Abstract
Introduction:
Pancreatic cancer (PC) is one of the most aggressive tumours, and better risk stratification among patients is required to provide tailored treatment. The meaning of radiomics and texture analysis as predictive techniques are not already systematically assessed. The aim of this study is to assess the role of radiomics in PC.
Methods:
A PubMed/MEDLINE and Embase systematic review was conducted to assess the role of radiomics in PC. The search strategy was ‘radiomics [All Fields] AND (“pancreas” [MeSH Terms] OR “pancreas” [All Fields] OR “pancreatic” [All Fields])’ and only original articles referred to PC in humans in the English language were considered.
Results:
A total of 123 studies and 183 studies were obtained using the mentioned search strategy on PubMed and Embase, respectively. After the complete selection process, a total of 56 papers were considered eligible for the analysis of the results. Radiomics methods were applied in PC for assessment technical feasibility and reproducibility aspects analysis, risk stratification, biologic or genomic status prediction and treatment response prediction.
Discussion:
Radiomics seems to be a promising approach to evaluate PC from diagnosis to treatment response prediction. Further and larger studies are required to confirm the role and allowed to include radiomics parameter in a comprehensive decision support system.
Keywords: decision supporting system, pancreatic cancer, radiomics
Introduction
Pancreatic cancer (PC) is one of the most aggressive tumours, representing the fourth cause of cancer-related deaths with 132,600 estimated new diagnoses, 128,000 deaths per year and accounting for 3.4–6.6% of all cancer cases, respectively.1,2 The incidence and mortality are slightly increasing during the years with a 5-year overall survival (OS) rate lower than 10%. 3 Males are more frequently affected than females and incidence in both sexes increases with age, reaching its peak in people older than 70 years. 4
Primary prevention is of utmost importance as there is currently no effective method of screening for the general population. Nevertheless, molecular or imaging tests can help early diagnosis in high-risk cohorts; for example, endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI) allowed to detect precursor or invasive pancreatic neoplasms in asymptomatic people with an inherited predisposition (5–10% of all patients), thus increasing the resectability of the tumours and the overall prognosis of these patients. 5 Further data are needed to establish the most adequate approach and to select patients who could benefit the most from screening programmes.
Signs and symptoms are indeed not disease-specific and usually appear when it has already become unresectable or distantly spread.
Computed tomography (CT) is the most commonly used imaging diagnostic tool, supported by EUS with fine needle biopsy or aspiration in the identification of small lesions and in providing a definitive diagnosis. 6 MRI and positron emission tomography (PET) may also contribute to the systemic staging of the disease and in characterizing the primary tumour as resectable, borderline resectable or unresectable.
PC is a heterogeneous group of diseases; nearly 90% of the total are adenocarcinomas. Exocrine tumours are known to have a more aggressive behaviour compared with endocrine ones, with a median survival of about 4 versus 27 months, as reported in a large population-based study. 7 Pancreatic adenocarcinoma can progress from different types of precursor lesions, such as pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasia (IPMN), under the push of consecutive genetic alterations whose significance is frequently unknown. 8 Some of these mutations have been recognized as having a role in different steps of carcinogenesis or even a prognostic significance, such as SMAD4 loss, which is linked to metastatic spread and lower survival rates. 9 These alterations may have, therefore, the potential to identify precursor lesions and serve as targets for new therapies. Currently, the first treatment option for PC is represented by radical surgery. Unfortunately, few more than 10% of patients are amenable to curative-intent surgery and prognosis remains poor even in this cluster of patients, with 5-year survival rates around 20%. 10
The quantitative analysis of medical images data and the extraction of imaging features, also called ‘radiomics’, represent an emerging approach in personalized medicine and advanced diagnostics, especially for disease characterization or outcome prediction.11–13 The interest towards radiomics is rapidly growing in the multidisciplinary cancer community as it shows an interesting pertinency and efficacy to answer several clinical questions arising in the management of patients affected by other gastrointestinal tumours.14–21
The aim of this study is to systematically assess and summarize evidence published in the scientific literature about the different applications of radiomics in PC.
Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations. 22 A systematic PubMed/MEDLINE and Embase search was performed using the following search strategy: ‘radiomics [All Fields] AND “pancreas” [MeSH Terms] OR “pancreas”[All Fields] OR “pancreatic” [All Fields])’.
Only original articles regarding radiomics applications in PC characterization were selected. Papers published between 1 January 2005 and 2 January 2021 were considered for this analysis. The exclusion criteria were as follows: (1) not original articles (e.g. reviews, editorials, letters, congress communications or posters, book chapters); (2) papers not referred to PC in humans (e.g. benign lesions, atrophy); (3) papers not referred to radiomics and (4) articles that were not in English, French, Spanish, Italian or German.
All the papers were selected and analyzed by a board of five radiation oncologists (ROs; CC, AD, FP, SM, AP), followed by an independent validation of three experts in PC (two ROs, GCM and FC, and one gastroenterologist, IB) and three experts on radiomics (two ROs, ND and LB, for the clinical point of view, and one physicist, DC, for the technical point of view). The whole process, the results and the discussion about potential discrepancies were validated by the other two different independent expert ROs (MAG and VV).
Results
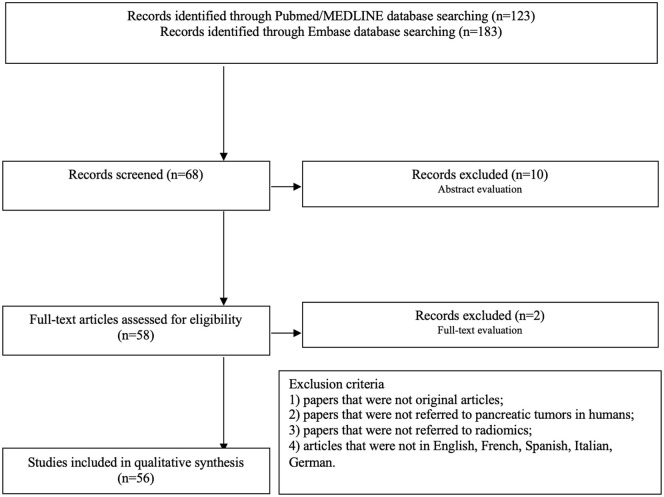
A total of 123 studies were obtained using the mentioned search strategy on PubMed/MEDLINE, and 183 articles were obtained on Embase. Of these, 68 papers were selected based on the title according to the previously described criteria. The selection process, shown in Figure 1, led to the identification of 58 potentially eligible studies according to the abstract (two papers discarded according to the exclusion criterion 1; seven papers discarded for the exclusion criterion 2, and one paper discarded according to the exclusion criterion 3). After full-text analysis, one paper was discarded for the exclusion criterion 1 and one paper according to criterion 2. After the complete selection process, a total of 56 papers were considered eligible for the analysis of the results. All the studies included in the analysis are retrospective; the range of publication year is from 2017 to 2021. The participant centres’ nations were China (n = 26; 46.4%), the United States (n = 13; 23.2%), Italy (n = 6; 10.7%), Canada and Germany (n = 3; 5.4%), Japan (n = 2; 3.6%), France, Russia and South Korea (n = 1; 1.78%). The radiomics analysis was performed on CT (n = 43; 76.8%), PET-CT (n = 6; 10.7%) or MR (n = 7; 12.5%) images. The median number of patients involved in the analysis was 110 (range 10–422); the median number of features analyzed was 410 (range 1–3328). Radiomics, as resulted from the analyzed works, plays a role in many aspects of PC management.
