Abstract
Kelp forests are declining in many regions globally with climatic perturbations causing shifts to alternate communities and significant ecological and economic loss. Range edge populations are often at most risk and are often only sustained through localised areas of upwelling or on deeper reefs. Here we document the loss of kelp forests (Ecklonia radiata) from the Sultanate of Oman, the only confirmed northern hemisphere population of this species. Contemporary surveys failed to find any kelp in its only known historical northern hemisphere location, Sadah on the Dhofar coast. Genetic analyses of historical herbarium specimens from Oman confirmed the species to be E. radiata and revealed the lost population contained a common CO1 haplotype found across South Africa, Australia and New Zealand suggesting it once established through rapid colonisation throughout its range. However, the Omani population also contained a haplotype that is found nowhere else in the extant southern hemisphere distribution of E. radiata. The loss of the Oman population could be due to significant increases in the Arabian Sea temperature over the past 40 years punctuated by suppression of coastal upwelling. Climate-mediated warming is threatening the persistence of temperate species and precipitating loss of unique genetic diversity at lower latitudes.
Subject terms: Biogeography, Conservation biology
Introduction
Climate change has precipitated loss and decline in kelp forests in many regions globally, threatening the vast ecological and economic values that these systems underpin1,2. Warming and extreme events have driven contemporary loss of kelp forests and transitions to alternate ecosystem states e.g.3–7. Marginal kelp populations at warm, equatorward range edges are often among the most vulnerable to climate change because they experience temperatures near or exceeding their thermal thresholds and may lack the genetic diversity to respond8. Such kelp populations inhabiting warm seascapes are often only able to persist due to localised regions of cooler water produced by upwelling or thermoclines at depth that create refugia from warming e.g.9–11. Concerningly, these marginal populations often harbour unique12,13 or functional14,15 genetic diversity.
The dominant and most widespread kelp in the southern hemisphere is Ecklonia radiata (C. Agardh) J. Agardh, which is also among the most warm-tolerant kelps16. This species has a shallow phylogeographic history with low genetic diversity17,18 suggestive of a recent evolutionary origin. Indeed, the genus Ecklonia may have crossed the equator from the northern Pacific < 5 mya19 and rapidly colonised southern temperate reefs17,20 during a period of cooling. The only northern hemisphere population of E. radiata that has been studied is in the Sultanate of Oman, hereafter Oman21. This population has been documented and studied in situ in a series of unpublished reports and herbarium specimens from the 1980’s22,23 from a single location on the southern Dhofar coastline (Sadah; 17.0420°N, 55.0796°E). Although possible northern hemisphere populations of E. radiata have been reported from Mauritania, Senegal and the Canary Islands16,21 and there are unconfirmed reports of E. radiata from Socotra, Yemen24, these reports are unreliable or unconfirmed. For example, inspection of photographs of the putative E. radiata in Socotra24 revealed a morphologically entirely distinct species that is unlikely to be E. radiata. Moreover, the Atlantic Ocean Mauritania and Canary Islands specimens have never been seen or reported in situ and are recorded as E. muratii on herbarium specimens. Reports of E. radiata in Senegal (Cabo Verde, a tropical archipelago) are most likely mislabelled samples from Cape Vert (Senegal, an upwelling zone; E.A. Serrão pers. obs.). Hence, we consider Oman to be the only likely northern hemisphere population of E. radiata but its identity remains to be confirmed genetically.
As with many low latitude kelp forests, the Omani population of E. radiata is thought to be sustained by localized cool water upwelling that allows this species to persist in an otherwise warm seascape. Intense upwelling occurs 20 km either side of Sadah23, where high nutrient levels and low temperatures (as low as 15.9 °C) facilitated the persistence of E. radiata populations between the months of July to mid-October25,26. As temperatures rise to > 27 °C in the post-monsoonal season (and occasionally > 30 °C), E. radiata sporophytes at Sadah senesce, adopting an annual life history22,23. Senescence and adoption of an annual life history has also been speculated to occur in E. radiata at the Abrolhos Islands off Western Australia27, the warmest location this species occurs in Australia. The adoption of an annual life history may occur because temperature tolerances of sporophytes, but not gametophytes, are exceeded in warmer months16,28.
Over the past few decades, the Dhofar coast has warmed and there have been periods of suppressed upwelling29–32. Although periodic suppression of upwelling and deepening of thermoclines can lead to temporary loss of kelp canopy cover33, the low latitude position of the Oman kelp forest, where summer temperatures often exceed the upper thermal limits of E. radiata sporophytes16 and the lack of nearby populations from which reefs could be reseeded following loss, renders this isolated population of E. radiata vulnerable to extinction. Thus, we conducted the first targeted surveys off the remote Sadah coast since the 1980s to confirm the contemporary presence of E. radiata. Our failure to find any extant E. radiata off Oman led us to analyse historical E. radiata herbarium specimens from Sadah to (a) confirm the species identity, (b) elucidate pathways of colonization and (c) compare patterns of genetic diversity with extant populations from throughout the Indian Ocean (Southern Africa and Western Australia).
Methods
Historical data and surveys
The only documented evidence of E. radiata from Oman are by34, who first noted the presence of E. radiata in 1982 during surveys of the distribution of the starfish Acanthaster planci in Dhofar, and by22,23. In particular, detailed studies (including temporal studies on productivity and herbivory) were conducted in 1983 and 1985 at a site they called ‘Ecklonia Bay’ at Wadi Haat, (misspelt as Haart), Sadah (17.0420°N, 55.0796°E, Fig. 1), which was the only place the authors found E. radiata from a number of sites along the southern Dhofar coast. This record of E. radiata was published in a short abstract on the Dhofar macroalgal community35.
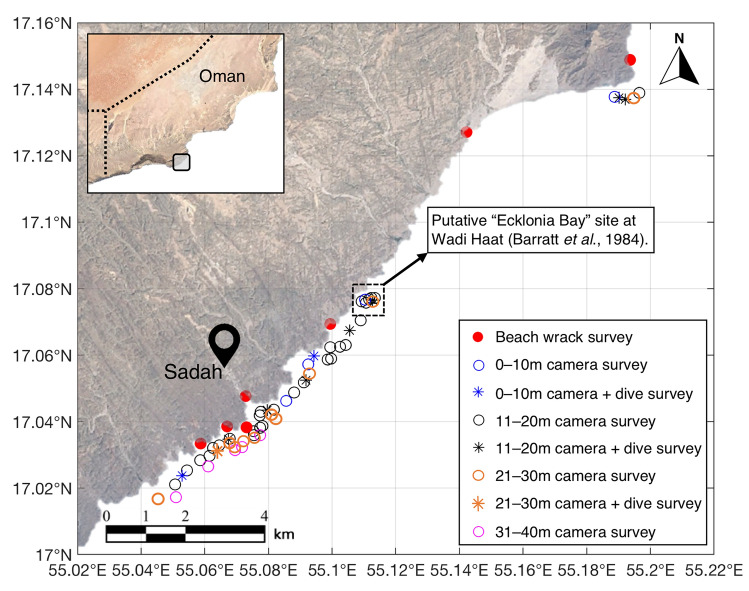
Figure 1.
