Abstract
PPM1A (magnesium-dependent phosphatase 1 A, also known as PP2Cα) is a member of the Ser/Thr protein phosphatase family. Protein phosphatases catalyze the removal of phosphate groups from proteins via hydrolysis, thus opposing the role of protein kinases. The PP2C family is generally considered a negative regulator in the eukaryotic stress response pathway. PPM1A can bind and dephosphorylate various proteins and is therefore involved in the regulation of a wide range of physiological processes. It plays a crucial role in transcriptional regulation, cell proliferation, and apoptosis and has been suggested to be closely related to the occurrence and development of cancers of the lung, bladder, and breast, amongst others. Moreover, it is closely related to certain autoimmune diseases and neurodegenerative diseases. In this review, we provide an insight into currently available knowledge of PPM1A, including its structure, biological function, involvement in signaling pathways, and association with diseases. Lastly, we discuss whether PPM1A could be targeted for therapy of certain human conditions.
Keywords: PPM1A, phosphatase, signal pathway, cancer, neurodegenerative diseases
Impact statement
Protein phosphatases reverse the role of protein kinases by catalyzing the removal of phosphate groups from proteins. In recent years, PPM1A, a magnesium-dependent member of the Ser/Thr protein phosphatase family, has received increasing attention, playing not only an important role in cellular physiology but also in the development and prognosis of many diseases. Here, we review the latest research progress assessing the structure and biological function of PPM1A including its signaling pathways and relationship with various diseases and discuss whether PPM1A could represent a therapeutic target in the future.
Introduction
Reversible protein phosphorylation is an essential post-transcriptional modification involved in the regulation of numerous cellular activities. Protein phosphatases remove phosphate groups from their substrates via dephosphorylation and, together with protein kinases, regulate protein activity. 1 Phosphorylation is involved in a broad range of cellular processes both in physiology and disease. Historically, protein kinases have received more attention than phosphatases, but recent evidence suggests that the latter play no lesser role in the regulation of physiological functions than protein kinases. 2
The PPM (metal-dependent protein phosphatase) family, also known as the PP2C family, are metal-dependent protein phosphatases which bind Mn2+ or Mg2+ and generally exist in a monomeric state. The PPM/PP2C family consists of many members, including PPM1A, PPM1B, PPM1E, and PPM1D, among others. 3 PPM1A has been found to participate in various vital cellular processes, and its dysfunction has been shown to contribute to the occurrence of certain diseases. 1 PPM1A is therefore now regarded as a promising target for therapeutic therapies. This review focuses on the structures and biological functions of the PPM phosphatase family and diseases associated with their dysfunction.
Structure of PPM1A
Barford et al. first reported the crystal structure of PPM1A in 1996. 4 PPM1A consists of two domains, an N-terminal catalytic domain and a 90-residue C-terminal protein domain. The center of its catalytic domain is a β-sandwich containing two manganese ions surrounded by α-helical structures. The manganese ions bind phosphate groups of the substrate in the binuclear enzymatic center. The activity of PPM1A depends Mn2+ or Mg2+, 5 although subsequent studies have suggested other metal ions such as ferrous ions may also activate PPM1A. Conversely, cadmium ions can inhibit PPM1A. 6 However, as the concentration of metal ions is very stable under normal physiological conditions, the abovementioned ions will generally not affect physiological functions of PPM1A and the regulation of PPM1A activity may therefore depend on tissue- or cell-specific expression, various post-translational modifications, subcellular localization, and degradation. 3 Research by the Debnath team found that binding of Mg2+ to the Asp-146/Asp-239 subunit is necessary for catalysis and limits the conformational mobility of the active site and the specific region of the flip subdomain, indicating that the third metal ion stabilizes the natural structure of the enzyme. 7 Similarly, Jackson et al. discovered the importance of Arg174 for the phosphatase activity of PPM1A. 8
Biological function of PPM1A
PPM1A is one of the most intensively studied members of the PPM phosphatase family. It not only plays a vital role in wound healing, inflammation, and neovascularization but also regulates bone morphogenetic protein signaling 1 and plays a critical function in the formation of the placenta, synthesis of oocytes, and differentiation of nerve cells.9–11 In short, PPM1A participates in a variety of physiological as well as pathological processes in vivo.
Virtually all proteins undergo some form of modification after synthesis, with phosphorylation representing one of the most common post-translational modifications. Targeted but reversible phosphorylation is crucial for physiological function of many proteins 12 and is achieved by the joint activity of protein and protein phosphatases which together regulate protein activity. PPM1A is expressed in nearly all tissues and is localized in both the cytoplasm and nucleus. 13 Due to its wide range of substrates, it participates in the regulation many important signaling pathways, predominantly as a negative regulator. Below, we have summarized some of the most important substrates of PPM1A.