Figure 1.
Flowchart of the systematic literature search process.
Diagnostic imaging features were found to correlate with diagnosis and differentiation of pancreatic ductal adenocarcinoma (PDAC) from noninvasive diseases in 10 studies (17.9%).23–32
CT-based features were found able to distinguish PC from normal pancreatic tissue23,28 and to detect cancer in the context of intraductal papillary mucinous neoplasm (IPMN)27,30; CT- and PET-CT-based radiomics could discriminate PC from pancreatitis,24–26,31,32 while MRI was able to discriminate pancreatic neuroendocrine tumours (pNETs) from solid pseudopapillary tumours in one study. 29 Six studies (10.7%) analyzed technical feasibility and reproducibility aspects of radiomics analysis. Yamashita et al. 33 demonstrated that the variation between contrast-enhanced CT scans (e.g. scanner model, pixel spacing and contrast administration rate) affects radiomic feature reproducibility more significantly than the variation in segmentation. Mori et al. 34 confirmed the minimal impact of delineation uncertainty of pancreatic neuroendocrine neoplasms (panNEN) on CT radiomics features extraction. Plautz et al. 35 observed statistically significant changes over the radiotherapy course time in patients’ radiomics feature values, describing interesting delta-radiomics applications also in PC. Chu et al. 36 compared the diagnostic performances between a commercial and an in-house radiomic software. Loi et al. 37 evaluated the impact of interpolation and discretization on the robustness of radiomics features. The reproducibility of radiomics features in patients affected by pNETs was analyzed by Gruzdev et al. 38
A correlation between cancer imaging features and treatment response prediction was found in seven studies (12.5%).39–45 Cozzi et al. 39 analyzed CT-based radiomic features and found a significant correlation with local control (LC) and OS after stereotactic body radiation therapy (SBRT). Nasief et al. 42 investigated the additional predictive power of combining delta-radiomics features with CA 19-9 levels in patients undergoing concomitant chemoradiation therapy, with this combination resulting in an earlier prediction of good and bad responders. Simpson et al. 43 investigated the role of 0.35T MRI-based delta radiomics in the prediction of treatment response. Parr et al. 44 predicted clinical outcomes (OS and recurrences) using a CT-based radiomics model. Cusumano et al. 45 built a 0.35T MRI-based delta radiomics to predict LC. Yue et al. 40 evaluated the prognostic value of PET-CT texture variations in predicting treatment response. Nasief et al. 41 predicted pathologic response to the treatment using CT delta radiomics.
Overall, 15 studies (26.8%) were focused on radiogenomics, correlating image phenotype with specific gene expression, mutations, molecular or pathological findings.46–60 Of these, 8 studies found correlations between CT or MRI features and pNETs grading.50–54,57–59 One study conducted by Chang et al. 60 defined and validated a radiomic model able to predict histological grade in patients affected by PDAC. Ren et al. 46 developed a CT-based radiomic model to perform differential diagnosis between pancreatic adenosquamous carcinoma and PDAC. Attiyeh et al. 49 demonstrated the possibility to predict SMAD4 status and tumour stromal content using CT-based features. Two studies assessed the role of machine learning algorithms, derived from both CT and MRI analysis, in correlating clinical outcomes predictions with molecular and pathological tumour pathways.47,48 Other two studies proposed PET-CT- or CT-based radiomics to predict mutational status or PD-L1 expression. 55
In total, 21 studies (37.5%) described the application of radiomics in PC risk group stratifications, mainly in preoperative setting.40,47,48,61–78
Within this heterogeneous group of papers, four articles62,66,72,74 analyzed the role of CT-based radiomics in lymph nodes (LNs) metastasis risk assessment before surgery. The preoperative evaluation of the risk of a positive resection margin using CT-based radiomics analysis was the aim of three papers.58,69,76 Zhang et al. 61 evaluated the risk of occurrence of pancreatic fistula using a CT-based radiomic model. Overall, 13 articles 40,47,48,63–65,68,70,71,73,75,77,78 analyzed the role of radiomics to predict clinical outcomes, such as OS, progression-free survival (PFS) and early relapse.
Table 1 summarizes the main characteristics of the analyzed studies, including aim, conclusion and the cluster area of radiomic correlation.
Table 1.
Quantitative synthesis of the 56 selected articles.
