Abstract
In childhood, a multitude of causes lead to pulmonary alveolar proteinosis (PAP), an excessive surfactant accumulation in the alveolar space, limiting gas exchange. Autoantibodies against granulocyte–macrophage colony-stimulating factor (GM-CSF) causing autoimmune PAP, the principal aetiology in adults, are rare.
In this first case series on autoimmune PAP, we detail the presentation and management issues of four children.
Whereas three children presented insidiously with progressive dyspnoea, one was acutely sick with suspected pneumonia. During management, one patient was hospitalised with coronavirus disease 2019, noninvasively ventilated, and recovered. All treatment modalities known from adults including whole-lung lavage, augmentation of GM-CSF by inhaled GM-CSF, removal of neutralising antibody by plasmapheresis and interruption of antibody production using rituximab were considered; however, not all options were available at all sites. Inhaled GM-CSF appeared to be a noninvasive and comfortable therapeutic approach.
The management with best benefit-to-harm ratio in autoimmune PAP is unknown and specialised physicians must select the least invasive and most effective treatment. To collect this cohort in a rare condition became feasible as patients were submitted to an appropriate registry. To accelerate the authorisation of novel treatments for autoimmune PAP, competent authorities should grant an inclusion of adolescents into trials in adults.
Short abstract
In children, management of autoimmune pulmonary alveolar proteinosis is very challenging. We need to consider all treatment options, as the most effective one with the best harm-to-benefit ratio is unknown. https://bit.ly/3IM220D
Introduction
Inappropriate catabolism of surfactant and consecutive filling of the alveoli defines pulmonary alveolar proteinosis (PAP). The progressive “occupation” of the alveolar spaces by an excessive amount of surfactant limits gas exchange and gradually exhausts respiratory reserve, leading to respiratory failure and, if untreated, death [1].
Granulocyte–monocyte colony-stimulating factor (GM-CSF) primarily drives the removal of surfactant by alveolar macrophages. Whereas in adults autoantibodies against GM-CSF are the principal cause of PAP, in children this cause is rarely identified [2]. Until now, <10 cases have been published [3–9], whereas a genetically caused interruption of signal transduction of the GM-CSF receptor due to mutations in downstream α- or β-subunit of the receptor has been more frequently described [2].
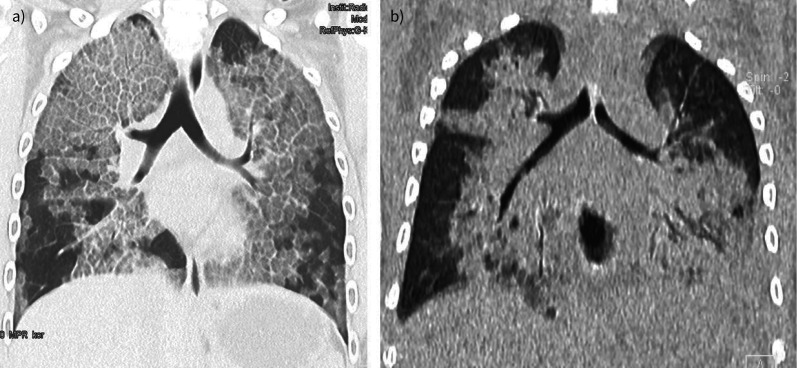
Independently of the aetiology, patients with PAP present with chronic, often dry cough and progressive dyspnoea when exercising and later on at rest. Often this remains unrecognised for many months [1]. In some children with PAP, particularly with GM-CSF receptor mutations, an acute presentation as a respiratory tract infection with fever or chest pain may be seen [10]. Then the characteristic but nonpathognomonic bilateral ground-glass opacification with reticulation from interlobar septal thickening (“crazy paving”) on computed tomography (CT) (figure 1a) needs to be differentiated. Crazy paving can be seen in a number of other diseases, including Pneumocystis pneumonia, bacterial pneumonia, lipoid pneumonia and acute respiratory distress syndrome, including coronavirus disease 2019 (COVID-19) [11]. In CT scans from children with COVID-19, crazy paving occurs in only 0.5% (adults 15–36%), ground-glass opacification in 37% (adult 68–83%) and consolidation in 22% (adults 33–44%) (figure 1b) [12]. For PAP, the history, a characteristic CT scan along with a viscid and milky bronchoalveolar lavage fluid, showing a crowded background of granular cellular and acellular debris, staining positive with periodic acid–Schiff, are diagnostic. Then, >100 different forms and causes of PAP in paediatrics need to be differentiated [2]. Increased levels of neutralising serum anti-GM-CSF autoantibodies further differentiate and diagnose the autoimmune form of PAP (aPAP) [13].
