Abstract
Rifapentine is a long-acting rifamycin which may be useful for intermittent drug therapy against tuberculosis. In this study we measured the efficacies of rifapentine-containing intermittent drug regimens for preventive therapy using the Cornell mouse model of latent tuberculosis. We infected groups of mice intravenously with Mycobacterium tuberculosis and then treated them with isoniazid and pyrazinamide for 12 weeks according to the Cornell latency development protocol. After a 4-week interval of no treatment, experimental preventive therapy was administered by esophageal gavage for 12 or 18 weeks. After equilibration and dexamethasone amplification treatment, mouse organs were analyzed by quantitative colony counts to measure the effectiveness of therapy. Our results showed that once-weekly isoniazid plus rifapentine combination therapy for 18 weeks was an effective preventive regimen with sterilizing potency and bacillary load reduction comparable to those of daily isoniazid therapy for 18 weeks. Monotherapy with rifapentine weekly or fortnightly or with rifampin twice weekly for up to 18 weeks did not offer advantages in reducing bacillary load or in sterilizing organs compared to the effects of a placebo. These results with the Cornell mouse model indicate that once-weekly, short-course preventive therapy with isoniazid plus rifapentine is effective and may warrant investigation in humans with latent tuberculosis infection.
Worldwide, tuberculosis remains a leading cause of death from any single infectious agent. There are approximately 8 million active cases of tuberculosis per year, with 3 million deaths annually, while about 1.7 billion people (one-third of the world’s population) are estimated to harbor latent Mycobacterium tuberculosis infection (18). Individuals with latent tuberculosis carry a 2 to 23% lifetime risk of developing reactivation disease later in life (25). In addition, immunosuppressive conditions including human immunodeficiency virus (HIV) infection dramatically increase the risk of reactivation of latent tuberculosis (1, 24, 26). Isoniazid (INH) treatment for 6 or 12 months has been recommended for many patients with latent tuberculosis on the basis of a series of randomized, placebo-controlled clinical trials which showed that it reduces the risk of active tuberculosis by 65% or more in immunocompetent individuals (11). Because adherence is poor and may limit the effectiveness of such prolonged treatment courses, shorter-course regimens have been studied as a means of improving efficacy. Four recent trials with HIV-infected, tuberculin-positive patients have shown the efficacy of short-course rifampin-based regimens for the prevention of tuberculosis. Whalen et al. (32) demonstrated that a 3-month course of INH plus rifampin (RIF) daily was as efficacious as a 6-month regimen of INH daily. Gordin and associates (12) achieved better adherence rates with only 2 months of preventive therapy with RIF plus pyrazinamide (PZA) daily and showed that this regimen was as effective in preventing active tuberculosis as treatment with INH daily for 12 months (12). Two additional studies with HIV-infected adults showed that treatment with RIF with PZA twice weekly for 2 to 3 months was as effective in the prevention of tuberculosis as treatment with INH twice weekly for 6 months (14, 23). Although significantly shorter in duration, the overall effectiveness of these RIF-containing regimens may still be limited by the innate difficulty of adherence to self-supervised therapy.
Rifapentine (RPT) is a long-acting rifamycin which is highly active against M. tuberculosis and which may be useful for intermittent, supervised dosing for preventive therapy (2–4, 10, 15). While the bioactivity of RIF is significantly reduced in animal models when it is taken three times weekly rather than six times weekly, significant bactericidal activity is still observed in mice treated with 10 mg of RPT per kg of body weight up to once fortnightly (7, 13, 17). Indeed, in M. tuberculosis-infected mice, RPT administered at a dose of 10 mg/kg once weekly was as effective as 10 mg of RIF per kg given daily (7, 17). Thus, RPT may be effective as an agent administered once weekly or even fortnightly in humans, and treatment with RPT may reduce the supervision costs of directly observed preventive therapy programs.
Previous studies of RPT-containing regimens for preventive chemotherapy against reactivation tuberculosis were conducted with a mouse model of chronic tuberculosis (5, 17). However, in this model mice do not enter a state in which bacilli appear to be absent. In contrast, the Cornell mouse model, originally described by investigators from Cornell University Medical School, generates an apparent sterile state in mouse tissues and may represent a closer approximation of latent tuberculosis in humans (6, 8, 20). Indeed, the two models often provide quite different assessments of the efficacies of preventive regimens (8, 17, 19). The purpose of this study was to measure the efficacies of RPT-containing intermittent drug regimens for preventive therapy against latent M. tuberculosis infection with the Cornell mouse model.
