Abstract
Background
There is growing evidence that lung function in early-life predicts later lung function. Adverse events over the lifespan might influence an individual's lung function trajectory, resulting in poor respiratory health. The aim of this study is to identify early-life risk factors and their impact on lung function trajectories to prevent long-term lung impairments.
Methods
Our study included participants from the Raine Study, a prospective pregnancy cohort, with at least two spirometry measurements. Lung function trajectories from the 6- to 22-year follow-ups were characterised using finite mixture modelling. Multinomial logistic regression analyses were used to evaluate the association between early-life predictors and lung function trajectories.
Main results
A total of 1512 participants (768 males, 744 females), representing 53% of the whole cohort, were included in this analysis. Four lung function trajectories of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC (z-scores) were identified. FEV1 and FVC trajectories were categorised as: “very low”, “low”, “average” and “above average”, respectively. Based on their shape, lung function trajectories of FEV1/FVC were categorised as “very low”, “low–average”, “average–low” and “average”. Asthma and maternal smoking were identified as risk factors for low lung function trajectories in this cohort, as well as early-life exposure to PM2.5Absorbance.
Conclusions
Early-life risk factors may influence lung function trajectories over time. Nonetheless, identifying children with a high risk of having low lung function trajectories should be prioritised to prevent deficits in later life.
Short abstract
Early-life risk factors may influence lung function trajectories over time. Nonetheless, identifying children with a high risk of having low lung function trajectories should be prioritised to prevent deficits in later life. https://bit.ly/3oYbgzr
Introduction
Chronic respiratory diseases are a leading cause of mortality worldwide, accounting for >10% of all the disability adjusted-life-years [1]. In the traditional lung growth model, lung development starts in utero and attains maximal lung function into early adulthood. This phase is followed by a slow decline with age [2]. An accumulating body of evidence suggests that chronic respiratory diseases have their roots in fetal and early postnatal life. The insults occurring during these vulnerable periods may have long-term consequences, influencing individual lung function over time [3–5].
Several population-based studies have explored the hypothesis that lung function varies over time along predictable trajectories [6–11]. For instance, Bui et al. [8] identified six lung function trajectories of forced expiratory volume in 1 s (FEV1) (z-score) from 7 to 53 years of age using the Tasmanian Longitudinal Health Study (TAHS). In the Manchester Asthma Study and Allergy Study (MAAS) and the Avon Longitudinal Study of Parents and Children (ALSPAC), Belgrave and colleagues [12] identified three lung function trajectories in 1051 individuals using specific airway resistance measurements from 3 to 11 years of age [6] and four lung function trajectories for FEV1 using two population-based birth cohorts. Two lung function trajectories of per cent of maximal predicted FEV1were found using data from the Tucson Children's Respiratory Study [7]. In the CAMP study, individuals with asthma were followed during young adulthood, and four trajectories of prebronchodilator FEV1 per cent predicted values within the group were identified [13]. Early-life factors that may diminish maximally attainable lung function included respiratory infections, preterm birth, allergies, childhood asthma, in utero smoke and environmental exposure [2]. Together with FEV1, forced vital capacity (FVC) and their ratio (FEV1/FVC) provide information on general respiratory health. There is the need to investigate lung function over the lifespan further and identify groups at higher risk of lung disease and modifiable exposures.
Identifying the complex multifactorial determinants of abnormal lung function requires longitudinal data with a long follow-up over periods of lung growth. Our aim was to characterise lung function trajectories of FEV1, FVC and FEV1/FVC over a 16-year period from childhood to young adulthood, and to investigate early-life factors associated with these trajectories. We hypothesised that early-life risk factors would be associated with low lung function trajectories in our cohort.
Methods
Study population
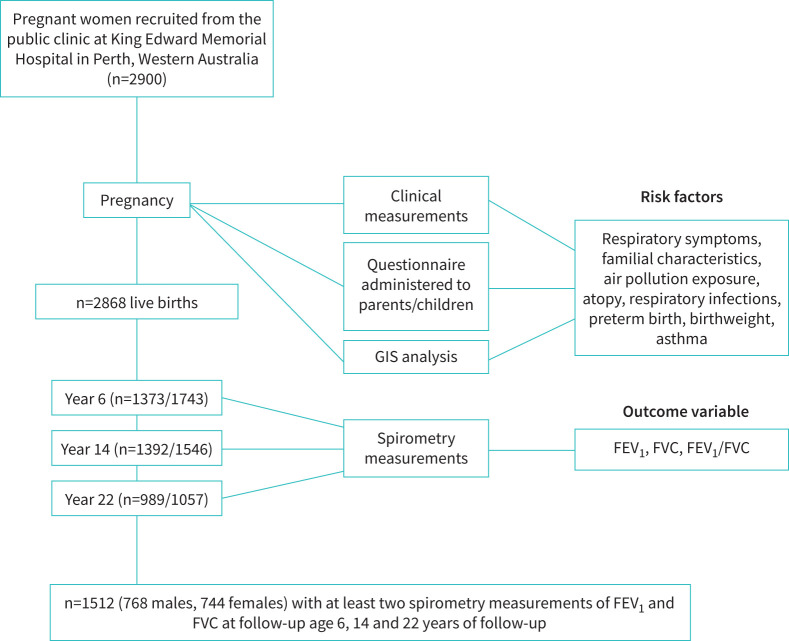
The Raine Study is a large prospective cohort of 2900 pregnant women recruited from King Edward Memorial Hospital in Perth, Western Australia between 1989 and 1991 [14]. Clinical and demographic data were collected during pregnancy and offspring assessed before birth and at 1, 2, 3, 5, 8, 10, 14, 17, 18, 20, 22, 27 and 28 years of age; detailed population recruitment information is presented elsewhere [15]. Children who had attended at least two or three visits with spirometry measurements within the 6-, 14- or 22-year follow-ups were included (figure 1). At each year of follow-up, participants completed a respiratory questionnaire adapted from the International Study on Asthma and Allergies in Childhood (ISAAC) questionnaire for school children [16]. This study was approved by the University of Western Australia Human Research Ethics approval committee (RA/4/1/2100). Each Raine follow-up used in this study received separate research ethics approval.
