Abstract
The medical care of patients with hematological malignancies who develop coronavirus disease 2019 (COVID-19) has been a major challenge during the current pandemic. We herein describe a patient in the blast phase of chronic myeloid leukemia who was hospitalized for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The patient was successfully treated with tocilizumab, and intubation was avoided. The severity of SARS-CoV-2 infection is mostly related to a severe acute respiratory distress syndrome that develops secondary to cytokine release syndrome, and interleukin 6 is the main cytokine involved in cytokine release syndrome. Very few reports have described the use of tocilizumab in patients with hematologic malignancies who develop SARS-CoV-2 infection, although a few cases of patients with multiple myeloma have been reported. To our knowledge, however, this is the first report of a SARS-CoV-2–infected patient in the blast phase of chronic myeloid leukemia who had a favorable response to treatment with tocilizumab. The management of patients with hematological malignancies who become infected with SARS-CoV-2 is a major challenge for practitioners, necessitating more specific research in this direction.
Keywords: Coronavirus disease 2019, tocilizumab, interleukin 6, hematological malignancy, blast phase, chronic myeloid leukemia
Introduction
Many cases of severe acute respiratory syndrome (SARS) began appearing secondary to infection with SARS coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), at the end of 2019. After the declaration of COVID-19 as a global pandemic, the management of patients with hematological malignancies has become challenging. This challenge is characterized by the simultaneous immunosuppression caused by the original disease and the immunosuppressive effect of treatment. We herein describe the successful treatment of a patient in the blast phase of chronic myeloid leukemia (CML) who developed SARS-CoV-2 infection.
Case presentation
A 41-year-old man was diagnosed with CML, initially treated with imatinib, and followed up for 4 years. His condition worsened 6 months before presentation to our hospital, entering the blast phase (49% blast rate). He was being treated with the Hyper-CVAD chemotherapy protocol in combination with nilotinib and had received three courses of chemotherapy (his last myelogram 2 months before presentation to our hospital showed a 28% blast rate). He was admitted to our emergency room because of a 2-day history of worsening dyspnea. He also had a 5-day history of a cough with myalgia and a fever of 38.4°C.
The initial evaluation revealed a conscious patient without deficits, blood oxygen saturation (SpO2) of 81% on room air, tachypnea at 27 breaths/minute, tachycardia at 115 beats/minute, blood pressure of 125/70 mmHg, body temperature of 38.7°C, body weight of 99.3 kg, height of 178 cm, and body mass index of 31.3 kg/m2.
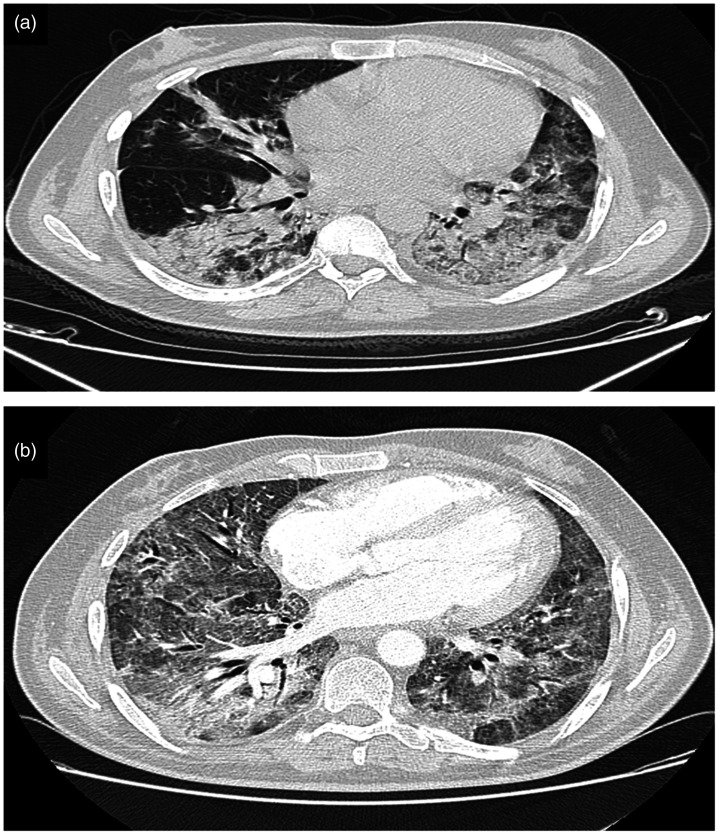
Given the current pandemic, reverse-transcription polymerase chain reaction for SARS-CoV-2 in a nasopharyngeal swab was performed, and the result was positive. Chest computed tomography with no contrast (Figure 1(a)) revealed typical peripheral ground-glass lesions suggestive of SARS-CoV-2 infection with 25% to 50% parenchymal involvement and no evidence of air effusion.
