Abstract
TAFRO syndrome is a systemic inflammatory disorder resembling multicentric Castleman disease; it is characterized by thrombocytopenia, anasarca, a fever, reticulin fibrosis, and organomegaly. Involvement of the adrenal glands, including adrenal infarction, hemorrhaging, and adrenomegaly, has recently been reported in several cases and been considered a characteristic early-stage symptom. We herein report a case of TAFRO syndrome initially presenting with bilateral adrenal infarctions and review the literature on TAFRO syndrome related to adrenal involvement. This case suggests that adrenal abnormalities as an early clinical feature of TAFRO syndrome may be useful for the early diagnosis.
Keywords: TAFRO syndrome, adrenal infarction, adrenal hemorrhaging
Introduction
TAFRO syndrome is a systemic inflammatory disorder first reported in Japan by Takai et al. in 2010 (1). It manifests as lymphadenopathy and resembles multicentric Castleman disease, but the other presentations are unique and characterized by thrombocytopenia, anasarca, a fever, reticulin fibrosis, and organomegaly. Involvement of the adrenal glands, including adrenal infarction, hemorrhaging, and adrenomegaly, has recently been reported in several cases and has been considered one of its early-stage characteristics (2).
We herein report a case of TAFRO syndrome that initially presented with bilateral adrenal infarctions and review the literature on TAFRO syndrome related to adrenal involvement.
Case Report
A 53-year-old woman presented to a clinic with a 3-day history of epigastric pain, nausea, and vomiting. Chest radiography, abdominal ultrasonography, and upper gastrointestinal tract endoscopy findings were unremarkable. The patient was referred to our hospital for a further investigation. Her medical history included pulmonary tuberculosis, which had been treated with appropriate regimens for 6 months, over 10 years earlier without any complications. She worked as a nurse, had no significant family history, and was not taking any medications.
On presentation, her temperature was 37.0℃, blood pressure was 151/78 mmHg, heart rate was 65 beats/min, respiratory rate was 18 breaths/min, and oxygen saturation was 98% on ambient air. She had no abdominal tenderness. Laboratory findings revealed leukocytosis, thrombocytopenia, and an inflammatory response (Table 1). Contrast-enhanced computed tomography (CT) of the abdomen showed decreased blood flow in the bilateral adrenal glands (Fig. 1). She was admitted with a diagnosis of bilateral adrenal infarction and was started on intravenous crystalloid and hydrocortisone (15 mg/day) therapy. The patient's epigastric pain and nausea improved, and she was discharged seven days after admission.
Table 1.
Laboratory Data of the Clinical Course.
| 1st admission | Outpatient follow-up | 2nd admission | ||||
|---|---|---|---|---|---|---|
| Complete blood cell | ||||||
| WBC (/μL) | 13,900 | 66,000 | 67,000 | |||
| Hb (g/dL) | 15.2 | 12.7 | 12.2 | |||
| Plt (/μL) | 77,000 | 52,000 | 51,000 | |||
| Biochemistry | ||||||
| TP (g/dL) | 8.3 | - | 7 | |||
| Alb(g/dL) | 4.5 | - | 2.6 | |||
| LDH (IU/L) | 284 | 247 | 299 | |||
| T-bil (g/dL) | 0.7 | 0.3 | 0.5 | |||
| AST (IU/L) | 23 | 26 | 30 | |||
| ALT(IU/L) | 29 | 16 | 23 | |||
| ALP (IU/L) | 345 | 335 | 755 | |||
| γ-GTP (IU/L) | 76 | 61 | 183 | |||
| BUN (mg/dL) | 15 | 15 | 16 | |||
| Cre (mg/dL) | 0.59 | 0.78 | 0.84 | |||
| Na (mEq/L) | 142 | 140 | 140 | |||
| Cl (mEq/L) | 100 | 106 | 103 | |||
| K (mEq/L) | 4.1 | 5.2 | 5 | |||
| CRP (mg/dL) | 6.69 | 12.2 | 27.68 | |||
| Coagulation test | ||||||
| PT-INR | 1.1 | 1.04 | 1.07 | |||
| APTT (s) | 35.7 | 40.6 | 47.1 | |||
| FIB (mg/dL) | 566 | - | - | |||
| FDP (μg/mL) | 5.0 | - | - | |||
| Hormonal data | ||||||
| Early morning cortisol (μg/dL) | 10.3 | - | - | |||
| Cortisol (60 min after ACTH loading test) (μg/dL) | 19.5 | - | - |
WBC: white blood cell, Hb: hemoglobin, Plt: platelet count, TP: total protein, Alb: albumin, LDH: lactate dehydrogenase, T-bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, Cre: creatinine, CRP: C-reactive protein, APTT: activated partial thromboplastin time, PT-INR: prothrombin time-international normalized ratio, FIB: fibrinogen, FDP: fibrin/fibrinogen degradation products, ACTH: adrenocorticotropic hormone
Figure 1.
