Summary
Prader-Willi syndrome arises as a consequence of absent paternal copies of maternally imprinted genes at 15q11-13. Such gender-of-origin imprinted genes are expressed in the brain and also in mammalian placenta where paternally expressed imprinted genes drive foetal nutritional demand. We hypothesise that the PWS phenotype is the result of the genotype impacting two pathways: first, directly on brain development and secondly, on placental nutritional pathways that results in its down-regulation and relative foetal starvation. The early PWS phenotype establishes the basis for the later characteristic phenotype. Hyperphagia. and other phenotypic characteristics arise as a consequence of impaired hypothalamic development. Hypothalamic feeding pathways become set in a state indicative of starvation, with a high satiety threshold and a dysfunctional neurophysiological state due to incorrect representations of reward needs, based on inputs that indicate a false requirement for food. Our hypotheses, if confirmed, would lead to novel and effective interventions.
Keywords: Prader-Wlli syndrome, Gender specific genomic imprinting, Hyperphagia, Foetal nutritional pathways
Introduction
This Personal View has its origins in an earlier hypothesis paper ‘The Paradox of Prader-Willi syndrome: a genetic model of starvation’1 in which we put forward two hypotheses. The first highlighted what we saw as a paradox: Prader-Willi syndrome (PWS), which was at that time considered to be a syndrome of obesity, we argued was in fact better conceptualised as a syndrome with a phenotype consistent with a permanent state of hunger, with the associated hormonal and behavioural consequences. The second hypothesis proposed that the core features of PWS are due to the absence of expression of a single gene. Both these views were contrary to widely held views at that time. Since 1956 PWS had been described as a syndrome of hypotonia, hypogonadism, hyperphagia and obesity (H3O), and considered to be a contiguous gene syndrome.
The aim of this paper is to explore these ideas further in light of recent research, and to set out an overarching testable theory of PWS. Our approach is primarily from a clinical perspective, seeking to explore underlying brain mechanisms that might explain the PWS phenotype and how such mechanisms might relate to the PWS genotype. We propose that such clinically-orientated conceptual models can challenge our current understanding, lay the foundation for new testable hypotheses, give direction to new research, and provide a framework for developing new interventions.
In the preparation of this paper we have relied on regular systematic literature searches on PWS undertaken by one of the authors (JW) on behalf of the Clinical and Scientific Advisory Board of the International PWS Organisation (IPWSO) (https://ipwso.org/information-for-medical-professionals/research-papers/), recent published reviews on different aspects of the neuropsychiatric and cognitive phenotype of PWS,2, 3, 4 and on reviews and focussed searches for research relevant to the hypotheses put forward. These have included reviews on foetal nutrition, placental function,5 hypothalamic function and development,6 and predictive models of brain decision-making.7
Background
The concept of PWS as a syndrome of starvation, we believe, is now widely accepted. We consider below how this idea of starvation manifests in-utero, but it is clear that children and adults with PWS have a persistent desire to eat and are unable to maintain energy homeostasis by limiting energy input closely in line with energy expenditure. If a person with PWS has free access to food, hyperphagia becomes apparent. The degree of this varies between individuals but under such circumstances people with PWS are unable to consistently regulate their food intake and are at risk for severe obesity. In addition to the above observed behaviour, there is evidence at different levels for this state of ‘starvation’: first, informant reports using standardised assessments, such as the Hyperphagia Questionnaire,8 record that, not only do people with PWS have hyperphagia, but they also have other behaviours associated with hunger, such as food stealing, preoccupation with food, and eating food normally considered inedible; secondly, at hormonal level, where, for example, blood levels of the orexigenic hormone, ghrelin, have been shown to be persistently high as would be expected when hungry9; and thirdly, at the level of the pattern of gene expression observed in hypothalamic tissue obtained from people with PWS at post-mortem.10
