Abstract
Background
In vivo studies of airway pathology in obstructive lung disease are limited by poor quality of specimens obtained with forceps. Obtainment of cryobiopsies has increased diagnostic yield in cancer and interstitial lung disease but has not been used in patients with asthma. In a recent pilot study, we found mucosal cryobiopsies to be larger and more intact than conventional forceps biopsies. The aim of the present study was to compare quality and safety of mucosal cryobiopsies versus conventional forceps biopsies in patients with asthma.
Methods
Endobronchial biopsies were obtained with forceps and cryoprobe from patients with asthma not currently treated with inhaled steroids and evaluated histologically.
Results
A total of 240 cryobiopsies and 288 forceps biopsies were obtained from 48 patients. Bleeding from the biopsy site was common but self-limiting. No major complications related to the procedure were seen. Cryobiopsy cross areas were four times larger compared with forceps. Stretches of intact epithelium were detected in all cryobiopsies compared to 33% in forceps biopsies. Further, the length of intact epithelium was on average four times longer in the cryobiopsies. Importantly, there was a good preservation of both antigens and mRNA in the cryobiopsies ensuring a suitability and robustness for immunohistochemistry and in situ hybridisation.
Conclusion
Obtainment of mucosal cryobiopsies in patients with asthma is safe and yields biopsies that are significantly larger and morphologically better preserved compared with traditional forceps biopsies. The cryotechnique thus seems to be a promising tool for future in vivo studies of airway pathology.
Short abstract
The cryotechnique is a promising, safe tool for obtaining mucosal biopsies in patients with asthma, yielding larger and better-preserved specimens compared with forceps biopsies, increasing utility for evaluating airway pathology https://bit.ly/3sHlVzd
Introduction
Asthma is a heterogeneous disease where understanding underlying disease mechanisms and applying clinically observed phenotypes to specific endotypes remains a challenge [1]. Since asthma is associated with both immunological and structural changes in most compartments of the airways including the epithelium, submucosal glands and airway smooth muscle, sampling at the site of disease is highly important to further unravel underlying pathology. Historically, endobronchial biopsies have been an important tool in such mechanistical research, and bronchoscopy in patients with asthma is generally safe, even in patients with severe disease [2–6]. In vivo studies of airway pathology are, however, often limited by the poor quality of specimens that can be obtained with endobronchial forceps. Biopsies are small with crushing artefacts and only limited content of deeper lying structures such as airway smooth muscle [7, 8]. Importantly, novel advanced techniques for studying pathology in airways disease in depth, such as single cell sequencing, require larger and more intact airway samples [9]. Clearly there is a need for better methods for obtaining high-quality samples, to further our understanding of disease mechanisms in obstructive airways diseases.
In suspected lung cancer, the use of a cryoprobe to obtain biopsies from both flat and exophytic endobronchial lesions has in recent years shown to be both safe and superior to forceps [10–12]. The use of transbronchial cryobiopsies has in parallel increased diagnostic yield in supposed interstitial lung disease and decreased the need of the more invasive video-assisted thoracoscopy [13, 14]. In a pilot study of patients with haemoptysis investigating the use of mucosal cryobiopsies to study airway histology, we showed that the method yields bigger biopsies with better preservation of the tissue sampled as well as higher contents of airway smooth muscle and submucosal glands [15].
Safety is paramount when using bronchoscopy as a research tool, and studies on transbronchial cryobiopsy for diagnosing interstitial lung disease report an increased risk of pneumothorax of up to 30% and bleeding as a common complication that needs to be handled, e.g. with a Fogerty balloon. Further, studies on endobronchial cryobiopsy in cancer diagnostics report an increase in non-severe bleeding compared to sampling with forceps [11, 12, 14]. This raises a concern regarding safety of the method as a research tool in patients with asthma. In the above-mentioned pilot study, one patient had a severe bleeding needing intervention from both the site of forceps and cryobiopsy, but the study was not powered to evaluate safety. Further, biopsies were not performed in patients with airway disease where mucosal vascularisation and fragility may be altered. Hence, we sought to further validate the usefulness and safety of bronchial cryobiopsies in a larger number of patients with inflammatory airway disease.
In the present study, we examined the use of mucosal cryobiopsies in patients with asthma, with the aim to compare the quality of mucosal cryobiopsies with conventional forceps biopsies, and to evaluate safety of performing mucosal cryobiopsies.