FIGURE 1.
Flow chart of participants from the Raine Study. The Raine Study participants attending at least two visits that included spirometry measurement within the 6-, 14- or 22-year follow-up were included in this study (n=1512). GIS: geographic information system; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Outcome variables
Lung function was assessed at 6, 14 and 22 years of age by spirometry according to the American Thoracic Society guidelines in place at each time point [17, 18]. FEV1 and FVC were measured from three forced expiratory curves with an acceptable start and end of test. The largest FEV1 and the largest FVC were recorded after examining the data from all acceptable curves. The FEV1/FVC ratio was then calculated. Reference values were derived from the Global Lung Function Initiative (GLI) reference equations [19] that has been validated in an Australasian population [20]. We calculated the z-scores for FEV1, FVC and FEV1/FVC in participants that successfully completed and had acceptable and repeatable spirometry measurements, using the GLI Tool Software developed by the GLI Task Force Group (ERS).
Covariates
Asthma
Current asthma was defined if the children: a) had wheezed in the past 12 months; b) “The child had taken asthma medications in the past 12 months”; and c) “The child had received a doctor-diagnosis of asthma ever?”.
Atopy
Atopy was defined as positive at age 6 years if the participants produced specific immunoglobulin (IgE) antibody levels >0.35 µg mL−1 in response to exposure to any of the following allergens: peanuts or food mix (food allergy), rye grass pollen, house dust mite, mould or cat fur (aeroallergens).
Wheeze, eczema and hay fever
Wheeze was defined as a positive response to “Has your child wheezed in the last 12 months?”. Eczema was defined as a positive response to “Child has eczema?” and a self-reported doctor diagnosis ever. Hay fever was defined as a positive response to “Child has hay fever?” and a self-reported doctor diagnosis.
Respiratory tract infections
Lower and upper respiratory tract infections during the first year of life were defined based on parental report through questionnaire. We defined the diseases using International Classification of Disease (ICD)-9 codes. Specifically, lower respiratory tract infections were defined if the child had pneumonia, bronchitis or tuberculosis, while upper respiratory tract infections were defined if the child had acute upper respiratory infections or chronic or allergic rhinitis.
Early-life exposure to air pollution
Geocodes of residential addresses were used to assign individual early-life air pollution exposures for all the participants. The address history was received from parents at each year of follow-up. Annual mean concentrations for nitrogen dioxide (NO2) (µg mg−1) and PM2.5Absorbance (proxy for elemental carbon) (10−5 m−1) were assigned for each participant's individual geocoded current residential history. Exposure estimates were derived from land use regression (LUR) models previously developed and validated for the Perth metropolitan area [21, 22]. The estimates were extrapolated to the years of follow-up using air monitoring data obtained from the WA Department of Water and Environmental Regulation.
Familial characteristics
Maternal smoke was defined as: in utero smoking if the mother had smoked only during pregnancy; smoking at age 6 if the mother smoked in the 6 years of follow-up, but not in pregnancy; smoking from pregnancy to age 6 if the mother smoked during pregnancy and was still smoking at the 6-year follow-up. Preterm birth was defined as birth before 37 weeks of gestation. Socioeconomic status when children were 6 years old was defined based on family income/year during pregnancy expressed in Australian dollars (AUD): a) <16 000; b) between 16 000 and 40 000; c) >40 000. Demographic variables were retrieved from the questionnaire completed by the parents when children were aged between 3 and 5 years. Parental asthma, eczema, wheeze, hay fever, smoking and maternal education were defined by parental self-report.
Statistical analysis
Lung function trajectories
Trajectories for FEV1, FVC and FEV1/FVC (z-scores) were modelled used group-based trajectory modelling, using “traj” plugin (STATA, version 16.0; StataCorp, College Station, TX, USA) [23]. Sensitivity analyses were performed to assess the robustness of results to various assumptions regarding lung function trajectories membership. Traj analyses longitudinal data using maximum likelihood estimation that accounts for missing data. Given the nature of FEV1 and FVC values (z-scores), a censored normal model was fitted. Within the “traj” plugin, the optimal number and shape of lung function trajectories were determined based on the smallest absolute Bayesian information criterion value and mean posterior probabilities. An average posterior probability of >0.7 was considered as an indicator of good model fit [24]. We started with a model consisting of one group with the highest polynomial order (cubic); the number of groups was then increased until the number that best fitted the data was identified [25]. Once the number of trajectories was identified, we reduced the polynomial orders until the highest order polynomial for each group was significant at the confidence level alpha (x)=0.05. Participants were assigned to one of the groups based on their highest estimated group membership probabilities resulting in a distribution over classes. Trajectories for FEV1, FVC and FEV1/FVC were modelled separately.
Early-life predictors
The distribution of participants’ characteristics by lung function trajectories were summarised using percentages, and Pearson's chi-squared tests were used to estimate differences between groups. We assessed the association of lung function trajectories with potential early-risk factors by multinomial logistic regression. Alpha was set at p<0.05.
Results
Participants with spirometry measurements at two or three time points were included for trajectory analysis (n=1512). The flow chart of participants in this study and their characteristics are shown in figure 1 and table 1. There was a higher proportion of childhood asthma or allergies in males, while low birthweight, wheezing and eczema in young adulthood were more prevalent in females. Compared with those included, excluded participants had lower socioeconomic status when they were age 6 years, fewer respiratory tract infections in the first year of life, less childhood and parental hay fever, asthma and atopy, and more of them were born preterm or exposed to parental smoke (supplementary table 2).
TABLE 1.
