Abstract
Background
Annona reticulata Linn, has been shown to possess antipyretic, antihelmintic, hypoglycemic, antiulcer and wound healing properties. However, its immunomodulatory role is yet to be explored.
Objective(s)
In the present study, we intended to investigate the effects of A. reticulata leaf ethanol extract on various components of the immune system.
Material and methods
The effects of A. reticulata leaf extract on human peripheral blood mononuclear cells, monocyte (THP1), and human macrophage (U937) cell lines were investigated. An animal study was conducted to observe the effect of the extract on humoral as well as cell mediated immunity.
Results
The extract stimulated proliferation of human PBMC, monocytes (THP1), and macrophages (U937) significantly in a dose dependent manner; expression of transforming growth factor-beta (TGF-β) increased in western blot analysis. Additionally, the extract treated macrophages exhibited features of activation under the microscope with a significant hike in the NO production. Flow cytometry of extract treated human PBMC revealed increased proliferation of lymphocytes (CD4, CD8 & B-cells) along with enhanced intracellular expression of IL-2, IL-6. Animal study data indicate a significant rise in the antibody titer as well as a strong delayed type hypersensitivity response in the extract (150 mg/kg and 300 mg/kg) treated mice; furthermore, the expression of IL-2 and IL-6 in mice PBMC was augmented.
Conclusion
The collective data evince the immunomodulatory potential of A. reticulata L. leaf.
Keywords: Annona reticulata L leaf extract, Macrophage, PBMC, Swiss albino mice, Immunomodulation
Abbreviations: BSA, Bovine serum albumin; DTH, Delayed type of hypersensitivity; FBS, Fetal Bovine Serum; FITC, Fluorescein isothiocyanate; HRP, Horseradish peroxidase; MTT, 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; MFI, Mean fluorescence intensity; PBMC, Peripheral blood mononuclear cells; PBS, Phosphate buffer saline; PE, Phycoerythrin; PHA, Phytohemagglutinin; PMA, Phorbolmyristate acetate; PVDF, Polyvinylidinedifluoride; SDS–PAGE, Sodium dodecyl sulfate polyacrylamide gel electrophoresis; SRBC, Sheep red blood cells; TBST, Tris buffered saline with tween; TGF-β, Transforming Growth factor beta; THP1, Human monocyte cell line; U937, Human macrophage cell line; CP, Cyclophosphamide
Highlights
-
•
Annona reticulata L. stimulates proliferation of human PBMC, monocytes, and macrophages significantly.
-
•
The extract activates cultured macrophages (U937).
-
•
The extract enhanced lymphocyte proliferation along with expression of interleukins in human PBMC.
-
•
Extract treated mice revealed a strong DTH response with significant rise in the antibody titer.
-
•
The expression of IL-2 and IL-6 in mice PBMC was augmented in the treated group.
1. Introduction
The immune system is a remarkably sophisticated and highly developed defense system with a simple mission to seek and kill all invaders and to protect the host. In the modern world, many diseases are the result of a defect in the immune system. Malnutrition, inadequate dilatory intake, stress, HIV, etc. have weakened the immune system making the host vulnerable to life threatening diseases [1]. Various drugs and modern synthetic molecules have been used to reinforce the host defense by modulating ‘induction, expression, amplification, or inhibition of any part or phase of the immune response’ [2]. However, the cost, their toxicity, and associated adverse effects have made them undesirable [3]. In response to this squeal, there is a rise in the utilization of medicinal herbs as immunomodulators. Researchers are now taking more interest in the idea of developing plant-derived immunomodulators as potential and safer alternatives.
Many biological substances have been shown to exert anti-inflammatory, anti-stress, anti-cancer effects by modifying the immune response [4]. Annona reticulata L (Bullock's heart) is one such popular plant that has been used in the alternative medicine to treat various diseases and to maintain good health. It is a member of the Annonaceae family. This highly valuable plant grows in the tropical and subtropical regions. In India, this fruit (also known as Bullock's heart or Ramphal) containing plant is cultivated widely in Bengal, Burma, and southern regions. Almost all parts (leaf, root, fruit, seed, stem bark, and root bark) of the plant have been used traditionally [5]. Multiple research studies have established its anticancer [6], anti-infective [7] and antioxidant [8], analgesic and anti-inflammatory [9], and wound healing activities [10].
