Abstract
Objective
Leg muscle strength (LMS) is decreased in early-stage Parkinson disease (PD) patients and is associated with slower walking and falls. However, LMS in advanced PD has not been well investigated. The purpose of this study was to evaluate LMS in advanced PD patients and its effects on gait performance, activities of daily living, and the cognitive function.
Methods
The medical records of 132 patients with idiopathic advanced PD [Hoehn and Yahr (H&Y) stages 3 and 4] with a mean disease duration of 9.6 years were retrospectively reviewed. Leg extensor muscle strength of the patients was measured using a Strength Ergo 240. The associations between the LMS and gait performance, Barthel index, H&Y stage, and Mini-Mental State Examination (MMSE) score were analyzed.
Results
A Spearman's correlation analysis showed that LMS was correlated with the sex, age, age of disease onset, H&Y stage, Barthel index, MMSE score, and gait parameters. A multivariable linear regression analysis for identifying predictors of LMS showed that the gait velocity (β=0.377), Barthel index (β=0.281), sex (β=-0.187), and disease duration (β=-0.155) were significant. A receiver operating characteristic curve analysis for discriminating between H&Y stage 3 and 4 was performed for LMS; the area under the curve was 0.774 (95% confidence interval=0.696-0.851).
Conclusions
LMS was strongly associated with multiple domains of clinical characteristics, especially gait velocity and the Barthel index. Our study also suggested that LMS can be a predictor of PD progression.
Keywords: Parkinson disease, leg muscle strength, gait, activity of daily living
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disease characterized by bradykinesia, muscular rigidity, resting tremor, and postural instability (1). PD motor symptoms are thought to be caused by loss of dopaminergic neurons in the substantia nigra pars compacta with the presence of Lewy bodies, intracellular inclusions containing the synaptic protein α-synuclein. The number of PD patients is expected to double from 6.9 million in 2015 to 14.2 million in 2040 globally (2). Because aging is the largest risk factor for developing PD, late-onset PD is expected to increase in the future (3).
In the general population, leg muscle strength (LMS) plays an important role in activities of daily living (ADL), including walking and standing up. LMS decreases progressively with age (4,5). A reduced LMS is associated with slower walking, falls, and trauma. In addition, a reduced leg muscle mass is associated with sarcopenia and frailty in elderly persons (6,7).
Koller and Kase first reported the quantitative assessment of leg muscle weakness in early-stage PD patients with Hoehn and Yahr (H&Y) stages 1 and 2 (8). It is now well-established that LMS is decreased in early- to moderate-stage PD. Furthermore, LMS is reported to be associated with the ability to get out of a chair (9), bradykinesia (10), and falls (11-13) in early- to moderate-stage PD. It has also been reported that leg muscle weakness is correlated a reduced gait velocity (11,14,15). Thus, leg muscle exercises for PD are effective for increasing the LMS, stabilizing balance, improving the motor function, and preventing falls (16,17).
Advanced PD patients with H&Y stage ≥3 have gait disturbance (small steps, slowness, and hesitation) and postural instability. In addition, dressing and other self-care tasks may become more difficult and increase the risk of falls (18). PD is associated with a high risk of hip fractures caused by falls owing to gait disturbance and balance impairment (19).
Since the significance of LMS evaluation in patients with advanced PD has not been fully investigated, we studied the relationships between LMS and gait performance, ADL, and the cognitive function in advanced PD patients.
Materials and Methods
Study design
A single-center, observational study was conducted to assess the relationships between leg muscle strength and demographic characteristics and other clinical symptoms in advanced PD patients. This study was approved by the Institutional Review Board of Fukuseikai Minami Hospital (registry number: 201901), and the protocol was consistent with the principles of the Declaration of Helsinki.
Subjects
The medical records of 132 inpatients with PD of H&Y stages 3 and 4 admitted to Fukuseikai Minami Hospital from January 2013 to October 2018 were retrospectively reviewed. The medical institution provided rehabilitation therapies and a physical evaluation for patients with neurodegenerative disorders.