Spatial distribution of sites surveyed (n = 64) throughout the Sadah region of south-western Oman to assess the contemporary occurrence of Ecklonia radiata. Map generated using MATLAB (ver. 9.8, The MathWorks, Inc., Natick, MA, USA, https://au.mathworks.com/products/matlab).
The Sadah Ecklonia site from22,23 was characterised as being bedrock with gullies extending to a depth of 20 m and approximately 200 m offshore, with large outcrops of bedrock on sand down to 30 m. Details of E. radiata surveys at these sites can be found in22,23, but briefly, Ecklonia forests were present at this one location at depths of 6.5–20 m, apparently below a thermocline. These surveys reported E. radiata as the dominant species at this site, especially between 9 and 20 m. These plants were mostly stage 2 sporophytes36 with an average length of 60–70 cm in the immediate post-monsoon period (mid-September). Ecklonia plants were found down to 30 m in densities similar to shallower waters but only a few plants reached stage 3 and these were mostly found below 20 m. Data on biomass, productivity and other parameters for Ecklonia at 12 m depth were measured in 1985 and found to be comparable with published data from similar depths for E. radiata in Australasia16. Seasonal growth data were collected and presumed to be maximal in July–August and slow to almost zero by November. The entire Ecklonia sporophyte population was reported to disappear in the monsoonal period by late January/early February as temperatures rose to and often exceeded 27 °C22.
Contemporary surveys
Targeted subtidal surveys for E. radiata (by Coleman & Wernberg) were done off the Sadah coast in October 2019 in and around the only site where E. radiata was found in23 (Fig. 1). The exact location of the single E. radiata population studied23 was only known from rough hand drawn maps and local site names, so locating the exact site was subjective. Hence, the putative Waadi Haat “Ecklonia Bay” site was surveyed as well as a range of sites and depths around the Sadah coast based on the maps, descriptions and names provided in the reports as well as additional sites to the north and south. Over a 5-day period in the middle of the post-monsoonal season when E. radiata was previously documented to be at peak abundance22,23 we conducted towed video surveys, SCUBA dives and shore-based surveys of wrack ~ 15 km either side of Sadah and between 1 and ~ 30 m depth in an attempt to locate E. radiata (Fig. 1). The middle of the post-monsoonal period was targeted because even if there had been shifts in phenology of this annual E. radiata population since the 1980s, this would most likely still have captured its presence in surveys. 57 towed video surveys of ~ 5 min in duration each were conducted (~ 200–400 m each). Towed video footage was watched in real time and also recorded to be viewed later and any potential E. radiata noted. We also conducted 10 dives across a range of sites and depths (chosen from video footage) to search for E. radiata. On each dive, we thoroughly searched for E. radiata along a 6 m wide belt on 30 m transects (n = 2), roving swimming surveys (~ 20 min each dive) and photographed the benthos in n = 30, 25 × 25 cm photo quadrats for later analysis and inspection for E. radiata. Finally, we conducted 6 × 1-h roving beach wrack surveys at different sites along the Sadah coast to search for E. radiata wrack (washed up sporophytes) which would show presence of E. radiata from previous years, seasons or unsampled sites.
Genetic analyses of historical and contemporary samples
Specimens of E. radiata from Sadah (collected in 1985 and 1987 by T. Wrathall and L. Barratt) deposited in herbaria of the University of Michigan (MICH613469, MICH613507 (n = 2 recruits), MICH613506; Fig. 2, high resolution photographs available at https://macroalgae.org/portal/), Sultan Qaboos University (SQUH00006214, TMRU #514) and the Oman Marine Science and Fisheries Centre, Ministry of Agriculture and Fisheries (MSFC#138) were accessed and shipped to the National Marine Science Centre in Australia. For these herbarium specimens, DNA was extracted from approximately 50 mg tissue from each sample using the combined CTAB and SDS protocol37. Extracted DNA was purified using a Mo-Bio PowerClean Pro DNA clean-up kit (Merck KGaA, Darmstadt, Germany) following the manufacturer’s protocol. Purified DNA was PCR amplified in 20 µL volumes using 0.5 µL of purified DNA extract in an Eppendorf Mastercycler Nexus Gradient thermocycler using the Thermo Scientific Phire Plant Direct PCR Master Mix kit (Thermo Fisher Scientific, Inc. Waltham, Massachusetts, USA), following the manufacturer’s ‘dilute and store’ protocol. Only 4 of these herbarium specimens were able to be amplified and sequenced.
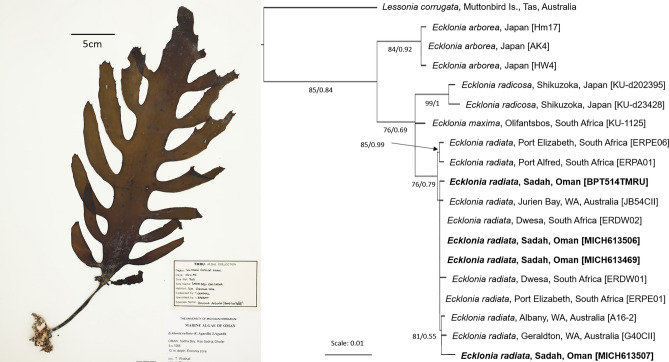
Figure 2.
Example herbarium specimen of E. radiata from Oman collected in the 1980s (MICH613469, high resolution photograph
available at https://macroalgae.org/portal/) and molecular phylogeny of Ecklonia rooted with Lessonia corrugata showing clear clustering of Omani Ecklonia sequences (in bold) within E. radiata confirming its species identity. Topology and branch lengths from maximum likelihood (ML) analysis of concatenated COI, Trnw1, Atp8 and rbcL sequences. Values provided as approximate Bayesian support (right) and ML bootstrap (left), with some intraclade values removed for clarity. DNA isolate numbers are provided to allow comparison with the text. Scale represents average number of nucleotide substitutions per site. See Supplementary Table 1 for Genbank accession numbers.
