Abstract
Despite the introduction of antiretroviral therapy (ART), HIV-associated pulmonary complications remain prevalent in children following perinatal HIV infection. In the post-ART era the incidence of opportunistic infections has decreased; however, non-infectious complications including diminished lung function are common. It is unclear whether early initiation of ART influences lung function later in life.
We performed a cross-sectional study examining pulmonary function tests (PFT) (spirometry, plethysmography, carbon monoxide diffusing capacity) in HIV-unexposed (HU), HIV-exposed-uninfected (HEU) and perinatally HIV-infected children on early ART (HIV+) recruited from the Cape Town arms of the CHER and IMPAACT 1060 trials. PFT was performed once children could participate (October 2013 to January 2020). Global Lung Initiative reference software was used for Z-standardisation of lung function by sex, age and height.
In total 394 children (HU n=90, HEU n=162, HIV+ n=142) underwent PFT, median age 8.7 (IQR 7.7–9.8) years. HIV+ had ART initiated at a median age of 17.6 (8.0–36.7) weeks. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC Z-scores were similar in all groups. Plethysmography demonstrated air-trapping with increased total lung capacity (TLC), functional residual capacity, residual volume (RV) and RV/TLC Z-scores in HIV+. There were no differences in alveolar volume; however, diffusing capacity was increased in HIV+.
Our findings indicate that following perinatal HIV infection, early ART may attenuate HIV-associated lung disease and is associated with normal childhood spirometry. However plethysmography demonstrates that small airway dysfunction is more pronounced in HIV+. Longitudinal follow-up is required to assess if these children are at risk of obstructive airway disease later in life.
Short abstract
Early ART benefits childhood lung function. Perinatally HIV-infected children with early ART have similar spirometry to HIV unexposed. However, they may be at risk of obstructive lung disease later in life due to small airway dysfunction. https://bit.ly/3oIBGow
Introduction
Lung disease remains a major complication of perinatal HIV infection. While antiretroviral therapy (ART) has decreased the incidence of opportunistic and recurrent infections, chronic lung disease and poor lung function persist in HIV-infected children [1–5]. Furthermore, the influence of perinatal HIV exposure on lung function later in life is not fully understood. HIV exposure without infection increases the risk of severe infection and immunological dysfunction early in life, which may disrupt lung function [6–8].
Lung involvement is thought to occur early in life with multiple mechanisms implicated. These include the human immunodeficiency virus itself, immunological dysfunction, pulmonary inflammation and respiratory infection [3, 9–11]. ART addresses several of these factors by decreasing the pulmonary viral reservoir and limiting recurrent lung infections. Thus the manifestation of HIV-associated lung disease has changed with the introduction of ART. Lung disease is now subtle, as structural changes and pulmonary infection are being replaced by chronic functional impairment. However, despite advances in ART, lung disease remains a significant morbidity in HIV+ [5, 12].
The effect of ART on HIV-associated lung disease and lung function remains unclear. There are no data examining the influence of the timing of ART initiation and childhood lung function. Available studies have examined children in whom ART was initiated late in childhood with lung function mostly performed in the second decade of life. These children had impaired lung function with small airway disease and airway obstruction being the predominant abnormalities [3, 13, 14]. This obstructive lung function phenotype tracked with age and did not improve over time [15].
Longitudinal studies of children with non-infectious lung disease suggest that a significant proportion of COPD has its origin early in life [16, 17]. Individuals who fail to reach peak expected lung function in the third decade of life are at increased risk for COPD. There is limited information on lung function in HIV+ children, and current data may not reflect lung function in those who began ART early in life. Similarly, there is insufficient data to demonstrate the lung function trajectory in HIV+ children and whether early ART influences this trajectory. Thus it remains to be seen whether the early introduction of ART allows for children with HIV to reach their full lung function potential.
To address the lack of knowledge on the effect of early ART and perinatal HIV exposure on childhood lung function, we compared childhood lung function in children with HIV and early ART, perinatal HIV exposure without infection, and children not exposed to HIV. Our hypothesis was that the early ART introduction would limit HIV-associated lung disease and permit normal development of childhood lung function in HIV+.
Methodology
We analysed cross-sectional data of baseline lung function in children with HIV (HIV+), HIV-exposed-uninfected (HEU) and HIV-unexposed (HU) children at Tygerberg Children's Hospital (Cape Town, South Africa), an academic referral hospital in a middle-income country with a high prevalence of HIV infection. Children were recruited from community clinics from a similar socioeconomic, demographic and geographic population in the Cape Town metropole.
HIV+: perinatal HIV infection diagnosed by a positive polymerase-chain-reaction (PCR) test for HIV-1 DNA and a plasma HIV-1 RNA viral load >1000 copies·mL−1 at 4 weeks of age for the participants from the CHER trial [2], or in the 1060/1104s trial HIV+ by having been diagnosed with HIV infection or having had an AIDS defining illness within 60 days of life, and a baseline HIV-1 RNA viral load above 5000 copies·mL−1 at enrolment [18].
