long track record of research and high-impact publications8,24. The granularity of these data made it possible to control for confounding and test for residual systematic bias. Linkage to additional data sources on RT-PCR and LFT tests allowed for a comprehensive assessment of testing rates and reliable SARS-CoV-2 infection rates. The data collection period covered a time when most cases of COVID-19 in Catalonia were attributable to the Delta variant of SARS-CoV-225,which is still the predominant variant worldwide. Our data are highly relevant for ongoing global vaccination strategies and future and current third-dose and booster campaigns.
In conclusion, by leveraging the potential of multiple data sources in parallel, our study confirmed that a heterologous vaccination schedule of ChAdOx1 and BNT162b2 was safe and provided better protection against COVID-19 than a homologous ChAdOx1 vaccination schedule in real-world settings experiencing the Delta variant. More research on other mixed-vaccine schedules with different prime-boost intervals are needed.
Methods
Study design and data sources
We performed a cohort study based on linked routinely collected data available to the Public Health Secretariat of Catalonia. Vaccine exposure was obtained from the Catalan Shared Clinical Records, a database with vaccine data covering the entire Catalan health system and all its vaccination centres. Additional linked data were obtained from the Catalan database of reverse transcription polymerase chain reaction (RT-PCR) tests and lateral flow tests (LFT) for SARS-CoV-2, from primary-care electronic health records and from a population-based administrative hospital admissions data (CMBD-AH for its acronym in Catalan language). Data from these linked databases have previously been used for multiple COVID-19 research studies and include information for nearly 90% of the Catalan population8.
Participants, cohorts and follow-up
For our primary analysis, we included all individuals aged 19–59 years old who received a first dose of the ChAdOx1 vaccine and a second dose of ChAdOx1 (homologous vaccination) or BNT162b2 (heterologous vaccination). We followed participants from the day they received their second dose of either vaccine until an outcome, death, third dose of the vaccine, or the end of data availability (5 December 2021).
We excluded people with a previous SARS-CoV-2 infection identified by a positive RT-PCR test or LFT and people assigned to one of the 10% of primary-care practices not contributing to our database.
Each participant receiving heterologous vaccination was matched 1:1 to one person receiving homologous vaccination using exact matching by age, sex, general practice centre and date of second dose. In a sensitivity analysis, we changed the matching ratio to 1:2 and 1:5 to increase sample size.
Study outcomes
The primary outcome for effectiveness analyses was SARS-CoV-2 infection, defined by the date of the earliest of a positive RT-PCR test or LFT, regardless of symptoms or clinical diagnosis. We measured the number of tests over time regardless of results as an additional outcome to account for diagnostic effort.
Safety outcomes included venous thromboembolism, venous thromboembolism with thrombocytopenia and myopericarditis within 21 days after the second vaccine dose, based on ChAdOx121 and BNT162b223 safety reports. Supplementary Table S2 includes the ICD-10-CM codes (international classification of diseases, 10th revision, clinical modification) used to ascertain when these events occurred.
We analysed the occurrence of a negative control outcome—low back pain—to identify potential unmeasured confounding. Negative control outcomes are health events not causally associated with the exposure of interest, here vaccination.
Additional covariates
Covariates used for confounding assessment included socio-demographics and clinical features assessed at the time of inclusion (day of the second vaccination), as recorded in primary-care electronic health records and linked administrative data: age (in years), sex, area of residence, rurality and socio-economic status, number of RT-PCR tests or LFT performed, pre-existing comorbidities and long-term medicine use. Supplementary Table 1 provides ICD-10-CM codes for comorbidities and Anatomical Therapeutic Chemical Classification (ATCC) codes used to identify previous medicine use. We assessed socio-economic status using a validated deprivation index based on census data (MEDEA deprivation index)26,27. Rurality of residence was measured, with rural areas defined by a population <10,000 inhabitants and a density <150 inhabitants/km2, as per regional guidance.
Statistical analysis
Exact matching (1:1) between heterologous ChAdOx1/BNT162b2 vaccination and homologous two-dose ChAdOx1 was performed using the following variables: age (±2 years), sex, general practice centre and day of the second vaccination (±2 days). As a sensitivity analysis, we generated additional study populations and repeated all analyses after matching with 1:2 and 1:5 ratios. We assessed confounding due to known variables by measuring covariate imbalance as the standardised mean difference (SMD) of all covariates listed above. We considered SMD > 0.1 to be imbalanced28.
Separately, we used propensity score matching as part of a post-hoc analysis requested by one of the manuscript reviewers. Propensity scores were estimated using logistic regression including all available confounders, and cohorts matched using propensity score values with a maximum caliper width of 0.2 SD, with additional exact matching on second-dose vaccination date (±2 days).
We plotted time-to-event Kaplan–Meier estimates according to vaccine exposure (homologous vs heterologous). Absolute risk reduction (ARR) was estimated as the difference in cumulative incidence of Covid-19 amongst those receiving homologous–heterologous vaccination. Cox regression models were then fitted to calculate hazard ratios and 95% confidence intervals for each of the study outcomes, according to vaccination schedule. Visual inspection of Schoenfeld residuals against the transformed time was used to evaluate the proportionality of hazards. A zero-inflated negative binomial regression model was used to calculate the incident rate ratio and 95% confidence interval for the number of tests.
All analyses were conducted using R version 4.0.0.