With respect to the genetics of PWS, in humans it has now been established that the maternally imprinted gene(s), whose absence of expression results in the core characteristics of PWS, lies in the vicinity of SNORD116, a gene cluster encoding small nucleolar RNAs.11 These are considered to be orphan C/D box snoRNAs since they do not target rRNAs or snRNAs. SNORD116 is highly expressed in the brain, lacks any significant complementarity with ribosomal RNA, and changes the expression levels of multiple genes.12 The proposition that there is a direct relationship between absent expression of particular maternally imprinted genes (e.g. Magel2, Necdin, IPW, SNORD116) and specific phenotypic characteristics remains the predominant model to date to explain the PWS phenotype.6,13
PWS as a disorder of brain development
In PWS, unlike many other genetically-determined neurodevelopmental syndromes, primary abnormalities of organ systems other than the brain are uncommon. Where they occur, it is as a consequence of downstream effects, for example, hypotonia leading to an increased risk of scoliosis, and growth hormone deficiency resulting in the physical phenotype.14 Early developmental delay and impairments in cognitive and intellectual abilities, communication, social cognition, and in general functioning indicate delayed and atypical brain development. In addition, hyperphagia, relative sex and growth hormone deficiencies of hypothalamic origin, and other phenotypic characteristics indicate wide-ranging hypothalamic dysfunction. Structural and functional neuroimaging has demonstrated a combination of subcortical and higher order brain structures being affected. These include those involved in processing reward, motivation, affect and higher order cognitive functions, with both anatomical and functional investigations indicating abnormalities. It appears likely that in PWS there is aberrant activity across distributed neural networks of which those involved in the control of eating behaviour have been the most extensively investigated.15
The questions are therefore: What are the main drivers of atypical brain development in PWS? Is it solely a direct consequence of the absence of expression of maternally imprinted gene(s) located at 15q11-13? Alternatively, given that gender-of-origin imprinted genes may influence foetal nutrition (see below), is atypical brain development wholly or partly a result of impaired nutritional transport to the foetus occurring at a critical time during mid to late gestation when the brain is rapidly growing and functional brain networks are differentially vulnerable to nutritional deficiencies?5 Whilst there will be other influences on brain development in people with PWS, such as the differences between the size of paternal deletions and the genetic differences between mUPD and deletions, we put forward the hypothesis that the above pathways are the primary drivers behind the phenotypic characteristic of PWS, and that these are shared across genotypes.
In addition to the above, given the fact that those with PWS due to the presence of a chromosome 15 mUPD have a much higher risk for psychotic illness compared to those individuals with a chromosome 15 paternal deletion,16 the risk for psychosis cannot be directly related to the core shared PWS genotype. Instead it must be what is unique about the genetics of mUPD that is critical: excess expression of genes of the opposite imprint located on chromosome 15 (Ube3a, ATP10). A two-hit model has been proposed,16 with the common PWS genotype predisposing to an increased risk for affective instability and affective disorder, and the additional effects arising with the presence of a mUPD resulting in the second hit. These two ‘hits’ in combination result in the high risk of what is an atypical affective psychotic illness.17
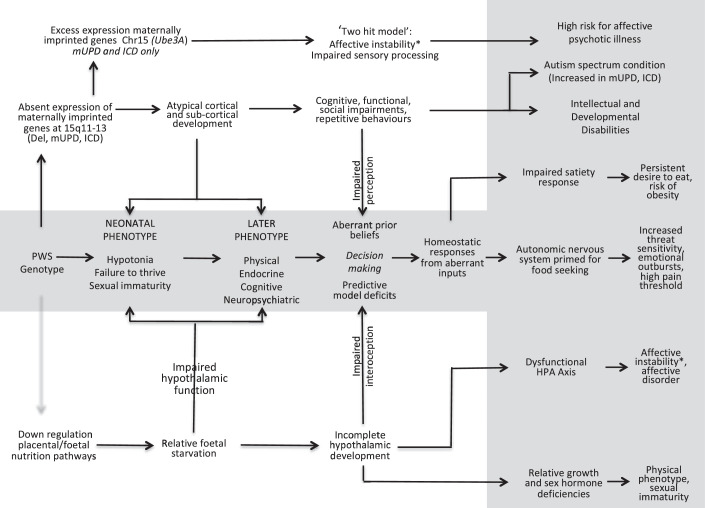
These three different causal pathways are illustrated in Figure 1, and the two pathways that we hypothesise give rise to the common PWS phenotypes are described in more detail below.
Figure 1.
The shaded area in the middle and the right of the figure illustrates the clinical manifestations of the proposed aetiological pathways that result in the early and then the later PWS phenotype. The pathway at the top of the figure is specific to those with the mUPD or imprinting centre defect form of PWS and, we hypothesis, this results in the increased risk of psychotic illness with age in this population. The next pathway down illustrates the direct effect of the absence of maternally imprinted gene expression on brain development and specifically the cortex and subcortical structures. The pathway at the bottom of the figure illustrates the hypothesised indirect effect of the PWS genotype, primarily on hypothalamic development, because the absence of SNORD116 results in the down regulation of placental nutritional pathways.