AMPK
AMP-activated protein kinase (AMPK) is an essential serine/threonine protein kinase which is considered a key coordinator of metabolism and energy. AMPK regulates short-term effects of the energy metabolism and can modify gene transcription and is characterized by its ability to bind to AMP, enabling its adjustment of enzymatic activity by sensing the cellular energy status and maintaining a balance between ATP production and consumption in eukaryotic cells. ATP depletion associated with a variety of stress responses, such as hypoxia, heat shock, metabolic poisoning, has been shown to activate AMPK.14,15
Davies et al. first reported that PPM1A could dephosphorylate AMPK and thereby inactivate the AMPK pathway. 16 In subsequent studies, PPM1A siRNA was shown to increase AMPKα phosphorylation levels in HeLa cells, indicating that PPM1A is involved in the dephosphorylation of AMPKα1 and α2. Moreover, PPM1A has been shown to inhibit HEK-293 cell proliferation. 17
MAPK (JNK/p38)
PPM1A is involved in the regulation of mitogen-activated protein kinase (MAPK) signaling which represents a central signaling module of the cell, composed of three layers of sequentially activated protein kinases: MAPKKKs, MAPKKs, and MAPKs. 3
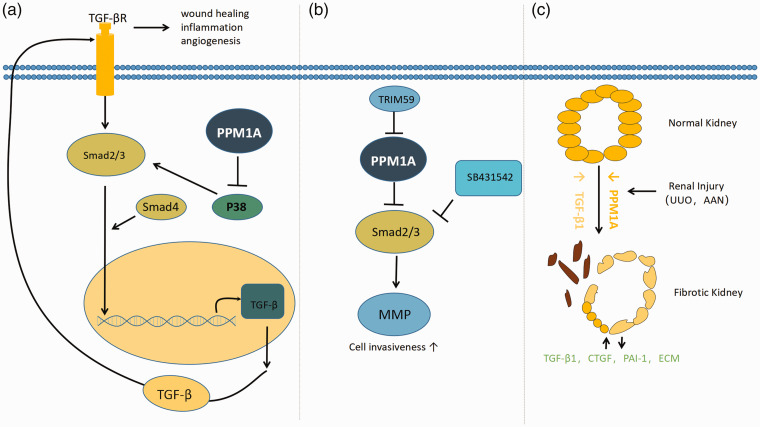
Moreover, studies have shown that PPM1A can inhibit stress-induced activation of the p38 and the JNK/MAPK kinase cascade. Similarly, the JNK and p38 upstream kinases MKK4 and MKK7, and MKK3b and MKK6b, respectively, can be dephosphorylated by PPM1A. 18 PPM1A participates in the regulation of the transforming growth factor signal transduction pathway through by dephosphorylation of p38, which is a marker of inflammation and angiogenesis 19 (Figure 1(a)). It has also been shown to control macrophage via JNK activation, and knockout of PPM1A promotes the selective apoptosis of macrophages infected by Mycobacterium tuberculosis. 20 Takekawa et al. found that PPM1A can also interact with p38 directly, 21 but this direct interaction is only detected under stress conditions. There is currently no evidence that PPM1A causes ERK pathway activation, suggesting that PPM1A inhibits only selected signaling pathways. 22
Figure 1.
Smad, as the substrate of PPM1A, regulates some diseases or physiological functions. (a) Model representing the role of PPM1A in the TGF-β signaling. PPM1A specifically dephosphorylates activated p38, making it easy to further phosphorylate in the presence of activation signal. However, p38 was over activated in the absence of PPM1A, resulting in enhanced transcription of TGF-related genes. TGF-β increased levels of activated TGF-β the receptor, in turn, activates its downstream Smad2/3, resulting in TGF-β. The lack of PPM1A in the adjusted cornea led to aberrant wound healing, inflammation, and neovascularization. (b) Model of Trim59 increases the invasion of endometrial stromal cells by ubiquitination of PPM1A. TRIM59 inhibits PPM1A through ubiquitination and activates TGF-β/Smad signaling to activate MMP (matrix metalloproteinase) and further promote the invasion of ectopic endometrial stromal cells in endometriosis. (c) Model for PPM1A involvement in renal fibrosis. PPM1A expression (orange) is readily evident in differentiated renal tubules. Kidney injury is accompanied by a progressive reduction (orange) of PPM1A in the tubular and interstitial regions, with increases in tissue TGF-b1 levels (yellow). Prolonged silencing of PPM1A promotes the secretion of PAI-1, CTGF, and extracellular matrix (ECM) and promotes the proliferation of fibroblasts through Smad3-dependent mechanism. (A color version of this figure is available in the online journal.)
CDK
Cell growth and cell cycle progression are controlled by the sequential activation and inactivation of cyclin-dependent kinases (CDK). 23 PPM1A participates in regulation of the cell cycle by binding and dephosphorylating CDKs. Cheng et al. showed that more than 99% of the phosphatase activity acting on Cdk2 is associated with PP2C-like phosphatases in HeLa cells. The authors also pointed out an evolutionary conservation of type 2C protein phosphatases in the control of cell cycle progression. They further described this interaction in a subsequent report, showing that ptc2 and ptc3 (members of the PP2C family in yeast) can dephosphorylate and inactivate the kinase cdc28p responsible for yeast budding and reproduction both in vivo or in vitro, and PPM1A has been shown to dephosphorylate both CDK2 and CDK6. These studies demonstrated that PPM1A plays an important role in the regulation of cell cycle progression and cell growth.24,25
CDK9 is the catalytic subunit of the general RNA polymerase II elongation factor called positive transcription elongation factor B (P-TEFb). PPM1A dephosphorylates the T-loop in vitro in both the core and 7SK snRNP P-TEFb complexes, and deletion of PPM1A in HeLa cells results in an increase in the total levels of CDK9 t-loop phosphorylation. Therefore, PPM1A dephosphorylates the T-loop of CDK9 and acts as a negative regulator of P-TEFb function. 26 In addition, in primary resting CD4+T cells, deletion of PPM1A increased the phosphorylation of the CDK9 t-loop, which also led to the increase of HIV-1 gene expression in these cells. Interestingly, although PPM1A can inhibit CDK9, it does not inhibit CDK8-mediated activation of -1LTR. 27
p53/MDM2
p53 plays a vital role in cell growth and the maintenance of genetic stability and prevents the growth of nutrient blood vessels in tumors. 28 Ofek et al. found that overexpression of PPM1A in 293T cells results in a failure of proliferate and clone formation. The authors show that overexpression of PPM1A results in a p53-dependent growth arrest in the G2/M phase and cellular apoptosis. Moreover, in HCT116 cells, PPM1A has been shown to increase the transcriptional activity of p53. 29 Using a cell line stably expressing human papillomavirus E6 protein, which induces p53 ubiquitination and degradation, it was also shown that the inhibition of clone formation caused by PPM1A overexpression is mediated via p53. 3 These studies therefore provide firm evidence that PPM1A is an important regulator of the p53 signaling pathway.
