Abstract
Triple-negative breast cancer is an aggressive subtype of breast cancer with poor clinical outcomes and poor prognosis. Hesperetin is an active component extracted from Citrus fruits and Traditional Chinese Medicine has a wide range of pharmacological effects. Here, we assessed the anti-migration and anti-invasive effects and explored inhibitory mechanisms of hesperetin on metastasis of human triple negative breast cancer MDA-MB-231 cells. Cell viability experiments revealed that 200 μM hesperetin has a clear inhibitory effect on MDA-MB-231 cells. TGF-β1 treatment induces apparent tumor progression in MDA-MB-231 cells including aberrant wound-healing and invasion ability, which is effectively suppressed by hesperetin co-treatment. Additionally, hesperetin inhibited the TGF-β1-mediated actin stress fiber formation. Western blot results showed that hesperetin suppressed the TGF-β1-mediated (i) activation of Fyn, (ii) phosphorylation of paxillin at Y31, Y88, and Y118 sites, (iii) the increased expression of RhoA, and (iv) activation of Rho-kinase. We demonstrated the increased interaction of Fyn with paxillin and RhoA protein in the TGF-β1-induced metastasis of MDA-MB-231 cells. Small interfering RNA Fyn inhibited phosphorylation of paxillin (Y31) and activation of Rho-kinase induced by TGF-β1. In conclusion, hesperetin has a significant inhibitory effect on migration and invasion of MDA-MB-231 cells induced by TGF-β1, which might be attributed to inhibiting the Fyn/paxillin/RhoA pathway.
Keywords: hesperetin, triple-negative breast cancer, Fyn, paxillin, RhoA, Rho-kinase
Introduction
Breast cancer has become the malignant tumor with the highest incidence and death among women worldwide. Approximately 20% of breast cancer patients have been diagnosed with triple-negative breast cancer (TNBC). 1 Because TNBC has special biological behaviors and clinicopathological features with a high metastasis risk. While among patients with adjuvant chemotherapy drugs have produced some curative effects in clinical practice, the prognosis of TNBC remains poor and the risk of death is still high. 2 Therefore, the adverse effects of traditional chemotherapy strategies have been driving the development of natural compounds as new therapeutic drugs and promoting the urgent exploration of other anticancer strategies.
Proto-oncogene tyrosine-protein kinase Fyn is the prototypical member of the Src family kinases (SFKs), which was identified in 1986.3,4 Fyn has been recognized as an important mediator and regulator in the signal transduction pathway associated with cell invasion, migration, and proliferation in various cancers, such as gastric cancer and prostate cancer.5,6 As a major participant in the regulation of cell movement, paxillin plays distinct roles in pathological conditions such as disruption of the endothelial cell barrier and cancer cell metastasis by recruiting cytoskeletal elements and signaling molecules involved in cell attachment, spreading, and migration. 7 The function of paxillin is tightly regulated by tyrosine phosphorylation. 8 Previously studies have revealed that the interaction of Fyn with paxillin leads to the tyrosine phosphorylation of the focal adhesion kinase. 9 Additionally, increased levels of activate SFK, is associated with Rho family of GTPases (including RhoA, Rac1, and Cdc42) regulate many aspects of intracellular actin dynamics. During regulating morphologic differentiation of oligodendrocyte cells, Fyn and Rho family of GTPases interaction have been confirmed in previous studies. 10 Additionally, it has been demonstrated that cytoskeletal reorganization and chemotaxis of mast cells are involved in interactions between Fyn and Rac1. 11 Although the role of Fyn and RhoA in tumorigenesis and development has been proved,6,12 the mechanism underlying interaction between Fyn and RhoA is not completely elucidated in the metastasis of TNBC yet. Hodge and Ridley have demonstrated that paxillin regulates the promotion of cell migration through binding to regulators and effectors of the Rho family of GTPases. 13 Moreover, most researchers agree that the RhoA/Rho-kinase (ROCK) signaling pathway plays a crucial role in the regulation of cell motility.12,14
Numerous studies discovered that flavonoids are able to inhibit cancer metastasis and delay cancer progression15,16 via suppressing the metastasis and angiogenesis-related signaling pathways. Hesperetin, a naturally occurring flavanone glycoside, can be obtained from Citrus fruits (C. sinensis and C.limon) and Traditional Chinese Medicine (Zhishi and Chenpi). It has been demonstrated that hesperetin has an anti-tumor effect on breast cancer cells by suppressing proliferation and inducing apoptosis in multiple cancer cell lines.17,18 However, the detailed anti-tumor efficacy and mechanisms of hesperetin as an anti-breast cancer agent are very limited.
In the present study, the aggressive TNBC MDA-MB-231 cell line is known to be resistant to several anti-cancer agents, 19 and was selected as the subject. The purpose of this study was to reveal a novel mechanism and pathway of hesperetin in TNBC migration and invasion induced by TGF-β1 in TNBC MDA-MB-231 cells through further elucidating the important role of the Fyn/paxillin/RhoA pathway in TNBC metastasis.
Materials and Methods
Cell Culture and Treatment
MDA-MB-231 cells were purchased from the Chinese Academy of Sciences (Shanghai, China). MDA-MB-231 cells were cultured in Leibovitz’s L-15 medium (GE Healthcare, USA) supplemented with 10% fetal bovine serum (Millipore, USA) at 37°C in a humidified atmosphere with 5% CO2. Cells were starved for 24 hour with serum-free L-15 medium before dosing. MDA-MB-231 cells were co-incubated with different concentrations of hesperetin and 20 ng/mL TGF-β1 for 24, 48, and 72 hour. Serum-free media lacking TGF-β1 or hesperetin was used as control. Serum-free medium only containing 20 ng/mL TGF-β1 was used as the positive control.