*affective instability and the risk of non-psychotic affective disorder common to all PWS genotypes.
HPA: Hypothalamic Pituitary Axis.
Hypothesis 1: atypical brain development in PWS is a consequence of the direct effects of the PWS genotype and indirect effects due to down-regulation of placental nutritional pathways.
Brain development is known to be a sequential process, which is tightly genetically regulated in humans5 and SnoRNAs are expressed in the developing brain with distinct patterns of expression of circular and linear forms.18 Given the absence of expression of the SnoRNA, SNORD116, and also the impact of this on the expression of other genes, it seems highly likely that this is one of the primary drivers for the atypical brain development seen in PWS, particularly in the cortex and sub-cortical structures. However, what has to be explained is the impact on hypothalamic development and functioning, which is so central to our understanding of much of the PWS phenotype. With specific regard to the hyperphagia, it has been argued, although not proven, that this arises as a consequence of the absence of expression of two maternally imprinted genes located at 15q11-13. First, the lack of expression of Magel2 leads to dysregulation of the leptin receptor, and therefore leptin cannot induce a fasting response. Secondly, the lack of expression of SNORD116 may result in impaired neuronal development and an imbalance of the hypothalamic feeding mechanisms.19 Others have proposed that the PWS genotype results in a diminished production of hypothalamic effector peptides that moderate energy balance and satiety.6 Such genetic models, however, fail to explain why there is evidence of widespread hypothalamic dysfunction in PWS.
What is particular to PWS is that the gene(s), whose absence of expression results in PWS, are only expressed when inherited from the paternal line. Kinship Theory20 proposes that such imprinted genes have arisen through evolution as a result of differential selection pressures. Imprinted genes in the foetus expressed only from the paternal line (patrigenes) drive the demand for maternal resources ensuring maximum nutrition for the foetus, at a cost to the mother both in-utero and in the infant prior to weaning. Over this period it is in the father's interest, in terms of successfully passing on his genes, to ensure that maternal resources are devoted to the growth and survival of the foetus and infant, albeit at some cost to the mother. After weaning, it is in the mother's interest that her offspring has a strong drive to seek out food, thereby reducing demand on her time and enabling her to distribute her resources across all of her offspring. This ensures survival of the maximum number of offspring and it is the optimum strategy for the ensuring the passing on of the genes of maternal origin. The reduction in foetal demand for maternal resources in-utero, the impaired suckling behaviour of infants with PWS, and the subsequent development of hyperphagia in early childhood observed in PWS could therefore be a direct consequence of the absence of paternally derived genetic influences. The consequences of the absence of an appropriate balance between the expression of imprinted paternally and maternally derived genes is extreme, as is illustrated by PWS, and places the survival of the infant at considerable risk.
One potential counter against this theory is that the switch to hyperphagia should be around the time of weaning. The median age when hyperphagia is reported to first develop, according to the stages set out by Miller et al.,21 is 54 months. This is later than weaning generally occurs. However, the above figure for the development of hyperphagia may be artificially high as a consequence of early diagnosis and the now careful management of the feeding of an infant with PWS, which might mask the onset of hyperphagia. Some support for an earlier age of onset of hyperphagia comes from a cross-sectional study of 42 children with PWS between the ages of seven months and five years. It was found that BMI was stable between 15 and 30 months, but by 30 months it had started to increase, as did the mothers’ ratings of their children's eating behaviour, suggesting that hyperphagia develops at this younger age.22
Regardless of the exact timing of this switch from hypophagia to hyperphagia, the questions that must be answered are: How is it that the absence of expression of a maternal imprinted gene results in both of the above states at different times in development? How to explain the other aspects of the PWS, such as relative sex and growth hormone deficiencies, and the neuropsychiatric phenotype?
The early PWS phenotype includes: reduced foetal movements as a consequence of hypotonia, low weight for gestational age, increased head to abdominal circumference, neonatal hypotonia and failure to thrive.23 With respect to the severe hypotonia and failure to thrive characteristic of the early PWS phenotype, the PWS genotype may specifically give rise to impaired development of the motor cortex and basal ganglia in the brain. This would result in the absence of centrally driven motor tone and would explain the hypotonia and also the inability to suckle normally after birth. Given advances in neonatal brain imaging,24 this could be readily investigated by comparing structure and function in different areas of the brain in neonates with PWS compared to other populations.
