Abstract
The activity of pleconaril in cell culture against prototypic enterovirus strains and 215 clinical isolates of the most commonly isolated enterovirus serotypes was examined. The latter viruses were isolated by the Centers for Disease Control and Prevention during the 1970s and 1980s from clinically ill subjects. Pleconaril at a concentration of ≤0.03 μM inhibited the replication of 50% of all clinical isolates tested. Ninety percent of the isolates were inhibited at a drug concentration of ≤0.18 μM. The most sensitive serotype, echovirus serotype 11, was also the most prevalent enterovirus in the United States from 1970 to 1983. Pleconaril was further tested for oral activity in three animal models of lethal enterovirus infection: coxsackievirus serotype A9 infection in suckling mice, coxsackievirus serotype A21 strain Kenny infection in weanling mice, and coxsackievirus serotype B3 strain M infection in adult mice. Treatment with pleconaril increased the survival rate in all three models for both prophylactic and therapeutic dosing regimens. Moreover, pleconaril dramatically reduced virus levels in target tissues of coxsackievirus serotype B3 strain M-infected animals. Pleconaril represents a promising new drug candidate for potential use in the treatment of human enteroviral infections.
Picornaviruses, in particular the enterovirus and rhinovirus groups, are responsible for the majority of human viral disease. Enteroviruses cause an estimated 10 million to 15 million symptomatic infections in the United States annually (49). Enterovirus infections result in myriad disease syndromes involving many organ systems of the body. They are the most common virus infection of the central nervous system (CNS) and the leading cause of viral meningitis (42). The annual number of viral meningitis cases in the United States has been estimated to be greater than 600,000 (47). Several thousand additional cases of enteroviral encephalitis are reported each year, and these often have devastating long-term sequelae (42). Passive surveillance reports to the Centers for Disease Control and Prevention (CDC), Atlanta, Ga., indicate that from 1970 to 1979, 46% of enterovirus isolations were from patients diagnosed with CNS disease including viral meningitis (35%) and encephalitis (11%) (6). Enteroviruses also cause significant respiratory disease. Viral respiratory infections (VRIs) associated with enterovirus infections include pharyngitis, croup, bronchitis, bronchiolitis, infectious asthma, pneumonia, pleurodynia, and the common cold (8). Other illnesses associated with enterovirus infection include neonatal enteroviral disease, paralytic disease, acute hemorrhagic conjunctivitis, myocarditis, pericarditis, herpangina, and juvenile-onset diabetes mellitus (41). Neonates and young children are at the greatest risk of developing severe and occasionally fatal enteroviral infections (2, 3, 32). Patients with compromised humoral immunity, such as those with agammaglobulinemia, who contract enteroviral infections may develop chronic meningitis or meningoencephalitis, often with a fatal outcome (30, 42, 45). Currently, there is no specific antiviral therapy to treat or prevent enterovirus disease.
Rhinoviruses are also responsible for VRIs and are the leading agent of the common cold. According to the National Center for Health Statistics, the U.S. population suffers 1 billion colds annually (36). In the United States in 1994, 66 million cases of the common cold required medical attention or resulted in restricted activity (36). Rhinoviruses are also a predominant cause of asthmatic exacerbations in both children and adults (7, 19) and are an underappreciated cause of severe lower respiratory tract disease (22). As with enterovirus infections, no specific antiviral medication exists to address rhinovirus disease.
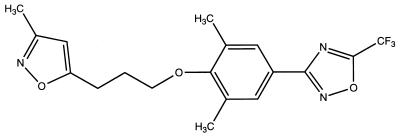
Pleconaril (Fig. 1) is a novel, orally bioavailable, and systemically acting small-molecule inhibitor of enteroviruses and rhinoviruses that is being developed for the treatment of diseases associated with picornavirus infections (1, 43, 46, 51). The drug is in late-stage clinical trials for the treatment of viral meningitis and VRIs. In support of the clinical development of pleconaril, we report here on the drug’s in vitro and in vivo activities against enteroviruses. The activity of pleconaril against rhinoviruses is described elsewhere (38).
FIG. 1.
Structure of pleconaril.
The enterovirus group of the picornavirus family comprises 67 distinct serotypes, including 3 strains of poliovirus, 23 group A and 6 group B coxsackieviruses, 31 echoviruses (EVs), and 4 “enteroviruses of humans” (31, 35). We have tested pleconaril in a virus cytopathic effect assay against prototypic enterovirus strains and against 215 clinical isolates representing the most commonly isolated enteroviruses. We further report on the in vivo protective efficacy, antiviral activity, and pharmacokinetics of the drug in mouse models of lethal enterovirus infection.
(Portions of this study were presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996 [38b].)
MATERIALS AND METHODS
Cells and viruses.
HeLa (WIS) cells were obtained from Roland Rueckert at the University of Wisconsin, Madison. LLC-MK2D rhesus monkey kidney cells and human embryonal rhabdomyosarcoma (RD) cells were obtained from the American Type Culture Collection, Rockville, Md. Enterovirus clinical isolates were obtained from Mark Pallansch at CDC. Prototypic enterovirus strains were purchased from the American Type Culture Collection. The following viruses were used in studies with animals and were obtained from the indicated investigators: coxsackievirus serotype A9 (CVA9; C. Wilfert, Duke University), CVA21 strain Kenny (M. Kenny, Merrell Dow Research Institute), and CVB3 strain M (C. Gauntt, University of Texas Health Science Center, San Antonio).
