Abstract
A 26-year-old Asian woman with persistent muscle weakness was diagnosed with polymyositis based on biopsy findings at another hospital 11 years ago. However, her symptoms fluctuated repeatedly under treatment with prednisone and immunosuppressive agents, and worsened 2 months prior to the current presentation. A second muscle biopsy suggested metabolic myopathy, and genetic testing revealed a novel c.1074C > T variant in the glycogen synthase 1 gene (GYS1), which is implicated in muscle glycogen storage disease type 0. However, no abnormalities in glycogen deposition were found by biopsy; rather, muscle fibers exhibited large intracellular lipid droplets. Furthermore, muscle strength was greatly restored and circulating levels of creatine kinase indicative of muscle degeneration greatly reduced by vitamin B2 treatment. Therefore, the final diagnosis was lipid storage myopathy.
Keywords: Lipid storage myopathy, polymyositis muscle, glycogen storage disease type 0, GYS1, case report, vitamin B2
Introduction
Lipid deposition myopathy (LSM) refers to a group of genetic metabolic myopathies in which lipids accumulate in skeletal muscle fibers because of abnormal metabolism, resulting in lesions and degeneration. The main clinical manifestations are progressive muscle weakness and movement intolerance, although the course of the disease can vary. These clinical manifestations are non-specific and resemble those of polymyositis (PM); indeed, early stage LSM is often misdiagnosed as PM.
This report documents a case of LSM initially misdiagnosed as PM based on initial muscle biopsy. A subsequent biopsy suggested metabolic myopathy, and genetic sequencing revealed a novel c.1074C > T variant in the glycogen synthase 1 gene (GYS1) indicative of GSD type 0. However, muscle strength was substantially improved by vitamin B2 treatment, consistent with lipid storage myopathy. This is the first report of an Asian patient with lipid storage myopathy initially misdiagnosed as PM but subsequently re-diagnosed as LSM based on biopsy findings at a later disease stage. The reporting of this study conforms to CARE guidelines. 1
Case report
A 26-year-old female patient with an 11-year history of proximal extremity weakness and difficulty swallowing presented at another hospital with exacerbated bilateral proximal muscle weakness that resulted in difficulty turning over, squatting, and going up and down stairs for the previous week, and was admitted for tests. In 2009, she had presented at the Rheumatology Department of another hospital with limb weakness, especially of the lower limbs, accompanied by difficulty walking and breathing. At that time, serum creatine kinase (CK) was elevated (6890 U/L), so both electromyography and muscle biopsy were performed to confirm possible PM. While the inflammatory antibody spectrum was negative, treatment with prednisone 60 mg once daily enhanced muscle strength and she was discharged with a diagnosis of PM. After treatment for 6 months with prednisone 50 mg once daily and methotrexate 15 mg once weekly, the prednisone dose was progressively reduced to 20 mg once daily. However, the condition relapsed, so the prednisone dose was increased again, resulting in improvement. In 2017, the patient presented to the Rheumatology and Immunology Department of our hospital with symptom recurrence, presumably from PM. In the weeks prior to this presentation, the patient experienced recurrence of severe muscle weakness and elevated serum CK and lactate dehydrogenase (LDH). A corticosteroid-dependent inflammatory muscle condition was assumed, so she was administered the immunosuppressive tacrolimus concomitant with prednisone and methotrexate, but again her condition relapsed after corticosteroid reduction, and was improved upon prednisone dosage increase, as evidenced by symptoms taking longer to emerge or reverse.
In August 2021, she again developed proximal weakness of both lower extremities, resulting in difficulty turning over, squatting, climbing stairs, and breathing, and sought treatment at another hospital. On admission, physical examination revealed stable vital signs, clear consciousness, full moon face, humped upper back, visible purple streaks on the lower abdomen and both sides of the thighs, grade 3 muscle strength of both lower limbs, and grade 4 strength of upper limbs, but no other obvious abnormalities. Family history revealed that the parents were close relatives, but there were no similar cases in the family. Routine blood examination revealed a high white blood cell count of 9.86 × 109/L, including a high neutrophil count of 6.84 × 109/L. Blood biochemical parameters included high levels of aminotransferase (318 U/L), aspartate aminotransferase (254 U/L), CK (1772 U/L), and LDH (2727 U/L). Tests for fungal infections (G and GM tests, tuberculosis (T-SPOT test), respiratory pathogen-related IgM, Epstein–Barr (EB) virus nucleic acid (quantitative) test, and EB virus antibody test were all negative (Table 1).
