Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible fibrotic interstitial lung disease. We performed size-based quantitation of pulmonary arterial remodelling in IPF and examined the role of endothelial-to-mesenchymal transition (EndMT) and effects on lung physiology.
Methods
Resected lung tissues from 11 normal controls (NCs), and 13 IPF patients were differentially stained using the Movat Pentachrome technique. Size-based classification for pulmonary arteries was conducted in NC and IPF tissues. For each pulmonary artery, arterial size, luminal diameter, thickness of the intima, media and adventitia, and elastin deposition were quantified using Image ProPlus7.0 software. In addition, immunohistochemical staining was performed for EndMT markers and collagen.
Results
Large and medium-size arterial numbers were significantly reduced in IPF compared to NCs (p<0.0001). Intima thickness was highest in the arterial range of 200–399 μm and 600–1000 μm (p<0.0001), while medial and adventitial thickness was significant across 200–1000 μm (p<0.05) compared to NC. Medial thickness was found to significantly affect the diffusing capacity of the lungs for carbon monoxide (DLCO) (r=−0.8, p=0.01). Total arterial elastin in IPF was higher across all arterial ranges except 100–199 μm in IPF than in NC, with the greatest differences in 200–399 μm (p<0.001) and 600–1000 μm (p<0.001). Total elastin also negatively correlated with DLCO (r’=−0.63, p=0.04) in IPF. An increase in EndMT markers and collagen type I/ IV was observed.
Conclusions
This is the first study demonstrating size-based differences in pulmonary arteries in IPF and its detrimental effect on lung physiology. The process of EndMT might be central to these vascular remodelling changes and could be a potential novel therapeutic target.
Short abstract
Pulmonary arterial remodelling occurs in IPF patients, affects lung function and may exaggerate pulmonary hypertension. Endothelial-to-mesenchymal transition appears decisive with vascular changes and could be a novel therapeutic target for IPF. https://bit.ly/3GG3qBa
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive chronic interstitial lung disease associated with irreversible lung fibrosis. IPF is a deadly disease, with mortality rates averaging 3.8 years from diagnosis [1, 2]. Currently, IPF affects >3 million people worldwide, which forms a substantial burden on healthcare [3]. Honeycombing and usual interstitial pneumonia (UIP) are some of the common observations of IPF lung when detected through high-resolution computed tomography [4]. Despite unknown aetiology, environmental, microbial factors and genetic susceptibility are associated with IPF pathogenesis [1, 4]. IPF pathology results from repeated injury to the lung interstitium, causing aberrant repair, leading to intense interstitial fibrosis and restricted gas exchange [5]. Several cells, including alveolar type II pneumocytes, endothelial cells, pericytes, fibrocytes, macrophages and mast cells, are crucial contributors to IPF pathogenesis. These cells promote the accumulation and proliferation of fibroblasts through several as yet undeciphered mechanisms and uneven deposition of extracellular matrix (ECM), culminating in irreversible lung parenchyma scarring and damage [6].
IPF patients are prone to abnormal structural changes in the pulmonary arteries, leading to pulmonary hypertension (PH) [7]. Past findings suggest lower blood vessel formation in fibrotic versus non-fibrotic areas, displaying considerable vascular heterogeneity across the IPF lung [8, 9]. Abnormal structural modifications of the vasculature such as complete occlusion or narrowing of the vessels by scarred tissues, plexiform lesions, proliferative intima and thickening of smooth muscle layers are some of the mainstays of IPF pathology [8, 10]. Interestingly, the low diffusing capacity of the lungs for carbon monoxide (DLCO) observed in IPF patients is also linked to PH development, a comorbidity observed in 30–80% of IPF patients [11]. Colombat et al. [12] suggested that occlusive venopathy in the non-fibrotic area of IPF lungs caused a reduction in pulmonary capillary blood volume, which was linked to lower DLCO in IPF-PH patients [13]. Furthermore, vascular remodelling was also observed to affect forced vital capacity (FVC) in IPF patients [14], though some studies have reported otherwise [11, 15].
Currently, there is little evidence on systematic morphometric analysis of arterial vascular remodelling in patients with IPF. For example, Parra et al. [14] morphometrically evaluated the internal luminal area and the perimeter of medium and large arteries in lung tissue from IPF patients. They suggested significant links between histological UIP patterns and vascular remodelling changes. Decreasing internal luminal area and increasing wall thickness of both medium and large arteries were mainly noted; but the study was semiquantitative [14]. A more recent study by Kinoshita et al. [16] used broadly classified arterial ranges to identify increases in individual layer thickness in IPF and idiopathic pleuroparenchymal fibroelastosis patients compared to control lungs tissues. The study, however, lacked association with physiological outcomes.
In the current study, we employed a comprehensive size-based classification approach to study normal and IPF arteries, and we provided data on absolute counts against a cohort of normal healthy controls. In addition, we analysed the thickness of each layer within arteries and have studied their effects on physiological function such as FVC, DLCO and smoking history in IPF patients. Our data indicate that increased deposition of elastin and collagen types I and IV could contribute to the arterial thickness in IPF lung. Finally, we illustrate the possible role of endothelial-to-mesenchymal transition (EndMT) activity in vascular remodelling.
Material and methods
Study population
Explant human lung tissues from 13 patients diagnosed with IPF were obtained during lung transplantation (Alfred Health Biobank Melbourne, ethics ID: 336–13); none were on anti-fibrotic treatment. All patients had pathologist-verified histopathology reports of UIP. In addition, tissues from 11 healthy normal control (NC) subjects consisting of small airways and parenchymal areas were provided by James Hogg Lung Registry, the University of British Columbia (ethics ID: H00-50110). This cohort consisted of patients who had died of causes other than pulmonary diseases. Detailed subject demographic information is provided in table 1. All functional data were collected before the lung transplantation.
TABLE 1.
Subject demographics and clinical characteristics
| Normal control | IPF | |
| Total number n | 11 | 13 |
| Age years | 41±15.1 | 64±5.06 |
| Sex (F/M) n | 6/5 | 6/7 |
| Body mass index kg·m−2 | NA | 26.69±3.03 |
| Smoking status: current/ex-smoker/never-smoker n | Nonsmokers | 0/7/6 |
| Smoking pack-years | 20.84±23.16 | |
| Lung physiology | ||
| FEV1 L | 1.70±0.40 | |
| FEV1 % predicted | 60.17±12.22 | |
| FVC L | 1.97±0.51 | |
| FVC % predicted | 53.5±12.98 | |
| DLCO mL·min−1·mmHg−1 | 5.91±2.92 | |
| DLCO corrected % predicted | 25.85±15.30 | |
Values given as mean±sd unless otherwise stated. IPF: idiopathic pulmonary fibrosis; F: female; M: male; NA: not available; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lungs for carbon monoxide.