We also used existing DNA extracted from fresh E. radiata material from Western Australia14 and dried material stored in silica gel from southern Africa to examine relationships among E. radiata from throughout the Indian Ocean. Specimens from Mozambique were collected from a reef off the coast of Zavora, Inhambane Province at 34 m depth at Tentação (24.4444°S, 35.3589°E). Voucher specimens are deposited in the herbarium of Meise Botanic Garden (www.botanicalcollections.be). For the southern African material, dried seaweed samples were ground into a fine powder using a Tissue lyser II (Qiagen, Hilden, Germany). Genomic DNA was extracted using the NucleoSpin® 96 Tissue kit (Macherey–Nagel, Duren, Germany) and diluted (1:100) before PCR amplification. For samples from Western Australia, DNA was extracted and cleaned as in14. All methods were performed in accordance with relevant guidelines and regulations and samples were collected under scientific collection permits issued by state or federal governments and herbarium specimens obtained under agreements with each herbarium/institution. Collection permits were RES2016/02 (Southern Africa), 6210/10/53 for Oman and CE005834, SW019697, #2050 and #3349 (Western Australia).
To confirm the species identity of Omani herbarium specimens, we amplified four DNA markers (COI, trnW-1, atp8 and rbcL) for a small subset of Australian, east African and Omani material to compare to published phylogenetic studies of Ecklonia (see supplementary material Table 1 for Genbank accession numbers). Two mitochondrial intergenic spacer regions (trnW-1 and atp8) were amplified using the primers trnW-trn1-F (5′ GGGGTTCAAATCCCTCTCTT 3′), trnW-trn1-R (5′ CCTACATTGTTAGCTTCATGAGAA 3′) for the trnWI marker and atp8-trnS-F (5′ TGTACGTTTCATATTACCTTCTTTAGC 3′) and atp8-trnS-R (5′ TAGCAAACCAAGGCTTTCAAC 3′) for the atp8 marker38. The RuBisCO large subunit (rbcL) chloroplast marker was amplified using the primers KL2 (5′ GATGCTGATTATAACGTTAAAG 3′) and KL8 (5′ GTTGGTGCATTTGACCACA 3′)39. Additionally, a portion of the mitochondrial cox1-5´ barcoding region (COI) was amplified using the previously published primers Gaz F2 (5′ CCAACCAYAAAGATATWGGTAC 3′) and Gaz R2 (5′ GGATGACCAAARAACCAAAA 3′)39 for all material (southern African, Australian and Omani). In accordance with the Thermo Scientific Phire Plant Direct PCR Master Mix kit manufacturer instructions, a gradient series PCR was performed, using purified DNA, to determine the optimal annealing temperature for each primer/template pair (southern African material: 55.6 °C and Australia/Oman material: 53.5 °C for Gaz F2 and Gaz R2; 53.0 °C for atp8-trnS-F and atp8-trnS-R; 53.0 °C for trnW-trn1-F and trnW-trn1-R; and 48.0 °C for KL2 and KL8). For each marker, PCR cycling consisted of an initial denaturation for 5 min at 98 °C, followed by 45 cycles of: 5 s at 98 °C, 5 s at the relevant annealing temperature for the primer/template pair and 45 s at 72 °C. A final extension was carried out for 1 min at 72 °C. Visualisation of PCR products was carried out using a 2% agarose gel and successful amplicons were purified and sequenced at the Australian Genomic Research Facility (AGRF), Sydney or the Central Services and Technologies of CCMAR, Portugal. Sequences were de novo assembled using Geneious Prime 11.1.5 (Biomatters Ltd., Auckland, New Zealand)40, and edited by eye. Primers were trimmed from alignments, and data quality checks were carried out with MegaBLAST (NCBI, Bethesda, Maryland, USA)41, and for the COI marker, protein translation. Concatenated and single gene alignments and a partition file (gene length) were imported into W-IQ-Tree for tree reconstruction42. W-IQ-Tree incorporates ModelFinder43, which automatically selects and applies a best-fit model to each partition44, which, in this study was identified as the HKY + F + I, F81 + F, TN + F and F81 + F models for the COI, trnW-1, atp8 and rbcL partitions respectively. W-IQ-Tree provides ultrafast bootstrap (BS)45 and approximate Bayesian branch support values. The resultant tree was visualised using FigTree 1.4.4 (Edinburgh, Scotland, UK)46 and rooted with Lessonia corrugata18.
To examine patterns of genetic diversity and relationships among E. radiata samples from throughout the Indian Ocean we amplified the CO1 marker from the full Indian Ocean dataset (n = 79; Table 1) as described above. CO1 haplotype number (H), diversity (Hd) and nucleotide diversity (π) were calculated using DnaSP version 6.12.03 (Barcelona, Catalonia, Spain)47. Haplotype TCS networks48 were reconstructed using PopArt (Dunedin, New Zealand)49. New sequences were deposited in Genbank under accession numbers in Supplementary Table 1.
Table 1.
CO1 diversity metrics for the E. radiata population in Oman compared to extant Western Australian and Southern African populations.
| Pops | N | K | S | Pi | H | Hd | |
|---|---|---|---|---|---|---|---|
| Oman | 1 | 4 | 0.500 | 1 | 0.00081 | 2 | 0.500 |
| Western Australia | 4 | 43 | 0.412 | 2 | 0.00069 | 3 | 0.401 |
| Southern Africa | 6 | 32 | 1.748 | 4 | 0.00284 | 4 | 0.708 |
| All regions | 79 | 1.334 | 7 | 0.00225 | 7 | 0.586 |
Pops, number of populations sampled; N, total sample size; k, average number of nucleotide differences; S, number of segregating sites; Pi, nucleotide diversity; H, number of haplotypes; Hd, haplotype diversity (corrected for sample size).