| First author (country) | No. of patients | Objectives | Cluster area | Study design | Imaging modality | No. of features | Conclusions |
|---|---|---|---|---|---|---|---|
| Chu et al. (China) 23 | 380 | To determine the utility of RF in differentiating CT cases of PDAC from normal pancreas | Diagnosis | Retrospective | CT | 40 | RF extracted from whole pancreas can be used to differentiate between CT cases from patients with PDAC and healthy control subjects with normal pancreas. |
| Zhang et al. (China) 24 | 111 | To investigate the value of radiomics method for noninvasively differentiating autoimmune pancreatitis from PDAC | Diagnosis | Retrospective | PET-CT | 251 | The quantified radiomics method could aid the noninvasive differentiation of autoimmune pancreatitis and PDAC in 18F-FDG PET-CT images and the integration of multidomain features is beneficial for the differentiation. |
| Park et al. (USA) 25 | 182 | To determine if machine learning of radiomics features could distinguish autoimmune pancreatitis from PDAC | Diagnosis | Retrospective | CT | 431 | The model obtained an accuracy of 95.2% and an AUC of 0.975 in distinguishing autoimmune pancreatitis from PDAC. |
| E et al. (China) 26 | 96 | To build a radiomics model able to distinguish PDAC from focal-type autoimmune pancreatitis | Diagnosis | Retrospective | CT | 1160 | The prediction model identifies PDAC from autoimmune pancreatitis with a sensitivity, specificity and accuracy of 93.3%, 96.1% and 94.8%, respectively. |
| Polk et al. (USA) 27 | 29 | To predict PC in patients with IPMNs | Diagnosis | Retrospective | CT | 39 | The model achieved an AUC of 0.93 and 0.90 for the training dataset and for the fivefold cross-validation, respectively. |
| Qiu et al. (China) 28 | 312 | To identify patients affected by PDCA against patients with healthy pancreas using a CT-based radiomics model | Diagnosis | Retrospective | CT | 26 | The proposed texture analysis architecture achieved an AUC of 0.88 and an accuracy of 81.19%. |
| Shi et al. (China) 29 | 66 | To identify patients affected by pNET from patients with solid pseudopapillary tumours using an MRI-based radiomics model | Diagnosis | Retrospective | MRI | 195 | The model achieved an AUC of 0.97 and 0.86 on the primary and validation cohort, respectively. |
| Tobaly et al. (France) 30 | 408 | To assess the performance of radiomic analysis to predict malignancy in IPMNs of pancreas | Diagnosis | Retrospective | CT | 85 | The radiomics model provided an AUC of 0.84 and 0.71 for training and external validation, respectively. |
| Ziegelmayer et al. (Germany) 31 | 86 | To evaluate the performance of deep convolutional neural network-assisted feature extraction against traditional radiomic features to predict the differentiation between autoimmune pancreatitis and PDAC | Diagnosis | Retrospective | CT | 1411 | Deep convolutional neural network-assisted feature extraction achieved a higher sensitivity, specificity and AUC in comparison with traditional radiomic features. |
| Ren et al. (China) 32 | 109 | To distinguish mass-forming pancreatitis from PDAC using radiomics | Diagnosis | Retrospective | CT | 396 | The model obtained a mean sensitivity, specificity and accuracy of 82.6%, 80.8% and 82.1%, respectively, at the leave group out cross-validation method. |
| Yamashita et al. (USA) 33 | 37 | To measure the reproducibility of radiomic features in pancreatic parenchyma and PDAC in patients who underwent consecutive CECT scans | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 266 | Variations between CECT scans (e.g. scanner model, pixel spacing and contrast administration rate) affected radiomic feature reproducibility to a greater extent than variation in segmentation. A smaller number of pancreatic tumour-derived radiomic features were reproducible compared with pancreatic parenchyma-derived radiomic features under the same conditions. |
| Mori et al. (Italy) 34 | 31 | To quantify the impact of CT delineation uncertainty of panNEN on RF | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 69 | The impact of inter-observer variability in delineating panNEN on RF was minimum, except for the neighbourhood intensity difference family and asphericity, showing a moderate agreement. |
| Plautz et al. (USA) 35 | 10 | To show that the values of texture features extracted from phantoms are stable over clinical timescales; that changes in patients’ feature values over the course of RT are treatment-induced and statistically significant | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 50 | The changes observed in features extracted from longitudinal patient CT data may be treatment-induced and demonstrate their potentiality for early assessment of treatment response. |
| Chu et al. (USA) 36 | 380 | To compare diagnostic performance between a commercial and an in-house radiomics software | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 478 | Similar diagnostic performances were achieved between commercially available and in-house radiomics softwares. |
| Loi et al. (Italy) 37 | 39 | To evaluate the impact of image interpolation and discretization in a radiomics-based prediction analysis of tumour grade, positive LNs, distant metastases and vascular invasion in patients affected by pancreatic neuroendocrine neoplasms | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 69 | The role of radiomic features is relatively invariant against image interpolation and discretization. |
| Gruzdev et al. (Russia) 38 | 12 | To evaluate reproducibility of radiomics features in patients affected by pNET | Technical feasibility and reproducibility aspects of radiomics analysis | Retrospective | CT | 52 | This study showed a high reproducibility of the results of the textural analysis of pNET. |
| Cozzi et al. (Italy) 39 | 100 | To appraise the ability of a radiomics signature to predict clinical outcome after SBRT for pancreas carcinoma | Treatment response prediction | Retrospective | CT | 41 | A CT-based radiomic signature was identified, which correlated with OS and LC after SBRT and allowed to identify low- and high-risk groups of patients. |
| Yue et al. (USA) 40 | 26 | To stratify risks of pancreatic adenocarcinoma patients using pre- and post-RT PET-CT images and to assess the prognostic value of texture variations in predicting therapy response of patients | Risk stratification and treatment response prediction | Retrospective | PET-CT | 48 | Locoregional metabolic texture response provides a feasible approach for evaluating and predicting clinical outcomes following the treatment of pancreatic adenocarcinoma with RT. |
| Nasief et al. (USA) 41 | 90 | To develop a delta-radiomic process based on ML to predict treatment pathologic response | Treatment response prediction | Retrospective | CT | 1300 | The results show that 13 DRFs passed the tests and demonstrated significant changes following 2–4 weeks of treatment. |
| Nasief et al. (USA) 42 | 24 | To investigate the predictivity power of combining different biomarkers (DRFs or CA19-9) in patients undergoing chemoRT | Treatment response prediction | Retrospective | CT | >1300 | The combination of CT delta radiomics and the clinical biomarker CA19-9 leads to improved prediction of treatment responses for chemoRT of PC, as compared with radiomics or CA19-9 alone. |
| Simpson et al. (USA) 43 | 20 | To predict treatment response in patients with PDAC who underwent SBRT using 0.35T MRI-based delta radiomics | Treatment response prediction | Retrospective | MRI | 42 | The model obtained an AUC of 0.81 in predicting treatment response. |
