In 2018 cycloserine was elevated to World Health Organization (WHO) group B status for multidrug-resistant tuberculosis (MDR-TB), and is recommended in longer MDR-TB treatment regimens [1]. Inclusion of cycloserine is associated with improved MDR-TB treatment success and reduced mortality, but is limited by treatment-associated depression, psychosis and neuropathy, forcing 9% of patients to stop therapy [1–3]. Cycloserine also demonstrates wide interindividual pharmacokinetic variation, with significant food and drug interactions, leaving nearly half of patients with inappropriate drug levels [4, 5]. Optimal dosing is unknown [6], but modelling studies suggest doses from 250 mg to 750 mg twice daily, with 500 mg twice daily for paucibacillary disease and 750 mg twice daily for cavitary pulmonary disease [7]. Therefore, clinicians must balance the known benefits of cycloserine with the dearth of susceptibility- and drug-monitoring capacity and the spectre of treatment-limiting side-effects. To evaluate the impact of cycloserine prescription and dose on incident depression during MDR-TB treatment, we analysed longitudinal cohort data from India.
Short abstract
In a longitudinal cohort of MDR-TB patients receiving individualised, DST-based treatment, neither the inclusion of cycloserine in a multidrug regimen nor the dose used (up to 750 mg daily) significantly increased incidence of depression during treatment https://bit.ly/3GtQmOH
To the Editor:
In 2018 cycloserine was elevated to World Health Organization (WHO) group B status for multidrug-resistant tuberculosis (MDR-TB), and is recommended in longer MDR-TB treatment regimens [1]. Inclusion of cycloserine is associated with improved MDR-TB treatment success and reduced mortality, but is limited by treatment-associated depression, psychosis and neuropathy, forcing 9% of patients to stop therapy [1–3]. Cycloserine also demonstrates wide interindividual pharmacokinetic variation, with significant food and drug interactions, leaving nearly half of patients with inappropriate drug levels [4, 5]. Optimal dosing is unknown [6], but modelling studies suggest doses from 250 mg to 750 mg twice daily, with 500 mg twice daily for paucibacillary disease and 750 mg twice daily for cavitary pulmonary disease [7]. Therefore, clinicians must balance the known benefits of cycloserine with the dearth of susceptibility- and drug-monitoring capacity and the spectre of treatment-limiting side-effects. To evaluate the impact of cycloserine prescription and dose on incident depression during MDR-TB treatment, we analysed longitudinal cohort data from India.
Outpatients aged ⩾15 years seeking MDR-TB care at a private hospital in Mumbai, India were recruited for a prospective observational cohort study approved by the institutional review boards of the P.D. Hinduja National Hospital and Medical Research Centre (Mumbai, India) and Johns Hopkins University School of Medicine (Baltimore, MD, USA) [8]. After informed consent (guardian consent for 15–18-year-olds), participants’ medical records were abstracted for demographic characteristics, treatment history, laboratory and imaging studies and treatment-associated side-effects. Due to additional drug resistance, most participants were ineligible for short-course regimens and received individualised, susceptibility-guided multidrug therapy for 24 months. This study pre-dates guidelines prioritising all-oral regimens, so injectable MDR-TB drugs were often included. Regimen was determined by resistance profile, not study participation or comorbidity. Cycloserine was initially prescribed at 250 mg daily, then increased as tolerated to 250 mg twice daily or 250 mg daily plus 500 mg nightly [1]. For simplicity, doses are presented as total mg per day (0 mg, 250 mg, 500 mg or 750 mg per day). Participants were followed for 1 year after treatment completion. At each visit, participants reported employment status, tobacco and alcohol use, social support [9], neuropathy, hallucinations and depression symptoms using the Patient Health Questionnaire-9 (PHQ-9) [10].