Assessment of historical environmental conditions
To investigate potential changing environmental conditions on E. radiata off Sadah, variables known to influence the physiology and distribution of kelp were quantified throughout a 20 km radius surrounding E. radiata’s historical occurrence off Sadah (17.0420°N, 55.0796°E) from January 1982 to January 2019. These included daily sea surface temperature (SST) from the reprocessed (level 4) Operational SST and Ice Analysis system (OSTIA) with 0.05° spatial resolution and the direction (i.e., positive, neutral or negative) and intensity of the Indian Ocean Dipole (IOD) mode index for the period January 1982 to January 2019. The IOD mode index is a measure of the gradient in SST between the western and eastern equatorial Indian Ocean. This climate-driver affects environmental conditions throughout the Indian Ocean, with a positive IOD mode associated with anomalously warm ocean temperatures and the suppression of upwelling in the western Indian Ocean including the coastal environment off Oman31. SST data were downloaded from the Copernicus Marine Environment Monitoring Service (https://marine.copernicus.eu; SST product #010_011) and IOD mode index data were downloaded from the National Oceanic and Atmospheric Administration’s Physical Sciences Laboratory (https://psl.noaa.gov/gcos_wgsp/Timeseries/DMI).
Time-series of SST and IOD mode index were plotted to assess for potential environmental drivers of change in E. radiata occurrence off Sadah between historical and contemporary surveys. Linear models were fitted to minimum, mean and maximum annual SST data to test for ocean warming trends off Sadah between January 1982 and January 2019. Plotting and analysis of environmental data was undertaken using the software R R Core50, with residual plots assessed visually to confirm that linear models fitted to SST data satisfied the assumptions of normality and homogeneity of variance.
Additional information
All samples were collected under scientific collection permits issued by state or federal governments in each country and herbarium specimens obtained under agreements with each herbarium/institution. Scientific collection permits were RES2016/02 (Southern Africa), 6210/10/53 for Oman and CE005834, SW019697, #2050 and #3349 (Western Australia).
Results
Failure to find extant E. radiata populations in Oman
We did not find any evidence of extant populations of E. radiata in Oman following 5 days of surveys off Sadah in the peak post-monsoon season, the period it was reported to be abundant in all surveys from the 1980s. This included 57 towed video surveys and 10 SCUBA dives at depths it was reported to be historically abundant around Sadah (Fig. 1). Moreover, surveys at deeper depths and on reefs surrounding Sadah also did not reveal any E. radiata. Moreover, no E. radiata was found during additional beach wrack surveys where washed up E. radiata plants may have accumulated from previous seasons or years or deeper depths (Fig. 1). Notably, since these 2019 surveys, Omani scientists have confirmed the absence of E. radiata at Sadah. They assessed benthic habitats (~ 200m2 per site) in 3 areas off Dhofar including Haat at Sadah, as part of a juvenile abalone seeding project. These surveys were done over 7 days in October/November 2020 for the abundance of macroalgae generally, but no Ecklonia was seen (S.A. Al-Ghassani pers. Obs.).
Species identification & genetic diversity
After trimming primer sequences, PCR amplification and sequencing yielded 655 bp of COI, 326 bp of trnW-1, 167 bp of atp8 and 1044 bp of rbcL. MegaBLAST searches of the NCBI database identified best matches for all sequences from the Omani specimens to be sequence data of E. radiata. This hypothesis was further supported by a phylogenetic reconstruction based on maximum likelihood (ML) using the concatenated dataset (COI, trnW-1, atp8 and rbcL), which recovered these sequences within a well-supported (BS = 99) monophyletic clade comprising E. radiata sequence data from samples spanning its range (Fig. 2).
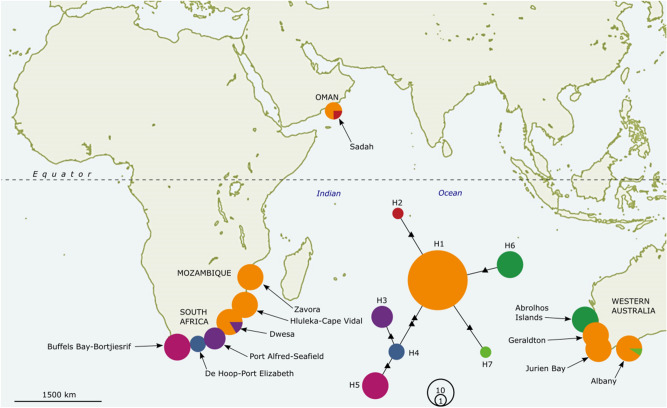
CO1 haplotype diversity (H = 0.586) was much greater than nucleotide diversity (π = 0.00225) indicative of only minor nucleotide differences between haplotypes (Table 1). A total of seven COI haplotypes were present among E. radiata samples from the Indian Ocean (H1-7, Table 1). A single widely distributed haplotype (H1) was found across all three major regions of the Indian Ocean (Oman, Australia and Africa) (Fig. 3). All regions had unique haplotypes including Oman, which had 1 unique haplotype (H2) from the 4 specimens we were able to amplify (Fig. 3). Haplotype diversity (Hd = 0.5) was high in Oman relative to other regions, despite only 4 individuals being able to be genotyped (Table 1).
Figure 3.
CO1 haplotype network of E. radiata from Oman and throughout the Indian Ocean. Colours represent different haplotypes and circle size represents sample size. See Supplementary Table 1 for Genbank accession numbers. Map created in Inkscape v1.1.1 (https://inkscape.org/release/inkscape-1.1.1/).
Trends and anomalies in environmental conditions
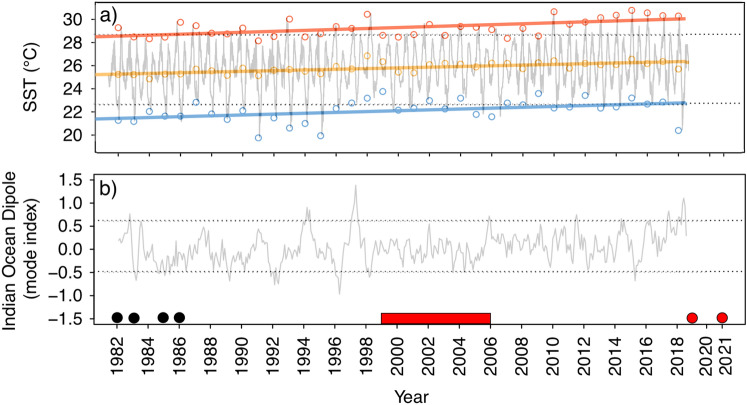
Minimum, mean and maximum annual SST was found to have significantly increased off Sadah, Oman, between 1982 and 2018 (min: F1,35 = 7.14, P = 0.01, + 1.36 °C ± 1.01 °C 95% CI; mean: F1,35 = 34.07, P < 0.001, + 1.12 °C ± 0.39 °C 95% CI; max: F1,35 = 17.76, P < 0.001, + 1.52 °C ± 0.72 °C 95% CI; Fig. 4a). The IOD index consistently varied between positive and negative mode between January 1982 and January 2019 (Fig. 4b). However, notable positive IOD events occurred during 1994 and 1997, which were associated with an intensity that exceeded all other positive or negative IOD events throughout the time-period analysed.