Movat's Pentachrome staining
Formalin-fixed, paraffin-embedded 3.5-micron tissue sections were cut. First, dewaxing of the tissue sections was done in xylene twice for 3 min, followed by gradual hydration using 100%, 95%, 70% ethanol, and running tap water. Next, tissue sections were stained using a Movat Pentachrome staining kit (Modified Russell-Movat-ab245884; Abcam, Melbourne, Australia) as per manufacturer instructions. The staining differentiates arterial structural morphology based on colour, such as collagen (yellow to red), elastin (black-blue) and nuclei (blue), muscle (red), mucin (bright blue) and fibrin (bright red) (figures 1 and 2).
FIGURE 1.
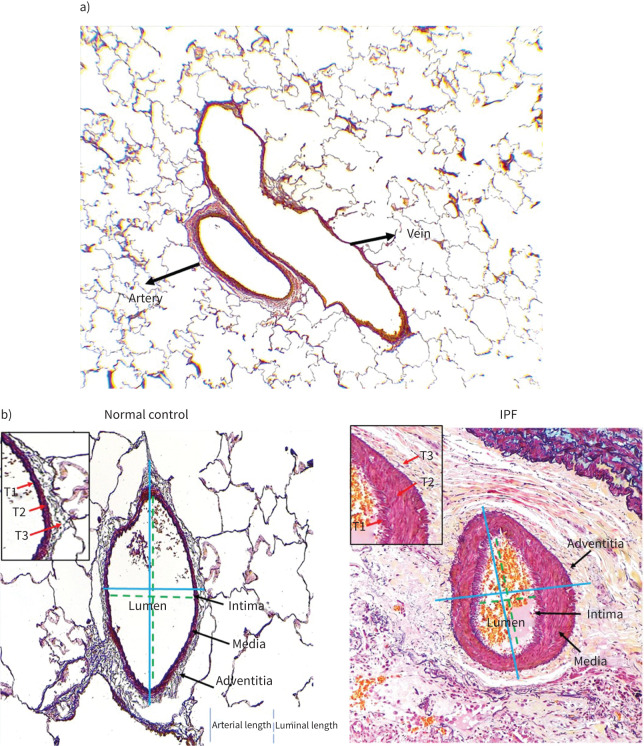
a) Illustration of the normal pulmonary artery and vein structure stained with Movat Pentachrome (4× magnification). The pulmonary arteries are well rounded in structure, while veins are elongated and irregular. b) Movat Pentachrome-stained normal control and idiopathic pulmonary fibrosis (IPF) of pulmonary arteries’ external and luminal length measurement (20× magnification). External length is measured from one end to the other end of the adventitia layer margin crossing the middle of the lumen, while the luminal length was measured from one end to another end of the intima layer margin. In the inset are layers of various thickness (T1 – intima, T2 – media and T3 – adventitia).
FIGURE 2.
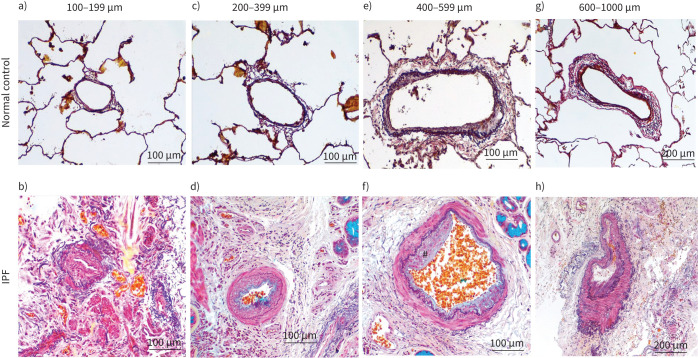
Representative images of Movat Pentachrome-stained pulmonary artery sizes for normal control and idiopathic pulmonary fibrosis (IPF): a, b) 100–199 μm, c, d) 200–399 μm, and e, f) 400–599 μm (20× magnification), and g, h) 600–1000 μm (10× magnification). The prominent intimal thickening and luminal narrowing in IPF patients across various sizes are noted. Also noted is the arterial adventitia area merging into the surrounding lung tissues in IPF patients. #: plexiform in part f.
Immunohistochemical staining for mesenchymal markers
Lung tissue sections were deparaffinised in xylene and antigen retrieval carried out using target retrieval citrate buffer pH 6.0 (Dako S2369) for 15 min. Tissues were immunostained with polyclonal rabbit anti-human S100A4 (1:1000; Dako A5114), mouse monoclonal VE-cadherin (CD144) (1:150; Thermofisher 14144982), vimentin, monoclonal mouse (1:200; Dako M7020), mouse monoclonal anti-N-cadherin (1:100; Abcam ab98952), monoclonal mouse anti-α-SMA (1:500, M0851, Dako), collagen type I (1:200, Abcam ab34710), rabbit polyclonal and collagen type IV (1:200, Abcam ab6586), rabbit polyclonal for 60 min followed by secondary HRP rabbit/mouse antibodies (Dako K5007) treatment for a further 30 min. The protein markers were visualised as brown after adding DAB substrate and counterstained for the nucleus with haematoxylin.
Pulmonary arterial, classification and counts
All images were captured using a Leica DM 500 microscope and Leica IC50W digital camera. Pulmonary arteries were first distinguished for morphometric analysis based on their rounded structure and thickness, with veins being thinner and elongated (figure 1a). The pulmonary arteries also had at least two defined elastin layers, while veins only had a single layer.
For arterial classification, measurements for at least five arteries of each size were carried out. Each arterial image from NC and IPF tissues was taken with a 4× objective following a vertical uni-direction, avoiding overlap. External length (one end to the other end of adventitia) and luminal length (one end to another end of intima layer margin) were measured (figure 1b) using measurement tools in the image ProPlus 7.0 software. Based on the size measurements, arteries were segregated into six groups, 100–1000 µm, interspaced at 100 µm, and total arterial length to luminal length ratios were calculated to determine the degree of vascular remodelling in IPF (figure 1b). In addition, the arterial numbers per mean per cent of tissue density were counted for all classified arterial sizes in IPF and NC.
