Abstract
Tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) are the first-line treatment for EGFR-mutant non-small cell lung cancer. Toxicities related to EGFR-TKIs include skin rash, paronychia, and diarrhea, which in some cases can lead to dose reductions or treatment interruptions. Herein, we report the case of a 51-year-old woman affected by advanced adenocarcinoma harboring an exon 19 deletion in the EGFR gene, who was treated with second-generation EGFR-TKI following a scheduled gradual dose reduction to better manage toxicities. Following prescription labeling, treatment was initiated at a dose of 40 mg daily. After a few months, the dose was reduced to 30 mg daily owing to grade 3 skin toxicity. A metabolic complete tumor response was observed after 1 year of treatment, then therapy was continued at 20 mg daily, enabling disease stabilization. In conclusion, low dose afatinib was effective in an EGFR-mutant non-small cell lung cancer patient who required dose reductions to better manage toxicities.
Keywords: Afatinib, non-small cell lung cancer, epidermal growth factor receptor mutation, tyrosine kinase inhibitor, adverse event, case report
Introduction
Lung cancer is one of the most common cancer types and a leading cause of cancer-related deaths worldwide.1,2 Lung tumors are generally classified as non-small cell lung cancer (NSCLC) and small cell lung cancer according to their histological features, and each subtype shows a characteristic molecular heterogeneity. 3
Among NSCLC cases is a subset in which members of the ErbB family, including epidermal grow factor receptor (EGFR/ErbB1), HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4 are often mutated; these transmembrane proteins are involved in various cellular pathways, and it has been observed that alterations in some of them are crucial for tumorigenesis. 3
The most common EGFR mutations occur in exons 18 through 21, which encode the intracellular tyrosine kinase (TK) domain. The classical oncogenic EGFR mutations are exon 19 deletion (Ex19Del) and exon 21 point mutations, which account for approximately 85% of all EGFR mutations. 4 EGFR Ex19Del mutations are the most prevalent, representing approximately 60% of all NSCLC-associated EGFR mutations. 4 These deletions include several molecular variants, including in-frame deletions, substitutions, and insertions.
Because EGFR somatic activating mutations are common drivers of cancer, EGFR protein has become a molecular target for personalized therapy over the past decade; several reversible and irreversible tyrosine kinase inhibitors (TKIs), i.e., gefitinib, erlotinib, afatinib, dacotinib, and osimertinib, have been developed and approved as first-line treatments for patients with somatic EGFR mutations.5 –9
Clinical application of the second-generation TKI afatinib has been spreading because of its efficacy;10 –15 nevertheless, it can cause adverse events (AEs) including cutaneous (rash or acne) and gastrointestinal (diarrhea or stomatitis) toxicities that necessitate treatment interruptions or dose reductions. 11
Herein, we report the case of a patient with advanced adenocarcinoma harboring a common EGFR Ex19Del mutation that is known to confer afatinib sensitivity. The patient was effectively treated with a gradually decreasing afatinib dose. Dose reduction from 40 mg to 20 mg allowed disease stabilization as well as better toxicity management. In our experience, afatinib at a reduced dose remains effective while allowing better tolerability than at higher doses.
Case report
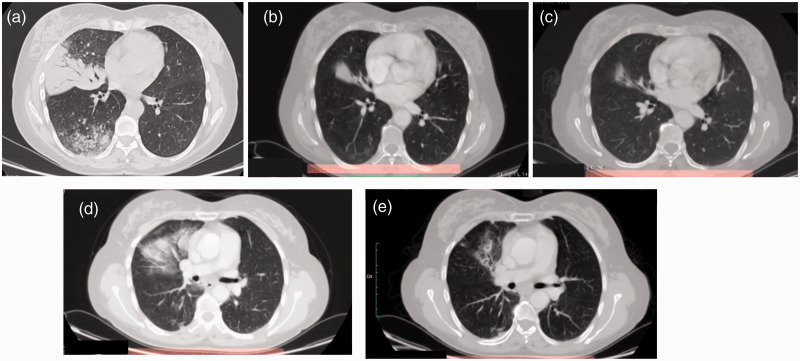
This study was compliant with all relevant ethical regulations involving human participants and was approved by the Istituto Oncologico del Mediterraneo Institutional Review Board (project ID code: n_1 of 24.09.2015). Signed informed consent was obtained from the patient. A 51-year-old woman with no smoking history presented at our hospital referring a 4-month history of cough and exertional dyspnea. The patient also had a medical history of hypertension, celiac disease, and dyslipidemia. Computed tomography (CT) imaging of the chest revealed a total consolidation of the middle lobe of the right lung, with ground glass effect and pleural diffusion (M1a) (Figure 1a).