Reagents and Antibodies
Hesperetin (purity ≥ 98%), purchased from Cayman Chemical Company (Michigan, USA), was dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. Transforming growth factor-β1 (TGF-β1) purchased from Wako (Tokyo, Japan) was made up at 40 μg/mL and stored at −80°C before use. The final concentration of 20 ng/mL of TGF-β1 was used in this study. Rhodamine phalloidin and DAPI (NucBlue® Fixed Cell Stain ReadyProbe™ reagent) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The primary and second antibodies used were as follows: Fyn (1:1000, BD Biosciences, USA), p-Src (Y416) (1:1000, Cell Signaling Technology, USA), paxillin (1:5000, BD Biosciences, USA), p-paxillin (Y31) (1:500, Santa Cruz, USA), p-paxillin (Y88) (1:500, Santa Cruz, USA), p-paxillin (Y118) (1:500, Santa Cruz, USA), RhoA (1:500, Santa Cruz, USA), MYPT-1(H-130) (1:500, Santa Cruz, USA), p-MYPT-1(Thr850) (1:500, Merck Millipore, USA). Anti-mouse IgG HRP Conjugate, anti-rabbit IgG HRP Conjugate, and anti-goat IgG HRP Conjugate (diluted 1:5000 with TBS-T) were purchased from Promega (Madison, WI, USA).
Cytotoxicity Assay
MDA-MB-231 cells were seeded at 5000 cells/well into 96-well culture plates for 24 hour, then cells were starved for 24 hour without serum before treatment with different concentrations (0, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μM) of hesperetin for 48 hour. The final concentration of DMSO (0.1% volume) was added to medium as a control. Cell Counting Kit-8 assay (Dojindo, Japan) was used to evaluate the viability of cells. The experiment was operated strictly following the instructions. The absorbance of the solutions in the wells was read at 450 nm in a microplate reader (iMark, Bio-Rad, USA).
Wound Healing Assay
The migration ability of MDA-MB-231 cells was examined by the wound-healing assay, which is a standard procedure in monitoring cell migration. 20 The cells were seeded into 35 mm dishes. When the cell confluence reached 70% to 80%, serum-free L-15 medium was changed to starve cells for 24 hour. A scratched area was created using a sterile 20 μL pipette tip on the cells. Then, the cells were incubated in serum-free medium containing 20 ng/mL TGF-β1 in the absence or presence of hesperetin (25, 50, and 100 μM). The cells migrated to the wound surface were observed and recorded under the microscope at 0, 24, 48, and 72 hour. The ratio of cell migration was calculated as the percentage of the remaining cell-free area compared with the area of the initial scratched area, 21 and analyzed using NIH Image-J software.
Transwell Invasion Assay
Cell invasion was assayed using Corning Matrigel invasion chamber 24-well plate (Corning BioCoat, USA). In brief, the Matrigel (BD Biosciences, USA) was diluted in serum-free L-15 medium in 1:1.6 ratio. The upper compartment of the transwell chamber was coated with 60 μL Matrigel and incubated for 30 min to gel at 37°C. Then, 5 × 104 cells of MDA-MB-231 were resuspended in serum-free L-15 medium and transferred into the upper chamber of each transwell. Lower chambers contained fresh L-15 medium with 20 ng/mL TGF-β1 in the absence or presence of hesperetin. After culturing for 24 hour, the cells remaining at the upper surface of the membrane were removed using a swab, whereas those cells that had invaded the lower membrane surface were fixed with 4% paraformaldehyde for 10 minute and stained with 0.5% crystal violet for 30 minute. The number of cells invaded through the filter was photographed using a Keyence microscope (Osaka, Japan) at a magnification of 10× and counted by NIH Image-J software.
Fluorescence Staining
Fluorescence studies were carried out as described previously. 22 In brief, the MDA-MB-231 cells were seeded on sterile coverslips that were previously coated with 200 μL 0.3% gelatin at room temperature for 30 minute. When the cells grew up to approximately 70% confluence, the serum-free L-15 medium was replaced for 24 hour. After the treatment with TGF-β1 in the absence or presence of hesperetin for 24 hour, the cells were fixed with 4% paraformaldehyde and stained with rhodamine phalloidin overnight at 4°C, then conjugated with DAPI for 1 h at room temperature after being washed with PBS (-) 3 times. Before observation, the cells were mounted with PermaFluor aqueous mounting medium (Thermo Fisher Scientific, Waltham, MA, USA). Stained cells were observed on a fluorescence microscope (Keyence, Osaka, Japan).
Immunoprecipitation
We performed Fyn and paxillin immunoprecipitation in MDA-MB-231 cells using Capturem IP & Co-IP Kit (Takara Bio, USA). Briefly, cell lysates were prepared as described previously 23 and clarified by centrifugation at 10 000×g and 4°C for 10 minute. About 100 µg total protein was removed for immunoprecipitation after the protein concentration was detected. 500 µL protein sample pre-incubated with primary antibody was loaded onto the equilibrated spin column, and centrifuged at 1000×g for 1 minute at room temperature. After being washed using the wash buffer in kits, the spin column was inserted into the collection tube containing 5 µL neutralization buffer. The eluted sample was collected by using 50 µL Elution Buffer and incubated with SDS sample buffer at 95°C for 5 minute for western blot analysis.
Fyn siRNA
MDA-MB-231 cells were cultured using antibiotic-free Leibovitz’s L-15 medium in 24-well plates. The cells were transfected with Fyn siRNA (80 nM) for 72 hour by using a ScreenFect Plus A Kit (Wako, Japan) according to the manufacturer’s recommendations. AllStars Negative Control siRNA was used as a control. The cells were subjected to serum starvation for 24 hour, then were incubated in serum-free medium containing 20 ng/mL TGF-β1 in the absence or presence of hesperetin (100 μM). After stimulation, cell lysates were collected using sample buffer, and western blot analysis was then carried out to confirm the knockdown efficacy of Fyn and to observe the expression of p-paxillin(Y31) and p-MYPT1 after transfection of MDA-MB-231 cells with Fyn siRNA.
Western Blot
Western blot was carried out as described previously. 22 Briefly, after treatment with TGF-β1 in the absence or presence of hesperetin for 48 hour, MDA-MB-231 cells were collected in a RIPA buffer (Wako, Japan) added with protein inhibitors by using a cell scraper. The proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes were blocked using 5% non-fat milk solution, then were treated with primary antibodies at 4°C overnight. Next, the membranes were washed 3 times in TBS-T and incubated with secondary antibodies for 1 hour at room temperature. Finally, Super Signal West Pico chemiluminescence substrates (Thermo Fisher Scientific, Waltham USA) were used to visualize the stained proteins. The protein bands were evaluated using Quantity One with ChemuDoc XRS-J software (Bio-Rad, USA).