Pulmonary total arterial and layer thickness measurement
The length of all three layers of arteries represented the total arterial thickness. To better assess arterial thickness across arterial sizes, separate magnifications were used; for instance, 63× objective for smaller 100–199 μm arteries, 40× for 200–399 μm, 20× for 400–599 μm and 10× for 600–1000 μm respectively. Similar magnifications were used to quantify thickness of individual layers. Subsequently, non-overlapping images were taken for all arterial sizes, and five images per arterial size per subject were randomly selected using an online random number generator. All the analysis was done with an observer (A.V.G.) blinded to subject and diagnosis.
Strategies for arterial thickness measurement were used as previously described [17]. In brief, the area of interest from each layer was manually drawn using imaging software tools. For intima measurements, thickness measurements from the outer luminal to the inner elastin layer were considered. Media layer thickness included the external layer of the inner elastin membrane and internal lining of the external elastin membrane, while areas from the media's external elastin layer to the arteries outermost connective tissue were considered adventitial thickness measurements. Based on arterial orientation, a horizontal, vertical or curved tool was selected using the software measurement tools, and the average distance between the selected layer margins was calculated using an automated distance calculator programme within the image ProPlus 7.0 software.
Pulmonary arterial elastin measurement
Image acquisition and randomisation were carried out similarly to thickness measurements. For total arterial measurements, the start of the outer end of the intima facing the lumen to the outer border of adventitia was manually selected using imaging software. For quantification of elastin, the total dark object was counted from the area of interest, and then elastin colour (black to blue/black) from the area of interest was counted. A similar strategy was used to measure intima, media and adventitia layer elastin. Percentage elastin was calculated using the following formula:
 |
Statistical analysis
All cross-sectional data were tested for their normal distributions using the D'Agostino–Pearson omnibus normality test. Analyses of variance were performed using ordinary one-way ANOVA using Bonferroni multiple comparison tests, which compared mean and standard deviation across all the groups of interest; specific group differences with correction for multiple comparisons were assessed using Dunn's test. Finally, for multi-variable correlations, we performed regression analyses using Spearman's rank test. All analysis was done using GraphPad prismV9, with a p-value ≤0.05 being considered significant.
Results
Morphological assessment of pulmonary arteries
Large and medium-size arterial numbers were significantly reduced in IPF compared to NC. Compared to arteries in control lungs, patients with IPF showed structural changes such as endothelial proliferation into the lumen, muscular hypertrophy of the intima and medial layer, proliferative intima and plexiform lesions. Also, excessive collagen and elastin deposition at adventitia was observed. Some of the arteries were completely remodelled and indistinguishably merged into surrounding tissues. Our data implicate that increased elastin and collagen deposition could contribute to the arterial thickness in IPF lung (figure 2).
Arterial structural changes and their number
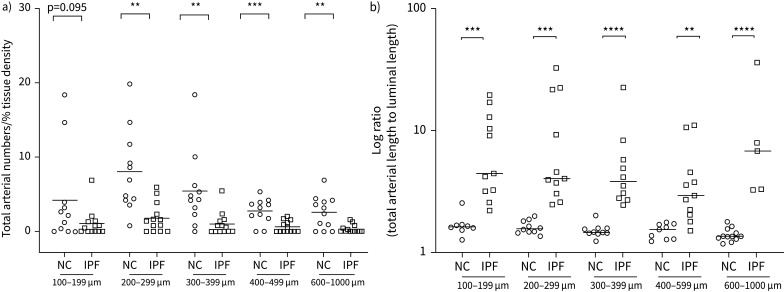
A significant decrease in arterial numbers was observed across medium and larger classified arterial ranges, i.e. 200–1000 µm range. Smaller artery numbers also trended lower in IPF but were non-significant compared to NC (figure 3a). The differences in total arterial length to lumen ratios in medium to larger 300–399 μm and 600–1000 μm size (p<0.0001) was more significant than 100–199 μm and 200–299 μm (p<0.001) arterial ranges compared to NC (figure 3b).
FIGURE 3.
a) Quantitative assessment of the total number of arteries in idiopathic pulmonary fibrosis (IPF) and normal control (NC) across classified arterial sizes and b) arterial length to luminal length log ratio of pulmonary arteries across the classified arterial sizes of IPF compared to NC. Data are presented as log ratio with unpaired t-test between each arterial classified size for NC and IPF. **p<0.01, ***p<0.001, ****p<0.0001 was considered significant.
We analysed relationships between arterial number, total arterial length to lumen ratios and DLCO. We noted that total arterial number positively correlated with DLCO % predicted (r’=0.56, p<0.05) (figure 4a), while the increase in arterial length to luminal ratios negatively correlated with DLCO % predicted (r’=−0.61, p=0.03) (figure 4b). Further, we also observed no significant differences between total arterial length to lumen ratios for arteries from fibrosed and non-fibrosed areas in IPF tissues (figure 4c).
FIGURE 4.
Correlations between a) total arterial numbers/mean per cent tissue density and % diffusing capacity of the lungs for carbon monoxide (DLCO); b) total arterial length to luminal length log ratio and % DLCO; and c) total arterial length to luminal length ratio for arteries from fibrosed and non-fibrosed area in idiopathic pulmonary fibrosis.