MATERIALS AND METHODS
Antibiotics.
RPT was provided by Hoechst-Marion-Roussel, Inc. (Kansas City, Mo.). INH, RIF, carbenicillin, polymyxin B, and trimethoprim were purchased from the Sigma Chemical Co., (St. Louis, Mo.), and amphotericin B was obtained from GIBCO Laboratories, (New York, N.Y.). PZA was provided by Wyeth-Ayerst-Lederle.
Bacterial cultivation.
The virulent CDC1551 (also known as CSU93 or Oshkosh) strain of M. tuberculosis (30) was grown at 37°C on Löwenstein-Jensen medium or in roller bottles in 7H9–albumin-dextrose complex (7H9-ADC) broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% glycerol and 0.05% Tween 80. For animal inoculation, liquid cultures were declumped by brief bath sonication and settling and were diluted in complete 7H9 medium. Colony counts from mouse organs were performed by using Middlebrook 7H10-ADC agar plates, made selective by adding carbenicillin, polymyxin B, trimethoprim, and amphotericin B to final concentrations of 100 μg/ml, 200 U/ml, 20 μg/ml, and 10 μg/ml, respectively.
Cornell mouse model and preventive therapies tested.
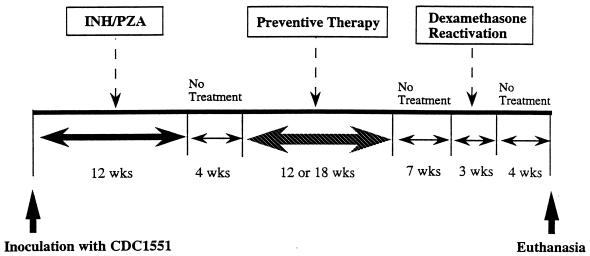
We used the unabridged Cornell mouse model as originally described to induce the apparent “sterile state” of tuberculosis (6, 8, 20). Outbred, female Swiss-Webster mice (age, 5 weeks; weight, 18 to 20 g) were purchased from Harlan Sprague-Dawley Inc. (Indianapolis, Ind.), housed in a pathogen-free, biosafety level 3 environment within microisolator cages, and allowed to acclimate to their new environment for 2 to 7 days prior to infection. Food and water were provided ad libitum. Infections were produced by intravenous inoculation into a tail vein of 0.1 ml of a cell suspension containing a declumped, diluted M. tuberculosis preparation whose titer was known (3.95 × 106 CFU per mouse). On the same day mice began receiving dietary treatment with INH (0.0125% [wt/wt] in chow) and PZA (0.5% [wt/wt] in chow) for a 12-week latency induction period (Fig. 1). By estimating a consumption of 4 g of chow/mouse/day, these concentrations resulted in daily doses of 25 mg of INH per kg and 1,000 mg of PZA per kg. Following the antibiotic-mediated latency induction, mice were kept untreated for 4 weeks to permit elimination of the INH and PZA and entry into a stable state of dormant infection. During the 4-week interval immediately following INH-PZA withdrawal, organs from mice have been shown to be culture negative by a number of methodologies including inoculation of tissue homogenates into guinea pigs (6, 8, 20).
FIG. 1.
Schematic diagram of the experimental design for preventive therapy of tuberculosis in the Cornell mouse model.
After the 12-week latency induction and a 4-week period of equilibration in the Cornell model sterile state, groups of eight mice were treated for 12 weeks with INH (25 mg/kg) daily, RIF (10 mg/kg) twice weekly, or RPT (10 mg/kg) twice weekly. Additional groups received 18 weeks of treatment with INH (25 mg/kg) daily, RIF (10 mg/kg) twice weekly, RPT (10 mg/kg) once weekly, RPT (10 mg/kg) once fortnightly, and INH (25 mg/kg) plus RPT (10 mg/kg) once weekly. Control groups received phosphate-buffered saline twice weekly. Drugs were administered by esophageal gavage; “daily” regimens were given 6 days per week. Following 12 or 18 weeks of preventive therapy, the mice were housed with no drug treatment for a 7-week interval to permit reactivation of the remaining mycobacteria. Finally, a 3-week course of intraperitoneal dexamethasone treatment (0.5 mg/mouse, six times a week) was given to amplify mycobacteria which may have reactivated. At the end of the experiment, following a 4-week interval, mice were killed and quantitative colony counts were performed.