TGF-β/Smad
Transforming growth factor-β (TGF-β) is an effective regulator of cellular growth, differentiation, and migration. In the early stages of tumorigenesis, TGF-β can directly act on cancer cells to inhibit tumor growth, but once mutations or epigenetic modifications are introduced during cancer development, the tumor suppressive function of the TGF-β signaling pathway is lost, and instead, it promotes tumorigenic processes such as cell proliferation, immunosuppression, angiogenesis, self-renewal of cancer stem cells, and epithelial-mesenchymal transformation. 30 Lin et al. showed that Smad2/3 dephosphorylation is mediated by a phosphatase insensitive to okadaic acid. Among 39 okadaic acid-insensitive phosphatases screened by the authors, they found that only PPM1A was able dephosphorylate Smad2 and Smad3. These experiments suggested that phosphorylated Smad2 is a direct substrate of Mg2+-dependent PPM1A. Dephosphorylation of the SXS motif of Smad via PPM1A terminates TGF-β signaling. Thus, PPM1A is proposed to Smad2/3 activity. 31 In the pathogenesis of endometriosis, TRIM59 (tripartite motif-containing 59) has been shown to inhibit PPM1A via ubiquitination and subsequent inactivation of the TGF-β/Smad signaling pathway, promoting the invasion of endometrial stromal cells 32 (Figure 1(b)). Based on the above results, it can be inferred that inhibition of the TGF-β signaling pathway may be related to the anti-tumoral and growth-limiting effects of PPM1A.
Wnt/Axin
Wnt genes are differentially regulated during development and encode secreted glycoproteins involved in cell signaling, cell fate determination, and oncogenesis. 33 PPM1A can dephosphorylate Axin, shorten its half-life, release Axin-mediated inhibition of LEF-1-dependent transcription, and activate the Wnt pathway. The Wnt signaling pathway and LEF-1-dependent transcription promote tumor proliferation and malignant transformation. Strovel et al. have shown that by directly dephosphorylating Axin and thereby decreasing its half-life, PPM1A alleviates the Axin-mediated repression of LEF-1-dependent transcription. 34 Taken together, PPM1A can therefore both inhibit and promote cell growth, depending on involved pathways.
NF-κB
NF-κB signaling contributes to several human diseases, notably inflammatory diseases and cancer. Lu and Yarbrough found that PPM1A inhibits both basal and TNFα- or IL-1β-stimulated transcriptional activity of endogenous NF-κB. The efficient inhibition NF-kB activity in IKKa/b double knockout MEFs suggests that IKKs are not the dominant mechanism of RelA inhibition by PPM1A. The authors of the above experiments concluded that PPM1A inhibits the NF-κB pathway in at least two distinct ways: (1) inhibition of upstream IKKs and (2) direct dephosphorylation of Re1A. 35 In addition, studies have also shown that knockout of PPM1A and PPM1B in HeLa cells resulted in higher TNFα-induced NF-κB activation. 36 Moreover, PPM1A acts as a phosphatase to IKKβ, the catalytic subunit required for activation of NF-κB in response to TNFα, dephosphorylating both Ser177 and Ser181 and thereby negatively regulating the NF-κB signaling pathway. The abovementioned pathways may be an important regulatory mechanism involved in the maintenance of the delicate balance of the inflammatory response mediated by TNFα. 36
The role of PPM1A in human diseases
Nephropathy
Over the last decade, PPM1A has been extensively researched in the context of several human diseases, and it is suggested that it may also represent a potential therapeutic target. For example, in unilateral ureteral obstruction (UUO) and aristolochic acid nephropathy (AAN) kidney injury models, the loss of PPM1A expression has been shown to initiate the production of fibrotic factors PAI-1 (plasminogen activator inhibitor-1), CTGF (connective tissue growth factor), and fibronectin. 37 PPM1A expression inhibited TGF-β1-induced SMAD3 phosphorylation and subsequently induced expression of fibrosis genes in renal epithelial cells and fibroblasts. 38 The long-term loss of PPM1A in kidney cells promotes the formation of a fibrotic phenotype. As PPM1A appears to act as an inhibitor of the SMAD3 pathway in renal fibrosis, decreased PPM1A expression in kidney injury promotes maladaptive repair, highlighted by epithelial growth arrest, dedifferentiation, fibrotic factor secretion, and fibroblast proliferation 38 (Figure 1(c)). Similarly, some studies have found that PPM1A may be an antifibrotic molecule in the liver. 39 In addition, the PPM1A/vitamin D receptor (VDR) complex has been shown to be recruited to phospho-Smad3 (pSmad3) in the presence of both TGF-β1 and OC, an analog of active vitamin D, which could promote the dephosphorylation of pSmad3 and attenuate the pSmad3-dependent production of TGF-β1. Taken together, these studies suggest that PPM1A may represent an anti-fibrotic target in the context of kidney injuries.
Diabetes/obesity
Recent studies have shown that show that PPM1A levels are increased significantly in keratocytes of diabetic rats, 40 and PPM family members have been shown to dephosphorylate the key molecule PPARγ. 41 ERK/Cdk5 have previously been shown to phosphorylate PPARγ on Ser273 residues, leading to a disruption of its expression 42 and the promotion of diabetes. 43 Khim et al. showed that PPM1A is a PPARγ protein phosphatase which can directly dephosphorylate PSer273 (Ser273 on PPARγ) and rescue the expression anti-diabetic genes. There is evidence that PPARγ phosphorylation at Ser273 is mediated by ERK and PPM1A affects PPARγ. 44 However, so far, the exact relationship between CDK5 and PPM1A in diabetes remains unexplored, but investigating the role of phosphorylation and PPM1A may help to further elucidate potential targets for the treatment of obesity-related metabolic disorders.