Figure 1.
Computed tomography (CT) imaging (a) at diagnosis, (b) 3 months after treatment with 40 mg afatinib daily, (c) 13 months after dose reduction to 30 mg afatinib daily, (d) 1 month after radiotherapy, and (e) 8 months after radiotherapy.
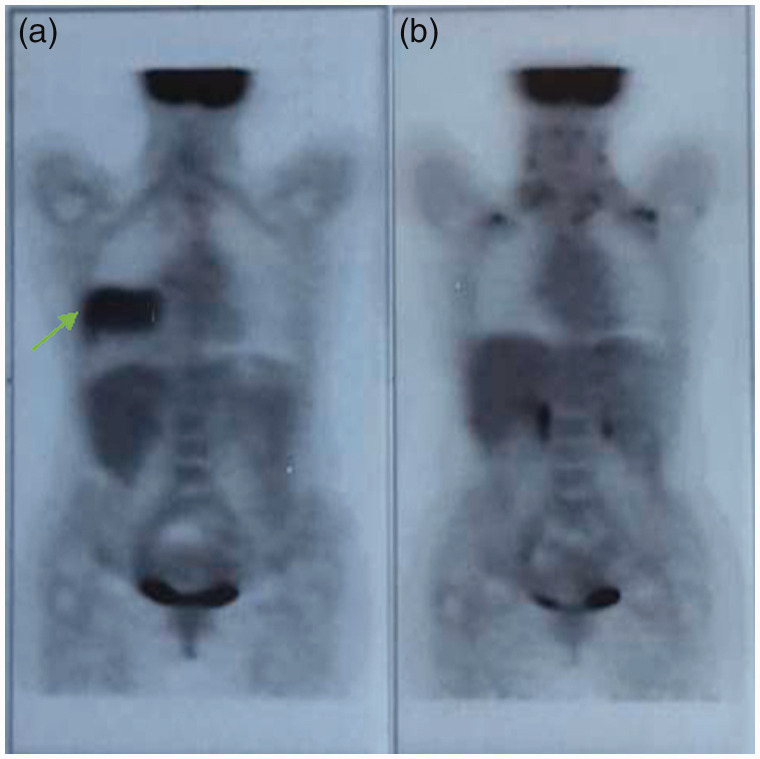
Positron emission tomography (PET) imaging (Figure 2a) confirmed the same lesions and hilar homolateral adenopathy; no evidence of distant metastases to other sites was noted. The patient underwent bronchoscopy with biopsy and brushing, and the diagnoses was NSCLC adenocarcinoma. EGFR gene sequencing showed the presence of an Ex19Del, activating E746-A750del mutation, and ALK negative. The patient was diagnosed as Stage IV (cT4 N0 M1a), according to the American Joint Committee on Cancer staging system, 7th edition. 16 On the basis of on histotype, tumor biology, and the patient’s good performance status, first-line therapy with afatinib (40 mg oral-daily) was initiated in September 2015.
Figure 2.
Positron emission tomography (PET) imaging (a) at diagnosis, and (b) after 13 months of afatinib treatment.
After 3 months of TKI therapy, a CT scan showed a good response to afatinib with partial reduction of the middle lobe lesion, with no more definable ground glass area (Figure 1b) and good tolerability. In April 2016, a CT scan revealed further reduction of the middle lobe lesion (not shown). Despite the good tumor response, the patient reported grade 3 skin rash and grade 1 diarrhea. As a consequence of the grade 3 skin toxicity, afatinib was interrupted, with gradual regression to grade 2 toxicity in 5 days. The patient started oral and topical antibiotic treatment according to dermatologic consultation. Six days after treatment interruption, the patient restarted afatinib at 30 mg daily. In October 2016, a CT scan revealed further reduction of middle lobe lesion and absence of hilar lymphadenopathy (Figure 1c). Aside from resolution of respiratory symptoms, the patient presented with improved cutaneous toxicity, with persistence of a grade 1 cutaneous rash and grade 1 paronychia requiring treatment with 2% sulfosalicylic cream, astringent gel, and polyethylene glycols ointment. Considering the good clinical response to treatment and limited extension of disease, the patient underwent a PET scan, which showed complete metabolic tumor response (Figure 2b).
