Abstract
Although hydroxyurea (HU) is an effective treatment for sickle cell anemia, uptake remains low. Shared decision making (SDM) is a recommended strategy for HU initiation to elicit family preferences; however, clinicians lack SDM training. We implemented an immersive virtual reality (VR) curriculum at eight pediatric institutions to train clinicians on SDM that included counseling virtual patients. Clinicians’ self-reported confidence significantly improved following the VR simulations on all communication skills assessed, including asking open-ended questions, eliciting specific concerns, and confirming understanding (ps ≤ 0.01 for all). VR may be an effective method for educating clinicians to engage in SDM for HU.
Keywords: virtual reality, shared decision making, hydroxyurea, virtual patients, virtual simulation
INTRODUCTION:
Although hydroxyurea (HU) is an effective disease-modifying treatment for sickle cell anemia (SCA), uptake remains low in pediatric populations due to parental concerns about safety and side-effects.1 The National Heart, Lung, and Blood Institute Guidelines recommend shared decision making (SDM) for HU initiation to elicit family preferences and values; however, most clinicians who care for children with SCA lack specific training in SDM.2,3 We developed a HU-SDM toolkit to facilitate such discussions (NCT03442114).3,4 It includes: 1) decision aids to support parents (educational brochure, booklet, video narratives, and in-visit issue card); 2) quality improvement tools to monitor SDM performance; and 3) a curriculum to train clinicians in advanced communication skills to engage caregivers in SDM.4,5
Prior strategies to train clinicians in SDM have consisted of distributing educational materials, educational meetings, audits with feedback, barrier assessments, and less frequently standardized patient encounters.6,7 To facilitate clinicians’ use of SDM in actual clinical visits, decision aids have been developed to present treatment options to patients in an inclusive, unbiased manner to promote meaningful exchanges between clinicians and patients.8 Decision aids have demonstrated efficacy in facilitating SDM; however, real-world implementation has been challenging.8,9 This may in part be due to the lack of opportunities to deliberately practice using decision aids in simulated, realistic settings.
Virtual reality (VR) is a three-dimensional computer-generated environment where users interact with graphical character representatives (avatars). The technology facilitates deliberate practice derived from Ericsson’s Theory on Expertise by allowing individuals to engage in realistic clinical scenarios in a safe, immersive environment that peers and experts can view to provide actionable feedback.10,11 Deliberate practice has been successfully utilized for VR-based communication training to address vaccine hesitancy resulting in enhanced confidence and skill demonstration.12,13 It has not been previously applied to training on SDM.13 Thus, we sought to use VR to train clinicians who specialize in pediatric hematology on SDM in the context of HU initiation. We examined the acceptability and tolerability of the approach and the impact of deliberate practice using VR simulations on clinicians’ confidence related to SDM communication skills.
MATERIALS AND METHODS:
Setting and Study Population
Cincinnati Children’s Hospital Medical Center (CCHMC) serves as the coordinating center for a multisite randomized trial of the HU-SDM toolkit (NCT03442114).5 The current study examines clinician-reported outcomes after participating in our immersive VR curriculum. Participating centers included Boston Medical Center, Children’s Hospital of Oakland, Children’s Hospital of Philadelphia, Lurie Children’s Hospital of Chicago, Nationwide Children’s Hospital, Nemours Alfred I. duPont Hospital for Children, St. Louis Children’s Hospital, and Texas Children’s Hospital. Eligible participants included clinicians (physicians and advanced practice providers) that care for patients with SCA. Training occurred in-person at the location of the participating institution. The CCHMC institutional review board approved this study that included a waiver of documentation of consent for participating clinicians.