FIGURE 1.
a) Autoimmune pulmonary alveolar proteinosis. Computed tomography scan of a child aged 14 years 8 months with 2 days of fever and dyspnoea. Note the ground-glass opacities and interlobar septal thickening giving the image of nonpathognomonic “crazy paving” pattern. b) Coronavirus disease 2019 pneumonia in a child aged 3 years 2 months with trisomy 21. Note the bilateral ground-glass and consolidating pattern. Both children had comparable degrees of respiratory insufficiency at the time of imaging.
We present four children with aPAP, among them one presenting acutely during the early COVID-19 pandemics and another one experiencing COVID-19. Medical treatment of PAP was very challenging in all children and appeared most successful with inhaled GM-CSF.
Case series
All patients presented with characteristic PAP symptoms and positive GM-CSF autoantibodies (table 1). All were included in the Kids Lung Register on the European Management Platform for Childhood Interstitial Lung Diseases (chILD-EU) after written informed consent. The register study and evaluation and reporting was approved by the ethics committee of Ludwig-Maximilians-University Munich (EK 111–13, 20–329).
TABLE 1.
Presentation and treatment of four children with autoimmune pulmonary alveolar proteinosis (PAP)
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age at presentation | 14 years | 14 years | 10 years | 15 years |
| Sex | Female | Male | Female | Male |
| Age at last follow-up | 14 years, 8 months | 16 years | 10 years, 6 months | 17 years |
| Previous history | Progressive dyspnoea on exertion for 7 months, two episodes of presumptive “community-acquired pneumonia” with fever, parenchymal lung infiltrates and dyspnoea during the past 18 months | Progressive dyspnoea with exercise for 1.5 years, weight loss, no appetite | No previous respiratory complaints. For 1 year, progressive dyspnoea on exertion, dry cough starting with a lower respiratory tract infection with fever over 2 weeks. Weight loss (3–4 kg). For 6 months, inhaled steroids, long-acting β-agonists | No previous relevant respiratory or other symptoms 3 months of progressive asthenia (very low weight: body mass index 15 kg·m−2; <3rd percentile), dry cough and dyspnoea |
| Initial presentation | 37.9°C, progressive dyspnoea, SpO2 89% in ambient air at rest, 94% on 4 L·min−1 oxygen, 25 breaths·min−1, expiratory crackles | No fever, no infections, pale, acrocyanosis, SpO2 at rest 88%, with slight movements <85% | No fever, no infections, pale, tachydyspnoea, SpO2 89% in ambient air at rest, inspiratory crackles | No fever, significant retractions, tachypnoea, inspiratory crackles SpO2 <89% |
| CT scan with crazy-paving pattern | Yes | Yes | Yes | Yes |
| BAL with milky appearance and cytology with acellular debris, no pathogenic organisms | Yes | Yes | Yes | Yes |
| Anti-GM-CSF antibody level (µg·mL−1) (reference <3 µg·mL−1) | 25.5 | 21.2 | Positive (Berlin and Hannover, Germany; not quantified) | Highly positive (Cambridge, UK; not quantified) |
| LDH (U·mL−1) at diagnosis (fold upper limit) | 1.6 | 1.3 | 0.9 | 0.5 |
| FVC (% pred) initial/last | 50/60 | 35/32 | 16.5/35 | 32/28 |
| DLCO (% pred) | 31 | Not done | Not done (55.3 after first WLL) | 20.4 |
| SARS-CoV-2 PCR test | Negative | Severe COVID-19 at age 15.3 years; 6 days hospitalisation, dexamethasone, 5 days NIV, increased oxygen need | Negative | Not done |
| SARS-CoV-2 serum antibody level (U·mL−1) | Not done | 228 (ref. <0.8) | Not done | Not done |
| WLL number (time period) | None | 13 (within 1 year) | 3 (within 5 months) | 6 (within 8 months) prior to rituximab/plasmapheresis over 1 month, followed by 1 WLL after 8 months |
| Inhaled GM-CSF (dose, duration) | Sargramostim (Leukine) 250 μg daily via an LC-STAR nebuliser with a manual interrupter valve connected to a PARI Turbo BOY compressor | Limited approval by insurance after 1 year of application and a court hearing | Sargramostim (Leukine) 250 μg daily via an e-flow nebuliser | Not available |
| Plasmapheresis-scheme, rituximab | Not done | Not done | Not done | 10 sessions of plasmapheresis followed by two doses of rituximab 375 mg·m−2 per dose; clinical improvement with less dyspnoea and need of oxygen |
