Abstract
Pulmonary artery sarcoma (PAS) is considered a very rare tumor with a poor prognosis. We herein report two cases of PAS that were diagnosed by positron emission tomography (PET)/computed tomography (CT). In both cases, PET was an effective option for diagnosing tumors, and surgical resection was a valid treatment for these diseases. If a pulmonary artery tumor is suspected, PET/CT is useful for diagnosing PAS and very helpful for choosing the surgical treatment strategy.
Keywords: pulmonary artery sarcoma, pulmonary hypertension, positron emission tomography/computed tomography
Introduction
Pulmonary artery sarcoma (PAS) is considered to be extremely rare, and its prognosis is very poor (approximately 1.5 months without surgical resection) (1). We herein report two cases of PAS diagnosed using positron emission tomography (PET)/computed tomography (CT) that underwent surgical resection.
Case Report
Case 1
A 77-year-old woman was admitted to the hospital with a history of dyspnea on exertion for 1 month. Contrast-enhanced CT (CECT) revealed a mass between the main pulmonary arterial (PA) and right PA (Fig. 1A). The patient was diagnosed with pulmonary embolism (PE) and received anticoagulant therapy for one month.
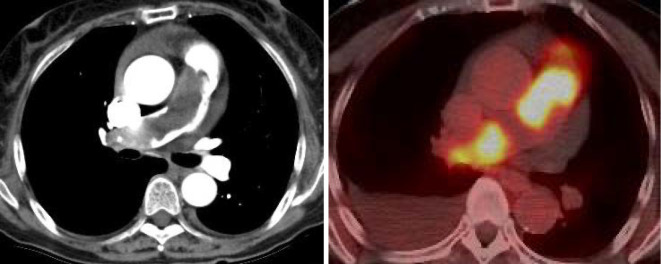
Figure 1.
A: Contrast-enhanced CT showed a mass between the main PA and the right PA. B: The abnormal accumulation of 18F-fluorodeoxyglucose was observed from the main PA to the branch of the right PA.
However, despite therapy, no improvement was observed, so she was referred to our hospital. The patient had severe dyspnea even with mild effort and was heavily fatigued. White blood cells were slightly increased (12,820 /μL). B-type natriuretic peptide (BNP) levels were increased (1,892.6 pg/dL).
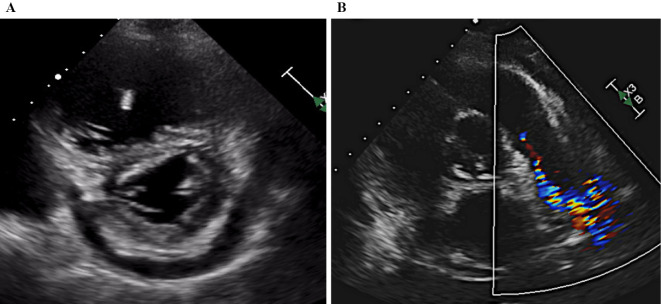
An electrocardiogram revealed sinus rhythm, right axis deviation, and negative T waves detected on II, III, and V2-6. Chest X-ray showed cardiac enlargement (CTR 0.63%) and pleural effusion. CECT revealed a mass from the main PA to the right PA, which was markedly obstructed. Echocardiography revealed a normal left ventricular function, but the left ventricle was excluded by the right ventricle (Fig. 2A). The estimated right ventricular pressure had increased from 59 to 82 mmHg over 14 days. A tumor with low echogenicity and irregular margins was detected from the main PA to the right PA. The tumor extended toward the pulmonary valve (Fig. 2B). The right ventricular load had increased rapidly. On PET/CT, the abnormal accumulation of 18F-fluorodeoxyglucose was observed from the main PA to the right PA; however, no metastases to other organs were detected (Fig. 1B).
Figure 2.
A: A D-shaped left ventricle was observed in systole and diastole due to the elevated pulmonary artery pressure. B: A tumor with low echogenicity and irregular margins was identified, extending from the main PA to the right PA. The tumor developed near the pulmonary valve.
