Abstract
Objective
To investigate the association between stroke and the risk for mortality among coronavirus disease 2019 (COVID-19) patients.
Methods
We performed systematic searches through electronic databases including PubMed, Embase, Scopus, and Web of Science to identify potential articles reporting adjusted effect estimates on the association of stroke with COVID-19-related mortality. To estimate pooled effects, the random-effects model was applied. Subgroup analyses and meta-regression were performed to explore the possible sources of heterogeneity. The stability of the results was assessed by sensitivity analysis. Publication bias was evaluated by Begg’s test and Egger’s test.
Results
This meta-analysis included 47 studies involving 7,267,055 patients. The stroke was associated with higher COVID-19 mortality (pooled effect = 1.30, 95% confidence interval (CI): 1.16–1.44; I2 = 89%, P < 0.01; random-effects model). Subgroup analyses yielded consistent results among area, age, proportion of males, setting, cases, effect type, and proportion of severe COVID-19 cases. Statistical heterogeneity might result from the different effect type according to the meta-regression (P = 0.0105). Sensitivity analysis suggested that our results were stable and robust. Both Begg’s test and Egger’s test indicated that potential publication bias did not exist.
Conclusion
Stroke was independently associated with a significantly increased risk for mortality in COVID-19 patients.
Keywords: COVID-19, Stroke, Mortality, Adjusted effect estimates, Meta-analysis
Introduction
A series of studies have explored the relationship between comorbid stroke and the risk of mortality in coronavirus disease 2019 (COVID-19), but the conclusions were inconsistent or conflicting. Two previous meta-analyses on the basis of un-adjusted effect estimates failed to observe that comorbid stroke was significantly related to an increased risk of mortality in COVID-19 patients [1, 2]. To our knowledge, several risk factors including age, gender, and other underlying conditions (diabetes, hypertension, chronic kidney disease, and chronic obstructive pulmonary disease and others) have been reported to have a significant impact on the clinical progression of patients with COVID-19 [3–8]. This meant that these factors might affect the association between stroke and COVID-19 mortality. For example, the results of univariate analysis in Muhammad et al.’s study [9] showed that stroke was a risk factor for the mortality of COVID-19 (odds ratio (OR) = 2.73, 95% confidence interval (CI): 1.08–6.88), while the results of multivariate analysis demonstrated that stroke was not significantly associated with COVID-19 mortality (adjusted OR = 1.34, 95% CI: 0.45–4.02). Similar findings were also observed by Lee et al. [10]. Therefore, a meta-analysis based on adjusted effect estimates was performed to investigate the association of stroke with fatal outcome in COVID-19 patients.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Checklist was used to improve the reporting of this meta‐analysis. Databases including PubMed, Web of Science, Scopus, and Embase were searched up to November 22, 2021, to find the relevant studies. The following search terms were used: (“COVID-19” OR “coronavirus disease 2019” OR “SARS-CoV-2” OR “novel coronavirus” OR “2019-nCoV”) AND (“stroke” OR “strokes” OR “cerebral stroke” OR “acute cerebrovascular accident” OR “cerebral infarction”) AND (“mortality” OR “death” OR “fatality” OR “deceased” OR “non-survivor”). Moreover, to find more grey literature on this topic, relevant references of the included studies were also taken into account.
The following inclusion criteria were defined for the selection of the papers: (1) articles should be published in English; (2) COVID-19 patients were diagnosed according to the World Health Organization (WHO) standards; (3) articles reported adjusted effect estimates on the association between stroke and the risk of mortality among COVID-19 patients. Repeated articles, case reports, reviews, comments, protocols, errata, and articles without sufficient information were excluded. Only the articles with the most complete data could be included if studies were based on the same data sources.
Two authors selected eligible studies independently and screened the titles and abstracts on the basis of the inclusion criteria. Any disagreement would be resolved through discussion. The primary message included the following data: author, country, cohort type, cases, numbers of stroke, mean or median age, proportion of males, setting, proportion of severe COVID-19 cases, adjusted effect estimate, and 95% CI.
Meta-analysis was performed using the R statistical software (Version 4.1.1, The R Foundation, Vienna, Austria) with “meta” package (Version 4.19-0) to calculate the pooled effect and 95% CI on the association between stroke and COVID-19 mortality. The I2 statistic was used to assess heterogeneity, which was considered significant if I2 > 50%. A random-effects model was adopted if heterogeneity was significant; otherwise, a fixed-effects model would be implemented. Subgroup analyses were conducted according to the area, age, cohort type, effect type, setting, cases, proportion of severe COVID-19 cases, and the proportion of males to explore the sources of heterogeneity. The robustness of the results was tested using sensitivity analysis by eliminating data one at a time. The authors also used the Begg’s and Egger’s tests to detect publication bias. Statistically significant difference was considered when P value was less than 0.05.
Results
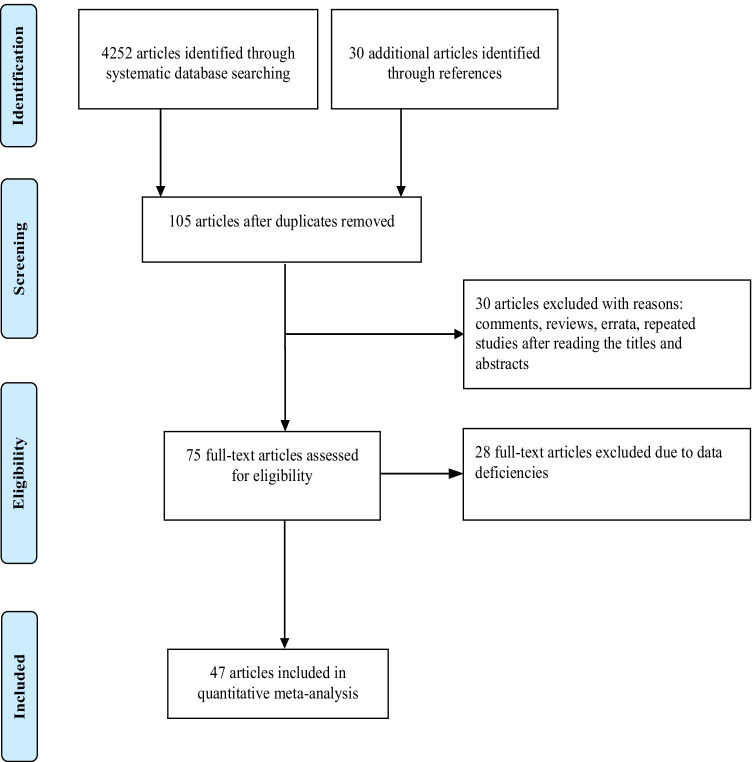
The literature search identified 4252 articles through database mining. A total of 4177 of them were excluded after screening the titles and abstracts, and 75 reports were reviewed for full-text evaluation. As a result, we included 47 studies of 7,267,055 patients [9–55]. A flowchart of the study search and selection was shown in Fig. 1. Basic characteristics of the included studies were presented in Table 1.