All clinical virus isolates were passaged once in the cell line used for their original isolation to establish working virus stocks and were then stored as aliquots in glass ampoules at −80°C. EVs were propagated in RD cells grown in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). CVA9 and CVB isolates were passaged in LLC-MK2D cells grown in MEM plus 5% FBS. Viruses were assayed for drug sensitivity in the cell line used for their original isolation with the exception of the CVA9 isolates, which were assayed in HeLa cells.
Virus cytopathic effect assay.
The sensitivities of enteroviruses to pleconaril were determined in a cell culture assay that measured the protection by the drug of an infected cell monolayer from the cytopathic effects of the viruses. Ninety-six-well tissue culture plates (Costar 3598) were seeded at a density of 2.8 × 104 cells/well for HeLa cells (in MEM plus 5% FBS), 3.6 × 104 cells/well for LLC-MK2D cells (in MEM plus 5% FBS), or 6 × 104 cells/well for RD cells (in MEM plus 10% FBS). The cells were incubated for 24 h at 37°C in a humidified, 5% CO2 atmosphere prior to their use in the assay.
To determine the virus inoculum in the assay, serial 0.5 log10 dilutions of individual viruses were plated in octuplicate onto their respective cell lines in medium 199 (M199) plus 5% FBS supplemented with 30 mM MgCl2 and 15 μg of DEAE dextran per ml (complete M199 medium). The plates were incubated for 3 days and were then fixed with 5% glutaraldehyde and stained with 0.1% crystal violet. After rinsing and drying, the optical density of the wells at a wavelength of 570 nm (OD570) was read on a Bio-Tek 300 plate reader. The highest dilution of virus that resulted in an OD570 reading of ≤15% of the cell culture control value was used for drug sensitivity testing.
To test for drug sensitivity, cells in 96-well plates were infected with the appropriate virus dilution at 37°C in 150 μl of complete M199 medium. During the 1-h virus attachment period, pleconaril was solubilized in dimethyl sulfoxide (DMSO) to 400 times the highest concentration to be tested in the assay and was then serially diluted twofold in DMSO in U-bottom, 96-well polypropylene plates (Costar 3790) to yield 10 compound dilutions. Two microliters of the DMSO compound dilutions were then diluted into 198 μl of complete M199 medium to effect a 100-fold dilution of compound. After virus attachment, 50 μl of this drug dilution was added to the 150-μl virus inoculum, resulting in a final 400-fold dilution of compound in 0.25% DMSO. Each drug concentration was run in quadruplicate. Uninfected cells and cells that received virus in the absence of compound were included on each plate. The plates were then incubated for 3 days at 37°C in a humidified, 2.5% CO2 atmosphere prior to fixation and staining. The 50% inhibitory concentration (IC50) was defined as the concentration of compound that protected 50% of the cell monolayer from virus-induced cytopathic effect.
Cell growth assay.
The cytotoxicity of pleconaril in cell culture was determined in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye-based assay (33). The cells were seeded at 1.3 × 104/ml (200 μl/well) in 96-well plates such that cell replication remained logarithmic for the 3-day incubation period. Four hours after seeding, the cells were overlaid with serial twofold dilutions of pleconaril in quadruplicate. After 3 days at 37°C, the overlay was removed, 100 μl of MTT at 1 mg/ml in phosphate-buffered saline was added to each well, and the incubation was continued for an additional 4 h. After careful aspiration of the unreacted MTT, the resulting blue formazan crystals were solubilized in 0.04 N HCl in isopropanol, and the OD570 was determined. The 50% cytotoxic concentration (CC50) of pleconaril was defined as the highest concentration of compound that resulted in ≥50% cell growth compared to that with the no-drug control.
Mouse studies.
The animals used in these studies were housed in an American Association for Laboratory Animal Care-accredited facility and were cared for in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (36a).
(i) Compound preparation and dosing.
Pleconaril was formulated as a suspension in a 0.5% xanthan gum–1% Tween 80 vehicle. The compound was administered intragastrically with a 0.5-ml Glaspak syringe fitted with a 24-gauge feeding needle (Popper and Sons, Inc., New York, N.Y.).
(ii) CVA9 infection of suckling mice.
Newborn ICR mouse pups of both sexes (weight, 1.2 to 1.9 g), born within 24 h of receipt, were obtained with their dams from Blue Spruce Farms, Altamont, N.Y. The pups were pooled, weighed, and distributed to the nursing dams in groups of 10. Three cages (30 pups) were included for each dosage group. Each mouse pup was infected subcutaneously over the right shoulder within 24 h of birth with CVA9. The amount of virus inoculated had been titrated to produce approximately 80% mortality in untreated animals over the 13-day observation period. Pleconaril was administered as a single, 0.03-ml dose 2.5 days postinfection. Mouse pups were checked daily for evidence of paralysis or death (paralyzed animals were killed).
(iii) CVA21 strain Kenny infection of weanling mice.
Weanling ICR mice (females; weight, 9 to 12 g) were obtained from Blue Spruce Farms. Four cages of five mice each were included for each dosage group. Mice were infected by the intraperitoneal route with 0.2 ml of CVA21 strain Kenny (21). The amount of virus inoculated had been titrated to produce approximately 80% mortality in untreated animals over the 14-day observation period. Pleconaril was administered as a 0.2-ml dose 2 h prior to infection and then 2 h postinfection on day 1. On days 2 through 5, the animals were dosed twice a day, at 9 a.m. and 3 p.m. Animals were checked daily for evidence of paralysis or death (paralyzed animals were killed).
(iv) CVB3 strain M infection of adult mice.