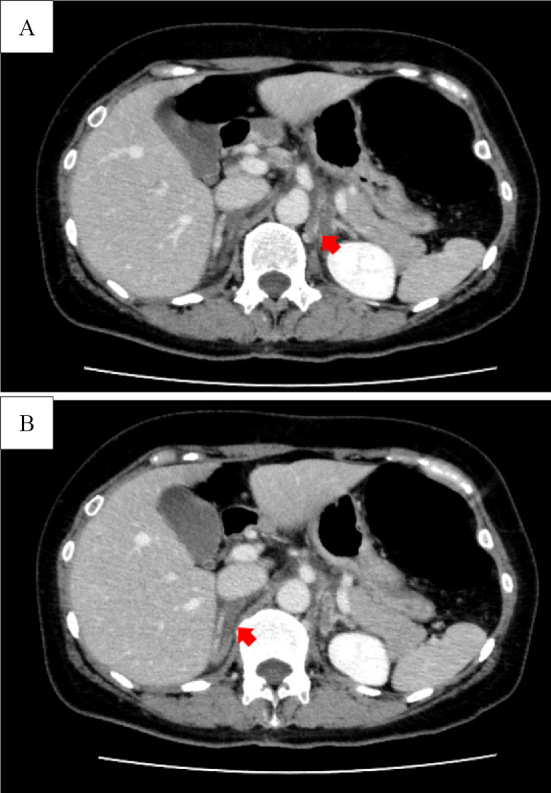
Contrast-enhanced CT of the abdomen. Decreased blood flow in the left adrenal gland (A: arrow) and the right adrenal gland (B: arrow).
Considering the adrenal infarctions and thrombocytopenia, we suspected antiphospholipid syndrome (APS) as a possible cause and followed her up in the outpatient clinic. On day 13 after discharge, her platelet count decreased to 52,000/mm3 (Table 1). Antiphospholipid antibodies were not detected, and she did not meet the diagnostic criteria for APS. Anti-SS-A and SS-B antibodies were positive, but there were no symptoms suggesting Sjogren's syndrome (Table 2). The results of the adrenocorticotropic hormone loading test were in the normal range, which ruled out adrenal insufficiency (Table 1). The remaining laboratory findings are shown in Table 2.
Table 2.
Laboratory Data of Differential Diagnosis.
| Serology | ||
| HIV antigen | 0.08 | |
| HIV antibody | 0.08 | |
| CMV IgM | 0.35 | |
| CMV IgG | <2.0 | |
| EBV anti-EA IgG | 0.4 | |
| EBV anti-VCA IgM | <10 | |
| EBV anti-VCA IgG | 160 | |
| T-SPOT | Negative | |
| HHV 8 type DNA | Negative | |
| Immunochemistry | ||
| IgG (mg/dL) | 1,262 | |
| IgG 4 (mg/dL) | 14.1 | |
| IgA (mg/dL) | 334 | |
| IgM (mg/dL) | 66 | |
| Antinuclear antibody | <40 (speckled) | |
| Anti-ds-DNA IgG antibody (IU/mL) | <10 | |
| Anti-RNP antibody | Negative | |
| Anti-Sm antibody | Negative | |
| Anti-SS-A antibody | 4 | |
| Anti-SS-B antibody | 1 | |
| Anti-cardiolipin β2GP1antibody (U/mL) | <1.2 | |
| Anti-cardiolipin antibody (IgG) (U/mL) | <8 | |
| Lupus anticoagulant | 0.75 | |
| MPO-ANCA (U/mL) | <1.0 | |
| PR3-ANCA (U/mL) | <1 |
HIV: human immunodeficiency virus, CMV: cytomegalovirus, EBV: Epstein-Barr virus, HHV-8: human herpes virus 8, Anti-ds-DNA: anti-double-stranded DNA, Anti-RNP: anti-ribonucleoprotein, Anti-SM: anti-Smith, Anti-SS: anti-Sjögren syndrome, MPO-ANCA: myeloperoxidase-anti neutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3-anti neutrophil cytoplasmic antibody
The following day, she noticed generalized edema on the face, arms, and legs. She also reported malaise. On day 18 after discharge, she presented at the outpatient clinic with a fever of 38.1℃. Laboratory tests revealed markedly elevated C-reactive protein (CRP) levels and progressive thrombocytopenia (Table 1). Repeated contrast-enhanced CT showed axillary and inguinal lymphadenopathy and small ascites (Fig. 2); however, the findings of bilateral adrenal infarction showed improvement. There was no hepatomegaly or splenomegaly on contrast-enhanced CT. The patient was admitted for a further investigation.