Fig. 1.
Flow chart of the process of study selection
Table 1.
Characteristics of the eligible studies included in this meta-analysis
| Author | Country | Cohort type | Cases (n) | No. of stroke (%) | Age (years) | Severe COVID-19 (%) | Male (%) | Setting | Adjusted-effect (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Atkins JL | UK | Retrospective | 507 | 23 (4.5) | 74.3 ± 4.5 | NA | NA | Hospitalized | OR = 1.16 (0.75–1.81) |
| Perez-Guzman PN | UK | Retrospective | 614 | NA | 69 ± 25 | 13.03 | 62.21 | Hospitalized | OR = 0.89 (0.48–1.65) |
| Kummer BR | USA | Retrospective | 3,248 | 387 (11.9) | 67.0 ± 16.2 | NA | 85.19 | Hospitalized | OR = 1.28 (1.01–1.63) |
| Gupta A | USA | Retrospective | 1,296 | 135 (10.4) | 69.8 ± 13.9 | 22.22 | 56.48 | Hospitalized | OR = 1.177 (0.821–1.688) |
| Nimkar A | USA | Retrospective | 327 | 91 (27.8) | 71 (59–82) | NA | 55.7 | Hospitalized | OR = 1.1 (0.6–1.9) |
| Reilev M | Denmark | Retrospective | 11,122 | 542 (4.9) | 48 (33–62) | 2.82 | 42 | Hospitalized | OR = 1.4 (1.1–1.8) |
| Miller J | USA | Retrospective | 3,633 | 1,758 (51.8) | 58.4 ± 18.1 | NA | 46.2 | All patients | OR = 0.94 (0.71–1.24) |
| Graziani D | Spain | Retrospective | 793 | 27 (3.4) | 75 ± 12 | NA | 83 | All patients | OR = 0.76 (0.58–1.71) |
| Clift AK* | UK | Prospective | 6,083,102 | 129,699 (2.13) | 48.2 ± 18.6 | NA | 49.9 | All patients | HR = 1.28 (1.19–1.39) |
| Liu J | China | Retrospective | 774 | 43 (5.6) | 64 (54–73) | 100 | 58.4 | Hospitalized | HR = 0.99 (0.58–1.7) |
| Sheshah E | Saudi Arabia | Retrospective | 300 | 5 (1.7) | 49.7 ± 13.2 | 7.0 | 86.3 | Hospitalized | OR = 1.5 (0.2–10.8) |
| Rossi L | Italy | Retrospective | 590 | 21 (3.6) | 76.2 (68.2–82.6) | 14.24 | 67.6 | All patients | HR = 1.721 (0.985–3.008) |
| FAI2R** | France | Retrospective | 694 | 25 (3.7) | 55.9 | 12.54 | 33.4 | All patients | OR = 1.52 (0.51–4.56) |
| Alguwaihes AM | Saudi Arabia | Retrospective | 439 | 17 (3.9) | 55 (19–101) | 28.02 | 68.3 | Hospitalized | HR = 1.3 (0.5–3.8) |
| Ling SF | UK | Retrospective | 444 | 40 (9.0) | 74 (63–83) | 7.6 | 55.2 | Hospitalized | OR = 1.09 (0.54–2.19) |
| Hobbs ALV | USA | Retrospective | 502 | 57 (11.4) | 62 (49–71) | 35.66 | 55.2 | Hospitalized | OR = 1.25 (0.55–2.72) |
| Ahlstrom B | Sweden | Retrospective | 1,981 | 59 (3.0) | 61 (52–69) | 100 | 74 | Hospitalized | HR = 1.04 (0.75–1.44) |
| Eskandar EN | USA | Retrospective | 4,711 | 27 (2.4) | 63.4 | NA | 53.3 | Hospitalized | HR = 1.75 (1.4–2.1) |
| Muhammad R | USA | Retrospective | 200 | 22 (11.0) | 58.9 ± 15.1 | 35.5 | 60.5 | Hospitalized | OR = 1.34 (0.45–4.02) |
| Kvale R | Norway | Retrospective | 8,809 | 125 (1.4) | NA | NA | 49.5 | All patients | OR = 1.5 (1.1–2.2) |
| Gonzalez-Fajardo JA | Spain | Prospective | 106 | 15 (14.15) | 65.66 ± 15.49 | 8.87 | 67.92 | Hospitalized | HR = 8.812 (2.25–34.516) |
| Efros O | Israel | Retrospective | 320 | 29 (9.06) | 63.66 ± 16.87 | NA | 64.06 | Hospitalized | HR = 1.90 (0.83–4.32) |
| Aoun M | Lebanon | Retrospective | 231 | 14 (6.1) | 61.46 ± 13.99 | 19.91 | 55.4 | Hospitalized | OR = 2.71 (0.89–8.28) |
| Caro-Codon J | Spain | Retrospective | 918 | 41 (4.5) | 63.2 ± 15.5 | 16.9 | 60.1 | Hospitalized | HR = 1.61 (0.89–2.9) |
| Galvez-Barron C | Spain | Retrospective | 103 | 13 (12.6) | 86.75 ± 4.65 | 57.28 | 40.8 | Hospitalized | OR = 0.85 (0.26–2.91) |
| Alwafi H | Saudi Arabia | Retrospective | 706 | 88 (12.5) | 48.0 ± 15.6 | 35.98 | 68.5 | Hospitalized | OR = 0.25 (0.06–1.01) |
| Fan FSY | China | Retrospective | 3,164 | NA | NA | NA | NA | Hospitalized | HR = 2.31 (1.35–3.96) |
| Cummins L | UK | Retrospective | 1,781 | 138 (7.7) | 59.77 | 8.53 | 55.2 | Hospitalized | OR = 1.93 (1.29–2.88) |
| Vogels Y | Netherlands | Retrospective | 114 | 7 (6.1) | 68.0 (59.0–73.3) | 100 | 76.3 | Hospitalized | HR = 1.82 (0.62–5.33) |
| Azarkar Z | Iran | Retrospective | 364 | 13(3.57) | 54.28 ± 18.81 | 18.13 | 56.9 | Hospitalized | OR = 5.56 (1.5–21.3) |
| Kelly JD | USA | Retrospective | 27,640 | 757 (2.7) | 57.59 | NA | 88.6 | All patients | OR = 0.98 (0.75–1.29) |
| Panagides V | France | Retrospective | 2,806 | 249 (8.98) | 66.4 ± 16.9 | 12.94 | 57.6 | Hospitalized | HR = 0.92 (0.67–1.26) |