Curriculum Design
The HU-SDM toolkit was co-created with parents via interviewing, clinical observations, feedback, and acceptability testing.4 The VR curriculum was similarly co-created with parents who in addition to reviewing the simulation plans, provided the voices for our parent avatars and participated in usability testing of the VR curriculum. The curriculum was approximately 3-hours long and took place in a conference room. It included a 2-hour workshop that discussed the HU-SDM toolkit as well as best-practice communication skills for SDM and motivational interviewing (e.g., active listening, open-ended questioning, confirming understanding through reflections, and using an ask-tell-ask approach to information sharing). Motivational interviewing skills were included given their relevance to the curricular objectives as motivational interviewing focuses on eliciting behavior change through exploration and resolution of ambivalence.14 The immersive VR simulations occurred during the final hour of the workshop, allowing participants to practice the SDM skills previously reviewed. The HU-SDM in-visit issue card, a decision aid that graphically presents common sources of hesitancy that parents report as key to decision-making about HU4, was incorporated into the virtual environment to reinforce practice with this aid. Clinicians participated in simulations via a 3D-mounted headset. After receiving the case history, clinicians verbally counseled an avatar family in the virtual environment around HU initiation (see video, Supplemental Digital Content 1: VR intervention). The VR environment replicated an outpatient clinic room, and we designed avatars based on common demographics of patients with SCA (e.g., Black/African American, Hispanic) (Figure 1). A facilitator (FJR, DD, BC) operated the avatars’ verbal and non-verbal responses in real-time to create a realistic experience for clinicians. Each simulation included three sources of hesitancy regarding HU initiation (e.g., risks, benefits, and impact on daily life such as school and work attendance). We piloted the VR curriculum with clinicians (2 hematologists, 2 nurse practitioners, 3 behavioral psychologists) at CCHMC and parents of a child with SCA and adapted the curriculum accordingly before use in this study.
Figure 1.

The virtual reality environment replicated a patient room that included the in-visit issue card to support providers in practicing shared decision with parent and patient avatars (A). Parent and patient avatars varied in appearance and could assume different body positions to indicate non-verbal cues to clinicians (B, C).
During site visits, we displayed the clinician’s view through the VR headset onto a projector screen so peers and workshop facilitators could observe the clinician-avatar interactions. After each simulation, clinicians and facilitators debriefed regarding the demonstrated SDM skills, including utilization of the in-visit issue card. We employed deliberate practice, an active learning process that is characterized by engaging in a task or behavior (e.g., counseling a family on HU) followed by receiving immediate feedback regarding areas for improvement.10 Each clinician participated in at least one simulation. The avatars, patient history, and sources of hesitancy were varied between clinicians at a single site to promote skill development through novel scenarios.
Survey Design
We collected demographic data, including age, gender, role (physician vs. advanced practice provider), and years of experience treating patients with SCA. To assess acceptability, we measured the level of immersion in the VR simulation using a tool with prior validity evidence among medical students and residents, the MEC-Spatial Presence Questionnaire.15,16 This tool assesses presence in a virtual environment using a 5-point Likert scale (strongly disagree to strongly agree). To evaluate tolerability, we assessed for VR side effects experienced by participants.17
To understand the impact of the curriculum, we assessed clinicians’ prior experience via a survey instrument prior to the workshop. This survey used a 5-point anchored scale based on experience (from no experience to the ability to coach others) to assess participants’ previous experiences related to addressing common HU initiation concerns (e.g., benefits, risks, costs, and impact on daily life) and SDM and motivational interviewing skills. Following the workshop, we assessed clinicians’ confidence related to HU initiation and communication via a retrospective pre-post survey on a 5-point scale (from not at all confident to very confident). Retrospective pre-post ratings have been shown to be a valid and sensitive approach to assessing faculty development programs.18 We piloted survey items with 7 clinicians with SCA experience and individuals with expertise in SDM and medical education before implementation.
Statistical Analysis
We conducted analyses in the R statistical environment, with the MICE package used as the imputation method for 4 missing item responses. We utilized descriptive and summary statistics for clinician demographics and scores on questionnaires. We assessed for differences in clinician confidence using Wilcoxon Signed-ranks tests, as they were ordinal questions, with r used as our measure of effect size. We calculated r by dividing the Z statistic by the square root of the sample size (Z/√(Npairs)). We interpreted r as the probability that differences in scores before and after the curriculum were greater than zero with effect sizes interpreted as small (0.01 to < 0.30), medium (0.30 to < 0.50), and large (≥ 0.50).19 Two-sample tests for the equality of proportions with continuity corrections assessed for change in counts of “very confident” responses.
RESULTS
Demographics
We implemented the VR curriculum between April 2019 to March 2020. Twenty-two (56%) of 39 eligible clinicians (2–4 participants/center) agreed to participate in the VR curriculum and complete evaluation metrics. Since VR training occurred on a single date, the most common reason for not participating was the inability to attend due to other responsibilities (e.g., clinical work). Most participants (91%) were female, and the most common age range was 35–44 years. The majority (73%) identified as physicians. The minimum years of experience caring for patients with SCA was 3, with a plurality (41%) reporting 6–10 years of experience caring for this population. (Table 1)
Table 1.