After radiotherapy consultation, afatinib was interrupted and the patient was admitted for radiation treatment between 12 December 2016 and 20 January 2017. Intensity modulated radiation therapy consisting of a total dose of 6020 cGy was focused on the right hemi-mediastinum plus homolateral lung lesion with a boost on the right pulmonary hilum. As a consequence of radiotherapy, the patient presented with post actinic pneumonia (Figure 1d), which was treated with corticosteroids, and then afatinib was restarted at 30 mg daily. In September 2017, a CT scan showed resolution of pneumonia and an additional tumor response (Figure 1e). Because the grade 2 cutaneous toxicity remerged in October 2017, the afatinib dosage was further reduced to 20 mg daily. The patient continued therapy with afatinib at 20 mg daily until May 2019 with good disease control and tolerability.
Discussion
Historically, platinum-based chemotherapy was the standard first-line treatment for NSCLC. In patients harboring EGFR mutations, EGFR-TKIs are now the standard of care and provide improved progression-free survival and overall response rates. 17 In fact, several studies have demonstrated the improved effectiveness of EGF-TKIs versus chemotherapy as a first-line therapy for metastatic NSCLCs harboring certain activating EGFR mutations, with fewer AEs than standard chemotherapy.18 –25 EGFR mutation status is the most important determining factor for clinical response to EGFR-TKI. 26 EGFR Ex19Del mutations account for approximately 60% of lung cancer-associated EGFR mutations and include a heterogeneous group of mutations. 27 The most frequently observed EGFR Ex19Del is E746-A750, which is between the third β-strand of the EGFR tyrosine kinase domain and its key regulatory αC helix, whereas many other EGFR Ex19Dels are complex insertion-deletions starting at leucine 747, in which the deleted amino acids are replaced with non-native residues (such as the L747-A750 > P and L747-P753 > S variants). Although it is known that EGFR Ex19Dels can constructively impact the sensitivity of TKI treatment by activating the TK region, potential differences in TKI sensitivity between individual EGFR Ex19Dels is not well estabilished. 27
Afatinib is an irreversible, second-generation EGFR-TKI that has been proven to provide significantly longer overall survival compared with platinum-based chemotherapy when used in lung cancer patients with EGFR Ex19Del mutations. 28 Second-generation TKIs have also demonstrated superior outcomes versus the first-generation TKIs, erlotinib and gefitinib. 29 Recent studies26,27,30 have investigated potential differences in the TKI sensitivity of common and uncommon EGFR Ex19Dels. Good clinical response to first-line afatinib monotherapy has been observed in each EGFR Ex19Del molecular variant. 30 However, patients harboring EGFR Ex19Dels starting at codon E746 had a better median progression-free survival (14.2 months) than those harboring Ex19Dels starting at codon L747 (6.5 months). 26 The recommended starting dose of afatinib is 40 mg daily for patients whose lung cancers harbor EGFR mutations, although this dose is often accompanied by side effects, with diarrhea and rash/acne being the most frequently reported AEs. In fact, more severe AEs were observed in patients who received the standard 40 mg afatinib daily compared with those who received a first-generation EGFR-TKI, such as gefitinib or erlotinib. 31 Therefore, in clinical practice, many clinicians prescribe a lower starting dose of afatinib32,33 or perform dose modifications34,35 to improve patient outcomes and adherence, 31 without compromising its beneficial effect.
