Abstract
A high temporal resolution, 4-chamber (4CH) cine is the standard method for determining cardiac rest periods during whole heart coronary magnetic resonance angiography (CMRA). We evaluated the image quality and reproducibility between the 4CH cine method and a novel approach using a velocity encoded mitral valve inflow cine (MVI). The goal of this study was to compare the quality of CMRAs utilizing MVI versus 4CH methods. Sharpness and vessel length for the LCA and RCA using each method were determined using Soap Bubble and two blinded observers independently assessed coronary image quality. Offline analysis on a separate, retrospective cohort (n = 25) was used to compare MVI and 4CH reproducibility. In the prospectively evaluated cohort there was no difference in overall vessel sharpness (4CH vs MVI mean ± SD) (31.0 ± 5.5% vs 30.5 ± 5.7%, p = .63), LCA vessel sharpness (30.0 ± 5.4% vs 31.1 ± 8.2%, p = .44), LCA length (4.7 ± 1.4 cm vs 4.6 ± 1.6 cm, p = .66), RCA vessel sharpness (32.1 ± 6.9% vs 31.1 ± 7.7%, p = .55), RCA length (5.51 ± 2.6 cm vs 5.95 ± 2.4 cm, p = .38), or image quality rating (2.66 vs 2.62, p = .80) between methods. In the retrospective cohort, the MVI method had 5.4% lower inter-observer variability (95% CI 3.7,7.2%, p < .0001) and 3.9% lower intra-observer variability (95% CI 2.4,5.4%, p < .0001) than the 4CH method.
MVI is a technically feasible and more reproducible method to determine cardiac rest periods compared to 4CH while preserving vessel sharpness, vessel length & image quality.
Keywords: CT and MRI, diagnostic testing, cardiology, cardiovascular imaging agents / techniques, diagnostic testing, cardiology, coronary angiography, coronary wall motion
Objectives
Whole heart coronary magnetic resonance angiography (CMRA) has become an invaluable addition in the evaluation of patients with congenital heart disease (CHD) by providing both detailed cardiac morphology and coronary anatomy.1–3 Data acquisition is usually performed while the heart is still, during the end-systolic rest period (ESRP) and mid-diastolic rest period (MDRP), to eliminate blurring from cardiac motion. The current reference standard for cardiac rest period determination during CMRA acquisition requires the reading physician to define the cardiac rest periods visually using a high-temporal resolution balanced steady-state free precession (SSFP) 4 chamber (4CH) cine as previously described in the literature.4–7 As a result, an experienced reading physician, specifically trained in the 4CH method is required to determine the cardiac rest periods as they can vary substantially from patient to patient. 8
Currently, automated detection techniques to determine cardiac rest periods are difficult to use in daily clinical practice as they require off-line analysis with specific software not integrated into commercially available MRI platforms. Studies evaluating cardiac rest period determination by automated detection have not demonstrated significant benefit over real time visual analysis. Some automated methods calculated much shorter shot durations, 9 were unable to accommodate real time changes in heart rate during scanning, or required increased processing time. 10 Sato et al. also recently developed an automated method using the mitral valve, tricuspid valve, and the apex of the heart, but the cardiac rest periods (both MDRP and ESRP) were found to start earlier than that determined by the physicians. 11 Overall, automated detection of cardiac rest periods remains a promising concept, but the application of 4CH and other methods of cardiac rest period determination have not clearly shown to provide any benefit over the current standard 4CH using real time visual analysis.
Otton et al. have previously illustrated the importance of choosing an accurate trigger time and used tissue doppler tracing as a method to clearly identify the cardiac rest phases. 12 In a similar vein, we developed a novel approach using velocity encoded mitral valve inflow cine (MVI) imaging. Utilizing spectral doppler echocardiographic principles, the MVI flow data when represented on a velocity graph represents the early diastolic, passive ventricular filling wave (or “E” wave in spectral doppler echocardiography) and the late diastolic, active ventricular filling wave secondary to atrial contraction (or “A” wave in spectral doppler echocardiography). Using this graph, the cardiac rest phases can be readily determined. 12
We hypothesized determination of MDRP and ESRP with MVI for CMRA acquisition may be superior to hitherto established visual analysis with a 4CH CINE. Purpose of this study was a systematical analysis of both methods with a comparison of image quality, reproducibility of resting phases and their evaluation time respectively.