Perinatal HIV exposure (HEU): maternal HIV infection without perinatal infection of the infant. HIV infection excluded between 12 and 24 weeks of age by a negative PCR for HIV-1 DNA.
Control-HIV unexposed (HU); no maternal HIV infection or postnatal HIV exposure. HIV infection was excluded by PCR testing for HIV-1 DNA at 24 weeks of age.
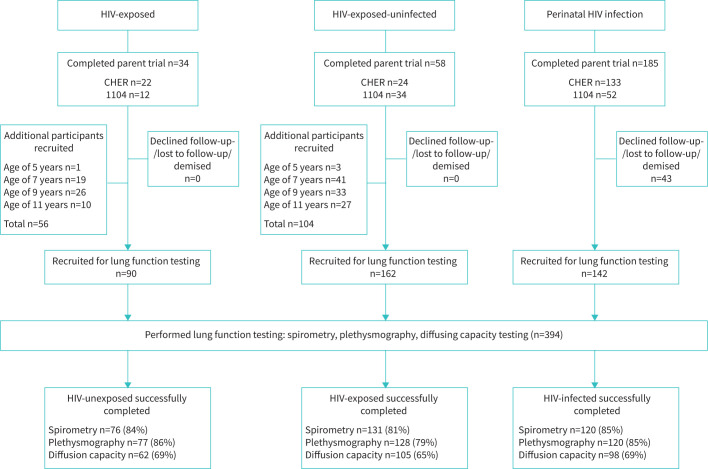
All HIV+ were previously enrolled in one of two randomised, controlled trials (CHER and IMPAACT 1060) and were recruited upon exit of the parent trial (CHER, September 2013; IMPAACT 1060, September 2015), and followed until able to participate in pulmonary function testing (PFT) [2, 18, 19]. 34 of the HU and 58 of the HEU were recruited from one of the parent trials (figure 1). Additional age-matched HU (n=56) and HEU (n=104) were recruited between October 2013 and December 2014. HU were recruited from vaccination centres and outpatient clinics that served the HIV+ participants and were without known lung disease or recent lung infection. HEU were enrolled from the same household, family or community as the HIV+ children; these children were included in a later neurocognitive substudy [20]. All HU and HEU participants had HIV infection excluded by ELISA for HIV antibodies upon recruitment to this study. PFT was performed annually from September 2013 to March 2020. Informed consent was obtained for all participants. In this study we report the results of the first PFT of these children. The analysis on longitudinal lung function data is ongoing.
FIGURE 1.
Participants recruited to perform pulmonary function tests and proportion who successfully completed spirometry, plethysmography and diffusing capacity testing.
The design, recruitment, methodology and outcomes of CHER and IMPAACT 1060 have previously been published [2, 19, 21, 22]. CHER compared early time-limited ART to ART deferred according to the 2006 World Health Organisation ART guidelines [23]. Those randomised to early time-limited arms began ART at <12 weeks of age. First-line ART was lopinavir-ritonavir, zidovudine and lamivudine. Of 133 participants completing the CHER trial (2011), 31 declined study participation, moved away or had died. Of the remaining participants, 12 were lost to follow-up prior to PFT.
IMPAACT 1060 compared nevirapine to lopinavir-ritonavir as a third antiretroviral in addition to zidovudine and lamivudine in children with HIV between 6 months and 3 years of age [18, 19]. These children then participated in a later neurocognitive substudy.
This study was performed under the approval and oversight of Stellenbosch Human Research Ethics Committee (#N12/11/076).
Anthropometry
Weight and height were measured at the time of PFT (supplementary methods). Weight-for-age, height-for-age Z-scores were calculated according to World Health Organization (WHO) Child Growth Standards using the WHO Anthro Survey Analyser (WHO AnthroPlus: https://www.who.int/growthref/tools/en/)
Haematological investigations
HIV viral load and CD4 count was performed annually in HIV+. HIV viral suppression was defined as a HIV viral load <400 viral copies·mL−1.
Pulmonary function testing
PFT was performed at Tygerberg Hospital by a single trained respiratory technologist, according to American Thoracic Society and European Respiratory Society standards and guidelines using the Jaeger MasterScreen-PFT and Master Screen Body with Labmanager version 5.32.0.5 (Carefusion Germany 234 GmbH, Hoechberg, Germany) [24–26]. All participants underwent spirometry to measure forced expiratory flow in 1 s (FEV1) and forced vital capacity (FVC). The best of a minimum of three acceptable spirometry manoeuvres is reported. Body plethysmography was performed to calculate lung volumes and capacities including total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC) and vital capacity (VCPLETH). Single-breath diffusion capacity testing was introduced from October 2013 to measure the alveolar volume (VA), diffusing capacity of the lung for carbon monoxide (DLCO)/VA and DLCO. DLCO was not normalised to haemoglobin concentration. The mean of a minimum of two technically acceptable body plethysmography and single-breath CO diffusing capacity tests is reported.