| Parr et al. (USA) 44 | 74 | To predict clinical outcomes (OS and recurrence) after SBRT | Treatment response prediction | Retrospective | CT | 841 | The combined clinical and radiomics model obtained an AUC of 0.68 in OS prediction and an AUC of 0.76 in recurrence prediction. |
| Cusumano et al. (Italy) 45 | 35 | To predict LC in patients affected by PDAC using 0.35T MRI-based delta-radiomics features | Treatment response prediction | Retrospective | MRI | 92 | This study demonstrates that low tesla MRI-based delta radiomics is adequate in 1-year LC prediction (AUC = 0.78, p = 0.005). |
| Ren et al. (China) 46 | 112 | To develop a model able to perform a differential diagnosis between pancreatic adenosquamous carcinoma and PDAC | Radiogenomics | Retrospective | CT | 792 | The proposed radiomics signature predicted the correct histology with 94.5% accuracy (76.4% accuracy in 10-times leave group out cross-validation method). |
| Kaissis et al. (Germany) 47 | 207 | To develop a ML CT-based algorithm capable to correlate preoperative CT to histopathological and molecular subsets and OS | Radiogenomics and risk stratification |
Retrospective | CT | 1474 | ML enables radiomic phenotyping of PDAC and the correlation with clinical outcomes. |
| Kaissis et al. (Germany) 48 | 132 | To develop supervised ML algorithm predicting above- versus below-median OS from DWI-derived radiomic features in patients with PDAC | Risk stratification and radiogenomics | Retrospective | MRI | 504 | ML application to ADC radiomics allowed OS prediction with a high diagnostic accuracy in an independent validation cohort. |
| Attiyeh et al. (USA) 49 | 35 | To determine whether radiomic analysis could accurately predict the genotype of PDAC driver genes and to use radiomics to predict stromal content in these tumours. | Radiogenomics | Retrospective | CT | 255 | RF extracted from clinical CT images is associated with genotype, the number of altered genes and stromal content in PDAC. |
| He et al. (China) 50 | 147 | To develop and validate an effective model to differentiate NF-pNET from PDAC | Radiogenomics | Retrospective | CT | 7 | The nomogram achieved an optimal preoperative, noninvasive differential diagnosis between atypical pNET and PDAC. |
| Gu et al. (China) 51 | 138 | To develop and validate a radiomics-based nomogram for preoperatively predicting grade 1 and grade 2/3 tumours in patients with pNET | Radiogenomics | Retrospective | CT | 853 | The proposed nomogram integrating the clinical predictor tumour margin and fusion radiomic signature had a powerful predictive capability for grade 1 and grade 2/3 in pNET patients. |
| Liang et al. (China) 52 | 137 | To develop and validate a nomogram model combining radiomics features and clinical characteristics to preoperatively differentiate grade 1 and grade 2/3 tumours in patients with pNET | Radiogenomics | Retrospective | CT | 467 | The combined nomogram model developed could be useful in differentiating grade 1 and grade 2/3 tumours in patients with pNETs. |
| McGovern et al. (USA) 53 | 121 | To identify imaging characteristics in patients with known pNET that predict the ALT phenotype by blinded retrospective review of preoperative multiphasic CT scans | Radiogenomics | Retrospective | CT | n.a. | Several preoperative CT features of pNET are associated with the ALT phenotype. CT findings of intratumoral calcifications and metastases predicted poor survival independent of the ALT status. |
| Zhao et al. (China) 54 | 99 | To establish a tumour grade prediction model for preoperative grade 1/2 NF-pNETs using radiomics for multislice spiral CT image analysis | Radiogenomics | Retrospective | CT | 585 | Radiomics developed with a combination of nonenhanced and portal venous phases shows good discrimination and calibration of preoperatively predicting tumour grading in patients with grade 1/2 NF-pNETs. |
| Lim et al. (South Korea) 55 | 48 | To determine if major gene mutations in KRAS, SMAD4, TP53 and CDKN2A were related to FDG PET-based radiomic features in PDAC | Radiogenomics | Retrospective | PET-CT | 35 | Genetic alterations of KRAS and SMAD4 had significant associations with FDG PET-based radiomic features in PDAC. |
| Iwatate et al. (Japan) 56 | 107 | To predict p53 status, PD-L1 expression and prognosis using radiomics | Radiogenomics | Retrospective | CT | 1037 | Radiomics could predict p53 mutation (AUC = 0.795) and PD-L1 expression (AUC = 0.683). Radiomics prediction of p53 mutation was associated with poor prognosis (p = 0.015). |
| Bian et al. (China) 57 | 102 | To predict nonfunctioning pNET grade using a CT-based radiomics score | Radiogenomics | Retrospective | CT | 1029 | The CT-based radiomics score was able to identify pNET grade with an AUC of 0.86. |
| Bian et al. (China) 58 | 157 | To predict pNET grades using an MRI-based radiomics score | Radiogenomics | Retrospective | MRI | 1409 | The MRI-based radiomics score identified grade 1 versus grade 2/3 nonfunctioning pNETs with an AUC of 0.775. |
| Bian et al. (China) 59 | 139 | To predict nonfunctional pNET grades using MRI-based radiomics features | Radiogenomics | Retrospective | MRI | 3328 | The model obtained an AUC of 0.769 and of 0.729 in the training and in the validation cohort, respectively. |
| Chang et al. (China) 60 | 401 | To define and validate a radiomics model to predict histological grade in patients affected by PDAC | Radiogenomics | Retrospective | CT | 1452 | The radiomics signature obtained an AUC of 0.961, 0.910 and 0.770, respectively, for training dataset, testing dataset and external validation dataset. |
| Zhang et al. (China) 61 | 117 | To develop and validate a radiomics-based formula for the preoperative prediction of POPF in patients undergoing pancreaticoduodenectomy | Risk stratification | Retrospective | CT | 1219 | A novel radiomics-based formula was developed and validated for predicting POPF in patients who underwent pancreaticoduodenectomy. |
| Li et al. (China) 62 | 159 | To develop a computational model integrating clinical data and imaging features extracted from CECT images to predict LN metastasis in patients with PDAC | Risk stratification | Retrospective | CT | 2041 | A noninvasive radiomics signature, extracted from CECT images, can be conveniently used to predict preoperative LN metastasis in patients with PDAC. |
| Tang et al. (China) 63 | 303 | To develop a preoperative radiomic nomogram to help identify patients with increased risk of ER | Risk stratification | Retrospective | MRI | 427 | The radiomic nomogram can effectively evaluate ER risks in patients with resectable PC preoperatively. |
| Xie et al. (China) 64 | 220 | To identify a CT-based radiomics nomogram for survival prediction in patients with resected PDAC | Risk stratification | Retrospective | CT | 330 | Rad-score was an independent prognostic factor in PDAC patients. |
| Zhang et al. (Canada) 65 | 520 (422 pz NSCLC) |
Using transfer learning, a CNN-based survival model was built and tested on preoperative CT images of resectable PDAC patients. | Risk stratification | Retrospective | CT | 1428 | The proposed CNN-based survival model outperforms traditional CPH-based radiomics and transfer learning pipelines in PDAC prognosis. |
| Bian et al. (China) 66 | 225 | To explore the relationship between the arterial rad-score and LN metastasis in PDAC | Risk stratification | Retrospective | CT | 1029 | The arterial rad-score is independently and positively associated with the risk of LN metastasis in PDAC. |