Childhood and parenthood characteristics in Raine Study participants with two or more spirometry measurements according to sex (n=1512)
| Va riable | Male | Female | p-value¶ |
| Sex | 768 (50.79) | 744 (49.21) | |
| Socioeconomic status# (income in AUD) | 0.713 | ||
| ≤16.000 | 67 (9.45) | 70 (10.14) | |
| 16 000–40 000 | 316 (44.57) | 293 (42.46) | |
| ≥40 000 | 326 (45.98) | 327 (47.39) | |
| Maternal education | 0.416 | ||
| Low | 145 (18.88) | 159 (21.37) | |
| Medium | 217 (28.26) | 200 (26.88) | |
| High | 345 (44.92) | 338 (45.43) | |
| Preterm birthweight (<37 weeks gestational age) | 45 (6.09) | 56 (7.94) | 0.167 |
| <0 . 001 | |||
| Low | 45 (5.86) | 77 (10.35) | |
| Normal | 723 (94.14) | 667 (89.65) | |
| Respiratory infections | 0.879 | ||
| URTI only | 245 (35.61) | 244 (36.31) | |
| LRTI or both | 182 (26.45) | 182 (27.08) | |
| Current asthma at age 6 years | 133 (17.32) | 99 (13.31) | 0 . 030 |
| Current asthma at age 14 years | 78 (10.16) | 69 (9.27) | 0.563 |
| Current asthma at age 22 years | 40 (5.21) | 63 (8.47) | 0 . 012 |
| Current wheeze at age 6 years | 172 (23.40) | 142 (20.03) | 0.120 |
| Current wheeze at age 14 years | 98 (13.78) | 94 (13.49) | 0.871 |
| Current wheeze at age 22 years | 84 (17.54) | 118 (22.96) | 0 . 034 |
| Current eczema at age 6 years | 166 (22.90) | 174 (24.96) | 0.361 |
| Current eczema at age 14 years | 127 (17.81) | 147 (21.06) | 0.123 |
| Current eczema at age 22 years | 45 (9.53) | 82 (16.43) | <0 . 001 |
| Current hay fever at age 6 years | 51 (6.64) | 53 (7.12) | 0.711 |
| Current hay fever at age 14 years | 156 (21.88) | 135 (19.37) | 0.244 |
| Current hay fever at age 22 years | 73 (15.37) | 104 (20.47) | 0.037 |
| Atopy at age 6 years | <0 . 001 | ||
| Food allergy only | 17 (2.21) | 15 (2.02) | |
| Aeroallergens only or both | 205 (26.69) | 127 (17.07) | |
| Maternal smoking | 0.287 | ||
| Only in pregnancy | 31 (4.41) | 35 (5.21) | |
| Only at age 6 years | 52 (7.41) | 54 (8.04) | |
| Both in pregnancy and at age 6 years | 130 (18.49) | 147 (21.88) | |
| Paternal smoking | 256 (33.33) | 253 (34.01) | 0.669 |
| Parental asthma | 0.268 | ||
| Mother only | 111 (16.82) | 104 (16.48) | |
| Father only | 53 (8.03) | 71 (11.25) | |
| Both | 18 (2.73) | 18 (2.85) | |
| Parental eczema | 0.445 | ||
| Mother only | 117 (17.67) | 106 (16.80) | |
| Father only | 42 (6.34) | 55 (8.72) | |
| Both | 21 (3.17) | 19 (3.01) | |
| Parental hay fever | 0.481 | ||
| Mother only | 231 (30.08) | 210 (28.23) | |
| Father only | 123 (16.02) | 116 (15.59) | |
| Both | 97 (12.63) | 114 (15.32) | |
| Parental wheeze | 313 (43.05) | 313 (44.78) | 0.512 |
Data presented as n (%). AUD: Australian dollars; LRTI: low respiratory tract infections in the first year of life; URTI: upper respiratory tract infections in the first year of life. #: socioeconomic status was defined by the family incomes, expressed in AUD. ¶: significance of differences was evaluated by Chi squared test and are given in bold.
Lung function trajectories
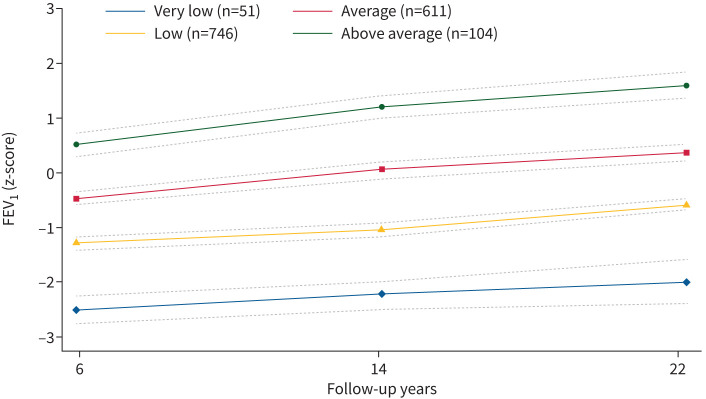
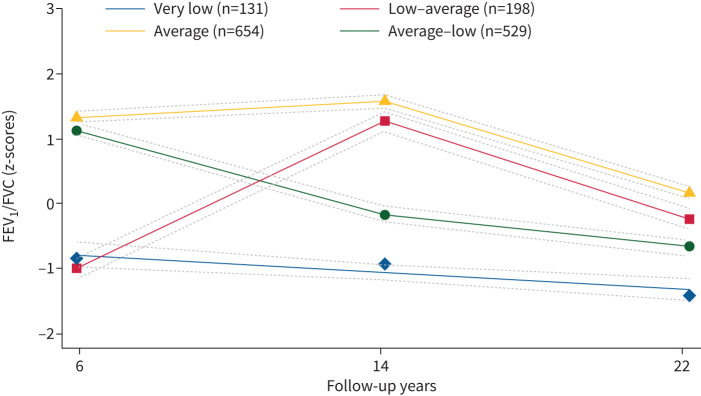
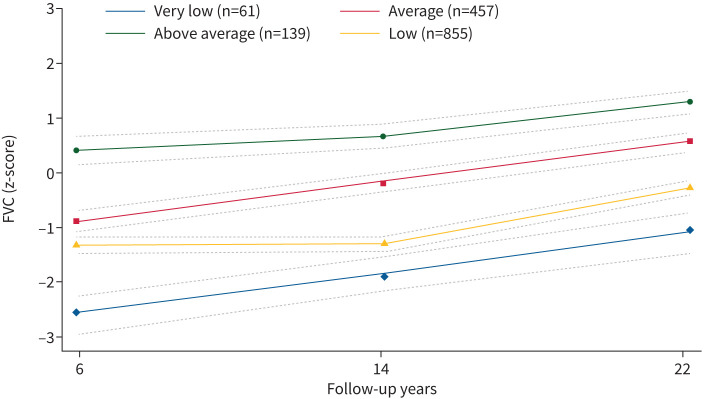
We identified four distinct lung function trajectories of FEV1, FVC and FEV1/FVC (figures 2–4) that best fitted our data (supplementary table 3). FEV1 and FVC trajectories were labelled respectively: “very low” (n=51, 3.37% for FEV1; n=61, 4.03% for FVC), “low” (n=746, 49.34% for FEV1; n=855, 56.55% for FVC), “average” (n=611, 40.41% for FEV1; n=457, 30.22% for FVC) and “above average” (n=104, 6.88% for FEV1; n=139, 9.19% for FVC). Based on their shape, lung function trajectories of FEV1/FVC were labelled as “very low” (n=131, 8.66%), “low–average” (n=198, 13.10%), “average” (n=654, 43.24%) and “average–low” (n=529, 34.99%). Most participants were in the low FEV1 (49.3%) and FVC (56.5%) trajectories, and in the average trajectories of FEV1/FVC (43.3%) (supplementary table 4).