Statistical analysis
Data were analysed using Prism 9.0.1 (GraphPad). Clinical and anthropometric data were summarised using descriptive statistics. Z-standardisation of lung function parameters was performed using Global Lung Initiative (GLI) reference data and software [27–29]. Z-scores of spirometry data wer estimated using the ‘Other’ ethnic reference population which has been demonstrated to best fit South African participants of African and mixed ethnic origin [30]. Plethysmography and diffusing capacity Z-scores were estimated using Caucasian reference values as no data for other ethnic groups are available. QQ plots were used to visually examine the distribution of quantitative lung function data. We performed multiple comparisons between all groups: HU to HEU, HU to HIV+ and HEU to HIV+. Normally distributed data were compared using one-way ANOVA with post hoc Tukey's correction for multiple comparisons and is summarised as mean±sd. Data which are not normally distributed were compared using the Kruskal–Wallis test with post hoc Dunn's correction for multiple comparisons and are reported as median with interquartile range. Where HIV+ pulmonary function Z-score differed significantly from HU or HEU, the mean difference and 95% confidence intervals or mean rank difference were calculated. Post hoc linear regression was performed to examine if an association between ART initiation and pulmonary function outcome was present.
Results
We recruited a total of 394 children for PFT (figure 1). Baseline demographic and clinical characteristics are summarised in table 1. HIV+were lighter for age than HU (p=0.0186) and shorter for age than HEU (p=0.0187). The anthropometry of HU and HEU was similar.
TABLE 1.
Characteristics of participants
| HIV-unexposed (HU) | HIV-exposed (HEU) | HIV-infected (HIV+) |
HU versus HIV+
(p-value) |
HEU versus HIV+
(p-value) |
|
| Subjects n | 90 | 162 | 142 | ||
| Age years | 8.8 (7.8–10.5) | 9.0 (7.9–10.6) | 8.4 (7.6–8.8) | p=0.0004 | p<0.0001 |
| Ethnicity n (%) | African 90 (100) | African 147 (91) Mixed 15 (9) |
African 129 (91) Mixed 13 (9) |
||
| Height cm | 128.3 (122.5–138.8) | 129.5 (124.1–139.0) | 123.8 (119.0–128.5) | p<0.0001 | p<0.0001 |
| Height-for-age Z-score | −0.56±1.1 | −0.53±1.09 | −0.87±1.00 | p=0.0848 | p=0.0187 |
| Weight kg | 27.75 (23.50–34.98) | 27.2 (23.9–33.2) | 24.3 (22.0–26.7) | p<0.0001 | p<0.0001 |
| Weight-for-age Z-score | −0.09±1.22 | −0.22±1.19 | −0.51±0.99 | p=0.0186 | p=0.0758 |
| Age at ART initiation weeks | 17.6 (8.0–36.7) | ||||
| Time interval years, first ART exposure to PFT | 7.65 (7.35–8.37) | ||||
| CD4 count at PFT (n=126) cells·mm−3 | 1065 (786–1298) | ||||
| HIV viral load at PFT (n=108) copies·mL−1 | 39 (19–302) | ||||
| HIV viral suppression at PFT (%) | 100 (92.6) |
Data are presented as mean±sd if normally distributed, median (interquartile range) if not normally distributed, unless otherwise stated. HIV viral suppression was defined as a HIV viral load <400 viral copies·mL−1. Compared with either one-way ANOVA with post hoc Tukey's multiple comparison test (normal distribution), Kruskal–Wallis with post hoc Dunn's multiple comparison test (not normally distributed) and Chi-square (categorical data). No statistically significant differences between HU and HEU. ART: antiretroviral therapy; PFT: pulmonary function test.
Vertical transmission prophylaxis and ART
Mothers with HIV and their infants received vertical transmission prophylaxis (VTP) according to local guidelines. Zidovudine was administered to mothers from 34 weeks’ gestation and to neonates for 7 days with a single dose of antepartum nevirapine during labour and to the newborn infant [31]. All HIV+ children received ART, and mothers with CD4 counts below 250 cells·mm−3 received combination ART. The median age of ART initiation was 17.6 (range 5.86–155.86, IQR 8.0–36.7) weeks and median duration from initiation of ART to PFT was 7.65 (IQR 7.35–8.37) years (table 1). Of the CHER participants, 46 had uninterrupted ART (median age 10.86 weeks, IQR 7.57–25.50); of these 32 had early ART initiation (<12 weeks of age) and 24 had deferred ART initiation (median age 33.79 weeks, IQR 22.25–41.07). 46 CHER participants had planned early interrupted ART according to the study protocol (median interruption duration 225 days, IQR 129–354) (figure 2). The median age of ART initiation of P1060 participants was 37.79 weeks (IQR 28.97–74.65) (figure 2). Viral load data were available in 108 (76.1%) with 100 being virologically suppressed (median viral load 39 copies·mL−1, IQR 19–302).
FIGURE 2.