Total arterial and layer thickness in IPF patients
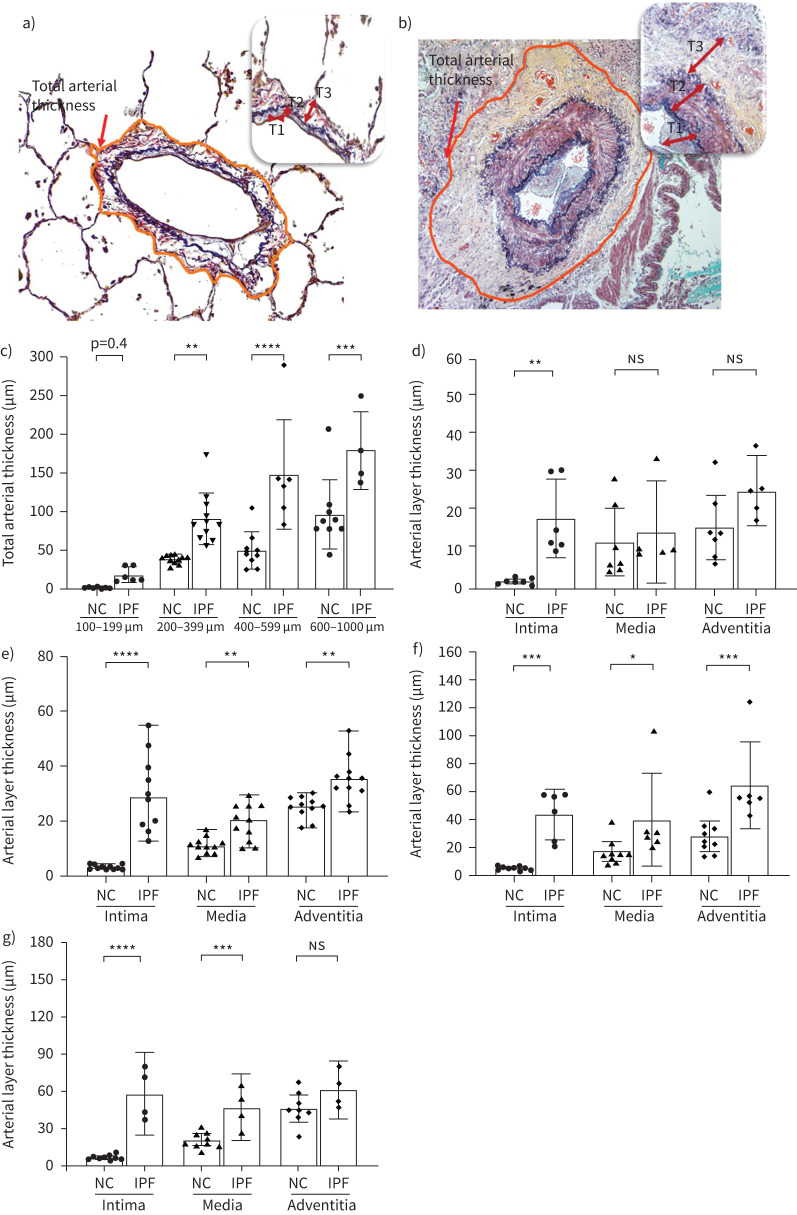
Thickness was measured across all the three arterial zones intima, media and adventitia in NC and IPF (figure 5a, b). Compared to NC, a two-fold increase in total thickness was found across arterial sizes, except the 100–199 μm range, wherein trends were higher, though insignificantly (figure 5c). Among individual arterial layers, intima showed a more significant fold difference in IPF than NC compared to media and adventitia. Intima was thicker across all measured arterial ranges in IPF patients, with greater significance observed in the arterial range of 200–399 μm and 600–1000 μm (p<0.0001), respectively, compared to NC (figure 5d–g). Within lower arterial ranges, both medial and adventitial thickness was distinguishably smaller in NC and IPF; however, the larger arterial range had significant increases in media with the greatest difference being in 600–1000 μm (p<0.001) and adventitia in 400–599 μm (p<0.001) in IPF arteries than NC.
FIGURE 5.
a, b) Arterial layer thickness measurement strategy in normal control (NC) and idiopathic pulmonary fibrosis (IPF). Total arterial thickness was measured by selecting total arterial circumference. Insets show layers of various thickness (T1 – intima, T2 – media and T3 – adventitia) thickness. Morphometric changes in arterial layers among c) total arterial thickness across arterial size 100–1000 µm, d) 100–199 µm, e) 200–399 µm, f) 400–599 µm and g) 600–1000 µm. All data are presented as multiple comparisons with ordinary one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 was considered significant. ns: nonsignificant.
Elastin deposition in arterial layers
The Movat Pentachrome stained elastin dark blue (figure 6a, b). In NC, elastin structures were well defined with inner and outer boundaries surrounding the media smooth muscle layer (figure 6a); however, elastin in IPF was disintegrated and scattered across and within the various layers (figure 6b). Quantitative analysis of the total arterial per cent elastin was significant across arterial ranges in IPF compared to NC except for 100–199 μm. The median value of per cent elastin in IPF subjects was 20% in small and mid-sized arteries, while larger arteries had close to 40% (figure 6c). In comparison, NC had significantly lower total elastin ranging from 5–10% across arterial sizes (figure 6c). Similar trends were observed in individual arterial layer analysis of elastin, with consistent increases seen across mid (200–399 µm (p<0.001), 400–599 µm (p<0.01)) and larger arteries (600–1000 µm; p<0.001); however, smaller arteries showed lower significance, probably due to considerable variability observed in both IPF and NC (figure 6d–g).
FIGURE 6.
a, b) Arterial layer elastin measurement strategy in normal control (NC) and idiopathic pulmonary fibrosis (IPF). The black colour represents the elastin count. Insets show various layers (E1 – intima, E2 – media and E3 – adventitia) of elastin. Morphometric changes in arterial layer elastin among c) total arterial elastin across arterial size 100–1000 µm, d) 100–199 µm, e) 200–399 µm, f) 400–599 µm and g) 600–1000 µm. Arterial elastin is expressed as a percentage. All data are presented as multiple comparisons with ordinary one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 was considered significant. ns: nonsignificant.