Infectious diseases
The relationship between PPM1A and infectious diseases has been thoroughly studied. Stimulator of interferon genes (STING, also known as MITA and ERIS) is a critical adaptor protein ubiquitously expressed in mammals and located on the endoplasmic reticulum membrane. It senses microorganisms such as viruses, bacteria, and parasites. Li et al. demonstrated that PPM1A physically interacts with and dephosphorylates both STING and TBK1, and thus negatively regulating antiviral signaling. Moreover, PPM1A regulates antiviral signaling by antagonizing TBK1-mediated STING phosphorylation and aggregation, maintaining innate immune homeostasis in the host cells. 45 The forced expression of PPM1A can inhibit the antiviral response to RNA viruses in cells and transgenic zebrafish expressing PPM1A displayed profoundly increased vulnerability to RNA viruses. PPM1A knockout effectively enhanced RNA perception and viral defense in 239T cells and mice which is thought to be mediated by the loss of dephosphorylation of mitochondrial antiviral signaling protein (MAVS) and TBK1 by PPM1A. 46 Sun et al. found that the upregulation of PPM1A impaired the antibacterial response of macrophages during HIV-1 and Mycobacterium tuberculosis infection. Interestingly, HIV-1 may have evolved an escape mechanism to inactivate the inherent antiviral response in macrophages by infection-induced upregulation of PPM1A. 47 Moreover, Schaaf et al. found that M. tuberculosis may block the apoptosis of host macrophages via the PPM1A signaling pathway. 20 In subsequent studies, the authors also revealed that PPM1A may be a negative regulator of M1-type monocyte-to-macrophage differentiation. 48 PPM1A can also inhibit the expression of HIV-1 gene in resting CD4T+ T cells. 27 Based on the above studies, we can infer that PPM1A seems to play a central role in the innate immune response of macrophages. Host-directed therapies against PPM1A may be very beneficial, in particular for patients with HIV or M. tuberculosis co-infections.
Cancer
Recent studies have suggested that PPM1A may play an important role in cancer development. As mentioned above, PPM1A has previously been shown to dephosphorylate SMAD2/3 downstream of TGF-β, thereby inhibiting the TGF-β pathway. Based on this finding, Geng et al. assessed PPM1A expression in 145 bladder cancer samples and found that PPM1A was decreased in samples from patients with muscle-invasive recurrence. Additionally, the authors found that PPM1A inhibition via lentivirus-mediated RNA interference significantly promoted urinary bladder cancer cell motility and epithelial–mesenchymal transition in vitro as well as metastasis in vivo. These effects were dependent on TGF-β/Smad signaling. In addition, loss of PPM1A expression in patients with bladder cancer has been shown to be related to poor differentiation, invasive muscle tumors, and poor prognosis. Therefore, PPM1A appears to promote tumorigenesis. 49 Other studies have found that PPM1A inhibits the invasive behavior of renal cancer cells stimulated by TGF-β1 in a Smad2/3-dependent manner and found that PPM1A overexpression in the tumor suppresses RCC (Renal cell carcinoma) xenograft tumor growth. 50
PPM1A has been shown to regulate cellular proliferation, cell invasion, and migration. However, how exactly PPM1A regulates these activities is not yet well understood.51 Abhijit et al. found that PPM1A is the most frequently lost phosphatase in ER (estrogen receptor)-negative breast cancer compared with ER-positive breast cancer. Loss of PPM1A moreover promotes the development of triple negative breast cancer (TNBC), suggesting that PPM1A is a vital tumor suppressive gene in aggressive breast cancers.
Nonstructural protein 3 (NS3) in HCV cells directly interacts with PPM1A promoting its ubiquitination and degradation. PPM1A inhibition via small interfering RNA significantly promoted hepatocellular carcinoma (HCC) cell migration, invasion, and epithelial mesenchymal transition (EMT), which were further intensified by TGF-β1 stimulation in vitro. Conversely, a rescue of PPM1A expression abrogated the NS3-mediated effects on HCC migration and invasion. 52 TRIM52 upregulation promoted proliferation, migration, and invasion of HCC cells via PPM1A ubiquitination, 53 while knockdown of endogenous PPM1A increased the proliferation of HepG2 human liver carcinoma cells. 54 It has been suggested that PPM1A immunohistochemistry may enable more detailed prognostic stratification of patients with pancreatic duct cancer. 55 Various studies have shown that PPM1A plays a vital role in cancer suppression, and a PPM1A-activating compound could represent a therapeutic target for certain cancers.
Autoimmune disease
PPM1A is involved in various autoimmune diseases. Kim et al. found that anti-PPM1A autoantibodies were higher in sera derived from individuals with ankylosing spondylitis (AS) than from individuals with other autoimmune diseases. In addition, the authors also found anti-PPM1A autoantibodies in HLA–B27-transgenic rats which spontaneously develop AS-like arthritis. PPM1A is highly expressed in the synovial tissue of AS patients and promotes osteoblast differentiation. These findings suggest that PPM1A may be involved in the pathogenesis of AS. 56 Further research by Kim et al. revealed that extracellular PPM1A-induced TNF production via TLR4 and MyD88 in macrophages and thereby may induce inflammation by acting as a damage-associated molecular patterns (DAMP). 57
Neurodegenerative diseases
Recent research suggests that PPM1A may play a role in neurodegenerative diseases. Degenerative brain disease is characterized, among other factors, by an abnormal activation of AMPK. For example, Ma et al. demonstrated that dysfunctional AMPK signaling forms the basis of AD-related synaptic dysfunction. 58 Coughlan et al. found that AMPK pathway activation delayed disease progression of amyotrophic lateral sclerosis (ALS) in SOD1 mice. 59 Ju et al. moreover showed that positive feedback regulation between elevated oxidative stress and AMPK-α1 activation contributes to the progression of Huntington’s disease (HD). 60 As mentioned above, PPM1A dephosphorylates and inactivates the AMPK pathway. 16 Dysregulated activation of AMPK may cause undesirable consequences, and therefore PPM1A may exert neuroprotective effects. However, the above results are predominantly based on laboratory studies and it is not yet known whether it is possible to activate or inhibit the AMPK pathway via PPM1A in a targeted manner. Nonetheless, this represents an interesting treatment strategy for neurodegenerative diseases.
Huntingtin (HTT) phosphorylation on serine-421 (S421) plays a neuroprotective role by suppressing mutated huntingtin protein (mHTT)-induced neurotoxicity in HD.61,62 Marion et al. discovered that PPM1A interacts with HTT. Using of cadmium chloride which selectively inhibits the PPM family the authors demonstrated that inhibition of the PPM/PP2C family prevented its striatal dopamine D2 receptor (D2R)-mediated biological role in HTT dephosphorylation. 63 However, the physiological role of PPM1A in D2R-mediated phosphorylation of HTT is still unclear.