Abdominal ultrasound revealed a fatty liver, and muscle magnetic resonance imaging showed abnormal signals in the muscles of the back, buttocks, and lower extremities. Electromyography (EMG) of muscles of the lower extremities showed myogenic damage in the form of a shortened mean motor unit potential duration, decreased amplitude, and increased polyphase wave ratio and fiber potential. Collectively, these signs were consistent with myositis. Additionally, computed tomography of the chest suggested interstitial lesions in the lower lobes of both lungs. Histopathological analysis of muscle biopsy samples revealed multiple vacuoles accompanied by an aggregation of lipid droplets and numerous atypical broken red fibers. Collectively, these findings suggested metabolic myopathy, so genetic testing was conducted. Further, muscle strength and dyspnea symptoms were greatly improved by empirically-dosed vitamin B2 (20 mg three times daily) and symptomatic liver protection for 3 days for suspected deposit myopathy. This occurred even after gradual prednisone dose reduction and withdrawing both methotrexate and tacrolimus, which resulted in increased levels of muscle enzymes and decreased muscle strength from grade 4 to grade 1 (Figures 1, 2).
Figure 1.
Medication prescribed to the patient from 2017 to 2021.
Figure 2.
Changes in CK and LDH from 2017 to 2021.
CK, creatine kinase; LDH, lactate dehydrogenase.
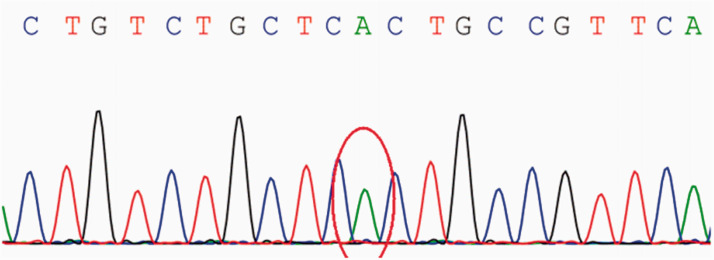
Genetic examination of the patient’s mother revealed a novel homozygous mutation in GYS1 (c.1074C > T p.S358S). Pedigree verification showed that the patient’s mother carried the heterozygous variant while the patient was homozygous for the variant (Figures 3, 4). According to American College of Medical Genetics and Genomics guidelines, this variant was classed as of unknown clinical significance (PM2_Supporting: normal population variance low-frequency variation of the database). At discharge, the muscle strength of both lower limbs was grade 4, the muscle strength of both upper limbs was grade 4+, serum CK levels were normal, lactate was low, and hydrogenase levels were close to normal. Two weeks after discharge, levels of serum enzymes indicative of muscle degeneration had returned to normal.
Figure 3.
Electropherogram of the patient’s variant showing a homozygous variation (antisense chain) of c.1074c > t.
Figure 4.
Electropherogram of the patient's mother’s variant showing a heterozygous variation (antisense chain) of c.1074c > t.
Discussion
LSM is a relatively rare disorder of muscle metabolism. Clinically, LSM manifests as persistent muscle weakness, muscle pain, and exercise intolerance. 2 These deficits result from muscle glycogen depletion when fatty acid metabolism cannot meet demands, so symptoms can be relieved by rest. 3 However, severe cases may be associated with lipid accumulation and subsequent muscle fiber damage. According to biochemical defects and genetic causes, LSM can be divided into four disease types: multiple acyl-CoA dehydrogenase defects (MADD), primary carnitine deficiency, neutral lipid deposition with ichthyosis, and neutral lipid deposition with myopathy. 4 To date, only about 700 MADD cases have been reported worldwide, of which most (95%) are symptomatic.5–7 However, only a few large series of patients have been reported, so most clinical information comes from single case reports and small case series.8,9
In China, the most common LSM type is late-onset MADD (RR-MADD), which is caused by a deficiency in the mitochondrial enzyme electron transfer flavoprotein-ubiquinone oxidoreductase (ETFDH). 10 Most patients respond positively to treatment with riboflavin (vitamin B2) alone, 11 although studies have also suggested supplementing with coenzyme Q11.12. In the current patient, vitamin B2 alone greatly improved muscle strength and reduced serum muscle enzymes levels, indicating reduced muscle degeneration, so coenzyme Q10 was not administered.
