Abstract
Acute ischemic stroke is currently a major cause of disability despite improvement in recanalization therapies. Stem cells represent a promising innovative strategy focused on reduction of neurologic sequelae by enhancement of brain plasticity. We performed a phase IIa, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Patients aged ≥60 years with moderate to severe stroke (National Institutes of Health Stroke Scale [NIHSS] 8–20) were randomized (1:1) to receive intravenous adipose tissue–derived mesenchymal stem cells (AD-MSCs) or placebo within the first 2 weeks of stroke onset. The primary outcome was safety, evaluating adverse events (AEs), neurologic and systemic complications, and tumor development. The secondary outcome evaluated treatment efficacy by measuring modified Rankin Scale (mRS), NIHSS, infarct size, and blood biomarkers. We report the final trial results after 24 months of follow-up. Recruitment began in December 2014 and stopped in December 2017 after 19 of 20 planned patients were included. Six patients did not receive study treatment: two due to technical issues and four for acquiring exclusion criteria after randomization. The final study sample was composed of 13 patients (4 receiving AD-MSCs and 9 placebo). One patient in the placebo group died within the first week after study treatment delivery due to sepsis. Two non-treatment-related serious AEs occurred in the AD-MSC group and nine in the placebo group. The total number of AEs and systemic or neurologic complications was similar between the study groups. No injection-related AEs were registered, nor tumor development. At 24 months of follow-up, patients in the AD-MSC group showed a nonsignificantly lower median NIHSS score (interquartile range, 3 [3–5.5] vs 7 [0–8]). Neither treatment group had differences in mRS scores throughout follow-up visits up to month 24. Therefore, intravenous treatment with AD-MSCs within the first 2 weeks from ischemic stroke was safe at 24 months of follow-up.
Keywords: Acute ischemic stroke, allogeneic adipose tissue–derived mesenchymal stem cells, clinical trial, stem cell therapy, safety
Introduction
Stroke is one of the most common causes of mortality worldwide and is a leading cause of disability. The estimated global incidence of stroke in 2016 was found to be between 189 and 218 cases per 100,000 inhabitants 1 . In the last two decades, vast improvements have been made in treatments for ischemic stroke, such as intravenous thrombolysis and mechanical thrombectomy. However, many patients suffer persistent neurological deficits and systemic complications. Current treatments are focused on reestablishing cerebral perfusion in the acute phase of stroke (usually limiting their applicability to the first 6–12 h from symptom onset), but do not favor regeneration once brain tissue is damaged. There is an urgent need for more widely available therapies focused on cerebral tissue repair, which would complement the current therapeutic options, extend the treatment time window, and help mitigate the disability and burden from stroke.
Cell therapies are innovative treatments that enhance brain tissue repair after ischemic damage through stimulation of neurogenesis, gliogenesis, oligodendrogenesis, synaptogenesis, and angiogenesis 2 . Stem cells are immature cells able to differentiate into diverse cell lines, which have also shown the capacity to secrete trophic factors and modulate the immune response, improving functional recovery after stroke in preclinical studies3,4. Although cell therapy in preclinical studies has shown beneficial effects, current challenges include finding suitable cell types, the best route of administration, and the timing and dose before translation to clinical practice is feasible3–6.
Many cell types have been used in preclinical stroke studies and clinical trials (eg, mononuclear cells7–9, neural stem cells, embryonic stem cells, induced pluripotent stem cells, mesenchymal cells)3,10–14. Among them, mesenchymal stem cells (MSCs) appear to be safe, possibly effective, and have advantages for clinical applicability because they are easy to obtain, have relatively low immunogenicity, and lack bioethical concerns. MSCs can derive from various sources, such as bone marrow, adipose tissue, dental pulp, and umbilical cord tissue. Adipose-derived MSCs in particular have the advantage of being abundant and easy to obtain by lipoaspiration techniques. Concerning mesenchymal cell origin, although autologous stem cells could initially appear safer, allogeneic MSCs lack human leucocyte antigen class II molecules, thus avoiding the risk of rejection. They also allow the possibility of having a cell bank readily available, which would permit earlier treatment administration.
Regarding the route of delivery, cell-based therapies have been dispensed by direct implantation into the central nervous system (intracerebral, intraventricular, or subarachnoid transplantation) based on the erroneous concept that stem cells’ basic mechanism of action was replacement of damaged brain tissue cells. However, less invasive systemic routes, such as intra-arterial, intravenous, and intranasal, are gaining more importance because the paracrine action of these cells is believed to play the primary role in brain regeneration. Preclinical studies have demonstrated that the intravenous infusion of stem cells is noninferior to other more invasive routes3,15, making this a propitious delivery route for translation to clinical trials.
In preclinical studies, cell therapy has typically been administered in the acute phase of stroke (within 24 h from symptom onset), with promising safety and efficacy results10–12. However, most clinical trials are performed in later phases of stroke when stem cells can only act on repair but not on brain protection mechanisms, with poor efficacy results, albeit no safety concerns 6 . The scarce trials in which an early treatment window has been explored have shown similar safety results, although trends toward a better functional status have been suggested7,8.
Cell dosages vary in animal models of stroke, at between 104 and 4.3 × 107 cells per kilogram of weight. Results appear to be more beneficial for lower rather than higher doses of MSCs, which could be due to a potential embolus formed with a higher dosage or slowing of cerebral blood flow10–13. In clinical trials, there is no standard dosage. The recommended dose is 1 to 2 × 106 cells per kilogram, although higher doses have been used without safety concerns.
Intravenous infusion of adipose-derived mesenchymal stem cells (AD-MSCs) has obtained good results in rat models of ischemic stroke. No safety issues have been observed, and positive results in functional recovery, reduction of brain tissue cell death, and increased cell proliferation in the peri-infarct zone have been described16–18. Currently, the RESSTORE 1 phase Ia/Ib clinical trial is recruiting patients with hemispheric nonlacunar stroke to be treated with various doses of intravenous AD-MSC solution or placebo. Safety and efficacy results after a 2-year follow-up period, including blood biomarkers and functional magnetic resonance imaging (MRI), are not yet available.