All patients met the United Kingdom Parkinson's Disease Society Brain Bank clinical diagnostic criteria (steps 1 and 2) (20). Studied patients met the following inclusion criteria: 1) stable medication usage for 4 weeks; 2) H&Y stages 3 or 4 during on time if patients had wearing-off phenomenon; and 3) ability to walk with or without a walking aid. The exclusion criteria, as assessed by a medical interview and physical examination, were 1) significant musculoskeletal conditions, including spondylosis or arthropathy that would interfere with testing; 2) diagnoses of other neurologic diseases (e.g., stroke, traumatic brain injury, or peripheral neuropathies); 3) other concomitant serious illnesses (e.g. cardiovascular disorders, psychiatric disorders) that precluded participation; or 4) difficulty of assessment due to severe freezing of gait. All patients were assessed during on time if patients had wearing-off phenomenon. The levodopa equivalent dose (LED) was calculated according to the previously reported formula (21).
Assessments
LMS was evaluated using a Strength Ergo 240 (SE240; Mitsubishi, Tokyo, Japan), in terms of the moment of maximum strength or peak torque (PT) of the extensor muscles of the lower limbs. The SE240 is a safe and simple tool for measuring the LMS, even in patients with severe motor impairment, because its seat has a backrest and fixed trunk with a seat belt (22,23). The muscle strength of the bilateral lower limbs was measured in the sitting position with the hips flexed at 110°. The position of the seat was adjusted to the angle of knee flexion at 20°. On the day of the assessment, the participants performed warm-up at submaximal levels to ensure a thorough familiarization with the equipment. Maximum PT (Nm) was recorded by a series of one performed 5 times at a rotary speed of 20 rounds/min. The average value of the maximum PT of the two test trials was calculated.
The 10-meter walk test was used to assess gait performance. The participants walked a total distance of 14 meters, with start time at the 2-meter mark and end-time at the 12-meter mark to remove the acceleration and deceleration phases. The time required was measured with a digital stopwatch, and the number of steps taken while walking was recorded. The average value of the required time and the number of steps of the two test trials was calculated. Stride length, stride time, gait velocity, and cadence were also calculated from the measured values.
Patients' gait performance was also evaluated with the timed up and go (TUG) test using an armchair. The turning point was marked on the floor 3 meters from the chair. The participants stood up from a chair, walked past a horizontal line marked with tape on the floor 3 meters from the start, turned around, walked back, and sat down at their fastest possible speed. Two trials were performed, and the time required was measured with a digital stopwatch and the results of the last two trials were averaged. Walking aids (stick or walker) were permitted in this test.
The Mini-Mental State Examination (MMSE) was used to assess global cognition, with scores ranging from 0 to 30 points.
The Barthel index, which is summed to give a score of 0 to 100 was used to measure the performance in ADL.
Statistical analyses
The demographic and clinical differences were compared between H&Y stage groups using the chi-square test and Mann-Whitney U test. The relationships between LMS [average PT-to-body weight ratio (PT/BW)] and clinical characteristics were assessed using Spearman's rank correlation analysis. Sex as a categorical variable was coded as 0 representing men and 1 representing women. A multiple linear regression analysis with stepwise elimination was used to identify the significant predictors of LMS and gait parameters (stride length, stride time, gait velocity, cadence, and TUG), including the age, sex, disease duration, Barthel index, and MMSE score. A receiver operating characteristic (ROC) curve analysis for discriminating between H&Y stages using the LMS was performed. The optimal cut-off values were defined as the point at which the Youden index (sensitivity+specificity-1) was maximal. A p value of <0.05 was considered significant.
All data were statistically analyzed using the SPSS software program, version 23 for Windows (SPSS, Chicago, USA).
Results
The demographic and clinical characteristics of the 132 PD patients are summarized in Table 1. The 132 patients were divided into 2 groups according to the H&Y stages as follows: one including 88 patients with H&Y3 and the other including 44 patients with H&Y4. The H&Y4 group had more women, an older age, a longer disease duration, lower Barthel index, and lower MMSE score than the H&Y3 group. There were no significant differences between the groups in the age of disease onset, body mass index (BMI), or LED.
Table 1.