Ethical considerations and information governance
All data were obtained from linked administrative sources after pseudonymisation in accordance with articles 6. e), 9.2. j) + 89 RGPD and 17.2.d of the LOPD-GDD. The proposed study was evaluated and approved by the Clinical Research Ethics Committee of the IDIAP Jordi Gol with Reference 21/269-PCV. This research was based on the agreement established in Regulation 2016/679 of the European Parliament and of the Council of April 27, 2016 on Data Protection and Organic Law 3/2018 of December 5 of protection of personal data and guarantee of digital rights.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We acknowledge English language editing by Jennifer A de Beyer of the Centre for Statistics in Medicine, University of Oxford. We thank Luis Garcia-Eroles for assistance and support with data management regarding linked hospital admissions data for the study of safety outcomes.
Author contributions
E.H., E.C., C.C. and D.P.A. designed the study. J.X., X.F., S.F., E.H., E.C. and D.P.A. drafted the first version of the manuscript. E.H., E.C., C.C., L.M.B., E.B., M.M., F.F., J.M.A. and M.M.P. contributed to data collection, extraction and/or curation. E.H. and E.C. led data analyses. E.H., E.C., J.X., S.F., C.C., L.M.B., F.F., E.B., M.M., M.M.P., J.M.A. and D.P.A. reviewed critically the content of the manuscript, edited further versions and approved the final version and revisions for submission.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Patient-level data utilised for the reported analyses are not accessible openly due to local information governance regulations as defined in the ‘Ethical considerations and information governance’ section. Aggregated data are provided for inspection as Supplementary Data 1-3, and R code used for analyses as Supplementary Code 1.
Competing interests
D.P.A.’s research group has received research grants from the European Medicines Agency, from the Innovative Medicines Initiative, from Amgen, Chiesi and from UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, Astra Zeneca and UCB Biopharma. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29301-9.
References
- 1.UNICEF COVID-19 Vaccine Market Dashboard. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard. (2021).
- 2.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Pollard AJ. ChAdOx1 nCoV-19 vaccine for SARS-CoV-2—Authors’ reply. Lancet. 2020;396:1486–1487. doi: 10.1016/S0140-6736(20)32267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabezas C, et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374:n1868. doi: 10.1136/bmj.n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupo de Trabajo Técnico de Vacunación COVID-19, de la Ponencia de Programa y Registro de Vacunaciones. Estrategia de vacunación frente a COVID-19 en España. 9 de febrero de 2021. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion3_EstrategiaVacunacion.pdf. (2021).
- 10.Borobia AM, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte-Salles T, Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398:94–95. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupo de Trabajo Técnico de Vacunación COVID-19, de la Ponencia de Programa y Registro de Vacunaciones. Estrategia de vacunación frente a COVID-19 en España. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/covid19/docs/COVID-19_Actualizacion8_EstrategiaVacunacion.pdf. (2021)
- 13.Liu X, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt T, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillus D, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenbusch M, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021;21:1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordström, P., Ballin, M. & Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg. Health Euro10.1016/j.lanepe.2021.100249 (2021). [DOI] [PMC free article] [PubMed]
- 18.Jin P, Li J, Pan H, Wu Y, Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: the evidence we have versus the evidence we need. Signal Transduct. Target. Ther. 2021;6:48. doi: 10.1038/s41392-021-00481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med.10.1038/s41591-021-01540-1 (2021). [DOI] [PMC free article] [PubMed]
- 20.Powell AA, et al. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March-June 2021, England. Eurosurveillance. 2021;26:2100634. doi: 10.2807/1560-7917.ES.2021.26.28.2100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ. 2021;373:n1435. doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Council for International Organizations of Medical Sciences. Guidelines for Preparing Core Clinical-Safety Information on Drugs, Second Edition, Report of CIOMS Working Group III and V. https://cioms.ch/wp-content/uploads/2018/03/Guidelines-for-Preparing-Core-Clinical-Safety-Info-Drugs-Report-of-CIOMS-Working-Group-III-and-V.pdf (2001).
- 23.Gargano JW, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, et al. Association of tramadol vs codeine prescription dispensation with mortality and other adverse clinical outcomes. JAMA. 2021;326:1504–1515. doi: 10.1001/jama.2021.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Català, M. et al. Analysis of new variants of SARS-CoV-2 in the Barcelona city and Northern Metropolitan area: success of the Delta and sinking of the B.1621 and the Gamma. in Analysis and prediction of COVID-19 for EU-EFTA-UK and other countries, #260. Research Report, 10–14 (Universitat Politècnica de Catalunya. 2021).
- 26.Domínguez-Berjón MF, et al. [Constructing a deprivation index based on census data in large Spanish cities (the MEDEA project)] Gac. Sanit. 2008;22:179–187. doi: 10.1157/13123961. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Gil M, et al. Linking of primary care records to census data to study the association between socioeconomic status and cancer incidence in Southern Europe: a nation-wide ecological study. PLoS ONE. 2014;9:e109706. doi: 10.1371/journal.pone.0109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TL, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 2017;17:78. doi: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Patient-level data utilised for the reported analyses are not accessible openly due to local information governance regulations as defined in the ‘Ethical considerations and information governance’ section. Aggregated data are provided for inspection as Supplementary Data 1-3, and R code used for analyses as Supplementary Code 1.