Therefore, in the Allogeneic Adipose Tissue–Derived Mesen-chymal Stem Cells In Acute Ischemic Stroke (AMASCIS) trial, our main objective was to demonstrate the safety of intravenous AD-MSC delivery within the first 2 weeks from stroke symptom onset and to explore the possible efficacy in reduction of stroke-associated disability. To our knowledge, this was the first clinical trial exploring intravenous use of AD-MSCs in patients with ischemic stroke. Preliminary safety and efficacy results after 6 months of follow-up have already been comunicated 19 ; here, we present the final clinical trial results.
Materials and Methods
Study Design
The AMASCIS trial was a phase IIa, pilot, single-center, prospective, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety of a single intravenous dose of 1 million AD-MSCs per kilogram of weight compared with placebo in the first 2 weeks after ischemic stroke. It was an academic trial, promoted by the Research Foundation from La Paz University Hospital, funded by the Spanish Ministry of Health and Social Policy, and approved by the La Paz University Hospital Ethics Committee and by the Spanish Agency of Medicines and Health Products in 2011 (EudraCT: 2011-003551-18). The clinical trial protocol was published before recruitment began 20 and is registered in Clinicaltrials.gov (NCT01678534).
Participants
Initially, we planned to enroll 20 patients aged 60 to 80 years with moderate to severe ischemic stroke in the region of the middle cerebral artery (MCA) with a National Institute of Health Stroke Scale (NIHSS) of 8 to 20 points, who had arrived at the hospital less than 12 h from stroke symptom onset. An initial computed tomography (CT) and/or MRI scan compatible with ischemic stroke in MCA was needed for inclusion. Patients received standard treatment for acute ischemic stroke, including recanalization therapies such as intravenous thrombolysis or mechanical thrombectomy. All patients (or their legal representative or relative) signed the informed consent document after being given a detailed explanation of the nature and purpose of this study and prior to the performance of any of the study-related procedures. Patients in a comatose state (score ≥2 on item 1a of the NIHSS); evidence of brain tumor, primary intraventricular, intracerebral, or subarachnoid hemorrhage on neuroimaging; cerebral edema with midline shift; or cerebellar or brainstem infarction were excluded. Clinical or laboratory signs suggestive of active infection, including human immunodeficiency virus and hepatitis B and C, were also considered exclusion criteria, along with a previous diagnosis of dementia or any health condition that could preclude appropriate diagnosis, treatment, or follow-up.
Recruitment began in March 2014, but due to inclusion difficulties, a protocol amendment was made to eliminate the upper age limit and to lengthen the time of arrival at the hospital to 24 h. The complete inclusion and exclusion list is included as supplemental material. This first amendment was approved by the ethics committee in December 2014, just after the first patient was enrolled. A second protocol amendment was approved in April 2016 to update information on standard acute ischemic stroke treatment, without inclusion and exclusion criteria modification.
Randomization and Masking
Each patient was assigned a unique number as they were enrolled in the study and was then allocated by a study nurse (nonblinded to the study treatment) to one of the two study groups (AD-MSCs or placebo) in a 1:1 ratio according to a computer-generated, simple randomization list prepared by an independent statistician. The supply for the study drug was then ordered by the same nurse from the cell’s manufacturer. After the study treatment was received at the Clinical Trials Unit, it was labeled with a unique identification number. To maintain blinding, vials containing placebo and AD-MSCs were identical and protocols were designed to ensure that physicians evaluating patient safety and efficacy outcomes would not have access to randomization codes.
Procedures
The experimental drug was a solution of allogeneic AD-MSCs, obtained by lipoaspiration techniques from a healthy donor, cultured in vitro, packed in cryovials with dimethyl sulfoxide and fetal bovine serum, and cryopreserved at −180°C as instructed by the manufacturer Histocell S.L. (Bizkaia, Spain). Before use, cryovials were thawed, washed, and finally packed in vials containing only cell product together with glucosaline solution, sodium bicarbonate, human albumin, and Ringer’s lactate. Details concerning the manufacturing processes and quality controls are specified in the investigator’s brochure, which is available at the study center and has been approved by the Spanish Agency of Medicines and Health Products.
The final experimental drug solution was composed of 10 million cells per milliliter of solution to be administered intravenously at a dose of 1 million cells per kilogram of the patient’s weight (0.1 ml per kilogram of the patient’s weight in the case of placebo solution) within 2 weeks from stroke symptom onset. All patients received conventional treatment for ischemic stroke according to the valid guidelines at that time.
The total study duration was 24 months and consisted of 10 scheduled patient visits (V0, screening; V1, pretreatment; V2, 2 h after treatment; V3, 24 h after treatment; V4, day 7 after treatment; V5, month 3; V6, month 6; V7, month 12; V8, month 18; V9, month 24).
Outcomes
The study’s primary outcome was safety, and it was assessed at each visit by evaluating adverse events (AEs) reported spontaneously or in response to prespecified questions at each visit, such as neurologic and systemic complications (deteriorating stroke, stroke recurrences, brain edema, seizures, hemorrhagic transformation, respiratory infections, urinary tract infections, deep vein thrombosis, pulmonary embolism, and gastrointestinal hemorrhage) and tumor development. Serious AEs (SAEs) were recorded at each visit and were immediately reported, following good clinical practice.
Secondary outcomes were included to explore the efficacy of the study treatment by using clinical parameters and neuroimaging. The modified Rankin Scale (mRS) was measured at baseline as well as at day 7 and month 3, whereas the NIHSS scale was measured at every scheduled visit. An mRS score of 0–2 and/or an NIHSS score of 0–1 were considered successful. Additional MRIs were performed at day 7 and month 3 to measure infarct size. An exploratory analysis was also performed to assess the influence of AD-MSC administration on serum levels of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP9), and extracellular vesicles (EVs). These biomarkers were collected before treatment administration and at 7 days and 3 months after. Blood was drawn from the antecubital vein, collected in BD Vacutainer Plus Plastic Serum tubes, and then centrifugated at 3,000 × g for 15 min. The serum levels of BDNF, VEGF, and MMP-9 were measured by enzyme-linked immunosorbent assay (ELISA), using protocols supplied by the manufacturer. EVs were isolated from the patient’s serum with an EV isolation kit (ExoQuick Ultra EV precipitation solution; System Biosciences, Palo Alto, CA, USA), following the manufacturer’s instructions. After isolation, EV levels from 1 ml of serum were analyzed by the EXOEL-CD63A-1 ELISA kit (System Biosciences), using protocols supplied by the manufacturer. Commercially available ELISA kits for measurement of all biomarkers were employed by an investigator who had no knowledge of the patients’ treatment allocation.