Design
Study population
Data from consecutive patients who were referred for a clinically indicated Cardiac MRI to include a CMRA at our institution between December 2017 and April 2018 were prospectively reviewed and analyzed. All patients were in sinus rhythm during the procedure. The UT Southwestern Institutional Review Board approved the study and there was a waiver of consent (STU 032016-009).
MRI technique- equipment set up
An Ingenia 1.5 Tesla scanner (Philips, Best, The Netherlands) with the 32-channel phased array digital receiver coil for signal reception was used to obtain the CMRA imaging by an experienced technician.
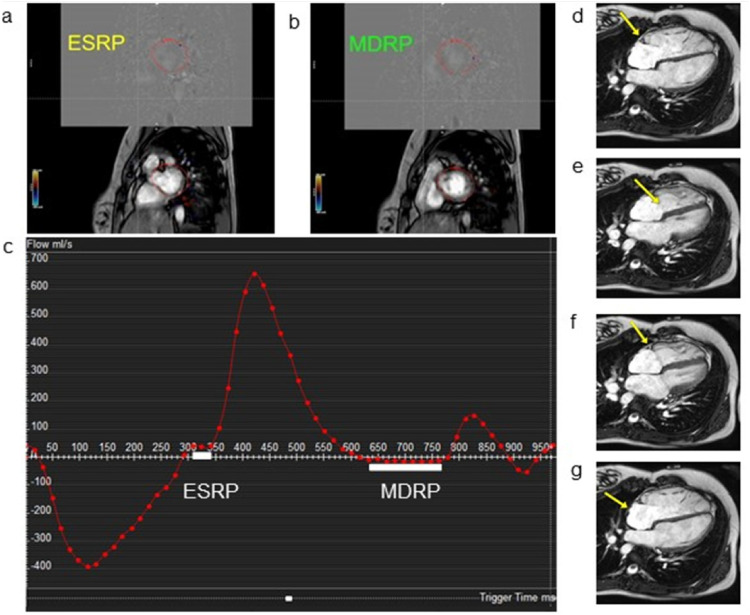
Each patient had a standard dual phase, high temporal resolution 4CH cine performed (60 phases, field of view (FOV) was 350 mm, slice thickness 8 mm, acquired voxel size was 2 × 2 mm, echo time (TE) was 1.49 and repetition time (TR) 3.0, Parallel imaging (SENSE factor = 2), Echo train length 9. The cardiac rest periods were determined using the 4CH cine slice as previously described in the literature4–7,13: ESRP was defined as starting from cessation of movement of the right coronary artery (RCA) in the right atrioventricular AV groove (i.e. pause in visible contraction of the Right ventricle (RV)) to just before the tricuspid valve opens; and MDRP was defined as starting from the cessation of movement of the RCA at the end of the rapid inflow until the beginning of atrial wall contraction, or atrial systole (Figure 1).
Figure 1.
Cardiac rest period determination by MVI and 4CH methods during CMRA. (a) and (b) represent single frames during a velocity-encoded mitral valve inflow (MVI) cine depicting ESRP and MDRP as viewed by reading physician when there is cessation of blood flow across mitral valve. (c) Graph of blood flow across the mitral valve (y-axis) over time (x-axis). White bars represent cardiac rest periods when there is cessation of blood flow. (d) In a 4CH cine, ESRP begins when the RCA (arrow) stops moving. (e) The end of ESRP is defined by the opening of the tricuspid valve (arrow). (f) After passive ventricular filling in early diastole, the RCA (arrow) movement will pause, representing the beginning of the MDRP. (g) The end of the MDRP occurs once the atrial wall (arrow) contracts indicating the onset of atrial systole. (ESRP: End-systolic rest period; MDRP: Mid-diastolic rest period).