Data were analysed per-participant and per-visit. PHQ-9 scores were modelled as an ordinal variable with scores indicating mild or no depression (<10), moderate depression (10–13) and moderately severe or severe depression (≥14). Alcohol use by Alcohol Use Disorders Identification Test (AUDIT) score [11] and body mass index (BMI) were calculated at each visit. Underweight was defined as BMI <18.5 kg·m−2. Differences in participant characteristics by depression status was assessed by Chi-squared test, with p<0.05 considered significant. Depression incident rate ratios (IRRs) were calculated for each covariate. Mixed-effects models with random intercepts, random slopes and unstructured correlation assessed univariable odds ratios for depression at each visit by covariate including cycloserine prescription, dose, weight-adjusted dose (mg·kg−1 per day) and treatment duration (months). Significant features from univariable models were included in a multivariable model.
From October 2017 to March 2021, 140 participants completed 881 PHQ-9 questionnaires over 200.4 person-years of treatment. Of these, 122 (87%) participants received cycloserine. Participants had a median age of 27 years (interquartile range 21–35 years), and 91 (65%) were female. Most had culture-positive (90%) pulmonary (89%) tuberculosis, 12% had diabetes and 3% were HIV-co-infected. Smoking was uncommon (4%), and no participants reported hazardous drinking (AUDIT ≥8). Tobacco use was more frequent among those with depression (15% and 2% for PHQ-9 ≥14 and <10, respectively; p=0.047). Though not significant, those with higher PHQ-9 scores more frequently stopped working due to MDR-TB (55% versus 26%; p=0.051), and underweight was more common among those with depression (median BMI 17.7 kg·m−2 and 21.1 kg·m−2 for PHQ-9 ≥14 and <10, respectively; p=0.509). No other clinical features differed significantly by depression status or cycloserine prescription.
Overall, 40 (28%) participants reported prevalent depression (PHQ-9 ≥10) at enrolment. Of those without prevalent depression, 38% reported incident depression during treatment; an incidence of 102.6 per 1000 person-years. Incidence increased with AUDIT score (IRR 2.98, 95% CI 1.86–4.77), but not with cycloserine prescription (IRR 1.11, 95% CI 0.46–2.68), duration of cycloserine use (monthly IRR 0.83, 95% CI 0.34–2.02), higher cycloserine dose (IRR 0.98, 95% CI 0.34–2.79 and IRR 2.03, 95% CI 0.48–8.66 for 500 mg and 750 mg per day, respectively), or higher doses in mg·kg−1 per day (IRR 1.03, 95% CI 0.93–1.15). In addition, 78 (55.7%) participants reported treatment-associated peripheral neuropathy. Of note, three participants taking cycloserine 250 g twice daily reported hallucinations (all ≥9 months into cycloserine), as did three participants never prescribed cycloserine and four participants previously taking cycloserine (all ≥2 months after discontinuation).
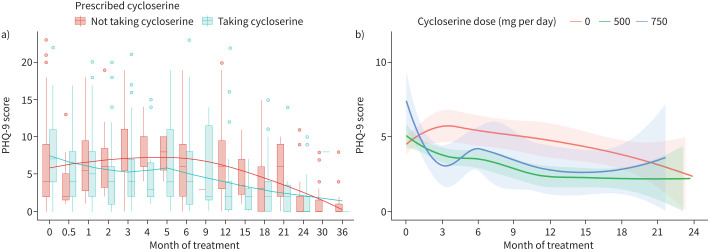
Moderately severe and severe depression declined for all participants during tuberculosis treatment, impacting 7.9% of participants at enrolment, 3.1% after 2 months and no participants at treatment completion (figure 1). PHQ-9 scores declined after 6 months, when injectable drugs (if prescribed) were discontinued. Though not significant, PHQ-9 scores were higher throughout treatment among participants not prescribed cycloserine than those prescribed cycloserine (figure 1a). Importantly, PHQ-9 scores were not higher among those prescribed higher cycloserine doses (figure 1b).
FIGURE 1.