Adult BALB/c mice (males; weight, 18 to 21 g) were obtained from Blue Spruce Farms. Four cages with three mice each were included for each dosage group. Mice were infected i.p. with 0.2 ml of the myocarditic strain M of CVB3. The amount of virus inoculated had been titrated to result in 80% mortality in untreated animals over the 17-day observation period. Pleconaril was administered as described above for the CVA21 model. Animals were checked daily for evidence of paralysis or death (paralyzed animals were killed). Fifty percent protective doses with 95% confidence limits were calculated by probit analysis in all efficacy models.
(v) Antiviral effect of pleconaril in CVB3 strain M-infected adult mice.
Adult male BALB/c mice were dosed orally with either 200 mg of pleconaril or vehicle alone per kg of body weight 2 h prior to infection with an 80% lethal inoculum of CVB3 strain M. Pleconaril was then readministered at 24-h intervals for a total of five daily doses. Two hours after the final drug dosing, groups of eight animals were killed and the virus titers in pancreatic and heart tissue were determined. Excised organs were weighed and homogenized as 20% (wt/vol) suspensions in M199 medium with a Duall tissue grinder (Kontes). The homogenate was frozen-thawed twice and then centrifuged at 200 × g in a Sorvall RT6000D centrifuge to remove debris. The resulting supernatant was collected and was stored at −80°C until titration by a plaque assay as described previously (16).
(vi) Determination of serum pleconaril levels in adult mice.
Adult male BALB/c mice were dosed orally with a single 2, 20-, or 200-mg/kg dose of pleconaril. Blood samples were obtained via cardiac puncture from five mice per time point for the 2- and 20-mg/kg doses and 15 mice per time point for the 200-mg/kg dose over a 24-h period. Blood from each time point was pooled and was allowed to clot, and serum was collected after centrifugation. Pleconaril was extracted from the serum with hexane, dried, and resuspended in methanol. Samples were assayed for pleconaril concentration determination by a validated gas chromatography method with electron-capture detection.
RESULTS
Activity of pleconaril in cell culture against prototypic enterovirus strains.
A panel of 15 prototypic enterovirus strains representing the most frequently isolated serotypes (49) has been widely used in diagnostic and research applications and has served as a general virus reference standard in the field (31). These strains have also been used for the identification and evaluation of enterovirus inhibitors (37, 52). The viruses in this panel were tested for their sensitivities to pleconaril in cell culture. However, to properly evaluate antiviral activity in cell culture assays, the CC50 of the test compound was first determined so that any inhibitory effects on virus replication could be distinguished from the effects due to compound cytotoxicity. We determined the CC50 of pleconaril using an MTT dye-based assay of cell growth (33). Since different enteroviruses were tested in different cells, we determined the CC50 of pleconaril for each cell culture system. The CC50 of pleconaril for the cell lines used in these assays ranged from 12.5 to 25 μM (data not shown). We have used the conservative value of 12.5 μM as the CC50 of pleconaril in estimating compound selectivity.
Pleconaril inhibited 14 of the 15 prototypic enterovirus strains in the cytopathic effect assay at a concentration range of 0.001 to 1.05 μM (Table 1). We included CVB3 strain M in our testing since this myocarditic virus is widely used in a mouse model of enteroviral disease (15). Interestingly, while CVB3 strain M was quite sensitive to pleconaril, CVB3 strain Nancy was not. The molecular basis for this insensitivity is discussed elsewhere (16).
TABLE 1.
Activity of pleconaril against prototypic enterovirus strainsa
| Enterovirus serotype and strain | IC50 (μM) |
|---|---|
| EV3 Morrisey | 0.31 ± 0.10 |
| EV4 Pesascek | 0.02 ± 0.005 |
| EV5 Noyce | 1.05 ± 0.42 |
| EV6 D’Amori | 0.05 ± 0.01 |
| EV7 Wallace | 0.05 ± 0.03 |
| EV9 Hill | 0.18 ± 0.03 |
| EV11 (Gregory) | 0.01 ± 0.005 |
| EV24 (DeCamp) | 0.01 ± 0.003 |
| EV30 (Bastianni) | 0.01 ± 0.005 |
| CVA9 Bozek | 0.005 ± 0.002 |
| CVB1 Conn-5 | 0.002 ± 0.0005 |
| CVB2 Ohio-1 | 0.003 ± 0.002 |
| CVB3 Nancy | >12.5 |
| CVB3 M | 0.02 ± 0 |
| CVB4 JVB | 0.05 ± 0.03 |
| CVB5 Faulkner | 0.001 ± 0.0003 |
Prototypic strains of the 15 most commonly isolated enteroviruses (49). The results are the mean ± standard deviation IC50 of at least two independent determinations.
Activity of pleconaril in cell culture against enterovirus clinical isolates.
Since many of the prototypic enterovirus strains were isolated in the 1940s and 1950s, we expanded our assessment of the antiviral activity of pleconaril to a larger panel of more recently recovered clinical isolates. We obtained 215 of the most commonly isolated enteroviruses collected from clinical samples by CDC during the 1970s and 1980s. All isolates were associated with clinical disease, including six fatal infections (Table 2). Each isolate was tested in the cytopathic effect assay for its sensitivity to pleconaril in at least two independent experiments. Pleconaril exhibited potent antiviral activity against 214 of the 215 clinical isolates (>99%) at a concentration range of 0.002 to 3.4 μM. A single isolate of EV serotype 24 (EV24) was insensitive to drug (IC50 >12.5 μM). For one-half of all isolates IC50s were ≤ 0.03 μM (the MIC at which 50% of isolates are inhibited [MIC50]). For 90% of all isolates the IC50 was ≤0.18 μM (MIC90).
TABLE 2.