| Bian et al. (China) 67 | 181 | To identify the relationship between a portal rad-score and SMV resection margin and to evaluate the diagnostic performance in patients with pancreatic head cancer | Risk stratification | Retrospective | CT | 1029 | The portal rad-score is significantly associated with the pathologic SMV resection margin, and it can accurately and noninvasively predict the SMV resection margin in patients with PC. |
| Khalvati et al. (Canada) 68 | 98 | To establish the prognostic value in terms of OS of CT-based radiomic features contoured by two human readers in patients affected by PDAC | Risk stratification | Retrospective | CT | 410 | The radiomic features predictive role was confirmed (hazard ratio = 1.56, p = 0.05 and hazard ratio = 1.35, p = 0.022 for the first and the second reader, respectively). |
| Hui (China) 69 | 86 | To predict, using preoperative CT radiomics, the resection margin after pancreaticoduodenectomy for pancreatic head PDAC | Risk stratification | Retrospective | CT | n.a. | The model obtained an AUC of 0.8614 in predicting margin status. |
| Mapelli et al. (Italy) 70 | 61 | To predict clinical outcomes and tumour aggressiveness in patients affected by pancreatic neuroendocrine neoplasms using Ga-DOTATOC and fluorine-18-fluorodeoxyglucose PET | Risk stratification | Retrospective | PET-CT | 9 | Specific texture features could noninvasively predict specific tumour characteristics and patients’ outcomes. |
| Mori et al. (Italy) 71 | 176 | To predict distant relapse FS in patients with locally advanced PDAC who underwent radiochemotherapy | Risk stratification | Retrospective | PET-CT | 198 | Distant relapse FS could be predicted by a PET-based radiomics model, p-value of 0.0005 and 0.03 for the training and the internal validation dataset, respectively. |
| Gao et al. (China) 72 | 172 | To build a radiomics-based nomogram to predict the risk of LN metastasis | Risk stratification | Retrospective | CT | 396 | The nomogram performances were AUC = 0.92 and AUC = 0.95 for training and internal validation cohort, respectively. |
| Toyama et al. (Japan) 73 | 161 | To evaluate the role of PET-based radiomics with machine learning in the prediction of prognosis in patients with PC | Risk stratification | Retrospective | PET-CT | 42 | It is possible to define patients’ risk category according to the radiomics feature. |
| Liu et al. (China) 74 | 85 | To develop a model able to predict preoperatively LN status in resectable PDAC | Risk stratification | Retrospective | CT | 1124 | The proposed model achieved an AUC of 0.841. |
| Salinas-Miranda et al. (Canada) 75 | 108 | To validate two previously published radiomic features as predictive of OS and time to progression | Risk stratification | Retrospective | CT | 2 | The predictive role was confirmed (hazard ratio of 1.27 and 1.25, p-value of 0.039 and 0.047, respectively). |
| Chen et al. (China) 76 | 146 | To develop a radiomics signature for predicting portal vein-superior mesenteric vein involvement in patients affected by PDAC | Risk stratification | Retrospective | CT | 869 | The radiomics signature achieved an AUC of 0.848 in the validation cohort. |
| Zaid et al. (USA) 77 | 207 | To evaluate if a quantitative score of CT contrast enhancement is comparable to previously published qualitative classification of patients affected by PDAC | Risk stratification | Retrospective | CT | 1 | This study showed that quantitative analysis is predictive of qualitative one and could be correlated to patients’ outcomes. |
| Zhou et al. (China) 78 | 106 | To develop a model to select appropriate candidates for irradiation stent placement among patients with UPC-MBO | Risk stratification | Retrospective | CT | 620 | The radiomics-based model had good performance for RFS prediction in patients with UPC-MBO who received an irradiation stent. Patients with slow progression should consider undergoing irradiation stent placement for a longer RFS. |
18F-FDG-PET/CT, 18 F-fluorodeoxyglucose positron emission tomography/computed tomography; ADC, apparent diffusion coefficient; ALT, alternative lengthening of telomeres; CECT, contrast-enhanced computed tomography; CNN, convolutional neural network; CPH, Cox proportional hazard model; CT, computed tomography; DRFs, delta-radiomic features; DWI, diffusion-weighted imaging; ER, early recurrence; LC, local control; LN, lymph node; ML, machine learning; MRI, magnetic resonance imaging; NF-pNET, nonfunctional neuroendocrine tumours; OS, overall survival; panNEN, pancreatic neuroendocrine neoplasms; PDAC, pancreatic ductal adenocarcinoma; pNET, pancreatic neuroendocrine tumours; POPF, postoperative pancreatic fistula; rad-score, radiomics score; RF, radiomic features; RFS, restenosis-free survival; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SMV, superior mesenteric vein; UPC-MBO, unresectable pancreatic cancer with malignant biliary obstruction.
Discussion
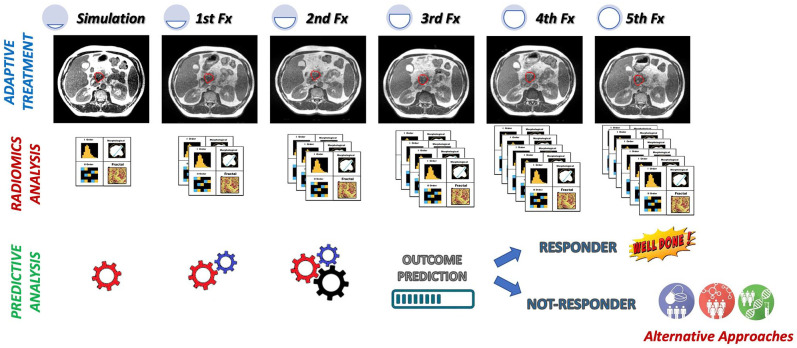
Radiomics seems to be an effective approach to evaluate patients affected by PC in several clinical settings and in different clinical contexts, from diagnosis to risk stratification, from biologic or genomic status prediction to treatment response evaluation or assessment. Figure 2 shows an example of clinical implementation of a delta radiomic model during a stereotactic radiotherapy treatment prescribed in five fractions.
Figure 2.
Example of clinical implementation of a delta radiomic model during a stereotactic radiotherapy treatment prescribed in five fractions. Before the start of the treatment, a radiomic model able to predict the LC 1 year from the end to the treatment was trained and tested on a retrospective cohort of patients. The model was based on the radiomic analysis of the MR images acquired during simulation and during fractions 1 and 2. Using the radiomic model, the RO can have a prediction of 1 year LC at the end of the fraction 2, so having the possibility to modify the radiation treatment for the remaining three fractions, increasing the dose or moving towards alternative approaches.
MR, magnetic resonance.
The common lack of technical standardization of the features extraction process and the necessity of external independent validation for each proposed predictive model hamper the feasibility of extensive radiomics studies in PC and impose the involvement of multidisciplinary research teams and the enrolment of larger patient samples.
Another limitation that reduces the generalizability of these observations is represented by the multiple kinds of image modalities and, for each of them, the variability of acquisition protocols. Moreover, the huge heterogeneity of features analyzed in the different studies poses a limitation in the possibility of creating a cluster of significant features for radiomics in this pathology. The same number of features analyzed, study by study, is very variable (from 1 to 3328 features per study) documenting that the resulting models are very much linked to the analysis experience of each centre.
Despite the above limitations, radiomics could potentially have an important role in providing reliable risk stratification (for both outcomes or complications), facilitating surgical choices, predicting clinical response after treatments, allowing differential diagnosis between cancer and other benign pancreatic abnormalities and predicting histological examination, disease differentiation grade or specific gene mutations.