A. reticulata leaves contain a wide range of phytochemicals such as alkaloids, amino acids, carbohydrates, steroids, flavonoids, proteins, tannins, glycosides, and phenolics [5]. The phytochemical profile of A. reticulata leaf implies it to be a rich source of immunomodulatory agents, though its effect on immunomodulation has not been elucidated scientifically till date, and literature regarding this is limited or inconspicuous. The leaves of another two species of the same family A. muricata and A. squamosa have already been shown to possess immunomodulatory activity both in vitro and in vivo [11,12]. Therefore, in the present study, the objective was to examine the immunomodulatory role of A. reticulata Linn leaf both in vitro and in vivo by using 32% ethanol extract. Varying percentages of ethanol for extraction starting from 30% to 50% were assess and it was observed that cell proliferation (MTT assay) was maximal at 32% ethanol extract of A. reticulata leaves. HPLC-MS analysis from a previous study [10] indicated that the extract contains two major compounds quercetin 84.02% (w/v) and β-sitosterol 15.98% (w/v) that promote cellular cross-talk and critically modulate various bioactive molecules and cytokines that are crucial in eliciting an immune response. Human PBMC were treated with the extract and its effects on the immune cells (CD4 cell, CD8 cell, and B-cells) were observed; effects on the macrophage and monocytes were also assessed (innate immunity). Apart from these cellular components, interleukins [13] and TGF-beta, a pleiotropic cytokine that can both suppress [14] and stimulate [15] the immune response,are the potential targets for immunomodulation. Therefore we also assessed the level of soluble factor TGF-beta and expression of intracellular interleukins (IL-2, IL-6) in cultured immune cells. Different cell lines were employed in these investigations to explore whether the plant extract interacts with a single or multiple cellular components of the immune system and alsotheir nature of interaction with different immune cells. In vivo animal study was designed to explore the effect of the leaf extract on both humoral and cell-mediated immunity. Again intracellular expression of IL-2, IL-6 in the extract treated mice PBMC was evaluated.
2. Materials and methods
2.1. Materials
RPMI 1640 media, Fetal Bovine Serum (FBS) and l-glutamine, phosphate buffer saline (PBS) were purchased from HiMedia (Mumbai, India). Penicillin and streptomycin were obtained from Life Technologies, USA. Antibodies against IL-2 (ab180780), IL-6 (ab208113), anti-mouse HRP linked secondary antibody, anti-rabbit HRP linked secondary antibody were purchased from Abcam (MA, USA). Antibodies against TGF-β, Actin were purchased from Santa Cruz Biotechnology (Dallas, Texas USA). Anti-CD4, anti-CD8, anti-CD19, anti CD3e antibody (FITC conjugated), anti Ki67 antibody (PE conjugated), anti-IL-2, and IL-6 antibodies (PE conjugated) were purchased from BD Biosciences, USA. PMA, Ionomycin, Brefeldin A, saponin were purchased from Sigma Aldrich, India. Vectashield mounting medium was from Vector Laboratories, Inc. (CA, USA). MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide), Bovine serum albumin (BSA) were purchased from SRL India. SRBC was procured from Indian Veterinary Research Institute, Eastern Regional Station, Kolkata. Cyclophosphamide was purchased from Abcam.
2.2. Cell lines and culture conditions
Peripheral blood mononuclear cells (PBMC) were grown in RPMI-1640 with 10% FBS, 2 mM glutamine, 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin and streptomycin. Human Monocyte cell line THP1 and Human macrophage cell line U937 were also maintained in RPMI supplemented with 10% FBS.
2.3. Collection and authentication of plant material
The fresh leaves of A. reticulata L (the plant name has been checked with http://www.theplantlist.org) were procured from Pandua (District-Hooghly, West Bengal, India) in the month of July 2018. The plant herbarium was authenticated by Mr. R. Gogoi, scientist, Central National Herbarium, Botanical Survey of India and a specimen voucher number (specimen no-SM-1; CNH/Tech.II/2018/24) was provided which is preserved in the Department of Life Science and Bio-Technology, Jadavpur University.
2.4. Extraction procedure
The detailed extraction process has been previously reported [10]. In short, A. reticulata Linn leaves (30 g) were meticulously cleaned with autoclaved water. The shade dried leaves were then smashed by a mixer grinder and were macerated in 32% ethanol. The mixture was passed through a Whatman filter paper (No 1). The suspension thus produced was sterilized by filtration technique (membrane pore size 0.22 μm). The filtrate was then put on a lyophilizer to obtain the crude dry extract (4.56 g). Finally, the crude extract was stored at −20 °C. Fresh stock solutions were prepared from the crude dry extract every time during the experiment.