With respect to the development of the hypothalamus and its networks we hypothesise that the impact of the PWS genotype may be indirect. Central to this hypothesis is the relevance of the placenta-foetal axis in the control of nutrient transfer to the foetus, including essential fatty acids, amino acids and vitamins,5,25,26 and the role of genes in which one or other allele is imprinted depending on the gender of parent of origin. As described earlier this class of genes have evolved to regulate maternal/foetal resource allocation, with paternally expressed genes favouring greater resource allocation to the foetus at a cost to the mother, and genes of the opposite imprint, having the opposite effect and limiting foetal growth. The placenta responds to different genetically-determined signals from the foetus and moderates the level of nutrition accordingly.27,28 Placentation and imprinted genes are believed to have evolved together, and of the 92 parent-of-origin specific imprinted genes known in the human genome, 75 are expressed in the placenta, 27 of which are imprinted solely in placental tissue. The most studied of these have been those associated with the maternal-foetal relationship in foetal nutrition and growth and, in humans, cases of pre-eclampsia or IUGR.29, 30, 31
In utero and early in life this ‘foetal starvation’ due to down regulation of the placental foetal pathways particularly impacts hypothalamic development, the later stages of which are normally triggered in mice by the leptin surge post-natally, depending on nutritional status,32 and in humans prenatally. One possibility is that resistance to the leptin surge, consequent upon already existing delayed hypothalamic development, would further impact on the development of the arcuate and other nuclei of the hypothalamus33 accounting for other hypothalamic related aspects of the PWS phenotype. The prediction that ensues from this hypothesis is that foetal ‘starvation’ impedes hypothalamic development and in PWS, as opposed to other genetic obesity syndromes, there will be small and poorly developed hypothalamic nuclei and aberrant connectivity to cortical areas that regulate feelings of fullness and satiety. Support for this comes from post-mortem observations.34
Although there are some phenotypic differences between the main genetic types of PWS that are apparent later in life they are similar in terms of the early characteristics. This indicates that any effects of the PWS genotypes on foetal nutrition were similar and determined by the shared core genotype. For example, in one study of 105 infants with deletion and 62 with mUPD, the two groups were similar in terms of foetal characteristics, the only difference observed was that there was a higher percentage who had had late deliveries in the mUPD group compared to those with a deletion,35 although mean gestational ages were similar in the two groups. In addition, given the higher rates of atypical births, which have been observed in several studies, it is possible that this factor might also contribute to impaired brain development, but in itself it would not explain the PWS-specific brain related impairments.
Importantly, this foetal starvation hypothesis could be tested in PWS mouse models and, if the data supports the hypothesis, high-dose nutritional supplements could be given to the pregnant mice to determine whether the early phenotype of the genetically modified pups could be ameliorated. In humans, levels of key nutrients could be measured in cord blood taken at birth in any hypotonic baby and levels determined in those subsequently shown to have PWS. One potentially counter-intuitive strategy could be to give additional leptin immediately after birth to the infant with PWS to determine whether further hypothalamic development might result. Below we consider how a combination of these direct and indirect effects of the genotype on early development could result in the later PWS phenotype.
Hypothesis 2: PWS can best be conceptualised as a disorder of homeostasis consequent upon impaired cerebral representations of internal and external environments.
Feelings, such as hunger and fullness, are a conscious manifestation of the homeostatic processes that have evolved to keep body weight within an optimal range to maintain reproductive fitness.36 As described above, we propose that hyperphagia and other phenotypic characteristics of hypothalamic origin in PWS are a result of impaired development of the foetal hypothalamus due to relative starvation in-utero. Hyperphagia subsequently becomes apparent in early childhood because of dysfunctional hypothalamic circuits37 as a result of impaired hypothalamic development and the presence of an excessively high threshold for the point at which conscious experiences shift from ‘hunger’ to ‘fullness’ and a sense of satiation is reached. The hypothalamus is resistant in its response to interoceptive messages and, as a result, changes in cortical activation patterns indicating ‘fullness’ do not occur and eating continues. Our original starvation hypothesis can be extended to account for the behavioural manifestations of PWS. These arise as a consequence of the regulatory systems of the brain being ‘locked’ in this state of ‘starvation’, although the need for food and the physical lack of nutrition are not the problem. People with PWS are physiologically and psychologically in what might be called ‘hunting mode’ as a consequence of the impaired hypothalamic development, which also results in relative growth and sex hormone deficiencies of hypothalamic origin, impaired temperature regulation, high pain threshold, dysautonomia, and an inability to effectively regulate mood and behaviour such that mood instability and emotional outbursts are common. Whilst the above phenomena relate primarily to the hypothalamus and its projections, atypical brain development also involves the cortex and sub-cortical structures resulting in specific impairments of social and general cognition and a reduced ability to interpret communication and environmental cues, such as facial expressions. These impairments contribute to what are likely to be sub-optimal inputs from the external environment.