The multidisciplinary management of these patients by dedicated teams of different specialists has proven to be a promising approach in terms of treatment quality and outcomes. 79
Radiation therapy (RT) can play a role in all possible scenarios, being particularly relevant in reducing the risk of relapse of resected cancer (adjuvant setting) and in pursuing LC in locally advanced disease (definitive setting).
Chemotherapy is also part of the management in all stages, and is administered in association with other treatment modalities (neoadjuvant, adjuvant therapy) or as a main part of the treatment for metastatic disease.
An important role to improve diagnostic and therapeutic options for PC patients is represented by the innovative approach of personalization through risk stratification, aiming to better define patients risk category and choose therapeutic patterns accordingly.80,81
Patients’ stratification is mainly performed based on the genotype, but the lack of a convergency in genomics models seems to reveal that the understanding of biological and clinical heterogeneity of PC is still far from completely understood.82,83 Recently, advances in imaging with the possibility of combining endoscopic modalities with image fusion techniques have provided new ways of approaching the diagnosis and stratification of PC.84–86
As part of an omics-guided care pathway, the integration of these modalities with artificial intelligence and deep-learning methods would seem to be a compelling prospect for pursuing personalized care in a highly heterogeneous disease.87–89 In this framework, radiomics is a promising method to be investigated that could provide more information about PC patients and its future integration in multiomics clinical support systems will allow more personalized and efficacious cancer care.
Footnotes
Author contributions: Calogero Casà: Conceptualization; Methodo-logy; Writing – original draft; Writing – review & editing.
Antonio Piras: Conceptualization; Methodology; Writing – review & editing.
Andrea D’Aviero: Conceptualization; Methodo-logy; Writing – original draft; Writing – review & editing.
Francesco Preziosi: Data curation; Writing – original draft.
Silvia Mariani: Data curation; Writing – original draft.
Davide Cusumano: Writing – review & editing.
Angela Romano: Writing – review & editing.
Ivo Boskoski: Conceptualization; Writing – review & editing.
Jacopo Lenkowicz: Writing – review & editing.
Nicola Dinapoli: Writing – review & editing.
Francesco Cellini: Supervision; Writing – review & editing.
Maria Antonietta Gambacorta: Conceptualiza-tion; Supervision; Writing – review & editing.
Vincenzo Valentini: Conceptualization; Super-vision; Writing – review & editing.
Gian Carlo Mattiucci: Conceptualization; Supervision; Writing – review & editing.
Luca Boldrini: Conceptualization; Methodo-logy; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Calogero Casà  https://orcid.org/0000-0001-5785-0549
https://orcid.org/0000-0001-5785-0549
Andrea D’Aviero  https://orcid.org/0000-0002-9194-620X
https://orcid.org/0000-0002-9194-620X
Ivo Boskoski  https://orcid.org/0000-0001-8194-2670
https://orcid.org/0000-0001-8194-2670
Luca Boldrini  https://orcid.org/0000-0002-5631-1575
https://orcid.org/0000-0002-5631-1575
Contributor Information
Calogero Casà, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Antonio Piras, UO Radioterapia Oncologica, Palermo, Italy.
Andrea D’Aviero, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Francesco Preziosi, Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
Silvia Mariani, Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
Davide Cusumano, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Angela Romano, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Ivo Boskoski, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCSS, Rome, Italy.
Jacopo Lenkowicz, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Nicola Dinapoli, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Francesco Cellini, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Maria Antonietta Gambacorta, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
Vincenzo Valentini, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
Gian Carlo Mattiucci, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
Luca Boldrini, UOC Radioterapia Oncologica, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Dipartimento Universitario di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Rome, Italy.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018; 103: 356–387. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Pancreatic Cancer: Cancer Stat facts, https://seer.cancer.gov/statfacts/html/pancreas.html (accessed 3 April 2020).
- 4. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016; 22: 9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am 2012; 41: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol 2011; 2: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 1766–1773. [DOI] [PubMed] [Google Scholar]
- 8. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 9. Xia X, Wu W, Huang C, et al. SMAD4 and its role in pancreatic cancer. Tumor Biol 2014; 36: 111–119. [DOI] [PubMed] [Google Scholar]
- 10. Shaib Y, Davila J, Naumann C, et al. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population-based study. Am J Gastroenterol 2007; 102: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 11. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dinapoli N, Alitto AR, Vallati M, et al. Moddicom: a complete and easily accessible library for prognostic evaluations relying on image features. Annu Int Conf IEEE Eng Med Biol Soc 2015; 2015: 771–774. [DOI] [PubMed] [Google Scholar]
- 13. Zwanenburg A, Vallières M, Abdalah MA, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020; 295: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinapoli N, Barbaro B, Gatta R, et al. Magnetic resonance, vendor-independent, intensity histogram analysis predicting pathologic complete response after radiochemotherapy of rectal cancer. Int J Radiat Oncol Biol Phys 2018; 102: 765–774. [DOI] [PubMed] [Google Scholar]
- 15. Dinapoli N, Casà C, Barbaro B, et al. Radiomics for rectal cancer. Transl Cancer Res 2016; 5: 424–431. [Google Scholar]
- 16. Gatta R, Vallati M, Dinapoli N, et al. Towards a modular decision support system for radiomics: a case study on rectal cancer. Artif Intell Med 2019; 96: 145–153. [DOI] [PubMed] [Google Scholar]
- 17. Cusumano D, Dinapoli N, Boldrini L, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med 2018; 123: 286–295. [DOI] [PubMed] [Google Scholar]
- 18. Boldrini L, Cusumano D, Chiloiro G, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med 2019; 124: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartoli M, Barat M, Dohan A, et al. CT and MRI of pancreatic tumors: an update in the era of radiomics. Jpn J Radiol 2020; 38: 1111–1124. [DOI] [PubMed] [Google Scholar]
- 20. Abunahel BM, Pontre B, Kumar H, et al. Pancreas image mining: a systematic review of radiomics. Eur Radiol 2021; 31: 3447–3467. [DOI] [PubMed] [Google Scholar]