Deficient lipid metabolism can affect all systems, so symptoms tend to be heterogenous. In the current patient, EMG showed myogenic damage or combined myogenic and neurogenic damage, serum muscle enzymes were greatly elevated, and recurrence was frequent. Several case studies have reported only slight recovery after glucocorticoid therapy.9,13,14 However, in this patient, increasing the glucocorticoid dose when the disease worsened induced relief, so an initial diagnosis of PM was assumed by many treating clinicians.
PM is an autoimmune disease that mainly affects skeletal muscle. The central pathology is aseptic muscle inflammation triggered by the infiltration of CD8+ T cells and the formation of immune synapses with major histocompatibility complex-I-positive muscle fibers, ultimately leading to muscle fiber destruction. The clinical onset can be acute or subacute, and the main symptoms are progressive proximal muscle weakness, elevated serum CK, signs of myogenic damage on the EMG, and inflammatory cell infiltration of skeletal muscle. These clinical manifestations are extremely similar to LSM so there is a risk of misdiagnosis in the early stage of LSM. Studies have demonstrated higher serum CK in PM than LSM, but differences may not easily be distinguished by routine clinical tests. 12 Alternatively, some PM patients are positive for autoantibodies, which can be used for differential diagnosis. Muscle pathology can also differ between LSM and PM. Under light microscopy, hematoxylin and eosin-stained sections of LSM muscle exhibit multiple fibers with large fused vacuoles and a broken appearance scattered throughout, while necrotic and regenerated fibers are rare. Masson’s trichrome staining also often reveals scattered red-dyed fibers, but not typical ruffled red fibers, while Oil Red O staining indicates that the vacuoles are fatty deposits. These appear mainly in type I fibers although type II fibers may also exhibit fatty deposits. Additionally, succinate dehydrogenase staining shows a diffuse reduction in enzyme activity, while coenzyme I tetrazolium reductase, cytochrome C oxidase, and periodic acid Schiff reaction staining are usually normal in LSM muscle. 11
Ultimately, the gold standard for distinguishing LSM from PM is genetic testing. Most LSM cases are associated with mutations in the ETFDH gene, electron transfer flavoprotein subunit alpha gene (ETFA), or electron transfer flavoprotein subunit beta gene (ETFB). To date, the most common variant found in patients with myopathy and RR-MADD is ETFDH c.1130T > C. 15 However, this was not identified in any of the 90 Chinese patients examined in a previous study. 13 Sixty-eight pathogenic ETFDH variants have been identified in Chinese patients, 33% of which were homozygous and 69% compound heterozygous.12,13,16 In contrast, the most common pathogenic gene for PM is HLA-DR52.
The case patient was a young female with an 11-year history of muscle weakness and muscle fiber degeneration (as evidenced by elevated CK and LDH). Initial biopsy revealed changes misinterpreted as signs of PM. However, PM is a heterogeneous disease with few specific signs. The patient was also negative for myositis antibodies and responsive to glucocorticoids. Some PM patients are prone to relapse, and, in this case, levels of muscle enzymes repeatedly increased and muscle strength decreased during drug tapering or withdrawal. Subsequent findings, including a muscle biopsy performed at another hospital, and a positive response to vitamin B2 and symptomatic liver protective treatment led to a change in diagnosis to LSM. Further, genetic testing identified a variant in an LSM-associated gene.
MADD is separable into mild and severe forms. Type III MADD is associated with a missense variant in ETFDH, is milder, and responds well to ribonucleic acid, while type I and type II forms are associated with ETFA, ETFB, or ETFDH variants that dramatically reduce enzyme expression or function.9,15,16 Our patient’s condition improved after 3 days, consistent with a mild form of the disease. The variant was inherited from the patient’s mother; we have no information on paternal inheritance. Theoretically, a GYS1 variant could be associated with muscle GSD type 0, but previous symptomatic cases exhibited homozygosity or an additional pathogenic gene. In this case, the patient harbored only one known chromosomal variant which was maternally inherited. According to ACMG guidelines, this variant should be graded as ‘of unknown clinical significance (PM2_Supporting: normal population variant data Low-frequency variation of the library).