In our case, the patient was treated with 40 mg afatinib daily owing to its ability to irreversibly block EGFR, which is different than first-generation EGFR-TKIs. After 6 months of treatment, the dose was reduced to 30 mg because of cutaneous toxicity; dose reduction to 30 mg daily resulted in a complete metabolic tumor response after another 5 months of therapy. A previous case report 36 and several clinical trials37,38 have reported the effectiveness and safety of the treatment strategy involving reduced doses of afatinib in patients with NSCLC adenocarcinoma, demonstrating that use of afatinib at reduced doses shows good tumor control and management of toxicities. Clinical studies37,38 have found no significant difference in the median progression-free survival of patients who received afatinib at reduced doses; there was also a reduction in the incidence and severity of AEs compared with those who received 40 mg or higher doses. Furthermore, a recent clinical trial that enrolled patients with metastatic lung adenocarcinoma who were treated with either 30 mg or 40 mg afatinib daily as first-line treatment demonstrated that patients who received an initial afatinib dose of 40 mg daily required dose reduction (or discontinuation) more frequently than those who initially received 30 mg daily (40% vs. 8%); however, this study did not discriminate between patients with Ex19Dels and those with exon 21 L858R point mutations. 31
In our case, a lower afatinib dose still allowed the patient to achieve a complete metabolic tumor response and then stable disease with a long lasting response (47 administrations from September 2015 to May 2019). Reducing the afatinib dose helps better manage AEs, including cutaneous toxicity. In our experience, afatinib at a reduced dose retains its efficacy with a better toxicity profile compared with higher doses.
Conclusion
This case demonstrated that low-dose afatinib was effective for disease control in a patient diagnosed with NSCLC harboring the EGFR Ex19Del mutation E746-A750del who developed unacceptable side effects at higher doses. However, more clinical trial and/or real-life data are required to find a reliable strategy for:
effectively treating patients harboring common and uncommon EGFR mutations, discriminating the use of different TKIs to reach the best clinical outcomes, while also reducing AEs;
reducing AEs associated with afatinib, while maintaining its clinical efficacy by using lower starting doses or dose reduction for the management of lung cancer.
The reporting of this study conforms with the CARE guidelines. 39
Acknowledgement
The authors would like to thank the patient involved the study.
Footnotes
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Francesca Esposito https://orcid.org/0000-0002-1597-9034
Paolo Giuffrida https://orcid.org/0000-0002-4628-1130
Claudia Caltavuturo https://orcid.org/0000-0001-7506-3223
Dario Giuffrida https://orcid.org/0000-0001-6404-2304
References
- 1.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011; 32: 605–644. doi: 10.1016/j.ccm.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019; 94: 1623–1640. doi: 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and molecular classification of lung cancer. Cancer Treat Res 2016; 170: 25–46. doi: 10.1007/978-3-319-40389-2_2 [DOI] [PubMed] [Google Scholar]
- 4.Xu CW, Lei L, Wang WX, et al. Molecular Characteristics and Clinical Outcomes of EGFR Exon 19 C-Helix Deletion in Non-Small Cell Lung Cancer and Response to EGFR TKIs. Transl Oncol 2020; 13: 100791. doi: 10.1016/j.tranon.2020.100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afatinib. Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2019/201292s015lbl.pdf (accessed 26 February 2020)
- 6.Osimertinib. Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2019/208065s013lbl.pdf (accessed 26 February 2020)
- 7.Tarceva (erlotinib) Label., 2020. Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf (accessed 26 February 2020)
- 8.Gefitinib. Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf (accessed 26 February 2020)
- 9.Dacomitinib. Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2018/211288s000lbl.pdf (accessed 12 February 2020)
- 10.Goss GD, Felip E, Cobo M, et al. Association of ERBB mutations with clinical outcomes of afatinib-or erlotinib-treated patients with lung squamous cell carcinoma: secondary analysis of the LUX-lung 8 randomized clinical trial. JAMA Oncol 2018; 4: 1189–1197. doi: 10.1001/jamaoncol.2018.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskens FALM, Mom CH, Planting AST, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 2008; 98: 80–85. doi: 10.1038/sj.bjc.6604108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuan FC, Li SH, Wang CL, et al. Analysis of progression-free survival of first-line tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring leu858Arg or exon 19 deletions. Oncotarget 2017; 8: 1343–1353. doi: 10.18632/oncotarget.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008; 27: 4702–4711. doi: 10.1038/onc.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897–907. doi: 10.1016/S1470-2045(15)00006-6 [DOI] [PubMed] [Google Scholar]