- 21. Chiloiro G, Rodriguez-Carnero P, Lenkowicz J, et al. Delta radiomics can predict distant metastasis in locally advanced rectal cancer: the challenge to personalize the cure. Front Oncol 2020; 10: 595012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu LC, Park S, Kawamoto S, et al. Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR Am J Roentgenol 2019; 213: 349–357. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Cheng C, Liu Z, et al. Radiomics analysis for the differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma in 18F-FDG PET/CT. Med Phys 2019; 46: 4520–4530. [DOI] [PubMed] [Google Scholar]
- 25. Park S, Chu LC, Hruban RH, et al. Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging 2020; 101: 555–564. [DOI] [PubMed] [Google Scholar]
- 26. E L, Xu Y, et al. Differentiation of focal-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma using radiomics based on multiphasic computed tomography. J Comput Assist Tomogr 2020; 44: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polk SL, Choi JW, McGettigan MJ, et al. Multiphase computed tomography radiomics of pancreatic intraductal papillary mucinous neoplasms to predict malignancy. World J Gastroenterol 2020; 26: 3458–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu J-J, Yin J, Qian W, et al. A novel multiresolution-statistical texture analysis architecture: radiomics-aided diagnosis of PDAC based on plain CT images. IEEE Trans Med Imaging 2021; 40: 12–25. [DOI] [PubMed] [Google Scholar]
- 29. Shi Y-J, Zhu H-T, Liu Y-L, et al. Radiomics analysis based on diffusion kurtosis imaging and T2 weighted imaging for differentiation of pancreatic neuroendocrine tumors from solid pseudopapillary tumors. Front Oncol 2020; 10: 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tobaly D, Santinha J, Sartoris R, et al. CT-based radiomics analysis to predict malignancy in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Cancers 2020; 12: 3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziegelmayer S, Kaissis G, Harder F, et al. Deep convolutional neural network-assisted feature extraction for diagnostic discrimination and feature visualization in pancreatic ductal adenocarcinoma (PDAC) versus autoimmune pancreatitis (AIP). J Clin Med 2020; 9: 4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren S, Zhao R, Zhang J, et al. Diagnostic accuracy of unenhanced CT texture analysis to differentiate mass-forming pancreatitis from pancreatic ductal adenocarcinoma. Abdom Radiol (NY) 2020; 45: 1524–1533. [DOI] [PubMed] [Google Scholar]
- 33. Yamashita R, Perrin T, Chakraborty J, et al. Radiomic feature reproducibility in contrast-enhanced CT of the pancreas is affected by variabilities in scan parameters and manual segmentation. Eur Radiol 2020; 30: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mori M, Benedetti G, Partelli S, et al. Ct radiomic features of pancreatic neuroendocrine neoplasms (panNEN) are robust against delineation uncertainty. Phys Med 2019; 57: 41–46. [DOI] [PubMed] [Google Scholar]
- 35. Plautz TE, Zheng C, Noid G, et al. Time stability of delta-radiomics features and the impact on patient analysis in longitudinal CT images. Med Phys 2019; 46: 1663–1676. [DOI] [PubMed] [Google Scholar]
- 36. Chu LC, Solmaz B, Park S, et al. Diagnostic performance of commercially available vs. in-house radiomics software in classification of CT images from patients with pancreatic ductal adenocarcinoma vs. healthy controls. Abdom Radiol (NY) 2020; 45: 2469–2475. [DOI] [PubMed] [Google Scholar]
- 37. Loi S, Mori M, Benedetti G, et al. Robustness of CT radiomic features against image discretization and interpolation in characterizing pancreatic neuroendocrine neoplasms. Phys Med 2020; 76: 125–133. [DOI] [PubMed] [Google Scholar]
- 38. Gruzdev IS, Zamyatina KA, Tikhonova VS, et al. Reproducibility of CT texture features of pancreatic neuroendocrine neoplasms. Eur J Radiol 2020; 133: 109371. [DOI] [PubMed] [Google Scholar]
- 39. Cozzi L, Comito T, Fogliata A, et al. Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS ONE 2019; 14: e0210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yue Y, Osipov A, Fraass B, et al. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol 2017; 8: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nasief H, Zheng C, Schott D, et al. A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol 2019; 3: 25–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasief H, Hall W, Zheng C, et al. Improving treatment response prediction for chemoradiation therapy of pancreatic cancer using a combination of delta-radiomics and the clinical biomarker CA19-9. Front Oncol 2020; 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simpson G, Spieler B, Dogan N, et al. Predictive value of 0.35 T magnetic resonance imaging radiomic features in stereotactic ablative body radiotherapy of pancreatic cancer: a pilot study. Med Phys 2020; 47: 3682–3690. [DOI] [PubMed] [Google Scholar]
- 44. Parr E, Du Q, Zhang C, et al. Radiomics-based outcome prediction for pancreatic cancer following stereotactic body radiotherapy. Cancers 2020; 12: 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cusumano D, Boldrini L, Yadav P, et al. Delta radiomics analysis for local control prediction in pancreatic cancer patients treated using magnetic resonance guided radiotherapy. Diagnostics 2021; 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren S, Zhao R, Cui W, et al. Computed tomography-based radiomics signature for the preoperative differentiation of pancreatic adenosquamous carcinoma from pancreatic ductal adenocarcinoma. Front Oncol 2020; 10: 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaissis GA, Ziegelmayer S, Lohöfer FK, et al. Image-based molecular phenotyping of pancreatic ductal adenocarcinoma. J Clin Med 2020; 9: 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaissis G, Ziegelmayer S, Lohöfer F, et al. A machine learning model for the prediction of survival and tumor subtype in pancreatic ductal adenocarcinoma from preoperative diffusion-weighted imaging. Eur Radiol Exp 2019; 3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Attiyeh MA, Chakraborty J, McIntyre CA, et al. CT radiomics associations with genotype and stromal content in pancreatic ductal adenocarcinoma. Abdom Radiol (NY) 2019; 44: 3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He M, Liu Z, Lin Y, et al. Differentiation of atypical non-functional pancreatic neuroendocrine tumor and pancreatic ductal adenocarcinoma using CT based radiomics. Eur J Radiol 2019; 117: 102–111. [DOI] [PubMed] [Google Scholar]
- 51. Gu D, Hu Y, Ding H, et al. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol 2019; 29: 6880–6890. [DOI] [PubMed] [Google Scholar]
- 52. Liang W, Yang P, Huang R, et al. A combined nomogram model to preoperatively predict histologic grade in pancreatic neuroendocrine tumors. Clin Cancer Res 2019; 25: 584–594. [DOI] [PubMed] [Google Scholar]
- 53. McGovern JM, Singhi AD, Borhani AA, et al. CT radiogenomic characterization of the alternative lengthening of telomeres phenotype in pancreatic neuroendocrine tumors. AJR Am J Roentgenol 2018; 211: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 54. Zhao Z, Bian Y, Jiang H, et al. CT-radiomic approach to predict G1/2 nonfunctional pancreatic neuroendocrine tumor. Acad Radiol 2020; 27: e272–e281. [DOI] [PubMed] [Google Scholar]