Methods
The RECONSTRUCT study is a single centre clinical prospective intervention study examining disease mechanisms in patients with asthma at Bispebjerg University Hospital in Copenhagen, Denmark. Patients underwent bronchoscopy before and after a 6-week course of high-dose inhaled corticosteroids. Airway mucosal biopsies were obtained using cryotechnique and standard forceps and evaluated histologically at the University of Lund, Sweden. To assess airway pathology and the quality of airway specimens without the effect of steroids, we enrolled steroid-free asthma patients with airway hyperresponsiveness to mannitol (ClinicalTrials.gov identifier: NCT03034005).
Population and ethics
In order to assess airway morphology without the effect of steroids, we enrolled patients with asthma not treated with either inhaled or oral corticosteroids for at least the past 3 months, who had signs of active asthma with airway hyperresponsiveness to mannitol (PD15 <635 mg). Exclusion criteria were:
forced expiratory volume in 1 s <70% of predicted
active smoking or a history of smoking with >10 pack-years
competing respiratory diseases including lower respiratory tract infections within the past 4 weeks
significant comorbidity (American Society of Anesthesiologists (ASA) grade <2) [16]
pregnancy or breastfeeding
hypersensitivity to inhaled corticosteroids
uncontrolled hypertension
acute myocardial infarction within past 6 months
aorta or cerebral aneurysms
recent abdominal operation
failure to comply with the study protocol in general
All participants were given oral and written information on the aims, contents and risks of the study and given the opportunity to bring an assessor if needed. All patients gave oral and written consent to participate in the study.
The study was approved by the Danish National Committee on Health Research Ethics (H-16043663), the Danish Medicines Agency (EUDRACT: 2016-03509-33) and the Danish Data protection Agency (BFH-2018-018) and monitored by the Danish Good Clinical Practice Unit (Project ID: 2016-898). ClinicalTrials.gov identifier: NCT03034005.
Bronchoscopy and tissue sampling
Bronchoscopy was performed as a standard outpatient procedure with a flexible bronchoscope (Olympus BF-1TQ180/BF-1TH190; Olympus, Hamburg, Germany) [17]. The sedatives midazolam and fentanyl were administered according to the operator in doses ranging from 5 to 20 mg and 50 to 150 µg, respectively. A single dose of 1000 mg tranexamic acid was administered in the beginning of the procedure as bleeding prophylaxis. Oral access with bronchoscopic guided placement of an orotracheal tube (BronchoFlex®; Rüsch, Duluth, GA, USA) was used for easy access and retrieval of the bronchoscope during sampling with the cryoprobe as well as for protection of the vocal cords. Supplemental oxygen was administered through a designated canal in the tube as needed.
Mucosal biopsies were obtained with both forceps (EndojawTM, Ø 1.9 mm; Olympus) and cryotechnique (Erbecryo® 2, Ø 1.9 mm; Erbe, Tübingen, Germany). The technique used for obtainment of cryobiopsies has been described elsewhere [15]. Briefly, mucosal cryobiopsies were obtained from longitudinal stretches at subsegmental levels at the discretion of the operator. The cryoprobe was placed adjacent to the airway wall before initiating a freeze cycle of 2–3 s, after which the probe and bronchoscope was gently pulled and retracted. Biopsies were immediately thawed in a saline bath, transferred to fixative and subjected to overnight fixation in 4% buffered formaldehyde before routine dehydration and paraffin embedment. Bleeding was scored as none, mild, moderate or severe according to British Thoracic Society guidelines [17].
Histological analyses
We used standard histological techniques to evaluate the size of the biopsies and to explore morphology. The preservation of mucosal structural integrity and architecture was evaluated by the presence and length of intact epithelium stretches at the biopsy border. Multiple 3-µm tissue paraffin sections were generated from the paraffin-embedded biopsies. Sections stained with standard haematoxylin-eosin were digitised by an Aperio slide scanner (Aperio AT2; Aperio, Buffalo Grove, IL, USA) and used for morphological assessment and quantitative morphometric analysis. Separate serial sections were also evaluated for suitability to perform routine protein visualisation by immunohistochemistry as well as visualisation of specific gene expression (mRNA) by in situ hybridisation.