While the exact mechanisms of Parkinson’s disease are unclear, research has previously focused on the following four aspects: (1) abnormal regulation of α-synuclein, (2) mitochondrial dysfunction, (3) oxidative stress, and (4) neuroinflammation.64–66 Schwarz et al. found that PPM1A is involved in fatty acid-induced apoptosis of nerve cells, 67 and PPM1A can also negatively regulate the DLK-1 pathway which is critical during development and synapse regeneration of mechanosensory neurons. The above results illustrate the importance of PPM/PP2C phosphatase in neuronal development. 68 Damage to specific subnuclei of the pars compacta of the substantia nigra, with severe obliteration of their neuromelanin neurons, is frequently considered the most important hallmark of PD. In a PD model, in addition to the most well-known substantia nigra, severe neuronal loss was also observed in the ventral tegmental area, locus coeruleus, and raphe nucleus.69–71 We speculate that PPM1A may affect neuronal apoptosis in PD patients, but further research is required to elucidate the specific mechanisms and how to target them therapeutically.
Targeting PPM1A for treatment of human diseases
It is reported that a third of human intracellular proteins are regulated by phosphorylation, and abnormal phosphorylation may be both the cause and consequence of a variety of human diseases. 72 PPM1A is a member of the Ser/Thr phosphatase family which exhibits half-maximal activation at physiological levels of Mg2+. Unfortunately, most inhibitors screened in a recent study did not inhibit PPM family phosphatases. 73 Interesting, in silico screening revealed a small molecule activator nplc0393 of PP2C which could prevent liver fibrosis by negatively regulating the transforming growth factor signaling pathway and cell cycle. 74 Moreover, Rogers et al. applied an in silico ligand screening of compounds in NCI database to identify micromolar inhibitors of the PPM family which revealed that a carboxynaphthyl moiety had a significant inhibitory effect on PP2C. 75
While the concentration of Mg2+ appears to be crucial for the activity of PPM1A, we must consider that Mg2+ is essential ion in vivo and it is not possible to directly interfere with the concentration of metal ions in vivo to affect PPM1A.
In the current studies, researchers used sanguinarine as a specific inhibitor of PPM1A to investigate the relationship between infections and PPM1A. PPM1A has been shown to inhibit macrophage apoptosis during M. tuberculosis infection via the JNK/AP-1 signaling pathway, which could be inhibited by sanguinarine. 20 A molecular complex composed of PPM1A and PPM1B has been shown to dephosphorylate SIRT2-S25 and was necessary for L. monocytogenes infection, while levels of phosphorylation were markedly reduced following sanguinarine treatment in L. monocytogenes infection. 76 While sanguinarine may be a key molecule for inhibition of PPM1A in response to infectious diseases, it is not clear how it influences PPM1A expression in vivo. Moreover, sanguinarine does not specifically inhibit PPM1A and has been shown to influence other pathways, such as the MAPK/p38 pathway. 77 Due to the complex nature and extensive interconnection of physiological pathways, further research is required to identify specific inhibitors or activators of PPM1A.
Conclusions
Protein kinases and phosphatase jointly regulate phosphorylation, the most well-studied post-translational modification. The reversible process of phosphorylation is tightly balanced and ensures intact signaling functions. PPM1A is an important member of the metal ion-dependent PPM protein phosphatase family which is emerging as an important regulator of a plethora of physiological functions and signaling pathways (Figure 2). In this role, it may also represent a therapeutic target (Table 1). However, current studies investigating the role of PPM1A are largely limited to animal or in vitro models, and the regulation of PPM1A expression, exact cellular localization, and phosphatase activity in humans is not yet fully explored, requiring further research.
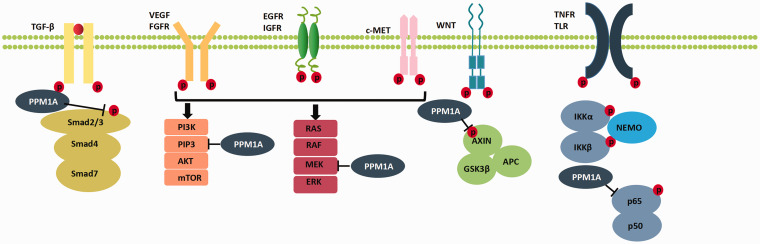
Figure 2.
A partial overview of the substrates and signaling pathways of PPM1A. Red P represents phosphorylation (or potential phosphorylation sites) of proteins. PPM1A may regulate these signaling pathways via dephosphorylation of corresponding substrates. (A color version of this figure is available in the online journal.)
Table 1.
Introduction of the correlation between PPM1A and various diseases and the substrates involved.
| Associated diseases | Substrates of PPM1A | Correlation between PPM1A and disease | |
|---|---|---|---|
| Nephropathy | Fibrotic kidney38 | TGF-β1/Smad2/3 | ↓ |
| Diabetes/obesity | Diabetes44 | PSer273 | ↓ |
| Infection with vesicular stomatitis virus46 | TBK1/IKKε MAV | ↑ | |
| Infectious diseases | Mycobacterium tuberculosis20 | JNK | ↑ |
| HIV infection27 | CDK9 | ↑ | |
| Bladder cancer49 | TGF-β1/Smad2/3 | ↓ | |
| Renal cell carcinoma50 | TGF-β1/Smad2/3 | ↓ | |
| Cancer | Triple negative breast cancer51 | CDK | ↓ |
| Hepatocellular carcinoma52–54 | PXR Smad2/3 | ↓ | |
| Pancreatic duct cancer55 | – | ↓ | |
| Autoimmune disease | Ankylosing spondylitis56 | – | ↑ |
| Neurodegenerative diseases | Alzheimer's disease58 | – | – |
| Amyotrophic lateral sclerosis59 | – | – | |
| Huntington’s disease63 | HTT Ser-421 | ↑ | |
| Parkinson's disease64–68 | – | – |
Note: In Table 1, the references corresponding to related diseases have been explained. In the column of correlation, “↑” represents positive correlation, “↓” represents negative correlation, and “−” represents unknown or unclear.