Sample Size Calculation, Statistical Analysis, and Data Management
The AMASCIS trial aimed to recruit 20 patients. A formal predetermination of sample size was not calculated because of the exploratory nature of this pilot study. This sample size should suffice to obtain enough information for the development of future phase IIb or III clinical trials according to the CONSORT (CONsolidated Standards of Reporting Trials) 2010 statement for the extension to randomized pilot and feasibility trials 21 .
Demographic and clinical characteristics were summarized in terms of median and interquartile range (IQR) or percentages as appropriate for each variable type. Due to the limited sample size, a univariate approach was employed for calculating nonparametric tests for the continuous variables (Wilcoxon rank-sum) and Fisher’s or chi-squared tests for the categorical variables. Only P values less than 0.05 were considered significant. Statistical computations were performed using SPSS version 21. The safety analysis performed for the patients who received study treatment included tables with all AEs and neurologic and systemic complications. For efficacy variables, a descriptive analysis of the “per protocol” population was performed, as well as an exploratory analysis comparing both treatment groups.
All data management followed the principles of the Spanish data protection Law “Ley Orgánica de Protección de datos” (LOPD 15/1999) and the European regulations/International Conference of Harmonization Good Clinical Practice guidelines. Independent Clinical Research Associates of the Clinical Trial Unit of La Paz University Hospital, not involved in any procedures, performed the data monitoring. An independent data monitoring committee periodically reviewed the unblinded data to identify any safety issues. Reports from this data monitoring committee were released in February and September 2016 and in March 2018; all of them concluded that there were no safety issues and recommended that the trial continue as planned.
Role of the Funding Source
The AMASCIS trial was an academic, investigator-initiated study funded by the Spanish Ministry of Health and Social Policy in a research call aimed to support independent study design, data collection, analysis, interpretation, and result reporting (project code EC 10/171). Most of the funding was used to acquire the study treatment from the cell manufacturer, which was not involved in any other study procedure. The corresponding authors and the writing group had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The first patient was enrolled in December 2014. Recruitment was transiently stopped several times during the summer and Christmas periods due to periodically planned qualifying works at the AD-MSC manufacturer. In addition, from January to June 2015, trial recruitment also stopped due to a relevant amendment in the manufacturing process that needed approval from the Spanish Agency for Medicines and Health Products. Recruitment was ultimately stopped on December 2017 after 19 of the 20 planned patients were included. The follow-up period of the last enrolled patient was finalized in September 2019.
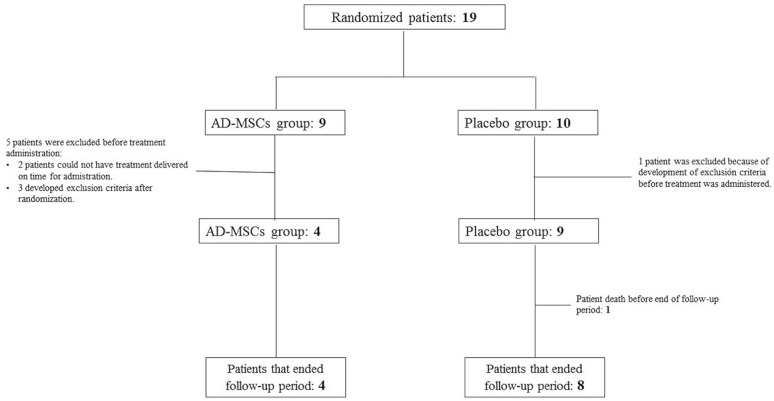
Of the 19 patients initially enrolled, 6 did not receive the trial treatment (2 because of technical issues during the manufacturing process and 4 due to development of exclusion criteria after being randomized). The final study sample was composed of 13 patients: 4 received AD-MSCs and 9 received placebo solution (Fig. 1). No differences in the baseline or demographic characteristics between the treatment groups were found (Table 1). The median time from stroke to treatment administration was 13 days (IQR, 13–13.75) in the AD-MSC treatment group and 12 (IQR, 10–13) days in the placebo group (P = 0.028).
Figure 1.
AMASCIS final study sample. Trial profile. Nineteen patients were initially randomized: 9 to the AD-MSC group and 10 to the placebo treatment group. In the AD-MSC group, two patients did not receive the study treatment because it could not be delivered on time for administration (2 weeks since symptom onset), and three developed exclusion criteria after randomization and before study treatment was applied. In the placebo group, only one patient developed exclusion criteria before treatment administration. Finally, AD-MSCs were administered to four participants and placebo to eight. One of these eight patients died before the end of the follow-up period. AD-MSCs: adipose tissue–derived mesenchymal stem cells; AMASCIS: Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke.
Table 1.
Baseline and Demographic Characteristics of the Study Population.