For immunohistochemistry, antigens were retrieved in a pretreatment module (PT-Link) machine (DakoCytomation, Glostrup, Denmark) before tissue sections were subjected to single and double immunostaining by an automated immunohistochemistry robot (Autostainer Plus, DakoCytomation, Glostrup, Denmark) and polymer horseradish peroxidase linked secondary antibodies (EnVision™ G2 Doublestain System Rabbit/Mouse, K5361, Dako) using diaminobenzidine (DAB) and Vina green as detection chromogens [18]. The following primary antibodies were used to test stain for selected structural and immunological cells: anti-α smooth muscle actin (clone 1A4, Dako, dilution 1:200), anti-human cluster of differentiation 31 (CD31, found in human endothelial cells) (clone JC70A, Dako; dilution1:50), anti-human interleukin-33 (IL-33, an epithelial alarmin) (clone Nessy-1, Alexis, Enzo Life Sciences, Farmingdale, NY, USA; dilution 1:500), anti-human mast cell tryptase (clone Mab 1222A, Millipore, Burlington, MA, USA; dilution 1:10 000), mouse anti-human cluster of differentiation (CD3, found on T-cells) (clone 2.38, Dako; dilution 1:50), anti-human cluster of differentiation 207 or Langerin (CD207, found in Langerhans cells) (langerin, Leica, Wetzlar, Germany; dilution 1:100). All primary and secondary antibodies used for immunohistochemistry have been extensively validated for use in clinical diagnostics and research.
For in situ hybridisation, messenger RNA (mRNA) probes against the human house-keeping gene ubiquitin C and the prokaryote gene DapB were used as positive and negative mRNA controls, respectively. The probes were visualised using the RNAscope 2.5 HD detection kit Brown assay kit (Advanced Cell Diagnostics, Hayward, CA, USA) according to the instructions supplied by the manufacturer [19, 20]. Sections were incubated with endogenous enzyme block, boiled in pretreatment buffer and treated with protease before target probe hybridisation. The target RNA was then amplified using a series of amplification solutions and visualised with coloured chromogen [19, 20].
Statistical analyses
Descriptive statistics were performed. Results are presented as medians with interquartile ranges and n (%). For comparison of size and histological quality parameters of biopsies, means with 95% confidence intervals are presented, and tests for differences were performed using paired t-tests.
All analyses were performed using IBM® SPSS statistics version 22 (IBM Software, Chicago, IL, USA).
Results
Population
50 patients were included in the study. Two patients withdrew consent before the first bronchoscopy due to personal reasons. A total of five mucosal cryobiopsies and six forceps biopsies were obtained from 48 patients resulting in a total of 240 and 288 biopsies, respectively. Clinical characteristics are presented in table 1.
TABLE 1.
Clinical characteristics
| Participants | 48 |
| Mucosal cryobiopsies | 240 |
| Forceps biopsies | 288 |
| Age years | 25 (7) |
| Female participants | 24 (50%) |
| Body mass index kg·m−2 | 23.4 (4.7) |
| ACQ-5 score | 1.42 (1.33) |
| FEV1 L | 3.77 (1.46) |
| FEV1 % predicted | 99 (15) |
| FEV1/FVC | 0.77 (0.12) |
| PD15 mannitol mg | 185 (337) |
| FENO ppb | 27.4 (46.7) |
| Former smoker | 9 (19%) |
| Age at asthma onset years | 12.5 (19) |
| Atopy | 28 (58%) |
Data are presented as n, n (%) and median (interquartile range). ACQ-5: Asthma Control Questionnaire-5; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FENO: exhaled nitric oxide fraction.
Biopsy size and tissue preservation
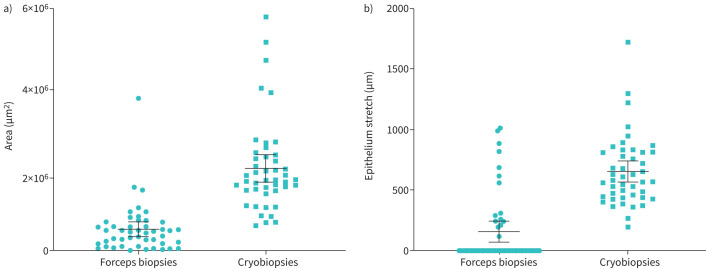
Mean cross-sectional area of the cryobiopsies was approximately four times larger than forceps biopsies: 2.05 mm2 (95% CI: 1.71–2.39) versus 0.50 mm2 (95% CI: 0.32–0.68) corresponding to a difference of 1.55 mm2 (95% CI: 1.16–19.4) (p<0.001; see figure 1).