Statistical Analyses
Statistical differences were detected using the unpaired Student’s t-test. Values are presented as mean ± SD and each experiment was performed at least 6 times. P values <0.05 were considered statistically significant. Calculations were performed using GraphPad Prism 8.4 for Windows-statistical software (GraphPad Prism software, California, USA).
Results
Cytotoxicity of Hesperetin to MDA-MB-231 Breast Cancer Cells
Hesperetin naturally presents in Citrus fruits and Traditional Chinese Medicine herbs, the chemical structure and sources of which are shown in Figure 1A. We first investigated the anti-proliferative effect of hesperetin on MDA-MB-231 cells. We treated the cells with various concentrations of hesperetin (0, 3.125, 6.25, 12.5, 25, 50,100, and 200 μM) for 48 hour, and then cell viability was measured. As shown in Figure 1B, hesperetin treatment at the concentration of ≤100 μM did not influence the viability of MDA-MB-231 cells compared to the control group. In contrast, 200 μM hesperetin treatment significantly inhibited the growth of MDA-MB-231 cells with 51.3% cell viability inhibition percentage. Based on this result, 25, 50, and 100 μM hesperetin was selected for the subsequent experiments to observe the effect of hesperetin on migration and invasion of MDA-MB-231 cells induced by TGF-β1.
Figure 1.
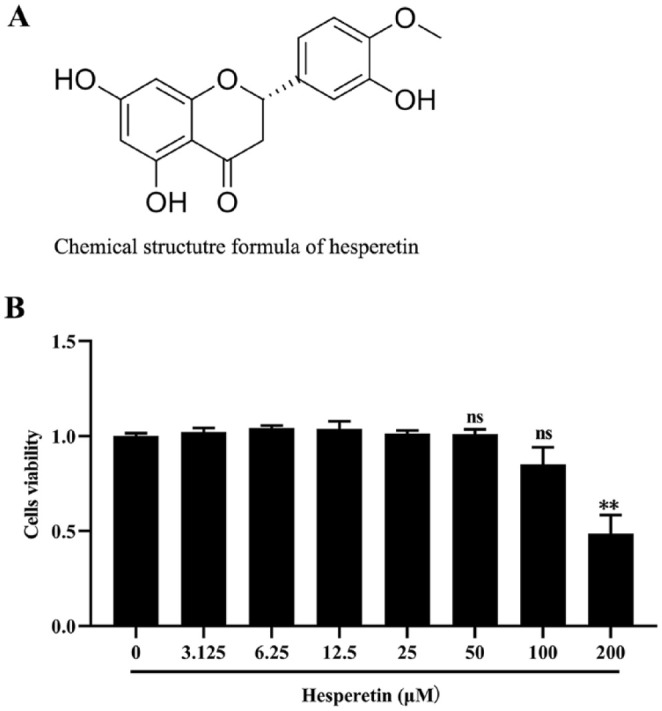
Chemical structure formula of hesperetin and the cytotoxicity effect of hesperetin on the MDA-MB-231 cells. (A) Chemical structure formula of hesperetin. (B) The cytotoxicity of hesperetin on MDA-MB-231 cells was detected by CCK-8 Kit assay. The result shows that hesperetin treatment at the concentration of ≤100 μM had no influence on the viability of MDA-MB-231 cells compared to the control group. By contrast, 200 μM hesperetin treatment obviously inhibited the growth of MDA-MB-231 cells with statistical significance. Each result represents mean ± SD of 6 independent experiments. ** P < 0.01 versus control group.
Hesperetin Inhibits Migration and Invasion in MDA-MB-231 Breast Cancer Cells
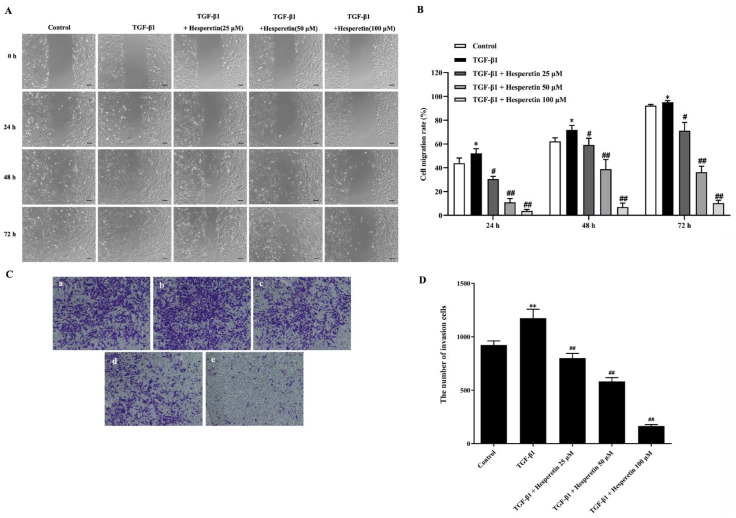
To determine whether hesperetin inhibits breast cancer metastasis, wound healing and transwell invasion assays were used to evaluate the effect of hesperetin on migration and invasion of MDA-MB-231 cells. As shown in Figure 2A and B, TGF-β1 treatment induced the migration of MDA-MB-231 cells, which were significantly inhibited by the hesperetin treatment. Additionally, hesperetin significantly inhibited TGF-β1-induced invasion of MDA-MB-231 cells (Figure 2C and D). Taken together, these results strongly suggest that hesperetin inhibited the TGF-β1-mediated migration and invasion of MDA-MB-231 cells.
Figure 2.