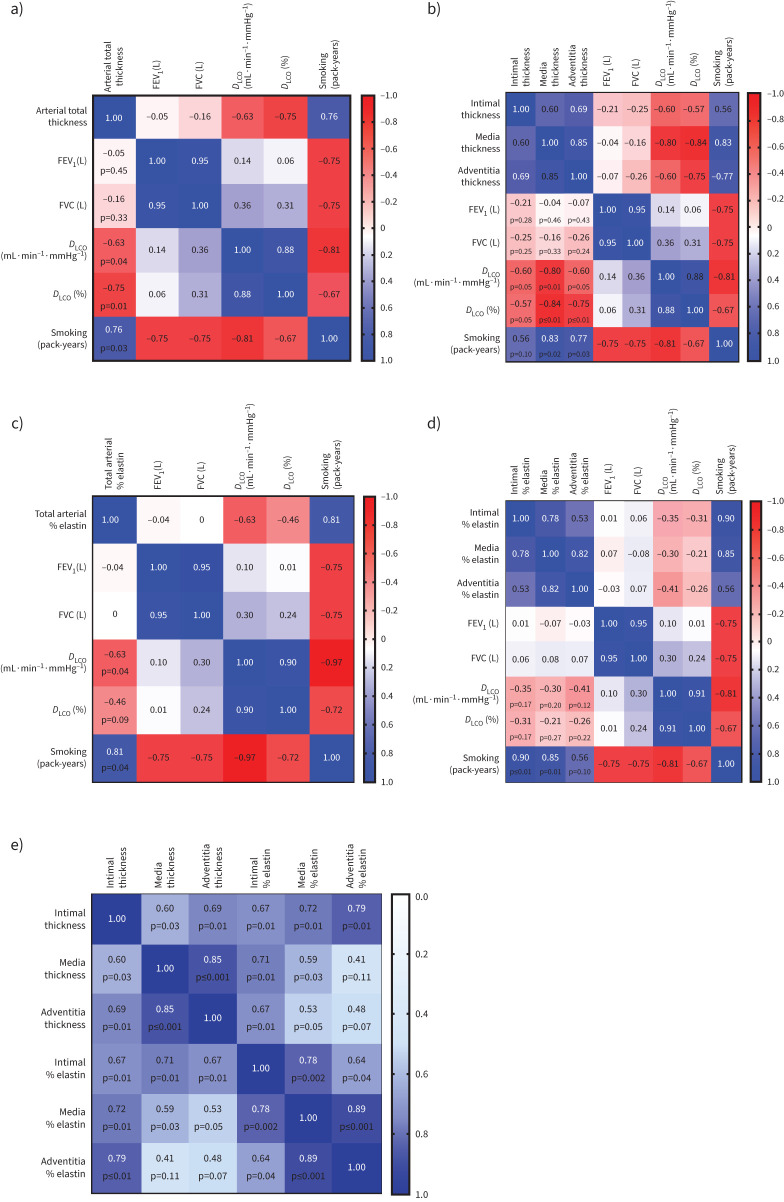
Arterial remodelling impact on lung physiology
We analysed relationships between arterial thickness, elastin deposition and lung physiological parameters through a multi-variable correlation matrix. We identified that both total thickness and total per cent elastin showed a significant negative correlation to DLCO % predicted (r’= −0.75, p=0.01) and (r’= −0.46, p=0.09), respectively (figure 7a, c). Individual layer thickness negatively correlated with DLCO; intima thickness versus DLCO % predicted (r’= −0.57, p=0.05); media thickness versus DLCO % predicted (r’= −0.84, p=<0.001) and adventitia thickness versus DLCO % predicted (r’= −0.75, p≤0.01). The total and individual layer thickness negatively correlated to FVC (L) insignificantly (figure 7b). Similar to total elastin, individual layers elastin also showed negative correlations DLCO % predicted (r’= −0.31, p=0.17) for intima, DLCO % predicted (r’= −0.21, p=0.27) for media and DLCO (%) predicted (r’= −0.26, p=0.22) for adventitia (figure 7d). Smoking (pack-years) affected both total and individual layer thicknesses and elastin deposition in IPF patients.
FIGURE 7.
Correlations matrix showing the impact on various measured indices on pulmonary function and smoking pack-years: a) pulmonary arterial total thickness, b) arterial individual layer thickness, c) pulmonary arterial total elastin, d) individual arterial layer elastin, and e) correlation between arterial thickness and arterial elastin across each arterial layer. Increased per cent elastin in each layer significantly correlated to their corresponding layer thickness. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lungs for carbon monoxide.
The correlation matrix between arterial elastin and thickness showed increased elastin percentage in each arterial layer correlated to their corresponding layer thickness (figure 7e).
Descriptive analysis of IPF arteries showed increase in EndMT marker expression
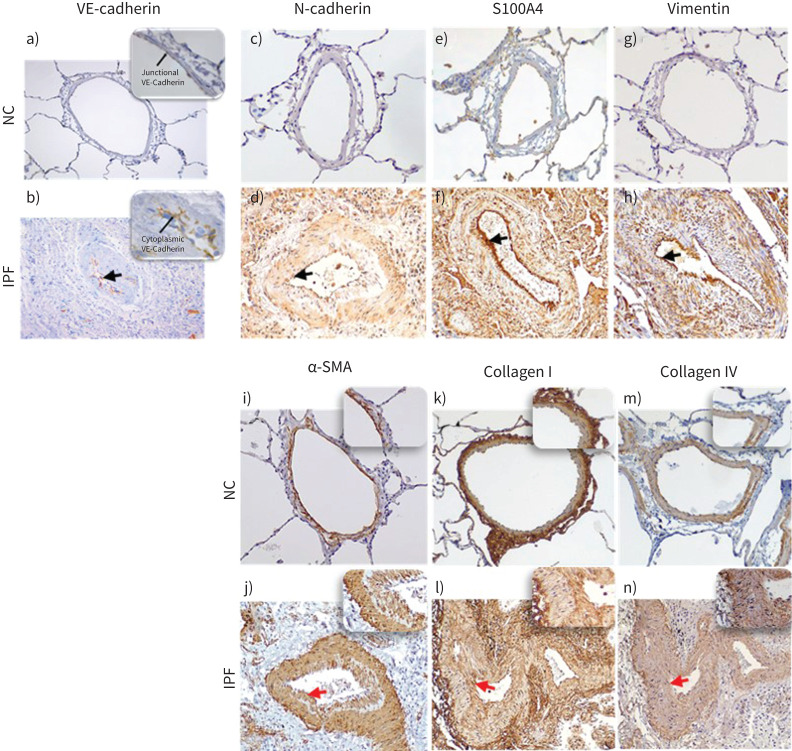
We observed increased expression of mesenchymal biomarkers N-cadherin, S100A4 and vimentin in the arterial layers (intima, media and adventitia) of IPF patients compared to NC, indicating the possible active transition of resident cells into mesenchymal traits (figure 8c–h). Furthermore, in NC, the endothelial VE-cadherin expression was explicitly observed at cell junctions, while in IPF, they were more cytoplasmic (figure 8a, b). We also observed in the arterial layers of IPF patients an increase in myofibroblast marker α-SMA and ECM proteins collagen type I and IV, especially pronounced in intimal areas compared to NC (figure 8i–n). In addition, the staining intensity of collagen type I was more enhanced and widespread across the tissue than collagen type IV, which was artery specific. Also, unlike IPF, the NC subject's collagen type IV was intact within arterial basement membrane (figure 8m, n).