Organ CFU counts.
Counting of the numbers of CFU of viable bacilli in the lungs and spleens was performed as described previously (21). Briefly, the lungs and spleens were removed aseptically and were homogenized in 1.0 ml of complete 7H9 broth in a Ten Broeck glass grinder. At least four serial 10-fold dilutions of the homogenates were plated onto selective 7H10 agar plates, and each dilution was plated in duplicate. Colony counts were recorded after incubation at 37°C in a CO2 incubator for 5 weeks. A culture was considered negative if no colonies appeared on plates inoculated with undiluted homogenates.
Statistical analysis.
Results were analyzed by the t test for samples of unequal variance or by the chi-square test. A P value of less than 5% denoted statistical significance. No adjustments were made for multiple comparisons.
RESULTS
Sterilizing potencies of preventive therapy regimens in the Cornell model of latent M. tuberculosis infection.
Sterilizing potency is a simple and reliable endpoint by which to evaluate the efficacy of a preventive therapy regimen. In mice receiving daily INH therapy for 12 weeks, negative cultures were observed in three of eight lung specimens and one of eight spleen specimens (Table 1). In contrast, neither RIF nor RPT monotherapy twice weekly for 12 weeks yielded negative cultures for either the spleens or the lungs. These differences in sterilizing potency at 12 weeks failed to reach statistical significance by the chi-square test.
TABLE 1.
Proportion of negative cultures in organ homogenates from mice treated with 12- or 18-week courses of preventive therapy
| Preventive therapy regimen | No. of negative cultures/no. of positive cultures (% of organs sterilized)
|
|
|---|---|---|
| Spleen | Lung | |
| 12-wk course | ||
| Placebo twice weekly | 1/7 (12.5) | 0/8 (0) |
| INH (25 mg/kg) daily | 3/5 (37.5) | 1/7 (12.5) |
| RIF (10 mg/kg) twice weekly | 0/7 (0) | 0/7 (0) |
| RPT (10 mg/kg) twice weekly | 0/8 (0) | 0/8 (0) |
| 18-wk course | ||
| Placebo, twice weekly | 1/6 (14.3) | 0/7 (0) |
| INH (25 mg/kg) daily | 4/4 (50.0) | 6/2 (75.0)a |
| RIF (10 mg/kg) twice weekly | 0/6 (0) | 0/6 (0) |
| RPT (10 mg/kg) once weekly | 0/6 (0) | 1/6 (14.3) |
| RPT (10 mg/kg) once fortnightly | 2/5 (28.6) | 2/5 (28.6) |
| INH + RPT once weekly | 5/3 (62.5) | 5/3 (62.5)b |
Significantly lower than the placebo group (P < 0.005).
Significantly lower than the placebo group (P < 0.02).
In contrast, both the lungs and spleens of five of eight (62.5%) mice treated with the combination of INH plus RPT for 18 weeks showed sterilization. Additionally, we found that an 18-week course of daily INH preventive therapy was sterilizing for the lungs and spleens of four of eight (50%) and six of eight (75%) mice, respectively. There were statistically significant differences in the proportion of negative cultures of lung specimens between mice in the placebo arm and mice in the arms receiving daily INH therapy (P < 0.005) or weekly INH plus RPT therapy (P < 0.02). Sterilization was observed in both the lungs and spleens of two of seven (28.6%) mice treated with RPT monotherapy fortnightly for 18 weeks. However, cultures of the lungs and spleens of zero of seven and one of seven mice receiving weekly RPT preventive therapy were negative, respectively. Hence, weekly INH plus RPT combination therapy appeared to be better than therapy with RPT alone administered weekly or fortnightly in achieving organ sterilization, although these differences were not statistically significant. Compared to the weekly INH-RPT regimen and daily INH regimen, the twice-weekly RIF regimen appeared to be less effective because we did not observe sterilization in any organ homogenates from mice treated with RIF alone.
Quantitative bacterial infectious burden per organ following preventive therapy for Cornell model of latent M. tuberculosis infection.