One way of conceptualising what is described above is through the framework of computational Bayesian models of brain function, decision-making and associated actions. These are seen as inferential processes in which prior beliefs are established based on previous internal and external sensory inputs.7 These processes, combined with a generative predictive model, seek to interpret the present sensory inputs and ultimately instigate appropriate behaviours. The application of this complex and challenging model to PWS is clearly speculative, but two of us as clinicians (AH and KM) consider that, in the case of PWS, this way of conceptualising the issues, based on the above theory, makes sense clinically and, as described below, helps to re-orientate our thinking therapeutically. People with PWS could be considered as having aberrant prior beliefs, which are a poor fit for the real internal and external worlds, which then result in deficits in understanding and in action (behaviour). Thus, inferences and actions may be conceptualised as ‘optimal’ but only on the basis of sub-optimal a priori beliefs. Essentially, the brain has a false representation of reward needs, which is largely based on an input that indicates a false need to obtain food and incomplete representations of the external social world. The responses are consistent with these incomplete and false inputs, both from the body and from the senses, but are sub-optimal when it comes to maintaining fitness.
How might such a speculative model help therapeutically? First, this way of conceptualising neural function and decision-making in people with PWS, if correct, re-orientates thinking towards correcting inputs rather than seeking to modify outputs (behaviours). It also provides potential insights into the experiences of people with PWS, suggesting that they may have to live their daily lives with a high degree of uncertainty, given that their perception of the world and their needs and the responses that follow may be based on false premises. This might help explain what informants often describe in people with PWS as episodes of anxiety. These are common and tend to be situational, such as at moments of change, and may in turn trigger emotional outbursts. These could be conceptualised as responses to a perceived threat for which thresholds may be lower or attentional and interpretational bias and flexibility altered given they are within this hypothesised ‘hunting mode’. The implication of this is that the model of care for people with PWS should aim to compensate for such uncertainty by limiting demand, increasing predictability in the environment, and optimising understanding. Examples include: the use of visual support to improve understanding of what will be happening and when; access to early intervention to help people with PWS develop a better understanding of the social world and to acquire more effective coping strategies; and a consistent approach to food security, both in terms of access to food to prevent obesity, and also to provide ‘emotional food security’ – the understanding that the appropriate amounts of food will always be available at the designated time and place. Secondly, such a model can be tested using neuroimaging and electrophysiological techniques. Examples include the use of the ACT-R general framework,38 which seeks to specify how the brain is organised. Such a framework can be used to interrogate neuroimaging data to make specific behavioural predictions. Alternatively, methodologies using EEG event-related potentials, such as the auditory Mismatched Negativity or P300,39 could be modified to determine similarities and differences in response to novel and familiar stimuli in people with PWS compared to others with typical or atypical patterns of development. As the sophistication of neuroimaging and analytical techniques continue to develop, it will be increasingly possible to investigate differences in both inputs and outputs as well as differences in activation patterns in the networks used under specific conditions, controlling the inputs and observing the outputs. Early examples of using neuroimaging to determine the neural correlates of decision-making by people with PWS have reported atypical patterns of cortical activation and deactivation when asked to switch attention40 and similarly when making choices about food before and after a calorie load.41
Conclusions
In Figure 1 we illustrate the genotype to phenotype pathways set out in this paper. The hypotheses are testable using PWS mouse models and in humans. The implications, if they are proven to be correct, are significant and open the door to new therapies. PWS differs from other causes of intrauterine growth retardation given the complete absence of expression in the foetus of nutrition promoting gene(s) and the potential additional direct effects of the absence of expression of such genes on subsequent intrauterine and early post-natal brain development. However, PWS may also provide a lens through which to explore the impact of impairment in foetal nutrition on intrauterine growth, development and later cognitive function and behaviours. We seek comment from those experts in the fields of placental function, foetal development, and in the neurosciences and encourage the testing of the hypotheses put forward.