FIGURE 8.
Descriptive images of immunohistochemically stained pulmonary arteries for VE-cadherin (magnification 20×): a) normal control (NC), b) idiopathic pulmonary fibrosis (IPF), in insets junctional and cytoplasmic expression of VE-cadherin in NC and IPF, respectively (100×). Staining images for: N-cadherin c) NC and d) IPF (20×); S100A4 e) NC and f) IPF; vimentin g) NC and h) IPF; α-SMA i) NC and j) IPF; collagen-I k) NC and l) IPF; and collagen-IV m) NC and n) IPF (all images taken in 20× magnification for medium-size arteries). The black arrows indicate mesenchymal protein expression in the intima, and the red arrows indicate α-SMA+ myofibroblast (in inset intima) and ECM protein: collagen I and collagen IV deposition (in inset intima).
Discussion
This novel study describes size-based morphological characteristics of the arterial vasculature in IPF, performed through systematic classification, using appropriate quantitative measurement strategies. Noticeably, IPF patients had extensive vascular remodelling changes through increased vascular wall thickening and disruptive ECM deposition. Interestingly, these arterial remodelling changes correlated inversely with lung physiological parameters such as DLCO and FVC, and IPF patients with smoking history had worse outcomes. Further, this study highlights a clear possibility that EndMT could have a critical contribution in driving these irreversible remodelling changes for the first time. Finally, the study highlights the noticeable impact of remodelled vasculature on DLCO, indicating PH complications in IPF patients.
The study noted key histopathological remodelling changes in IPF arteries, including extensive luminal occlusion, intimal proliferation, unevenly dispersed elastin fibres, collagen fragmentation and deposition across arterial layers. In addition, the adventitia was repeatedly found to be merged into surrounding fibrotic areas. Such features are classically reminiscent of arteries in IPF lungs and very similar as noted in PH-COPD and PH-IPF lung [18] pathology, suggesting a strong relationship between the two pathologies [12, 18]. Furthermore, we identified regression in numbers of medium and large muscular arteries in IPF patients, likely resulting from intense fibrotic remodelling damage or lack of physical space for further arterial expansion. Previous similar findings of such arterial obliteration have been featured in emphysema patients with severe PH [19]. Interestingly, our observation of a much lower decline in smaller arteries illustrates heterogeneity in arterial formation. Earlier findings on vascular formation in IPF are contradictory, with Keane et al. [20] demonstrating augmented angiogenic activity. In contrast, others have documented a decline in vessel formation, identified primarily through lower vascular endothelial growth factor levels in bronchoalveolar lavage of IPF patients [21, 22]. Also, our study found no apparent differences between total arterial thickness across the sizes in fibrotic areas and the more normal non-fibrotic areas. Although only a few areas with corresponding arterial sizes were available, our analysis suggests that such remodelling change, at least in the late stage of IPF, does not differ based on the fibrotic status and would represent the pathological changes in their entirety. Future studies with tissues from comparing early and late-stage fibrotic versus non-fibrotic areas could better predict pathological arterial remodelling.
The arterial architecture comprises three layers, the inner intima constituting endothelial cells, the medial smooth muscle layer bounded by elastin laminas and the outer adventitial, mainly connective tissues [23]. Any configurational change to these layers affects the overall oxygenation, leading to severe hypoxic conditions. Our assessment of the arteries revealed thickening of the layer across arterial sizes in IPF lungs compared to healthy controls. Specifically, our data suggest a more considerable intimal thickening than the media and adventitia layer across the arterial sizes than seen in healthy subjects. Few previous studies have made such extensive and critical analyses of IPF arteries. Our findings are in contrast to a previous study by Kinoshita et al. [16], who identified significantly more thickening in adventitia than in intima and media in their patient cohort, and, to an extent, are in concurrence with findings by Hoffman et al. [24] who identified an increase in intima and media but not in adventitia in the IPF-PF cohort of patients. However, both studies lacked essential correlation to human lung physiological parameters, which is critical in the clinical understanding of disease progression and has been addressed in the current study.
The heightened activity of the endothelium in the intima and their aggressive encroachment into luminal space can be attributed to their transformative ability into a more proliferative phenotype; however, molecular exploration into such endothelial cell changes in IPF is still warranted [24]. Several growth factors and signalling mechanisms, including transforming growth factor-β, hedgehog (SHH) and notch, are highly active in IPF tissues and are crucial to EndMT and proliferation [25, 26]. Similar factors could be contributing to medial and adventitial thickening, for which smooth muscle hypertrophy and fibroblast hyperplasia were exhibited in our study of IPF arteries [23, 27]. An interesting study by Arribas et al. [28] in a rat hypertension model showed that the adventitial thickening occurred well before changes in intima or media occurred, actively causing arterial remodelling and PH. The inter-dynamics of arterial layers and their cause-and-effect relationship with overall vascular thickening remain underexplored and require further understanding in IPF.
Elastin fibres maintain the structural integrity of the vasculature. In normal arteries, the internal and external elastin laminas consist of mature elastic fibres that offer elasticity to the vascular tissue. Their fragmentation or aberrant deposition can adversely affect elastic recoil and other vascular mechanical properties [29, 30]. Our study identified increased accumulation and elastin breakdown across different arterial layers and sizes in IPF patients. Our data support the observation of Kinoshita et al. [16] of medial elastosis in IPF patients; however, unlike their findings, our study showed more significant elastin deposition in the intimal layer than in media and adventitia, especially in medium and large arteries. The increased presence of elastin, either as fragments or elastic laminal thickening, can aid endothelial migration and enhance metalloprotease activities [31, 32]. Minkin et al. [33] demonstrated the elastin degradation products such as desmosine and isodesmosine in urine and blood samples from patients diagnosed with pulmonary arterial hypertension (PAH). The presence of elastin fragments in the circulation activates cytokines and associated signalling pathways that can further degrade elastic fibres [31]. Both elastin and collagen contribute to the arterial thickening and PAH development in humans [34]. Poiani et al. [35] in hypoxia-induced PH rat models showed increased accumulation of elastin and collagen in pulmonary arteries. Several in vitro studies have previously demonstrated that a poor oxygen environment is conducive to fibroblast proliferation and ECM accumulation [36, 37]
Lung physiological tests such as FVC and DLCO are used to assess IPF patient's lung function. In IPF patients, these lung function parameters are adversely affected. Reduced DLCO out of proportion to the underlying pulmonary fibrosis is a sign of concomitant PH [27]. Nadrous et al. [38] also observed frequent PH in advanced IPF correlated with low DLCO and low resting and arterial oxygen tension. Our study data confirm significant vascular remodelling changes such as increased arterial thickness, and elastin negatively influenced DLCO and FVC in IPF patients. Reduction in arterial numbers and luminal size was also observed to impact DLCO adversely. Occlusive venopathy has also been observed in non-fibrotic lung areas of IPF patients, suggesting that the reduction in pulmonary capillary blood volume is due to occlusive venopathy and related to low DLCO values in the IPF-PH patients [12]. We also identified that smoking impacted arterial thickness and elastin deposition similar to that reported in COPD patients associated with PH comorbidities [39].