As an alternative endpoint for preventive therapy efficacy after 12 or 18 weeks, we also quantitated the bacterial CFU burdens per organ. As shown in Table 2, in this experiment placebo treatment resulted in 3.86 ± 1.84 and 3.24 ± 2.20 log10 CFU counts in the spleens at 12 and 18 weeks, respectively, and 5.56 ± 1.36 and 5.77 ± 0.11 log10 CFU counts in the lungs at 12 and 18 weeks, respectively. The organ CFU counts confirmed that the combination of INH and RPT administered weekly for 18 weeks was effective, resulting in 1.46 ± 2.04 log10 CFU counts in the spleen and 1.78 ± 2.49 log10 CFU counts in the lung. The log10 CFU counts in the lungs of mice treated with INH plus RPT were significantly lower than those in the lungs of the mice in the placebo group (P < 0.05). We found no significant difference in the organ CFU counts between the weekly RPT plus INH group and the daily INH monotherapy group. However, when compared with RPT monotherapy, combination therapy with RPT plus INH was significantly more potent in reducing the organ bacterial burden over 18 weeks (P < 0.05). Monotherapy with INH (25 mg/kg) daily for 18 weeks also resulted in significant reductions of organ bacillary loads, with 1.93 ± 2.11 and 0.71 ± 1.34 log10 CFU counts in spleens and lungs, respectively. A statistically significant difference was observed in the CFU counts between the INH arm and the placebo arm (P < 0.001). The CFU counts in the spleens and lungs of mice receiving a 12-week course of INH daily showed approximately 1.5- and 2.0-log-unit reductions, respectively, compared with those in the spleens and lungs of mice in the placebo arm; however, these differences did not achieve statistical significance by the t test. Using bacillary count reduction as the endpoint, we found that in comparison with the 12-week INH regimen, INH treatment for 18 weeks showed a distinct superiority, with a reduction in lung CFU counts of greater than 2.5 log units (0.71 ± 1.34 versus 3.56 ± 2.10; P < 0.01).
TABLE 2.
CFU counts in organ homogenates from mice treated with 12- or 18-week courses of preventive therapy
| Preventive therapy regimen | Log10 CFU count
|
|
|---|---|---|
| Spleen | Lung | |
| 12-wk course | ||
| Placebo twice weekly | 3.86 ± 1.84 | 5.56 ± 1.36 |
| INH (25 mg/kg) daily | 2.37 ± 2.01 | 3.56 ± 2.10 |
| RIF (10 mg/kg) twice weekly | 4.26 ± 0.62 | 5.18 ± 0.69 |
| RPT (10 mg/kg) twice weekly | 3.49 ± 0.96 | 4.91 ± 0.73 |
| 18-wk course | ||
| Placebo, twice weekly | 3.24 ± 2.20 | 5.77 ± 0.11 |
| INH (25 mg/kg) daily | 1.93 ± 2.11 | 0.71 ± 1.34a |
| RIF (10 mg/kg) twice weekly | 4.11 ± 0.40 | 5.39 ± 0.49 |
| RPT (10 mg/kg) once weekly | 3.63 ± 1.18 | 4.67 ± 2.35 |
| RPT (10 mg/kg) once fortnightly | 2.13 ± 2.15 | 2.86 ± 3.14 |
| INH + RPT once weekly | 1.46 ± 2.04b | 1.78 ± 2.49ab |
Significantly lower than the placebo group (P < 0.005).
Significantly lower than the group of mice treated with RPT once weekly (P < 0.05).
In contrast, twice-weekly RIF monotherapy showed no organ bacterial load reduction in comparison with the load in the organs of mice in the placebo arm even if the treatment period was extended to 18 weeks. In addition, the log10 CFU counts in the spleens and lungs of the mice treated with an 18-week course of once-weekly RPT therapy were as high as those in the spleens and lungs of the placebo-treated mice, with values of 3.63 ± 1.18 and 4.67 ± 2.35 log10 CFU, respectively. Fortnightly treatment with RPT over 18 weeks was modestly effective, resulting in 2.13 ± 2.15 log10 CFU counts in the spleen and 2.86 ± 3.14 log10 CFU counts in the lung, although this reduction was not statistically significant compared with the counts in the spleens and lungs of mice receiving placebo or weekly RPT therapy.