Figure 4.
Time-series of satellite-derived (a) sea surface temperature (SST) within a 15 km radius surrounding historical occurrences of Ecklonia radiata off Sadah, Oman (17.0420°N, 55.0796°E) and (b) the intensity of the Indian Ocean Dipole (IOD) mode index, which is determined by the gradient in SST between the western equatorial Indian Ocean (50°E–70°E and 10°S–10°N) and the eastern equatorial Indian Ocean (90°E–110°E and 10°S–10°N). N.B. strong positive IOD mode indices are associated with anomalously warm ocean temperatures and the suppression of upwelling in the Western Indian Ocean, including coastal environments off Oman. Trends in minimum (blue data), mean (orange data) and maximum (red data) annual SST have been overlaid in panel a), which show significant (at alpha level 0.05) positive increases in minimum (+ 1.36 °C ± 1.01 °C 95% CI), mean (+ 1.12 °C ± 0.39 °C 95% CI) and maximum (+ 1.52 °C ± 0.72 °C 95% CI) SST from 1982 to 2018 throughout the spatial extent assessed. Dashed horizontal lines on all plots denote 90% confidence intervals for each time-series. Black circles on panel B represent dates when E. radiata was documented in Oman and red lines and circles represent dates of surveys when it was not seen in surveys.
Discussion
Marginal, warm edge populations of kelp are declining in many regions globally3,6,51 threatening the unique genetic diversity such marginal populations often support13,14,52. Here, we report the loss of the E. radiata population from Oman, the only confirmed northern hemisphere population of this species. Of concern is that this local extinction implies the loss of haplotype diversity that is found nowhere else in the species’ extant range. Significant ocean warming trends punctuated by periods of suppressed upwelling that supported the existence of this species in a shallow, low latitude seascape, are likely to have driven the loss of this kelp population.
Warming, upwelling and kelp loss
It is apparent that Ecklonia radiata was thriving off Sadah, Oman in the 1980s22,23 but was not found in contemporary surveys. Although there had not been any targeted surveys done at Sadah since the 1980s, the only site that reported E. radiata off the Dhofar coast23, several other non-targeted surveys (e.g. for fish, abalone or taxonomy) were done at other sites but did not report any E. radiata. For example, the Oman Seaweed Project (OSP; 1998/99), surveyed seaweeds off Dhofar but did not find any E. radiata. Similarly, the Algal Biodiversity Project of Oman (1999–2002) led to several publications53,54, but also did not report E. radiata. Tom Schils and Klaas Pauly of the Phycology Research Group, Ghent University, Belgium, carried out surveys mainly around Masirah Island and Barr Al Hikman on the Arabian Sea and the Sea of Oman coastlines over the period 1999 to 2006 e.g.55–57. Although the latter publication lists Ecklonia radiata in Dhofar this is presumed to be the record from54 which is listed in their references. None of these authors found any evidence of E. radiata in Oman. Most notable, however, is that the local Omani scientists and abalone divers that regularly work along the Dhofar coastline conducting surveys for abalone where E. radiata could be present, have not seen any E. radiata over the past few decades (pers. comm. S A Al.-Ghassani). Hence, although many of the above surveys did not specifically visit Sadah or look for E. radiata, it appears that this species was likely lost from Oman many decades ago.
While we cannot definitively determine the cause of E. radiata loss from Sadah, we suggest that long-term ocean warming in the region, punctuated by short-term environmental events that have substantially altered the physical environment, such as periodic cessation of upwelling31, caused the documented extinction of Ecklonia off Oman. The Western Indian Ocean has been continually warming since the start of twentieth century, with the rate of warming accelerating during the last five decades30. Our primary data analysis specific to the coastal ocean off Sadah supports the gradual warming of this region, with maximum annual temperatures increasing by ~ 1.5 °C since 1982, to an average maximum of over 28 °C in 2019, which would have persistently challenged the upper thermal limit (26 °C) of E. radiata16.
Despite being a warm-tolerant kelp with an upper extreme thermal tolerance range between 21.2–26.5 °C in other parts of its range16, E. radiata is unlikely to have survived such temperatures. Warming would have caused the thermocline described by Barratt et al.23 at 6.5 m likely extended to deeper water + 15m58, to include the whole E. radiata forest including any fully grown (stage 3) plants. Therefore, ocean temperatures are likely to have exceeded the upper thermal limit of E. radiata sporophytes throughout the complete depth distribution of the population off Sadah. These high temperatures (i.e., > ~ 28 °C and occasionally > 30 °C) are in excess of any known tolerances for both sporophytes and gametophytes in all other parts of the E. radiata distribution. Even if Omani kelp forests had a higher thermal tolerance than elsewhere in its range, such extreme temperatures in excess of 30 °C would likely have caused the loss of E. radiata from Oman. A similar disappearance was observed of an Ecklonia cava population in 1997 to 1999 in Tosa Bay, Japan, with only urchin barrens and coralline algae found in 20007,59. Anomalously high sea surface temperatures recorded in this region during 1997–98 El Niño Southern Oscillation event were implicated in this disappearance of E. cava7. It is possible that E. radiata persists in Oman in much deeper (cooler) areas than what was sampled in the 1980s or the present study (> 40 m) and Autonomous Underwater Vehicle (AUV) surveys could clarify if this is the case. However, the complete lack of Ecklonia plants in our beach wrack surveys and the poor water clarity off Oman during monsoonal upwelling (and hence low light penetration at depth) makes this unlikely relative to other low latitude areas where Ecklonia persists in much deeper, clearer water e.g. 50–80m9,60–62.
Genetic confirmation and possible origin of Omani E. radiata
Our analysis of historical herbarium specimens confirmed Omani kelp as E. radiata and represent the first genetic sequences for this population. We also reveal that its evolutionary origins are from a single common CO1 haplotype that is present throughout the southern hemisphere suggesting rapid historical colonization. However, the Omani population also contained a CO1 haplotype that is found nowhere else in the Indian (this paper) or Pacific Ocean (M.A. Coleman unpbl data) distribution of this species. Indeed, diversification from the common haplotype was found in all marginal populations sampled here (H3/4/5 from South Africa and H6 from the Abrolhos Islands, Western Australia) indicative of isolation following rapid colonisation e.g. Abrolhos14, and consistent with known genetic breaks63. The genetic diversity of E. radiata in Oman in the 1980s was likely higher than that reported here given that one unique haplotype was found from only 4 herbarium specimens (Hd = 0.5) which is higher than the diversity reported for extant Western Australian populations (Hd = 0.401, n = 43 specimens).