Contributors
AH and JW led on the development of the various ideas put forward in this paper. KM contributed to the section on the second hypothesis and theories of decision-making. AH and JW led on the writing and revisions of different versions and all authors commented on the final manuscript. All authors approved the final version of the paper.
Funding
No funding required for the work undertaken in the preparation of this paper.
Ethics approval
Not required.
Declaration of interests
None.
Acknowledgments
We would like to thank many colleagues, families of people with PWS, and people with PWS who have helped with our research into PWS over many years and who have inspired us with their ideas, commitment and enthusiasm.
References
- 1.Holland A.J., Whittington J.E., Hinton E.C. The paradox of Prader-Willi syndrome: a genetic model of starvation. Lancet. 2003;362:989–991. doi: 10.1016/S0140-6736(03)14370-X. [DOI] [PubMed] [Google Scholar]
- 2.Whittington J., Holland A. A review of psychiatric conceptions of mental and behavioural disorders in Prader-Willi syndrome. Neurosci Biobehav Rev. 2018;95:396–405. doi: 10.1016/j.neubiorev.2018.10.006. DecEpub 2018 Oct 28. PMID: 30392879. [DOI] [PubMed] [Google Scholar]
- 3.Thuilleaux D., Laurier V., Copet P., et al. A model to characterize psychopathological features in adults with Prader-Willi syndrome. Am J Med Genet A. 2018;176(1):41–47. doi: 10.1002/ajmg.a.38525. JanEpub 2017 Nov 17. PMID: 29150898. [DOI] [PubMed] [Google Scholar]
- 4.Whittington J., Holland A. Cognition in people with Prader-Willi syndrome: insights into genetic influences on cognitive and social development. Neurosci Biobehav Rev. 2017:153–167. doi: 10.1016/j.neubiorev.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Georgieff M.K., Ramel S.E., Cusick S.E. Nutritional influences on brain development. Acta Paediatr. 2018;107(8):1310–1321. doi: 10.1111/apa.14287. AugEpub 2018 Mar 22. PMID: 29468731; PMCID: PMC6045434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa-da-Silva F., Fliers E., Swaab D.F., Yi C.X. Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome. J Neuroendocrinol. 2021;33(7):e12994. doi: 10.1111/jne.12994. JulEpub 2021 Jun 22. PMID: 34156126; PMCID: PMC8365683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parr T., Rees G., Friston K.J. Computational neuropsychology and Bayesian inference. Front Hum Neurosci. 2018;12:61. doi: 10.3389/fnhum.2018.00061. Feb 23PMID: 29527157; PMCID: PMC5829460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dykens E.M., Maxwell M.A., Pantino E., Kossler R., Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity. 2007;15(7):1816–1826. doi: 10.1038/oby.2007.216. (Silver Spring)JulPMID: 17636101. [DOI] [PubMed] [Google Scholar]
- 9.Cummings D.E., Clement K., Purnell J.Q., et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–644. doi: 10.1038/nm0702-643. JulPMID: 12091883. [DOI] [PubMed] [Google Scholar]
- 10.Bochukova E.G., Lawler K., Croizier S., et al. A Transcriptomic signature of the hypothalamic response to fasting and BDNF deficiency in Prader-Willi syndrome. Cell Rep. 2018;22(13):3401–3408. doi: 10.1016/j.celrep.2018.03.018. Mar 27PMID: 29590610; PMCID: PMC5896230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y., AlHumaidi S.S., Faqeih E.A., Pitel B.A., Lundquist P., Aypar U. A novel deletion of SNURF/SNRPN exon 1 in a patient with Prader-Willi-like phenotype. Eur J Med Genet. 2017;60(8):416–420. doi: 10.1016/j.ejmg.2017.05.003. AugEpub 2017 May 26. PMID: 28554868. [DOI] [PubMed] [Google Scholar]