| Lugon JR | Brazil | Retrospective | 741 | 26 (3.5) | 57 ± 16 | 28.48 | 61 | All patients | HR = 1.37 (0.67–2.82) |
| Bonnet G | France | Retrospective | 28,778 | 253 (8.9) | 66.6 ± 17.0 | 0 | 57.9 | Hospitalized | HR = 1.96 (1.47–2.62) |
| Semenzato L | France | Retrospective | 87,809 | 5,620 (6.4) | 67 ± 19 | NA | 53.3 | Hospitalized | HR = 1.39 (1.32–1.47) |
| Chai C | China | Ambispective | 166 | 4 (2.4) | 65 (59–70) | NA | 49 | Hospitalized | HR = 3.7 (1.1–12.9) |
| Işık F | Turkey | Retrospective | 1,897 | 80 (4.2) | 62 (50–72) | 25.83 | 52.7 | Hospitalized | OR = 1.23 (0.608–2.486) |
| Bushman D | USA | Retrospective | 1,029 | 50 (4.9) | 56 (23–64) | 32.8 | 65.5 | Hospitalized | OR = 1.43 (0.78–2.62) |
| Bandera A | Italy | Retrospective | 1,018 | NA | 65 ± 16 | NA | NA | Hospitalized | OR = 1.15 (0.6–2.19) |
| Lee JH | Korea | Retrospective | 7,162 | 200 (2.79) | 47.7 ± 18.7 | 11.45 | 40.1 | All patients | OR = 0.894 (0.634–1.262) |
| Zerbo O | USA | Retrospective | 219,001 | 1,125 (0.5) | 37.45 | 1.1 | 47.3 | All patients | HR = 1.59 (1.29–1.97) |
| Wang B | USA | Retrospective | 16,504 | 2,301 (13.9) | 67.6 ± 12.0 | 17.89 | 47.7 | All patients | HR = 1.06 (0.96–1.18) |
| Zagidullin NS | Russia | Retrospective | 386 | 4 (1.04) | 59 (49–66) | 12.5 | 40.16 | Hospitalized | OR = 1.09 (0.55–2.18) |
| Puebla Neira DA | USA | Retrospective | 31,526 | 9,308 (29.52) | 66.23 ± 12.27 | 23.2 | 53.36 | Hospitalized | OR = 1.12 (1.01–1.21) |
| Lu Y | USA | Retrospective | 608,251 | 10,949 (1.8) | 83 | 2.46 | 32.7 | All patients | HR = 0.94 (0.87–1.01) |
| Marques M | Spain | Retrospective | 2,112 | 132 (6.3) | 66.6 ± 17.4 | 16.29 | 42.9 | Hospitalized | OR = 0.8 (0.51–1.24) |
| Ouattara E | France | Retrospective | 98,336 | 6,192 (6.3) | 71 (56–83) | 26.19 | 53.8 | All patients | HR = 2.91 (2.62–3.23) |
* indicates combined effects based on subgroups; ** published by FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors; The age (years) was presented as mean ± standard deviation or median (interquartile range, IQR); COVID-19, coronavirus disease 2019; CI, confidence interval; NA, not available; OR, odds radio; HR, hazard radio; USA, the United States of America; UK, the United Kingdom
Overall, comorbid stroke was significantly linked to an increased risk of mortality in patients with COVID-19 (pooled effect = 1.30, 95% CI: 1.16–1.44, random-effects model, Fig. 2). This significant association was also observed in the subgroup analyses based on cases (23 studies, pooled effect = 1.27, 95% CI: 1.08–1.48 for < 1000 cases; 24 studies, pooled effect = 1.29, 95% CI: 1.13–1.46 for ≥ 1000 cases), mean age (16 studies, pooled effect = 1.26, 95% CI: 1.19–1.34 for < 60 years old; 29 studies, pooled effect = 1.30, 95% CI: 1.13–1.51 for ≥ 60 years old), proportion of males (13 studies, pooled effect = 1.16, 95% CI: 1.00–1.33 for < 50%; 31 studies, pooled effect = 1.36, 95% CI: 1.18–1.57 for ≥ 50%), setting (34 studies, pooled effect = 1.31, 95% CI: 1.18–1.45 for hospitalized patients; 13 studies, pooled effect = 1.25, 95% CI: 1.01–1.56 for all patients), area (11 studies, pooled effect = 1.48, 95% CI: 1.01–2.18 for Asia; 22 studies, pooled effect = 1.34, 95% CI: 1.14–1.58 for Europe; 14 studies, pooled effect = 1.18, 95% CI: 1.04–1.35 for Americas), effect estimates (20 studies, pooled hazard ratio (HR) = 1.51, 95% CI: 1.26–1.80; 27 studies, pooled OR = 1.14, 95% CI: 1.07–1.21), and proportion of severe COVID-19 cases (13 studies, pooled effect = 1.25, 95% CI: 1.03–1.51 for < 14%; 20 studies, pooled effect = 1.32, 95% CI: 1.08–1.62 for ≥ 14%) (Table 2). As we implemented subgroup analysis by cohort type, the association was still significant in the subgroups of retrospective studies (44 studies, pooled effect = 1.27, 95% CI: 1.14–1.42) and ambispective study (1 study, pooled effect = 3.70, 95% CI: 1.08–12.67), while no significant association was found in the subgroup of prospective studies (2 studies, pooled effect = 2.96, 95% CI: 0.45–19.31) (Table 2).
Fig. 2.
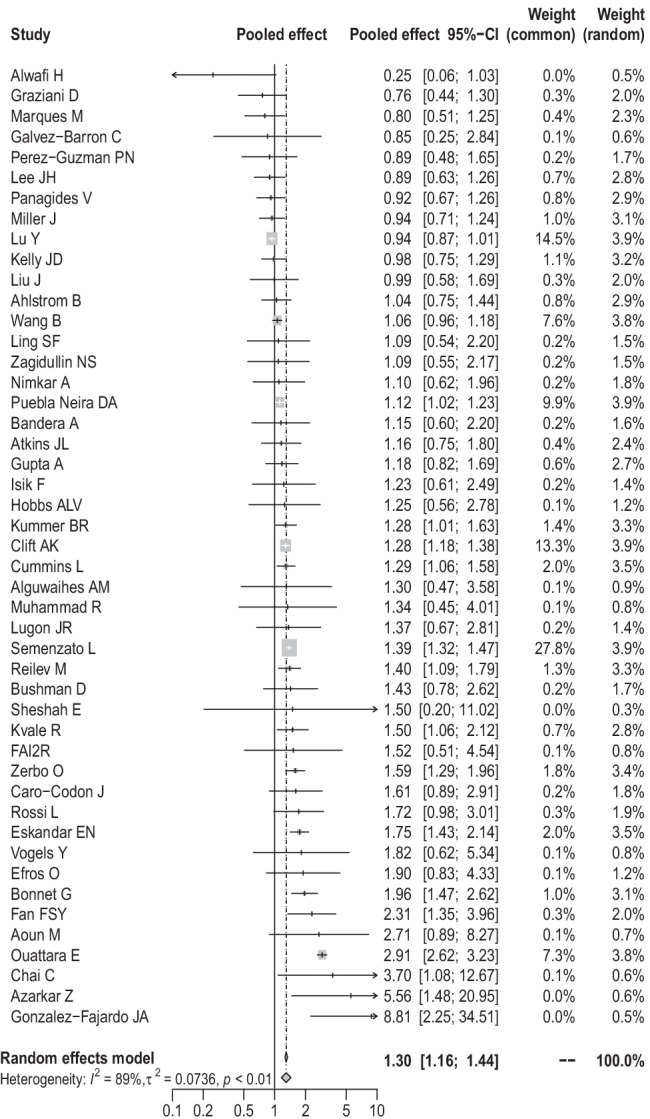
Forest plot on the association of stroke with mortality of COVID-19 patients
Table 2.