DISCUSSION
The Cornell model of murine tuberculosis has been shown to be useful for demonstrating and studying dormancy (20). Our protocol, although somewhat elaborate, is a direct extension of more than a decade of work of W. McDermott and colleagues in developing this latency model. Unlike the chronic tuberculosis model in which the numbers of viable tubercle bacilli and bacterial metabolic activity remain appreciable throughout, the Cornell mouse model produces a state of infection in which bacteria appear to be metabolically quiescent (6, 8). Indeed, the two models often provide different assessments of the efficacy of the same drug regimen. For example, a 6-week course of RIF monotherapy was effective in the chronic tuberculosis model (19), while RIF alone for 6 weeks was not active in the Cornell mouse model (8). Moreover, RIF plus PZA was superior to RIF alone in the chronic tuberculosis model (17), whereas RIF plus PZA and RIF alone had similar efficacies in the Cornell mouse model (8). These discrepancies may result from the differences in the growth rates of tubercle bacilli in the mouse model used. RPT has been tested previously in the chronic tuberculosis model (5, 17). Our study is the first assessment of the drug with the Cornell mouse model.
RIF has been reported to be effective in preventive therapy regimens in humans when it is used for 3 to 6 months alone or for 2 months in combination with PZA (14, 16). A somewhat unexpected result from this study with mice was the lack of efficacy of twice-weekly RIF therapy for 12 or 18 weeks as preventive therapy for latent M. tuberculosis infection. In this regard it would have been useful to have included daily RIF and twice-weekly INH plus RIF therapeutic arms for comparative purposes. Previous studies have shown that preventive therapy with RIF daily for 6 weeks is efficacious and has potency similar to those of RIF plus INH and RIF plus PZA when efficacy is evaluated in the Cornell mouse model (8) and that intermittently administered RIF is less active than RIF given daily in the chronic tuberculosis infection model (17). On the basis of these previous results, the poor potency of twice-weekly RIF therapy in our study is more likely due to intermittent administration of the drug rather than to the poor sterilizing activity of RIF in the Cornell mouse model. As the CDC1551 strain used in this study has been reported to be sensitive to RIF in vitro (this has also been confirmed in our laboratory), RIF resistance is unlikely to account for the poor efficacy of the intermittent RIF regimen (30).
The chronic murine tuberculosis model has demonstrated that the bactericidal activity of RPT given at 10 mg/kg twice weekly for 12 weeks was comparable to that of RIF given at 10 mg/kg six times weekly for 12 weeks (17). The efficacy of intermittent RPT stems from its favorable pharmacokinetics, with an elimination half-life five times longer than that of RIF (13), and prolonged in vitro activity of up to 4 weeks after a single exposure (22). Despite the previous favorable results with the chronic infection model, intermittent RPT monotherapy produced only a modest preventive therapy benefit in this study with the Cornell mouse model. Our Cornell mouse model data did not show that RPT tended to provide a better reduction of bacillary load than twice-weekly RIF therapy in both the 12- and 18-week treatment courses.
In these Cornell mouse model experiments, we used strain CDC1551, which is a recent clinical isolate of proven virulence in humans (30). As some recent studies with the Cornell mouse model with laboratory isolates have had to use a foreshortened latency induction period of less than 12 weeks to achieve bacterial reactivation (8), we reasoned that a highly virulent strain might be more likely to survive the full 12-week latency induction originally described (20). At the end of the experiment we used dexamethasone to induce an immunodeficient state, similar to that seen in nude mice, during which mycobacteria not eliminated by preventive therapy may amplify. In nude mice the response of M. tuberculosis infection to intermittent RPT regimens was less favorable than that in normal mice because virtually all nude mice had relapses within 12 weeks after the cessation of chemotherapy (5). Moreover, RPT monotherapy was also associated with the selection of RIF-resistant mutants in the mouse chronic tuberculosis model (13). These data may merit consideration in determining whether intermittent RPT monotherapy is appropriate for a fixed-duration preventive therapy regimen for immunosuppressed patients including those with HIV infection. Our data seem to suggest that fortnightly treatment with RPT over 18 weeks was more effective in reducing bacillary counts than once-weekly treatment with RPT for the same duration. This trend, which did not achieve statistical significance, was probably due to animal variability. The relatively small sizes of our mouse groups (six to eight animals) may have limited our ability to detect fine differences in efficacy among the different rifamycin monotherapy protocols.