Differences in Demographic Characteristics, Leg Muscle Strength and Gait Performance between Patients by Parkinson Disease Severity.
| H&Y 3 (n=88) |
H&Y 4 (n=44) |
p value | ||||
|---|---|---|---|---|---|---|
| Female, n (%) | 35 (39.8) | 26 (59.1) | 0.036 | |||
| Age (years) | 71.9±5.6 | 76.2±6.1 | 0.007 | |||
| Age of disease onset (years) | 62.8±11.2 | 65.6±7.3 | 0.530 | |||
| Disease duration (years) | 9.1±5.7 | 10.4±4.7 | 0.046 | |||
| BMI (kg/m2) | 22.0±3.5 | 21.2±3.6 | 0.238 | |||
| Barthel index | 80.1±16.5 | 61.1±21.5 | <0.001 | |||
| Levodopa equivalent dose (mg/day) | 784±352 | 837±318 | 0.305 | |||
| MMSE score | 26.5±3.1 | 24.3±4.2 | 0.002 | |||
| Weaker leg PT/BW (Nm/kg) | 1.13±0.52 | 0.66±0.29 | <0.001 | |||
| Stronger leg PT/BW (Nm/kg) | 1.29±0.55 | 0.79±0.30 | <0.001 | |||
| Average PT/BW (Nm/kg) | 1.21±0.53 | 0.73±0.29 | <0.001 | |||
| Stride length (cm) | 54.1±16.9 | 42.6±15.5 | <0.001 | |||
| Stride time (s) | 0.48±0.13 | 0.53±0.11 | <0.001 | |||
| Gait velocity (m/s) | 1.19±0.46 | 0.83±0.32 | <0.001 | |||
| Cadence (steps/min) | 129.9±23.6 | 116.7±18.1 | <0.001 | |||
| Timed up and go test (s) | 16.2±13.0 | 26.4±17.4 | <0.001 |
The chi-square test and Mann-Whitney U test were used. Data are presented as the means±standard deviation. H&Y: Hoehn and Yahr stage, BMI: body mass index, MMSE: Mini-Mental State Examination, PT/BW: peak torque-to-body weight ratio
The data for LMS and the gait parameters of the two groups are shown in Table 1. In the H&Y4 patients, the weaker leg, stronger leg, and average PT/BW values were all lower than those in the H&Y3 patients. In addition, the H&Y4 patients had a shorter stride, longer time, and slower gait velocity than the H&Y3 patients.
Correlation analyses of LMS and clinical characteristics are shown in Table 2 and Figure. The results showed that LMS was negatively correlated with sex (r=-0.369), age (r=-0.290), age of disease onset (r=-0.258), and H&Y stage (r=-0.447) and positively correlated with the Barthel index (r=0.451) and MMSE score (r=0.304). Furthermore, there were correlations between LMS and all gait parameters (stride length: r=0.562, stride time: r=-0.387, gait velocity: r=0.611, cadence: r=0.388, TUG: r=-0.652). There were no significant correlations between LMS and disease duration, BMI, or LED.
Table 2.
Results of a Correlation Analysis of Leg Muscle Strength and Clinical Characteristics.
| All patients (n=132) | r | p value | ||
|---|---|---|---|---|
| Sex (male: 0, female: 1) | -0.369 | <0.001 | ||
| Age | -0.290 | 0.001 | ||
| Age of disease onset | -0.258 | 0.003 | ||
| Disease duration | 0.149 | 0.087 | ||
| H&Y stage | -0.447 | <0.001 | ||
| BMI | 0.033 | 0.704 | ||
| Barthel index | 0.451 | <0.001 | ||
| Levodopa equivalent dose | 0.076 | 0.384 | ||
| MMSE score | 0.304 | <0.001 | ||
| Stride length | 0.562 | <0.001 | ||
| Stride time | -0.387 | <0.001 | ||
| Gait velocity | 0.611 | <0.001 | ||
| Cadence | 0.388 | <0.001 | ||
| Timed up and go test | -0.652 | <0.001 |
Spearman’s rank correlation analysis was performed.
Figure.