FIGURE 1.
a) Cross-sectional biopsy area and b) length of intact stretches of epithelium in forceps biopsies (circles) versus cryobiopsies (squares). Individual measures with mean and 95% confidence interval.
Stretches of intact epithelium was detected in all cryobiopsies compared with in 33% of the forceps biopsies. The mean length of intact epithelium in cryobiopsies was approximately four times longer compared with forceps biopsies: mean length 653 µm (95% CI: 567–653) versus 166 µm (95% CI: 77–255) corresponding to a difference of 487 µm (95% CI: 368–606), p<0.001; see figure 1. Representative micrographs of the cryobiopsies are presented in figure 2.
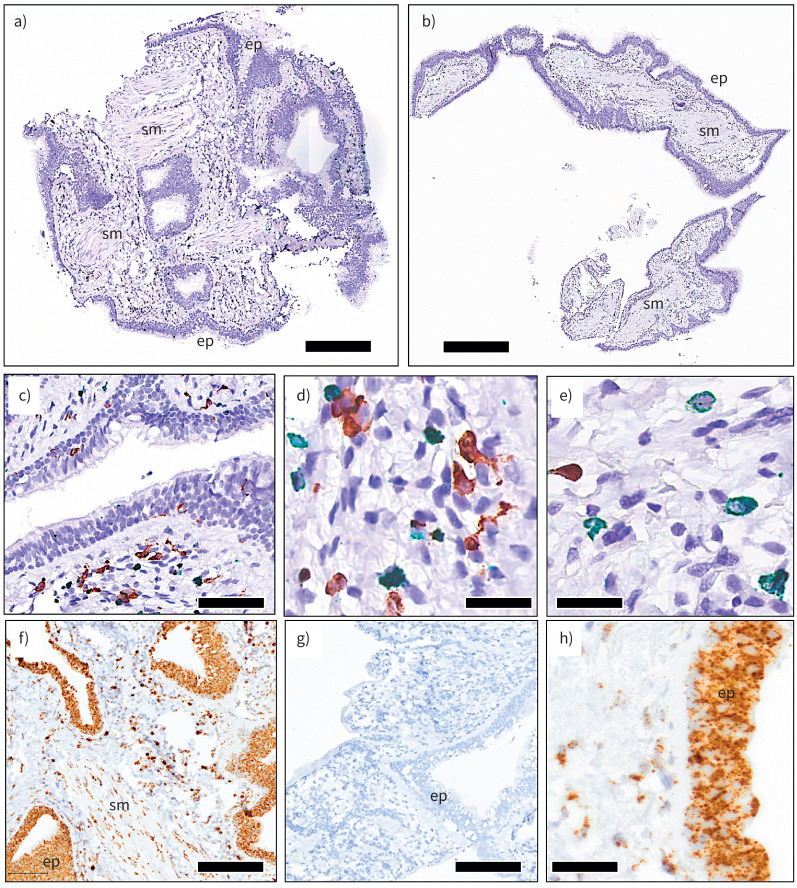
FIGURE 2.
a, b) Representative overview micrographs of cryobiopsies from asthmatic bronchi. c–e) Examples of robust immunohistochemical identification of airway mucosal immune cells (c, d: green, MPO+ neutrophils; brown, CD68+ macrophages; e: green MCt and brown MCtc mast cells). f, h) Detection of the positive control gene UBC mRNA in situ hybridisation (ISH). g) Negative ISH control with probe against the soil bacteria gene DapB. Ep: epithelium; sm: smooth muscle. Scale bars: a, 250 µm; b, 350 µm; c, 70 µm; d–e, 25 µm; f–g, 120 µm; h, 50 µm.
Because of the larger biopsy size, it was also evident that the cryobiopsies had larger areas of cross-sectioned subepithelial lamina propria tissue, airway smooth muscle bundles and subepithelial glands.
Immunohistochemistry and in situ hybridisation
The cryobiopsies were evaluated for their suitability for detection of protein cell markers and mRNA expression with immunohistochemistry and in situ hybridisation, respectively. Paraffin sections from cryobiopsies displayed good intensity of immunoreactivity (figure 2). Likewise, mRNA detection by in situ hybridisation resulted in clear and robust staining, as evidenced by staining for negative and positive control mRNA probes (figure 2).