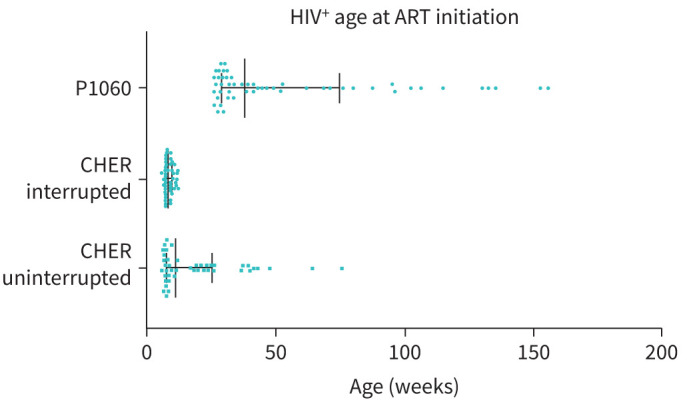
Age at antiretroviral therapy (ART) initiation in perinatally HIV-infected (HIV+) children. Data displayed as median (interquartile range).
Lung function tests
Of the 394 recruited participants, 327 (83%) successfully completed spirometry, 325 (82%) completed plethysmography and 265 (67%) diffusing capacity (figure 1). Z-scores were calculated for lung function outcomes to account for the influence of age, height and sex and allow comparison between HU, HEU and HIV+.
Spirometry (table 2, figure 3)
TABLE 2.
Successfully completed lung function tests of HIV-unexposed (HU), HIV-exposed-uninfected (HEU) and perinatally HIV-infected children on early ART (HIV+)
| HU | HEU | HIV+ | HU versus HIV+ (p-value) | HEU versus HIV+ (p-value) | |
| Spirometry | |||||
| Subjects n | 76 | 131 | 120 | ||
| FEV1 absolute L | 1.380 (1.205–1.648) | 1.430 (1.200–1.710) | 1.250 (1.110–1.450) | p=0.0057 | p=0.0001 |
| FEV1 Z-score | −0.892±0.932 | −0.786±1.125 | −0.570±1.083 | p=0.1001 | p=0.2456 |
| FVC absolute L | 1.550 (1.32–1.813) | 1.570 (1.340–1.860) | 1.355 (1.195–1.598) | p=0.0009 | p=0.0002 |
| FVC Z-score | −0.894±0.898 | −0.953±1.196 | −0.669±1.207 | p=0.3695 | p=0.1199 |
| FEV1/FVC absolute | 0.89 (0.853–0.940) | 0.914 (0.863–0.962) | 0.909 (0.879–0.954) | p=0.1771 | p>0.9999 |
| FEV1/FVC Z-score | −0.031±1.284 | 0.301±1.205 | 0.1551±1.076 | p=0.5297 | p=0.5891 |
| Plethysmography | |||||
| Subjects n | 77 | 128 | 120 | ||
| Total lung capacity (TLC) L | 2.481±0.487 | 2.467±0.561 | 2.258±0.3524 | p=0.0041 | p=0.0018 |
| TLC Z-score | −0.2194±1.050 | −0.406±1.178 | 0.2906±0.9855 | p=0.0039 | p<0.0001 |
| Residual volume (RV) L | 0.8612±0.2584 | 0.8746±0.2537 | 0.8734±0.2257 | p=0.9373 | p=0.9992 |
| RV Z-score | 0.6125±0.7607 | 0.6251±0.7185 | 0.9497±0.6517 | p=0.0034 | p=0.001 |
| RV/TLC | 34.75±8.153 | 35.56±9.210 | 38.69±8.441 | p=0.0058 | p=0.0139 |
| RV/TLC Z-score | 1.399±0.8341 | 1.489±1.059 | 1.733±0.8190 | p=0.0371 | p=0.0995 |
| Functional residual capacity (FRC) L | 1.28 (1.13–1.55) | 1.33 (1.15–1.59) | 1.18 (1.06–1.36) | p=0.0135 | p=0.0001 |
| FRC Z-score | 0.3676±0.9391 | 0.4456±0.8829 | 0.7156±0.8926 | p=0.0234 | p=0.0504 |
| Vital capacity (VCPLETH) L | 1.540 (1.285–1.860) | 1.530 (1.290–1.825) | 1.380 (1.195–1.598) | p=0.0011 | p=0.0008 |
| VCPLETH Z-score | −2.179±0.8135 | −2.281±1.061 | −2.157±1.077 | p=0.9884 | p=0.6037 |
| Diffusing capacity | |||||
| Subjects n | 62 | 105 | 98 | ||
| D LCO | 14.75 (13.22–16.87) | 14.77 (12.69–17.01) | 13.93 (12.46–16.09) | p=0.1802 | p=0.2837 |
| DLCO Z-score | 0.231 (−0.25–0.76) | 0.176 (−0.38–0.82) | 0.50 (0.01–1.28) | p=0.0375 | p=0.0026 |
| Alveolar volume (VA) L | 2.370 (2.068–2.800) | 2.380 (2.095–2.790) | 2.11 (1.938–2.315) | p=0.0005 | p<0.0001 |
| VA Z-score | −0.44 (−1.15–0.18) | −0.34 (−1.0–0.52) | −0.19 (−0.68–0.36) | p=0.1480 | p>0.9999 |
| DLCO/VA | 6.400 (5.788–6.783) | 6.010 (5.395–6.680) | 6.565 (6.005–7.230) | p=0.1564 | p<0.0001 |
Data are presented as mean±sd if normally distributed, median (interquartile range) if not normally distributed, unless otherwise stated. No statistically significant differences between HU and HEU. ART: antiretroviral therapy; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide.