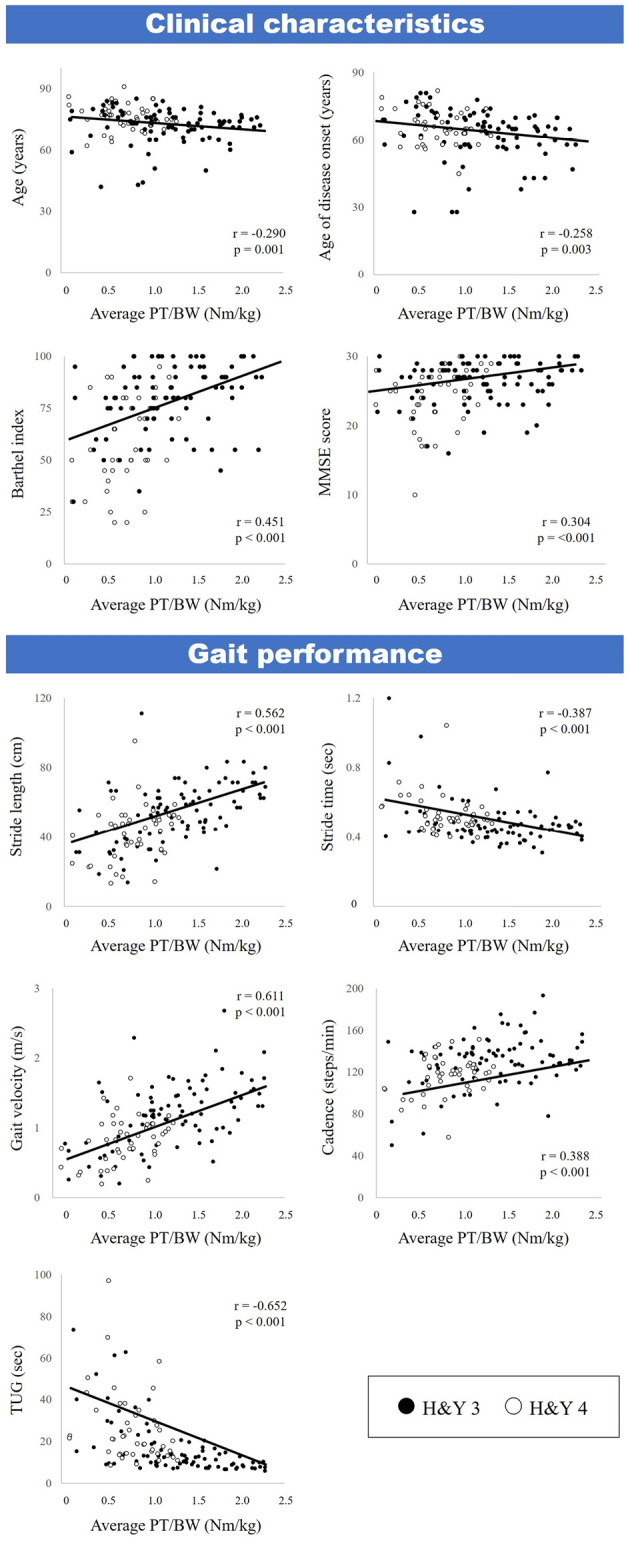
A correlation analysis of the leg muscle strength and demographic characteristics. Black circles indicate H&Y 3 patients, and white circles indicate H&Y 4 patients. Spearman’s rank correlation analysis was performed.
In the multivariate linear regression model, gait velocity (β=0.377, p<0.001), Barthel index (β=0.281, p<0.001), sex (β=-0.187, p=0.012) and disease duration (β=-0.155, p=0.030) were independently associated with LMS (Table 3).
Table 3.
Results of a Multivariate Linear Regression Analysis of the Leg Muscle Strength and Gait Performance, Including the Age, Sex, Disease Duration, Barthel Index, and MMSE Score.
| Unstandardized B | β | 95%CI | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Gait velocity | 0.431 | 0.377 | 0.242, 0.621 | <0.001 | ||||
| Barthel index | 0.007 | 0.281 | 0.003, 0.011 | <0.001 | ||||
| Sex | -0.194 | -0.187 | -0.344, -0.044 | 0.012 | ||||
| Disease duration | -0.015 | -0.155 | 0.001, 0.028 | 0.030 |
R=0.667, adjusted R2=0.427
The AUC for average PT/BW when comparing H&Y 3 and 4 patients was 0.774 (95% confidence interval=0.696-0.851). The optimal cut-off value was 1.045 using the Youden index, and the sensitivity and specificity were 0.602 and 0.886, respectively.
Discussion
This is the first study to investigate the relationship between LMS and gait performance, ADL, and the cognitive function in advanced PD patients with H&Y stages 3 and 4. LMS was shown to be strongly associated with not only gait performance but also patients' ADL. Previous studies in PD patients have not focused on LMS or ADL except for gait performance, probably because the subjects had mild to moderate PD, so their ADL was often independent.