Figure 1.
(a) Axial nonenhanced chest computed tomography image (lung window) showing bilateral ground-glass opacities typical of SARS-CoV-19 infection with pulmonary involvement estimated between 25% and 50%. (b) Axial contrast-enhanced chest computed tomography image (lung window) showing worsening of the lesions with estimated pulmonary involvement of >75%.
The biological workup (Table 1) performed on admission showed an inflammatory reaction with marked ferritinemia at 560 ng/mL (reference range, 30–300 ng/mL), lactate dehydrogenase (LDH) concentration of 656 IU/L (reference range, 125–143 IU/L), D-dimer concentration of 3.6 mg/L (reference range, 0.0–0.5 mg/L), high C-reactive protein (CRP) concentration of 257 mg/L (reference range, 0–6 mg/L), and fibrinogen concentration of 6.3 g/L (reference range, 2–4 g/L). The blood count showed lymphopenia at 100/µL (reference range, 1000–4000/µL) with thrombocytopenia at 90/L (reference range, 150–450/L). Arterial blood gas measurement showed severe hypoxemia with a partial pressure of oxygen (PaO2) of 33 mmHg.
Table 1.
Laboratory findings.
| Variable | Reference range | Day 1 | Day 3 | Day 6 | Day 8 | Day 10 and TCZ administration | Day 13 | Day 20 | Day 25 | Day 27 |
|---|---|---|---|---|---|---|---|---|---|---|
| White blood cells (/µL) | 4000–10,000 | 6520 | 13,648 | 9830 | 6409 | 4795 | 7529 | 7920 | 5098 | 5783 |
| Segmented neutrophils (/µL) | 1500–7000 | 3578 | 9007 | 7630 | 5900 | 4830 | 5690 | 5390 | 4970 | 4850 |
| Lymphocytes (/µL) | 1000–4000 | 100 | 201 | 320 | 350 | 290 | 590 | 870 | 740 | 780 |
| Hemoglobin (g/dL) | 12–16 | 12.4 | 11.5 | 12.9 | 11.4 | 11.1 | 12.8 | 12.2 | 11.3 | 11.5 |
| Platelets (×104/µL) | 15–40 | 9 | 11.3 | 12.3 | 11.4 | 10.9 | 8.5 | 5.4 | 7.8 | 10.3 |
| ALT (IU/L) | 0–55 | 76 | 69 | 93 | 78 | 63 | 54 | 61 | 55 | 72 |
| AST (IU/L) | 5–34 | 27 | 37 | 30 | 32 | 29 | 56 | 42 | 30 | 32 |
| LDH (IU/L) | 125–143 | 656 | 783 | 876 | 890 | 994 | 703 | 378 | 208 | 196 |
| IL-6 (pg/mL) | 0–7 | – | – | 217 | 255 | 262 | 26 | – | – | – |
| Serum ferritin (µg/L) | 20–300 | 560 | 654 | 876 | 987 | 1128 | 659 | 479 | 483 | 380 |
| Hematocrit (%) | 37–49 | 40 | 38 | 42 | 39 | 48 | 51 | 43 | 47 | 38 |
| Glucose (G/L) | 0.7–1.15 | 0.8 | 1.1 | 1.2 | 0.85 | 0.9 | 1 | 0.73 | 0.92 | 0.9 |
| CRP (mg/L) | 0–6 | 257 | 303 | 376 | 389 | 394 | 340 | 76 | 104 | 98 |
| Procalcitonin (ng/mL) | 0.0–0.05 | 0.01 | 0.3 | 0.07 | 0.03 | 0.02 | 0.02 | 0.01 | 0.03 | 0.03 |
| Total protein (g/L) | 64–83 | 76 | 70 | 64 | 68 | 69 | 65 | 69 | 72 | 69 |
| Albumin (mg/L) | 35–50 | 38 | 37 | 35 | 41 | 40.3 | 46 | 42 | 40 | 39 |
| High-sensitivity cardiac troponin (pg/mL) | 0–26 | 3 | 5 | 1.9 | 13 | 19 | 10 | 9 | 4 | 3 |
| Prothrombin time (seconds) | 11–13 | 11 | 11.8 | 11.9 | 12 | 12.1 | 11.9 | 11.8 | 11.6 | 11.7 |
| D-dimer (mg/L) | 0.0–0.5 | 3.6 | 4.3 | 4.9 | 5.8 | 6.4 | 5.3 | 4.7 | 3.2 | 2.1 |
| Fibrinogen (G/L) | 2–4 | 6.3 | 7.2 | 7.8 | 8.9 | 9.1 | 7.3 | 5.6 | 4.5 | 3.9 |
| Partial thromboplastin time (seconds) | 30–50 | 44 | 43 | 49 | 53 | 57 | 52 | 43 | 42 | 49 |
| Urea (mmol/L) | 2.5–7.6 | 3.65 | 4.76 | 4.38 | 3.98 | 5.67 | 4.98 | 5.68 | 4.98 | 3.87 |
| Creatinine (mg/L) | 6–12 | 8.3 | 9.7 | 10.1 | 10.3 | 8.8 | 8.4 | 7.9 | 9.1 | 10.2 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CRP, C-reactive protein; TCZ, tocilizumab; IL-6, interleukin 6.