Illnesses associated with 215 enterovirus clinical isolates
| Clinical diagnosis | No. of isolates |
|---|---|
| Meningitis or encephalitis | 95a |
| Gastroenteritis | 10 |
| VRI | 6 |
| Fever of unknown origin | 3 |
| Pericarditis | 2 |
| Paralysis | 2 |
| Poliomyelitis | 2 |
| Sepsis | 2 |
| Sudden infant death syndrome | 1a |
| Hemorrhagic pneumonia | 1a |
| Croup | 1 |
| Exanthem | 1 |
| Guillian-Barré syndrome | 1 |
| Pancreatitis | 1 |
| Pleurodynia | 1 |
| Pneumonitis | 1 |
| Seizures | 1 |
| Other infection | 7 |
| Undiagnosed infection | 77b |
One fatality.
Three fatalities.
Table 3 summarizes the antiviral activity of pleconaril and the cell culture selectivity index (CC50/MIC90) for pleconaril. For all isolates, pleconaril exhibited a selectivity index of ≥34, indicating that inhibition of virus replication occurred well below the CC50 of the drug. EV11 isolates, the most prevalent of the virus isolates (12.2% of 23,481 isolates) (49), were collectively the most sensitive to pleconaril (MIC90, 0.02 μM; selectivity index, 625).
TABLE 3.
Summary of antiviral activity of pleconaril and selectivity index for pleconaril against clinical enterovirus isolates in cell culture
| Clinical isolates | % of all isolatesa | No. of isolates testedb | MIC90 (IC50 range) (μM) | Selectivity indexc |
|---|---|---|---|---|
| CVA9 | 4.5 | 14 | 0.06 (0.005–0.15) | 208 |
| CVB1 | 1.6 | 13 | 0.04 (0.008–0.04) | 313 |
| CVB2 | 4.8 | 14 | 0.07 (0.005–1.89) | 179 |
| CVB3 | 4.5 | 15 | 0.37 (0.02–0.51) | 34 |
| CVB4 | 4.6 | 10 | 0.26 (0.08–0.30) | 48 |
| CVB5 | 8.7 | 16 | 0.03 (0.01–0.10) | 417 |
| EV3 | 3.2 | 15 | 0.33 (0.02–1.21) | 38 |
| EV4 | 6.3 | 18 | 0.13 (0.005–0.54) | 96 |
| EV5 | 2.0 | 8 | 0.26 (0.03–0.39) | 48 |
| EV6 | 5.5 | 19 | 0.04 (0.005–0.07) | 313 |
| EV7 | 3.0 | 11 | 0.19 (0.013–3.29) | 66 |
| EV9 | 11.3 | 9 | 0.10 (0.01–0.15) | 125 |
| EV11 | 12.2 | 20 | 0.02 (0.002–0.02) | 625 |
| EV24 | 1.8 | 12 | 0.07 (0.002–>12.5) | 179 |
| EV30 | 6.8 | 21 | 0.05 (0.005–0.11) | 250 |
Percentage of 23,481 enteroviruses isolated from 1970 to 1983 (49).
Number of clinical isolates of the indicated enterovirus serotype tested in the study described in this report.
Selectivity index in cell culture (CC50/MIC90; see text for details).
Protective efficacy of pleconaril in mouse models of enterovirus infection.
The results presented above indicate that pleconaril has potent and broad-spectrum activity in cell culture against clinically relevant enterovirus isolates. To extend these results to the in vivo setting, we examined the oral efficacy of pleconaril in several well-established mouse models of lethal picornavirus infection: CVA9 infection of suckling mice (52), CVA21 strain Kenny infection of weanling mice (21), and CVB3 strain M infection of adult mice (15).
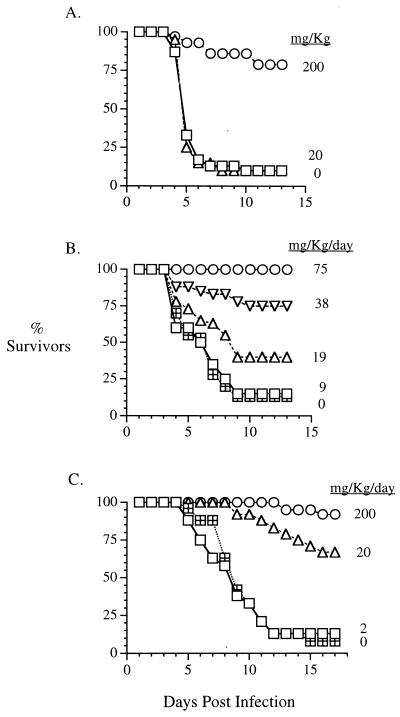
As shown in Fig. 2A, 90% of placebo-treated suckling mice died within 10 days of CVA9 infection. In contrast, a single oral therapeutic dose of 200 mg of pleconaril per kg given 2.5 days after virus challenge protected animals from lethal infection. Eighty percent of pleconaril-treated, CVA9-infected animals survived the entire 14-day observation period.
FIG. 2.
Activity of pleconaril in mouse models of enterovirus infection. (A) CVA9 in suckling mice; (B) CVA21 strain Kenny in weanling mice; (C) CVB3 strain M in adult mice.
CVA21 strain Kenny infection of weanling mice resulted in greater than 85% mortality within 9 days of challenge (Fig. 2B). Administration of a single oral dose of pleconaril prior to virus challenge followed by 5 days of twice-daily oral dosing resulted in the dose-dependent protection of animals from lethal disease. Dosages as low as 19 mg/kg/day resulted in a statistically significant increase in survival relative to that for placebo-treated animals (P = 0.04 by chi-square analysis). One hundred percent of animals survived the CVA21 strain Kenny infection when they were treated with pleconaril at a dosage of 75 mg/kg/day.