- 12.Falaleeva M., Surface J., Shen M., de la Grange P., Stamm S. SNORD116 and SNORD115 change expression of multiple genes and modify each other's activity. Gene. 2015;572(2):266–273. doi: 10.1016/j.gene.2015.07.023. Nov 10Epub 2015 Jul 26. PMID: 26220404; PMCID: PMC5586535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salles J., Eddiry S., Lacassagne E., et al. Patients with PWS and related syndromes display differentially methylated regions involved in neurodevelopmental and nutritional trajectory. Clin Epigenetics. 2021;13(1):159. doi: 10.1186/s13148-021-01143-0. Aug 13PMID: 34389046; PMCID: PMC8361855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza M.A., McAllister C., Suttie M., et al. Growth hormone, gender and face shape in Prader-Willi syndrome. Am J Med Genet Part A. 2013;161(10):2453–2463. doi: 10.1002/ajmg.a.36100. [DOI] [PubMed] [Google Scholar]
- 15.Manning K.E., Holland A.J. Puzzle pieces: neural structure and function in Prader-Willi syndrome. Diseases. 2015;3(4):382–415. doi: 10.3390/diseases3040382. Dec 17PMID: 28943631; PMCID: PMC5548261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soni S., Whittington J., Holland A.J., et al. The phenomenology and diagnosis of psychiatric illness in people with Prader-Willi syndrome. Psychol Med. 2008 Oct;38(10):1505–1514. doi: 10.1017/S0033291707002504. Epub 2008 Jan 4. PMID: 18177526. [DOI] [PubMed] [Google Scholar]
- 17.Aman L.C.S., Manning K.E., Whittington J.E., Holland A.J. Mechanistic insights into the genetics of affective psychosis from Prader-Willi syndrome. Lancet Psychiatry. 2018;5(4):370–378. doi: 10.1016/S2215-0366(18)30009-9. AprEpub 2018 Jan 16. PMID: 29352661. [DOI] [PubMed] [Google Scholar]
- 18.Chen B.J., Huang S., Janitz M. Changes in circular RNA expression patterns during human foetal brain development. Genomics. 2019;111(4):753–758. doi: 10.1016/j.ygeno.2018.04.015. JulEpub 2018 Apr 27. PMID: 29709512. [DOI] [PubMed] [Google Scholar]
- 19.Salminen I.I., Crespi B.J., Mokkonen M. Baby food and bedtime: evidence for opposite phenotypes from different genetic and epigenetic alterations in Prader-Willi and Angelman syndromes. SAGE Open Med. 2019;7 doi: 10.1177/2050312118823585. Jan 282050312118823585PMID: 30728968; PMCID: PMC6350130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haig D., Wharton R. Prader-Willi syndrome and the evolution of human childhood. Am J Hum Biol. 2003;15(3):320–329. doi: 10.1002/ajhb.10150. May-JunPMID: 12704708. [DOI] [PubMed] [Google Scholar]
- 21.Miller J.L., Lynn C.H., Driscoll D.C., et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A(5):1040–1049. doi: 10.1002/ajmg.a.33951. MayEpub 2011 Apr 4. PMID: 21465655; PMCID: PMC3285445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstone A.P., Holland A.J., Butler J.V., Whittington J.E. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes. 2012;36:1564–1570. doi: 10.1038/ijo.2011.274. [DOI] [PubMed] [Google Scholar]
- 23.Srebnik N., Gross Even-Zohar N., Salama A., et al. Recognizing the unique prenatal phenotype of Prader-Willi syndrome (PWS) indicates the need for a diagnostic methylation test. Prenat Diagn. 2020;40(7):878–884. doi: 10.1002/pd.5712. JunEpub 2020 May 21. PMID: 32297338. [DOI] [PubMed] [Google Scholar]
- 24.Kwon S.H., Vasung L., Ment L.R., Huppi P.S. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol. 2014;41(1):257–283. doi: 10.1016/j.clp.2013.10.003. MarEpub 2013 Dec 12. PMID: 24524459. [DOI] [PubMed] [Google Scholar]
- 25.Gaccioli F., Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. 2016;7:40. doi: 10.3389/fphys.2016.00040. Feb 16PMID: 26909042; PMCID: PMC4754577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleal J.K., Lewis R.M. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20(4):419–426. doi: 10.1111/j.1365-2826.2008.01662.x. AprEpub 2008 Feb 8. PMID: 18266945. [DOI] [PubMed] [Google Scholar]