Hesperetin inhibits the migration and invasion of MDA-MB-231 cells induced by TGF-β1. (A) Wound healing assay was applied to observe the inhibitory effect of hesperetin on the migration of MDA-MB-231 cells induced by TGF-β1. Cells were treated with TGF-β1 positive control or co-incubation of TGF-β1 and (25, 50, and 100 μM) hesperetin. Photos were taken with Keyence fluorescent microscope at 10× magnification at 0, 24, 48, and 72 hour after treatment. Scale bar = 100 μm. (B) The treatment of hesperetin inhibits dose-dependently migration induced by TGF-β1 in MDA-MB-231 cells. Cell migration rate was analyzed using NIH ImageJ software. Each result represents mean ± SD of 6 independent experiments. *P < 0.05 versus control group; #P < 0.05 versus TGF-β1 group; ## P < 0.01 versus TGF-β1 group. (C) Transwell invasion assay was used to evaluate the inhibitory effect of hesperetin on the invasion of MDA-MB-231 cells induced by TGF-β1. a: Control; b: TGF-β1; c: TGF-β1 + hesperetin 25 μM; d: TGF-β1 + hesperetin 50 μM; e: TGF-β1 + hesperetin 100 μM. Photos were taken with Keyence microscope at 10 × magnification at 48 hour after treatment. (D) The statistical figure results show that the TGF-β1-mediated invasion was significantly decreased in a dose-dependent manner in MDA-MB-231 cells after treatment with hesperetin. The number of invading cells was counted using NIH ImageJ software, and statistical analysis of results was analyzed with Graphpad Prism 8.4 software. Data are expressed as the mean ± SD, n = 6. **P < 0.01 versus control group; ##P < 0.01 versus TGF-β1 group.
Hesperetin Inhibits Actin Stress Fiber Formation in MDA-MB-231 Cells
Actin stress fiber formation has been reported to regulate migration and invasion of tumor cells. 24 TGF-β1 is demonstrated to induce actin stress fiber formation. 25 We observed changes of the actin stress fiber formation in the absence or presence of hesperetin by immunofluorescence. As shown in Figure 3, the treatment with TGF-β1 induced remarkable actin stress fiber formation compared with control cells. By contrast, hesperetin treatment significantly inhibited TGF-β1-induced actin stress fiber formation (Figure 3).
Figure 3.
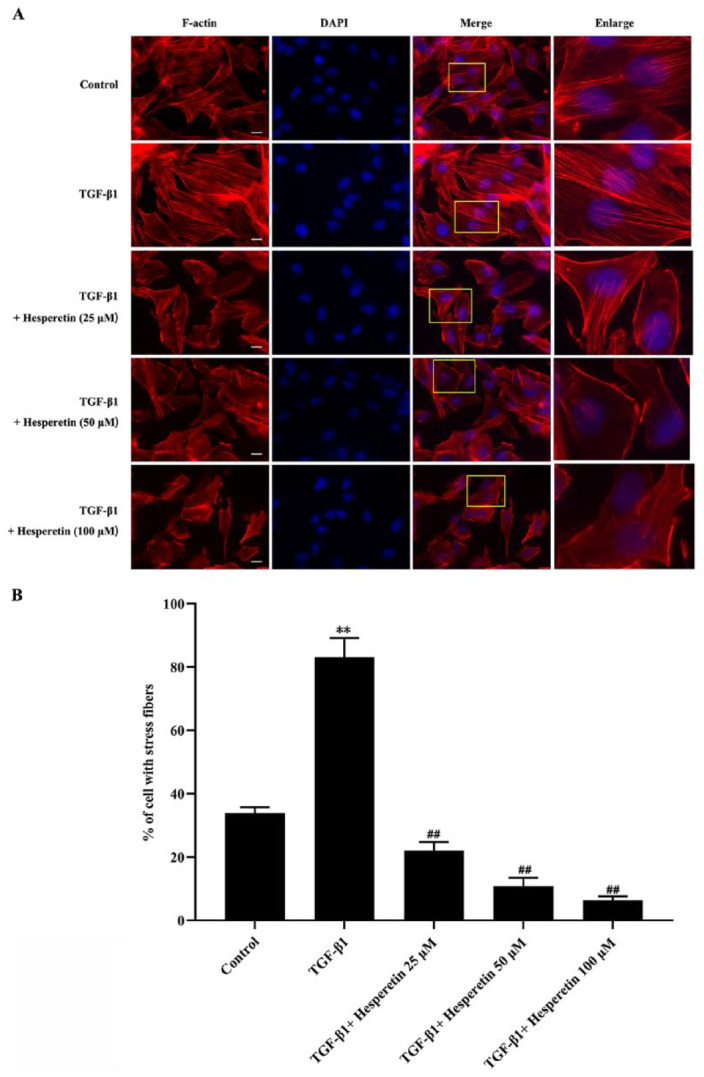
The inhibitory effect of hesperetin on the actin stress fiber formation in MDA-MB-231 breast cancer cells. (A) MDA-MB-231 cells were serum-starved for 24 hour, followed by TGF-β1 stimulation in different concentrations hesperetin. Immunofluorescence results were obtained by Keyence fluorescent microscope at 60 × magnification at 24 hour after hesperetin treatment. F-actin was stained by rhodamine phalloidin (red). Nuclear was stained by DAPI (blue). The boxed images were enlarged in the right panels. Scale bar = 10 μm. (B) Actin stress fiber formation was quantified by the percentage of cells with stress fibers in the experiments described above. Data are the means of 3 experiments in which at least 40 cells were counted per experiment. Data are expressed as the mean ± SD **P < 0.01 versus control group; ## P < 0.01 versus TGF-β1 group.