The cardiac rest periods with MVI were determined as follows. First, the standard 2- and 4-chamber cine planes were used to define the mitral valve plane which was aligned to also include part of the left ventricular outflow tract. A velocity-encoded phase contrast cine was obtained in this mitral valve plane (60 phases, Parallel imaging (SENSE factor = 2)), FOV was: 280 mm, slice thickness 8 mm, Acquired Voxel 2 × 2 mm, velocity encoding limit 120 cm/s, Echo train length = 2). On the scanner itself, flow analysis was performed, with the region of interest drawn to include the left ventricular outflow and the entirety of the mitral valve. ESRP was defined as starting at the end of systole immediately after the cessation of flow through the aortic valve and ending with the beginning of flow through the mitral valve. MDRP was defined as starting at the end of rapid ventricular filling and ending at the start of atrial systole when there is no flow across the mitral valve (Figure 1).
Sequential patients undergoing CMRA for clinical purposes were enrolled. In addition to the clinically indicated CMRI sequences chosen by the reading physician, each patient underwent two isotropic dual phase whole heart 3D SSFP CMRAs, as previously described, 14 using prospectively determined cardiac rest periods from either a MVI or 4CH cine. Vessel sharpness and length for both the Left coronary artery (LCA), which included the left anterior descending artery, and Right coronary artery (RCA) were determined in both phases using coronary reformatting software (SoapBubble) for both 3D CMRAs performed on each patient using both the MVI or 4CH method (Figure 2). Vessel sharpness and length in the LCA and RCA in both phases were averaged for each method. 15 Left circumflex branch was not assessed given the variable size and morphology in our pediatric cohort. Two observers (GG, TH) independently assessed coronary image quality using scoring system (0–4) as previously described by McConnell et al. with 0 = coronary artery not visible, 1 = coronary artery was visible, 2 = coronary artery was visible with moderately blurred borders, 3 = coronary artery visible with mildly blurred borders, 4 = coronary artery visible with sharp borders) of the MPR image corresponding to the LCA and RCA in diastole and systole for each method. Consensus scoring was utilized when scores between observers differed by 2 or greater. 16
Figure 2.
Multiplanar reformatted images from same patient using both techniques. (a) left image and (b) right image are transverse plane reformatted images showing similar image quality of the left coronary artery system utilizing MVI and 4CH techniques on the same patient A:MVI; B:4CH.
To assess reproducibility, additional analysis was performed on 25 cardiac MRI studies. Two independent pediatric cardiologists each with >10 years of experience in CMR (GG, TH) then retrospectively determined the ESRP and MDRP using both methods to evaluate reproducibility in this off-line patient cohort. An offline methodology was also chosen to minimize disruption to the clinical workflow and to ensure adequate blinding. Both observers were blinded to patient information, underlying cardiac pathology, the results of the CMRA studies, as well as to each other's results. One observer (TH) evaluated both methods twice to assess the intra-observer variability. Specifically, for each patient, we computed , where and are the difference and average of the two measurements by the same observers using the same method, respectively. To quantify inter-observer variability, we similarly computed , where and are the difference and average of measurements by the same observers using two different methods. For the purposes of the study, the images were transferred to an available work station and retrospectively evaluated and independently scored for image quality. 16
Statistical analysis
In the prospective cohort, the parametric variables including the RCA and LCA Vessel sharpness and lengths were compared with paired student t-test and reported as mean ± standard deviation. The non-parametric variable of image quality was compared by Wilcoxon matched pairs signed rank test
In the offline analysis on the retrospective cohort, a linear mixed-effect model was used to compare inter- and intra-observer variability between methods in the retrospective cohort. The dependent variables were or . The covariates included methods and measurement sites. Patient random effects were also included to account for within-patient correlation. Inter-observer and intra-observer variability for each method was analyzed. Image quality scoring of the LCA in this cohort was compared by interquartile range.