Looking forward, in-depth investigation of PPM1A may not only promote our understanding of phosphatases but may also provide future targets for diseases.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, interpretation, and analysis of the data and review of the article; ML wrote the article, YLZ, YS, XFX, XYS collected and analyzed the literature, and JGY modified the article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC, No. 81860246, 82160517), the Natural Science Foundation of Guangxi Province (grant number 2018GXNSFAA138050, 2018GXNSFBA138017), the Scientific Research and Technology Development Program of Guangxi (grant number AD18281009, AD18281010), and Thousands of Young and Middleaged Backbone Teachers in Guangxi Colleges and Universities Training Plan.
ORCID iDs: Jianguo Yan  https://orcid.org/0000-0003-4601-4830
https://orcid.org/0000-0003-4601-4830
Yali Zhou  https://orcid.org/0000-0001-7571-5316
https://orcid.org/0000-0001-7571-5316
References
- 1.Kamada R, Kudoh F, Ito S, Tani I, Janairo JIB, Omichinski JG, Sakaguchi K. Metal-dependent Ser/Thr protein phosphatase PPM family: evolution, structures, diseases and inhibitors. Pharmacol Ther 2020; 215:107622. [DOI] [PubMed] [Google Scholar]
- 2.Krzyzosiak A, Sigurdardottir A, Luh L, Carrara M, Das I, Schneider K, Bertolotti A. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell 2018; 174:1216–28 e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammers T, Lavi S. Role of type 2C protein phosphatases in growth regulation and in cellular stress signaling. Crit Rev Biochem Mol Biol 2007; 42:437–61 [DOI] [PubMed] [Google Scholar]
- 4.Das AK, Helps NR, Cohen PT, Barford D. Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 a resolution. Embo J 1996; 15:6798–809 [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Pandey A, Srivastava AK, Tran LS, Pandey GK. Plant protein phosphatases 2C: from genomic diversity to functional multiplicity and importance in stress management. Crit Rev Biotechnol 2016; 36:1023–35 [DOI] [PubMed] [Google Scholar]
- 6.Pan C, Liu HD, Gong Z, Yu X, Hou XB, Xie DD, Zhu XB, Li HW, Tang JY, Xu YF, Yu JQ, Zhang LY, Fang H, Xiao KH, Chen YG, Wang JY, Pang Q, Chen W, Sun JP. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci Rep 2013; 3:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debnath S, Kosek D, Tagad HD, Durell SR, Appella DH, Acevedo R, Grishaev A, Dyda F, Appella E, Mazur SJ. A trapped human PPM1A-phosphopeptide complex reveals structural features critical for regulation of PPM protein phosphatase activity. J Biol Chem 2018; 293:7993–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson MD, Fjeld CC, Denu JM. Probing the function of conserved residues in the serine/threonine phosphatase PP2Calpha. Biochemistry 2003; 42:8513–21 [DOI] [PubMed] [Google Scholar]
- 9.Chuderland D, Dvashi Z, Kaplan-Kraicer R, Ben-Meir D, Shalgi R, Lavi S. De novo synthesis of protein phosphatase 1A, magnesium dependent, alpha isoform (PPM1A) during oocyte maturation. Cell Mol Biol Lett 2012; 17:433–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Zhou Z, Lin H, Lv X, Fu J, Lin P, Zhu C, Wang H. Protein phosphatase 1A (PPM1A) is involved in human cytotrophoblast cell invasion and migration. Histochem Cell Biol 2009; 132:169–79 [DOI] [PubMed] [Google Scholar]
- 11.Shohat M, Ben-Meir D, Lavi S. Protein phosphatase magnesium dependent 1A (PPM1A) plays a role in the differentiation and survival processes of nerve cells. PloS One 2012; 7:e32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Lee J, Oh B, Kimm K, Koh I. Prediction of phosphorylation sites using SVMs. Bioinformatics 2004; 20:3179–84 [DOI] [PubMed] [Google Scholar]
- 13.Lifschitz-Mercer B, Sheinin Y, Ben-Meir D, Bramante-Schreiber L, Leider-Trejo L, Karby S, Smorodinsky NI, Lavi S. Protein phosphatase 2Calpha expression in normal human tissues: an immunohistochemical study. Histochem Cell Biol 2001; 116:31–9 [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ, Chern Y. AMPK-mediated regulation of neuronal metabolism and function in brain diseases. J Neurogenet 2015; 29:50–8 [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature 2009; 459:1146–9 [DOI] [PubMed] [Google Scholar]
- 16.Davies SP, Helps NR, Cohen PT, Hardie DG. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 1995; 377:421–5 [DOI] [PubMed] [Google Scholar]
- 17.Chida T, Ando M, Matsuki T, Masu Y, Nagaura Y, Takano-Yamamoto T, Tamura S, Kobayashi T. N-Myristoylation is essential for protein phosphatases PPM1A and PPM1B to dephosphorylate their physiological substrates in cells. Biochem J 2013; 449:741–9 [DOI] [PubMed] [Google Scholar]
- 18.Hanada M, Kobayashi T, Ohnishi M, Ikeda S, Wang H, Katsura K, Yanagawa Y, Hiraga A, Kanamaru R, Tamura S. Selective suppression of stress-activated protein kinase pathway by protein phosphatase 2C in mammalian cells. FEBS Lett 1998; 437:172–6 [DOI] [PubMed] [Google Scholar]
- 19.Dvashi Z, Sar Shalom H, Shohat M, Ben-Meir D, Ferber S, Satchi-Fainaro R, Ashery-Padan R, Rosner M, Solomon AS, Lavi S. Protein phosphatase magnesium dependent 1A governs the wound healing-inflammation-angiogenesis cross talk on injury. Am J Pathol 2014; 184:2936–50 [DOI] [PubMed] [Google Scholar]
- 20.Schaaf K, Smith SR, Duverger A, Wagner F, Wolschendorf F, Westfall AO, Kutsch O, Sun J. Mycobacterium tuberculosis exploits the PPM1A signaling pathway to block host macrophage apoptosis. Sci Rep 2017; 7:42101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. Embo J 1998; 17:4744–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem 2002; 277:31818–25 [DOI] [PubMed] [Google Scholar]