FIGURE 2.
Lung function trajectories (forced expiratory volume in 1s (FEV1)) from 6 to 22 years of follow-up in the Raine participants with two or more spirometry measurements (n=1512). The dashed lines represent the 95% confidence intervals.
FIGURE 4.
Lung function trajectories (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)) from 6 to 22 years of follow-up in the Raine participants with two or more spirometry measurements (n=1512). The dashed lines represent the 95% confidence intervals.
FIGURE 3.
Lung function trajectories (forced vital capacity (FVC)) from 6 to 22 years of follow-up in the Raine participants with two or more spirometry measurements (n=1512). The dashed lines represent the 95% confidence intervals.
Early-life predictors and lung function trajectories
The distribution of participants’ characteristics by lung function trajectories are shown in supplementary table 6. Adjusted associations between childhood, parental and environmental factors and lung function trajectories compared with the average trajectory (reference group) show that participants in the low trajectory of FEV1 and in the very low and low–average trajectory of FVC were more likely to have childhood asthma when compared with the reference group (table 2). Being in the average–low trajectory of FEV1/FVC was associated with being less likely to have respiratory allergies alone or combined with food allergy during childhood; participants in the very low trajectory of FEV1/FVC were 2.47 times more likely to be exposed to both pre- and postnatal maternal smoke. With respect to the average trajectory, being in the low trajectory of FEV1 was associated with a higher risk of being exposed to PM2.5Absorbance in early life, while being in the average–low trajectory of FEV1/FVC was associated with a lower risk of being exposed to PM2.5Absorbance and NO2.
TABLE 2.
Association expressed as risk relative ratio (RRR) between childhood, parental factors, air pollution exposure and forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC lung function trajectories in Raine participants with two or more spirometry measurements
| Variable | FEV1 (n=1512) RRR (95% CI) | FVC (n=1512) RRR (95% CI) | FEV1/FVC (n=1512) RRR (95% CI) | ||||||
| Average (reference category) | Average (reference category) | Average (reference category) | |||||||
| Very low (n=51) | Low (n=746) | Above average (n=104) | Very low (n=61) | Low (n=855) | Above average (n=139) | Very low (n=131) | Low-average (n=198) | Average-low (n=529) | |
| Females | 0.61 (0.34–1.10) | 1.03 (0.84–1.29) | 0.88 (0.58–1.33) | 0.67 (0.39–1.15) | 1.04 (0.83–1.31) | 0.88 (0.60–1.29) | 0.83 (0.57–1.22) | 1.17 (0.85–1.61) | 0.80 (0.63–1.01) |
| SES | |||||||||
| 16 000–40 000 | 2.24 (0.66–7.61) | 0.97 (0.65–1.43) | 1.33 (0.60–2.98) | 2.13 (0.62–7.35) | 0.84 (0.54–1.29) | 0.67 (0.35–1.28) | 1.05 (0.52–2.13) | 0.60 (0.33–1.07) | 0.77 (0.51–1.18) |
| ≥40 000 | 1.09 (0.66–7.61) | 1.06 (0.72–1.55) | 1.11 (0.50–2.51) | 1.43 (0.41–5.01) | 0.82 (0.53–1.25) | 0.62 (0.33–1.19) | 0.91 (0.45–1.85) | 0.95 (0.54–1.67) | 0.74 (0.48–1.21) |
| Prete rm | 1.04 (0.31–3.51) | 1.30 (0.84–2.01) | 0.97 (0.30–2.37) | 1.51 (0.60–3.79) | 1.03 (0.38–1.62) | 0.85 (0.38–1.90) | 0.94 (0.38–2.30) | 2.03 (1.11–3.71) | 1.92 (1.19–3.08) |
| Birthweight | 1.59 (0.80–3.17) | 0.80 (0.59–1.08) | 0.63 (0.29–1.37) | 1.66 (0.74–3.76) | 0.95 (0.63–1.44) | 0.67 (0.31–1.48) | 0.97 (0.44–2.13) | 1.77 (1.02–3.08) | 1.63 (0.90–2.50) |
| Respiratory infections | |||||||||
| URTI only | 0.41 (0.18–3.56) | 0.82 (0.64–1.06) | 1.25 (0.76–2.04) | 0.55 (0.28–1.99) | 1.02 (0.78–1.34) | 1.30 (0.84–2.02) | 0.82 (0.52–1.29) | 1.07 (0.74–1.56) | 0.93 (0.71–1.22) |
| LRTI or both | 1.49 (0.75–2.93) | 1.06 (0.79–1.43) | 1.30 (0.72–2.34) | 1.02 (0.52–1.99) | 1.08 (0.79–1.48) | 1.04 (0.61–1.77) | 0.99 (0.60–1.63) | 1.04 (0.67–1.62) | 1.00 (0.73–1.38) |
| Asthma at age 6 years | 2.07 (0.90–4.74) | 1.68 (1.20–2.36) | 0.89 (0.42–1.90) | 0.97 (0.44–2.16) | 1.02 (0.72–1.44) | 0.93 (0.51–1.67) | 3.31 (2.01–5.47) | 1.98 (1.21–3.24) | 1.57 (1.08–2.28) |
| Atopy (IgE) at age 6 | |||||||||
| Food allergy (only) | 1.20 (1.15–9.75) | 1.16 (0.52–2.56) | 1.20 (0.26–2.57) | –# | 1.41 (0.59–3.41) | 1.42 (0.36–6.64) | –# | 2.35 (0.82–6.77) | 1.90 (0.81–4.46) |