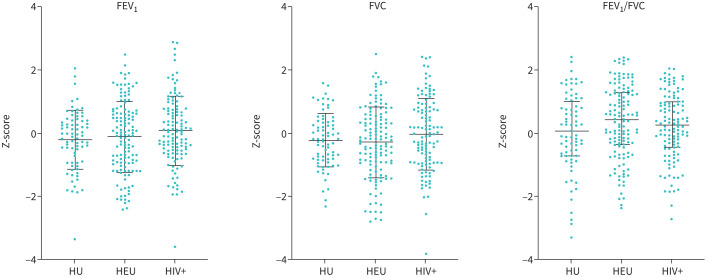
FIGURE 3.
Spirometry (FEV1, FVC, FEV1/FVC) Z-scores of HIV-unexposed (HU), HIV-exposed-uninfected (HEU) and perinatally HIV-infected (HIV+) children. Z-scores calculated using Global Lung Initiative reference data and software. Normally distributed (mean±sd), compared with ordinary one-way ANOVA with Tukey post hoc multiple comparison test, non-normally distributed data (median (interquartile range)) compared with Kruskal–Wallis test and post hoc Dunn's correction for multiple comparisons. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Spirometry outcomes were similar for HU, HEU and HIV+ with similar mean Z-score of FEV1, FVC or FEV1/FVC and no statistically significant difference between any of the groups for FEV1, FVC or FEV1/FVC.
Plethysmography (table 2, figure 4)
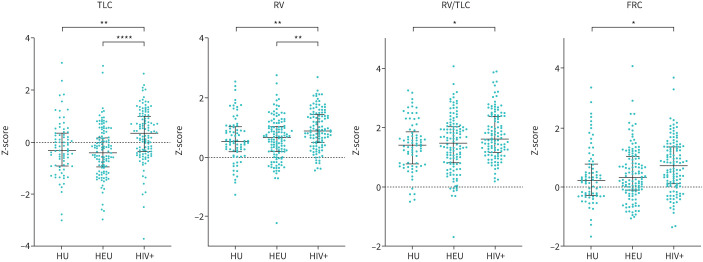
FIGURE 4.
Plethysmography Z-scores for HIV-unexposed (HU), HIV-exposed-uninfected (HEU) and HIV+ children. Data displayed as mean±sd, comparison of groups with ordinary one-way ANOVA and Tukey post hoc multiple comparison test. Single data point of HEU Z-score for TLC and RV/TLC is not included in the figure as Z<−5 and >Z+7, respectively. TLC: total lung capacity; RV: residual volume, FRC: functional residual capacity. *p<0.05; **p<0.01; ****p<0.0001.
TLC Z-score was higher in HIV+ than HU (mean difference 0.5101, 95% CI 0.1381–0.8820, p=0.0039) and HEU (mean difference 0.6966, 95% CI 0.3722–1.021, p<00001). RV Z-score was higher in HIV+ than HU (mean difference 0.3372, 95% CI 0.09434–0.5801, p=0.0034) and HEU (mean difference 0.3246, 95% CI 0.1127–0.5365, p=0.001). The mean RV/TLC Z-score was significantly higher in HIV+ than HU (mean difference 0.3341, 95% CI 0.01584–0.6524, p=0.0371) but not HEU (mean difference 0.2431, 95% CI −0.03450–0.5208, p=0.0995). RV/TLC Z-score was similar in HU and HEU. Furthermore, FRC Z-scores were higher in HIV+ than HU (mean difference 0.3840, 95% CI 0.03799–0.6579, p=0.0234). The increase in TLC, RV and FRC Z-scores, along with significantly higher RV/TLC in HIV+, indicates air-trapping (figure 4). Earlier ART initiation was associated with increased TLC Z-score (R2=0.081, p=0.0016); however, there was no correlation between the timing of ART initiation and Z-scores of RV (R2=0.028, p=0.071), RV/TLC (R2=0.027, p=0.073) or FRC (R2=0.017, p=0.155) (supplementary figure S1).
Single-breath diffusion capacity testing (table 2)
HIV+ had significantly higher DLCO Z-score than HU (mean rank difference 31.05, p=0.0376) and HEU (mean rank difference 35.81, p=0.0026). The DLCO Z-score was similar in HU and HEU. No correlation between timing of ART initiation and DLCO Z-score in HIV+ was present (r=−0.125, p=0.22). There was no statistically significant difference in VA Z-scores between groups.