- 23.Morgan DO. Principles of CDK regulation. Nature 1995; 374:131–4 [DOI] [PubMed] [Google Scholar]
- 24.Cheng A, Kaldis P, Solomon MJ. Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms. J Biol Chem 2000; 275:34744–9 [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Ross KE, Kaldis P, Solomon MJ. Dephosphorylation of cyclin-dependent kinases by type 2C protein phosphatases. Genes Dev 1999; 13:2946–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Dow EC, Liang YY, Ramakrishnan R, Liu H, Sung TL, Lin X, Rice AP. Phosphatase PPM1A regulates phosphorylation of thr-186 in the Cdk9 T-loop. J Biol Chem 2008; 283:33578–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budhiraja S, Ramakrishnan R, Rice AP. Phosphatase PPM1A negatively regulates P-TEFb function in resting CD4(+) T cells and inhibits HIV-1 gene expression. Retrovirology 2012; 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408:307–10 [DOI] [PubMed] [Google Scholar]
- 29.Ofek P, Ben-Meir D, Kariv-Inbal Z, Oren M, Lavi S. Cell cycle regulation and p53 activation by protein phosphatase 2C alpha. J Biol Chem 2003; 278:14299–305 [DOI] [PubMed] [Google Scholar]
- 30.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006; 6:506–20 [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, Shi Y, Chen YG, Meng A, Feng XH. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006; 125:915–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Wang H, Sun L, Niu C, Xu J. TRIM59 inhibits PPM1A through ubiquitination and activates TGF-β/Smad signaling to promote the invasion of ectopic endometrial stromal cells in endometriosis. Am J Physiol Cell Physiol 2020; 319:C392–c401 [DOI] [PubMed] [Google Scholar]
- 33.Nusse R, Varmus HE. Wnt genes. Cell 1992; 69:1073–87 [DOI] [PubMed] [Google Scholar]
- 34.Strovel ET, Wu D, Sussman DJ. Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem 2000; 275:2399–403 [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Yarbrough WG. Negative regulation of RelA phosphorylation: emerging players and their roles in cancer. Cytokine Growth Factor Rev 2015; 26:7–13 [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, Pass AK, Chu M, Zhang D, Lu X, Fu S, Lin X, Yang J. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell Signal 2009; 21:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J, Goldschmeding R, Samarakoon R, Higgins PJ. Protein phosphatase Mg(2+)/Mn(2+) dependent-1A and PTEN deregulation in renal fibrosis: novel mechanisms and co-dependency of expression. Faseb J 2020; 34:2641–56 [DOI] [PubMed] [Google Scholar]
- 38.Samarakoon R, Rehfuss A, Khakoo NS, Falke LL, Dobberfuhl AD, Helo S, Overstreet JM, Goldschmeding R, Higgins PJ. Loss of expression of protein phosphatase magnesium-dependent 1A during kidney injury promotes fibrotic maladaptive repair. Faseb J 2016; 30:3308–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Lan Q, Li W, Yang L, You J, Zhang YM, Ni W. Tripartite motif protein 52 (TRIM52) promoted fibrosis in LX-2 cells through PPM1A-mediated Smad2/3 pathway. Cell Biol Int 2019. DOI: 10.1002/cbin.11206 [DOI] [PubMed] [Google Scholar]
- 40.Lee JE, Lee JS, Hwang SH. Microarray for genes associated with signal transduction in diabetic OLETF keratocytes. Korean J Ophthalmol 2007; 21:111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasdelen I, van Beekum O, Gorbenko O, Fleskens V, van den Broek NJ, Koppen A, Hamers N, Berger R, Coffer PJ, Brenkman AB, Kalkhoven E. The serine/threonine phosphatase PPM1B (PP2Cβ) selectively modulates PPARγ activity. Biochem J 2013; 451:45–53 [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 2010; 466:451–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, Shulman GI, Gygi SP, Spiegelman BM. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 2015; 517:391–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khim KW, Choi SS, Jang HJ, Lee YH, Lee E, Hyun JM, Eom HJ, Yoon S, Choi JW, Park TE, Nam D, Choi JH. PPM1A controls diabetic gene programming through directly dephosphorylating PPARγ at Ser273. Cells 2020; 9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z, Liu G, Sun L, Teng Y, Guo X, Jia J, Sha J, Yang X, Chen D, Sun Q. PPM1A regulates antiviral signaling by antagonizing TBK1-mediated STING phosphorylation and aggregation. PLoS Pathog 2015; 11:e1004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang W, Zhang Q, Lin X, Wu S, Zhou Y, Meng F, Fan Y, Shen T, Xiao M, Xia Z, Zou J, Feng XH, Xu P. PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1. Sci Adv 2016; 2:e1501889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J, Schaaf K, Duverger A, Wolschendorf F, Speer A, Wagner F, Niederweis M, Kutsch O. Protein phosphatase, Mg2+/Mn2+-dependent 1A controls the innate antiviral and antibacterial response of macrophages during HIV-1 and Mycobacterium tuberculosis infection. Oncotarget 2016; 7:15394–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith SR, Schaaf K, Rajabalee N, Wagner F, Duverger A, Kutsch O, Sun J. The phosphatase PPM1A controls monocyte-to-macrophage differentiation. Sci Rep 2018; 8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng J, Fan J, Ouyang Q, Zhang X, Zhang X, Yu J, Xu Z, Li Q, Yao X, Liu X, Zheng J. Loss of PPM1A expression enhances invasion and the epithelial-to-mesenchymal transition in bladder cancer by activating the TGF-β/Smad signaling pathway. Oncotarget 2014; 5:5700–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong Y, Gong L, Yu B, Dong Y. PPM1A suppresses the proliferation and invasiveness of RCC cells via Smad2/3 signaling inhibition. J Receptor Signal Transd Res 2021; 41:245–54 [DOI] [PubMed] [Google Scholar]