The patient was admitted to the COVID-19 intensive care unit. His respiratory condition was treated with a high-concentration oxygen mask with an oxygen flow rate of 13 L/minute and a target SpO2 of >92%. He also underwent the following therapeutic protocol: zinc (45-mg capsule every 12 hours), vitamin C (2 g/day), dexamethasone (6 mg/day), therapeutic dosing of low-molecular-weight heparin (8000 IU every 12 hours), and gastric protection with proton pump inhibitors (20 mg/day) in conjunction with his tyrosine kinase inhibitor nilotinib. This therapy resulted in stabilization of the patient’s clinical condition.
On day 3, the patient developed chills and produced green sputum. Biological assessment showed a white blood cell count of 13,648/µL (reference range, 4000–10,000/µL), neutrophil count of 9007/µL (reference range, 1500–7000/µL), and procalcitonin concentration of 0.3 ng/mL (reference range, 0.00–0.05 ng/mL). A protected distal bronchial sample was taken, and the patient was administered probabilistic antibiotic therapy with amoxicillin/clavulanic acid and levofloxacin. His clinical course was favorable, and his biological assessment results normalized 3 days later. The bacteriological study indicated a Streptococcus pneumoniae infection sensitive to amoxicillin/clavulanic acid. The levofloxacin was stopped and the 7-day course of amoxicillin/clavulanic acid was completed.
On day 6, the patient reported more severe respiratory difficulties even under high-concentration oxygen mask therapy, and his PaO2 was 37 mmHg on oxygen. A chest computed tomography scan with and without injection (Figure 1(b)) showed an increased number of ground-glass lesions with an estimated involvement of >85% and no signs of pulmonary embolism or gas effusion. We administered high-flow nasal oxygen therapy with a flow rate of 50 L/minute and a fraction of inspired oxygen (FiO2) of 70%, and the patient underwent prone sessions lasting 16 hours per day.
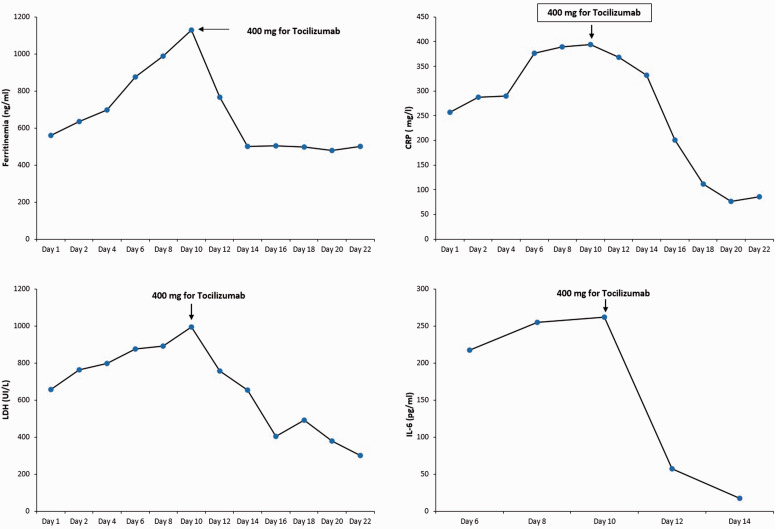
The patient’s SpO2 was stabilized at 86% to 90%. The kinetics of ferritin, LDH, CRP, and D-dimer were increasing, and the interleukin 6 (IL-6) concentration was measured for the first time and was found to be very high (Figure 2).