| Aeroallergens only or both | 0.46 (0.17–1.24) | 0.96 (0.72–.1.27) | 1.37 (0.81–2.31) | 0.94 (0.46–2.92) | 0.95 (0.59–3.41) | 1.23 (0.76–1.97) | 0.74 (0.45–1.22) | 0.77 (0.50–1.19) | 0.70 (0.51–0.95) |
| Maternal smoking | |||||||||
| Smoking in pregnancy only | 1.07 (0.24–4.83) | 0.72 (0.40–1.32) | 1.75 (0.75–4.06) | 1.38 (0.38–5.01) | 0.69 (0.37–1.30) | 1.57 (0.70–3.56) | 2.22 (0.90–5.50) | 1.25 (0.51–3.02) | 1.38 (0.74–2.58) |
| Smoking at age 6 only | 0.34 (0.04–2.70) | 1.07 (0.70–1.62) | 0.35 (0.11–1.18) | 1.61 (0.57–4.56) | 1.42 (0.88–2.31) | 0.45 (0.15–1.33) | 1.05 (0.47–2.34) | 0.92 (0.47–1.79) | 0.90 (0.56–1.43) |
| Both | 1.40 (0.66–2.96) | 1.12 (0.83–1.51) | 0.57 (0.29–1.13) | 1.23 (0.61–2.47) | 0.73 (0.53–1.14) | 0.69 (0.37–1.30) | 2.47 (1.52–2.50) | 1.35 (0.85–2.14) | 0.77 (0.55–1.09) |
| Parental asthma | |||||||||
| Mother asthma only | 1.29 (0.52–3.20) | 1.02 (0.70–1.49) | 0.67 (0.31–1.47) | 1.94 (0.79–4.80) | 0.91 (0.60–1.35) | 0.62 (0.32–1.21) | 0.92 (0.48–1.75) | 0.88 (0.49–1.57) | 0.74 (0.50–1.10) |
| Father asthma only | 1.04 (0.31–3.47) | 1.14 (0.73–1.80) | 0.34 (0.10–1.19) | 1.99 (0.67–5.91) | 1.13 (0.69–1.84) | 0.61 (0.25–1.45) | 0.99 (0.46–2.11) | 0.84 (0.41–1.71) | 1.49 (0.91–2.45) |
| Both | –# | 1.82 (0.80–4.12) | 0.56 (0.07–4.73) | 2.41 (0.43–3.49) | 1.19 (0.50–2.80) | 0.27 (0.03–2.28) | 0.99 (0.25–3.93) | 1.27 (0.37–4.35) | 1.76 (0.73–4.26) |
| Parental hay fever | |||||||||
| Mother hay fever | 1.06 (0.59–1.92) | 0.84 (0.62–1.13) | 1.06 (0.59–1.92) | 0.97 (0.45–2.07) | 0.79 (0.46–1.37) | 1.06 (0.62–1.81) | 0.59 (0.34–1.03) | 0.89 (0.57–1.37) | 0.96 (0.70–1.32) |
| Father hay fever | 0.56 (0.21–1.49) | 0.73 (0.52–1.02) | 1.04 (0.54–2.03) | 0.82 (0.47–1.43) | 0.97 (0.43–2.23) | 1.04 (0.57–1.88) | 1.27 (0.72–2.23) | 1.17 (0.71–1.92) | 1.26 (0.87–1.32) |
| Both | 0.49 (0.17–1.43) | 0.72 (0.50–1.04) | 1.38 (0.70–2.71) | 0.82 (0.31–2.17) | 0.76 (0.51–1.12) | 1.60 (0.87–2.95) | 0.76 (0.40–1.45) | 0.99 (0.58–1.68) | 0.99 (0.67–1.46) |
| Parental eczema | |||||||||
| Mother eczema only | 0.41 (0.12–1.40) | 1.06 (0.78–1.46) | 0.73 (0.37–1.45) | 0.81 (0.46–1.41) | 0.88 (0.40–1.94) | 0.75 (0.54–1.04) | 1.35 (0.79–2.31) | 0.72 (0.44–1.18) | 0.97 (0.69–1.37) |
| Father eczema only | 1.59 (0.52–4.88) | 1.47 (0.92–2.36) | 0.98 (0.36–2.62) | 0.71 (0.28–1.80) | 1.66 (0.63–4.39) | 1.10 (0.67–1.80) | 2.38 (1.20–4.72) | 0.88 (0.42–1.83) | 1.26 (0.76–2.08) |
| Both | 0.41 (0.12–1.40) | 1.06 (0.78–1.46) | 0.73 (0.37–1.45) | 0.81 (0.46–1.41) | 0.88 (0.40–1.94) | 0.75 (0.54–1.04) | 1.35 (0.79–2.31) | 0.72 (0.44–1.18) | 0.97 (0.69–1.37) |
| PM2.5Abs | 0.29 (0.09–1.06) | 1.63 (1.06–2.51) | 1.09 (0.57–2.08) | 0.74 (0.29–1.88) | 0.99 (0.54–1.81) | 0.97 (0.65–1.44) | 0.62 (0.33–1.17) | 0.58 (0.33–1.01) | 0.52 (0.35–0.79) |
| NO2 | 0.78 (0.66–1.10) | 0.95 (0.90–1.01) | 1.04 (0.93–1.17) | 0.91 (0.79–1.06) | 1.04 (0.94–1.15) | 1.02 (0.95–1.08) | 0.96 (0.86–1.06) | 0.94 (0.86–1.02) | 0.90 (0.84–0.96) |
RRR (95% CI) and significant differences from the average trajectory were derived from a multinomial logistic regression. Bold values indicate a significant association at α=0.05. For asthma, model was adjusted for maternal smoking, parental asthma, sex and socioeconomic status. For atopy, model was adjusted for childhood asthma, parental asthma, parental hay fever, parental wheezing, sex and socioeconomic status. For respiratory tract infections, model was adjusted for maternal smoking, sex and socioeconomic status. For maternal smoke, model was adjusted for childhood asthma, maternal asthma and socioeconomic status. For parental asthma, model was adjusted for parental hay fever, parental eczema, parental wheezing, sex and socioeconomic status. For parental hay fever, model was adjusted for parental asthma, parental wheezing, sex and socioeconomic status. For parental eczema, model was adjusted for parental asthma, parental hay fever, sex and socioeconomic status. For PM2.5Abs and nitrogen dioxide (NO2), model was adjusted for childhood asthma, childhood hay fever, parental eczema, parental wheezing, sex and socioeconomic status. The average trajectory of FEV1, FEV1/FVC and FVC were used as reference category. SES: socioeconomic status; LRTI: low respiratory tract infections in the first year of life; URTI: upper respiratory tract infections in the first year of life; Ig: immunoglobulin. #: low number of participants in the category.