The dose-dependent efficacy of pleconaril was also observed in the CVB3 strain M-infected adult mouse model. Greater than 85% of placebo-treated animals died within 12 days from a lethal CVB3 strain M infection (Fig. 2C). By using the same dosing regimen used in the CVA21 model described above, pleconaril protected animals from lethal disease at dosages as low as 20 mg/kg/day. With the 200-mg/kg/day dosage of pleconaril, 90% of animals survived the infection. Control mice used to assess toxicity (drug- or placebo-treated, mock-infected mice) were monitored daily for changes in behavior and/or growth. No overt toxicity was observed in mice under any of these dosing regimens.
On the basis of the results from these mouse efficacy models, the 50% protective doses (with 95% confidence intervals) for pleconaril were 93 mg/kg/day (24 to 157 mg/kg/day), 26 mg/kg/day, (20 to 31 mg/kg/day), and 12 mg/kg/day (2 to 30 mg/kg/day) in the models of CVA9 infection of suckling mice, CVA21 infection of weanling mice, and CVB3 infection of adult mice, respectively.
Effect of pleconaril on viral titers in tissues of CVB3 strain M-infected mice.
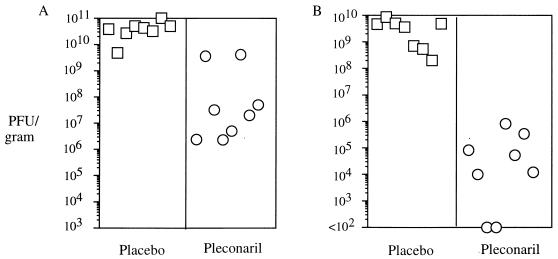
To determine if the protective efficacy of pleconaril observed in mice was attributable to its antienterovirus activity, we examined virus levels in target tissues of adult mice infected with CVB3 strain M. Adult male BALB/c mice were dosed orally with either 200 mg of pleconaril per kg or vehicle alone 2 h prior to infection with an 80% lethal inoculum of CVB3 strain M. Pleconaril was then readministered at 24-h intervals for a total of five daily doses. Organs were harvested 2 h after the final drug dosing. As shown in Fig. 3A, viral titers in the pancreatic tissues of animals treated with pleconaril were 1 to 4 log10 lower than those in the pancreatic tissues of untreated control animals (P = 0.0002 by the Mann-Whitney nonparametric test). The reduction in the titer in the heart was even more dramatic, with the hearts of pleconaril-treated animals exhibiting 4- to >7-log10 reductions in virus titer (P = 0.0002; Fig. 3B). Two of the eight animals treated with pleconaril had undetectable levels of virus in the heart on day 4.
FIG. 3.
CVB3 strain M titers in pancreases (A) and hearts (B) of placebo- or pleconaril-treated adult mice.
To rule out a drug carryover effect, a known quantity of virus was spiked into homogenized tissues from mock-infected, drug- or placebo-treated animals, and the titer was determined by plaque assay. No difference in virus titer was observed between the two groups, indicating that pleconaril binding to CVB3 strain M was completely reversible by dilution and that no drug carryover effects influenced the experimental viral titer data (data not shown). Although histopathologic examination of tissues was not conducted, these results strongly suggest that pleconaril’s ability to protect mice from lethal disease (Fig. 2) is due to its profound antiviral effect in treated animals.
Serum pleconaril levels in adult mice.
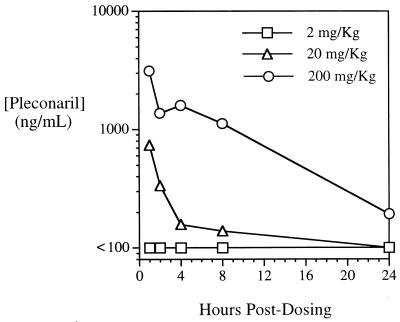
To correlate the in vivo protective effects of pleconaril and its antiviral activity with serum drug levels, we examined the pharmacokinetics of pleconaril in adult mice. Animals were administered a single 2-, 20-, or 200-mg/kg dose of pleconaril by oral gavage, and serum samples were obtained over the course of 24 h. A dose-related increase in serum drug levels was observed (Fig. 4). The concentrations in serum 1 h after administration (the first time point analyzed) of a 2-, 20-, or 200-mg/kg dose were <100, 738, and 3,140 ng/ml, respectively. These serum drug levels substantiate the data that indicate that pleconaril protects mice from lethal disease in the CVB3 infection model (Fig. 2C). The animals were protected from lethal disease when they were administered pleconaril at the 20- and 200-mg/kg/day dosages, with which significant concentrations of pleconaril were observed in serum, but not when they were administered pleconaril at a dosage of at 2 mg/kg/day, with which pleconaril concentrations were below the minimum quantifiable level. The serum half-lives of pleconaril after administration of the two higher dosages were 5.3 and 6.5 h, respectively. The areas under the curve for the 20- and 200-mg/kg doses were 1,994 and 22,739 ng · h/ml, respectively, indicating that the oral bioavailability of pleconaril is dose proportional at this dosage range. These serum drug levels are consistent with the pharmacokinetics of pleconaril observed both in species tested in preclinical trials (rats and dogs; unpublished data) and in humans (1).
FIG. 4.
Serum pleconaril levels in adult mice after single oral dosing.
DISCUSSION
For a drug to successfully manage and control enterovirus diseases, several significant challenges must be met. First, an antienterovirus drug must exhibit potent activity against a broad spectrum of enterovirus serotypes. There are over 67 distinct serotypes of enteroviruses. Any one of these serotypes is potentially capable of causing any of the many diseases associated with enterovirus infection. In fact, no single enterovirus serotype is exclusively associated with any particular disease. For example, of the 93 patients with clinical diagnoses of viral meningitis or encephalitis reported in Table 2, 3 patients were infected with CVA, 31 were infected with CVB, and 56 were infected with EV.