2.5. In vitro study
2.5.1. Cell proliferation assay
Standard MTT assay was performed in human monocyte cell line (THP1), human macrophage cell line (U937), and human PBMC, isolated by method reported earlier [17]. Twenty-four well plates were seeded with a seeding density of 5 × l04 cells per well and allowed to adhere for 24 h before treatment. At three different incubation periods, different concentrations of the plant extract were added to the test wells. PHA (10 μg/ml) was also used as a positive control in case of PBMCs. Control wells contained only medium (RPMI with 10% FBS in case of THP1 and U937 cell lines and l-glutamine with RPMI and 10% FBS in case of PBMC cells). After 24 h, 48 h, and 72 h of incubation MTT solution (0.5 mg/ml) was added to all the wells. The plates were incubated for 3 h at 37 °C and 5% CO2. Then extraction buffer was added to dissolve the formazan product and absorbance was measured at 570 nm using a spectrophotometer [Synergy H1, BioTek Instruments, Highland Park, Winooski, USA.]. An initial screening with the extract and MTT reagent in a 24 well culture plate without any cells was performed to exclude false positive results.
2.5.2. Western blot
Standard protocol was followed using actin as the housekeeping protein (Detailed in Supplement-1). Expression of TGF-β, with and without extract, in human monocytes, macrophages and PBMC was observed.
2.5.3. Activation of macrophages & NO production
Human macrophages (U937) were grown and maintained in RPMI with 10% FBS. 24 well plates were seeded with U937 cells (5 × l04 cells per well) and allowed to adhere. Following 24 h incubation period, different concentrations (10, 25, 50, 75, 100, 200 μg/ml) of the plant extract were added to the wells. Control wells contained only RPMI with 10% FBS. Wells stimulated with 10 μg/ml of lipopolysaccharide (LPS) served as the positive control. The cells were incubated for another 24 h. Cells (U937) from control, positive control, and different treatment groups were then put on a glass slide, stained with hematoxylin and eosin, and observed under a microscope for the morphological changes indicating activation. NO production was ascertained by Griess reaction followed by quantification of its more stable form, nitrite. The method has been described elsewhere [16]. The absorbance was measured at 550 nm and nitrite concentrations (μmol/L) were calculated against a sodium nitrite standard curve.
2.5.4. Flow cytometry
2.5.4.1. Proliferation assay
Human PBMCs, isolated using previously reported method [17], were diluted in complete RPMI 1640 medium to a final concentration of 106/ml. The PBMC suspension was then transferred to 24-well flat-bottom culture plates and cells were incubated with medium only or phytohemagglutinin (PHA; 10 μg/ml; Sigma) or plant extract (150 μg/ml) for 5 days at 37 °C in presence of 5% CO2. On day 5, the PBMC were further stimulated with PMA (5 ng/ml), Ionomycin (1 μg/ml), and Brefeldin A (10 mg/ml) for another 4 h. PMA and ionomycin lead to T cell activation and production of a plethora of cytokines whereas Brefeldin A inhibits protein secretion resulting in accumulation of cytokines within the activated cell. This was followed by surface and intracellular staining using anti-CD4, anti-CD8, anti-CD19 antibody (FITC conjugated), anti Ki67 antibody (PE conjugated) following the manufacturer's protocol. Ki67 is a nuclear protein which is exclusively expressed during all the active phases of cell proliferation and can be used as a safe and reliable flow cytometry marker to assess lymphocyte proliferation [18]. Flow cytometry was performed on BD FACSVerse flow cytometer using BD FACSuite software (BD Biosciences).
2.5.4.2. Intracellular interleukin expression
Human PBMC were stimulated with PMA (5 ng/ml), Ionomycin (1 μg/ml), and Brefeldin A (10 mg/ml) as described in section 2.5.4.1. This was followed by intracellular staining with PE conjugated anti IL-2 and IL-6 antibodies (BD Biosciences, USA) following the manufacturer's protocol. Flow cytometry was performed on BD FACSVerse flow cytometer. Mean Fluorescent Intensity (MFI) and relative fold increase in MFI were measured.