It is interesting to speculate on the origin of the Omani E. radiata population and how it may have crossed the equator. The presence of E. radiata populations on isolated islands (e.g. Lord Howe Island, Houtman Abrolous Islands) elsewhere within its range suggests that long distance dispersal is possible despite the lack of floating structures. Thus, we suggest that colonization in Oman may have been via “stepping stone” dispersal among cooler regions of upwelling (Somalia, Yemen, Oman) and/or cooler deep reefs in more tropical locations e.g. Mozambique64,65. Indeed, there are unconfirmed reports of deepwater E. radiata off Madagascar, and Sodwana Bay, South Africa (50 m, K. Sink Pers. Comm.), and in this study we confirmed the presence of the species in Inhambane, Mozambique (29–35 m) suggesting that this may have been a dispersal pathway during periods of cooling. Confirming this hypothesis could benefit from analysis of seaweed biogeography from other parts of the Arabian and African coast including Somalia where there is also significant monsoon-induced upwelling56,66.
An alternative hypothesis for the presence of a single, isolated population of E. radiata in Oman is an introduction from east Africa, potentially via historical frankincense67 or general maritime trade routes. The Omani empire was centred on the island of Zanzibar and maritime trade with East African ports further south such as Sofala on the coast of Mozambique and on Madagascar was common. The Omani vessels could have carried algal fragments from this region back to Oman after pulling up anchors or fishing gear. Indeed, stone ballast, anchors and pots associated with shipwrecks found in Zanzibar, Mombasa and Kilwa (Tanzania) are similar to those also found in Oman and other Arabian ports and date back to the ninth century68,69. The presence of a unique CO1 haplotype in Oman suggests that if this were the case, it was likely an old and single introduction followed by isolation or that the source population was not sampled (e.g. the unique haplotype may be present in unconfirmed/unsampled populations off Somalia or Yemen).
The E. radiata population in Oman was likely living on the edge, only sustained by the cooler waters that came with the localized monsoonal upwelling along this small section of the Dhofar coast. Globally, many isolated kelp populations persist at low latitudes only on deeper reefs where waters are cooler9,10,12,70 and further retreat to depth of kelp populations is predicted under future ocean warming62. Continued warming of our oceans and climate-induced change to oceanographic processes such as upwelling threatens these populations and the genetic diversity they can harbor. While it is unlikely that in situ conservation actions will be enough to protect these marginal populations against ongoing warming, we can take proactive measures to secure their diversity in germplasm or culture banks71. Protecting this diversity ex situ may one day present new opportunity to proactively restore or boost resilience of declining kelp forests72.
Supplementary Information
Acknowledgements
We thank the PADI Foundation for funding to support this work and the Oman Ministry of Agriculture and Fisheries Wealth and Sultan Qaboos University for vessel and personnel support in Oman. The expedition to Mozambique was funded by the Belgian Directorate-General for Development Cooperation through the CEBioS Programme, partim GTI, The King Léopold III Fund for Nature Exploration and Conservation, and the Research Foundation – Flanders. Scientific collection permits were granted by the Ministry of Environment and Climate Affairs, Director General of Nature Conservation, Oman (Permit Number: 6210/10/53), and the Ministry of Land and Environment, National Administration of Conservation Areas, Mozambique (Permit Number: 04/2018). Genomic analyses were also funded through an Australian Research Council grant to TW and MAC (DP200100201). The genetic work on African samples was funded by a Pew Marine Fellowship to EA Serrao and FCT – Foundation for Science and Technology (Portugal) through UIDB/04326/2020, PTDC/MAR-EST/6053/2014 (Genekelp).
Author contributions
MAC and TW conceived the idea, received primary funding and conducted the fieldwork. AM, SAA and BPJ contributed local knowledge and field assistance. MR, MAC, MJN, JJB, ODC, FL, GAP, EAS and PM contributed samples and conducted parts of the laboratory work. CC conducted environmental conditions analysis and produced figures. MJN, MAC and MR conducted the analyses. MAC wrote the manuscript. All authors contributed to funding parts of the work and editing the manuscript.
Data availability
Genbank accession numbers for CO1 sequences generated available in Supplementary Table 1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08264-3.