Clinician characteristics and years of experience caring for patients with sickle cell anemia.
| Participant characteristics (n=22) | |
|---|---|
| Characteristic | n (%) |
| Current role | |
| Attending physician | 16 (73) |
| Nurse practitioner | 6 (27) |
| Age | |
| 25–34 y | 3 (14) |
| 35–44 y | 11 (50) |
| 45–54 y | 3 (14) |
| 55–64 y | 5 (23) |
| Sex | |
| Male | 2 (9) |
| Female | 20 (91) |
| Years of practice | |
| < 3 y | 1 (5) |
| 3–5 y | 5 (23) |
| 6–10 y | 7 (32) |
| 11–15 y | 2 (9) |
| > 15 y | 7 (32) |
| Years of experience caring for patients with sickle cell anemia | |
| < 3 y | 0 (0) |
| 3–5 y | 4 (18) |
| 6–10 y | 9 (41) |
| 11–15 y | 3 (14) |
| > 15 y | 6 (27) |
Acceptability
All participants (100%) strongly agreed or agreed that the VR curriculum captured their senses, and 95% strongly agreed or agreed that they felt as though they were physically present in the environment. Ninety-one percent strongly agreed or agreed that the objects in the simulation gave them the feeling they could do things with them. Following the VR experience, most (86%) strongly agreed or agreed that they still had a concrete mental image of the spatial environment (Figure 2).
Figure 2.
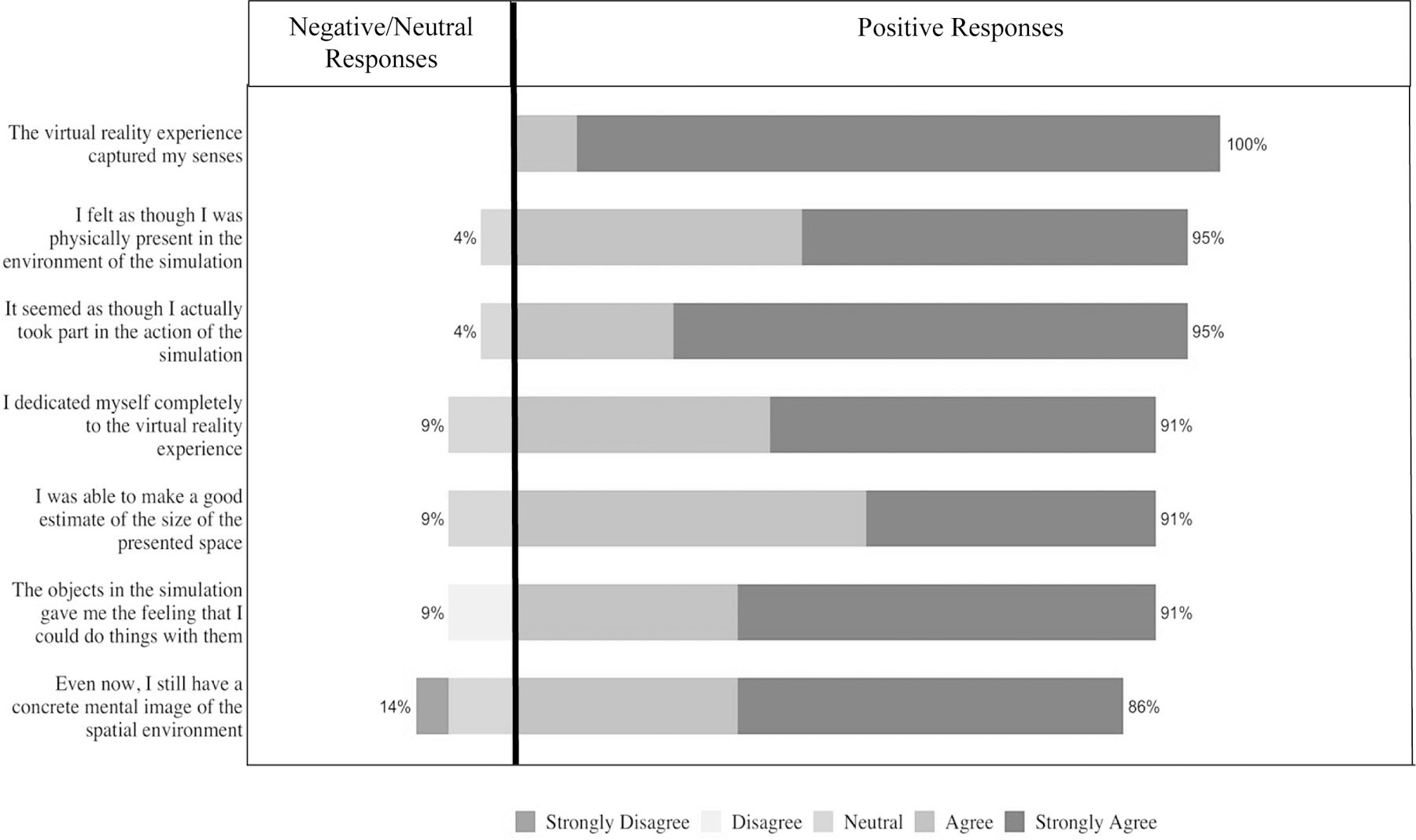
Clinician responses to specific items on the MEC-Spatial Presence Questionnaire. Items are rated on a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree)
Tolerability
The majority of participants tolerated the VR intervention well. The most common side effects were blurred vision (23%), disorientation (23%), dizziness (18%), and eye strain (14%).
Impact
At baseline, the majority (>67%) of participants felt that they could coach other clinicians on discussing the risks and benefits of HU with families. Only one individual (5%) felt that they could coach another clinician on discussing costs related to the medication initiation. More than half (59%) of participants felt they could use more experience discussing costs with families. In terms of motivational interviewing skills, clinicians reported the most experience asking open-ended questions, with 36% expressing the ability to coach others and 55% reporting sufficient experience. Clinicians reported the least experience using an ask-tell-ask approach to share information, with 41% having no experience in this skill or requesting more experience.