Discussion
Using data collected prospectively from the Raine Study, we characterised lung function trajectories from 6 to 22 years of age and identified four trajectories for FEV1, FVC and FEV1/FVC, respectively. Childhood asthma and maternal smoke were identified as risk factors for low lung function trajectories, as well as early-life exposure to air pollutants. To the best of our knowledge, this is the first study to characterise lung function trajectories for FEV1, FVC and FEV1/FVC in z-scores in the same cohort and consider the impact of early-life predictors.
At present, studies investigating lung function trajectories report a variety of outcomes, with FEV1 being the most common [8, 12, 13]. Only one study explored FEV1, FVC and FEV1/FVC trajectories from 10 to 26 years, separately for males and females [9], but this study reported raw values not adjusted for age. Similar to our study, trajectories of FEV1/FVC showed a stable decline from puberty to young adulthood, although the authors did not cover the early childhood period. The Tasmanian Longitudinal Health Study cohort, however, found six trajectories of FEV1, of which two – the early below average, accelerated decline and early low, accelerated growth, normal decline – were newly identified compared with previous studies. This may be because the age of participants extended to middle age and they were older than those in our cohort; thus, we did not detect lung function decline with age in early adulthood. These results together suggest that the number of trajectories highly differ according to the study population and the statistical method used to identify trajectories.
The results of our study confirmed our a priori hypothesis that poor lung function trajectories are associated with early-life factors. In our cohort, participants with asthma were more likely to belong to the low lung function trajectories. In our cohort, children at 6 years of age had lower GLI z-scores for FEV1 and FVC. Nonetheless, the results continue to show that lung function tracks throughout time. Specifically, having asthma was associated with almost twofold risk of having a low lung function trajectory compared to an average trajectory for FEV1. A similar risk is associated with the very low and low–average trajectories of FEV1/FVC. This suggests that participants in the lowest trajectories have an increased risk of asthma diagnosis in childhood.
Compared with the average trajectory, participants in the very low trajectory of FEV1/FVC were threefold as likely to be exposed to maternal smoking during gestation or up to the age of 6 years. The association was not seen if mothers only smoked in pregnancy or when the child was 6 years old. Although there was a lack of association with FEV1, it is likely that maternal smoking increases airway obstruction risk, as defined by a low FEV1/FVC trajectory. Previously reported associations between maternal smoke and low lung function trajectories support our findings [8, 26], except for the Isle of Wight birth cohort, where exposure to in utero smoke was not a risk factor for low lung function trajectories [9].
Our findings suggest that early-life exposure to PM2.5Absorbance was associated with the low FEV1 trajectory. Inhalation of particles in early life might affect the airway epithelium, worsening the children's lung function and trajectory. These results have not been previously described. Only few studies reported information on the longitudinal effects of childhood exposure [27], despite a wide range of studies demonstrating the cross-sectional link between adverse effects of air pollution and lung function in children. Perth is an area with low concentrations of air pollutants. However, Dirgawati et al. [28] reported an association between PM2.5Absorbance and an increased risk of all-cause mortality, hypothesising that concentrations lower than those recommended by the World Health Organisation might affect respiratory health. It is possible that PM2.5Absorbance, defined as a surrogate for black carbon, may act on the respiratory system leading to early alterations in airway size or impaired gas exchange and lung function. Children are particularly vulnerable to the effects of air pollution, as respiratory airways development occurs during in utero and continues until 6–8 years of age when the lungs reach full functionality [29]. Cohort participants were born between 1989 and 1990, years in which the air monitoring networks for the Perth area were not initiated. Thus, we defined early-life air pollution exposure at the first available time, when children were aged between 3 and 4 years, being unable to include periods in which most of the lung development occur. Previous studies reported adverse association between lung function and early-life exposure to NO2 [30–32], while no association was seen in our study. It may be possible that the strongest effects of NO2 on lungs occur during intrauterine life and early postnatal period, which were not included in our study.
Our low–average trajectory of FEV1/FVC was associated with low birthweight, while the average–low trajectory of FEV1/FVC was associated with childhood respiratory allergies alone or combined with food allergies. According to other studies [33, 34], our results suggest that a food allergy response can lead to clinical symptoms, including airway inflammation that might decrease lung function over time. However, it is still unclear if having food allergies in childhood is an independent risk factor for decreasing lung function despite asthma status. Further, males were more likely to belong to the very low lung function trajectories of FVC. Socioeconomic status, respiratory infections, preterm birth, paternal eczema and parental hay fever were not associated with the lung function trajectories of FEV1, FVC or FEV1/FVC. While FEV1 gives an indication of airway calibre, FVC is indicative of lung capacity. Previous research showed a lack of association between children's respiratory health and socioeconomic status [35], while others supported the hypothesis that low educational level can be a disadvantageous factor for respiratory outcomes in children, such as the diagnosis of asthma [36], with different effects depending on race and ethnicity [37]. Respiratory infections in our study were also recorded based on parental recall, which is not ideal in the absence of molecular detection. A potential explanation for the average–low trajectory is a limitation of spirometry. Spirometry is reliable and acceptable when performed at preschool age, although children might have difficulty exhaling completely. As such, the FVC measured at 6 years of age in our population sample may be underestimated, potentially leading to an artificially high FEV1/FVC ratio because they did achieve a full FEV1, but a suboptimal FVC. We also observed a low–average trajectory in our cohort. The GLI have previously reported that predicted values of FEV1/FVC fell from 3–10 years of age in healthy children but increased up to 16 years followed by a linear decline into adulthood [38]. Changes in FEV1/FVC ratio in childhood and adolescence are not completely unexpected since this is a period of lung growth. The participants in this trajectory may have had an adolescent growth spurt prior to 14 years, leading to the observed catch-up growth.