References
- 1.Wernberg T, Krumhansl KA, Filbee-Dexter K, Pedersen M. Status and trends for the world’s kelp forests. In: Sheppard C, editor. World Seas: An Environmental Evaluation. Elsevier; 2019. pp. 57–78. [Google Scholar]
- 2.Smale DA, et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change. 2019;9:306–312. doi: 10.1038/s41558-019-0412-1. [DOI] [Google Scholar]
- 3.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MA, Minne AJP, Vranken S, Wernberg T. Genetic tropicalisation following a marine heatwave. Sci. Rep. UK. 2020;10:12726. doi: 10.1038/s41598-020-69665-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumhansl KA, et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. 2016;113:13785–13790. doi: 10.1073/pnas.1606102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arafeh-Dalmau N, et al. Extreme marine heatwaves alter kelp forest community near its equatorward distribution limit. Front. Mar. Sci. 2019 doi: 10.3389/fmars.2019.00499. [DOI] [Google Scholar]
- 7.Tanaka K, Taino S, Haraguchi H, Prendergast G, Hiraoka M. Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol. Evol. 2012;2:2854–2865. doi: 10.1002/ece3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wernberg T, et al. Genetic diversity and kelp forest vulnerability to climatic stress. Sci. Rep. UK. 2018;8:1851. doi: 10.1038/s41598-018-20009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham MH, Kinlan BP, Druehl LD, Garske LE, Banks S. Deep-water kelp refugia as potential hotspots of tropical marine diversity and productivity. Proc. Natl. Acad. Sci. 2007;104:16576. doi: 10.1073/pnas.0704778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzinelli EM, et al. Large-scale geographic variation in distribution and abundance of Australian deep-water kelp forests. PLoS ONE. 2015;10:e0118390. doi: 10.1371/journal.pone.0118390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varela R, Rodríguez-Díaz L, de Castro M, Gómez-Gesteira M. Influence of Eastern Upwelling systems on marine heatwaves occurrence. Glob. Planet Change. 2021;196:103379. doi: 10.1016/j.gloplacha.2020.103379. [DOI] [Google Scholar]
- 12.Assis J, et al. Deep reefs are climatic refugia for genetic diversity of marine forests. J. Biogeogr. 2016;43:833–844. doi: 10.1111/jbi.12677. [DOI] [Google Scholar]
- 13.Lourenço CR, et al. Upwelling areas as climate change refugia for the distribution and genetic diversity of a marine macroalga. J. Biogeogr. 2016;43:1595–1607. doi: 10.1111/jbi.12744. [DOI] [Google Scholar]
- 14.Vranken S, et al. Genotype-environment mismatch of kelp forests under climate change. Mol. Ecol. 2021;30:3730–3746. doi: 10.1111/mec.15993. [DOI] [PubMed] [Google Scholar]
- 15.Wood G, et al. Genomic vulnerability of a dominant seaweed points to future-proofing pathways for Australia's underwater forests. Glob. Change Biol. 2021;27:2200–2212. doi: 10.1111/gcb.15534. [DOI] [PubMed] [Google Scholar]
- 16.Wernberg T, et al. Biology and ecology of the globally significant kelp Ecklonia radiata. Oceanogr. Mar. Biol.: An Annu. Rev. 2019;57:265–324. doi: 10.1201/9780429026379-6. [DOI] [Google Scholar]
- 17.Durrant HMS, Barrett NS, Edgar GJ, Coleman MA, Burridge CP. Shallow phylogeographic histories of key species in a biodiversity hotspot. Phycologia. 2015;54:556–565. doi: 10.2216/15-24.1. [DOI] [Google Scholar]
- 18.Rothman MD, et al. A molecular investigation of the genus Ecklonia (Phaeophyceae, Laminariales) with special focus on the southern hemisphere. J. Phycol. 2015;51:236–246. doi: 10.1111/jpy.12264. [DOI] [PubMed] [Google Scholar]
- 19.Starko S, et al. A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol. Phylogenetics Evol. 2019;136:138–150. doi: 10.1016/j.ympev.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd, S. A. & Edgar, G. J. In (eds Shepherd, S. A. & Edgar, G. J.) (CSIRO Publishing, 2013).
- 21.Guiry, M. D. et al. AlgaeBase: An on-line resource for algae. Cryptogam. Algol.35, 105–115, 111 (2014).
- 22.Barratt, L., Ormond, R. F. G. & Wrathall, T. J. Ecological studies of southern Oman kelp communities. Part 1. Ecology and productivity of the sublittoral algae Ecklonia radiata and Sargassopsis zanardinii (Council for the conservation of the environment and water resources, and regional organisation for the protection of the marine environment, Muscat and Kuwait, 1986).
- 23.Barratt, L. et al.An ecological study of the rocky shores on the south coast of Oman. Report of IUCN to UNEP's regional seas programme, Vol. 104 (Tropical Marine Research Unit, York, 1984).
- 24.Klaus R, Turner JR. The marine biotopes of the Socotra Archipelago. Fauna Arab. 2004;20:45–116. [Google Scholar]
- 25.Claereboudt, M. R. Oman. In World Seas: An Environmental Evaluation, (ed. Sheppard, C.) 25–47 (Academic Press, 2019).
- 26.Savidge, G., Lennon, H. J. & Matthews, A. D. A shore based survey of oceanographic variables in the Dhofar region of southern Oman, August–October 1985. In Ecological Studies of Southern Oman Kelp Communities. Summary Report, 4–21. ROPME/GC-6/001 (1988).
- 27.Hatcher BG, Kirkman H, Wood WF. Growth of the kelp Ecklonia-radiata near the northern limit of its range in Western-Australia. Mar. Biol. 1987;95:63–73. doi: 10.1007/Bf00447486. [DOI] [Google Scholar]
- 28.Veenhof, R. et al. Kelp gametophytes in changing oceans. Oceanogr. Mar. Biol. Annu. Rev.60(in press).
- 29.Goes JI, Thoppil PG, Gomes HDR, Fasullo JT. Warming of the Eurasian landmass is making the Arabian sea more productive. Science. 2005;308:545. doi: 10.1126/science.1106610. [DOI] [PubMed] [Google Scholar]
- 30.Roxy MK, et al. A reduction in marine primary productivity driven by rapid warming over the tropical Indian Ocean. Geophys. Res. Lett. 2016;43:826–833. doi: 10.1002/2015GL066979. [DOI] [Google Scholar]
- 31.Watanabe TK, Watanabe T, Yamazaki A, Pfeiffer M, Claereboudt MR. Oman coral δ18O seawater record suggests that Western Indian Ocean upwelling uncouples from the Indian Ocean Dipole during the global-warming hiatus. Sci. Rep. UK. 2019;9:1887. doi: 10.1038/s41598-018-38429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe TK, et al. Past summer upwelling events in the Gulf of Oman derived from a coral geochemical record. Sci. Rep. UK. 2017;7:4568. doi: 10.1038/s41598-017-04865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards M, Estes JA. Catastrophe, recovery and range limitation in NE Pacific kelp forests: a large-scale perspective. Mar. Ecol. Prog. Ser. 2006;320:79–87. doi: 10.3354/meps320079. [DOI] [Google Scholar]
- 34.Glynn PW. Monsoonal upwelling and episodic Acanthaster predation as probable controls of coral reef distribution and community structure in Oman, Indian Ocean. Atoll Res. Bull. 1993;379:1–66. doi: 10.5479/si.00775630.379.1. [DOI] [Google Scholar]
- 35.Hiscock S, Barratt L, Ormond R. The marine algae of Dhofar, Oman-an upwelling system in the Arabian Sea. Br. Phycol. J. 1984;19:194. doi: 10.1080/00071618400650081. [DOI] [Google Scholar]
- 36.Kirkman H. The 1st year in the life-history and the survival of the juvenile marine macrophyte, Ecklonia-radiata (Turn) J Agardh. J. Exp. Mar. Biol. Ecol. 1981;55:243–254. doi: 10.1016/0022-0981(81)90115-5. [DOI] [Google Scholar]
- 37.Maeda T, Kawai T, Nakaoka M, Yotsukura N. Effective DNA extraction method for fragment analysis using capillary sequencer of the kelp, Saccharina. J. Appl. Phycol. 2013;25:337–347. doi: 10.1007/s10811-012-9868-3. [DOI] [Google Scholar]
- 38.Voisin M, Engel CR, Viard F. Differential shuffling of native genetic diversity across introduced regions in a brown alga: Aquaculture vs. maritime traffic effects. Proc. Natl. Acad. Sci. USA. 2005;102:5432. doi: 10.1073/pnas.0501754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane CE, Lindstrom SC, Saunders GW. A molecular assessment of northeast Pacific Alaria species (Laminariales, Phaeophyceae) with reference to the utility of DNA barcoding. Mol. Phylogenetics Evol. 2007;44:634–648. doi: 10.1016/j.ympev.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Kearse M, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson M, et al. NCBI BLAST: A better web interface. Nucl. Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucl. Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chernomor O, von Haeseler A, Minh BQ. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016;65:997–1008. doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rambaut A, Drummond A. FigTree: Tree Figure Drawing Tool, Version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh; 2008. [Google Scholar]
- 47.Rozas J, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 48.Clement M, Posada D, Crandall KA. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 49.Leigh JW, Bryant D. popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 50.Team, R. C. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
- 51.Vergés A, et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. 2016;113:13791–13796. doi: 10.1073/pnas.1610725113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood G, et al. Using genetics to test provenance effects and to optimise seaweed restoration. J. Appl. Ecol. 2020 doi: 10.1111/1365-2664.13707. [DOI] [Google Scholar]
- 53.Wynne, M. J. A checklist of the benthic marine algae of the Northern Arabian Sea coast of the Sultanate of Oman. Bot. Mar.61, 481–498. 10.1515/bot-2018-0035 (2018).