Following participation in the curriculum, clinicians’ self-reported confidence significantly improved after VR simulations on discussing benefits related to HU with families, Z = −2.01, p = .03, r = .50, discussing costs, Z = −2.44, p = .01, r = .55 and discussing the impact of HU on daily life, Z = −2.33, p = .02, r = .52. Confidence in discussing risks with families approached statistical significance, Z = −1.8, p = .07, r = .40. In terms of motivation interviewing skills, clinicians’ self-reported confidence significantly improved after VR simulations on all communication skills assessed including asking open-ended questions, Z = −3.16, p = .001, r = .71, eliciting specific concerns, Z = −3.60, p = .0003, r = .81, confirming understanding, Z = −3.31, p = .0009, r = .74, and using an ask-tell-ask approach to information sharing, Z = −3.85, p = .0001, r = .86. With the exception of confidence in discussing benefits and risks, clinician responses of “very confident” significantly increased following the VR curriculum for all survey items (Table 2).
Table 2.
Change in clinician “very confident” response for communication skills prior to and following participation in the virtual reality curriculum
| Pre (N=22) |
Post (N=22) |
χ 2 | p-value* | |
|---|---|---|---|---|
| N (%) | ||||
| Confidence in counseling on common hydroxyurea initiation concerns: | ||||
| Benefits | 16 (72) | 21 (95) | 3.84 | .05 |
| Risks | 16 (72) | 20 (91) | 2.82 | .09 |
| Costs | 5 (23) | 13 (59) | 14.23 | < .001 |
| Impact of daily life | 14 (64) | 21 (95) | 6.48 | .01 |
| Confidence in using shared decision making and motivational interviewing skills: | ||||
| Asking open-ended questions | 10 (45) | 22 (100) | 13.87 | < .001 |
| Eliciting specific concerns | 7 (32) | 20 (91) | 17.78 | < .001 |
| Confirming understanding | 9 (41) | 20 (91) | 13.67 | < .001 |
| Using an ask-tell-ask approach to information sharing | 4 (18) | 17 (77) | 21.91 | < .001 |
Note: p-value determined using 2-sample tests for the equality of proportions.