| AD-MSC n = 9 | Placebo n = 10 | P value | |
|---|---|---|---|
| Sex | |||
| Male | 1 (11.1%) | 3 (30.0%) | 0.582 |
| Female | 8 (88.8%) | 7 (70.0%) | |
| Age (years) | 78 (70.5–82) | 76 (69–80.25) | 0.437 |
| Hypertension | 6 (66.7%) | 9 (90%) | 0.303 |
| Diabetes | 2 (22.2%) | 2 (20%) | 1.000 |
| Dyslipidemia | 5 (55.6%) | 6 (60%) | 1.000 |
| Smoker | 0.929 | ||
| Current | 1 (11.1%) | 1 (10%) | |
| Former | 2 (22.2%) | 3 (30%) | |
| Metabolic syndrome | 1 (11.1%) | 2 (20%) | 1.000 |
| Coronary arterial disease | 0 (0%) | 1 (10%) | 1.000 |
| Atrial fibrillation | 5 (55.6%) | 8 (80%) | 0.350 |
| Previous stroke | 2 (2.22%) | 2 (20%) | 1.000 |
| Weight (kg) | 65 (61–73.5) | 71 (65–86) | 0.164 |
| Modified Rankin Scale (mRS) at baseline | 0.582 | ||
| mRS 0 points | 7 (77.8%) | 9 (90%) | |
| mRS 1 point | 2 (22.2%) | 1 (10%) | |
| NIHSS baseline (V1, screening) | 12 (9–17.5) | 10.5 (9.5–14) | 0.325 |
| NIHSS pretreatment (V2) | 9.5 (6.75–13.75) | 9 (6–11.5) | 0.697 |
| Capillary glucose levels at baseline | 99 (85–144) | 124 (98.5–142.5) | 0.453 |
| Reperfusion therapy | 0.189 | ||
| None | 7 (77.8%) | 4 (40%) | |
| Intravenous thrombolysis (IVT) | 1 (11.1%) | 1 (10%) | |
| Mechanical thrombectomy (MT) | 0 (0%) | 4 (40%) | |
| Both (IVT and MT) | 1 (11.1%) | 1 (10%) | |
| Infarct size (ml) | 43.22 (37.57–94.01) | 88.165 (55.06–130.75) | 0.414 |
| Stroke etiological subtype | 0.131 | ||
| Large vessel disease | 1 (11.1%) | 0 (0%) | |
| Cardioembolic | 5 (55.5%) | 10 (100%) | |
| Unknown | 1 (11.1%) | 0 (0%) | |
| Uncommon etiology | 2 (22.2%) | 0 (0%) | |
| Time (days) since stroke symptom onset to study treatment administration | 13 (13–13.75) | 12 (10–13) | 0.028 |
AD-MSCs: adipose-derived mesenchymal stem cells; NIHSS: National Institute of Health Stroke Scale.
Regarding the primary outcome, a total of 124 AEs were reported throughout the 24 months of follow-up (complete list of AEs in supplementary material): 50 in the AD-MSC treatment group and 74 in the placebo arm (P = 0.074). Eleven SAEs were reported (full details provided in Table 2): two in the cell treatment arm and nine in the placebo group. The most frequent AEs were depression (present in all the patients treated with AD-MSCs and in 22.2% of the patients in the placebo arm), urinary tract infections (75% of the AD-MSC treatment group and 55.5% of the placebo group), and respiratory infections (25% of the AD-MSC group and 44.4% of the placebo group). Only one patient in the placebo treatment group died within the first 7 days of treatment administration, due to a multiorgan failure. There was no AE considered to be related to AD-MSC administration. No injection-related AEs or tumor developments were reported. No differences were found in the frequency of predefined systemic or neurological complications between the study groups (Table 3).
Table 2.
List of SAEs.
| SAE | Treatment arm | Time since treatment administration to SAE | Relation between study treatment and SAE | Outcome |
|---|---|---|---|---|
| Multiorgan failure | Placebo | 24 hours | Possibly related | Death |
| Aspiration pneumonia | Placebo | 24 months | Not related | Recovered |
| Ischemic stroke recurrence | Placebo | 18 months | Not related | Recovered with sequelae |
| Functional decline | Placebo | 18 months | Not related | Recovered with sequelae |
| Neurological decline | Placebo | 24 months | Not related | Recovered |
| Decompensated cardiac insufficiency | Placebo | 24 months | Not related | Recovered |
| Sepsis due to urinary infection | Placebo | 24 months | Not related | Recovered |
| Acute pancreatitis of biliary origin | Placebo | 3 months | Not related | Recovered |
| Recurrent epileptic seizures with cardiac arrest | Placebo | 5 months | Not related | Recovered |
| Hematuria | AD-MSCs | 3 months | Not related | Recovered |
| Hypernatremia | AD-MSCs | 3 months | Not related | Recovered |
AD-MSCs: adipose-derived mesenchymal stem cells; SAE: serious adverse event.
Table 3.
Predefined Systemic and Neurological Complications.
| AD-MSCs n = 4 |
Placebo n = 9 |
P value | |
|---|---|---|---|
| Deaths | 0 (0%) | 1 (11.1%) | 1.0 |
| No. of patients with SAEs | 1 (25%) | 4 (44.4%) | 1.0 |
| Patients with neurological complications | |||
| Deteriorating stroke | 0 (0%) | 0 (0%) | |
| Stroke recurrence | 1 (25%) | 1 (11.1%) | 1.0 |
| Brain edema | 0 (0%) | 0 (0%) | |
| Seizures | 0 (0%) | 2 (22.2%) | 1.0 |
| Symptomatic hemorrhagic transformation | 0 (0%) | 0 (0%) | |
| Patients with systemic complications | |||
| Respiratory infection | 1 (25%) | 3 (33.3%) | 1.0 |
| Urinary tract infection | 3 (75%) | 5 (55.6%) | 1.0 |
| Deep vein thrombosis | 0 (0%) | 1 (11.1%) | 1.0 |
| Pulmonary embolism | 0 (0%) | 0 (0%) | |
| Gastrointestinal hemorrhage | 0 (0%) | 0 (0%) | |
| Tumor development | 0 (0%) | 0 (0%) | |
AD-MSCs: adipose-derived mesenchymal stem cells; SAE: serious adverse event.
Results from the efficacy analysis showed no significant differences between treatment groups regarding mRS (Fig. 2), NIHSS scores, or infarct size in neuroimaging during the predefined visits for their evaluation. The analysis of the NIHSS scale during all scheduled visits showed no differences in obtaining successful scores between the treatment groups; nor were there any differences found when NIHSS score distributions were compared (Table 4, Fig. 3). The median NIHSS score at 3 months was 5.5 points in both treatment arms, and at month 24, patients treated with AD-MSCs showed nonsignificantly lower median scores (3 [3–6.75] vs 7 [0–12.5]) compared with the placebo group.
Figure 2.