2.6. In vivo animal study
2.6.1. Ethical approval
All mouse experiments were conducted at Ballygunge Science College, University of Calcutta, India, following the guidelines of the Institutional Animal Ethics Committee (885/GO/Re/S/05/CPCSEA) in complance with the ARRIVE guidelines regarding the handling of laboratory animals in scientific experiments.
2.6.2. Experimental animals
Healthy adult male Swiss albino mice (8 week old, weighing 28–30 g) were used in the study. They were nurtured in pathogen free condition with free access to food and water with a 12 h light–dark cycle at a temperature of 27 ± 2 °C, relative humidity of 50–70%. As described before [19] the animals were weighed twice weekly, monitored regularly for food and water intake, general activity, panting pattern, and fur condition. All attempts were made to lessen the suffering of the mice. They were observed for 2 weeks for acclimatization.
2.6.3. Antigens and drugs
For this study, sheep RBCs (SRBC) were used as antigen. Cyclophosphamide (CP) 50 mg/kg of body weight (BW) was used as immunosuppressing agent.
2.6.4. Acute toxicity assay
Acute toxicity testing using the up-and-down (UDP) method [20] was performed using intraperitoneal route of administration. No toxicity or mortality was observed up to the dose of 1500 mg/kg. Thus the extract could be regarded to have an LD50 of about 3000 mg/kg or higher [21].
2.6.5. Experimental design
Thirty animals (N = 30) were divided randomly (simple randomization; using online randomization software; https://www.graphpad.com/quickcalcs/randomize1/) into 6 treatment groups (I–VI) each containing 5 mice. The first group (group I) served as the control and received only vehicle (normal saline) treatment. The treating agents, their dose, and the schedule of treatment in different groups is presented in Table 1. Overall the animals were treated for 7 days. All injections were given intraperitoneally maintaining sterility and trauma was minimized to the extent possible. The reason behind choosing the intraperitoneal route is described previously [22]. In a preliminary screening, the plant extract was administered intraperitoneally in normal healthy Swiss albino mice at fixed doses of 150, 200, 250, 300 mg/kg of BW (between 1/10th and 1/20th of LD50) and the increase in total leukocyte count was measured. The optimum results were obtained with the dose of 150 mg/kg and 300 mg/kg (Supplement A). Further exploration was done with these two doses. After 9 days, the animals were anaesthetized and sacrificed by cervical dislocation. The following variables in different treatment groups were assessed: paw thickness as a measure of delayed type of hypersensitivity; antibody titer; expression of interleukins in mouse PBMCs; immunohistochemical analysis of spleen tissue. Group allocation, conduction of the experiments, outcome measurement, and data analysis were performed by different individuals in the same research team to minimize confounding bias.
Table 1.
Different experimental groups and the treatment they were given.
| Groups | Treatment received on different day of the experiment |
|---|---|
| Group I (Saline group) | Saline (ip) for 7 days + SRBC (ip) on 5th day + SRBC (rf, sc) on 7th day |
| Group II (CP group) | Saline (ip) for 7 days + SRBC (ip) on 5th day + CP injection on 6th day + SRBC (rf, sc) on 7th day |
| Group III (CP + Extract 150 group) | Extract (ip) (150 mg/kg) for 7 days + SRBC (ip) on 5th day + CP on 6th day + SRBC (rf, sc) on 7th day |
| Group IV (CP + Extract 300 group) | Extract (ip) (300 mg/kg) for 7 days + SRBC (ip) on 5th day + CP on 6th day + SRBC (rf, sc) on 7th day |
| Group V (Extract 150 group) | Extract (ip) (150 mg/kg) for 7 days + SRBC (ip) on 5th day + SRBC (rf, sc) on 7th day |
| Group VI (Extract 300 group) | Extract (ip) (300 mg/kg) for 7 days + SRBC (ip) on 5th day + SRBC (rf, sc) on 7th day |
All the animals were randomly divided into 6 experimental groups. Each row represents a single treatment group and the treatment received by each experimental unit (animal) in that group on various day of the experiment. All the agents were administered by injection. ip-intraperitoneal injection; rf-right foot pad; sc-subcutaneous injection; SRBC-sheep RBC; CP-cyclophosphamide.
2.6.5.1. Cellular immune response
Delayed type of hypersensitivity (DTH) was assessed. After measuring the footpad thickness on the 7th day, SRBC (0.025 × 109 cells) was injected subcutaneously in the right paw and 25 μl of saline was injected into the left paw of animals of all the groups. Footpad thickness was measured again after 24 h. Increase in the right foot thickness was taken as the index of cell-mediated immunity. All the measurements were taken by digital calipers (Mitutoyo America).