Figure 2.
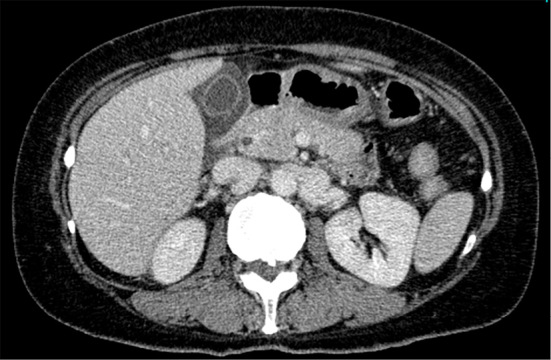
Contrast-enhanced CT of the abdomen revealed small ascites.
On hospital day 3, the leg edema started to gradually worsen. Blood tests revealed a decline in the renal function and thrombocyte count from 51,000/mm3 to 47,000/mm3. Chest radiography and abdominal ultrasonography showed pleural effusion and ascites, respectively; both conditions gradually became exacerbated. Blood and urine cultures were negative. Laboratory tests for tuberculosis, human herpes virus 8, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus infection were negative (Table 2). Bone marrow aspiration and a biopsy performed on hospital day 3 showed only hypercellular bone marrow and no reticulin myelofibrosis or megakaryocytes. A right axillary lymph node biopsy performed on hospital day 9 showed highly atrophic germinal centers, expanded interfollicular areas, and small vessel proliferation (Fig. 3). The patient was finally diagnosed with TAFRO syndrome.
Figure 3.
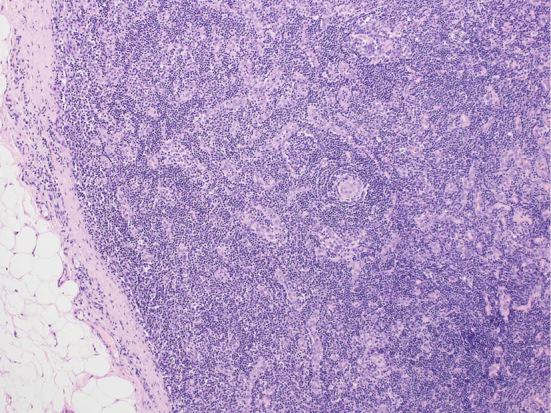
A right axillary lymph node biopsy showed highly atrophic germinal centers, expanded interfollicular areas, and small vessel proliferation.
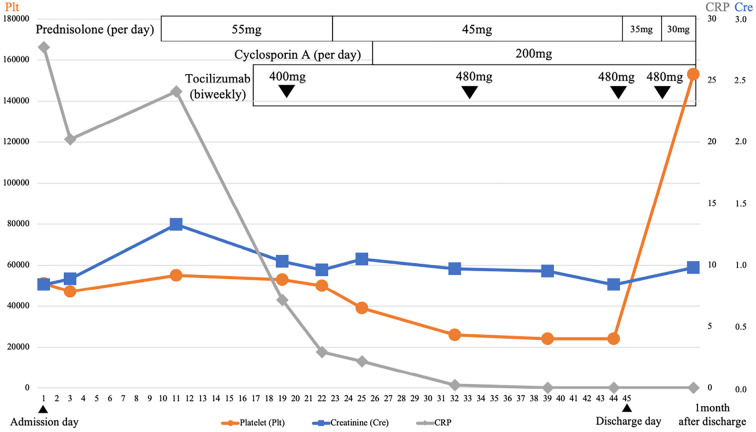
On hospital day 10, after the lymph node biopsy, prednisolone therapy was started at a dose of 55 mg/day. Because the findings of thrombocytopenia, pleural effusion, and ascites had not improved, tocilizumab at 400 and 480 mg was administered on hospital days 19 and 33, respectively. Cyclosporin A at 200 mg/day was subsequently added on hospital day 26. After the combination therapy was started, the patient's condition gradually improved, and prednisolone was tapered to 35 mg/day at day 45. The pleural effusion and ascites diminished, and the CRP levels decreased to 0.02 mg/dL. The patient was discharged on day 45 after readmission.