This study’s major observation was that intermittent therapy with RPT plus INH given once weekly for 18 weeks provided both a high proportion of negative cultures of mouse organs (P < 0.02) and also an excellent bacillary load reduction (P < 0.05) compared with those for animals receiving placebo preventive therapy. The efficacy of weekly RPT plus INH therapy for 18 weeks was comparable to that of daily INH therapy for 18 weeks. A comparison of the outcomes between RPT alone versus RPT plus INH indicates that INH contributes significantly to the efficacy of the combination regimen. In fact, INH has been shown to be effective as intermittent therapy, despite its short half-life, even when it is given only once weekly (9). Unfortunately, a once-weekly INH monotherapy arm, which would have permitted an assessment of the relative contribution of INH to the success of the INH plus RPT regimen, was not included in our study. However, a previous study showed that once-weekly therapy with INH plus RPT eradicated M. tuberculosis from mice, while once-weekly therapy with INH plus RIF did not. Furthermore, the once-weekly regimens with INH were not as effective as the twice-weekly ones (29). Hence, there are data to support the conclusion that RPT is essential for the preventive efficacy of weekly therapy with RPT plus INH in combination.
Improved intermittent treatment regimens for both active tuberculosis and latent M. tuberculosis infection would have important public health implications. Improved intermittent treatment regimens might increase the rate of patient adherence in the unsupervised setting and would diminish the costs of supervised therapy. Our study indicates that, on the basis of its effectiveness in mice, RPT plus INH may be a promising candidate as a once-weekly preventive therapy regimen in humans. Human trials of weekly RPT plus INH therapy in the continuation phase of active tuberculosis therapy reveal that the combination is well tolerated, although its efficacy has not been fully established (27, 28, 31). In view of the fact that many of the adverse effects of short-course preventive therapy with RIF plus PZA are attributable to PZA, further study of RPT plus INH as an alternative, more tolerable preventive therapy regimen which offers weekly intermittent dosing may be warranted in humans.
ACKNOWLEDGMENTS
This work was supported by contract 200-93-0636 from the Centers for Disease Control and Prevention and by grants from the Heiser Foundation, the Johns Hopkins School of Public Health Faculty Development Program, and Hoechst-Marion-Roussel.
We thank Caryn Good for technical contributions and Jennifer Doetsch for helpful assistance in the preparation of the manuscript.
REFERENCES
- 1.Antonucci G, Girardi E, Raviglione M C, Ippolito G. Risk factors for tuberculosis in HIV-infected persons: a prospective cohort study. JAMA. 1995;274:143–148. doi: 10.1001/jama.274.2.143. [DOI] [PubMed] [Google Scholar]
- 2.Arioli V, Berti M, Carniti G, Randisi E, Rossi E, Scotti R. Antibacterial activity of DL473, a new semisynthetic rifamycin derivative. J Antibiot (Tokyo) 1981;34:1026–1029. doi: 10.7164/antibiotics.34.1026. [DOI] [PubMed] [Google Scholar]
- 3.Assandri A, Ratti B, Christina T. Pharmacokinetics of rifapentine, a new long lasting rifamycin, in the rat, the mouse and the rabbit. J Antibiot (Tokyo) 1984;37:1066–1069. doi: 10.7164/antibiotics.37.1066. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham A T, Coleman A J, Orme M L S, Park B K, Pearson N J, Short A H, Southgate P J. Antibacterial activity in serum and urine following oral administration in man of DL473 (a cyclopentyl derivative of rifampicin) Br J Clin Pharmacol. 1978;6:455P–456P. doi: 10.1111/j.1365-2125.1978.tb04626.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapuis L, Ji B, Truffot-Pernot C, O’Brien R J, Raviglione M C, Grosset J H. Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice. Am J Respir Crit Care Med. 1994;150:1355–1362. doi: 10.1164/ajrccm.150.5.7952564. [DOI] [PubMed] [Google Scholar]