Our descriptive observations indicate the increased presence of mesenchymal cells in the intima, media and adventitia layers, implying mesenchymal transformation in vascular cells [7]. The intimal mesenchymal marker expression was the highest among the three layers, indicating possible endothelial cell transition through the EndMT process [40–43]. In media and adventitia, the presence of mesenchymal cells markers such as N-cadherin, Vimentin and S100A4 was intriguing and pointed towards either resident cell transformation to mesenchymal cells or active inward migration of transitioning endothelial cells; however, these observations need further in-depth verification. Interestingly, we also observed increased cytoplasmic expression rather than junctional of VE-cadherin in IPF endothelial cells, indicating possible active cellular uptake of this protein. Furthermore, we demonstrate an increase in α-SMA+ expression prominently in the intimal layer of IPF arteries, suggesting inadvertent myofibroblast proliferation. The myofibroblasts are a highly active form of fibroblast known to aberrantly secrete ECM protein, causing thickening and stiffening of tissues [17]. The increase in collagen type I and IV's aberrant and disruptive deposition is possibly linked to αSMA+ myofibroblasts; however, the source of these cells requires further investigation. Together with other cell types, transitioning endothelial cells could majorly contribute to the arterial remodelling and fibrotic pathology observed in IPF. Thus, we postulate the substantial involvement of EndMT in arterial remodelling and its possible role in PH and other lung complications.
A limitation of this study is that the number of IPF and NC samples varied for size-based comparison, as some arterial sizes were not found in IPF and NC tissues. Another limitation is the non-availability of cardiac function data or a clinical diagnosis of PH for the IPF patients studied, and as such, these associations could not be established in this study. However, the negative correlation of vascular remodelling with DLCO indicates possible PH in IPF patients.
In summary, this study provides important morphometric data on the remodelling of muscular arteries in the IPF lung. More importantly, we have identified that such change directly resulted in physiological change, deficient oxygen absorption and reduced lung capacity. EndMT seems central to IPF pathology and could be a novel therapeutic target. Thus, our data would support the need for diagnosis and therapeutic options for vascular remodelling, which might occur with IPF prognosis and future complications.
Acknowledgements
Archana Vijay Gaikwad, Wenying Lu and Mathew Suji Eapen are thankful to the National Health and Medical Research Council Centre of Research Excellence in Pulmonary Fibrosis (Australia) for the CREATE Fellowship.
Provenance: Submitted article, peer reviewed.
Conflict of interest: S.S. Sohal reports personal fees for lectures from Chiesi outside the submitted work. All the other authors do not have any conflict of interest to declare.
Support statement: This work was supported by research grants from Clifford Craig Foundation, Launceston General Hospital and Lung Foundation Australia. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018; 378: 1811–1823. doi: 10.1056/NEJMra1705751 [DOI] [PubMed] [Google Scholar]
- 2.Eapen MS, Gaikwad AV, Thompson IE, et al. . The effectiveness of immunosuppressive cyclosporin in attenuating the progression of interstitial lung diseases. J Thorac Dis 2019; 11: S1139–S1142. doi: 10.21037/jtd.2019.04.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glassberg MK. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am J Manag Care 2019; 25: Suppl. 11, S195–S203. [PubMed] [Google Scholar]
- 4.Raghu G, Remy-Jardin M, Myers JL, et al. . Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389: 1941–1952. doi: 10.1016/S0140-6736(17)30866-8 [DOI] [PubMed] [Google Scholar]
- 6.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015; 24: 102–114. doi: 10.1183/09059180.00003214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghigna M-R, Dorfmüller P. Pulmonary vascular disease and pulmonary hypertension. Diagn Histopathol 2019; 25: 304–312. doi: 10.1016/j.mpdhp.2019.05.002 [DOI] [Google Scholar]
- 8.Farkas L, Gauldie J, Voelkel NF, et al. . Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 2011; 45: 1–15. doi: 10.1165/rcmb.2010-0365TR [DOI] [PubMed] [Google Scholar]
- 9.Barratt S, Millar A. Vascular remodelling in the pathogenesis of idiopathic pulmonary fibrosis. QJM 2014; 107: 515–519. doi: 10.1093/qjmed/hcu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan SD, Shlobin OA, Ahmad S, et al. . Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration 2008; 76: 288–294. doi: 10.1159/000114246 [DOI] [PubMed] [Google Scholar]
- 11.Lettieri CJ, Nathan SD, Barnett SD, et al. . Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. doi: 10.1378/chest.129.3.746 [DOI] [PubMed] [Google Scholar]
- 12.Colombat M, Mal H, Groussard O, et al. . Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol 2007; 38: 60–65. doi: 10.1016/j.humpath.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Sakao S, Tanabe N, Tatsumi K. Hypoxic pulmonary vasoconstriction and the diffusing capacity in pulmonary hypertension secondary to idiopathic pulmonary fibrosis. J Am Heart Assoc 2019; 8: e013310.doi: 10.1161/JAHA.119.013310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra ER, David YR, Costa L, et al. . Heterogeneous remodeling of lung vessels in idiopathic pulmonary fibrosis. Lung 2005; 183: 291–300. doi: 10.1007/s00408-004-2542-z [DOI] [PubMed] [Google Scholar]