In the heart team discussion, because of the risk of sudden death, she was referred for surgical resection at the main PA. Extensive pneumonectomy was considered before the surgery; however, due to concerns about the increased burden on the patient, the tumor in the PA was resected as much as possible, followed by chemotherapy. The procedure was initiated via standard median sternotomy, and cardiopulmonary bypass was established. The anterior surface of the main PA was hard and degenerated due to the tumor, so an incision was made from the left PA. Tumor progression was observed in the left PA, but no infiltration was observed in the surrounding area. The tumor extended to the distal right PA and was difficult to remove because of adhesions and invasion of the vessel wall. Therefore, part of the tumor was left in the right PA. It was also difficult to preserve the pulmonary valve, and reconstruction of the main PA with a bioprosthetic valve (Inspiris 23 mm) and prosthetic graft replacement was needed.
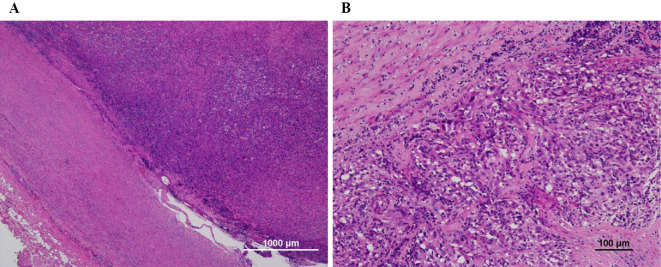
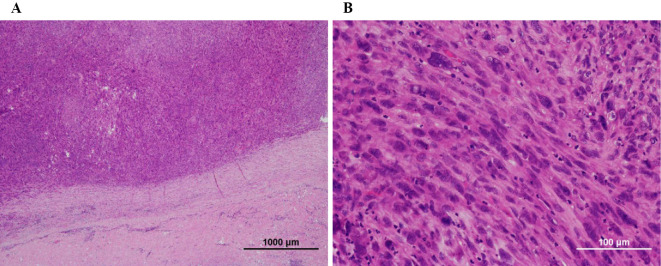
Histopathology revealed proliferative anaplastic spindle-shaped cells that had extended to the vascular lumen (Fig. 3). Immunohistochemical staining showed that neoplastic cells were slightly positive for alpha-smooth muscle actin and desmin. Finally, the patient was diagnosed with intimal undifferentiated sarcoma. Postoperatively, echocardiography showed no pulmonary hypertension, and the symptoms improved.
Figure 3.
A: Tumor cells lining the intimal surface of the pulmonary artery [Hematoxylin and Eosin (H&E) staining]. B: Histopathology findings of cardiac spindle cell sarcoma (H&E staining).
Case 2
A 57-year-old-woman was admitted to a previous hospital with a chief complaint of general fatigue and a slight fever for the past 2 months.
A physical examination revealed an ejection murmur Levine III/VI on the second left sternal border and a body temperature of 37.2 °C. Regarding laboratory findings, the C-reactive protein level was high at 7.228 mg/dL, whereas the BNP level was slightly elevated at 52 pg/dL. The D-dimer level was not elevated (0.9 μg/mL).
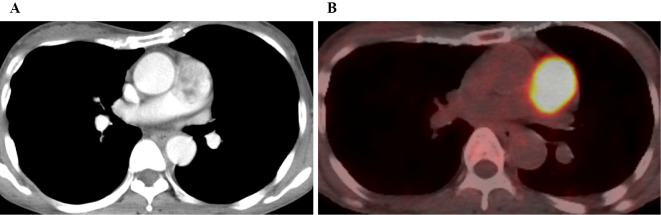
An electrocardiogram revealed a sinus rhythm, normal axis, and no ST-T change. Chest X-ray showed no cardiac enlargement and no pleural effusion. Echocardiography revealed that the main PA was occupied by a large mobile mass attached to the vascular wall, while no pulmonary hypertension was present. CECT showed a large, heterogeneous mass occupying the main PA, and there were no remarkable changes suggesting other tumors and deep-vein thrombosis (Fig. 4A). Considering the possibility of a malignant tumor, she was transferred to our hospital. PET/CT was performed to distinguish malignant tumors and showed an increased uptake at the mass in PA (Fig. 4B). There was no abnormal accumulation of 18F-fluorodeoxyglucose in other organs. Therefore, a pulmonary artery tumor was suspected.