Results
The prospective study included 24 patients (mean age 16; range 5 to 52 years) (Table 1). There was no difference in overall vessel sharpness (4CH vs MVI mean ± SD) (31.04 ± 5.49% vs 30.49 ± 5.74%, p = .63), LCA vessel sharpness (30.0 ± 5.4% vs 31.1 ± 8.2%, p = .44), RCA vessel sharpness (32.1 ± 6.9% vs 31.1 ± 7.7%, p = .55), RCA vessel length (5.51 ± 2.6 cm vs 5.95 ± 2.4 cm, p = .38), LCA vessel length (4.7 ± 1.4 cm vs 4.6 ± 1.6 cm, p = .66) or image quality rating (2.66 vs 2.62, p = .80) (Table 2).
Table 1.
Demographics.
| CMRA patients (n = 24) | |
|---|---|
| Sex, M/F | 11/13 |
| Age (years)* | 16 (5–52) |
| Weight (Kg)** | 58.8 ± 24 |
| General Anesthesia | 2 |
| History Of CHD | 14 |
*median (range); **mean ± SD; M: male; F: female; Kg: kilogram; CHD: congenital heart disease.
Table 2.
Prospective cohort results.
| 4CH | MVI | CI | p-value (∝ <.05) | |
|---|---|---|---|---|
| Vessel wall sharpness* | 31.0% ± 5.5% | 30.5% ± 5.7% | −1.8%–2.9% | .63 |
| RCA vessel length* | 5.5 cm ± 2.6 cm | 6.0 cm ± 2.3 cm | −1.5–0.6 cm | .38 |
| LCA vessel length* | 4.7 cm ± 1.4 cm | 4.6 cm ± 1.6 cm | −0.5–0.8 cm | .66 |
| Overall Image Quality# | 2.7 (1.0–3.9) | 2.6 (0.3–3.6) | .80 |
*mean ± SD; #median (range); RCA: right coronary artery; LCA: left coronary artery.
In the retrospective study cohort, we included 25 patients (mean age 14, range 0.8–26 years) (Table 3).The diagnoses varied and included tetralogy of Fallot, hypoplastic left heart syndrome, double outlet right ventricle, hypertrophic cardiomyopathy, coarctation of the aorta, and Marfan syndrome. Results of the linear mixed-effect models showed that inter-observer variability of the MVI method was 5.4% lower (95% CI 3.7–7.2%, p < .0001) than the 4CH cine view orientation method. The intra- observer variability was 3.9% lower with the MVI method compared to the 4CH cine view method (95% CI 2.4–5.4%, p < .0001). MDRP and ESRP were determined using both methods and recorded. The time needed to determine the rest periods did not differ significantly in this small cohort for both methods (4CH 57.6 ± 26.0 s, MVIPC 52.5 ± 36.3 s, p = .4) (Table 4 ). Image quality scores for LCA were comparable to the published literature (median = 3, IQR 3–4).
Table 3.
Demographics.
| CMRA patients (n = 25) | |
|---|---|
| Sex, M/F | 8/17 |
| Age (years)* | 14 (0.8–26) |
| Weight (Kg)** | 54 ± 21 |
| History of CHD | 17 |
*median (range); **mean ± SD; M: male; F: female; Kg: kilogram; CHD: congenital heart disease.
Table 4.
Retrospective cohort for reproducibility calculations.