- 15.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 2007; 175: 875–880. doi: 10.1164/rccm.200608-1153CC [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita Y, Ishii H, Kushima H, et al. . Remodeling of the pulmonary artery in idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2020; 10: 306. doi: 10.1038/s41598-019-57248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eapen MS, Lu W, Hackett TL, et al. . Increased myofibroblasts in the small airways, and relationship to remodelling and functional changes in smokers and COPD patients: potential role of epithelial–mesenchymal transition. ERJ Open Res 2021; 7: 00876-2020. doi: 10.1183/23120541.00876-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958; 18: 533–547. doi: 10.1161/01.CIR.18.4.533 [DOI] [PubMed] [Google Scholar]
- 19.Adir Y, Shachner R, Amir O, et al. . Severe pulmonary hypertension associated with emphysema: a new phenotype? Chest 2012; 142: 1654–1658. doi: 10.1378/chest.11-2816 [DOI] [PubMed] [Google Scholar]
- 20.Keane MP, Belperio JA, Burdick MD, et al. . ENA-78 is an important angiogenic factor in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001; 164: 2239–2242. doi: 10.1164/ajrccm.164.12.2104106 [DOI] [PubMed] [Google Scholar]
- 21.Meyer KC, Cardoni A, Xiang Z-Z. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med 2000; 135: 332–338. doi: 10.1067/mlc.2000.105618 [DOI] [PubMed] [Google Scholar]
- 22.Tzouvelekis A, Anevlavis S, Bouros D. Angiogenesis in interstitial lung diseases: a pathogenetic hallmark or a bystander? Respir Res 2006; 7: 82. doi: 10.1186/1465-9921-7-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomashefski JF, Dail DH. Da il and Hammar's Pulmonary Pathology, Vol. 1. New York, Springer, 2008. [Google Scholar]
- 24.Hoffmann J, Wilhelm J, Marsh LM, et al. . Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med 2014; 190: 98–111. doi: 10.1164/rccm.201401-0037OC [DOI] [PubMed] [Google Scholar]
- 25.Gaikwad AV, Eapen MS, McAlinden KD, et al. . Endothelial to mesenchymal transition (EndMT) and vascular remodeling in pulmonary hypertension and idiopathic pulmonary fibrosis. Expert Rev Respir Med 2020; 14: 1027–1043. doi: 10.1080/17476348.2020.1795832 [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Philp AM, Corte T, et al. . Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther 2021; 225: 107839. doi: 10.1016/j.pharmthera.2021.107839 [DOI] [PubMed] [Google Scholar]
- 27.Ruffenach G, Hong J, Vaillancourt M, et al. . Pulmonary hypertension secondary to pulmonary fibrosis: clinical data, histopathology and molecular insights. Respir Res 2020; 21: 303. doi: 10.1186/s12931-020-01570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arribas SM, Hillier C, González C, et al. . Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension 1997; 30: 1455–1464. doi: 10.1161/01.HYP.30.6.1455 [DOI] [PubMed] [Google Scholar]
- 29.Karnik SK, Brooke BS, Bayes-Genis A, et al. . A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003; 130: 411–423. doi: 10.1242/dev.00223 [DOI] [PubMed] [Google Scholar]
- 30.Roger Parra E, Adib Kairalla R, de Carvalho CRR, et al. . Abnormal deposition of collagen/elastic vascular fibres and prognostic significance in idiopathic interstitial pneumonias. Thorax 2007: 62: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cocciolone AJ, Hawes JZ, Staiculescu MC, et al. . Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol 2018; 315: H189–H205. doi: 10.1152/ajpheart.00087.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skeie JM, Mullins RF. Elastin-mediated choroidal endothelial cell migration: possible role in age-related macular degeneration. Invest Ophthalmol Vis Sci 2008; 49: 5574–5580. doi: 10.1167/iovs.08-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minkin R, Grosu H, Tartell L, et al. . Detection of elastin degradation products in the blood and urine of patients with pulmonary arterial hypertension. Chest 2010; 138: Suppl. 4, 891A. doi: 10.1378/chest.10663 [DOI] [Google Scholar]
- 34.Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 2001; 22: 433–449. doi: 10.1016/S0272-5231(05)70282-3 [DOI] [PubMed] [Google Scholar]
- 35.Poiani GJ, Tozzi CA, Yohn SE, et al. . Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res 1990; 66: 968–978. doi: 10.1161/01.RES.66.4.968 [DOI] [PubMed] [Google Scholar]
- 36.Das M, Dempsey EC, Bouchey D, et al. . Chronic hypoxia induces exaggerated growth responses in pulmonary artery adventitial fibroblasts: potential contribution of specific protein kinase c isozymes. Am J Respir Cell Mol Biol 2000; 22: 15–25. doi: 10.1165/ajrcmb.22.1.3536 [DOI] [PubMed] [Google Scholar]
- 37.Falanga V, Kirsner RS. Low oxygen stimulates proliferation of fibroblasts seeded as single cells. J Cell Physiol 1993; 154: 506–510. doi: 10.1002/jcp.1041540308 [DOI] [PubMed] [Google Scholar]
- 38.Nadrous HF, Pellikka PA, Krowka MJ, et al. . The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest 2005; 128: Suppl. 6, 616s–617s. doi: 10.1378/chest.128.6_suppl.616S [DOI] [PubMed] [Google Scholar]
- 39.Peinado VI, Pizarro S, Barberà JA. Pulmonary vascular involvement in COPD. Chest 2008; 134: 808–814. doi: 10.1378/chest.08-0820 [DOI] [PubMed] [Google Scholar]
- 40.Sohal SS. Endothelial to mesenchymal transition (EndMT): an active process in chronic obstructive pulmonary disease (COPD)? Respir Res 2016; 17: 20. doi: 10.1186/s12931-016-0337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohal SS. Epithelial and endothelial cell plasticity in chronic obstructive pulmonary disease (COPD). Respir Investig 2017; 55: 104–113. doi: 10.1016/j.resinv.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 42.Eapen MS, Myers S, Lu W, et al. . sE-cadherin and sVE-cadherin indicate active epithelial/endothelial to mesenchymal transition (EMT and EndoMT) in smokers and COPD: implications for new biomarkers and therapeutics. Biomarkers 2018; 23: 709–711. doi: 10.1080/1354750X.2018.1479772 [DOI] [PubMed] [Google Scholar]
- 43.Eapen MS, Lu W, Gaikwad AV, et al. . Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur Respir J 2020; 56: 2003167. doi: 10.1183/13993003.03167-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]