Subgroup analysis and meta-regression
| Variables | No. of studies | Meta-regression | Subgroup analysis | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| Tau2 | z value | P value | Pooled ES (95% CI) | I2 | Tau2 | P value | ||
| Cases | - | 0.0759 | − 0.2204 | 0.8255 | - | - | - | - |
| < 1000 | 23 | - | - | - | 1.27 (1.08–1.48) | 32% | 0.0126 | 0.07 |
| ≥ 1000 | 24 | - | - | - | 1.29 (1.13–1.46) | 94% | 0.0825 | < 0.01 |
| Age (years) | - | 0.0743 | - | 0.3530 | - | - | - | - |
| < 60 | 16 | - | - | - | 1.26 (1.19–1.34) | 45% | 0.0217 | 0.03 |
| ≥ 60 | 29 | - | 0.5354 | 0.5924 | 1.30 (1.13–1.51) | 93% | 0.0948 | < 0.01 |
| NA | 2 | - | 1.4386 | 0.1503 | 1.70 (1.27–2.28) | 43% | 0.0399 | 0.19 |
| Male (%) | - | 0.0714 | - | 0.3402 | - | - | - | - |
| < 50 | 13 | - | - | - | 1.16 (1.00–1.33) | 80% | 0.0356 | < 0.01 |
| ≥ 50 | 31 | - | 1.3648 | 0.1723 | 1.36 (1.18–1.57) | 89% | 0.0882 | < 0.01 |
| NA | 3 | - | 0.9429 | 0.3457 | 1.44 (1.06–1.94) | 54% | 0.0874 | 0.11 |
| Area | - | 0.0743 | - | 0.4816 | - | - | - | - |
| Asia | 11 | - | 0.9885 | 0.3229 | 1.48 (1.01–2.18) | 60% | 0.2077 | < 0.01 |
| Europe | 22 | - | 1.0192 | 0.3081 | 1.34 (1.14–1.58) | 91% | 0.0964 | < 0.01 |
| America | 14 | - | - | - | 1.18 (1.04–1.35) | 76% | 0.0340 | < 0.01 |
| Cohort type | - | 0.0780 | - | 0.2055 | - | - | - | - |
| Retrospective | 44 | - | − 1.5469 | 0.1219 | 1.27 (1.14–1.42) | 89% | 0.0736 | < 0.01 |
| Prospective | 2 | - | − 1.1170 | 0.2640 | 2.96 (0.45–19.31) | 87% | 1.6176 | < 0.01 |
| Ambispective | 1 | - | - | - | 3.70 (1.08–12.67) | - | - | - |
| Effect | - | 0.0645 | − 2.5578 | 0.0105 | - | - | - | - |
| HR | 20 | - | - | - | 1.51 (1.26–1.80) | 95% | 0.1112 | < 0.01 |
| OR | 27 | - | - | - | 1.14 (1.07–1.21) | 17% | 0.0027 | 0.21 |
| Setting | - | 0.0766 | 0.4118 | 0.6805 | - | - | - | - |
| Hospitalized | 34 | - | - | - | 1.31 (1.18–1.45) | 56% | 0.0290 | < 0.01 |
| All patients | 13 | - | - | - | 1.25 (1.01–1.56) | 96% | 0.1257 | < 0.01 |
| Severe COVID-19 (%) | - | 0.0782 | - | 0.9113 | - | - | - | - |
| < 14 | 13 | - | - | - | 1.25 (1.03–1.51) | 81% | 0.0663 | < 0.01 |
| ≥ 14 | 20 | - | 0.4223 | 0.6728 | 1.32 (1.08–1.62) | 93% | 0.1140 | < 0.01 |
| NA | 14 | - | 0.3038 | 0.7613 | 1.30 (1.13–1.49) | 62% | 0.0326 | < 0.01 |
ES, effect sizes; CI, confidence interval; NA, not available; OR, odds radio; HR, hazard radio; COVID-19, coronavirus disease 2019
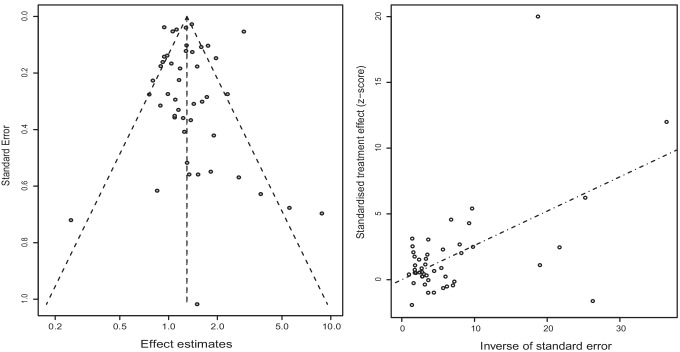
And the outputs of meta-regression demonstrated that effect type might be the source of heterogeneity (P = 0.0105) (Table 2). Sensitivity analysis by omitting each eligible study one by one demonstrated that our findings were stable and robust (Fig. 3). Publication bias was not found in the Begg’s test (P = 0.0864) and Egger’s test (P = 0.8776) (Fig. 4).
Fig. 3.