| Overall outcome of PAP | Gradual improvement, complete remission of respiratory failure at rest at the end of the first month of treatment. Treatment was tapered to 4 days on, 1 day off at 3 months of further improvement. At 4 months after treatment initiation, CT of the chest demonstrated amelioration of the radiological findings and PFTs showed an increase of FVC to 58% predicted and DLCO to 49% predicted | With monthly WLL, just stable; deterioration to baseline before next WLL | Improved after first lung lavage (no oxygen dependency since then) and initiation of GM-CSF inhalation. No dyspnoea at rest or low physical activity, but no reconstitution of lung function since first WLL | WLL insufficiently treating respiratory failure; invasive off-label plasmapheresis and rituximab resulted in less dyspnoea, need of oxygen and WLL. CT and lung function improved, but did not normalise |
CT: computed tomography; BAL: bronchoalveolar lavage; GM-CSF: granulocyte–macrophage colony-stimulating factor; LDH: lactate dehydrogenase; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; WLL: whole-lung lavage; SpO2: peripheral oxygen saturation; COVID-19: coronavirus disease 2019; NIV: noninvasive ventilation; PFT: pulmonary function test.
Diagnosis was confounded in patient 1, a 14-year-old female adolescent, who came to the emergency department with the potential diagnosis of COVID-19, due to a 2-day history of temperature up to 37.9°C, progressive dyspnoea, low peripheral oxygen saturation (SpO2; 89% at room air) and expiratory crackles. Chest radiography revealed bilateral infiltrates. Diagnostics for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were negative. As there was no response to treatment of community-acquired pneumonia, further diagnostic work-up was performed and aPAP was diagnosed. On the basis of the existing Greek legislation, to initiate “off-label” rescue inhaled (i)GM-CSF therapy, the patient was registered at the National Drug Administration, and her parents provided written informed consent. 250 μg GM-CSF (sargramostim (Leukine); Genzyme, Cambridge, MA, USA) were inhaled daily via an LC-STAR nebuliser with a manual interrupter valve connected to a PARI Turbo BOY compressor [13]. The patient improved gradually and attained complete remission of respiratory failure at rest at the end of the first month of treatment. Treatment was tapered to 4 days on, 1 day off at 3 months. 4 months after treatment initiation, chest CT showed a decrease in radiological findings and lung function improved with forced vital capacity (FVC) (58% predicted) and diffusing capacity of the lung for carbon monoxide (49% predicted).
Patient 2 had progressive dyspnoea with exercise for 1.5 years; he had no appetite, and a weight loss of several kilograms. From the diagnosis onward, he was in need of monthly whole-lung lavage (WLL). Each lavage improved respiratory failure, in that the boy was able to attend school and sustain everyday life activities; however, he experienced desaturation of SpO2 <90% with slight exercise. Severe COVID-19 complicated his course, which rendered noninvasive ventilation and a hospital stay necessary. Treatment with inhaled GM-CSF was suggested early; however, the insurance provided refused to cover the costs until recently, when permission for 1 year of treatment was granted.
Patient 3, aged 10 years, had no relevant pre-existing conditions. 12 months prior to diagnosis, symptoms began with progressive dyspnoea on exertion and a persistent dry cough following an infection with fever for 2 weeks, as well as weight loss. Asthma therapy (fluticasone/salmeterol) was started without clear effect. At the age of 10 years, she was admitted to hospital because of hypoxaemia (SpO2 90% at rest while breathing room air) without any signs of an infection. aPAP was diagnosed subsequently and after the first WLL, the girl no longer needed oxygen supplementation, but pulmonary function remained limited. Inhaled GM-CSF, started at the age of 10.3 years, led to stabilisation. To facilitate improvement, two further WLLs were performed.