Second, the ideal drug must be available systemically. Enteroviruses cause systemic infections and, because of this, affect many different organ systems of the body, including the CNS, heart, lungs, and skin. Consequently, to adequately address enterovirus disease in all its manifestations, the antiviral drug must distribute throughout the body. In particular, the drug must be able to cross the blood-brain barrier, since a common clinical syndrome associated with enterovirus infection is viral meningitis (6).
Third, any drug to be used to treat patients afflicted with enterovirus diseases must be safe. Enteroviruses cause diseases in all age groups, from enterovirus disease in neonates, to viral meningitis (predominantly in children) and myocarditis in young adults, to viral respiratory disease in people of all ages and chronic and life-threatening infections in immunocompromised and elderly individuals. A drug with an unblemished safety profile will provide the broadest utility in the treatment of enterovirus disease.
Pleconaril is the result of the successful application of rational drug design. A combination of X-ray crystallography, computer modeling, genetic algorithm methodologies, and traditional medicinal chemistry was used to chemically design and optimize the drug to exhibit the desired antiviral activity spectrum, in vivo pharmacokinetic properties, and safety profile (4, 5, 9–13, 17, 20, 26, 27, 48).
The mechanism by which pleconaril exerts its antiviral effect involves inhibition of picornavirus capsid protein function. Specifically, pleconaril integrates within a hydrophobic pocket inside the virion, leading to rigidification and compression of the viral capsid (14, 25, 28, 37, 38, 38a, 39, 40, 48, 52). In doing so, pleconaril prevents virus attachment to cells and uncoating of viral RNA (50), thus interrupting the infection cycle.
The picornavirus capsid structure is relatively conserved among the enteroviruses and rhinoviruses. However, on the basis of X-ray crystallographic analyses, there do exist subtle differences in the sizes and shapes of the hydrophobic pockets among these viruses (4, 18, 23, 24, 34, 48). By optimizing the chemical nature of the compound’s ring systems, as well as the spacer between the rings, pleconaril was designed to provide the best overall pocket fit for the majority of enteroviruses and rhinoviruses. As a result, both the antiviral potency and antipicornavirus activity spectrum were maximized. As reported here, pleconaril indeed has broad-spectrum and potent antienterovirus activity. As is described elsewhere, pleconaril also shows potent, broad-spectrum activity against rhinoviruses (50).
Pleconaril exhibits excellent oral bioavailability, approaching 70% in both dogs and humans (1; unpublished data). This high level of bioavailability was achieved by incorporating into the drug chemical modifications that minimized metabolism of the molecule by the liver. In particular, introduction of a trifluoromethyl substituent onto the oxadiazole ring resulted in global protection of the compound from degradation by enzymes involved in oxidative metabolic processes (13). The result of this metabolic stabilization is reflected in the drug’s long serum half-life after oral dosing. Pleconaril also readily penetrates the blood-brain barrier. In rats, drug levels in brain tissues exceeded those in serum after oral dosing (unpublished data). This feature of the drug is likely due to its lipophilic character.
Another consequence of the metabolic stabilization of the drug is that fewer and lower levels of drug metabolites are present in vivo. The presence of fewer metabolites affords fewer opportunities for encounters with molecules with significant toxicity. Moreover, given pleconaril’s high antiviral potency and its long serum half-life, low drug doses are sufficient to achieve target drug levels in the clinical situation. These features, coupled with the specific and selective antiviral mechanism of pleconaril, suggest that pleconaril should have an excellent safety profile. This indeed appears to be the case. To date, pleconaril has been administered to over 1,700 individuals and has shown no significant drug-related adverse events over those caused by a placebo.
The results of several trials to evaluate the clinical utility of pleconaril have been reported. In a double-blind, placebo-controlled, CVA21 challenge model of VRI, pleconaril-treated adult volunteers experienced significant improvements in all measured clinical endpoints (46). Similar positive results were observed in double-blind, placebo-controlled studies with adults and children suffering from enteroviral meningitis (29, 51). Finally, among agammaglobulinemic children suffering from chronic CNS enterovirus infection, the majority of patients have experienced significant clinical benefit from a single course of pleconaril therapy (43, 44).
On the basis of the clinical benefits observed in human trials to date and on the basis of pleconaril’s broad spectrum of antipicornavirus activity, the near-term prospects for a therapeutic agent for treatment of enterovirus diseases appear promising.
ACKNOWLEDGMENTS
We thank Graham Lockwood and Nancy O’Neil for assistance with the in vivo studies. We also thank Mark McKinlay and Marc Collett for critical reviews of the manuscript.