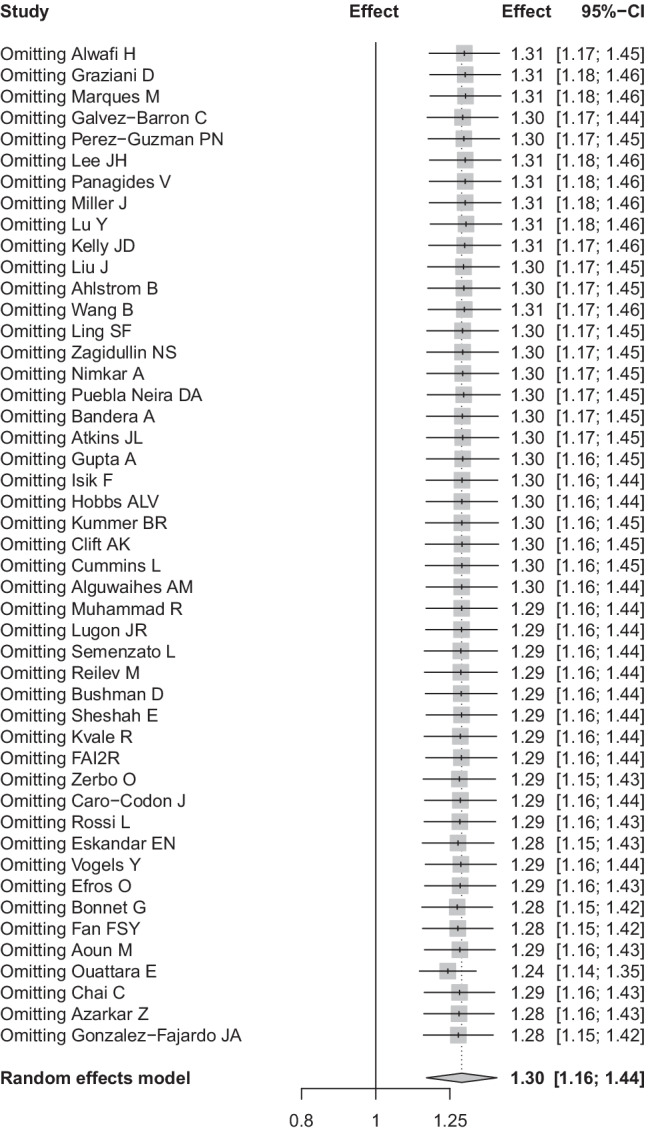
Sensitivity analysis
Fig. 4.
Publication bias based on funnel plot and radial plot
Discussion
Two previous meta-analyses did not observe the significant relationship between comorbid stroke and the increased risk of mortality in COVID-19 patients on the basis of un-adjusted effect estimates [1, 2]. In order to exclude the influence of factors such as gender, age, and comorbidities, we conducted a meta-analysis of 47 articles involving 7,267,055 patients that contained the results of multivariate analysis. The results showed that comorbid stroke was significantly associated with an increased risk of mortality in COVID-19 patients on the basis of confounders-adjusted effect estimates, which demonstrated that stroke might be an independent risk factor for COVID-19-related mortality. The outputs of subgroup analyses stratified by age, proportion of male patients, area, cases, setting, effect type, and proportion of severe COVID-19 infection were in consistence with the conclusion above. According to the results of meta-regression, heterogeneity may originate from the different effect indicators used in the included studies.
Stroke has been confirmed increasing the risk of pulmonary complications like pneumonia, which could lead to the fatal outcome of COVID-19 patients [56–58]. It might partly explain why COVID-19 patients with stroke displayed a higher mortality in our analysis. From another perspective, infection was a vital risk factor for stroke, especially systemic upper respiratory disease [59, 60]. The spike protein surface unit of SARS-CoV-2 highly binds to human ACE-2 receptor, which affects the normal physiological function of the ACE-2 to degrade ANG II , causing neuronal damage and endothelial cell apoptosis [61]. The dysfunction of endothelial cell, leading to fibrinolysis inhibition and excessive thrombin production [62], plays an important role in the occurrence of thrombotic events [63]. The human immune system will produce amounts of proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor-alpha (TNF-α), and IL-6 when SARA-CoV-2 infects the body [64]. This phenomenon referred to as “cytokine storm” results in the degradation of the extracellular matrix (ECM), which is responsible for maintaining the integrity and stability of vascular endothelial cells. Degradation of the ECM contributes to the extravasation of blood components, and leads to hemorrhagic brain injury [65]. Worse still, stroke patients suffering from COVID-19 were more susceptible to undesirable outcomes and greater mortality rates compared with those without [66, 67]. A higher risk of large vessel occlusion and hemorrhagic transformation among ischemic stroke patients with COVID-19, combined with COVID-19 patients being more prone to intracerebral hemorrhage, might contribute to this difference [68–70]. Previous study has shown a past history of cerebrovascular disease might be associated with an increased stroke risk in COVID-19 patients [71]. Thus, we suspected that patients with a history of stroke are more likely to trigger above-mentioned mechanisms, leading to higher mortality of COVID-19 patients with pre-existing stroke. During clinical treatment, more attention should be paid to the possible two-way relationship between stroke and COVID-19 infection.
However, several limitations should be acknowledged. First, our study was limited due to lack of explanation of how stroke was defined in the literature and therefore we could not explore the relationship between different stroke types and COVID-19 mortality. Furthermore, it needs to be acknowledged that most of the selected articles were retrospective observational studies, which were not able to completely explain the casual link between the stroke and COVID-19 mortality, implying that more prospective studies with larger samples are necessary to verify the findings. Lastly, the pooled effects were estimated based on risk factors-adjusted effects, but the adjusted risk factors were not fully consistent across the included researches.
In conclusion, our data indicated that stroke was related to a significantly increased risk for COVID-19 mortality. These findings were supposed to provide a basis for risk scales in the care of patients with COVID-19 in emergency services or intensive care units.
Acknowledgements
We would like to thank Ruiying Zhang, Mengke Hu, Xuan Liang, Wenwei Xiao, Ying Wang, Yang Li, Peihua Zhang, and Jian Wu (all are from Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis.
Author contribution
Yadong Wang, Haiyan Yang, and Guangcai Duan designed the study. Shuwen Li, Jiahao Ren searched articles and extracted the data. Shuwen Li, Hongjie Hou, Jie Xu, and Xueya Han analyzed the data. Shuwen Li wrote and reviewed the manuscript. All the authors approved the final manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (grant number 81973105).
Data availability
All data relevant to this study are included in this article.
Declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Informed consent
Not applicable.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gao Y, Chen Y, Liu M, Niu M, Song Z, Yan M, Tian J. Nervous system diseases are associated with the severity and mortality of patients with COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2021;149:e66. doi: 10.1017/S0950268821000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 3.Liang X, Shi L, Wang Y, Xiao W, Duan G, Yang H, Wang Y. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infect. 2020;81(3):e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesas AE, Cavero-Redondo I, Alvarez-Bueno C, Sarria Cabrera MA, Maffei de Andrade S, Sequi-Dominguez I, Martinez-Vizcaino V. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15(11):e0241742. doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, Jamshidi P, Murthi M, Mirsaeidi M. COVID-19 Clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med (Lausanne) 2020;7:459. doi: 10.3389/fmed.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Xiao W, Liang X, Zhang P, Shi L, Wang Y, Wang Y, Yang H. The association of cerebrovascular disease with adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. J Stroke Cerebrovasc Dis. 2020;29(11):105283. doi: 10.1016/j.jstrokecerebrovasdis.2020.105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Liang X, Hou H, Xu J, Shi L, Wang Y. The association of dementia with COVID-19 mortality: Evidence based on adjusted effect estimates. J Infect. 2021;82(5):e6–e10. doi: 10.1016/j.jinf.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Xu J, Liang X, Shi L, Wang Y. Autoimmune diseases are independently associated with COVID-19 severity: evidence based on adjusted effect estimates. J Infect. 2021;82(4):e23–e26. doi: 10.1016/j.jinf.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhammad R, Ogunti R, Ahmed B, Munawar M, Donaldson S, Sumon M, Kibreab A, Thomas AN, Mehari A. Clinical characteristics and predictors of mortality in minority patients hospitalized with COVID-19 infection. J Racial Ethn Health Disparities. 2022;9(1):335–345. doi: 10.1007/s40615-020-00961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Hwang YM, Cho Y, Oh IY. Prognostic impact of atrial fibrillation in patients with severe acute respiratory syndrome coronavirus 2 infection. Medicine (Baltimore) 2021;100(33):e26993. doi: 10.1097/MD.0000000000026993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.B. Ahlstrom, R. Frithiof, M. Hultstrom, I.M. Larsson, G. Strandberg, M. Lipcsey (2021) The swedish covid-19 intensive care cohort: Risk factors of ICU admission and ICU mortality, Acta Anaesthesiol Scand [DOI] [PMC free article] [PubMed]
- 12.Alguwaihes AM, Al-Sofiani ME, Megdad M, Albader SS, Alsari MH, Alelayan A, Alzahrani SH, Sabico S, Al-Daghri NM, Jammah AA. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alwafi H, Naser AY, Qanash S, Brinji AS, Ghazawi MA, Alotaibi B, Alghamdi A, Alrhmani A, Fatehaldin R, Alelyani A, Basfar A, AlBarakati A, Alsharif GF, Obaid EF, Shabrawishi M. Predictors of length of hospital stay, mortality, and outcomes among hospitalised COVID-19 patients in Saudi Arabia: a cross-sectional study. J Multidiscip Healthc. 2021;14:839–852. doi: 10.2147/JMDH.S304788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoun M, Khalil R, Mahfoud W, Fatfat H, Bou Khalil L, Alameddine R, Afiouni N, Ibrahim I, Hassan M, Zarzour H, Jebai A, Khalil NM, Tawil L, Mechref Z, El Imad Z, Chamma F, Khalil A, Zeidan S, El Ghoul B, Dahdah G, Mouawad S, Azar H, Chahine KA, Kallab S, Moawad B, Fawaz A, Homsi J, Tabaja C, Delbani M, Kallab R, Hoballah H, Haykal W, Fares N, Rahal W, Mroueh W, Youssef M, Rizkallah J, Sebaaly Z, Dfouni A, Ghosn N, Nawfal N, Jaoude WA, Bassil N, Maroun T, Bassil N, Beaini C, Haddad B, Moubarak E, Rabah H, Attieh A, Finianos S, Chelala D. Age and multimorbidities as poor prognostic factors for COVID-19 in hemodialysis: a Lebanese national study. BMC Nephrol. 2021;22(1):73. doi: 10.1186/s12882-021-02270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azarkar Z, Salehiniya H, Kazemi T, Abbaszadeh H. Epidemiological, imaging, laboratory, and clinical characteristics and factors related to mortality in patients with COVID-19: a single-center study. Osong Public Health Res Perspect. 2021;12(3):169–176. doi: 10.24171/j.phrp.2021.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet G, Weizman O, Trimaille A, Pommier T, Cellier J, Geneste L, Panagides V, Marsou W, Deney A, Attou S, Delmotte T, Ribeyrolles S, Chemaly P, Karsenty C, Giordano G, Gautier A, Chaumont C, Guilleminot P, Sagnard A, Pastiero J, Ezzouhairi N, Perin B, Zakine C, Levasseur T, Ma I, Chavignier D, Noirclerc N, Darmon A, Mevelec M, Duceau B, Sutter W, Mika D, Fauvel C, Pezel T, Waldmann V, Cohen A, Critical C-FI. Characteristics and outcomes of patients hospitalized for COVID-19 in France: the Critical COVID-19 France (CCF) study. Arch Cardiovasc Dis. 2021;114(5):352–363. doi: 10.1016/j.acvd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D. Bushman, A. Davidson, P. Pathela, S.K. Greene, D. Weiss, V. Reddy, N. Team, J. Latash (2021) Risk Factors for death among hospitalized patients aged 21–64 years diagnosed with COVID-19-New York city, March 13-April 9, 2020, J Racial Ethn Health Disparities [DOI] [PMC free article] [PubMed]
- 19.Caro-Codon J, Rey JR, Buno A, Iniesta AM, Rosillo SO, Castrejon-Castrejon S, Merino C, Marco I, Martinez LA, Garcia-Veas JM, Martin-Polo L, Rodriguez-Sotelo L, Martinez-Cossiani M, Gonzalez-Valle L, Herrero A, Lopez-de-Sa E, Merino JL, Investigators C-C. Characterization of myocardial injury in a cohort of patients with SARS-CoV-2 infection. Med Clin (Barc) 2021;157(6):274–280. doi: 10.1016/j.medcli.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai C, Feng X, Lu M, Li S, Chen K, Wang H, Wang W, Tang Z, Cheng G, Wu X, Li Y, Wen Y, Da B, Fan H, Wang L, Ai F, Li W, Peng C, Zhang H, Wen S, Zhang J, Weng Y, Tang Z. One-year mortality and consequences of COVID-19 in cancer patients: a cohort study. IUBMB Life. 2021;73(10):1244–1256. doi: 10.1002/iub.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, Hayward A, Hemingway H, Horby P, Mehta N, Benger J, Khunti K, Spiegelhalter D, Sheikh A, Valabhji J, Lyons RA, Robson J, Semple MG, Kee F, Johnson P, Jebb S, Williams T, Hippisley-Cox J. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.F.R.S.S.S.C.I (2020) consortium, contributors, Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients, Ann Rheum Dis [DOI] [PMC free article] [PubMed]