The present study results showed that, in advanced PD patients, LMS decreased with increasing severity of the disease. Lima et al. (24) showed that the muscle power of the trunk, hip, knee flexors and extensors, and ankle plantar flexors was lower in L-dopa-naïve early-stage PD (H&Y 1 and 2) than in the control group. Nogaki et al. (25) reported that leg muscle weakness was seen more often on the affected side as disease progression in early-stage PD (H&Y 1 and 2) than less affected side. Nocera et al. (26) demonstrated that LMS (knee extensor muscles) was negatively correlated with H&Y stage (spearman rho=-0.484, p=0.002). Our results support these findings showing that leg muscle weakness was seen in early-stage PD and worsened with disease progression.
The pathophysiology of reduced muscle strength in PD is still poorly understood, although several mechanisms have been suggested. Reduced cortical activation of the muscles occurs due to the nigral dopaminergic deficit, resulting in an increase in tonic inhibition of the thalamus. In addition to motor circuit, motor-cognitive neural network may also contribute the reduced LMS. Impaired motor unit recruitment can cause abnormal muscle activation patterns, altered agonist burst frequency and intensity, and a reduced torque generation rate (19,27,28). Some reports have shown that anti-parkinsonian drug therapy (15) and subthalamic nucleus deep brain stimulation (29,30) increased LMS. These results suggest that muscle weakness can be caused by a deficit of central origin in the pathophysiology of PD. In addition, muscle strength in PD may also be associated with bradykinesia (27,28), malnutrition (31), and physical inactivity.
Three previous studies reported that LMS was correlated with gait velocity in PD. Pedersen et al. (14) reported that dorsiflexor strength and the 10-meter walk test were evaluated in H&Y stage ≤3 PD compared with age-matched controls. Concentric isokinetic strength was correlated well with gait velocity only in men. In addition, the stride length and stride frequency increased with gait velocity; however, these findings were not seen in women or controls. They concluded that impaired LMS might be an important factor for adequate gait performance. In contrast, the present study clearly identified a correlation between LMS and gait performance in advanced PD. Nallegowda et al. (15) showed that isokinetic ankle muscle strength was positively correlated with gait velocity in the on (r=0.393, p<0.05) and off states (r=0.397, p<0.05) in subjects with an average H&Y stage of 2.7. Allen et al. (11) examined leg extensor muscle strength measured by a leg press machine in mild to moderate PD. They found that a reduced muscle power was related to reduced gait velocity and an increased fall risk. Compared with previous studies, the participants in the present study had a higher H&Y stage, older age, and longer disease duration. Similar to the present study, LMS was correlated with gait velocity in patients reported in the previous literature (11,14,15). Furthermore, the present study showed correlations between LMS and ADL and the cognitive function. The association between LMS and the cognitive function in elderly people has been investigated (32,33), but not in PD patients specifically. In the multivariate linear regression model, gait velocity, Barthel index, sex, and disease duration were independently associated with LMS. These findings suggested that LMS is correlated with not only localized leg dysfunction but also systemic disease severity. Thus, LMS may be a valuable rating scale for assessing the severity in advanced PD.
Several limitations associated with the present study warrant mention. First, this was a single-center, retrospective design, and we did not have a control group for a comparison. Second, only advanced PD patients with or without walking aids were analyzed, which might have led to selection bias. Third, patients were not evaluated with more disease-specific assessment batteries, such as the Montreal Cognitive Assessment for the assessment of the cognitive function and Movement Disorders Society Unified Parkinson's Disease Rating Scale for the assessment of motor symptoms. In addition, neuropsychiatric symptoms such as depression, anxiety and apathy, which can affect muscle strength measurement, were not evaluated. Fourth, each patient was evaluated cross-sectionally, so longitudinal changes in the weakness of lower limbs were not evaluated. Fifth, only the lower limbs, not the whole body, were evaluated. Axial muscles, such as the paraspinal muscles, which are important for gait and posture, were not studied. Sixth, information on other factors potentially affecting gait performance, such as daily meal contents and the amount of daily exercise, was not examined in this study. Large-scale studies are needed to better understand the mechanisms underlying LMS in PD.