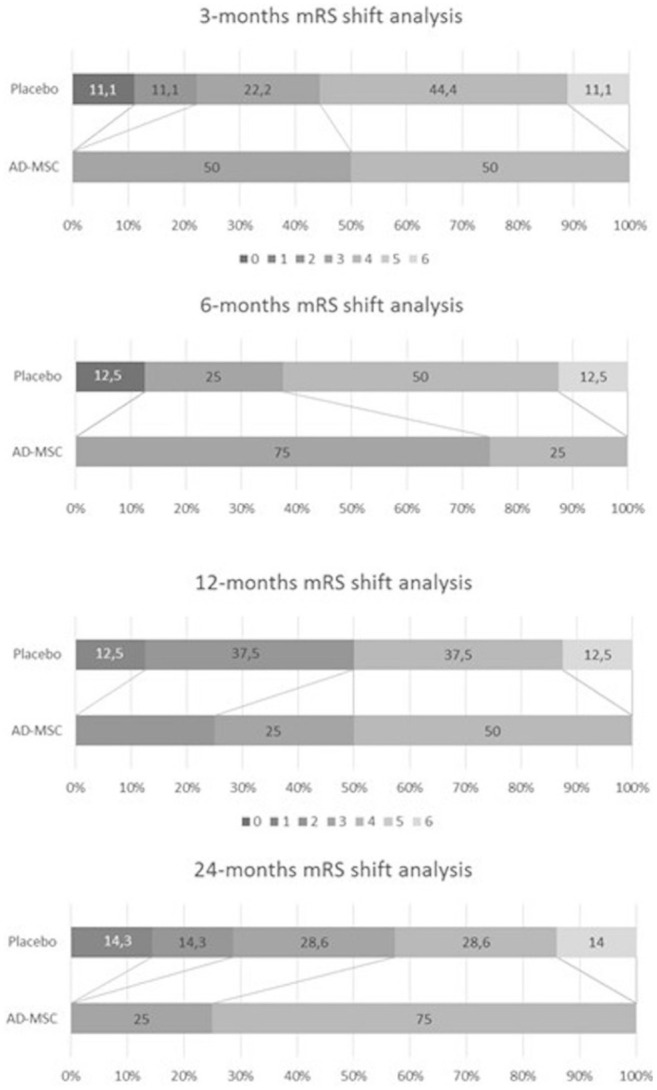
mRS shift analysis. AD-MSCs: adipose-derived mesenchymal stem cells; mRS: modified Rankin Scale.
Table 4.
NIHSS Scores.
| NIHSS score | AD-MSCs | Placebo | P value |
|---|---|---|---|
| Screening (V0) | |||
| Median [IQR] | 10.5 [8.25–18] | 11 [9–14] | 1.000 |
| Max | 20 | 17 | |
| Min | 8 | 8 | |
| Pretreatment (V1) | |||
| Median [IQR] | 9.5 [6.75–13.75] | 9 [6–11.5] | 0.697 |
| Max | 15 | 17 | |
| Min | 6 | 1 | |
| 2 h post-treatment (V2) | |||
| Median [IQR] | 9.5 [6.75–13.75] | 9 [6–11.5] | 0.697 |
| Max | 15 | 17 | |
| Min | 6 | 1 | |
| 24 h post-treatment (V3) | |||
| Median [IQR] | 8 [5.5–13.5] | 9 [5–14.5] | 0.699 |
| Max | 15 | 17 | |
| Min | 5 | 1 | |
| 7 days (V4) | |||
| Median [IQR] | 8.5 [5–12.75] | 8 [2.25–10.5] | 0.608 |
| Max | 14 | 13 | |
| Min | 4 | 0 | |
| Month 3 (V5) | |||
| Median [IQR] | 5.5 [2–6] | 5.5 [1.25–6.75] | 0.861 |
| Max | 6 | 8 | |
| Min | 1 | 0 | |
| Month 6 (V6) | |||
| Median [IQR] | 2.5 [1.25–4.5] | 5 [3–8.5–8] | 0.199 |
| Max | 5 | 10 | |
| Min | 1 | 0 | |
| Month 12 (V7) | |||
| Median [IQR] | 2.5 [1.25–5.25] | 3 [0.75–5.75] | 1.000 |
| Max | 6 | 8 | |
| Min | 1 | 0 | |
| Month 18 (V8) | |||
| Median [IQR] | 2.5 [2–5.25] | 7 [1.5–9.5] | 0.306 |
| Max | 6 | 10 | |
| Min | 2 | 0 | |
| Month 24 (V9) | |||
| Median [IQR] | 3 [3–6.75] | 7 [0–12.5] | 0.900 |
| Max | 8 | 17 | |
| Min | 3 | 0 | |
AD-MSCs: adipose-derived mesenchymal stem cells; NIHSS: National Institute of Health Stroke Scale.
Figure 3.
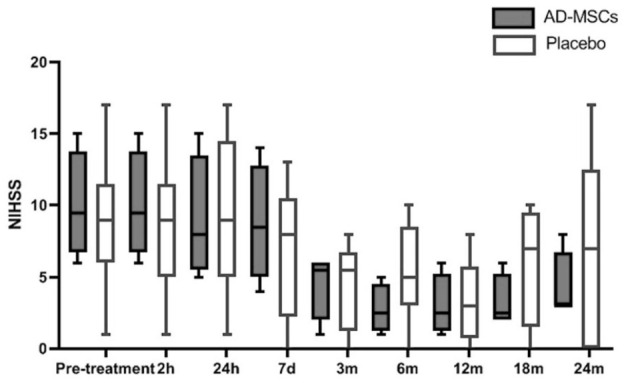
NIHSS scores up to 24 months of follow-up. AD-MSCs: adipose-derived mesenchymal stem cells; NIHSS: National Institute of Health Stroke Scale.