Patient 4 was a 15-year-old boy, who was malnourished (body mass index 14 kg·m−2, <3rd percentile), with no history of respiratory tract infections or environmental exposure. 4 months after symptoms began, he presented with acute respiratory failure (high-flow oxygen, inspiratory oxygen fraction (FiO2) 0.6–0.75). Six WLLs were performed; the first one under extracorporeal membrane oxygenation (ECMO) without significant clinical improvement. To improve the poor clinical condition, the child was treated with 10 sessions of plasmapheresis followed by two doses of rituximab. Dyspnoea, need for oxygen supply and WLLs improved and only one WLL was necessary within a period of 8 months. Currently he is on high-flow oxygen (FiO2 0.3) during sleep, and overall condition and body mass index (16 kg·m−2) have improved.
Discussion
aPAP is an ultra-rare condition in children and adolescents, with only few cases described worldwide; here we present the first series of four cases of paediatric aPAP and highlight in detail up-to-date management problems.
Diagnosis of aPAP is difficult and necessitates a high degree of awareness, as the disease often progresses slowly. The hallmark feature of chronic interstitial lung disease is desaturation upon exercise, and this should prompt referral to specialised paediatric pneumology care. The COVID-19 pandemic has added another layer of complexity, as the combination of fever and ground-glass opacities on chest imaging (figure 1b) are compatible with SARS-CoV-2-related pneumonia in any age group [14]. Thus, not only the initial diagnosis, but also an exacerbation in a patient with established PAP and residual ground glass on imaging constitutes a challenge and prompts the clinician to repeat PCR testing or consider early SARS-CoV-2 monoclonal antibody treatment.
Treatment of PAP in children is very challenging and the results are not satisfying. Due to its extreme rarity, there is no “standard treatment” and there are no consensus recommendations on how to treat paediatric PAP. The few specialised centres apply different treatments based on locally available techniques, personal expertise, experience collected in adult subjects and published reports. The cases reported here reflect this heterogeneity and are an important starting point to gauge management recommendations.
Available treatment options for aPAP include WLL, augmentation of GM-CSF by iGM-CSF or subcutaneous GM-CSF, removal of neutralising antibodies by plasmapheresis and interruption of antibody production by drugs, including B-cell-depleting antibodies. Lung transplant is not a real option, as recurrent disease has been described [1].
WLL is an invasive procedure taking 3–7 h of general anaesthesia, often associated with post-interventional mechanical ventilation and initially sometimes requiring ECMO [15]. In children, monthly procedures under general anaesthesia for several hours, sometimes over years, have a huge psychosocial impact on the developing child, in addition to the risk of medical complications, including airway injury, particularly in small infants, hypoxaemia and cerebral insult from frequent use of anaesthetics [16]. This technique may be used for rescue of severe respiratory failure and initially, when it is not yet clear how many WLLs will be necessary or when no other treatment option is available. In milder cases, lavage of all lobes by flexible bronchoscopy has been feasible [17].
Competitive binding of the disease-causing, neutralising endogenous GM-CSF autoantibodies by inhaled or subcutaneously applied recombinant GM-CSF is an elegant pathophysiological approach to treat aPAP, and was successfully used for the first time 25 years ago [1]. Aerosolised GM-CSF is most promising, and supported by several clinical trials, with response rates of 62% long-term treatment effects [18]. A review summarised that iGM-CSF treatment was more effective than s.c. GM-CSF therapy, including a higher response rate (89% versus 71%, p=0.023) [19]. Almost all evidence was derived in adults; however, similar responses to iGM-CSF are expected in adolescents with aPAP, as shown in one of the cases reported here.
Elimination of the disease-causing GM-CSF antibodies from the circulation and consecutively from affected deeper tissue compartments by plasmapheresis or similar procedures, in combination with inhibition of novel antibody production by antibodies against B- or plasma cells is rather invasive due to the necessity of a large-bore central vascular access and induction of long-lasting general immunodeficiency [1].
Summarising our experience in the management of four children as presented here, WLLs were not as efficient as reported in adult patients, with a success rate of up to 70% for long-lasting cure [1]. In one of our patients, a very intense regimen with monthly or more frequent lavages was necessary. The techniques and volumes applied per kilogram body weight were the same as in adult subjects. Combining WLL with debilitating plasmapheresis cycles and rituximab treatment prolonged the interval between lavages, at the expense of significant immunosuppression. Inhaled GM-CSF extended its powerful therapeutic potential and safety to the clinical setting of young paediatric patients with aPAP. Clearly, iGM-CSF is the least invasive and most comfortable therapeutic approach, avoiding repetitive long-lasting anaesthesia, risky procedures like WLL and plasmapheresis or long-term immunosuppression in the treatment of such young adolescents. Considering overall costs for a treatment over 3 months, iGM-CSF (sargramostin 75 ampules) costs EUR 26 170; three WLLs with intensive care support costs EUR 33 400.