Serial measurement of Patient Health Questionnaire (PHQ)-9 depression scores over the course of treatment for multidrug-resistant tuberculosis. a) Histograms and smoothed regression curves of participants’ PHQ-9 scores among those taking cycloserine during the visit and those not taking cycloserine. b) Smoothed regression curves (lines) and 95% confidence interval ranges (shading) for participants’ PHQ-9 scores, stratified by their daily total dose of cycloserine (mg per day) at each visit. There were no significant differences in PHQ-9 scores by either prescription of cycloserine or cycloserine dose, although participants who did not take cycloserine tended to have higher PHQ-9 scores throughout the course of treatment.
Unadjusted odds of depression were significantly associated with pulmonary disease, underweight, alcohol use, social support, injectable MDR-TB drugs (OR 2.71, 95% CI 1.66–4.42) and shorter cycloserine treatment (monthly OR 0.91, 95% CI 0.86–0.95). Peripheral neuropathy was associated with a nonsignificant increase in depression (OR 1.78, 95% CI 0.98–3.23). Importantly, current cycloserine treatment and dose were not associated with increased odds of depression. Adjusted (a) odds of depression were associated with weight and social support, but not with current cycloserine use (aOR 0.85, 95% CI 0.48–1.51), while longer cycloserine treatment reduced odds of depression (monthly aOR 0.95, 95% CI 0.90–1.00).
This longitudinal cohort study had several important findings. While depression was common, odds of moderate, moderately severe and severe depression were not significantly increased by prescription of cycloserine, cycloserine dose, weight-adjusted dose or dosing frequency. Underweight, social support, alcohol use and prescription of injectable tuberculosis drugs were associated with depression, and rates of depression were similar to those reported elsewhere for MDR-TB [12, 13]. Not surprisingly, neuropathy and unemployment increased depression. PHQ-9 scores declined over time, independent of cycloserine prescription. This may reflect the stress of diagnosis and treatment initiation, with improvement as symptoms resolve, treatment is simplified and injections cease. Importantly, these findings shift blame from cycloserine as the cause of depression in MDR-TB treatment and creates an opportunity for cycloserine dose-intensification.
This observational, single-site study had several limitations. Findings from this young, female-predominant cohort may not be generalisable to older patients; those with comorbidities such as HIV, diabetes complicated by neuropathy or meningitis with functional impairment; or to populations with higher rates of tobacco and alcohol use, as reported elsewhere in India [14]. Similarly, changing MDR-TB treatment guidelines may impact the durability of these results as guidelines prioritise shorter, all-oral regimens to avoid injections.
While cycloserine is recommended, data on optimised dosing are lacking, and wide interindividual pharmacokinetic variability makes cycloserine exposure unpredictable [1–6]. Cycloserine clearance and trough concentrations are associated with clinical neuropathy, but the individual dose has not been consistently associated with the neuropsychiatric side-effects studied in this cohort [15]. Until dose-specific outcomes data are available, higher cycloserine doses suggested by modelling studies should be considered [7]. Our data suggest that the challenges of MDR-TB diagnosis and treatment initiation were larger drivers of depression than cycloserine use alone. While clinicians should remain vigilant to the impact of depression on MDR-TB treatment, total doses up to and potentially above 750 mg are likely to be reasonably tolerated.
Shareable PDF
Footnotes
Support statement: This work was supported by the NIH/DBT RePORT India Consortium with funding in whole or in part from the Government of India's (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global. Additional support came from the National Health and Education Society, and the Indo-South African collaboration with funding from DBT and the Indian Department of Science and Technology (DST), as well as NIAID (K23AI135102, R21AI122922 and R01AI134430 to J.A. Tornheim, and UM1AI069465 to A. Gupta), the Office of the Director, Fogarty International Center, and Office of AIDS Research of the National Institutes of Health through the Fogarty Global Health Fellows Program Consortium (R25TW009340 to J.A. Tornheim), the Johns Hopkins University School of Medicine Clinician Scientist Career Development Award (to J.A. Tornheim), the Ujala Foundation, Gilead Foundation and Wyncote Foundation (to A. Gupta). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, DST, ICMR, NIH or CRDF Global. Any mention of trade names, commercial projects, or organisations does not imply endorsement by any of the sponsoring organisations. The funding sources had no role in the study design, data collection, data analysis, data interpretation or writing of the report. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: J.A. Tornheim reports, during the conduct of the study, salary support from NIH/NIAID (K23AI135102, R21AI122922 and R01AI134430), salary support from the NIH Fogarty International Center (R25TW009340) and salary support from the Johns Hopkins Clinician Scientist Career Development Award; salary support for a study clinician during the first year of the project was provided by the Frederick Mulder Foundation and the Pogge Tong Foundation. C. Rodrigues reports payment for manuscript writing, honoraria for lectures, educational events from Pfizer; honoraria for lectures from Cipla, Glenmark and Novartis; outside the submitted work. All other authors have nothing to disclose.