Safety
Mild bleeding from the biopsy site after cryobiopsy was a common complication seen in all patients. There were no cases of severe complications including moderate or severe bleeding, pneumothorax or procedure-related pneumonia. All patients were discharged the same day after standard observation in the recovery ward without any post-procedural complications. Two patients (4%) withdrew from the study after the bronchoscopy due to discomfort related to the procedure.
Time needed for each biopsy was not recorded specifically, but it is the opinion of the operators that this does not differ notably between the two techniques, ∼5 min for a total of five cryobiopsies. Preparation and placement of the orotracheal tube increases procedural time slightly.
Discussion
This study has for the first time showed that the obtainment of endobronchial cryobiopsies in patients with asthma yields specimens of better quality compared with biopsies obtained with forceps. Cryobiopsies are bigger and better preserved and can be used at standard outpatient setup without a noteworthy increase in procedural time taken and importantly without introducing significant risk of severe complications for the patients. We believe that this technique can set a new standard for obtainment of airway specimens ensuring biopsies of better quality to increase applicability and improve opportunities in future in vivo studies of asthma.
The current study is the first study to evaluate the potential of cryobiopsies for studying airway pathology in airway disease. We have showed that biopsies obtained with the cryotechnique are bigger and with better preservation of the morphology. The results are in line with results from our previous study in patients with haemoptysis as well as what has been published on endobronchial cryobiopsies in other fields, e.g. suspected lung cancer [15, 21]. Mucosal cryobiopsies in this study were on average four times larger than biopsies obtained with forceps. Endobronchial cryobiopsies obtained from malignant lesions have in some circumstances been shown to be even bigger [21]. It is our experience that extending freezing cycles beyond 3 s results in difficulties of retracting the cryoprobe, supposedly because of involvement of underlying cartilage structures which we believe limits the size of biopsies obtained. If the cryoprobe was undetachable, we have successfully stopped the freezing cycle and waited until the frozen area has thawed before initiating a new cycle. All cryobiopsies contained stretches of intact epithelium, whereas this was only the case in 33% of the forceps biopsies. In comparison a quality study by Labonté et al. [7] showed that intact epithelium was only present in 41% of forceps biopsies. Indeed unintended mechanical desquamation of the epithelium is a common crushing artefact present in specimens obtained with forceps from both patients with airway disease and healthy controls [22]. A reason for a slightly lower rate of forceps biopsies containing intact stretches of epithelium in the current study could be the application of more strict evaluation criteria. In the current study the epithelium should be firmly attached to the basement membrane and with preserved tight junctions before considered intact. Nevertheless, this does not change the main message regarding a comparison of epithelial preservation between forceps and cryobiopsies, where stretches of intact epithelium were not only longer but also present in all specimens obtained with the cryomethod. The more preserved epithelium is not only due to larger biopsy size. The main reason could be the fact that once frozen, the epithelium and other tissue structures are spatially fixated, and this prevents the type of elastic tissue-damaging stretching that may occur during the withdrawal of traditional forceps after closing the jaws.
Safety was a big concern, and mild bleeding from the cryobiopsy site was seen in all patients. Bleeding was, however, self-limiting in all instances, and we saw no other procedure-related adverse events. A low complication rate was also the finding in our pilot study in patients with haemoptysis but has now been validated after obtainment of 240 cryobiopsies [15]. A similar safety profile has been observed in a trial with 600 patients with suspected lung cancer where the rate of severe bleeding needing intervention after obtainment of cryobiopsies was comparable to what was seen after forceps biopsies [11]. In this study, systemic tranexamic acid was administered for bleeding prophylaxis. The evidence behind the use of tranexamic acid for this purpose is limited though a recent study showed less bleeding after transbronchial lung biopsy when tranexamic acid was instilled locally [23]. Considering the low complication rate seen in this study, the need of prophylactic tranexamic acid for prophylaxis is uncertain and needs further investigation.
Pulmonary arterial hypertension is generally a contraindication for performing transbronchial cryobiopsy as it may increase risk of bleeding [14]. In this study, pulmonary arterial pressure was not evaluated in line with other studies evaluating endobronchial cryobiopsies in cancer diagnostics. Whether pulmonary arterial hypertension increases the risk of bleeding when obtaining endobronchial cryobiopsies therefore needs to be further investigated [11].
Sedatives needed to perform the procedures were within standard dosing regimens as also reported previously [11, 15].