- 6.de Wit D, Wootton M, Dhillon J, Mitchison A. The bacterial DNA content of mouse organs in the Cornell model of dormant tuberculosis. Tuber Lung Dis. 1995;76:555–562. doi: 10.1016/0962-8479(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon J, Dickinson J M, Guy J A, Ng T K, Mitchison D A. Activity of two long-acting rifamycins, rifapentine and FCE 22807, in experimental murine tuberculosis. Tuber Lung Dis. 1992;73:116–123. doi: 10.1016/0962-8479(92)90066-S. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon J, Dickinson J M, Sole K, Mitchison D A. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother. 1996;40:552–555. doi: 10.1128/aac.40.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson J M, Ellard G A, Mitchison D A. Suitability of isoniazid and ethambutol for intermittent administration in the treatment of tuberculosis. Tubercle. 1968;49:351–366. doi: 10.1016/s0041-3879(68)80016-9. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson J M, Mitchison D A. In vitro properties of rifapentine (MDL473) relevant to its use in intermittent chemotherapy of tuberculosis. Tubercle. 1987;68:113–118. doi: 10.1016/0041-3879(87)90026-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferebee S H. Controlled chemoprophylaxis trials in tuberculosis: a general review. Adv Tuberc Res. 1970;17:28–106. [PubMed] [Google Scholar]
- 12.Gordin F M and the CPCRA 004/ACTG 177 Investigators. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Preliminary analysis of short-course preventive therapy for tuberculosis in HIV-infected adults. [Google Scholar]
- 13.Grosset J, Lounis N, Truffot-Pernot C, Brien R J O-, Raviglione M C, Ji B. Once-weekly rifapentine-containing regimens for treatment of tuberculosis in mice. Am J Respir Crit Care Med. 1998;157:1436–1440. doi: 10.1164/ajrccm.157.5.9709072. [DOI] [PubMed] [Google Scholar]
- 14.Halsey N A, Coberly J S, Desormeaux J, Losikoff P, Atkinson J, Moulton L H, Cantave M, Johnson M, Davis H, Geiter L, Johnson E, Huebner R, Boulos R, Chaisson R E. Randomised trial of isoniazid versus rifampin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–791. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 15.Heifets L B, Lindholm-Levy P J, Flory M A. Bactericidal activity in vitro of various rifamycins against M. avium and M. tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 16.Hong Kong Chest Service and British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis. 1992;145:36–41. doi: 10.1164/ajrccm/145.1.36. [DOI] [PubMed] [Google Scholar]
- 17.Ji B, Truffot-Pernot C, Lacroix C, Raviglione M C, O’Brien R J, Olliaro P, Roscigno G, Grosset J H. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 18.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 19.Lecoeur H F, Truffot-Pernot C, Grosset J H. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am Rev Respir Dis. 1989;140:1189–1193. doi: 10.1164/ajrccm/140.5.1189. [DOI] [PubMed] [Google Scholar]
- 20.McCune R M, Feldmann F M, Lambert H P, McDermott W. Microbial persistance. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki E, Miyazaki M, Chen J, Chaisson R E, Bishai W R. Moxifloxacin (BAY 12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor N, Simon B, Mezo N, Heifets L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob Agents Chemother. 1995;39:2073–2077. doi: 10.1128/aac.39.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala B N, Nyirenda O, Luo N, Pobee J, Elliott A M, McAdam K P, Porter J D. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–2457. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Narain J P, Raviglione M C, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuber Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 25.Parrish N M, Dick J D, Bishai W R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 26.Selwyn P A, Hartel D, Lewis V A, Schoenbaum E E, Vermund S H, Klein R S, Walker A T, Friedland G H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 27.Tam C M, Chan S L, Lam C W, Dickinson J M, Mitchison D A. Bioavailability of Chinese rifapentine during a clinical trial in Hong Kong. Int J Tuberc Lung Dis. 1997;1:411–416. [PubMed] [Google Scholar]
- 28.Tam C M, Chan S L, Lam C W, Leung C C, Kam K M, Morris J S, Mitchison D A. Rifapentine and isoniazid in the continuation phase of treating pulmonary tuberculosis: initial report. Am J Respir Crit Care Med. 1998;157:1726–1733. doi: 10.1164/ajrccm.157.6.9707037. [DOI] [PubMed] [Google Scholar]
- 29.Tuberculosis Chemotherapy Centre. A controlled comparison of a twice-weekly and three once-weekly regimens in the initial treatment of pulmonary tuberculosis. Bull W H O. 1970;43:143. [PMC free article] [PubMed] [Google Scholar]
- 30.Valway S E, Sanchez M P C, Shinnick T F, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 31.Vernon A, Villarino E, Geiter L, O’Brien R. Design of U.S. Public Health Service (U.S.P.H.S) Study 22: a trial of once weekly isoniazid (INH) and rifapentine (Rpt) in the continuation phase of TB treatment. Am J Respir Crit Care Med. 1997;155(Part 2):A255. [Google Scholar]
- 32.Whalen C C, Johnson J L, Okwera A, Hom D L, Huebner R, Mugyenyi P, Mugerwa R D, Ellner J J. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]