GSD results from congenital defects in glycogen metabolizing enzymes that cause glycogen to accumulate in the lysosomes and cytoplasm of muscles, liver cells, and cardiomyocytes, leading to cell death and ultimately to multi-organ dysfunction. GSD is divided into 13 types numbered according to the glucose-metabolizing enzyme mutated. 17 Among these, the most common type is GSD-II, which manifests as slowly progressive proximal muscle atrophy. It can be difficult to distinguish from PM, and the main treatment is enzyme replacement therapy. Glycogen depletion caused by GYS1 variants leads to elevated glucose. GSD type 0 manifests as increased glycogen particles in muscle cells; however, PAS staining of muscle biopsy tissue from our patient revealed no such increase. Moreover, the patient responded to vitamin B2, without requiring enzyme replacement therapy, and evidence for gene pathogenicity was only medium according to the frequency of insertions or deletions revealed by high-throughput sequencing.
This is the first reported case of LSM with a GSD type 0-related variant misdiagnosed as PM. When muscle disease is mild, the muscle biopsy may not contain any lipid droplets. 13 The absence of lipid droplets in the first biopsy from our patient may therefore be explained by the progressive nature of the disorder. Our patient was responsive to glucocorticoids and immunosuppressive agents, but always relapsed upon drug tapering or withdrawal. Therefore, we suggest that when treatment fails to prevent myopathy, testing for PM, GSD, mitochondrial disease, and progressive muscular dystrophy should be carried out. 18 Given the family history of consanguineous marriage and muscle biopsy revealing numerous muscle cells with lipid droplets in this case, genetic LSM was considered a possibility, and successful vitamin B2 treatment was consistent with this. However, Sanger sequencing did not identify a mutation in a typical LSM gene. Further, the patient’s condition improved without enzyme replacement therapy, so the diagnosis appeared inconsistent with genetic analysis. However, the negative LSM gene test cannot rule out the disease because the patient did not undergo a large fragment gene mutation test, so it is still possible that the disorder of lipid metabolism is secondary to mitochondrial disease. In such cases with equivocal findings, a comprehensive diagnosis should consider the patient’s medical history, family history (e.g., of consanguineous marriage), pathology, and treatment effects. If necessary, further large fragment gene detection should be conducted.
This article describes identification of the first known Asian patient with LSM and a GSY1 variant. Despite this variant, muscle biopsy tissue exhibited no glycogen accumulation. The main lesson to be learned from this case is that treatment should be re-evaluated if there are doubts about the diagnosis and treatment regimen, even those based on past pathological examinations. Follow-up observations should be examined to consider multiple possible diseases, and the diagnosis altered if necessary. For patients with myasthenia or elevated CK showing repeated symptom exacerbation upon withdrawal of corticosteroids or immunosuppressants, multiple muscle biopsies combined with genetic testing are necessary. In our patient, clinical manifestations, muscle biopsy findings, and therapeutic effects suggested a lipid deposition disease, so the GSY1 variant may be a nonpathological polymorphism. Because no pathogenic variant has been confirmed, the pathomechanism requires further study.
Declaration of conflicting interest: All authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Mei Tian https://orcid.org/0000-0002-1633-2988
Statement of ethics
The patient provided written informed consent for publication of this article (including the external hospital examination results). The study protocol was approved by the Medical Ethics Committee of Affiliated Hospital of Zunyi Medical University.
Table 1.
Initial laboratory values.
| Parameters | Patient measurement | Normal reference range |
|---|---|---|
| White blood cells (×109/L) | 9.86 | 3.5–9.5 |
| Neutrophil count (×109/L) | 6.84 | 1.8–6.3 |
| Hemoglobin (g/L) | 132 | 130–175 |
| Platelets (×109/L) | 262 | 100–300 |
| Alanine aminotransferase (U/L) | 318 | 7–40 |
| Aspartate aminotransferase (U/L) | 254 | 13–35 |
| CK (U/L) | 1772 | 26–140 |
| LDH (U/L) | 2727 | 140–271 |
| G and GM tests/T-SPOT test/EB virus nucleic acid quantitative/ EB virus antibody/Respiratory pathogen IgM Jiu Lian | Negative | Negative |
CK, creatine kinase; LDH, lactate dehydrogenase; RV, residual gas volume; TCL, total lung volume; IgG, immunoglobulin G; IgM: immunoglobulin M.