Hesperetin Inhibits Activation of Fyn and Tyrosine Phosphorylation of Paxillin
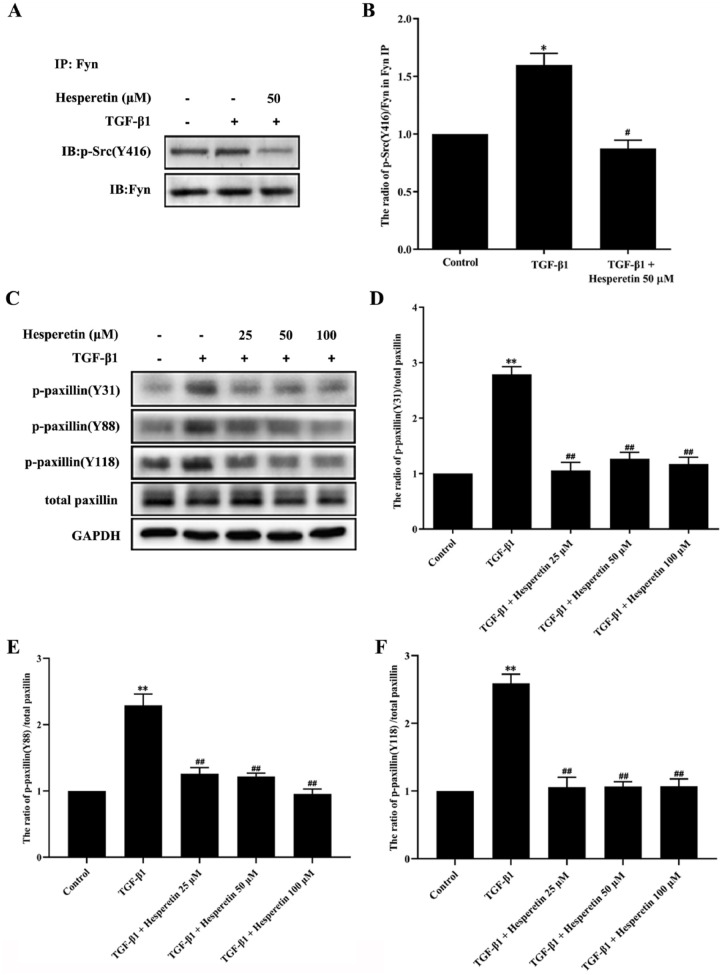
Fyn was shown to be involved in the migration and invasion of different cell types.5,6 Like other members of SFK, activation of Fyn is regulated by tyrosine phosphorylation and dephosphorylation of Fyn at 2 sites Y420 and Y531 (in humans and pigs). 26 While phosphorylation at Y420 upregulates enzyme activity, phosphorylation at Y531 reduces enzyme activity. 26 The phosphorylation of paxillin tyrosine at Tyr31, Tyr88 and Tyr118 sites plays a vital role in the migration and invasion of various tumors.23,27 To determine whether hesperetin inhibits TGF-β1-induced Fyn activation and paxillin phosphorylation during the migration and invasion of MDA-MB-231 cells, we analyzed Fyn activation via western blot analysis using a phospho-Src family (Y416) polyclonal antibody (Cell Signaling Technology, USA) after Fyn immunoprecipitation, and investigated the phosphorylation of paxillin at Tyr31, Tyr88, and Tyr118 sites in MDA-MB-231 cells. As shown in Figure 4A and B, western blot analysis revealed that the phosphorylation of Src (Y416) induced by TGF-β1 was markedly blocked by 100 μM hesperetin in MDA-MB-231 cells (P < 0.01), suggesting that hesperetin inhibited the activation of Fyn induced by TGF-β1. Compared with the TGF-β1 treatment group, hesperetin (25, 50, and 100 μM) reduced TGF-β1-induced phosphorylation of paxillin at Y31, Y88, and Y118 with a statistical significance (P < 0.05) (Figure 4C–F).
Figure 4.
The inhibitory effect of hesperetin on the activation of Fyn and phosphorylation of paxillin in MDA-MB-231 breast cancer cells. (A and B) Hesperetin inhibited the activation of Fyn in MDA-MB-231 cells. (A) Analysis of Fyn activation via p-Src immunoblotting after Fyn immunoprecipitation from MDA-MB-231 cell samples. Representative western blot showed the variation of p-Src (Y420) in different groups. (B) Statistical analysis revealed that Fyn activation (proportion of Fyn phosphorylation at Y420 to total Fyn after Fyn immunoprecipitation). Data were presented as the mean ± SD, n = 6. *P < 0.05 versus control group; #P < 0.05 versus TGF-β1 group. (C–F) Hesperetin inhibited the tyrosine phosphorylation of paxillin at Y31, Y88, and Y118 sites induced by TGF-β1 in MDA-MB-231 cells. (C) The tyrosine phosphorylation of paxillin in MDA-MB-231 cells was analyzed by western blot. (D–F) Statistical analysis revealed tyrosine phosphorylation of paxillin (proportion of paxillin phosphorylation at Y31, Y88, and Y118 sites to total paxillin, respectively). Total paxillin was used as control. Data are expressed as the mean ± SD, n = 6. **P < 0.01 versus control group; ##P < 0.01 versus TGF-β1 group.
Hesperetin Inhibits the Expression of RhoA and Activation of Rho-kinase
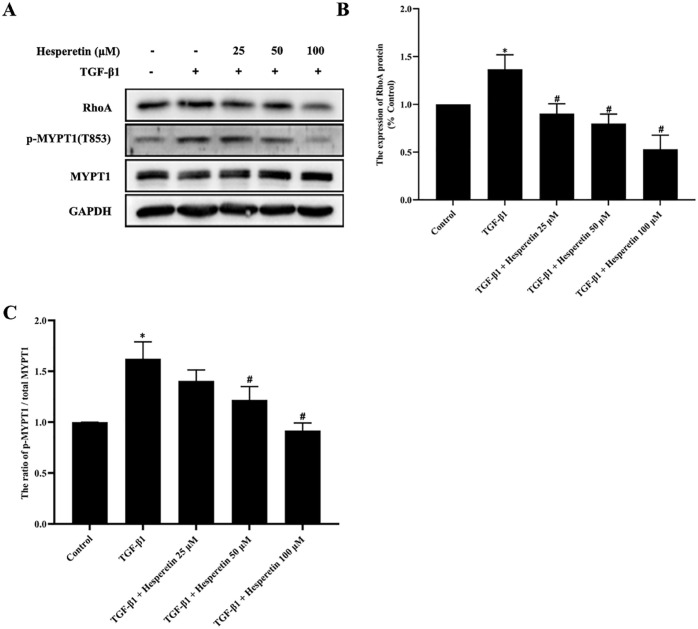
The RhoA/Rho-kinase (ROCK) signaling pathway plays an essential role in TGF-β1-induced migration and invasion of cancer cells. 28 Our results showed that hesperetin significantly increased RhoA expression through TGF-β1 treatment (P < 0.05), as shown in Figure 5A and B. We observed the expression level of phosphorylated MYPT1 (an indicator of ROCK activation) in the MDA-MB-231 cells. As shown the Figure 5A and C, 50, and 100 μM hesperetin treatment significantly downregulated the expression of p-MYPT1 in the MDA-MB-231 cells compared with the TGF-β1 group.