Discussion
In this study, we demonstrate that following perinatal HIV infection, forced expiratory lung function and diffusing capacity are preserved in HIV+ children in whom ART is initiated early in life. However, plethysmography findings indicate that small airway obstruction continues to occur in children with HIV despite early ART.
Spirometry was similar in all groups indicating that the lung health was generally good without severe lung disease or function impairment. Expiratory flow and volume were similar in HU and HEU demonstrating that perinatal HIV and antiretroviral exposure for VTP does not disrupt the development of childhood lung function despite an increased risk of pneumonia in childhood [7]. Encouragingly, unlike previous PFT studies where ART was initiated later in childhood, there was no indication of expiratory airflow limitation in HIV+ [13, 32, 33]. In contrast to our study, participants in previous childhood PFT studies were older at HIV diagnosis (5 to 12 years), ART initiation (5 to 12 years) and lung function testing (11 to 14 years) [13, 14, 32–34]. Airflow limitation with decreased FEV1 and FEV1/FVC was more common and severe in children with delayed HIV diagnosis [33]. Our study findings suggest that in low- and middle-income countries, early diagnosis of perinatal HIV infection and initiation of ART may attenuate airflow limitation later in life in children with HIV infection.
The lack of expiratory flow and volume abnormalities may be due to the limited sensitivity of spirometry to detect small airway obstruction in children [35, 36]. Plethysmography can detect air-trapping in the absence of abnormal expiratory flow and may be more sensitive to detect small airway dysfunction in children than spirometry [37]. The use of plethysmography allowed our study to identify increased static RV and RV/TLC, features of small airway obstruction not found on spirometry. The mean VCPLETH of all groups was lower than expected. It is unclear whether this represents airflow obstruction in all groups or whether this is a limitation of the reference values that were used for this study. Small airway obstruction may be due to small airway injury or reactive airways. Air-trapping was significantly more severe in HIV+. Apart from air-trapping due to small airway obstruction, increased RV/TLC is found with certain structural abnormalities including cysts and bullae. However, given the limited abnormal spirometry and increased RV/TLC in all groups, significant structural lung disease is unlikely. We speculate that environmental factors such as exposure to air pollution or the burning of bio-fuels may have led to small airway injury in our study population and that HIV+ exaggerates this injury. Furthermore, our findings indicate that in children at risk of obstructive airway pathology, spirometry may not detect early small airway disease; therefore advanced PFT such as plethysmography should be considered.
The results of diffusing capacity testing were unexpected. DLCO was higher in HIV+ despite a similar VA. A potential explanation for this is increased pulmonary blood volume, either due to raised haemoglobin or increased alveolar capillary density. Given the good overall lung health in our population, pulmonary haemorrhage and intra-alveolar blood is highly unlikely. In contrast to our study, DLCO in HIV+ children with late ART initiation (mean age 5 years) was significantly lower than HU [13]. Interpretation of DLCO in HIV+ children is complicated by the paucity of data. Future studies should examine the diffusing capacity in HIV+ and include measurements of alveolar capillary volume and haemoglobin at the time of testing.
The longitudinal lung function trajectory in children with HIV is unclear. It is not known whether children with HIV follow a lower trajectory throughout life, have a plateau or have a more rapid decline of lung function. A longitudinal study in a similar population to ours, median age of 4.3 years at ART initiation, demonstrated that children with HIV follow a similar, albeit lower, spirometry trajectory to HU children [15]. However, these children started with diminished lung function, and to date, no longitudinal studies have examined lung function following the early initiation of ART. Our study adds valuable lung function data of HIV+ children in the first decade of life. We found that spirometry is not disrupted by HIV infection, provided ART is initiated in the first years of life. Thus, the children in our study have a similar start of their lung function trajectory to HU and the opportunity to follow a normal trajectory, potentially allowing children with HIV to reach their full lung function potential.
ART was initiated early relative to practice at the time of the parent studies. However, a number of children in the CHER deferred ART arm and P1060 trial initiated ART in the second and third year of life and may have suffered long-term sequelae. Owing to the limited number of children in our study initiating ART after the first year of life, we were not able to compare lung function of children initiated on ART in the first year of life to those with later ART initiation. Future cohort trials should compare lung function of children with HIV with early ART initiation to those with initiation in the second year of life to provide a better understanding of the influence of timing of ART initiation and childhood lung function. Specifically studies should examine whether extremely early initiation of ART, within the first days of birth, improves childhood lung function. Studies of extremely early ART have shown encouraging results to decrease the HIV viral reservoir; however, there are no data on the lung function of these children [38, 39].
In order to account for the influence of age, height and sex on pulmonary function we performed Z-standardisation using GLI reference data. The Z-standardisation of PFT parameters allows for the comparison of lung function between groups and potentially between different studies. The generalisation of normal lung function as calculated by the GLI reference data is limited by the lack of reference data for all ethnic groups. However, we were cautious to define lung disease based on the upper and lower limits of normal (Z<−1.64, Z>1.64) of a reference group different to our study population. Furthermore, only Caucasian reference data are available for plethysmography and diffusing capacity, limiting the capability to comment on normality in these lung functions.