- 27.Eggermann T., Davies J.H., Tauber M., et al. Growth restriction and genomic imprinting-overlapping phenotypes support the concept of an imprinting network. Genes. 2021;12(4):585. doi: 10.3390/genes12040585. (Basel)Apr 17PMID: 33920525; PMCID: PMC8073901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N., Leverence J., Bick D., Sampath V. Ontogeny of growth-regulating genes in the placenta. Placenta. 2012;33(2):94–99. doi: 10.1016/j.placenta.2011.11.018. FebEpub 2011 Dec 10. PMID: 22154689. [DOI] [PubMed] [Google Scholar]
- 29.Monk D. Genomic imprinting in the human placenta. Am J Obstet Gynecol. 2015;213(4 Suppl):S152–S162. doi: 10.1016/j.ajog.2015.06.032. OctPMID: 26428495. [DOI] [PubMed] [Google Scholar]
- 30.Keverne E.B. Epigenetically regulated imprinted genes and foetal programming. Neurotox Res. 2010;18(3–4):386–392. doi: 10.1007/s12640-010-9169-z. NovEpub 2. [DOI] [PubMed] [Google Scholar]
- 31.Tucci V., Isles A.R., Kelsey G., Ferguson-Smith A.C., Erice Imprinting Group Genomic imprinting and physiological processes in mammals. Cell. 2019;176(5):952–965. doi: 10.1016/j.cell.2019.01.043. Feb 21PMID: 30794780.010. [DOI] [PubMed] [Google Scholar]
- 32.Skowronski A.A., Shaulson E.D., Leibel R.L., LeDuc C.A. The postnatal leptin surge in mice is variable in both time and intensity and reflects nutritional status. Int J Obes. 2021 doi: 10.1038/s41366-021-00957-5. (Lond)Sep 2Epub ahead of print. PMID: 34475504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouret S.G., Simerly R.B. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70(4):295–301. doi: 10.1111/j.1399-0004.2006.00684.x. OctPMID: 16965320. [DOI] [PubMed] [Google Scholar]
- 34.Swaab D.F. Prader-Willi syndrome and the hypothalamus. Acta Paediatr Suppl. 1997;423:50–54. doi: 10.1111/j.1651-2227.1997.tb18369.x. NovPMID: 9401539. [DOI] [PubMed] [Google Scholar]
- 35.Butler M.G., Sturich J., Myers S.E., Gold J.A., Kimonis V., Driscoll D.J. Is gestation in Prader-Willi syndrome affected by the genetic subtype? J Assist Reprod Genet. 2009;26(8):461–466. doi: 10.1007/s10815-009-9341-7. AugEpub 2009 Sep 17. PMID: 19760168; PMCID: PMC2767487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damasio A., Carvalho G.B. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14(2):143–152. doi: 10.1038/nrn3403. FebPMID: 23329161. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Wang J., Zhang G., et al. The neurobiological drive for overeating implicated in Prader-Willi syndrome. Brain Res. 2015;1620:72–80. doi: 10.1016/j.brainres.2015.05.008. Sep 16Epub 2015 May 18. PMID: 25998539. [DOI] [PubMed] [Google Scholar]
- 38.Dimov C., Khader P.H., Marewski J.N., et al. How to model the neurocognitive dynamics of decision making: a methodological primer with ACT-R. Behav Res. 2020;52:857–880. doi: 10.3758/s13428-019-01286-2. [DOI] [PubMed] [Google Scholar]
- 39.Chennu S., Noreika V., Gueorguiev D., Shtyrov Y., Bekinschtein T.A., Henson R. Silent expectations: dynamic causal modeling of cortical prediction and attention to sounds that weren't. J Neurosci. 2016;36(32):8305–8316. doi: 10.1523/JNEUROSCI.1125-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodcock K.A., Humphreys G.W., Oliver C., Hansen P.C. Neural correlates of task switching in paternal 15q11-q13 deletion Prader-Willi syndrome. Brain Res. 2010;1363:128–142. doi: 10.1016/j.brainres.2010.09.093. DecEpub 2010 Oct 1. PMID: 20920489. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwpoort I.C.V., Slagboom T.N.A., Jakobsdóttir S., et al. Food-related brain activation measured by fMRI in adults with Prader-Willi syndrome. J Clin Med. 2021;10(21):5133. doi: 10.3390/jcm10215133. OctPMID: 34768651; PMCID: PMC8584580. [DOI] [PMC free article] [PubMed] [Google Scholar]