- 15.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 2010; 28: 3965–3972. doi: 10.1200/JCO.2009.26.7278 [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer, 2010. [Google Scholar]
- 17.Myers DJ, Wallen JM. Lung adenocarcinoma. Treasure Island (FL): StatPearls Publishing, 2020. https://www.ncbi.nlm.nih.gov/books/NBK519578/ [PubMed]
- 18.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. doi: 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 19.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. doi: 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 20.Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: A meta-analysis. J Natl Cancer Inst 2013; 105: 595–605. doi: 10.1093/jnci/djt072 [DOI] [PubMed] [Google Scholar]
- 21.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 22.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 23.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. doi: 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–1889. doi: 10.1093/annonc/mdv270 [DOI] [PubMed] [Google Scholar]
- 25.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–742. doi: 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 26.Lee VH, Tin VP, Choy TS, et al. Association of exon 19 and 21 EGFR mutation patterns with treatment outcome after first-line tyrosine kinase inhibitor in metastatic non-small-cell lung cancer. J Thorac Oncol 2013; 8: 1148–1155. doi: 10.1097/JTO.0b013e31829f684a [DOI] [PubMed] [Google Scholar]
- 27.Truini A, Starrett JH, Stewart T, et al. The EGFR Exon 19 Mutant L747-A750>P Exhibits Distinct Sensitivity to Tyrosine Kinase Inhibitors in Lung Adenocarcinoma. Clin Cancer Res 2019; 25: 6382–6391. doi: 10.1158/1078-0432.CCR-19-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 29.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577–589. doi: 10.1016/s1470-2045(16)30033-x. [DOI] [PubMed] [Google Scholar]
- 30.Tokudome N, Koh Y, Akamatsu H, et al. Differential significance of molecular subtypes which were classified into EGFR exon 19 deletion on the first line afatinib monotherapy. BMC Cancer 2020; 20: 103. doi: 10.1186/s12885-020-6593-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YC, Tsai MJ, Lee MH, et al. Lower starting dose of afatinib for the treatment of metastatic lung adenocarcinoma harboring exon 21 and exon 19 mutations. BMC Cancer 2021; 21: 495. doi: 10.1186/s12885-021-08235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama T, Yoshioka H, Fujimoto D, et al. A phase II study of low starting dose of afatinib as first-line treatment in patients with EGFR mutation-positive non-small-cell lung cancer (KTORG1402). Lung Cancer 2019; 135: 175–180. doi: 10.1016/j.lungcan.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 33.Yang CJ, Tsai MJ, Hung JY, et al. The clinical efficacy of Afatinib 30 mg daily as starting dose may not be inferior to Afatinib 40 mg daily in patients with stage IV lung Adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol Toxicol 2017; 18: 82. doi: 10.1186/s40360-017-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halmos B, Tan EH, Soo RA, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: Results from a global real-world study (RealGiDo). Lung Cancer 2019; 127: 103–111. doi: 10.1016/j.lungcan.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 35.Imai H, Kaira K, Suzuki K, et al. A phase II study of afatinib treatment for elderly patients with previously untreated advanced non-small-cell lung cancer harboring EGFR mutations. Lung Cancer 2018; 126: 41–47. doi: 10.1016/j.lungcan.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 36.Giusti R, Mazzotta M, Iacono D, et al. Complete tumor response with afatinib 20 mg daily in EGFR-mutated non-small cell lung cancer: a case report. Clin Drug Investig 2017; 37: 581–585. doi: 10.1007/s40261-017-0515-2 [DOI] [PubMed] [Google Scholar]
- 37.Hirsh V, Yang JCH, Tan EH, et al. First-line afatinib (A) vs gefitinib (G) for patients (pts) with EGFR mutation positive (EGFRm?) NSCLC (LUX-Lung 7): patient-reported outcomes (PROs) and impact of dose modifications on efficacy and adverse events (AEs). J Clin Oncol 2016; 34: 9046–9046. doi: 10.1200/JCO.2016.34.15_suppl.9046 [Google Scholar]
- 38.Nakamura A, Tanaka H, Saito R, et al. Phase II Study of Low-Dose Afatinib Maintenance Treatment Among Patients with EGFR-Mutated Non-Small Cell Lung Cancer: North Japan Lung Cancer Study Group Trial 1601 (NJLCG1601). Oncologist 2020; 25: e1451–e1456. doi: 10.1634/theoncologist.2020-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]