She continued to receive a biweekly injection of tocilizumab 480 mg and oral prednisolone and cyclosporin A at 200 mg/day. One month after discharge, the thrombocyte count returned to normal levels. The clinical course during the second admission is shown in Fig. 4.
Figure 4.
Clinical course during the second admission. The patient was first treated with high-dose glucocorticoids. After tocilizumab and cyclosporin A were added, the patient’s condition gradually improved.
Discussion
We report the case of a 53-year-old woman who presented with gastric pain and nausea caused by bilateral adrenal infarctions. Subsequently, she developed generalized edema (anasarca) and a fever. These presentations and the results of the lymph node biopsy led to the diagnosis of TAFRO syndrome. The bilateral adrenal infarctions were not accompanied by adrenal insufficiency and spontaneously improved before the initiation of the treatments for TAFRO syndrome.
Adrenal infarction is a rare clinical manifestation that has been reported in APS, essential thrombocythemia, polycythemia vera, Crohn's disease, and pregnancy (3-5). It has also been reported in patients with TAFRO syndrome. In our literature search, we found 14 cases of TAFRO syndrome with adrenal abnormalities (2,4,6-9). We have summarized these cases in Table 3. The adrenal abnormalities included adrenal infarction (7/14), adrenal hemorrhaging (4/14), and adrenomegaly (3/14). The lesions were predominantly located bilaterally. Kurokawa et al. suggested that adrenal CT abnormalities are observed at the early stage of TAFRO syndrome, within 30 days of the symptom onset (2). In the present case, consistent with the previous report, bilateral adrenal infarctions were the initial presentation of TAFRO syndrome.
Table 3.
Case Reports of TAFRO Syndrome with Adrenal Abnormalities.
| Case No | Age | Sex | Adrenal abnormality | Unilateral or bilateral | ||||
|---|---|---|---|---|---|---|---|---|
| 1 (2) | 24 | Female | Adrenal infarction | Bilateral | ||||
| 2 (2) | 50 | Male | Adrenal infarction | Unilateral | ||||
| 3 (2) | 71 | Male | Adrenomegaly | Unilateral | ||||
| 4 (2) | 33 | Male | Adrenal infarction | Bilateral | ||||
| 5 (2) | 55 | Male | Adrenal infarction | Bilateral | ||||
| 6 (2) | 53 | Male | Adrenal infarction | Bilateral | ||||
| 7 (2) | 35 | Male | Adrenomegaly | Bilateral | ||||
| 8 (4) | 46 | Male | Adrenal infarction | Unilateral | ||||
| 9 (6) | 48 | Male | Adrenal hemorrhage | Unilateral | ||||
| 10 (7) | 43 | Male | Adrenomegaly | Unilateral | ||||
| 11 (8) | 19 | Male | Adrenal hemorrhage | Bilateral | ||||
| 12 (8) | 31 | Female | Adrenal hemorrhage | Bilateral | ||||
| 13 (9) | 48 | Male | Adrenal hemorrhage | Bilateral | ||||
| 14 (Our case) | 53 | Female | Adrenal infarction | Bilateral |
The mechanism by which adrenal infarction occurs is not fully understood; however, it is hypothesized that it may be caused by venous and lymphatic edema (2) or hypercoagulability (4). The adrenal glands have multiple arteries but only one draining vein; thus, abnormalities in the venous system are directly linked to adrenal abnormalities (2,3). One of the mechanisms underlying adrenal hemorrhaging is venous congestion (10). Therefore, the various adrenal lesions (infarction, hemorrhaging, and enlargement) in TAFRO syndrome may be caused by the same mechanism, i.e. an adrenal venous flow impaired by edema or hypercoagulation.