Our study had numerous strengths. We recruited a large number of participants from the same population and were able to compare lung function of HIV+ to HE and HU to account for the effect of perinatal HIV and VTP exposure on childhood lung function. We performed extensive lung function testing to assess forced expiratory manoeuvres, static lung volumes and diffusing capacity testing giving unique insights into lung physiology and function.
Limitations include the lack of data of clinical information including respiratory symptoms and disease, antenatal and second-hand tobacco smoke exposure and the limited HU participants recruited in infancy, preventing examination of the influence of early life pulmonary infection on childhood lung function. A further limitation is that HIV PCR testing was not performed within the first 24 h of birth, inhibiting the discrimination between intrauterine and intra-partum HIV infection. Finally, the heterogeneous HIV+ population of our study due to the different inclusion and ART initiation criteria of CHER and IMPAACT 1060 limits the insight into the correlation of timing of ART initiation and childhood lung function and may have introduced selection and survival bias. Specifically, this study is not able to account for the influence of lung infection and tuberculosis, for which HIV+ are at increased risk, on lung function.
Conclusion
In this study we demonstrate that early ART initiation, following perinatal HIV infection, is associated with normal childhood spirometry with no forced expiratory airflow deficit. However, despite early ART, small airway dysfunction is more pronounced in HIV+ than HIV-exposed and HIV-unexposed children. Longitudinal examination of lung function is required to evaluate whether airflow limitation develops later in life.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure 00691-2021.SUPPLEMENT (1.1MB, tiff)
Supplementary methods 00691-2021.SUPPL_METHODS (72.7KB, pdf)
Acknowledgements
We wish to express our gratitude to each of the study participants and their parents for their contribution to this study.
Provenance: Submitted article, peer reviewed.
Conflict of interest: A. Gie reports grants from Fogharty International Center during the conduct of the study.
Conflict of interest: J. Morrison has nothing to disclose.
Conflict of interest: D. Maree has nothing to disclose.
Conflict of interest: B. Laughton has nothing to disclose.
Conflict of interest: S.H. Browne has nothing to disclose.
Conflict of interest: M.F. Cotton reports grants from the NIH during the conduct of the study.
Conflict of interest: P. Goussard has nothing to disclose.
Conflict of interest: S. Innes has nothing to disclose.
Support statement: This study was supported by University of California San Diego Center for AIDS Research grants S9000412 and P30-AI036214, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 1R01-HD083042, Fogarty International Center grants R24-TW007988 and D43TW010937, and Collaborative Initiative for Paediatric HIV Education and Research grant 158-INN. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Nesheim SR, Kapogiannis BG, Soe MM, et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the perinatal AIDS collaborative transmission study, 1986-2004. Pediatrics 2007; 120: 100–109. doi: 10.1542/peds.2006-2052 [DOI] [PubMed] [Google Scholar]
- 2.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359: 2233–2244. doi: 10.1056/NEJMoa0800971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calligaro GL, Gray DM. Lung function abnormalities in HIV-infected adults and children. Respirology 2015; 20: 24–32. doi: 10.1111/resp.12385 [DOI] [PubMed] [Google Scholar]
- 4.Shearer WT, Jacobson DL, Yu W, et al. Long-term pulmonary complications in perinatally HIV-infected youth. J Allergy Clin Immunol 2017; 140: 1101–1111.e7. doi: 10.1016/j.jaci.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol 2008; 43: 1–10. doi: 10.1002/ppul.20676 [DOI] [PubMed] [Google Scholar]
- 6.Gray DM, Wedderburn CJ, MacGinty RP, et al. Impact of HIV and antiretroviral drug exposure on lung growth and function over 2 years in an African Birth Cohort. Aids 2020; 34: 549–558. doi: 10.1097/QAD.0000000000002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slogrove AL, Goetghebuer T, Cotton MF, et al. Pattern of infectious morbidity in HIV-exposed uninfected infants and children. Front Immunol 2016; 7: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalbert E, Williamson KM, Kroehl ME, et al. HIV-exposed uninfected infants have increased regulatory T cells that correlate with decreased T cell function. Front Immunol 2019; 10: 595. doi: 10.3389/fimmu.2019.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Martino M, Veneruso G, Gabiano C, et al. Airway resistance and spirometry in children with perinatally acquired human immunodeficiency virus-type 1 infection. Pediatr Pulmonol 1997; 24: 406–414. doi: [DOI] [PubMed] [Google Scholar]
- 10.Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev 2020; 100: 603–632. doi: 10.1152/physrev.00039.2018 [DOI] [PubMed] [Google Scholar]