References
- 1.World Health Organization (WHO) . WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva, WHO, 2020. [PubMed] [Google Scholar]
- 2.Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e93–e142. doi: 10.1164/rccm.201909-1874ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang TJ, Wares DF, Jafarov A, et al. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2013; 17: 1257–1266. doi: 10.5588/ijtld.12.0863 [DOI] [PubMed] [Google Scholar]
- 4.van der Galiën R, Boveneind-Vrubleuskaya NV, Peloquin C, et al. Pharmacokinetic modeling, simulation, and development of a limited sampling strategy of cycloserine in patients with multidrug-/extensively drug-resistant tuberculosis. Clin Pharmacokinet 2020; 59: 899–910. doi: 10.1007/s40262-020-00860-8 [DOI] [PubMed] [Google Scholar]
- 5.Zhu M, Nix DE, Adam RD, et al. Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy 2001; 21: 891–897. doi: 10.1592/phco.21.11.891.34524 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) . Technical Report on the Pharmacokinetics and Pharmacodynamics (PK/PD) of Medicines used in the Treatment of Drug-Resistant Tuberculosis. Geneva, WHO, 2018. [Google Scholar]
- 7.Deshpande D, Alffenaar JC, Köser CU, et al. d-Cycloserine pharmacokinetics/pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis 2018; 67: Suppl. 3, S308–S316. doi: 10.1093/cid/ciy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udwadia ZF, Tornheim JA, Ganatra S, et al. Few eligible for the newly recommended short course MDR-TB regimen at a large Mumbai private clinic. BMC Infect Dis 2019; 19: 94. doi: 10.1186/s12879-019-3726-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocalevent RD, Berg L, Beutel ME, et al. Social support in the general population: standardization of the Oslo social support scale (OSSS-3). BMC Psychol 2018; 6: 31. doi: 10.1186/s40359-018-0249-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007; 22: 1596–1602. doi: 10.1007/s11606-007-0333-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction 1993; 88: 791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 12.Walker IF, Khan AM, Khan AM, et al. Depression among multidrug-resistant tuberculosis patients in Punjab, Pakistan: a large cross-sectional study. Int J Tuberc Lung Dis 2018; 22: 773–778. doi: 10.5588/ijtld.17.0788 [DOI] [PubMed] [Google Scholar]
- 13.Redwood L, Mitchell EMH, Viney K, et al. Depression, stigma and quality of life in people with drug-susceptible TB and drug-resistant TB in Vietnam. Int J Tuberc Lung Dis 2021; 25: 461–467. doi: 10.5588/ijtld.20.0952 [DOI] [PubMed] [Google Scholar]
- 14.Thomas BE, Thiruvengadam K, Rani S, et al. Smoking, alcohol use disorder and tuberculosis treatment outcomes: a dual co-morbidity burden that cannot be ignored. PLoS One 2019; 14: e0220507. doi: 10.1371/journal.pone.0220507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Court R, Centner CM, Chirehwa M, et al. Neuropsychiatric toxicity and cycloserine concentrations during treatment for multidrug-resistant tuberculosis. Int J Infect Dis 2021; 105: 688–694. doi: 10.1016/j.ijid.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02511-2021.Shareable (332KB, pdf)