- 54.Richards, G. & Wynne, M. J. 57 (2003).
- 55.Schils, T. Marine Plant Communities of Upwelling Areas Within the Arabian Sea: A Taxonomic, Ecological ABD Biogeographic Case Study on the Marine Flora of the Socotra Archipelago (Yemen) and Masirah Island (Oman). PhD thesis (2002).
- 56.Schils T, Coppejans E. Phytogeography of upwelling areas in the Arabian Sea. J. Biogeogr. 2003;30:1339–1356. doi: 10.1046/j.1365-2699.2003.00933.x. [DOI] [Google Scholar]
- 57.Schils T, Wilson SC. temperature threshold as a biogeographic barrier in northern Indian Ocean Macroalgae. J. Phycol. 2006;42:749–756. doi: 10.1111/j.1529-8817.2006.00242.x. [DOI] [Google Scholar]
- 58.Wiggert JD, Hood RR, Banse K, Kindle JC. Monsoon-driven biogeochemical processes in the Arabian Sea. Prog. Oceanogr. 2005;65:176–213. doi: 10.1016/j.pocean.2005.03.008. [DOI] [Google Scholar]
- 59.Serisawa Y, Imoto Z, Ishikawa T, Ohno M. Decline of the Ecklonia cava population associated with increased seawater temperatures in Tosa Bay, southern Japan. Fish. Sci. 2004;70:189–191. doi: 10.1111/j.0919-9268.2004.00788.x. [DOI] [Google Scholar]
- 60.Nelson W, Duffy C, Trnski T, Stewart R. Mesophotic Ecklonia radiata (Laminariales) at Rangitāhua, Kermadec Islands, New Zealand. Phycologia. 2018;57:534–538. doi: 10.2216/18-9.1. [DOI] [Google Scholar]
- 61.Richmond S, Stevens T. Classifying benthic biotopes on sub-tropical continental shelf reefs: How useful are abiotic surrogates? Estuar. Coast. Shelf Sci. 2014;138:79–89. doi: 10.1016/j.ecss.2013.12.012. [DOI] [Google Scholar]
- 62.Davis TR, Champion C, Coleman MA. Climate refugia for kelp within an ocean warming hotspot revealed by stacked species distribution modelling. Mar. Environ. Res. 2021;166:105267. doi: 10.1016/j.marenvres.2021.105267. [DOI] [PubMed] [Google Scholar]
- 63.Jooste CM, Oliver J, Emami-Khoyi A, Teske PR. Is the Wild Coast in eastern South Africa a distinct marine bioregion? Helgol. Mar. Res. 2018;72:6. doi: 10.1186/s10152-018-0509-3. [DOI] [Google Scholar]
- 64.Bolton JJ, et al. Where is the western limit of the tropical Indian Ocean seaweed flora? An analysis of intertidal seaweed biogeography on the east coast of South Africa. Mar. Biol. 2004;144:51–59. doi: 10.1007/s00227-003-1182-9. [DOI] [Google Scholar]
- 65.Bolton JJ. The biogeography of kelps (Laminariales, Phaeophyceae): A global analysis with new insights from recent advances in molecular phylogenetics. Helgol. Mar. Res. 2010;64:263–279. doi: 10.1007/s10152-010-0211-6. [DOI] [Google Scholar]
- 66.Bolton JJ, De Clerck O, John DM (2003). Seaweed diversity patterns in Sub-Saharan Africa. In Proceedings of the Marine Biodiversity in Sub-Saharan Africa: The Known and the Unknown. (eds. Decker, C. et al. ) Cape Town, South Africa, pp. 229–241 (2003).
- 67.Wood M, et al. Zanzibar and Indian Ocean trade in the first millennium CE: The glass bead evidence. Archaeol. Anthropol. Sci. 2017;9:879–901. doi: 10.1007/s12520-015-0310-z. [DOI] [Google Scholar]
- 68.Pollard E, Bates R, Ichumbaki EB, Bita C. Shipwreck evidence from Kilwa, Tanzania. Int. J. Naut. Archaeol. 2016;45:352–369. doi: 10.1111/1095-9270.12185. [DOI] [Google Scholar]
- 69.Staples, M. In Oman. A Maritime History (eds Al Salimi, A. & Staples, E.) Chap. 4, 81–116 (Georg Olms Verlag, 2017).
- 70.Assis J, et al. Past climate changes and strong oceanographic barriers structured low-latitude genetic relics for the golden kelp Laminaria ochroleuca. J. Biogeogr. 2018;45:2326–2336. doi: 10.1111/jbi.13425. [DOI] [Google Scholar]
- 71.Wade R, et al. Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biol. 2020;18:e3000641. doi: 10.1371/journal.pbio.3000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coleman MA, et al. Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 2020 doi: 10.3389/fmars.2020.00237. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genbank accession numbers for CO1 sequences generated available in Supplementary Table 1.