REFERENCES
- 1.Abdel-Rahman S M, Kearns G L. Single oral dose escalation pharmacokinetics of pleconaril (VP 63843) capsules in adults. J Clin Pharmacol. 1999;39:613–618. doi: 10.1177/00912709922008227. [DOI] [PubMed] [Google Scholar]
- 2.Abzug M J, Levin M J, Rotbart H A. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–824. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Abzug M J. Perinatal enterovirus infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: ASM Press; 1995. pp. 221–238. [Google Scholar]
- 4.Badger J, Minor I, Kremer M J, Oliveira M A, Smith T J, Griffith J P, Guerin D M A, Krishnaswamy S, Luo M, Rossmann M G, McKinlay M A, Diana G D, Dutko F J, Fancher M, Rueckert R R, Heinz B A. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci USA. 1988;85:3304–3308. doi: 10.1073/pnas.85.10.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey T R, Diana G D, Kowalczyk P F, Akullian V, Eissenstat M A, Cutliffe D, Mallamo J P, Carabateas P M, Pevear D C. Antirhinoviral activity of heterocyclic analogs of Win 54954. J Med Chem. 1992;35:4628–4633. doi: 10.1021/jm00102a017. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Enterovirus surveillance report, 1970-1979. Atlanta, Ga: Centers for Disease Control; 1981. [Google Scholar]
- 7.Chidekel A S, Rosen C L, Buzzy A R. Rhinovirus infection associated with serious lower respiratory illness in patients with bronchopulmonary dysplasia. Pediatric Infect Dis J. 1997;16:43–47. doi: 10.1097/00006454-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Chonmaitree T, Mann L. Respiratory infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: ASM Press; 1995. pp. 255–270. [Google Scholar]
- 9.Diana G D, Treasurywala A M, Bailey T R, Oglesby R C, Pevear D C, Dutko F J. A model for compounds active against human rhinovirus-14 based on X-ray crystallography data. J Med Chem. 1990;33:1306–1311. doi: 10.1021/jm00167a006. [DOI] [PubMed] [Google Scholar]
- 10.Diana G D, Kowalczyk P, Treasurywala A M, Oglesby R C, Pevear D C, Dutko F J. CoMFA analysis of the interactions of antipicornavirus compounds in the binding pocket of human rhinovirus-14. J Med Chem. 1992;35:1002–1008. doi: 10.1021/jm00084a005. [DOI] [PubMed] [Google Scholar]
- 11.Diana G D, Cutcliffe D, Volkots D L, Mallamo J P, Bailey T R, Vescio N, Oglesby R C, Nitz T J, Wetzel J, Giranda V, Pevear D C, Dutko F J. Antipicornavirus activity of tetrazole analogues related to disoxaril. J Med Chem. 1993;36:3240–3250. doi: 10.1021/jm00074a004. [DOI] [PubMed] [Google Scholar]
- 12.Diana G D, Volkots D L, Nitz T J, Bailey T R, Long M A, Vescio N, Aldous S, Pevear D C, Dutko F J. Oxadiazoles as ester bioisosteric replacements in compounds related to disoxaril. Antirhinovirus activity. J Med Chem. 1994;37:2421–2436. doi: 10.1021/jm00041a022. [DOI] [PubMed] [Google Scholar]
- 13.Diana G D, Rudewicz P, Pevear D C, Nitz T J, Aldous S C, Aldous D J, Robinson D T, Draper T, Dutko F J, Aldi C, Gendron G, Oglesby R C, Volkots D L, Reuman M, Bailey T R, Czerniak R, Block T, Roland R, Oppermann J. Picornavirus inhibitors: trifluoromethyl substitution provides a global protective effect against hepatic metabolism. J Med Chem. 1995;38:1355–1371. doi: 10.1021/jm00008a014. [DOI] [PubMed] [Google Scholar]
- 14.Diana G D, Pevear D C. Antipicornavirus drugs: current status. Antivir Chem Chemother. 1997;8:401–408. [Google Scholar]
- 15.Gauntt C J, Trousdale M D, LaBadie D R, Paque R E, Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3:207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- 16.Groarke J M, Pevear D C. Attenuated virulence of pleconaril-resistant coxsackievirus B3 variants. J Infect Dis. 1999;179:1538–1541. doi: 10.1086/314758. [DOI] [PubMed] [Google Scholar]
- 17.Guiles J W, Diana G D, Pevear D C. [(Biaryloxy)alkyl]isoxazoles: picornavirus inhibitors. J Med Chem. 1995;38:2780–2783. doi: 10.1021/jm00014a029. [DOI] [PubMed] [Google Scholar]
- 18.Hadfield A T, Lee W-M, Zhao R, Oliveira M A, Minor I, Rueckert R R, Rossmann M G. The refined structure of human rhinovirus 16 at 2.15 Å resolution: implications for the viral life cycle. Structure. 1997;5:427–441. doi: 10.1016/s0969-2126(97)00199-8. [DOI] [PubMed] [Google Scholar]
- 19.Halonen P, Rocha E, Hierholzer J, Holloway B, Hyypia T, Hurskainen P, Pallansch M. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–653. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeger E P, Pevear D C, Felock P J, Russo G R, Treasurywala A M. Genetic algorithm based method to design a primary screen for antirhinovirus agents. In: Reynolds C H, Holloway M K, Cox H K, editors. Computer-aided molecular design: applications in agrochemicals, materials, and pharmaceuticals. Washington, D.C: American Chemical Society; 1995. pp. 139–155. [Google Scholar]
- 21.Kenny M T, Dulworth J K, Barger T M, Daniel J K. Antipicornavirus activity of some diaryl methanes and aralkylaminopyridines. Antivir Res. 1987;7:87–97. doi: 10.1016/0166-3542(87)90024-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim J O, Hodinka R L. Serious respiratory illness associated with rhinovirus infection in a pediatric population. Clin Diagn Virol. 1998;10:57–65. doi: 10.1016/s0928-0197(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim K H, Willingmann P, Gong Z X, Kremer M J, Chapman M S, Minor I, Oliveira M A, Rossmann M G, Andries K, Diana G D, Dutko F J, McKinlay M A, Pevear D C. A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A. J Mol Biol. 1993;230:206–227. doi: 10.1006/jmbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Smith T J, Chapman M S, Rossmann M G, Pevear D C, Dutko F J, Felock P J, Diana G D, McKinlay M A. Crystal structure of human rhinovirus serotype 1A (HRV1A) J Mol Biol. 1989;210:91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 25.Lewis J K, Bothner B, Smith T J, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proc Natl Acad Sci USA. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallamo J P, Diana G D, Pevear D C, Dutko F J, Chapman M S, Kim K H, Minor I, Oliveira M, Rossmann M G. Conformationally restricted analogues of disoxaril: a comparison of the activity against human rhinovirus types 14 and 1A. J Med Chem. 1992;35:4690–4695. doi: 10.1021/jm00103a006. [DOI] [PubMed] [Google Scholar]
- 27.McKinlay M A, Dutko F J, Pevear D C, Woods M G, Diana G D, Rossmann M G. Rational design of antipicornavirus agents. In: Brinton M A, Heinz F X, editors. New aspects of positive-strand RNA viruses. Washington, D.C: American Society for Microbiology; 1990. pp. 366–372. [Google Scholar]
- 28.McKinlay M A, Pevear D C. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu Rev Microbiol. 1992;46:635–654. doi: 10.1146/annurev.mi.46.100192.003223. [DOI] [PubMed] [Google Scholar]
- 29.McKinlay, M. A. Personal communication.