Figure 2.
Evolution of inflammatory marker kinetics before and after the introduction of tocilizumab.
On day 8, the patient’s SpO2 dropped again to 76% despite high-flow nasal oxygen therapy in the prone position. His PaO2 was 36 mmHg. The patient’s condition was too unstable to perform thoracic imaging. Transthoracic ultrasound showed no abnormalities, and pleural ultrasound showed no evidence of pneumothorax. The patient was put on noninvasive ventilation with a positive end-expiratory pressure of 8 cmH2O, asynchrony index of 8 cmH2O, and FiO2 of 90%. Blood gas analysis 3 hours later showed a PaO2 of 57.7 mmHg and PaO2/FiO2 of 64.1; thus, the patient was in a very critical state.
Another biological workup showed continuous elevation of ferritin, LDH, CRP, and IL-6 (Figure 2). The decision was made to introduce tocilizumab despite scant reports of its use in patients with leukemia. The patient received 400 mg of tocilizumab on day 10.
This treatment resulted in an improvement in the patient’s respiratory level, and his PaO2/FiO2 ratio increased to 167. He was successfully weaned from the NIV on day 13 and placed on high-flow nasal oxygen therapy with a flow rate of 55 L/minute and FiO2 of 60%. Biologically, the kinetics of ferritin, LDH, CRP, and IL-6 were decreasing (Figure 2).
On day 20, we were able to treat the patient with a high-concentration oxygen mask with a flow rate of 15 L/minute associated with sessions of the prone position with an SpO2 of 87%. Clinically, the patient was well at this point. His biological workup showed a slight increase in the ferritin, CRP, D-dimer, and LDH concentrations. The blood count still showed lymphopenia at 870/µL with thrombocytopenia at 50/L, requiring a reduction in the anticoagulation therapy to a preventive dose. On day 27, the patient was transferred to the hematology department with a very good respiratory condition using only oxygen goggles with a flow rate of 5 L/minute, and his SpO2 was 90%.
Discussion
In December 2019, the world experienced the emergence of SARS-CoV-2, a new strain in the family of beta-coronaviruses that causes COVID-19. This virus caused millions of deaths due to SARS, causing the world to enter a state of high alert. In February 2020, the World Health Organization declared SARS-CoV-2 infection as a pandemic. 1 This novel coronavirus has spread to 216 countries, requiring the implementation of general containment.
The world has already experienced two epidemics caused by coronaviruses: SARS-CoV in 2002 and 2003 and Middle East respiratory syndrome-coronavirus (MERS-CoV) in 2013. However, SARS-CoV-2 is characterized by higher transmission, a greater variety of clinical manifestations, and higher mortality compared with SARS-CoV and MERS-CoV, and this is explained by the biological and genomic structure of SARS-CoV-2.1,2
SARS-CoV-2 is an RNA virus that is mainly transmitted from human to human. Its cell receptor is angiotensin-converting enzyme 2, which is bound to the spike (S) protein present on the outer surface of beta coronaviruses. 2 Angiotensin-converting enzyme 2 is preferentially expressed in the lung and intestinal epithelium, which explains the pulmonary tropism of the virus. To simplify, the pathophysiology of severe SARS-CoV-2 infection is secondary mainly to an abnormal host response or to overexpression of inflammatory cytokines, free radicals, and chemokines. Indeed, cytokine release syndrome (CRS), which causes an acute respiratory distress syndrome subsequent to SARS-CoV-2 infection, remains the main cause of mortality in patients with SARS-CoV-2.2,3
Among the key elements of CRS is IL-6. This well-known cytokine is endowed with both anti-inflammatory and proinflammatory effects, and it is secreted mainly by macrophages, monocytes, endothelial cells, and T lymphocytes. 3 Among the functions of IL-6 is maturation and differentiation of hematopoietic stem cells. 3
In another sense, patients with hematological malignancies represent a major challenge during this pandemic because of the high infectious risk in this group of patients, on one hand by the disease itself and on the other hand by the immunosuppressive treatments.4,5 According to He et al., 6 patients with hematological malignancies do not have a high risk of developing SARS-CoV-2 infection, but their clinical course is more severe than that of other patients. This can be explained by either the progression of their hematological malignancy or the occurrence of bacterial superinfection during hospitalization. Notably, after the introduction of a tyrosine kinase inhibitor as a mainstay of CML treatment, the prognosis of these patients becomes almost identical to that of the general population. Despite this therapeutic progress, however, the management of patients followed for CML in the blast phase remains a challenge, and thus the prognosis remains poor. 7