| 4CH Mean ± STDEV | MVI Mean ± STDEV | P-value | Mean Absolute difference (MVI – 4CH) ± STDEV | |
|---|---|---|---|---|
| MDRP1 beginning (msec) | 317.21 ± 39.34 | 308.37 ± 36.40 | 0.16 | 35.04 ± 20.73 |
| MDRP2 ending (msec) | 399.47 ± 50.01 | 386.68 ± 47.53 | 0.11 | 38.72 ± 39.8 |
| ESRP1 beginning (msec) | 596.33 ± 83.41 | 576.35 ± 63.91 | 0.10 | 48.76 ± 37.00 |
| ESRP2 ending (msec) | 737.83 ± 157.83 | 724.85 ± 145.20 | 0.60 | 45.88 ± 44.03 |
| Time needed to measure rest periods (MDRP and ESRP) (Sec) | 57.6 ± 26.0 | 52.5 ± 36.3 | 0.37 |
Discussion
The study systematically evaluated two methods for determination of cardiac resting periods for acquisition of dual phase CMRA. We found that the mitral valve inflow method was more reproducible in both inter- and intra-observer variability than the 4CH method. There was no difference in the vessel sharpness, vessel length or image quality of the LCA or RCA between both methods. The time needed to measure the rest periods was similar between both methods as well.
Our analysis did not show a significant difference in RCA sharpness compared to LCA sharpness despite the RCA having closer proximity to the surface coils. 17 As the cardiac MRI field continues to advance at a rapid pace, variability in MRI techniques and quality across institutions poses a significant challenge. Creating methods such as the MVI which are based upon physiologic principles readily understood by physicians and MRI technicians alike and which require minimal post processing will allow more centers to utilize dual phase cardiac imaging with minimal training and preservation of image quality between institutions. The 4CH method has been previously validated as a highly reproducible technique for assessment of coronary artery dimensions between two large institutions. 17 Nevertheless, the 4CH method requires both a high-quality image and a reading physician and MRI technician who have extensive experience with this method for it to achieve commensurate image quality in a highly reproducible fashion as previously described. 17 In our opinion, the MVI technique provides a more illustrative graph of the cardiac rest periods, which are largely responsible for the decreased inter- and intra-observer variability demonstrated in our study cohort and produced CMRAs with comparable image quality to the 4CH method. MVI is also an attractive method for cardiac rest period determination as it is based upon phase contrast quantification, which provides an objective measure that lends itself to future automation and integration into available CMRA software. Mitral-aortic continuity allows for easier imaging of inflow-outflow phase-contrast in a single slice, therefore mitral valve inflow plane was used in our study. However, tricuspid valve inflow imaging could be an alternative method. Because the MVI method is based on the assessment of a simple 1D signal, this process can be easily automatized by simple thresholding algorithms. Such software is attractive for its potential to decrease scan time, but also for its theoretical capacity to improve image quality further through real time adjustments in the acquisition window as a patient's heart rate changes and cardiac rest periods vary throughout the sequence. The future aspirations are for a widely available multi-phase CMRA which will allow for reformatting of images in multiple cardiac phases with no need for determination of resting phases prior to CMRA acquisition. 18 As multi-phase CMRA is yet not widely available, our simple method of using MVI provides a practical alternative.
Limitations
The 3D MRAs were completed predominately towards the end of each patient's CMRI study to ensure that the clinically indicated sequences were obtained. While all patients enrolled completed all clinically indicated sequences, the research sequences used in our study data may have been adversely impacted by increased patient motion or decreased cooperation resulting in our overall decreased image quality and vessel sharpness compared to previous studies. The study cohort did not include healthy volunteers.
Conclusions
Mitral valve inflow technique for determination of systolic and diastolic cardiac resting phases for acquisition of CMRA is feasible and has indicated higher reproducibility compared to hitherto established 4CH CINE visual determination. Mitral valve inflow technique may be used for further standardization and automatization of CMRA planning.