Despite a few minutes of extra preparation time in placing the protective orotracheal tube, the cryomethod does not increase procedural time when compared with obtaining biopsies with forceps as has also been demonstrated for endobronchial sampling with the cryoprobe in patients with suspected lung cancer where time spent obtaining each biopsy was estimated to be ∼5 min for both forceps biopsies and cryobiopsies [11].
Importantly, freezing artefacts were not a problem for either standard histological evaluation or cellular and molecular explorations by immunohistochemistry or in situ hybridisation. That said, the cryoprocedure will inevitably cause mechanical damage at a micro scale due to ice crystal formation. For this reason, cryobiopsies cannot be used for ultrastructural electron-microscopic studies.
Since it is not possible with standard bronchoscopes to retract the cryoprobe along with the bronchoscope without such placement to protect the vocal cords and ensure easy access and retrieval, an orotracheal tube is mandatory, increasing procedural time slightly. In rare events of uncontrolled bleeding or other emergencies, the orotracheal tube may become an advantage ensuring easy introduction of, for example, tamponade balloons or suction catheters for establishing bleeding control.
The current study was performed in patients not currently treated with inhaled corticosteroids, presumably with most patients having mild to moderate disease. Though safety of performing research bronchoscopies in patients with severe disease does not seem to be increased, safety and applicability of the cryomethod in these patients need to be further validated [3].
Being able to obtain bigger biopsies from the airways without increasing procedural risk increases applicability and utility for studying airway disease. We believe that the ability to perform in vivo studies investigating pathology and response to, for example, treatment with specimens of better quality that are not affected by the sampling process itself can push our understanding of underlying disease mechanisms in asthma further. With biopsies containing a high degree of well-preserved epithelium and deeper lying structures, the opportunity to investigate these compartments as secluded entities or perhaps more importantly as complex networks improves. Obtaining cryobiopsies from the airways can therefore complement further research in both the important role of the airway epithelium in maintaining both health and airway disease and airway smooth muscle [24]. Indeed, studying airway smooth muscle pathology in asthma has been limited due to the low content in forceps biopsies increasing both numbers of study participants and the number of specimens obtained from each individual to make meaningful investigations [8]. The need of well-preserved large biopsies is also evident when using more novel techniques including transcriptomics and single cell sequencing [25]. Whether mucosal cryobiopsies can be used for this needs further exploration though. While post mortem samples can give valuable information, studies including such samples remain those of observational character, limiting the ability to draw causal conclusions, and improving the quality of airway specimens with new sampling techniques to further our understanding of airway pathology through in vivo studies is therefore much needed [26].
Besides applicability in a research setting, this new method may also become valuable in clinical practice. Though bronchoscopy is not a part of the standard diagnostic approach in airway disease, it may be needed in specific cases with diagnostic challenges or severe refractory disease [27]. With increasing requirement for assessing underlying disease drivers and sub-phenotyping patients before initiating targeted treatment, we expect this need to increase in the future.
Conclusion
This study shows that mucosal cryobiopsies obtained from patients with asthma are both bigger and better preserved than biopsies obtained with forceps. Importantly the method is safe and can be performed at a standard outpatient setup. The method therefore appears to be superior to forceps with the potential to improve in vivo studies and our understanding of airway pathology in asthma.
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03034005.
Conflict of interest: L. Frøssing reports receiving payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from GSK for a spirometry brochure. C. Porsbjerg reports receiving grants or contracts from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK, outside the submitted work; consulting fees from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK, outside the submitted work; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events received from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK, outside the submitted work. The remaining authors have nothing to disclose.