Because IL-6 is the main mediator of CRS that induces the acute respiratory distress syndrome, the use of an IL-6 inhibitor is a therapeutic measure that became very important at the beginning of the pandemic to avoid fatalities due to SARS-CoV-2 infection. The IL-6 inhibitor tocilizumab is a recombinant humanized IL-6 receptor monoclonal antibody of the IgG1 subtype. 3 Its mechanism of action is inhibition of signal transduction mediated by IL-6 receptors, 3 and it is effective in CRS. 8
The published results regarding the efficacy of tocilizumab in severe cases of SARS-CoV-19 infection to date are highly divergent. A randomized, double-blind, placebo-controlled trial of patients with severe SARS-CoV-19 infection conducted by Stone et al. 9 showed that the use of tocilizumab did not prevent intubation or reduce mortality; however, this trial could not rule out a benefit of tocilizumab because of the wide confidence intervals. In contrast, another randomized controlled trial by Gordon et al. 10 showed that the use of tocilizumab improved survival, including in patients with severe infection. There are few published data regarding the use of tocilizumab in patients with hematologic malignancy and severe SARS-CoV-2 infection. Zhang et al. 11 was the first to describe a patient with multiple myeloma who was successfully treated with tocilizumab for severe SARS-CoV-2 infection. Ranger et al. 12 described a patient with uncontrolled CML who was favorably treated with tocilizumab for severe SARS-CoV-2 infection. Although these two reports are insufficient to conclude whether tocilizumab is effective in this group of patients, there are still exceptional and special cases. To our knowledge, this is the first reported case of therapeutic success of tocilizumab in a patient in the blast phase of CML.
Regarding the risk of infection after the use of tocilizumab in patients with hematologic malignancy who develop severe SARS-CoV-2 infection, Ranger et al. 12 concluded that the use of tocilizumab does not increase the risk of infection in patients with hematologic malignancy. However, because the sample size of the study was very small, further studies with larger sample sizes are needed.
The use of tocilizumab in our patient allowed us to avoid intubation and improve his prognosis. Additionally, our patient did not present clinical or biological evidence of bacterial surinfection or other opportunistic infection after the introduction of tocilizumab.
The reporting of this study conforms to the CARE guidelines. 13
Conclusion
Patients with hematologic malignancies represent a major challenge for practitioners during this pandemic because SARS-CoV-2 infection and hematologic malignancies overlap in several common points, mainly that of immunity.
Footnotes
Consent for publication and ethics approval: The patient described in this report provided both verbal and written consent for publication. The requirement for ethics approval was waived because of the nature of this study (case report).
Declaration of conflict of interest: The authors declare no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ORCID iDs: Amine Bouchlarhem https://orcid.org/0000-0001-5750-6932
Leila Haddar https://orcid.org/0000-0003-4579-1275
Oussama Lamzouri https://orcid.org/0000-0001-5100-5280
References
- 1.Mohamadian M, Chiti H, Shoghli A, et al. COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med 2021; 23: 0–2. doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortaz E, Tabarsi P, Varahram M, et al. The immune response and immunopathology of COVID-19. Front Immunol 2020; 11: 1–9. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wu Z, Li JW, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 2020; 55: 105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchlarhem A, Haddar L, Lamzouri O, et al. Multiple cranial nerve palsies revealing blast crisis in patient with chronic myeloid leukemia in the accelerated phase under nilotinib during severe infection with SARS-COV-19 virus: case report and review of literature. Radiol Case Reports 2021; 16: 3602–3609. doi: 10.1016/J.RADCR.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020; 136: 2881–2892 [Online]. Available: www.epigear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia 2020; 34: 1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer 2017; 123: 4391–402. doi: 10.1002/cncr.30864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler U, Jensen M, Manzke O, et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999; 94: 2217–2224. doi: 10.1182/BLOOD.V94.7.2217.419K02_2217_2224. [PubMed] [Google Scholar]
- 9.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383: 2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384: 1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Song K, Tong F, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv 2020; 4: 1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranger A, Haji R, Kaczmarski R, et al. Interleukin-6 blockade treatment for COVID-19 associated cytokine release syndrome in a patient with poorly controlled chronic myeloid leukaemia. Br J Haematol 2020; 190: e128–e130. doi: 10.1111/bjh.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]