Figure 5.
The inhibitory effect of hesperetin on the expression of RhoA and the activation of Rho-kinase in MDA-MB-231 breast cancer cells. (A) The expression of RhoA and ROCK activation (proportion of MYPT1 phosphorylation at T853 site to total MYPT1) in MDA-MB-231 cells were analyzed by western blot. (B and C) Quantification of experiments as in (A). The results showed that TGF-β1-induced increased expression of RhoA and p-MYPT1 were significantly blocked by hesperetin (50 and 100 μM). Data are expressed as the mean ± SD, n = 6. *P < 0.05 versus control group; # P < 0.05 versus TGF-β1 group.
The Interaction Between Fyn, Paxillin and RhoA, and the Effect of Fyn siRNA on p-paxillin (Y31) and p-MYPT1
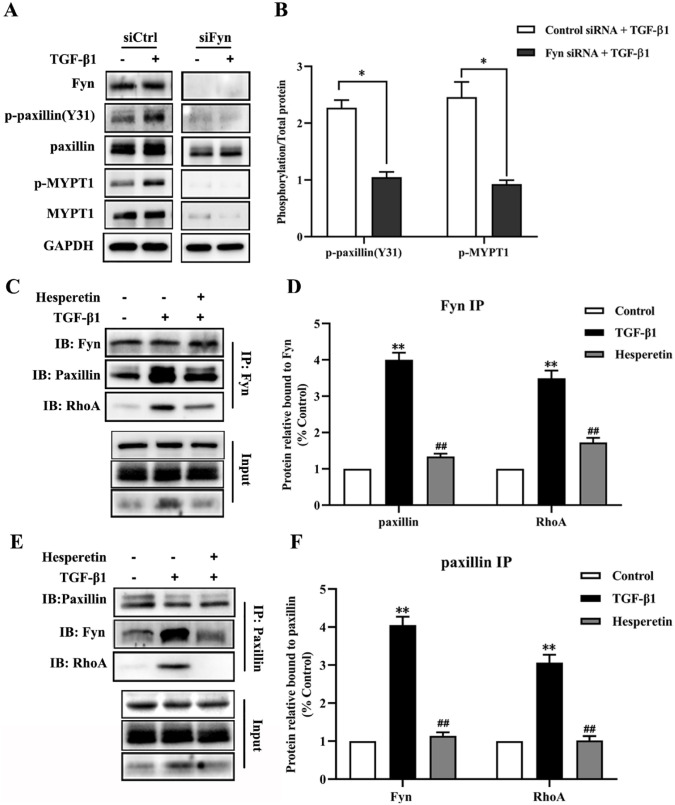
To further investigate the role of Fyn in metastasis induced by TGF-β1, we utilized siRNA to knock down Fyn in MDA-MB-231 cells. As the Figure 6A and B show, siRNA-mediated knockdown of Fyn abrogated the TGF-β1-induced phosphorylation of paxillin(Y31) and MYPT1. Stimulation of MDA-MB-231 cells in the control siRNA group with TGF-β1 obviously increased the expression of p-paxillin(Y31) and p-MYPT1 (Figure 6A and B). Importantly, the TGF-β1-induced p-paxillin(Y31) and p-MYPT were significantly suppressed in the group of Fyn-knockdown MDA-MB-231 cells compared with the group of control siRNA-transfected MDA-MB-231 cells (P < 0.05).
Figure 6.
The interaction between Fyn, paxillin and RhoA and the role of Fyn in paxillin/RhoA pathway. (A) Western blot analysis of lysates from MDA-MB-231 cells transfected with Fyn siRNA or control siRNA with or without TGF-β1 treatment. (B) Quantitative statistical analysis of immunoblots (A) indicates the reduced phosphorylation of paxillin (Y31) and MYPT1 at T853 after knockdown of Fyn. Data are expressed as the mean ± SD, n = 6. *P < 0.01 Fyn siRNA group versus control siRNA group. (C) Immunoprecipitation of Fyn in MDA-MB-231 cells reveals the interaction between Fyn, paxillin and RhoA. (D) Quantitative statistical analysis of immunoblots in (C) shows relative paxillin and RhoA levels after Fyn immunoprecipitation. Data are expressed as the mean ± SD, n = 6. **P < 0.01 versus control group; ## P < 0.01 versus TGF-β1 group. (E) Immunoprecipitation of paxillin in MDA-MB-231 cells reveals the interaction between Fyn, paxillin and RhoA. (F) Quantitative statistical analysis of immunoblots in (E) shows relative Fyn and RhoA levels after paxillin immunoprecipitation. Data are expressed as the mean ± SD, n = 6. **P < 0.01 versus control group; ##P < 0.01 versus TGF-β1 group.
We next applied immunoprecipitation to investigate the interaction of Fyn, paxillin, and RhoA in migration and invasion of MDA-MB-231 cells induced by TGF-β1, considering that all of them play a vital role in the metastasis of breast cancer. Western blot results showed the interaction between the Fyn, paxillin and RhoA in TGF-β1 treatment group was obviously increased both after Fyn immunoprecipitation (Figure 6C and D) and paxillin immunoprecipitation (Figure 6E and F), compared with the control group. By contrast, the increased interaction between the Fyn, paxillin, and RhoA were suppressed by hesperetin.
Discussion
It has been well confirmed that TNBC treatment is hampered by metastasis. However, the mechanism of TNBC metastasis remains unclear. The current study demonstrated that hesperetin prevents migration and invasion of human triple-negative breast cancer cell line MDA-MB-231 induced by TGF-β1. These inhibitory effects are attributed to hesperetin reducing TGF-β1-induced Fyn activation, tyrosine phosphorylation of paxillin at Y31, Y88, and Y118 sites, the increased expression of RhoA, and ROCK activation, suggesting that hesperetin has an inhibitory effect on migration and invasion via the Fyn/paxillin/RhoA pathway. This research strongly showed that hesperetin is valuable as a therapeutic tool for treating TNBC metastasis.