Further limitations of our study are that we had a high dropout rate and respiratory symptoms were more prevalent in included participants. This implies that the children under study were more likely to attend more visits for lung function testing. Although we investigated many risk factors, inclusion of pregnancy complications or chronic diseases among parents or children were not included in our analysis as there were small numbers and the results would not be meaningful. Lastly, when we investigated the association between lung function trajectories and early-life exposure to air pollution, we back-extrapolated air pollution data for 19 years of follow-up. Although LUR models have been shown to be stable over a period of 35 years [39, 40], we might overestimate the results as some, but not all, areas in Perth may have undergone re-development or expansion and air pollution levels may have varied over time, leading to prediction error from our models. Another limitation is that, ideally, we would have liked to back-extrapolate the LUR models to the pregnancy timepoints. However, modelling historical air pollution exposure depends on the availability of monitored concentrations, which were not available during the antenatal period and the first 3 years of life. Nonetheless, our study makes a unique contribution to the literature on long-term pollution at relatively low exposure levels in early life and lung function. The availability of detailed demographics and clinical information for children and their parents for developmental periods enabled us to establish lung function trajectories over 16 years from early life to young adulthood. The study is also strengthened by adopting a finite mixture model identifying lung function trajectories that were not based on any a priori hypothesis.
In conclusion, we found discrete trajectories in our study population from childhood to young adulthood with low lung function trajectories characterised by asthma, maternal smoke, low birthweight and early-life exposure to air pollution. Our findings reinforce the hypothesis that there are groups in the population that follow similar respiratory paths and that events occurring in the first years of life have a role in influencing lung function trajectories. These trajectories may be partly established before 6 years of age. Special attention should be given to participants belonging to the very low trajectory of FEV1 who might be potentially at higher risk. This study's practical relevance is to provide the opportunity to identify those children who are at higher risk of low lung function trajectories and, consequently, prevent deficits in later life, allowing individuals to maximise their lung function potential throughout life fully.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00072-2022.SUPPLEMENT (435.8KB, pdf)
Supplementary material 00072-2022.SUPPLEMENTARY_CLEAN_FS (67.7KB, docx)
Acknowledgements
We would like to acknowledge the Raine Study participants and their families for their ongoing participation in the study, and the Raine Study team for study coordination and data collection. We also thank the National Health and Medical Research Council (NHMRC) for their long-term contribution to funding the study over the last 30 years. The core management of the Raine Study is funded by The University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. The Raine Study Gen2 14-year follow-up was funded by NHMRC project grant 211192, NMHRC Program Grant 003209 and the Raine Medical Research Foundation. The Raine Study Gen2-22 year follow-up was funded by NHMRC project grants 1027449, 1044840 and 1021858. We further acknowledge Jackie Joseph Bowen for the contribution on the sixth-year lung function measurements, and Marie Deverall and Lisha Van Reyk for the 14th year lung function measurements.
Provenance: Submitted article, peer reviewed.
Author contributions: F. Sanna, R.E. Foong and G.L. Hall conceived and designed the study. D. Blake and J. Heyworth provided support on the air pollution data gathering, GIS analysis and back-extrapolation analysis. F. Sanna performed the statistical analysis. F. Sanna, R.E. Foong and G.L. Hall interpreted the results. F. Sanna wrote the first draft of the manuscript. All the authors contributed to the interpretation of the results and critically reviewed and approved the final version of the manuscript.
Conflict of interest: R.E. Foong reports support for the present manuscript received from the Australian National Health and Medical Research Council (NHMRC) and grants or contracts outside the submitted work received from Raine Foundation Priming. P.D. Sly, J. Heyworth and G.L. Hall report support for the present manuscript received from NHMRC. The remaining authors have nothing to disclose.