- 23.Cummins L, Ebyarimpa I, Cheetham N, Tzortziou Brown V, Brennan K, Panovska-Griffiths J. Factors associated with COVID-19 related hospitalisation, critical care admission and mortality using linked primary and secondary care data. Influenza Other Respir Viruses. 2021;15(5):577–588. doi: 10.1111/irv.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efros O, Barda N, Meisel E, Leibowitz A, Fardman A, Rahav G, Klempfner R, Grossman E. Myocardial injury in hospitalized patients with COVID-19 infection-risk factors and outcomes. PLoS One. 2021;16(2):e0247800. doi: 10.1371/journal.pone.0247800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eskandar EN, Altschul DJ, de la Garza Ramos R, Cezayirli P, Unda SR, Benton J, Dardick J, Toma A, Patel N, Malaviya A, Flomenbaum D, Fernandez-Torres J, Lu J, Holland R, Burchi E, Zampolin R, Hsu K, McClelland A, Burns J, Erdfarb A, Malhotra R, Gong M, Semczuk P, Gursky J, Ferastraoaru V, Rosengard J, Antoniello D, Labovitz D, Esenwa C, Milstein M, Boro A, Mehler MF. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96(11):e1527–e1538. doi: 10.1212/WNL.0000000000011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvez-Barron C, Arroyo-Huidobro M, Minarro A, Ananos G, Chamero A, Martin M, Gris C, Avalos JL, Capielo AM, Ventosa E, Tremosa G, Rodriguez-Molinero A, C.-r.g.o. CSAPG COVID-19: clinical presentation and prognostic factors of severe disease and mortality in the oldest-old population: a cohort study. Gerontology. 2022;68(1):30–43. doi: 10.1159/000515159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D. Graziani, J.B. Soriano, C. Del Rio-Bermudez, D. Morena, T. Diaz, M. Castillo, M. Alonso, J. Ancochea, S. Lumbreras, J.L. Izquierdo (2020) Characteristics and prognosis of COVID-19 in patients with COPD, J Clin Med 9(10) [DOI] [PMC free article] [PubMed]
- 28.Hobbs ALV, Turner N, Omer I, Walker MK, Beaulieu RM, Sheikh M, Spires SS, Fiske CT, Dare R, Goorha S, Thapa P, Gnann J, Wright J, Nelson GE. Risk factors for mortality and progression to severe COVID-19 disease in the Southeast region in the United States: a report from the SEUS Study Group. Infect Control Hosp Epidemiol. 2021;42(12):1464–1472. doi: 10.1017/ice.2020.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.F. Isik, M. Cap, A. Akyuz, O. Bilge, B. Aslan, U. Inci, I. Kaya, E. Tastan, M. Oksul, N.K. Cap, A. Karagoz, E. Baysal (2021) The effect of resistant hypertension on in-hospital mortality in patients hospitalized with COVID-19, J Hum Hypertens [DOI] [PMC free article] [PubMed]
- 30.Kelly JD, Bravata DM, Bent S, Wray CM, Leonard SJ, Boscardin WJ, Myers LJ, Keyhani S. Association of social and behavioral risk factors with mortality among US veterans with COVID-19. JAMA Netw Open. 2021;4(6):e2113031. doi: 10.1001/jamanetworkopen.2021.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kummer BR, Klang E, Stein LK, Dhamoon MS, Jette N. History of stroke is independently associated with in-hospital death in patients with COVID-19. Stroke. 2020;51(10):3112–3114. doi: 10.1161/STROKEAHA.120.030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.S.F. Ling, E. Broad, R. Murphy, J.M. Pappachan, S. Pardesi-Newton, M.F. Kong, E.B. Jude (2020) High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study, Nutrients 12(12) [DOI] [PMC free article] [PubMed]
- 33.Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, Cai J, Huang S, Guo J, Zhang L, Chen Y, Zhu W, Du H, Liu Y, Wang T, Chen L, Wen Z, Annane D, Qu J, Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques M, Correig E, Ibarretxe D, Anoro E, Antonio Arroyo J, Jerico C, Borrallo RM, Miret M, Naf S, Pardo A, Perea V, Perez-Bernalte R, Ramirez-Montesinos R, Royuela M, Soler C, Urquizu-Padilla M, Zamora A, Pedro-Botet J, S.-X.r. group. Masana L, Domingo JL. Long-term exposure to PM10 above WHO guidelines exacerbates COVID-19 severity and mortality. Environ Int. 2022;158:106930. doi: 10.1016/j.envint.2021.106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller J, Fadel RA, Tang A, Perrotta G, Herc E, Soman S, Nair S, Hanna Z, Zervos MJ, Alangaden G, Brar I, Suleyman G. The impact of sociodemographic factors, comorbidities, and physiologic responses on 30-day mortality in coronavirus disease 2019 (COVID-19) patients in metropolitan detroit. Clin Infect Dis. 2021;72(11):e704–e710. doi: 10.1093/cid/ciaa1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimkar A, Naaraayan A, Hasan A, Pant S, Durdevic M, Suarez CN, Elenius H, Hambardzumyan A, Lakshmi K, Mandel M, Jesmajian S. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):687–695. doi: 10.1016/j.mayocpiqo.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.E. Ouattara, A. Bruandet, A. Borde, X. Lenne, F. Binder-Foucard, M. Le-Bourhis-Zaimi, J. Muller, P. Tran Ba Loc, F. Seguret, S. Tezenas du Montcel, V. Gilleron (2020) Risk factors of mortality among patients hospitalised with COVID-19 in a critical care or hospital care unit: analysis of the French national medicoadministrative database, BMJ Open Respir Res 8(1) [DOI] [PMC free article] [PubMed]
- 38.Panagides V, Vincent F, Weizman O, Jonveaux M, Trimaille A, Pommier T, Cellier J, Geneste L, Marsou W, Deney A, Attou S, Delmotte T, Fauvel C, Ezzouhairi N, Perin B, Zakine C, Levasseur T, Ma I, Chavignier D, Noirclerc N, Darmon A, Mevelec M, Karsenty C, Duceau B, Sutter W, Mika D, Pezel T, Waldmann V, Ternacle J, Cohen A, Bonnet G, Critical C-FI. History of heart failure in patients with coronavirus disease 2019: Insights from a French registry. Arch Cardiovasc Dis. 2021;114(5):415–425. doi: 10.1016/j.acvd.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Guzman PN, Daunt A, Mukherjee S, Crook P, Forlano R, Kont MD, Lochen A, Vollmer M, Middleton P, Judge R, Harlow C, Soubieres A, Cooke G, White PJ, Hallett TB, Aylin P, Ferguson N, Hauck K, Thursz MR, Nayagam S. Clinical characteristics and predictors of outcomes of hospitalized patients with coronavirus disease 2019 in a multiethnic London national health service trust: a retrospective cohort study. Clin Infect Dis. 2021;73(11):e4047–e4057. doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puebla Neira DA, Watts A, Seashore J, Duarte A, Nishi SP, Polychronopoulou E, Kuo YF, Baillargeon J, Sharma G. Outcomes of patients with COPD hospitalized for coronavirus disease 2019. Chronic Obstr Pulm Dis. 2021;8(4):517–527. doi: 10.15326/jcopdf.2021.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reilev M, Kristensen KB, Pottegard A, Lund LC, Hallas J, Ernst MT, Christiansen CF, Sorensen HT, Johansen NB, Brun NC, Voldstedlund M, Stovring H, Thomsen MK, Christensen S, Gubbels S, Krause TG, Molbak K, Thomsen RW. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi L, Malagoli A, Biagi A, Zanni A, Sticozzi C, Comastri G, Pannone L, Gandolfi S, Vergara P, Villani GQ. Renin-angiotensin system inhibitors and mortality in patients with COVID-19. Infection. 