As there is no “standard treatment” for paediatric PAP, including aPAP, it is important to consider all treatment options in every child, as the most effective one with best harm-to-benefit ratio is unknown. Depending on the presentation, specialised physicians must select the least invasive and most effective treatment; health insurance ought to cover the costs.
One major limitation of this work relates to the few cases presented of this ultra-rare condition; thus, we recommend referring all cases with paediatric PAP to an appropriate registry. In the near future, register-based observational trials may yield new insights. Lastly, competent authorities must take care to guarantee inclusion of adolescents with ultra-rare conditions into adult trials [20] to accelerate access to novel treatments.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: M. Griese reports grants or contracts received from Böhringer Ingelheim paid to their institution; participation on a Data Safety Monitoring Board or Advisory Board for Böhringer Ingelheim, personal fees received. Unpaid Head chILD-EU; all disclosures made outside the submitted work.
Conflict of interest: P. Panagiotou has nothing to disclose.
Conflict of interest: E.D. Manali reports receiving grants or contracts, paid to their institution, from Hoffmann La Roche, Boehringer Ingelheim and Savara; personal payments for lectures, presentations, speakers' bureaus, manuscript writing or educational events received from Hoffmann La Roche and Boehringer Ingelheim; support for attending meetings and/or travel paid to the institution received from Hoffmann La Roche and Boehringer Ingelheim; all disclosures made outside the submitted work.
Conflict of interest: M. Stahl has nothing to disclose.
Conflict of interest: N. Schwerk has nothing to disclose.
Conflict of interest: V. Costa has nothing to disclose.
Conflict of interest: K. Douros has nothing to disclose.
Conflict of interest: M. Kallieri has nothing to disclose.
Conflict of interest: R.M. Urbantat has nothing to disclose.
Conflict of interest: H. von Bernuth reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from CSL Behring and Octapharma; participation on an advisory board for Takeda; and is an associate member of the Standing committee on vaccinations. All disclosures made outside the submitted work.
Conflict of interest: L. Kolilekas has nothing to disclose.
Conflict of interest: L. Morais has nothing to disclose.
Conflict of interest: A. Ramos has nothing to disclose.
Conflict of interest: K. Landwehr has nothing to disclose.
Conflict of interest: K. Knoflach has nothing to disclose.
Conflict of interest: F. Gothe has nothing to disclose.
Conflict of interest: K. Reiter has nothing to disclose.
Conflict of interest: V. Papaevangelou has nothing to disclose.
Conflict of interest: A.G. Kaditis has nothing to disclose.
Conflict of interest: C. Kanaka-Gantenbein has nothing to disclose.
Conflict of interest: S.A. Papiris reports receiving grants or contracts paid to their institution from Hoffmann La Roche, Boehringer Ingelheim and Savara; personal payments received from Hoffmann La Roche and Boehringer Ingelheim for attending meetings and/or travel; all disclosures made outside the submitted work.