Multicentric Castleman disease is another disease that needs to be differentiated from TAFRO syndrome. Adrenal lesions have also been reported in Castleman disease, but they included adrenal tumors, and there have been no reports of adrenal hemorrhaging or infarction (11,12). Furthermore, adrenal biopsies of patients with Castleman disease have shown tissue comparable to that found in the lymph nodes, and the pathogenesis is different from that of adrenal lesions in TAFRO syndrome (2,4,11). Although most reports of adrenal tumors have been in patients with unicentric Castleman disease, the type of adrenal lesion may be useful for differentiating this disease from TAFRO syndrome.
The standard treatment for TAFRO syndrome is high-dose glucocorticoids. In refractory cases, cyclosporin A and tocilizumab are usually combined with glucocorticoids (13-16). In the present case, the findings of thrombocytopenia, pleural effusion, and ascites did not improve with high-dose glucocorticoids, so we added tocilizumab and cyclosporin A. The patient responded well to the combination treatment and has been stable during the outpatient follow-up.
Conclusion
Bilateral adrenal infarctions or hemorrhaging are useful clinical features indicating early-stage TAFRO syndrome. Adrenal abnormalities should be widely recognized as a symptom of TAFRO syndrome.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N, Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report of five cases. J Clin Exp Hematop 53: 63-68, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Kurokawa R, Gonoi W, Yokota H, et al. Computed tomography findings of early-stage TAFRO syndrome and associated adrenal abnormalities. Eur Radiol 30: 5588-5598, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Alshahrani MA, Bin Saeedan M, Alkhunaizan T, Aljohani IM, Azzumeea FM. Bilateral adrenal abnormalities: imaging review of different entities. Abdom Radiol 44: 154-179, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara Y, Ito K, Takamura A, Nagata K. The first case of thrombocytopenia, anasarca, fever, renal impairment or reticulin fibrosis, and organomegaly (TAFRO) syndrome with unilateral adrenal necrosis: a case report. J Med Case Rep 12: 295, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandelwal A, Krishna JS, Khandelwal K, Virmani V, Ryan J. Bilateral adrenal infarction in Crohn′s disease. Indian J Endocrinol Metab 17: 933-935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nara M, Komatsuda A, Itoh F, et al. Two cases of thrombocytopenia, anasarca, fever, reticulin fibrosis/renal failure, and organomegaly (TAFRO) syndrome with high serum procalcitonin levels, including the first case complicated with adrenal hemorrhaging. Intern Med 56: 1247-1252, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono S, Yoshimoto K, Nishimura N, et al. Complete resolution of a case of TAFRO syndrome accompanied by mediastinal panniculitis, adrenal lesion, and liver damage with hyperbilirubinemia. Intern Med 60: 1303-1309, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducoux G, Guerber A, Durel CA, Asli B, Fadlallah J, Hot A. Thrombocytopenia, anasarca, fever, reticulin fibrosis/renal failure, and organomegaly (TAFRO) syndrome with bilateral adrenal hemorrhaging in two Caucasian patients. Am J Case Rep 21: e919536, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito F, Kameoka Y, Nara M, et al. TAFRO syndrome with bilateral adrenal hemorrhaging. J Jpn Soc Intern Med 106: 288-294, 2017(in Japanese). [PubMed] [Google Scholar]
- 10.Tan GXV, Sutherland T. Adrenal congestion preceding adrenal hemorrhaging on CT imaging: a case series. Abdom Radiol 41: 303-310, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Otto M, Wieprzowski Ł, Dzwonkowski J, Ziarkiewicz-Wróblewska B. Castleman's disease - an unusual indication for laparoscopic adrenalectomy. Wideochir Inne Tech Maloinwazyjne 7: 50-54, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müssig K, Horger M, Wehrmann M. Adrenal Castleman's disease. Ann Hematol 86: 63-65, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Yamaga Y, Tokuyama K, Kato T, et al. Successful treatment with cyclosporin A in tocilizumab-resistant TAFRO syndrome. Intern Med 55: 185-190, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara S, Mochinaga H, Nakata H, et al. Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol 103: 718-723, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi T, Shimizu T, Toyama T, Abe R, Okamoto S. Successful treatment of TAFRO syndrome with tocilizumab, prednisone, and cyclophosphamide. Intern Med 56: 2205-2211, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]