DISCUSSION
In this multisite educational study, experienced clinicians who completed an immersive VR curriculum on SDM related to HU initiation reported enhanced communication skills. Confidence was used as a proxy for skill acquisition. Specifically, there was a significant improvement in confidence related to asking open-ended questions, eliciting specific concerns, confirming understanding, and using an ask-tell-ask approach to information sharing. Furthermore, as VR simulations included several hesitancy sources regarding HU initiation, clinicians reported increased confidence when discussing topics such as benefits, costs, and impact of HU on daily life following workshop participation. Notably, most clinicians reported that they felt capable of coaching other clinicians on discussing the benefits of HU before workshop participation, indicating an opportunity for using VR to advance the skills of even highly experienced clinicians. These findings confirm prior evidence that most clinicians who care for children with SCA lack training in SDM, an important communication strategy that can be applied to many clinical scenarios.3 VR may represent an effective and scalable strategy to train clinicians on key communication skills to promote the successful implementation of SDM in real-life clinical encounters.
Previous curricula related to SDM exist;6,7 however, these approaches have had variable effects with prompting the use of SDM in actual patient encounters, perhaps, in part, because of the lack of opportunity for realistic, deliberate practice of SDM. Deliberate practice refers to strategic and goal-oriented activities that improve skills and behavior.10 Given the time limitations of clinicians participating in our simulations, we allowed clinicians to view others’ simulations in real-time and participate in debriefing rather than have each clinician independently participate in all scenarios. Given the increased confidence reported by clinicians, our methodology might be a time-efficient and effective strategy of utilizing VR for communication training.
Prior VR curricula have primarily targeted students and medical trainees.11,16,20 Most of the population included in this study were ≥ 35 years and cared for patients with SCA for over 5 years. Still, most participants tolerated the VR experience well and reported the environment as immersive. More side effects were reported by clinicians participating in this study than in previous communication-based VR curricula targeting trainees.12 Future VR interventions targeting clinicians, novel to VR, might consider strategies to optimize the experience, such as extended periods of orientation to the virtual environment and/or shorter scenarios. Non-immersive virtual environments might also have a role in enhancing clinicians’ communication skills.13 Still, the reported increased confidence in communication skills is particularly striking among this population given their many years of experience. It demonstrates the potential opportunities for VR and deliberate practice learning strategies in continuing medical education.
This study had limitations. First, we implemented the VR curriculum with a specific clinician population caring for pediatric patients with SCA limiting the sample size and statistical power. However, among the multisite population, the VR curriculum effectively improved clinicians’ confidence with medium to large effects demonstrated. Self-selection bias might have impacted those who chose to participate, although our sample represented our targeted population of experienced clinicians. Second, we developed our survey assessing clinicians’ confidence de novo given the lack of previously validated questionnaires. However, we utilized a well-established approach to survey design (retrospective pre-post) for evaluation and piloted our survey with multiple clinicians and experts before implementation to establish content and response-process validity.18 Finally, we reported the impact of our VR curriculum on clinicians’ self-assessed confidence as a surrogate for skill acquisition which may not reflect actual expertise. Next steps include evaluating the impact of the HU-SDM toolkit on caregiver report of decisional uncertainty and perception of shared-decision making following real-world visits with clinicians before and following our VR training. As a secondary outcome, we plan to assess patient uptake and adherence to HU.5
Despite its limitations, the initial results of this curriculum suggest that VR may be an effective approach to training clinicians on SDM. Given its impact on experienced clinicians, this VR curriculum may also be beneficial for fellowship trainees to inform future practice. VR has become more accessible to users due to the decreasing costs of equipment. Though our VR program is not currently publicly available as it is under investigation, we are hopeful that the results of this study will support widespread dissemination of our approach. Advances in artificial intelligence might support further scalability of effective VR curricula by removing the need for a human facilitator and decreasing implementation costs. Given its ability to replicate unique clinical scenarios in a safe, immersive environment that promotes deliberate practice without patient risk, we anticipate continued growth of VR curricula for healthcare providers.
Supplementary Material
Supplemental Digital Content 1.mp4 [video]
Acknowledgement:
We would like to thank the sickle cell anemia programs at Boston Medical Center, Children’s Hospital of Oakland, Children’s Hospital of Philadelphia, Lurie Children’s Hospital of Chicago, Nationwide Children’s Hospital, Nemours Alfred I. duPont Hospital for Children, St. Louis Children’s Hospital, and Texas Children’s Hospital and for their participation in this curriculum.