Support statement: This study was supported by Bundesministerium für Bildung und Forschung grant HCQ4SurfDefect, the German Center for Lung Research (DZL), and Deutsche Forschungsgemeinschaft grant Gr 970/9-1. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Trapnell BC, Nakata K, Bonella F, et al. . Pulmonary alveolar proteinosis. Nat Rev Dis Primers 2019; 5: 16. doi: 10.1038/s41572-019-0066-3 [DOI] [PubMed] [Google Scholar]
- 2.Griese M. Pulmonary alveolar proteinosis: a comprehensive clinical perspective. Pediatrics 2017; 140: e20170610. doi: 10.1542/peds.2017-0610 [DOI] [PubMed] [Google Scholar]
- 3.Alasiri AM, Alasbali RA, Alaqil MA, et al. . Autoimmune pulmonary alveolar proteinosis successfully treated with lung lavage in an adolescent patient: a case report. J Med Case Rep 2021; 15: 340. doi: 10.1186/s13256-021-02906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld L, Jennings J, Fiorino EK, et al. . Pulmonary alveolar proteinosis: a case of profound hypoxemia in a previously healthy teenager. Pediatr Emerg Care 2021; 37: e571–e573. doi: 10.1097/PEC.0000000000001820 [DOI] [PubMed] [Google Scholar]
- 5.Meka SG, Mohr M, Nair GB, et al. . Autoimmune pulmonary alveolar proteinosis mimicking Mycoplasma pneumonia in an adolescent. Respir Med Case Rep 2020; 30: 101100. doi: 10.1016/j.rmcr.2020.101100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AL, Schissel S, Levy BD, et al. . Clinical problem-solving. A crazy cause of dyspnea. N Engl J Med 2011; 364: 72–77. doi: 10.1056/NEJMcps0901689 [DOI] [PubMed] [Google Scholar]
- 7.Sirin Kose S, Asilsoy S, Uzuner N, et al. . Pulmonary alveolar proteinosis in hereditary and autoimmune forms with 2 cases. Pediatr Emerg Care 2020; 36: e470–e472. doi: 10.1097/PEC.0000000000001536 [DOI] [PubMed] [Google Scholar]
- 8.Strickler A, Boza ML, Koppmann A, et al. . Autoimmune pulmonary proteinosis in a Chilean teenager, a rare aetiology of interstitial lung disease. BMJ Case Rep 2014; 2014: bcr2012006987. doi: 10.1136/bcr-2012-006987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trukalj M, Perica M, Ferenčić Z, et al. . Successful treatment of autoimmune pulmonary alveolar proteinosis in a pediatric patient. Am J Case Rep 2016; 17: 641–645. doi: 10.12659/AJCR.897868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildebrandt J, Yalcin E, Bresser HG, et al. . Characterization of CSF2RA mutation related juvenile pulmonary alveolar proteinosis. Orphanet J Rare Dis 2014; 9: 171. doi: 10.1186/s13023-014-0171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb WR, Müller NL, Naidich DP. High-resolution CT of the Lung. 4th Edn. Philadelphia, Lippincott Williams & Wilkins, 2009. [Google Scholar]
- 12.Nino G, Zember J, Sanchez-Jacob R, et al. . Pediatric lung imaging features of COVID-19: a systematic review and meta-analysis. Pediatr Pulmonol 2021; 56: 252–263. doi: 10.1002/ppul.25070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papiris SA, Tsirigotis P, Kolilekas L, et al. . Pulmonary alveolar proteinosis: time to shift? Expert Rev Respir Med 2015; 9: 337–349. doi: 10.1586/17476348.2015.1035259 [DOI] [PubMed] [Google Scholar]
- 14.Duzgun SA, Durhan G, Demirkazik FB, et al. . COVID-19 pneumonia: the great radiological mimicker. Insights Imaging 2020; 11: 118. doi: 10.1186/s13244-020-00933-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campo I, Luisetti M, Griese M, et al. . Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis 2016; 11: 115. doi: 10.1186/s13023-016-0497-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Ji J, Zhao GQ. General anesthesia affecting on developing brain: evidence from animal to clinical research. J Anesth 2020; 34: 765–772. doi: 10.1007/s00540-020-02812-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z, Jing J, Wang H, et al. . Pulmonary alveolar proteinosis in China: a systematic review of 241 cases. Respirology 2009; 14: 761–766. doi: 10.1111/j.1440-1843.2009.01539.x [DOI] [PubMed] [Google Scholar]
- 18.Papiris SA, Tsirigotis P, Kolilekas L, et al. . Long-term inhaled granulocyte-macrophage-colony stimulating factor in autoimmune pulmonary alveolar proteinosis: effectiveness, safety, and lowest effective dose. Clin Drug Investig 2014; 34: 553–564. doi: 10.1007/s40261-014-0208-z [DOI] [PubMed] [Google Scholar]
- 19.Sheng G, Chen P, Wei Y, et al. . Better approach for autoimmune pulmonary alveolar proteinosis treatment: inhaled or subcutaneous granulocyte-macrophage colony-stimulating factor: a meta-analyses. Respir Res 2018; 19: 163. doi: 10.1186/s12931-018-0862-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapnell BC, Inoue Y, Bonella F, et al. . Inhaled molgramostim therapy in autoimmune pulmonary alveolar proteinosis. N Engl J Med 2020; 383: 1635–1644. doi: 10.1056/NEJMoa1913590 [DOI] [PMC free article] [PubMed] [Google Scholar]