- 51.Mazumdar A, Tahaney WM, Reddy Bollu L, Poage G, Hill J, Zhang Y, Mills GB, Brown PH. The phosphatase PPM1A inhibits triple negative breast cancer growth by blocking cell cycle progression. NPJ Breast Cancer 2019; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Zhao Y, Gao Y, Hu W, Qu Y, Lou N, Zhu Y, Zhang X, Yang H. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J Exp Clin Cancer Res 2017; 36:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Tao R, Wu SS, Xu CC, Wang JL, Chen J, Yu YS, Tang ZH, Chen XH, Zang GQ. TRIM52 up-regulation in hepatocellular carcinoma cells promotes proliferation, migration and invasion through the ubiquitination of PPM1A. J Exp Clin Cancer Res 2018; 37:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pondugula SR, Flannery PC, Apte U, Babu JR, Geetha T, Rege SD, Chen T, Abbott KL. Mg2+/Mn2+-dependent phosphatase 1A is involved in regulating pregnane X receptor-mediated cytochrome p450 3A4 gene expression. Drug Metab Dispos 2015; 43:385–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan J, Yang MX, Ouyang Q, Fu D, Xu Z, Liu X, Mino-Kenudson M, Geng J, Tang F. Phosphatase PPM1A is a novel prognostic marker in pancreatic ductal adenocarcinoma. Hum Pathol 2016; 55:151–8 [DOI] [PubMed] [Google Scholar]
- 56.Kim YG, Sohn DH, Zhao X, Sokolove J, Lindstrom TM, Yoo B, Lee CK, Reveille JD, Taurog JD, Robinson WH. Role of protein phosphatase magnesium-dependent 1A and anti-protein phosphatase magnesium-dependent 1A autoantibodies in ankylosing spondylitis. Arthritis Rheumatol 2014; 66:2793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee B, Song YS, Rhodes C, Goh TS, Roh JS, Jeong H, Park J, Lee HN, Lee SG, Kim S, Kim M, Lee SI, Sohn DH, Robinson WH. Protein phosphatase magnesium-dependent 1A induces inflammation in rheumatoid arthritis. Biochem Biophys Res Commun 2020; 522:731–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid β. J Neurosci 2014; 34:12230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coughlan KS, Mitchem MR, Hogg MC, Prehn JH. Preconditioning" with latrepirdine, an adenosine 5'-monophosphate-activated protein kinase activator, delays amyotrophic lateral sclerosis progression in SOD1(G93A) mice. Neurobiol Aging 2015; 36:1140–50 [DOI] [PubMed] [Google Scholar]
- 60.Ju TC, Chen HM, Chen YC, Chang CP, Chang C, Chern Y. AMPK-α1 functions downstream of oxidative stress to mediate neuronal atrophy in Huntington's disease. Biochim Biophys Acta 2014; 1842:1668–80 [DOI] [PubMed] [Google Scholar]
- 61.Colin E, Zala D, Liot G, Rangone H, Borrell-Pagès M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. Embo J 2008; 27:2124–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warby SC, Chan EY, Metzler M, Gan L, Singaraja RR, Crocker SF, Robertson HA, Hayden MR. Huntingtin phosphorylation on serine 421 is significantly reduced in the striatum and by polyglutamine expansion in vivo. Hum Mol Genet 2005; 14:1569–77 [DOI] [PubMed] [Google Scholar]
- 63.Marion S, Urs NM, Peterson SM, Sotnikova TD, Beaulieu JM, Gainetdinov RR, Caron MG. Dopamine D2 receptor relies upon PPM/PP2C protein phosphatases to dephosphorylate huntingtin protein. J Biol Chem 2014; 289:11715–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson's disease. J Immunol Res 2018; 2018:4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443:787–95 [DOI] [PubMed] [Google Scholar]
- 66.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Ijms 2018; 19:3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarz S, Hufnagel B, Dworak M, Klumpp S, Krieglstein J. Protein phosphatase type 2Calpha and 2Cbeta are involved in fatty acid-induced apoptosis of neuronal and endothelial cells. Apoptosis 2006; 11:1111–9 [DOI] [PubMed] [Google Scholar]
- 68.Tulgren ED, Baker ST, Rapp L, Gurney AM, Grill B. PPM-1, a PP2Cα/β phosphatase, regulates axon termination and synapse formation in Caenorhabditis elegans. Genetics 2011; 189:1297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seidel K, Mahlke J, Siswanto S, Krüger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf HW, den Dunnen W, Rüb U. The brainstem pathologies of Parkinson's disease and dementia with lewy bodies. Brain Pathol 2015; 25:121–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 2003; 60:337–41 [DOI] [PubMed] [Google Scholar]
- 71.Alberico SL, Cassell MD, Narayanan NS. The vulnerable ventral tegmental area in Parkinson's disease. Basal Ganglia 2015; 5:51–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen P. The role of protein phosphorylation in human health and disease. The sir Hans Krebs medal lecture. Eur J Biochem 2001; 268:5001–10 [DOI] [PubMed] [Google Scholar]
- 73.McCluskey A, Sim AT, Sakoff JA. Serine-threonine protein phosphatase inhibitors: development of potential therapeutic strategies. J Med Chem 2002; 45:1151–75 [DOI] [PubMed] [Google Scholar]
- 74.Zhang M, Yogesha SD, Mayfield JE, Gill GN, Zhang Y. Viewing serine/threonine protein phosphatases through the eyes of drug designers. Febs J 2013; 280:4739–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogers JP, Beuscher AE, Flajolet M, McAvoy T, Nairn AC, Olson AJ, Greengard P. Discovery of protein phosphatase 2C inhibitors by virtual screening. J Med Chem 2006; 49:1658–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira JM, Chevalier C, Chaze T, Gianetto Q, Impens F, Matondo M, Cossart P, Hamon MA. Infection reveals a modification of SIRT2 critical for chromatin association. Cell Rep 2018; 23:1124–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem 2005; 280:19078–86 [DOI] [PubMed] [Google Scholar]