Source of Funding Source:
Research reported in this work was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (CDR-1609–36055). Anna Hood was supported in part by a grant from the National Heart, Lung, and Blood Institute (1F32HL143915)
Footnotes
Conflicts of Interest None of the authors have any financial relationships relevant to this article or conflicts of interest to disclose.
References
- 1.Smith AW, Bodas P, Sidebotham L, et al. Improving Uptake of Hydroxyurea in Patients with Sickle Cell Disease: A Retrospective Study of a Clinic-based Change in Consenting Practices. J Natl Med Assoc. 2019;111(2):169–175. [DOI] [PubMed] [Google Scholar]
- 2.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. Jama. 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 3.Crosby LE, Shook LM, Ware RE, et al. Shared decision making for hydroxyurea treatment initiation in children with sickle cell anemia. Pediatr Blood Cancer. 2015;62(2):184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crosby LE, Walton A, Shook LM, et al. Development of a Hydroxyurea Decision Aid for Parents of Children With Sickle Cell Anemia. J Pediatr Hematol Oncol. 2019;41(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood AM, Strong H, Nwankwo C, et al. Engaging Caregivers and Providers of Children With Sickle Cell Anemia in Shared Decision Making for Hydroxyurea: Protocol for a Multicenter Randomized Controlled Trial. JMIR Res Protoc. 2021;10(5):e27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews. 2014(9). [DOI] [PubMed]
- 7.Muller E, Strukava A, Scholl I, et al. Strategies to evaluate healthcare provider trainings in shared decision-making (SDM): a systematic review of evaluation studies. BMJ Open. 2019;9(6):e026488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkman WB, Lipstein EA, Taylor J, et al. Design and implementation of a decision aid for juvenile idiopathic arthritis medication choices. Pediatr Rheumatol Online J. 2017;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsulamy N, Lee A, Thokala P, et al. What Influences the Implementation of Shared Decision Making: An Umbrella Review. Patient Education and Counseling. 2020;103(12):2400–2407. [DOI] [PubMed] [Google Scholar]
- 10.Ericsson KA, Krampe R, Tesch-Römer CJPR. The role of deliberate practice in the acquisition of expert performance. 1993;100:363–406. [Google Scholar]
- 11.Real FJ, DeBlasio D, Ollberding NJ, et al. Resident perspectives on communication training that utilizes immersive virtual reality. Educ Health (Abingdon). 2017;30(3):228–231. [DOI] [PubMed] [Google Scholar]
- 12.Real FJ, DeBlasio D, Beck AF, et al. A Virtual Reality Curriculum for Pediatric Residents Decreases Rates of Influenza Vaccine Refusal. Acad Pediatr. 2017;17(4):431–435. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Kim H, Kim KH, et al. Effective virtual patient simulators for medical communication training: A systematic review. Med Educ. 2020;54(9):786–795. [DOI] [PubMed] [Google Scholar]
- 14.Rollnick S, Miller W. What is Motivational Interviewing? Behavioural and Cognitive Psychotherapy. 1995;23(4):325–334. [DOI] [PubMed] [Google Scholar]
- 15.Vorderer P, Wirth W, Gouveia FR, et al. Mec spatial presence questionnaire. 2004;18:2015. [Google Scholar]
- 16.Zackoff MW, Real FJ, Cruse B, et al. Medical Student Perspectives on the Use of Immersive Virtual Reality for Clinical Assessment Training. Acad Pediatr. 2019;19(7):849–851. [DOI] [PubMed] [Google Scholar]
- 17.Golding JF. Motion sickness. Handb Clin Neurol. 2016;137:371–390. [DOI] [PubMed] [Google Scholar]
- 18.Skeff KM, Stratos GA, Bergen MR. Evaluation of a Medical Faculty Development Program:A Comparison of Traditional Pre/Post and Retrospective Pre/Post Self-Assessment Ratings. Evaluation & the Health Professions. 1992;15(3):350–366. [Google Scholar]
- 19.Patil Indrajeet. Test and effect size details. February 19, 2021. Available at: https://cran.r-project.org/web/packages/statsExpressions/vignettes/stats_details.html#parametric-1. Accessed March 4, 2021.
- 20.Carrard V, Bourquin C, Orsini S, et al. Virtual patient simulation in breaking bad news training for medical students. Patient Educ Couns. 2020;103(7):1435–1438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1.mp4 [video]