- 55. Lim CH, Cho YS, Choi JY, et al. Imaging phenotype using 18F-fluorodeoxyglucose positron emission tomography–based radiomics and genetic alterations of pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 2020; 47: 2113–2122. [DOI] [PubMed] [Google Scholar]
- 56. Iwatate Y, Hoshino I, Yokota H, et al. Radiogenomics for predicting p53 status, PD-L1 expression, and prognosis with machine learning in pancreatic cancer. Br J Cancer 2020; 123: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bian Y, Jiang H, Ma C, et al. CT-based radiomics score for distinguishing between grade 1 and grade 2 nonfunctioning pancreatic neuroendocrine tumors. AJR Am J Roentgenol 2020; 215: 852–863. [DOI] [PubMed] [Google Scholar]
- 58. Bian Y, Li J, Cao K, et al. Magnetic resonance imaging radiomic analysis can preoperatively predict G1 and G2/3 grades in patients with NF-pNETs. Abdom Radiol 2021; 46: 667–680. [DOI] [PubMed] [Google Scholar]
- 59. Bian Y, Zhao Z, Jiang H, et al. Noncontrast radiomics approach for predicting grades of nonfunctional pancreatic neuroendocrine tumors. J Magn Reson Imaging 2020; 52: 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang N, Cui L, Luo Y, et al. Development and multicenter validation of a CT-based radiomics signature for discriminating histological grades of pancreatic ductal adenocarcinoma. Quant Imaging Med Surg 2020; 10: 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang W, Cai W, He B, et al. A radiomics-based formula for the preoperative prediction of postoperative pancreatic fistula in patients with pancreaticoduodenectomy. Cancer Manag Res 2018; 10: 6469–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li K, Yao Q, Xiao J, et al. Contrast-enhanced CT radiomics for predicting lymph node metastasis in pancreatic ductal adenocarcinoma: a pilot study. Cancer Imaging 2020; 20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang TY, Li X, Zhang Q, et al. Development of a novel multiparametric mri radiomic nomogram for preoperative evaluation of early recurrence in resectable pancreatic cancer. J Magn Reson Imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie T, Wang X, Li M, et al. Pancreatic ductal adenocarcinoma: a radiomics nomogram outperforms clinical model and TNM staging for survival estimation after curative resection. Eur Radiol 2020; 30: 2513–2524. [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y, Lobo-Mueller EM, Karanicolas P, et al. CNN-based survival model for pancreatic ductal adenocarcinoma in medical imaging. BMC Med Imaging 2020; 20: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bian Y, Guo S, Jiang H, et al. Relationship between radiomics and risk of lymph node metastasis in pancreatic ductal adenocarcinoma. Pancreas 2019; 48: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bian Y, Jiang H, Ma C, et al. Performance of CT-based radiomics in diagnosis of superior mesenteric vein resection margin in patients with pancreatic head cancer. Abdom Radiol 2020; 45: 759–773. [DOI] [PubMed] [Google Scholar]
- 68. Khalvati F, Zhang Y, Baig S, et al. Prognostic value of CT radiomic features in resectable pancreatic ductal adenocarcinoma. Sci Rep 2019; 9: 5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hui B. Identification of pancreaticoduodenectomy resection for pancreatic head adenocarcinoma: a preliminary study of radiomics. Comput Math Methods Med 2020; 2020: 2761627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mapelli P, Partelli S, Salgarello M, et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: an endearing tool for preoperative risk assessment. Nucl Med Commun 2020; 41: 896–905. [DOI] [PubMed] [Google Scholar]
- 71. Mori M, Passoni P, Incerti E, et al. Training and validation of a robust PET radiomic-based index to predict distant-relapse-free-survival after radio-chemotherapy for locally advanced pancreatic cancer. Radiother Oncol 2020; 153: 258–264. [DOI] [PubMed] [Google Scholar]
- 72. Gao J, Han F, Jin Y, et al. A Radiomics nomogram for the preoperative prediction of lymph node metastasis in pancreatic ductal adenocarcinoma. Front Oncol 2020; 10: 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Toyama Y, Hotta M, Motoi F, et al. Prognostic value of FDG-PET radiomics with machine learning in pancreatic cancer. Sci Rep 2020; 10: 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu P, Gu Q, Hu X, et al. Applying a radiomics-based strategy to preoperatively predict lymph node metastasis in the resectable pancreatic ductal adenocarcinoma. J Xray Sci Technol 2020; 28: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 75. Salinas-Miranda E, Khalvati F, Namdar K, et al. Validation of prognostic radiomic features from resectable pancreatic ductal adenocarcinoma in patients with advanced disease undergoing chemotherapy. Can Assoc Radiol J 2020; 72: 605–613. [DOI] [PubMed] [Google Scholar]
- 76. Chen F, Zhou Y, Qi X, et al. Radiomics-assisted presurgical prediction for surgical portal vein-superior mesenteric vein invasion in pancreatic ductal adenocarcinoma. Front Oncol 2020; 10: 523543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zaid M, Widmann L, Dai A, et al. Predictive modeling for voxel-based quantification of imaging-based subtypes of pancreatic ductal adenocarcinoma (PDAC): a multi-institutional study. Cancers 2020; 12: 3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou HF, Han YQ, Lu J, et al. Radiomics facilitates candidate selection for irradiation stents among patients with unresectable pancreatic cancer. Front Oncol 2019; 9: 973–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Malafa MP, Chair V, Al-Hawary M, et al. Cassadie Moravek ¥ pancreatic cancer action network continue NCCN guidelines version 1.2020 Pancreatic Adenocarcinoma 2019, https://www2.tri-kobe.org/nccn/guideline/archive/pancreas2020/english/pancreatic.pdf
- 80. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52. [DOI] [PubMed] [Google Scholar]
- 81. Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011; 17: 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer 2016; 16: 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vanella G, Capurso G, BoÅ¡koski I, et al. How to get away with COVID-19: endoscopy during post-peak pandemic. Therap Adv Gastroenterol 2020; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Acri G, Testagrossa B, Sestito A, et al. CT and MRI slice separation evaluation by LabView developed software. Z Med Phys 2018; 28: 6–13. [DOI] [PubMed] [Google Scholar]
- 86. Renard Y, Hossu G, Chen B, et al. A guide for effective anatomical vascularization studies: useful ex vivo methods for both CT and MRI imaging before dissection. J Anat 2018; 232: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boldrini L, Bibault J-E, Masciocchi C, et al. Deep learning: a review for the radiation oncologist. Front Oncol 2019; 9: 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fionda B, Boldrini L, D’Aviero A, et al. Artificial intelligence (AI) and interventional radiotherapy (brachytherapy): state of art and future perspectives. J Contemp Brachytherapy 2020; 12: 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Valentini V, Boldrini L, Damiani A, et al. Recommendations on how to establish evidence from auto-segmentation software in radiotherapy. Radiother Oncol 2014; 112: 317–320. [DOI] [PubMed] [Google Scholar]