Figure 4.
A: Contrast-enhanced CT showed a mass in the main PA. B: PET/CT revealed a higher-level uptake at the mass in the PA.
After the heart team held a discussion, she received PA resection and prosthetic graft replacement. The operation time was 3 hours and 25 minutes, and an artificial cardiopulmonary was used. An incision was made from the anterior surface of the main PA. The tumor extended from the vicinity of the pulmonary valve to just before the bifurcation of the main PA. The tumor was resected extensively along with the attachment. The main PA was reconstructed, and a prosthetic graft was replaced.
Histopathology revealed proliferation of anaplastic spindle-shaped cells originating from the medial smooth muscle layer to the luminal mass, and focal necrosis of the neoplastic tissue was found (Fig. 5A, B). Immunohistochemical staining revealed that neoplastic cells were slightly positive for alpha-smooth muscle actin, calponin, and desmin. The patient was diagnosed with intimal undifferentiated sarcoma based on the histopathological findings.
Figure 5.
A: Tumor cells lining the intimal surface of the pulmonary artery [Hematoxylin and Eosin (H&E) staining]. B: Histopathological findings of cardiac spindle cell sarcoma (H&E staining).
Her postoperative course was good, and she was discharged after 14 days after the surgery. She was subsequently referred to the oncology team for adjuvant chemotherapy and radiotherapy. Three cycles of adjuvant chemotherapy (doxorubicin plus ifosfamide) and adjuvant radiotherapy (total dose: 66 Gy in 33 fractions) were performed. She has survived for 15 months since the initial diagnosis.
Discussion
We reported two cases of PAS. The median survival time of patients with PAS is reportedly short (2). Early and primary surgical resection is the preferred treatment to prolong patients' lives. In both of the present cases, PET/CT was effective for the diagnosis, and emergency surgical resection improved the prognosis.
PAS was first reported by Mandelstamm in 1923 and is a rare disease with an incidence of only 0.001-0.028% in lung tumors. PAS is always a sarcoma of high malignant potential, and its incidence in women is twice that in men (3). One-third of PAS cases are undifferentiated PAS. The other sarcoma types are fibrosarcoma (20%) and leiomyosarcoma (20%) (4). PAS can be divided into two types (5): intimal and intramural. Intimal sarcomas exhibit a polypoidal growth pattern in the lumen and usually show fibroblast or myofibroblast differentiation (6). Intramural sarcoma is considered distinct from intimal sarcoma and classified separately according to the histological subtype (7). In both cases, the tumor consists of spindle cells and arises from the intimal wall of the pulmonary artery. The tumors stain slightly for calponin, alpha-smooth muscle actin, and desmin as leiomyosarcoma markers. However, the staining is not limited to the leiomyosarcoma; fibroblasts and myofibroblasts are often stained as well. Therefore, these tumors are classified as intimal undifferentiated sarcomas.
A recent, population-based study surveying the database between 1997 and 2010 showed that the overall 1-, 3-, and 5-year survival rates of patients with PAS were 63%, 29%, and 22%, respectively (1). In terms of the treatment modality, surgical curative resection has significantly improved the overall survival rate compared with other methods (36.5±20.2 months vs. 11±3 months) (8). Although previous reports have suggested that the detection of PAS and surgical resection at an early stage are crucial because the prognosis is poor without surgery, the number of patients with localized stages in those studies was less than 50% of the study population (9). The difficulty of early detection is attributed to the fact that symptoms such as dyspnea, cough, and chest pain mimic those of pulmonary thromboembolism (10). Although the most effective way to diagnose a PAS is yet to be determined, some reports have established that the use of PET/CT can aid in distinguishing between clots and masses. An increased 18F-fluorodeoxyglucose uptake in masses visualized using PET is suggestive of a malignant tumor (11,12). Asabella et al. compared the ability of PET/CT and CECT to detect early recurrence in patients treated for retroperitoneal sarcoma. The sensitivity and specificity of PET/CT were higher than those of CECT (66.7% and 100% vs. 58.3% and 50%, respectively) (13).