- 30.McKinney R E, Jr, Katz S L, Wilfert C M. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 31.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 655–712. [Google Scholar]
- 32.Modlin J F. Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis. 1986;8:918–926. doi: 10.1093/clinids/8.6.918. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Muckelbauer J K, Kremer M, Minor I, Diana G, Dutko F J, Groarke J, Pevear D C, Rossmann M G. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure. 1995;3:653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 35.Muir P, Kammerer U, Korn K, Mulders M N, Poyry T, Weissbrich B, Kandolf R, Cleator G M, van Loon A M. Molecular typing of enteroviruses: current status and future requirements. Clin Microbiol Rev. 1998;11:202–227. doi: 10.1128/cmr.11.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institute of Allergy and Infectious Diseases. Common cold fact sheet. Bethesda, Md: National Institutes of Health; 1998. [Google Scholar]
- 36a.National Institutes of Health. Guide for the care and use of laboratory animals. Bethesda, Md: National Institutes of Health; 1988. [Google Scholar]
- 37.Otto M J, Fox M P, Fancher M J, Kuhrt M F, Diana G D, McKinlay M A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985;27:883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pevear, D. C., E. M. Jaeger, and T. M. Tull. Activity of pleconaril against rhinoviruses. Submitted for publication.
- 38a.Pevear D C, Fancher M J, Felock P J, Rossmann M G, Miller M S, Diana G, Treasurywala A M, McKinlay M A, Dutko F J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989;63:2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38b.Pevear D C, Seipel M E, Pallansch M, McKinlay M A. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of VP 63843 against field isolates of non-polio enteroviruses, abstr. H99; p. 181. [Google Scholar]
- 39.Phelps D K, Post C B. A novel basis for capsid stabilization by antiviral compounds. J Mol Biol. 1995;254:544–551. doi: 10.1006/jmbi.1995.0637. [DOI] [PubMed] [Google Scholar]
- 40.Phelps D K, Rossky P J, Post C B. Influence of an antiviral compound on the temperature dependence of viral protein flexibility and packing: a molecular dynamics study. J Mol Biol. 1998;276:331–337. doi: 10.1006/jmbi.1997.1542. [DOI] [PubMed] [Google Scholar]
- 41.Rewers M, Atkinson M. The possible role of enteroviruses in diabetes mellitus. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: ASM Press; 1995. pp. 353–385. [Google Scholar]
- 42.Rotbart H A. Meningitis and encephalitis. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: ASM Press; 1995. pp. 271–289. [Google Scholar]
- 43.Rotbart H A, Jones A, Novelli V, Blanche S, Webster A D B. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pleconaril treatment of chronic enteroviral central nervous system infections in children with agammaglobulinemia, abstr. H-126; p. 236. [Google Scholar]
- 44.Rotbart H A the Pleconaril Treatment Registry. Abstracts of the 36th Annual Meeting of the Infectious Diseases Society of America. 1998. Pleconaril therapy of potentially life-threatening enterovirus infections, abstr. 791; p. 249. [DOI] [PubMed] [Google Scholar]
- 45.Rudge P, Webster A D, Revesz T, Warner T, Espanol T, Cunningham-Rundles C, Hyman N. Encephalomyelitis in primary hypogammaglobulinaemia. Brain. 1996;119:1–15. doi: 10.1093/brain/119.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Schiff G M, McKinlay M A, Sherwood J R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Oral efficacy of VP 63843 in coxsackievirus A21 infected volunteers, abstr. H-43; p. 171. [Google Scholar]
- 47.Scott Levin. Physician drug & diagnosis audit. Newtown, Pa: Scott-Levin, a Division of PMSI Scott-Levin, Inc.; 1997. [Google Scholar]
- 48.Smith T J, Kremer M J, Luo M, Vriend G, Arnold E, Kamer G, Rossmann M G, McKinlay M A, Diana G D, Otto M J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986;233:1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- 49.Strikas R A, Anderson L J, Parker R A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 50.Tull, T. M., G. A. Skochko, and D. C. Pevear. The mechanism of action of pleconaril against human picornaviruses. Submitted for publication.
- 51.Weiner L B, Rotbart H A, Gilbert D L, Hayden F G, Mynhardt J H, Dwyer D E, Trocha H, Rogers J M, McKinlay M A. Program addendum of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Treatment of enterovirus meningitis with pleconaril (VP 63843), an antipicornaviral agent, abstr. LB-27; p. 14. [Google Scholar]
- 52.Woods M G, Diana G D, Rogge M C, Otto M J, Dutko F J, McKinlay M A. In vitro and in vivo activities of WIN 54954, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1989;33:2069–2074. doi: 10.1128/aac.33.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]