Support statement: R.E. Foong is a recipient of a National Health and Medical Research Council Early Career Fellowship and received a Raine Priming Grant to support this work. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Forum of International Respiratory Societies . The Global Impact of Respiratory Disease. 2nd Edn. Sheffield, European Respiratory Society, 2017. [Google Scholar]
- 2.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019; 7: 358–364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 3.Carraro S, Scheltema N, Bont L, et al. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. Eur Respir J 2014; 44: 1682–1696. doi: 10.1183/09031936.00084114 [DOI] [PubMed] [Google Scholar]
- 4.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis 2013; 7: 161–173. doi: 10.1177/1753465813479428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015; 385: 899–909. doi: 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 6.Belgrave DC, Buchan I, Bishop C, et al. Trajectories of lung function during childhood. Am J Respir Crit Care Med 2014; 189: 1101–1109. doi: 10.1164/rccm.201309-1700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016; 194: 607–612. doi: 10.1164/rccm.201604-0753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 9.Karmaus W, Mukherjee N, Janjanam VD, et al. Distinctive lung function trajectories from age 10 to 26 years in men and women and associated early life risk factors – a birth cohort study. Respir Res 2019; 20: 98. doi: 10.1186/s12931-019-1068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lødrup Carlsen KC, Mowinckel P, Hovland V, et al. Lung function trajectories from birth through puberty reflect asthma phenotypes with allergic comorbidity. J Allergy Clin Immunol 2014; 134: 917–923. doi: 10.1016/j.jaci.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 11.Turner S, Fielding S, Mullane D, et al. A longitudinal study of lung function from 1 month to 18 years of age. Thorax 2014; 69: 1015–1020. doi: 10.1136/thoraxjnl-2013-204931 [DOI] [PubMed] [Google Scholar]
- 12.Belgrave DCM, Granell R, Turner SW, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med 2018; 6: 526–534. doi: 10.1016/S2213-2600(18)30099-7 [DOI] [PubMed] [Google Scholar]
- 13.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016; 374: 1842–1852. doi: 10.1056/NEJMoa1513737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newnham JP, Evans SF, Michael CA, et al. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 1993; 342: 887–891. doi: 10.1016/0140-6736(93)91944-H [DOI] [PubMed] [Google Scholar]
- 15.Straker LM, Hall GL, Mountain J, et al. Rationale, design and methods for the 22 year follow-up of the Western Australian Pregnancy Cohort (Raine) Study. BMC Public Health 2015; 15: 663. doi: 10.1186/s12889-015-1944-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher MI, Weiland SK. The International Study of Asthma and Allergies in Childhood (ISAAC). Clin Exp Allergy 2008; 28: Suppl. 5, 52–66. doi: 10.1046/j.1365-2222.1998.028s5052.x [DOI] [PubMed] [Google Scholar]
- 17.Crapo RO, Hankinson JL, Irvin C, et al. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152: 1107–1136. doi: 10.1164/ajrccm.152.3.7663792 [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall GL, Thompson BR, Stanojevic S, et al. The Global Lung Initiative 2012 reference values reflect contemporary Australasian spirometry. Respirology 2012; 17: 1150–1151. doi: 10.1111/j.1440-1843.2012.02232.x [DOI] [PubMed] [Google Scholar]
- 21.Dirgawati M, Barnes R, Wheeler AJ, et al. Development of land use regression models for predicting exposure to NO2 and NOx in metropolitan Perth, Western Australia. Environl Model Softw 2015; 74: 258–267. doi: 10.1016/j.envsoft.2015.07.008 [DOI] [Google Scholar]
- 22.Dirgawati M, Heyworth JS, Wheeler AJ, et al. Development of Land Use Regression models for particulate matter and associated components in a low air pollutant concentration airshed. Atmos Environ 2016; 144: 69–78. doi: 10.1016/j.atmosenv.2016.08.013 [DOI] [Google Scholar]
- 23.Haviland AM, Jones BL, Nagin DS. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociol Methods Res 2011; 40: 367–390. doi: 10.1177/0049124111400041 [DOI] [Google Scholar]
- 24.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6: 109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 25.Peristera P, Platts LG, Magnusson Hanson LL, et al. A comparison of the B-spline group-based trajectory model with the polynomial group-based trajectory model for identifying trajectories of depressive symptoms around old-age retirement. Aging Ment Health 2020; 24: 445–452. doi: 10.1080/13607863.2018.1531371 [DOI] [PubMed] [Google Scholar]
- 26.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010; 65: 14–20. doi: 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 27.Schultz ES, Hallberg J, Bellander T, et al. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med 2016; 193: 171–177. doi: 10.1164/rccm.201505-0928OC [DOI] [PubMed] [Google Scholar]
- 28.Dirgawati M, Hinwood A, Nedkoff L, et al. Long-term exposure to low air pollutant concentrations and the relationship with all-cause mortality and stroke in older men. Epidemiology 2019; 30: Suppl. 1, S82–S89. doi: 10.1097/EDE.0000000000001034 [DOI] [PubMed] [Google Scholar]
- 29.Bateson TF, Schwartz J. Children's response to air pollutants. J Toxicol Environ Health A 2008; 71: 238–243. doi: 10.1080/15287390701598234 [DOI] [PubMed] [Google Scholar]
- 30.Mölter A, Agius RM, de Vocht F, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect 2013; 121: 1232–1238. doi: 10.1289/ehp.1205961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004; 351: 1057–1067. doi: 10.1056/NEJMoa040610 [DOI] [PubMed] [Google Scholar]
- 32.Nordeide Kuiper I, Svanes C, Markevych I, et al. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ Int 2021; 146: 106219. doi: 10.1016/j.envint.2020.106219 [DOI] [PubMed] [Google Scholar]
- 33.Friedlander JL, Sheehan WJ, Baxi SN, et al. Food allergy and increased asthma morbidity in a School-based Inner-City Asthma Study. J Allergy Clin Immunol Pract 2013; 1: 479–484. doi: 10.1016/j.jaip.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherenian MG, Singh AM, Arguelles L, et al. Association of food allergy and decreased lung function in children and young adults with asthma. Ann Allergy Asthma Immunol 2018; 121: 588–593. doi: 10.1016/j.anai.2018.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehring U, Gruzieva O, Agius RM, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect 2013; 121: 1357–1364. doi: 10.1289/ehp.1306770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong T, Lundholm C, Rejnö G, et al. Parental socioeconomic status, childhood asthma and medication use – a population-based study. PLoS One 2014; 9: e106579. doi: 10.1371/journal.pone.0106579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakur N, Oh SS, Nguyen EA, et al. Socioeconomic status and childhood asthma in urban minority youths. The GALA II and SAGE II studies. Am J Respir Crit Care Med 2013; 188: 1202–1209. doi: 10.1164/rccm.201306-1016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quanjer PH, Stanojevic S, Stocks J, et al. Changes in the FEV₁/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J 2010; 36: 1391–1399. doi: 10.1183/09031936.00164109 [DOI] [PubMed] [Google Scholar]
- 39.Beelen R, Hoek G, Fischer P, et al. Estimated long-term outdoor air pollution concentrations in a cohort study. Atmos Environ 2007; 41: 1343–1358. doi: 10.1016/j.atmosenv.2006.10.020 [DOI] [Google Scholar]
- 40.Gulliver J, de Hoogh K, Hansell A, et al. Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain. Environ Sci Technol 2013; 47: 7804–7811. doi: 10.1021/es4008849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00072-2022.SUPPLEMENT (435.8KB, pdf)
Supplementary material 00072-2022.SUPPLEMENTARY_CLEAN_FS (67.7KB, docx)