Support statement: The study was funded by an unrestricted grant from the Lundbeck foundation
References
- 1.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol 2019; 144: 1–12. doi: 10.1016/j.jaci.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 2.Bergeron C, Tulic MK, Hamid Q. Tools used to measure airway remodelling in research. Eur Respir J 2007; 29: 596–604. doi: 10.1183/09031936.00019906 [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE, Busse WW. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119: 14–21. doi: 10.1016/j.jaci.2006.10.025 [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Evans MD, Bleecker ER, et al. Safety of investigative bronchoscopy in the Severe Asthma Research Program. J Allergy Clin Immunol 2011; 128: 328–336.e3. doi: 10.1016/j.jaci.2011.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elston WJ, Whittaker AJ, Khan LN, et al. Safety of research bronchoscopy, biopsy and bronchoalveolar lavage in asthma. Eur Respir J 2004; 24: 375–377. doi: 10.1183/09031936.04.00063003 [DOI] [PubMed] [Google Scholar]
- 6.Tapanainen L, Lindqvist A, Halme M, et al. Investigative bronchoscopy and endobronchial biopsy is well tolerated in hyperreactive asthma patients. Respir Med 2002; 96: 466–468. doi: 10.1053/rmed.2002.1304 [DOI] [PubMed] [Google Scholar]
- 7.Labonté I, Laviolette M, Olivenstein R, et al. Quality of bronchial biopsies for morphology study and cell sampling: a comparison of asthmatic and healthy subjects. Can Respir J 2008; 15: 431–435. doi: 10.1155/2008/202615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brightling CE, Bradding P, Symon FA, et al. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002; 346: 1699–1705. doi: 10.1056/NEJMoa012705 [DOI] [PubMed] [Google Scholar]
- 9.Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol 2021; 21: 347–362. doi: 10.1038/s41577-020-00477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aktas Z, Gunay E, Hoca NT, et al. Endobronchial cryobiopsy or forceps biopsy for lung cancer diagnosis. Ann Thorac Med 2010; 5: 242–246. doi: 10.4103/1817-1737.69117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetzel J, Eberhardt R, Herth FJF, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012; 39: 685–690. doi: 10.1183/09031936.00033011 [DOI] [PubMed] [Google Scholar]
- 12.Schumann C, Hetzel J, Babiak AJ, et al. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg 2010; 140: 417–421. doi: 10.1016/j.jtcvs.2009.12.028 [DOI] [PubMed] [Google Scholar]
- 13.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016; 193: 745–752. doi: 10.1164/rccm.201504-0711OC [DOI] [PubMed] [Google Scholar]
- 14.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration 2018; 95: 188–200. doi: 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 15.Hvidtfeldt M, Pulga A, Hostrup M, et al. Bronchoscopic mucosal cryobiopsies as a method for studying airway disease. Clin Exp Allergy 2019; 49: 27–34. doi: 10.1111/cea.13281 [DOI] [PubMed] [Google Scholar]
- 16.Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications: a study of consistency of ratings. Anesthesiology 1978; 49: 239–243. doi: 10.1097/00000542-197810000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013; 68: Suppl. 1, i1-i44. doi: 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 18.Roos AB, Sethi S, Nikota J, et al. IL-17A and the promotion of neutrophilia in acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 428–437. doi: 10.1164/rccm.201409-1689OC [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012; 14: 22–29. doi: 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver JS, Kearley J, Copenhaver AM, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol 2016; 17: 626–635. doi: 10.1038/ni.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetzel J, Hetzel M, Hasel C, et al. Old meets modern: the use of traditional cryoprobes in the age of molecular biology. Respiration 2008; 76: 193–197. doi: 10.1159/000135934 [DOI] [PubMed] [Google Scholar]
- 22.Ordoñez C, Ferrando R, Hyde DM, et al. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med 2000; 162: 2324–2329. doi: 10.1164/ajrccm.162.6.2001041 [DOI] [PubMed] [Google Scholar]
- 23.Kuint R, Levy L, Cohen GP, et al. Prophylactic use of tranexamic acid for prevention of bleeding during transbronchial lung biopsies: a randomized, double-blind, placebo-controlled trial. Respir Med 2020; 173: 106162. doi: 10.1016/j.rmed.2020.106162 [DOI] [PubMed] [Google Scholar]
- 24.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 2020; 145: 1499–1509. doi: 10.1016/j.jaci.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers DC, Carew AM, Lukowski SW, et al. Transcriptomics and single-cell RNA-sequencing. Respirology 2019; 24: 29–36. doi: 10.1111/resp.13412 [DOI] [PubMed] [Google Scholar]
- 26.Agache I, Eguiluz-Gracia I, Cojanu C, et al. Advances and highlights in asthma in 2021. Allergy 2021; 76: 3390–3407. doi: 10.1111/all.15054 [DOI] [PubMed] [Google Scholar]
- 27.Kuo CW, Liao XM, Huang YC, et al. Bronchoscopy-guided bronchial epithelium sampling as a tool for selecting the optimal biologic treatment in a patient with severe asthma: a case report. Allergy Asthma Clin Immunol 2019; 15: 76. doi: 10.1186/s13223-019-0378-6 [DOI] [PMC free article] [PubMed] [Google Scholar]