Conclusion
The present results suggest that LMS is strongly associated with multiple domains of clinical features, especially gait performance. Thus, LMS may be a valuable tool for the assessment of gait performance and ADL in advanced PD patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181-184, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey ER, Bloem BR. The Parkinson pandemic - a call to action. JAMA Neurol 75: 9-10, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev 14: 19-30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrucci L, Guralnik JM, Buchner D, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the women's health and aging study. J Gerontol A Biol Sci Med Sci 52: M275-M285, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Med Sci 67: 28-40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc 64: 144-150, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreland JD, Richardson JA, Goldsmith CH, et al. Muscle weakness and falls in older adults: a systematic review and metaanalysis. J Am Geriatr Soc 52: 1121-1129, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Koller W, Kase S. Muscle strength testing in Parkinson's disease. Eur Neurol 25: 130-133, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Inkster LM, Eng JJ, MacIntyre DL, et al. Leg muscle strength is reduced in Parkinson's disease and related to the ability to rise from a chair. Mov Disord 18: 157-162, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen NE, Canning CG, Sherrington C, et al. Bradykinesia, muscle weakness and reduced muscle power in Parkinson's disease. Mov Disord 24: 1344-1351, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Allen NE, Sherrington C, Canning CG, et al. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson's disease. Parkinsonism Relat Disord 16: 261-264, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Durmus B, Baysal O, Altinayar S, et al. Lower extremity isokinetic muscle strength in patients with Parkinson's disease. J Clin Neurosci 17: 893-896, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Moreno Catalá M, Woitalla D, Arampatzis A. Central factors explain muscle weakness in young fallers with Parkinson's disease. Neurorehabil Neural Repair 27: 753-759, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen SW, Oberg B, Larsson LE, et al. Gait analysis, isokinetic muscle strength measurement in patients with Parkinson's disease. Scand J Rehabil Med 29: 67-74, 1997. [PubMed] [Google Scholar]
- 15.Nallegowda M, Singh U, Handa G, et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: a pilot study. Am J Phys Med Rehabil 83: 898-908, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Chung CL, Thilarajah S, Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson's disease: a systematic review and meta-analysis. Clin Rehabil 30: 11-23, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Mak MK, Wong-Yu IS, Shen X, et al. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13: 689-703, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Evans JR, Mason SL, Williams-Gray CH, et al. The natural history of treated Parkinson's disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 82: 1112-1118, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Ali Hosseinzadeh, Malahat Khalili, Behnaz Sedighi, et al. Parkinson's disease and risk of hip fracture: systematic review and meta-analysis. Acta Neurol Belg 118: 201-210, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Daniel SE, Lees AJ. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm (Suppl) 39: 165-172, 1993. [PubMed] [Google Scholar]
- 21.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649-2653, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara T, Liu M, Chino N. Effect of pedaling exercise on the hemiplegic lower limb. Am J Phys Med Rehabil 82: 357-363, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Yamada S, Omori Y, et al. The relationships between muscle strength torque measured by pedaling type isokinetic machine and that by conventional isokinetic dynamometer. Rigakuryouhougaku 28: 338-342, 2001(in Japanese). [Google Scholar]
- 24.Lima LO, Cardoso F, Teixeira-Salmela LF, et al. Work and power reduced in L-dopa naïve patients in the early-stages of Parkinson's disease. Arq Neuropsiquiatr 74: 287-292, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Nogaki H, Kakinuma S, Morimatsu M. Muscle weakness in Parkinson's disease: a follow-up study. Parkinsonism Relat Disord 8: 57-62, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Nocera JR, Buckley T, Waddell D, et al. Knee extensor strength, dynamic stability, and functional ambulation: are they related in Parkinson's disease? Arch Phys Med Rehabil 91: 589-595, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berardelli A, Rothwell JC, Thompson PD, et al. Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131-2146, 2001. [DOI] [PubMed] [Google Scholar]
- 28.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson's disease: a review of potential mechanisms. Parkinsons Dis 2012: 124527, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturman MM, Vaillancourt DE, Metman LV, et al. Effects of five years of chronic STN stimulation on muscle strength and movement speed. Exp Brain Res 205: 435-443, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Vaillancourt DE, Prodoehl J, Sturman MM, et al. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson's disease. Mov Disord 21: 50-58, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaafar AF, Gray WK, Porter B, et al. A cross-sectional study of the nutritional status of community-dwelling people with idiopathic Parkinson's disease. BMC Neurol 10: 124, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamoto H, Yoshitake Y, Takai Y, et al. Knee extensor strength is associated with Mini-Mental State Examination scores in elderly men. Eur J Appl Physiol 112: 1945-1953, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Pedrero-Chamizo R, Albers U, Tobaruela JL, et al. Physical strength is associated with Mini-Mental State Examination scores in Spanish institutionalized elderly. Geriatr Gerontol Int 13: 1026-1034, 2013. [DOI] [PubMed] [Google Scholar]