Although PET/CT is difficult to perform for all patients with masses in the PA due to costs, CECT may also be able to suggest the presence of a PA tumor. In previous reports, CECT showed PAS with heterogeneous contrast and a low-attenuation filling defect occupying the entire luminal diameter of PA (14). In addition, in PE, it is very unusual for the symptoms to persist despite adequate anticoagulation and to have massive blood flow obstruction distributed unilaterally. In cases with these findings, the patient should be examined for a PA tumor (15). Endobronchial ultrasound-guided transbronchial needle aspiration is used to evaluate the tumor tissue (16). However, this may be difficult for patients in the advanced stages of disease owing to the risk associated with the procedure. In both of our cases, the patients' condition was deemed serious; therefore, a low-risk yet highly effective examination was selected.
In cases of suspected PAS, there is a possibility of the tumor growing and rapidly increasing the PA pressure, eventually leading to death. Therefore, complete tumor resection, including lung resection, should be urgently performed if possible. Some earlier studies have reported that patients with unresectable tumors and those in a serious condition were able to be successfully treated with adjuvant radiotherapy or chemotherapy (17). However, these therapeutic modalities have been shown to not increase the survival times of the patients.
Conclusion
PET/CT may be the best option for distinguishing PA thrombosis from tumors in case of PAS, which can lead to early decision-making concerning surgical resection, the only effective treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Mussot S, Ghigna MR, Mercier O, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardio-Thorac Surg 43: 787-793, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Krüger I, Borowski A, Horst M, de Vivie ER, Theissen P, Gross-Fengels W. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg 38: 91-95, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Levy E, Korach A, Amir G, Milgalter E. Undifferentiated sarcoma of the pulmonary artery mimicking pulmonary thromboembolic disease. Heart Lung Circ 15: 62-63, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Parish JM, Rosenow EC, Swensen SJ, Crotty TB. Pulmonary artery sarcoma clinical features. Chest 110: 1480-1488, 1996. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Classification of Tumours. Pathology & Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, Eds. IARC Press, Lyon, 2004. [Google Scholar]
- 6.Tavora F, Miettinen M, Fanburg-Smith J, Franks TJ, Burke A. Pulmonary artery sarcoma: a histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 32: 1751-1761, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cox JE, Chiles C, Aquino SL, Savage P, Oaks T. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Compu Assist Tomogr 21: 750-755, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Blackmon SH, Rice DC, Correa AM, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 87: 977-984, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Qin BD, Jiao XD, Zang YS. Primary pulmonary leiomyosarcoma: a population-based study. Lung Cancer 116: 67-72, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Minakata K, Konishi Y, Matsumoto M, Auta M, Nonaka M, Yamada N. Primary leiomyosarcoma of the pulmonary artery mimicking massive pulmonary thromboembolism. Jpn Circ J 64: 783-784, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Lesar MS, Travis WD, et al. RM and CT findings in pulmonary artery sarcoma. J Comput Assist Tomogr 13: 906-909, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Scheffel H, Stolzmann P, Plass A, et al. Primary intimal pulmonary artery sarcoma: a diagnostic challenge. J Thorac Cardiovasc Surg 135: 949-950, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Niccoli-Asabella A, Altini C, Notaristefano A, et al. A retrospective study comparing contrast-enhanced computed tomography with 18F-FDG-PET/CT in the early follow-up of patients with retroperitoneal sarcomas. Nucl Med Commun 34: 32-39, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Yi CA, Lee KS, Choe YH, Han D, Kwon OJ, Kim S. Computed tomography in pulmonary artery sarcoma: distinguishing features from pulmonary embolic disease. J Comput Assist Tomogr 28: 34-39, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Olsson HE, Spitzer RM, Erston WF. Primary and secondary pulmonary artery neoplasia mimicking acute pulmonary embolism. Radiology 118: 49-53, 1976 Jan. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Chung JH, Jheon S, et al. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J 38: 1480-1482, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Uchida A, Tabata M, Kiura K, et al. Successful treatment of pulmonary artery sarcoma by a two-drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn J Clin Oncol 35: 417-419, 2005. [DOI] [PubMed] [Google Scholar]