The rearrangement of the actin cytoskeleton is the first step in tumor migration and invasion.29,30 It has been demonstrated that TGF-β1 induced mobilization of the actin cytoskeleton. 25 Because hesperetin inhibited migration and invasion in both wound healing and transwell assays, we hypothesized that hesperetin might affect actin cytoskeleton rearrangement. As we expected, hesperetin treatment significantly reduced TGF-β1-induced actin stress fibers formation (Figure 3A and B). The Rho family of GTPases is associated with the migration and invasion of cancer cells. 31 It is generally recognized that the expression and activation of RhoA and ROCK affect actin stress fiber formation. 32 Our results showed the increased RhoA expression in MDA-MB-231 cells treated with TGF-β1, while that of the hesperetin-treated samples was clearly reduced. Simultaneously, TGF-β1-induced ROCK activation was obviously suppressed after treatment with hesperetin compared with the TGF-β1 group. These results suggested that hesperetin inhibited TGF-β1-induced migration and invasion via restraining the RhoA/ROCK signal pathway.
The tyrosine phosphorylation of paxillin plays a crucial role in the migration and invasion of tumor cells. In fact, a large number of experimental studies on tumors have shown that the increased phosphorylation of paxillin at Tyr31 and Tyr118 residues were considered as a marker of metastasis. 7 Previous study suggested that paxillin may play an important role in regulating signals from Rho family of GTPases to the actin cytoskeleton. 33 Paxillin has also been shown to coordinate the spatiotemporal activation of the Rho family of GTPases, thus allowing the activation of various signaling pathways related to cell migration.13,34 Evidence showed that the phosphorylation of paxillin Tyr 31 and Tyr 118 causes the activation of RhoA/ROCK pathway to regulate migration and invasion of tumor cells. 35 Additionally, previous study has confirmed that paxillin phosphorylated in different tissues and cells can enhance RhoA activity or expression. 23 The present study confirmed that hesperetin reduced the TGF-β1-induced phosphorylation of paxillin Tyr31, Tyr88, and Tyr118 in MDA-MB-231 cells.
Fyn phosphorylates tyrosine residues on key targets involved in a variety of different signaling pathways. 36 Fyn is known to interact with some molecular signals including paxillin, 11 which possibly lends insight into its role in tumor metastasis. Moreover, it has been observed that some factors can regulate the tyrosine phosphorylation of paxillin by regulating SFK, and induce migration and invasion of tumor cells. 27 It has been reported that Src directly phosphorylates paxillin at Y88. 37 We speculated that Fyn is involved in the metastasis of TNBC and attributed to regulate the tyrosine phosphorylation of paxillin. Our results demonstrated that hesperetin inhibited the TGF-β1-induced activation of Fyn in MDA-MB-231 cells. The knockdown of Fyn abolished the TGF-β1-induced phosphorylation of paxillin and ROCK activation in MDA-MB-231 cells. Additionally, the present results indicated that the interaction between Fyn, paxillin and RhoA plays a key role in the metastasis of MDA-MB-231 cells induced by TGF-β1, which was inhibited by hesperetin. Taken together, our results suggest that the key role of Fyn as an upstream molecule of the paxillin/RhoA pathway is clarified for the first time in the metastasis of MDA-MB-231 cells induced by TGF-β1. These data highlight the importance of Fyn in regulation of TNBC metastasis.
Hesperetin, as a natural flavonoid, belongs to the class of polyphenols. Our study demonstrated for the first time that hesperetin plays an anti-tumor role in TNBC MDA-MB-231cells by inhibiting the Fyn/Paxillin/RhoA signaling pathway. It has been reported that some of polyphenols have obvious antitumor activity with different mechanisms of action.38-41 It is expected to explore more polyphenolic compounds with this activity. Although we aimed to investigate the anti-metastasis effect and mechanism of hesperetin on TNBC, to some extent, the lack of in vivo experiments may be a limitation of our study. In order to further confirm the effect and mechanism of hesperetin and whether hesperetin or other polyphenols can be used as a therapeutic or protective agent of TNBC in the future, many in vivo studies will be needed.
Conclusions
In summary, the present study demonstrated that hesperetin inhibits the TGF-β1-mediated migration and invasion of TNBC MDA-MB-231 cells, and the potential mechanism involved in the Fyn/paxillin/RhoA signaling pathway. Our studies confirmed that the Fyn/paxillin/RhoA signaling pathway plays an important role in TNBC metastasis. Importantly, we confirmed for the first time the interaction of Fyn, paxillin and RhoA in the metastasis of MDA-MB-231 cells induced by TGF-β1. Our research suggested that the Fyn/paxillin/RhoA signaling pathway serves as a pathway of therapeutic targets for TNBC therapy. Moreover, our data can accelerate the development of hesperetin as a therapeutic agent for the treatment of TNBC, although the present study lacked in vivo experiments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Scientific Research Fund of Jilin Provincial Education Department, China [JJKH20210485KJ].
Ethics Statement: The protocol for this research project has been approved by the Institutional Ethics Committee of Jilin Medical University.