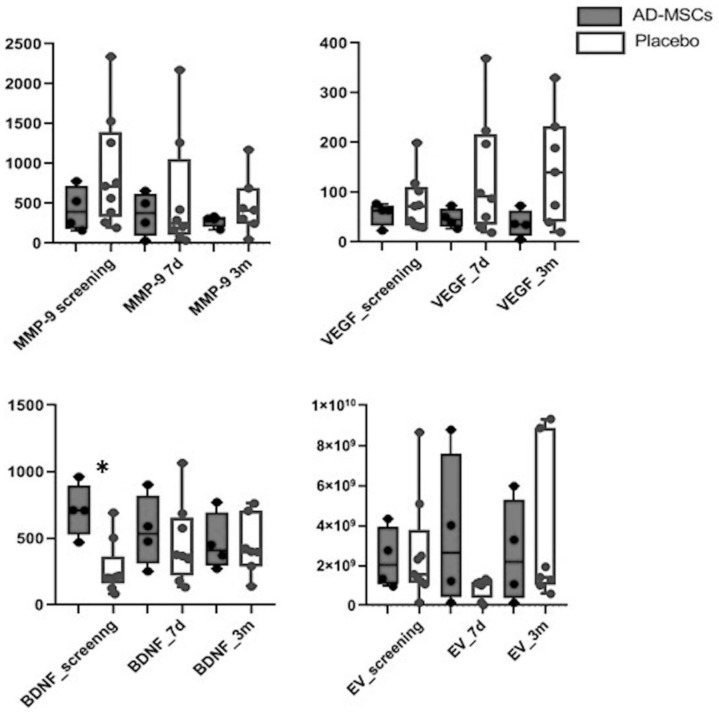
An analysis of the serum biomarkers showed higher median basal values of BDNF in the AD-MSC treatment group (710 [589.85–836.4] UI/ml vs 204.6 [193.3–219] UI/ml, P = 0.014). No significant differences were detected in BDNF levels at the other measured time points (7 days and 3 months); however, increasing BDNF values from baseline were observed in the placebo group, whereas these values in the AD-MSC group decreased, and this different trend was significant (P = 0.014). The same significant trend was observed in VEGF levels, although no differences between treatment groups in any of the measured time points were detected (Fig. 4). Regarding MMP-9 and EVs, there were no differences in their levels between treatment groups at any of the measured time points.
Figure 4.
Levels of serum biomarkers. BDNF: brain-derived neurotrophic factor; EV: extracellular vesicle; MMP9: matrix metalloproteinase-9; VEGF: vascular endothelial growth factor.
Discussion
According to the results of the AMASCIS trial, intravenous infusion of allogeneic AD-MSCs within the first 2 weeks from ischemic stroke symptom onset is safe and well tolerated at a unique dose of 1 million cells per kilogram. No infusion reactions occurred, and there was no observed association between the reported AEs and AD-MSC administration. To our knowledge, this is the first clinical trial evaluating treatment with intravenous allogeneic AD-MSCs in patients with ischemic stroke. This type of cell therapy has subsequently been tested in other nonneurologic diseases, such as acute respiratory distress 22 and treatment-refractive rheumatoid arthritis 23 , without significant safety issues.
Although the beneficial effect of intravenous AD-MSCs in ischemic stroke has been already demonstrated in animal models, even with stroke-associated comorbidities16,17,24,25, the AMASCIS trial was underpowered to detect significant differences in secondary outcome efficacy due to its exploratory nature and limited sample size. Although there appeared to be a trend toward lower NIHSS scores in the AD-MSC treatment allocation group, no differences in functional outcome as measured by the mRS were noted. Recently, the positive effect of intravenous cell therapy using allogeneic multipotent adult progenitor cells has been suggested in “Multistem in Acute Stroke Treatment to Enhance Recovery Study (MASTERS)”, in which 129 patients were randomized to receive 1,200 million cells between 24 and 48 h from symptom onset 7 . No dose-limiting toxicity, infusional, or allergic reactions were detected during follow-up, and there was no difference in global stroke recovery at day 90 between the treatment and placebo groups. A post hoc analysis, however, showed an improvement in the mRS distribution at 90 days in an early window of the treatment administration group (<36 h) and also a higher percentage of excellent outcomes (defined as a composite end point of mRS ≤1, NIHSS ≤1, and Barthel index ≥95). After 1 year of follow-up, the early treatment group showed significant differences in several clinical outcomes. Compared with the MASTERS trial population, the participants in the AMASCIS trial were older and with a predominance of women. We consider this to prove the safety of MSC therapy in populations often underrepresented in stroke trials, contributing to current knowledge in this field. The “Intravenous stem cells after ischemic stroke-Heuristic value of multimodal MRI to assess MEsenchymal stem cell therapy in Stroke (ISIS-HERMES)” clinical trial 26 , in which 16 of 31 patients were treated with autologous bone marrow–derived MSCs in the 2 weeks after stroke onset, showed significant improvements in NIHSS motor scores and Fugl-Meyer motor scores, although no differences were observed with the placebo group regarding mRS, full NIHSS score, or Barthel Index at 6 months and 2 years after treatment administration. Long-term efficacy of MSC therapy in ischemic stroke has also been suggested in an open-label, observer-blinded clinical trial 27 in which 16 patients were treated with autologous bone marrow–derived MSCs (vs 36 patients in the control group) within 7 weeks from stroke onset and follow-up for 5 years. No differences in neurologic complications occurred between the treatment groups, and a significant improvement in mRS scores was reported. Therefore, there appears to be a tendency toward efficacy in the short and long term with MSC therapies; however, the most appropriate cell type, as well as the manner and time of administration is yet to be determined.
Currently, there are various suggested mechanisms to explain the possible therapeutic effect of mesenchymal-derived stem cells in stroke, such as cell migration, modulation of the immune response, brain protection, and stimulation of endogenous brain repair processes 28 . When MSCs are implanted by intravenous injection, the mechanisms that are probably most relevant in brain repair would be those derived from their paracrine function, involving secretion of cytokines and growth factors that favor reformation of the neurovascular units, endogenous neurogenesis, neuroblast migration, and axonal growth and plasticity. Among various trophic factors implicated in these functions are VEGF and BDNF. In our study, we observed slightly increasing values of both VEGF and BDNF in the placebo treatment group in contrast to the subtle decrease in values in the AD-MSC group, a fact that appears contradictory. However, taking into account the small sample size and intragroup variability, we consider this observation scarcely reliable. EVs secreted by MSCs are spherical cytoplasmatic components of 50 to 150 nm in size that contain large numbers of bioactive components, such as proteins, lipids, growth factors, cytokines, chemokines, messenger RNAs (mRNAs), and microRNAs that function as messengers between the MSCs and the injured brain tissue cells 29 and contribute to regeneration of the neurovascular unit. In a recent preclinical study in rats with subcortical ischemic stroke 30 performed by our study group, the early intravenous administration of EVs derived from AD-MSCs showed significant functional recovery, a reduction in white matter injury, generation of neurovascular unit cells, and a reduction in cell death compared with the placebo group, demonstrating their importance in stroke recovery. In the AMASCIS trial, no differences were observed in EV secretion between treatment groups, but again, this result is limited by the sample size.
