Abstract
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed and administered worldwide. There have been reports of neurological adverse events following immunization (AEFIs). We herein report a case of refractory longitudinally extensive transverse myelitis in a 75-year-old Japanese man following the first dose of the BNT162b2 vaccine. The patient developed total sensory loss below the umbilicus and complete paralysis in both legs. Although he was treated with steroid therapy and plasma exchange, his recovery was limited, and severe sequelae remained. Further studies, including large epidemiological studies, are required to understand the association between SARS-CoV-2 vaccines and neurological AEFI.
Keywords: COVID-19, SARS-CoV-2, vaccine, longitudinally extensive transverse myelitis, transverse myelitis, adverse event following immunization
Introduction
Several different types of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines have been developed and administered worldwide. While SARS-CoV-2 vaccines are expected to control the coronavirus disease (COVID-19) pandemic, a range of neurological adverse events following immunization (AEFIs), including stroke, Guillain-Barré syndrome, facial palsy, transverse myelitis, and acute disseminated encephalomyelitis, have been reported (1).
We herein report a case of refractory longitudinally extensive transverse myelitis (LETM) following the first dose of a SARS-CoV-2 vaccine.
Case Report
A 75-year-old Japanese man developed ascending paresthesia starting in the soles of his feet 3 days after receiving the first dose of the BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine. Ten days after the onset of his symptoms, he developed lower back pain and noticed reduced sensation during urination and defecation. The next day, he developed severe weakness in both legs and was admitted to a hospital.
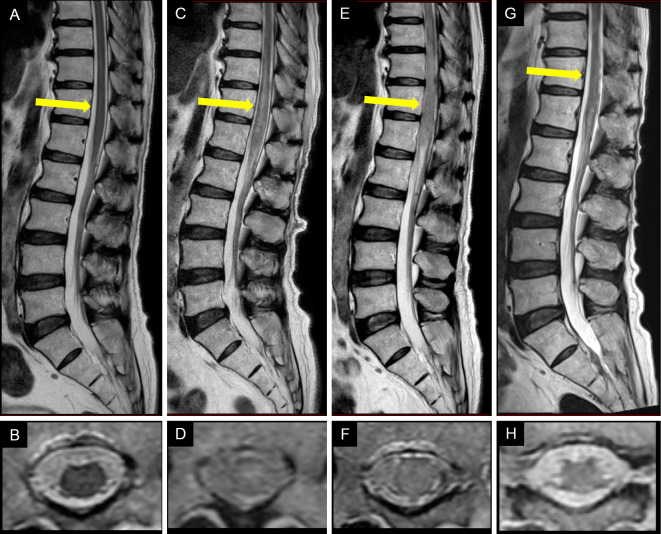
A neurological examination revealed total sensory loss below the level of the umbilicus and complete paralysis and loss of deep tendon reflexes in both legs. He had a medical history of hypertension and hyperlipidemia as well as prostate cancer, for which he had received radiation therapy followed by hormonal therapy. Thoracolumbar spine magnetic resonance imaging (MRI) on admission showed no abnormalities (Figure A, B); however, repeat MRI performed the following day revealed a longitudinally extensive hyperintense lesion from the lower thoracic to lumbar spine (Figure C, D), which showed no gadolinium enhancement. Brain and cervical spine MRI revealed not notable abnormalities.
Figure.
Sagittal (A, C, E, G) and axial (B, D, F, H) T2-weighted magnetic resonance imaging (MRI) of the thoracolumbar spine at the Th12 level (indicated by arrows). (A, B) MRI performed 11 days after the symptom onset shows no abnormalities. (C, D) MRI performed 12 days after the symptom onset shows a longitudinally extensive hyperintense lesion in the lower thoracic and lumbar region. (E, F) MRI performed 16 days after the symptom onset shows expansion and more marked hyperintensity of the lesion. (G, H) Follow-up MRI performed 50 days after the symptom onset shows a reduction in the intensity and extent of the lesion compared with previous MRI findings.
Routine blood tests, including coagulation tests, detected no abnormalities. Tests for serum autoantibodies, including antinuclear, anti-SSA, and anti-SSB antibodies, were negative. An initial cerebrospinal fluid (CSF) examination revealed a mildly elevated protein level (80 mg/dL) without pleocytosis. There were no oligoclonal bands on CSF electrophoresis, and myelin basic protein was within the normal limits (49.3 pg/mL; normal: <102 pg/mL).
The patient was provisionally diagnosed with myelitis, and intravenous methylprednisolone at a dose of 1 g/day was administered for 3 days; however, no improvement was observed in his symptoms. Repeat thoracolumbar spine MRI performed 16 days after the onset revealed the further expansion of the lesion (Figure E, F). The patient was therefore transferred to our hospital for a further evaluation and treatment on day 17.
Cell-based assays of serum anti-aquaporin-4 (anti-AQP4) antibodies and anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibodies were also negative. A panel diagnostic for paraneoplastic neurologic syndrome that tested for antibodies to amphiphysin, CV2, PNMA2 (Ma2/Ta), Ri, Yo, Hu, recoverin, SOX1, titin, zic4, GAD65, and Tr (DNER) was also negative. A CSF examination on admission revealed a moderately elevated protein level (155 mg/dL) and markedly elevated myelin basic protein (8,580 pg/mL) with pleocytosis (33 cells/μL with 54% lymphocytes). CSF glial fibrillary acidic protein-IgG using immunohistochemistry and a cell-based assay were negative. The antibody index for varicella zoster virus was not elevated.