- 11.Neff CP, Chain JL, MaWhinney S, et al. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med 2015; 191: 464–473. doi: 10.1164/rccm.201408-1521OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rylance J, McHugh G, Metcalfe J, et al. Chronic lung disease in HIV-infected children established on antiretroviral therapy. Aids 2016; 30: 2795–2803. doi: 10.1097/QAD.0000000000001249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Githinji LN, Gray DM, Hlengwa S, et al. Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Ann Am Thorac Soc 2017; 14: 722–729. doi: 10.1513/AnnalsATS.201612-1018OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio A, Monpoux F, Bailly C, et al. Pulmonary function in HIV-1 vertically infected children. J AIDS Clin Res 2012; 3: 3–6. doi: 10.4172/2155-6113.1000146 [DOI] [Google Scholar]
- 15.Githinji LN, Gray DM, Hlengwa S, et al. Longitudinal changes in spirometry in South African adolescents perinatally infected with human immunodeficiency virus who are receiving antiretroviral therapy. Clin Infect Dis 2020; 70: 483–490. doi: 10.1093/cid/ciz255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh SB, Lodge CJ, Burgess JA, et al. Childhood predictors and adult COPD risk of lung function trajectories: a prospective cohort study from the first to the sixth decade. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 17.Belgrave DCM, Granell R, Turner SW, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med 2018; 6: 526–534. doi: 10.1016/S2213-2600(18)30099-7 [DOI] [PubMed] [Google Scholar]
- 18.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; 363: 1510–1520. doi: 10.1056/NEJMoa1000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366: 2380–2389. doi: 10.1056/NEJMoa1113249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boivin MJ, Chernoff M, Fairlie L, et al. African multi-site 2-year neuropsychological study of school-age children perinatally infected, exposed, and unexposed to human immunodeficiency virus. Clin Infect Dis 2020; 71: E105–E114. doi: 10.1093/cid/ciz1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow-Mosha L, Angelidou K, Lindsey J, et al. Nevirapine- versus lopinavir/ritonavir-based antiretroviral therapy in HIV-infected infants and young children: long-term follow-up of the IMPAACT P1060 randomized trial. Clin Infect Dis 2016; 63: 1113–1121. doi: 10.1093/cid/ciw488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Innes S, Laughton B, van Toorn R, et al. Recovery of HIV encephalopathy in perinatally infected children on antiretroviral therapy. Dev Med Child Neurol 2020; 62: 1309–1316. doi: 10.1111/dmcn.14639 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access. Geneva, WHO Press, 2006; 1–52. www.who.int/hiv/pub/guidelines/paediatric020907.pdf?ua=1%0Ahttp://apps.who.int/iris/bitstream/10665/85322/3/WHO_HIV_2013.7_ara.pdf%0Apapers2://publication/uuid/2CC31691-F302-4856-9799-571DD463F67E [PubMed]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005: 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 25.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 26.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. doi: 10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 27.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 29.Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021: 57: 2000289. doi: 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 30.Smith SJ, Gray DM, MacGinty RP, et al. Choosing the better global lung initiative 2012 equation in South African population groups. Am J Respir Crit Care Med 2021; 202: 1724–1727. doi: 10.1164/rccm.202005-2085LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draper B, Abdullah F. A review of the prevention of mother-to-child transmission programme of the Western Cape provincial government, 2003–2004. S Afr Med J 2008; 98: 431–434. [PubMed] [Google Scholar]
- 32.Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatr Infect Dis Soc 2016; 5: 162–169. doi: 10.1093/jpids/piv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2012; 55: 145–152. doi: 10.1093/cid/cis271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rylance S, Rylance J, McHugh G, et al. Effect of antiretroviral therapy on longitudinal lung function trends in older children and adolescents with HIV-infection. PLoS One 2019; 14: e0213556. doi: 10.1371/journal.pone.0213556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukic KZ, Coates AL. Does the FEF25-75 or the FEF75 have any value in assessing lung disease in children with cystic fibrosis or asthma? Pediatr Pulmonol 2015; 50: 863–868. doi: 10.1002/ppul.23234 [DOI] [PubMed] [Google Scholar]
- 36.Quanjer PH, Weiner DJ, Pretto JJ, et al. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J 2014; 43: 1051–1058. doi: 10.1183/09031936.00128113 [DOI] [PubMed] [Google Scholar]
- 37.Mahut B, Bokov P, Delclaux C. Abnormalities of plethysmographic lung volumes in asthmatic children. Respir Med 2010; 104: 966–971. doi: 10.1016/j.rmed.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 38.Maswabi K, Ajibola G, Bennett K, et al. Safety and efficacy of starting antiretroviral therapy in the first week of life. Clin Infect Dis 2021; 72: 388–393. doi: 10.1093/cid/ciaa028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luzuriaga K, Tabak B, Garber M, et al. HIV Type 1 (HIV-1) Proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210: 1529–1538. doi: 10.1093/infdis/jiu297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure 00691-2021.SUPPLEMENT (1.1MB, tiff)
Supplementary methods 00691-2021.SUPPL_METHODS (72.7KB, pdf)