One of the main remaining questions regarding stem cell therapy is the optimal time window for their administration. Most preclinical studies are performed in acute stages of stroke, but clinical trials are usually conducted at later stages. Taking into account the proposed mechanisms of action of MSCs that imply modulation of inflammation responses of the ischemic tissue, the benefit of stem cell administration would appear greater in the earliest phases of ischemic stroke. The promising efficacy results in the early treatment administration group of the MASTERS trial support this theory.
Therefore, considering the safety results from the AMASCIS study, we are currently recruiting patients for the AMASCIS-02 trial (NCT04280003), in which an earlier time window will be explored for safety and efficacy results in 30 patients with ischemic stroke 31 .
The main limitation of the AMASCIS study is the small sample size due to its pilot clinical trial design and the low number of patients in the treatment group as a consequence of technical issues during the manufacturing process and the development of exclusion criteria after randomization.
In conclusion, the AMASCIS pilot phase IIa clinical trial results suggest that the intravenous administration of AD-MSCs within the first 2 weeks of ischemic stroke onset is safe at 24 months of follow-up. Although no efficacy end points were statistically significant between treatment groups, a trend toward improvement was observed in the NIHSS scores in the AD-MSC treatment arm compared with placebo.
Supplemental Material
Supplemental material, sj-doc-1-cll-10.1177_09636897221083863 for Final Results of Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial by Elena de Celis-Ruiz, Blanca Fuentes, María Alonso de Leciñana, María Gutiérrez-Fernández, Alberto M. Borobia, Raquel Gutiérrez-Zúñiga, Gerardo Ruiz-Ares, Laura Otero-Ortega, Fernando Laso-García, Mari Carmen Gómez-de Frutos and Exuperio Díez-Tejedor in Cell Transplantation
Supplemental material, sj-doc-2-cll-10.1177_09636897221083863 for Final Results of Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial by Elena de Celis-Ruiz, Blanca Fuentes, María Alonso de Leciñana, María Gutiérrez-Fernández, Alberto M. Borobia, Raquel Gutiérrez-Zúñiga, Gerardo Ruiz-Ares, Laura Otero-Ortega, Fernando Laso-García, Mari Carmen Gómez-de Frutos and Exuperio Díez-Tejedor in Cell Transplantation
Acknowledgments
We would like to acknowledge our patients who participated in the trial and their families. We would also like to acknowledge our clinical site investigators (Patricia Martínez, Borja Sanz-Cuesta, Melisa Báez) and Alberto Sánchez Funes (medical student) for his contributions to data analysis. We greatly appreciate the support of Morote Traducciones for editing assistance.
Footnotes
Contributors: EdC-R wrote the first draft of the manuscript and was involved in data interpretation and statistical analysis; BF was involved in the writing of the manuscript, study design, data interpretation, and patient enrollment. RG-Z, MAdL, and GR-A enrolled patients. MG-F, LO-O, FL-G, and MCGdF performed the biomarker analysis and interpretation. AB contributed to the statistical analysis. ED-T performed the study design, data interpretation, and writing of the manuscript.
Ethical Approval: Ethical approval to perform this clinical trial was obtained from La Paz University Hospital Ethics Committee (approval code: 3479) and by the Spanish Agency of Medicines and Health Products in 2011 (EudraCT: 2011-003551-18).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the La Paz University Hospital Ethics Committee’s approved protocols (approval code: 3479). This article does not contain any studies with animal subjects.
Statement of Informed Consent: Written informed consent was obtained from the patient or their legally authorized representative for anonymized patient information to be published in this article.
Trial Registry Name and Number: Reparative Therapy in Acute Ischemic Stroke With Allogenic Mesenchymal Stem Cells From Adipose Tissue, Safety Assessment, a Randomized, Double Blind Placebo Controlled Single Center Pilot Clinical Trial (AMASCIS-01). NCT01678534.
EudraCT: 2011-003551-18
Data Sharing: The data that support the findings of this study are available from the corresponding author on request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BF reports grants from Spanish Ministry of Health and European Commission (Horizon2020 program) during the conduct of the study and from Daichi-Sankyo, Bayer, BMS-Pfizer, Novonordisk, and Novartis outside the submitted work. MG-F (CP15/00069; CPII20/00002) reports grants from the Spanish Ministry of Health and European Commission (Horizon2020 program) during the conduct of the study; nonfinancial support was received from Cellerix/Tigenix and Histocell, outside the submitted work. AMB reports grants from Instituto de Salud Carlos III, during the conduct of the study; personal fees from Mundipharma and Menarini outside the submitted work; and was Principal Investigator in Clinical Trials for GSK, Daiichi Sankyo, and Janssen. ED-T reports grants from the Spanish Ministry of Health and European Commission (Horizon2020 program) during the conduct of the study and nonfinancial support from Cellerix/Tigenix, Histocell, Daichi-Sankyo, Bayer, Sanofy Aventis, and Novartis outside the submitted work. EdC-R, RG-Z, MAdL, JCMV, GR-A, MCGdF (FI17/00188), FL-G (FI18/00026), and LO-O (CP20/00024) report nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was funded by the Spanish Ministry of Health and Social Policy (EC10/171).
ORCID iD: Elena de Celis-Ruiz  https://orcid.org/0000-0002-9224-9422
https://orcid.org/0000-0002-9224-9422
Supplemental Material: Supplemental material for this article is available online.