A second course of methylprednisolone was administered intravenously at a dose of 1 g/day for 3 days, followed by oral prednisolone at an initial dose of 1 mg/kg/day. The patient recovered some sensation in the area between the umbilicus and the groin, but his legs remained paralyzed. Therapeutic plasma exchange was administered daily for 7 days, starting on day 28. Although follow-up T2-weighted thoracolumbar spine MRI performed 50 days after the onset showed a reduction in the axial and longitudinal extent of the hyperintense lesion (Figure G, H), the patient's symptoms remained almost unchanged. A nerve conduction study revealed that compound muscle action potentials were not evoked in the tibial or peroneal nerves, but conduction in the median, ulnar, and sural nerves was normal. The patient experienced no obvious improvement in the paralysis of his legs or the dysfunction of his bladder and bowel and was transferred to a rehabilitation hospital 70 days after the symptom onset.
Discussion
LETM is defined as a spinal cord lesion that extends over three or more vertebral segments on spinal MRI. Neuromyelitis optica spectrum disorder is the most common cause of LETM. Other causes include myelin oligodendrocyte glycoprotein antibody-associated disease, vascular disease, infectious disease, and paraneoplastic neurological syndrome (2). Another case of LETM after vaccination with the AZD1222 (AstraZeneca) SARS-CoV-2 vaccine was reported recently (3).
The World Health Organization has developed a classification system to assess the causal relationship between vaccines and AEFIs (4). However, this classification makes it practically difficult to assess cases related to new vaccines, such as SARS-CoV-2 vaccines. Recently, criteria for neurological AEFIs have been proposed that take into account the temporal relationships, risk factors, and possibility of other etiologies (5). Based on these criteria, this case meets the criteria for a “probable” AEFI, considering the following features: (i) the onset of neurological symptoms occurred within a week after vaccination; (ii) the patient had no previous neurological symptoms after other vaccines, nor symptoms suggestive of prior infection; and (iii) no other cause of LETM was identified on a thorough diagnostic evaluation.
Transverse myelitis following vaccination has been reported with several types of SARS-CoV-2 vaccines (3,6-9). In this patient, the symptoms started 3 days after the first dose of SARS-CoV-2 vaccination, which is consistent with other cases reported, which started within 10 days of vaccination. However, there is no proven causal relationship between myelitis and SARS-CoV-2 vaccination, including in this patient, and the benefits of vaccination are thought to outweigh the risks of COVID-19.
Notably, initial thoracolumbar spine MRI on admission showed no abnormalities, but repeat MRI the following day revealed an obvious lesion, which was further enlarged three days later. There have been other reports that abnormal lesions on repeat MRI performed after a negative spinal MRI may occur in patients with idiopathic transverse myelitis and myelitis associated with anti-MOG antibodies (10,11). In addition, some patients with anti-AQP4 antibodies showed spinal cord atrophy without a clinical history of myelitis or any spinal cord lesions on MRI (12). Although the initial MRI scan was performed 11 days after the symptom onset in this patient, considering the subsequent exacerbation of CSF inflammatory findings, the lesion may have still been in the early stage of development at the time of the initial MRI procedure. In patients with clinically suspected transverse myelitis but no abnormalities on MRI, repeat MRI is important.
This patient was treated with steroid therapy and plasma exchange; however, his clinical improvement was limited, and he experienced severe sequelae. A nerve conduction study showed that compound muscle action potentials were not elicited in the motor nerves of the legs with normal sensory nerve findings, suggesting that severe anterior horn cell damage associated with myelitis led to axonal degeneration of the motor nerves of the legs or that the patient suffered from radiculomyelitis. Urinary sphincter dysfunction on admission and LETM that is visible on MRI, both of which were present in this patient, are associated with a poorer functional recovery in patients with idiopathic acute transverse myelitis than those without these symptoms (10).
To date, there have been only a few reported cases of LETM following SARS-CoV-2 vaccination, and to our knowledge, this is the first report of such a case in Japan. Although there have been multiple reports on neurological AEFI associated with the SARS-CoV-2 vaccine, there are only a few published individual case reports of cases of myelitis, including LETM. Further studies, including large-scale epidemiological studies, will be required to obtain a better understanding of the association between SARS-CoV-2 vaccines and neurological AEFIs.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Akio Kimura from Gifu University for the measurements of CSF GFAP-IgG.
References
- 1.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol 89: 856-857, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 27: 279-289, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J Neuroimmunol 358: 577606, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. 2nd ed. 2019 update, World Health Organization, Geneva. [Google Scholar]
- 5.Butler M, Tamborska A, Wood GK, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry 92: 1144-1151, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alshararni A. Acute transverse myelitis associated with COVID-19 vaccine: a case report. J Res Pharm Sci 12: 2083-2087, 2021. [Google Scholar]
- 7.Malhotra HS, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM 114: 591-593, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahir N, Koorapati G, Prasad S, et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus 13: e16624, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vegezzi E, Ravaglia S, Buongarzone G, et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J Neuroimmunol 359: 577686, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobo Calvo A, Mañé Martínez MA, Alentorn-Palau A, Bruna Escuer J, Romero Pinel L, Martínez-Yélamos S. Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol 13: 135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sechi E, Krecke KN, Pittock SJ, et al. Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult Scler 27: 303-308, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventura RE, Kister I, Chung S, Babb JS, Shepherd TM. Cervical spinal cord atrophy in NMOSD without a history of myelitis or MRI-visible lesions. Neurol Neuroimmunol Neuroinflamm 3: e224, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]