ORCID iD: Qian Lu  https://orcid.org/0000-0001-6742-907X
https://orcid.org/0000-0001-6742-907X
References
- 1. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25-32. [DOI] [PubMed] [Google Scholar]
- 2. Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res. 2018;8:1483-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson D, Fournel M, Veillette A. Oncogenic activation of p59fyn tyrosine protein kinase by mutation of its carboxyl-terminal site of tyrosine phosphorylation, tyrosine 528. J Biol Chem. 1994;269:10956-10963. [PubMed] [Google Scholar]
- 4. Semba K, Nishizawa M, Miyajima N, et al. Yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci USA. 1986;83:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posadas EM, Al-Ahmadie H, Robinson VL, et al. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu J, Zhou Z, Wei Z, et al. FYN promotes gastric cancer metastasis by activating STAT3-mediated epithelial-mesenchymal transition. Transl Oncol. 2020;13:100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Webb DJ, Schroeder MJ, Brame CJ, et al. Paxillin phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4925-4929. [DOI] [PubMed] [Google Scholar]
- 9. Mizutani T, Shiraishi K, Welsh T, Ascoli M. Activation of the lutropin/choriogonadotropin receptor in MA-10 cells leads to the tyrosine phosphorylation of the focal adhesion kinase by a pathway that involves Src family kinases. Mol Endocrinol. 2006;20:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang X, Draghi NA, Resh MD. Signaling from integrins to fyn to rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samayawardhena LA, Kapur R, Craig AW. Involvement of fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan J, Chen L, Xiao J, et al. SHROOM2 inhibits tumor metastasis through RhoA-ROCK pathway-dependent and – independent mechanisms in nasopharyngeal carcinoma. Cell Death Dis. 2019;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodge RG, Ridley AJ. Regulating rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496-510. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Gallo KA. MLK3 regulates paxillin phosphorylation in chemokine-mediated breast cancer cell migration and invasion to drive metastasis. Cancer Res. 2012;72:4130-4140. [DOI] [PubMed] [Google Scholar]
- 15. Androutsopoulos VP, Tsatsakis AM. Benzo[a]pyrene sensitizes MCF7 breast cancer cells to induction of G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via activation of cell signaling proteins and CYP1-mediated metabolism. Toxicol Lett. 2014;230:304-313. [DOI] [PubMed] [Google Scholar]
- 16. Park EJ, Min HY, Chung HJ, et al. Down-regulation of c-Src/EGFR-mediated signaling activation is involved in the honokiol-induced cell cycle arrest and apoptosis in MDA-MB-231 human breast cancer cells. Cancer Lett. 2009;277:133-140. [DOI] [PubMed] [Google Scholar]
- 17. Androutsopoulos V, Arroo RR, Hall JF, Surichan S, Potter GA. Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res. 2008;10:R39-NaN12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salmela AL, Pouwels J, Kukkonen-Macchi A, et al. The flavonoid eupatorin inactivates the mitotic checkpoint leading to polyploidy and apoptosis. Exp Cell Res. 2012;318:578-592. [DOI] [PubMed] [Google Scholar]
- 19. Gest C, Joimel U, Huang L, et al. Rac3 induces a molecular pathway triggering breast cancer cell aggressiveness: differences in MDA-MB-231 and MCF-7 breast cancer cell lines. BMC Cancer. 2013;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossen NS, Hansen AJ, Selhuber-Unkel C, Oddershede LB. Arachidonic acid randomizes endothelial cell motion and regulates adhesion and migration. PLoS One. 2011;6:e25196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okamoto T, Akita N, Kawamoto E, Hayashi T, Suzuki K, Shimaoka M. Endothelial connexin32 enhances angiogenesis by positively regulating tube formation and cell migration. Exp Cell Res. 2014;321:133-141. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Zhang M, Lyu B, Kishi H, Kobayashi S. Omega-3 and omega-6 DPA equally inhibit the sphingosylphosphorylcholine-induced Ca2+-sensitization of vascular smooth muscle contraction via inhibiting Rho-kinase activation and translocation. Sci Rep. 2017;7:36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. Cell Physiol. 2007;213:740-749. [DOI] [PubMed] [Google Scholar]
- 25. Edlund S, Landström M, Heldin CH, Aspenström P. Transforming growth factor-beta-induced mobilization of Actin Cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunter T. A tail of Two Src’s: Mutatis Mutandis. Cell. 1987;49:1-4. [DOI] [PubMed] [Google Scholar]
- 27. Aponte M, Jiang W, Lakkis M, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Res. 2008;68:5839-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maeda D, Kubo T, Kiya K, et al. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-β1 secretion in abnormal scar formation. J Plast Surg Hand Surg. 2019;53:288-294. [DOI] [PubMed] [Google Scholar]
- 29. Zhao H, Jiao Y, Zhang Z. Deguelin inhibits the migration and invasion of lung cancer A549 and H460 cells via regulating actin cytoskeleton rearrangement. Int J Clin Exp Pathol. 2015;8:15582-15590. [PMC free article] [PubMed] [Google Scholar]
- 30. Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261-272. [DOI] [PubMed] [Google Scholar]
- 31. Schmitz AA, Govek EE, Böttner B, Van Aelst L. Rho GTPases: signaling, migration, and Invasion. Exp Cell Res. 2000;261:1-12. [DOI] [PubMed] [Google Scholar]
- 32. Katoh K. Activation of rho-kinase and focal adhesion kinase regulates the organization of stress fibers and focal adhesions in the central part of fibroblasts. PeerJ. 2017;5:e4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen GC, Turano B, Ruest PJ, Hagel M, Settleman J, Thomas SM. Regulation of rho and rac signaling to the actin cytoskeleton by paxillin during Drosophila development. Mol Cell Biol. 2005;25:979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23-32. [DOI] [PubMed] [Google Scholar]
- 35. Iwasaki T, Nakata A, Mukai M, et al. Involvement of phosphorylation of TYR-31 and TYR-118 of paxillin in MM1 cancer cell migration. Int J Cancer. 2002;97:330-335. [DOI] [PubMed] [Google Scholar]
- 36. Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn, a novel molecular target in cancer. Cancer. 2010;116:1629-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Scott A, Zhang P, et al. Regulation of paxillin-p130-PI3K-AKT signaling axis by src and PTPRT impacts colon tumorigenesis. Oncotarget. 2017;8:48782-48793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin BW, Gong CC, Song HF, Cui YY. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 2017;174:1226-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng HL, Hsieh MJ, Yang JS, et al. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget. 2016;7:35208-35223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernández-Palanca P, Fondevila F, Méndez-Blanco C, Tuñón MJ, González-Gallego J, Mauriz JL. Antitumor effects of Quercetin in Hepatocarcinoma in vitro and in vivo models: A systematic review. Nutrients. 2019;11:2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Q, Huang H, Zheng F, et al. Resveratrol exerts antitumor effects by downregulating CD8+CD122+ Tregs in murine hepatocellular carcinoma. OncoImmunology. 2020;9:1829346. [DOI] [PMC free article] [PubMed] [Google Scholar]