References
- 1.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 2.Angelini C, Tavian D, Missaglia S. Heterogeneous phenotypes in lipid storage myopathy due to ETFDH gene mutations. JIMD Rep 2017; 38: 33–40. DOI: 10.1007/8904_2017_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohkuma A, Noguchi S, Sugie H, et al. Clinical and genetic analysis of lipid storage myopathies. Muscle Nerve 2009; 39: 333–342. DOI: 10.1002/mus.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen RKJ, Koňaříková E, Giancaspero TA, et al. Riboflavin-responsive and non-responsive mutations in FAD synthase cause multiple acyl-CoA dehydrogenase and combined respiratory-chain deficiency. Am J Hum Genet 2016; 98: 1130–1145. DOI: 10.1016/j.ajhg.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Hong D, Zhang W, et al. Severe sensory neuropathy in patients with adult-onset multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 2016; 26: 170–175. DOI: 10.1016/j.nmd.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Xue Y, Zhou Y, Zhang K, et al. Compound heterozygous mutations in electron transfer flavoproteinn dehydrogenase identified in a young Chinese woman with late-onset glutaric aciduria type II. Lipids Health Dis 2017; 16:185. DOI: 10.1186/s12944-017-0576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu XY, Wang ZQ, Wang DN, et al. A historical cohort study on the efficacy of glucocorticoids and riboflavin among patients with late-onset multiple acyl-CoA dehydrogenase deficiency. Chin Med J (Engl) 2016; 129: 142–146. DOI: 10.4103/0366-6999.173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, Zhu X, Qi X, et al. Riboflavin-responsive multiple Acyl-CoA dehydrogenation deficiency in 13 cases, and a literature review in mainland Chinese patients. J Hum Genet 2014; 59: 256–261. DOI: 10.1038/jhg.2014.10. [DOI] [PubMed] [Google Scholar]
- 9.Olsen R, Olpin SE, Andresen BS, et al. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain: A Journal of Neurology 2007; 130: 2045–2054. DOI: 10.1093/brain/awm135. [DOI] [PubMed] [Google Scholar]
- 10.Wen B, Dai T, Li W, et al. Riboflavin-responsive lipid-storage myopathy caused by ETFDH gene mutations. J Neurol Neurosurg Psychiatry 2010; 81: 231–236. DOI: 10.1136/jnnp.2009.176404. [DOI] [PubMed] [Google Scholar]
- 11.Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring flavoprotein dehydrogenase (ETFDH) gene. Brain 2007; 130: 2037–2044. DOI: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi J, Wen B, Lin J, et al. Clinical features and ETFDH mutation spectrum in a cohort of 90 Chinese patients with late-onset multiple acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 2014; 37: 399–404. DOI: 10.1007/s10545-013-9671-6. [DOI] [PubMed] [Google Scholar]
- 13.Fu HX, Liu XY, Wang ZQ, et al. Significant clinical heterogeneity with similar ETFDH genotype in three Chinese patients with late-onset multiple acyl-CoA dehydrogenase deficiency[J]. Neurol Sci 2016; 37: 1099–1105. DOI: 10.1007/s10072-016-2549-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Huang JJ, Wang ZQ, et al. Value of muscle enzyme measurement in evaluating different neuromuscular diseases[J]. Clin Chim Acta 2011; 413: 520–524. DOI: 10.1016/j.cca.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Olsen RK, Andresen BS, Christensen E, et al. Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat 2003; 22: 12–23. DOI: 10.1002/humu.10226. [DOI] [PubMed] [Google Scholar]
- 16.Grunert SC. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme A dehydrogenase deficiency. Orphanet J Rare Dis 2014; 9: 117. DOI: 10.1186/s13023-014-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldfors A, Di Mauro S. . New insights in the field of muscle glycogenoses. Curr Opin Neurol 2013; 26: 544–553. DOI: 10.1093/brain/awm135. [DOI] [PubMed] [Google Scholar]
- 18.Beresford MW, Pourfarzam M, Turnbull DM, et al. So doctor, what exactly is wrong with my muscles? Glutaric aciduria type II presenting in a teenager. Neuromuscul Disord 2006; 16: 269–273. DOI: 10.1016/j.nmd.2006.07.003. [DOI] [PubMed] [Google Scholar]