2021;49(2):287–294. doi: 10.1007/s15010-020-01550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenzato L, Botton J, Drouin J, Cuenot F, Dray-Spira R, Weill A, Zureik M. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheshah E, Sabico S, Albakr RM, Sultan AA, Alghamdi KS, Al Madani K, Alotair HA, Al-Daghri NM. Prevalence of diabetes, management and outcomes among Covid-19 adult patients admitted in a specialized tertiary hospital in Riyadh. Saudi Arabia, Diabetes Res Clin Pract. 2021;172:108538. doi: 10.1016/j.diabres.2020.108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogels Y, Pouwels S, van Oers J, Ramnarain D. Characteristics and risk factors associated with mortality in critically ill patients with COVID-19. Cureus. 2021;13(4):e14442. doi: 10.7759/cureus.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.B. Wang, B.S. Glicksberg, G.N. Nadkarni, D. Vashishth (2021) Evaluation and management of COVID-19-related severity in people with type 2 diabetes, BMJ Open Diabetes Res Care 9(1) [DOI] [PMC free article] [PubMed]
- 47.Zagidullin NS, Motloch LJ, Musin TI, Bagmanova ZA, Lakman IA, Tyurin AV, Gumerov RM, Enikeev D, Cai B, Gareeva DF, Davtyan PA, Gareev DA, Talipova HM, Badykov MR, Jirak P, Kopp K, Hoppe UC, Pistulli R, Pavlov VN. J-waves in acute COVID-19: a novel disease characteristic and predictor of mortality? PLoS One. 2021;16(10):e0257982. doi: 10.1371/journal.pone.0257982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerbo O, Lewis N, Fireman B, Goddard K, Skarbinski J, Sejvar JJ, Azziz-Baumgartner E, Klein NP. Population-based assessment of risks for severe COVID-19 disease outcomes. Influenza Other Respir Viruses. 2022;16(1):159–165. doi: 10.1111/irv.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lugon JR, Neves P, Pio-Abreu A, do Nascimento MM, Sesso R, C.-H.-B. Investigators Evaluation of central venous catheter and other risk factors for mortality in chronic hemodialysis patients with COVID-19 in Brazil. Int Urol Nephrol. 2022;54(1):193–199. doi: 10.1007/s11255-021-02920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandera A, Nobili A, Tettamanti M, Harari S, Bosari S, Mannucci PM, C.-N.W. Group Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: comment. Intern Emerg Med. 2022;17(1):299–302. doi: 10.1007/s11739-021-02797-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Jiao Y, Graham DJ, Wu Y, Wang J, Menis M, Chillarige Y, Wernecke M, Kelman J, Forshee RA, Izurieta HS. Risk factors for COVID-19 deaths among elderly nursing home medicare beneficiaries in the prevaccine period. J Infect Dis. 2022;225(4):567–577. doi: 10.1093/infdis/jiab515. [DOI] [PubMed] [Google Scholar]
- 52.R. Kvale, K.H. Bonaa, R. Forster, K. Gravningen, P.B. Juliusson, T.A. Myklebust Does a history of cardiovascular disease or cancer affect mortality after SARS-CoV-2 infection?, Tidsskr Nor Laegeforen 140(2) (2021). [DOI] [PubMed]
- 53.Gonzalez-Fajardo JA, Ansuategui M, Romero C, Comanges A, Gomez-Arbelaez D, Ibarra G, Garcia-Gutierrez A. Mortality of COVID-19 patients with vascular thrombotic complications. Med Clin (Engl Ed) 2021;156(3):112–117. doi: 10.1016/j.medcle.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan FSY, Yip TCF, Yiu B, Lam B, Au L, Lau AY, Ip B, Soo Y, Leung TW, Li T, Lui G, Wong GLH, Mok V. Neurological diseases and risk of mortality in patients with COVID-19 and SARS: a territory-wide study in Hong Kong. J Neurol Neurosurg Psychiatry. 2021;92(12):1356–1358. doi: 10.1136/jnnp-2021-326286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, Eckhardt C, Bikdeli B, Platt J, Nalbandian A, Elias P, Cummings MJ, Nouri SN, Lawlor M, Ranard LS, Li J, Boyle C, Givens R, Brodie D, Krumholz HM, Stone GW, Sethi SS, Burkhoff D, Uriel N, Schwartz A, Leon MB, Kirtane AJ, Wan EY, Parikh SA. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–418. doi: 10.1016/S1474-4422(19)30030-4. [DOI] [PubMed] [Google Scholar]
- 57.Ho CH, Lin WC, Hsu YF, Lee IH, Hung YC. One-year risk of pneumonia and mortality in patients with poststroke dysphagia: a nationwide population-based study. J Stroke Cerebrovasc Dis. 2018;27(5):1311–1317. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 58.Singh RJ, Chen S, Ganesh A, Hill MD. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke. 2018;13(8):787–796. doi: 10.1177/1747493018798526. [DOI] [PubMed] [Google Scholar]
- 59.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 60.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5(4):456–463. doi: 10.1002/acn3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pranata R, Huang I, Lim MA, Wahjoepramono EJ. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bibas M, Biava G, Antinori A. HIV-Associated venous thromboembolism. Mediterr J Hematol Infect Dis. 2011;3(1):e2011030. doi: 10.4084/mjhid.2011.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.P.A. Lapchak, Q. Wu (2011) Vascular dysfunction in brain hemorrhage: translational pathways to developing new treatments from old targets, J Neurol Neurophysiol 2011 [DOI] [PMC free article] [PubMed]
- 66.Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, Tan BYQ. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50(3):587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, Sanger M, Kim S, Scher E, Dehkharghani S, Wachs M, Tanweer O, Volpicelli F, Bosworth B, Lord A, Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ladopoulos T, Zand R, Shahjouei S, Chang JJ, Motte J, Charles James J, Katsanos AH, Kerro A, Farahmand G, Vaghefi Far A, Rahimian N, Ebrahimzadeh SA, Abedi V, Papathanasiou M, Labedi A, Schneider R, Lukas C, Tsiodras S, Tsivgoulis G, Krogias C. COVID-19: neuroimaging features of a pandemic. J Neuroimaging. 2021;31(2):228–243. doi: 10.1111/jon.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.M.S. Dhamoon, A. Thaler, K. Gururangan, A. Kohli, D. Sisniega, D. Wheelwright, C. Mensching, J.T. Fifi, M.G. Fara, N. Jette, E. Cohen, P. Dave, A.C. DiRisio, J. Goldstein, E.M. Loebel, N.A. Mayman, A. Sharma, D.S. Thomas, R.D. Vega Perez, M.R. Weingarten, H.H. Wen, S. Tuhrim, L.K. Stein, I. Mount Sinai Stroke, Acute cerebrovascular events with COVID-19 infection, Stroke 52(1) (2021) 48–56. [DOI] [PubMed]
- 71.Siepmann T, Sedghi A, Simon E, Winzer S, Barlinn J, de With K, Mirow L, Wolz M, Gruenewald T, Schroettner P, von Bonin S, Pallesen LP, Rosengarten B, Schubert J, Lohmann T, Machetanz J, Spieth P, Koch T, Bornstein S, Reichmann H, Puetz V, Barlinn K. Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur J Neurol. 2021;28(1):238–247. doi: 10.1111/ene.14535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this study are included in this article.