References
- 1. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gutiérrez-Fernández M, Fuentes B, Rodríguez-Frutos B, Ramos-Cejudo J, Vallejo-Cremades MT, Díez-Tejedor E. Trophic factors and cell therapy to stimulate brain repair after ischaemic stroke. J Cell Mol Med. 2012;16(10):2280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7(5):378–87. [DOI] [PubMed] [Google Scholar]
- 4. Savitz SI. Developing cellular therapies for stroke. Stroke. 2015;46(7):2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang OY, Kim EH, Cha JM, Moon GJ. Adult stem cell therapy for stroke: challenges and progress. J Stroke. 2016;18(3):256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krause M, Phan TG, Ma H, Sobey CG, Lim R. Cell-based therapies for stroke: are we there yet? Front Neurol. 2019;10:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, Sen S, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–68. [DOI] [PubMed] [Google Scholar]
- 8. Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I, Garcia-Solis D, Cayuela A, Montaner J, Boada C, Rosell A, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242–44. [DOI] [PubMed] [Google Scholar]
- 9. Vahidy FS, Haque ME, Rahbar MH, Zhu H, Rowan P, Aisiku IP, Lee DA, Juneja HS, Alderman S, Barreto AD, Suarez JI, et al. Intravenous bone marrow mononuclear cells for acute ischemic stroke: safety, feasibility, and effect size from a phase I clinical trial. Stem Cells. 2019;37(11):1481–91. [DOI] [PubMed] [Google Scholar]
- 10. Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014. 8;82(14):1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lalu MM, Montroy J, Dowlatshahi D, Hutton B, Juneau P, Wesch N, Y Zhang S, McGinn R, Corbett D, Stewart DJ, A Fergusson D. From the lab to patients: a systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Transl Stroke Res. 2020;11(3):345–64. [DOI] [PubMed] [Google Scholar]
- 12. Sarmah D, Agrawal V, Rane P, Bhute S, Watanabe M, Kalia K, Ghosh Z, Dave KR, Yavagal DR, Bhattacharya P. Mesenchymal stem cell therapy in ischemic stroke: a meta-analysis of preclinical studies. Clin Pharmacol Ther. 2018;103(6):990–98. [DOI] [PubMed] [Google Scholar]
- 13. Cui L li, Golubczyk D, Tolppanen AM, Boltze J, Jolkkonen J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann Neurol. 2019;86(1):5–16. [DOI] [PubMed] [Google Scholar]
- 14. Xue P, Wang M, Yan GH. Mesenchymal stem cell transplantation as an effective treatment strategy for ischemic stroke in Asia: a meta-analysis of controlled trials. Ther Clin Risk Manag. 2018;14:909–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Expósito-Alcaide M, Merino J, Roda JM, Díez-Tejedor E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011. 23;175:394–405. [DOI] [PubMed] [Google Scholar]
- 16. Gutiérrez-Fernández M, Reodríguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Fuentes B, Díez-Tejedor E. Adipose tissue-derived stem cells in stroke treatment: from bench to bedside. Discov Med. 2013;16:37–43. [PubMed] [Google Scholar]
- 17. Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S, Díez-Tejedor E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013. 28;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutiérrez-Fernández M, Otero-Ortega L, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Díez-Tejedor E. Adipose tissue-derived mesenchymal stem cells as a strategy to improve recovery after stroke. Expert Opin Biol Ther. 2015;15(6):873–81. [DOI] [PubMed] [Google Scholar]
- 19. Short-term safety of allogeneic adipose-tissue derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): a phase II, randomised, double-blind, placebo-controlled, single-centre, pilot clinical trial. doi: 10.2139/ssrn.3420416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Díez-Tejedor E, Gutiérrez-Fernández M, Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML, Gimeno BF. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23(10):2694–700. [DOI] [PubMed] [Google Scholar]
- 21. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA; PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016. 24;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, Blanco F, Martínez-Taboada VM, Taylor P, Martín-Martín C, DelaRosa O, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196–202. [DOI] [PubMed] [Google Scholar]
- 24. Gómez-de Frutos MC, Laso-García F, Diekhorst L, Otero-Ortega L, Fuentes B, Jolkkonen J, Detante O, Moisan A, Martínez-Arroyo A, Díez-Tejedor E, Gutiérrez-Fernández M, et al. Intravenous delivery of adipose tissue-derived mesenchymal stem cells improves brain repair in hyperglycemic stroke rats. Stem Cell Res Ther. 2019;10(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46(9):2599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, Vadot W, Marcel S, Lamalle L, Grand S, Detante O, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11(5):910–23. [DOI] [PubMed] [Google Scholar]
- 27. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Zhang Q, Wang W, Lin F, Wang S, Zhao J. Mesenchymal stem cell therapy for ischemic stroke: a look into treatment mechanism and therapeutic potential. J Neurol. 2021;268(11):4095–107. [DOI] [PubMed] [Google Scholar]
- 29. Davis C, Savitz SI, Satani N. Mesenchymal stem cell derived extracellular vesicles for repairing the neurovascular unit after ischemic stroke. Cells. 2021. 31;10(4):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Otero-Ortega L, Laso-García F, Frutos MCG, Diekhorst L, Martínez-Arroyo A, Alonso-López E, García-Bermejo ML, Rodríguez-Serrano M, Arrúe-Gonzalo M, Díez-Tejedor E, Fuentes B, et al. Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res Ther. 2020. 19;11(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Celis-Ruiz E, Fuentes B, Moniche F, Montaner J, Borobia AM, Gutiérrez-Fernández M, Díez-Tejedor E. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open. 2021;11(8):e051790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-cll-10.1177_09636897221083863 for Final Results of Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial by Elena de Celis-Ruiz, Blanca Fuentes, María Alonso de Leciñana, María Gutiérrez-Fernández, Alberto M. Borobia, Raquel Gutiérrez-Zúñiga, Gerardo Ruiz-Ares, Laura Otero-Ortega, Fernando Laso-García, Mari Carmen Gómez-de Frutos and Exuperio Díez-Tejedor in Cell Transplantation
Supplemental material, sj-doc-2-cll-10.1177_09636897221083863 for Final Results of Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial by Elena de Celis-Ruiz, Blanca Fuentes, María Alonso de Leciñana, María Gutiérrez-Fernández, Alberto M. Borobia, Raquel Gutiérrez-Zúñiga, Gerardo Ruiz-Ares, Laura Otero-Ortega, Fernando Laso-García, Mari Carmen Gómez-de Frutos and Exuperio Díez-Tejedor in Cell Transplantation