Abbreviations
- CMRA
coronary magnetic resonance angiography
- CHD
congenital heart disease
- 4CH
4-chamber
- MVI
mitral valve inflow phase contrast
- SSFP
steady-state free precession
- MDRP
mid-diastolic rest period
- RCA
right coronary artery
- RV
right ventricle
- LAD
left anterior descending artery
- ESRP
end-systolic rest period
- FOV
field of view
- TR
repetition time
- TE
Echo time
- IQR
image quality score
- ESRP1
beginning of end systolic rest period
- ESRP2
end of systolic rest period
- MDRP1
beginning of mid-diastolic rest period
- MDRP2
end of mid-diastolic rest period
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Animesh Tandon https://orcid.org/0000-0001-9769-8801
Sravani Avula https://orcid.org/0000-0003-4924-5175
References
- 1.Beerbaum P, Sarikouch S, Laser KT, et al. Coronary anomalies assessed by whole-heart isotropic 3D magnetic resonance imaging for cardiac morphology in congenital heart disease. J Magn Reson Imaging 2009; 29: 320–327. [DOI] [PubMed] [Google Scholar]
- 2.Prakash A, Powell AJ, Geva T, et al. Multimodality noninvasive imaging for assessment of congenital heart disease. Circ Cardiovasc Imaging 2010; 3: 112–125. [DOI] [PubMed] [Google Scholar]
- 3.Banka P, Geva T. Advances in pediatric cardiac MRI. Curr Opin Pediatr 2016; 28: 575–583. [DOI] [PubMed] [Google Scholar]
- 4.Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 2013; 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain T, Mathur S, Peel SA, et al. Coronary artery size and origin imaging in children: a comparative study of MRI and trans-thoracic echocardiography. BMC Med Imaging 2015; 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangcharoen T, Bell A, Hegde S, et al. Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology 2011; 259: 240–247. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Stuber M, Kissinger KV, et al. Impact of bulk cardiac motion on right coronary MR angiography and vessel wall imaging. J Magn Reson Imaging 2001; 14: 383–390. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Vidan E, Bergman GW. Cardiac motion of coronary arteries: variability in the rest period and implications for coronary MR angiography. Radiology 1999; 213: 751–758. [DOI] [PubMed] [Google Scholar]
- 9.Jahnke C, Paetsch I, Nehrke K, et al. A new approach for rapid assessment of the cardiac rest period for coronary MRA. J Cardiovasc Magn Reson 2005; 7: 395–399. [DOI] [PubMed] [Google Scholar]
- 10.Ustun A, Desai M, Abd-Elmoniem KZ, et al. Automated identification of minimal myocardial motion for improved image quality on MR angiography at 3T. AJR Am J Roentgenol 2007; 188: W283–W290. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Okada T, Kuhara S, et al. Automatic identication of the cardiac rest period using template updating for magnetic resonance coronary angiography. Adv Biomed Eng 2016; 5: 26–31. [Google Scholar]
- 12.Otton JM, Phan J, Feneley M, et al. Defining the mid-diastolic imaging period for cardiac CT - lessons from tissue Doppler echocardiography. BMC Med Imaging 2013; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain T, Lossnitzer D, Bellsham-Revell H, et al. Three-dimensional dual-phase whole-heart MR imaging: clinical implications for congenital heart disease. Radiology 2012; 263: 547–554. [DOI] [PubMed] [Google Scholar]
- 14.Henningsson M, Hussain T, Vieira MS, et al. Whole-heart coronary MR angiography using image-based navigation for the detection of coronary anomalies in adult patients with congenital heart disease. J Magn Reson Imaging 2016; 43: 947–955. [DOI] [PubMed] [Google Scholar]
- 15.Etienne A, Botnar RM, van Muiswinkel AM, et al. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 2002; 48: 658–666. [DOI] [PubMed] [Google Scholar]
- 16.McConnell MV, Khasgiwala VC, Savord BJ, et al. Comparison of respiratory suppression methods and navigator locations for MR coronary angiography. AJR Am J Roentgenol 1997; 168: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 17.Greil G., Desai M, Fenchel M, et al. Reproducibility of free breathing cardiovascular magnetic resonance coronary angiography. J Cardiovasc Magn Reson 2007; 9: 49–56. [DOI] [PubMed] [Google Scholar]
- 18.Zitzelsberger T, Krumm P, Hornung A, et al. Multi-phase coronary magnetic resonance angiography improves delineation of coronary arteries. Acta Radiol 2019; 60: 1422. [DOI] [PubMed] [Google Scholar]