2.6.5.2. Humoral immune response
We followed the method reported by Bin-Hafeez et al. 2001 with modification [23]. In short, all groups were challenged with an injection of 0.2 ml of 10% SRBC suspension in normal saline (v/v) intraperitoneally, on day 5. On day 8, blood was collected in a microcentrifuge tube from each mouse by retro-orbital vein puncture and was subjected to hemagglutination assay to determine the antibody titer (Detailed in Supplement 2).
2.6.5.3. Flow cytometry analysis
As described above (section 2.5.4.1), the mice PBMC from different treatment groups were stimulated with PMA (5 ng/ml), Ionomycin (1 μg/ml), and Brefeldin A (10 mg/ml). Following surface staining with FITC conjugated anti-CD3e and anti-CD14 antibody, cells were permeabilized (0.2% saponin) and stained with PE conjugated anti IL-2 and IL-6 antibodies respectively, and then analyzed on BD FACSVerse flow cytometer (Detailes provided in Supplement 3).
2.6.5.4. Immunohistochemistry
Immunostaining of the formalin-fixed, paraffin-embedded mice spleen tissue from each group was started with overnight incubation with primary antibodies against IL-2 (ab180780; Abcam) and IL-6 (ab208113; Abcam) and was completed according to the protocol (MP-7601; IHC Guide, Vector Laboratories). Bright field images were captured with a microscope (Leica).
2.7. Statistical analysis
Study data represented as mean ± SD. Variance among groups was evaluated using two factors ANOVA, single factor ANOVA, followed by post Hoc Tukey's range test for multiple comparisons. Kruskal–Wallis test followed by Dunn's test was performed to evaluate the difference of antibody titers in different treatment groups. P < 0.05 was accepted as statistically significant. P < 0.05, P < 0.01 and P < 0.001 are represented by ∗, ∗∗, and ∗∗∗ respectively. (Software: Graph pad Prism version-8 GraphPad Software, Inc., San Diego, California, USA).
3. Results
3.1. Effect of A. reticulata L. leaf extract on immune cell proliferation and TGF-β expression
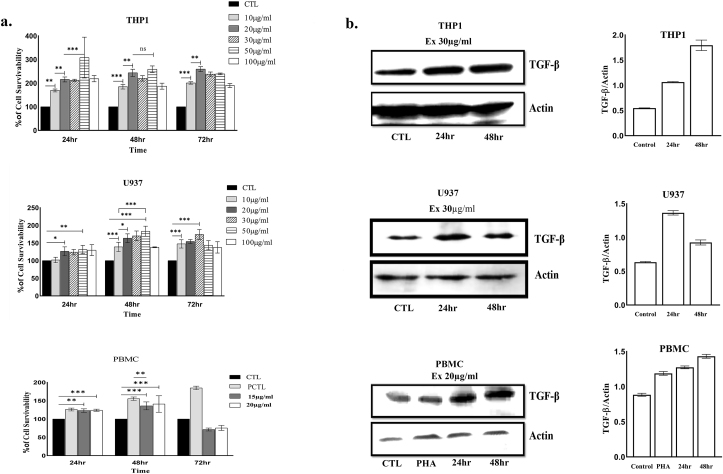
Significant proliferation of human PBMC was observed with increasing concentration of the extract, maximum (141 ± 21%) being at 48 h of incubation with the concentration of 20 μg/ml. In case of monocytes (THP1), maximal proliferation was obsrved at the extract concentration of 50 μg/ml both at 24 h and 48 h (308 ± 80%, 259 ± 12.7% respectively). Macrophages (U937) also showed a hike in proliferation in a dose-dependent manner. Maximal proliferation was noted at 50 μg/ml both at 24 h (131.5 ± 10.9%) and 48 h (183.3 ± 13.2%) (Fig. 1a). We also obsrved that the expression of TGF-β was enhanced in all the three cell lines with increasing time up to 48 h (Fig. 1b).
Fig. 1.
a. Graphical representation of the MTT assay showing the effect of the increasing concentrations (10, 20, 30, 50, 100 μg/ml) of A. reticulata leaf extract on THP1, U937, and human PBMC cells at 24 h, 48 h, and 72 h. The results expressed as mean ± S.D. of three independent experiments. The percentage of cell growth was calculated using untreated cells as 100%. ∗(P < 0.05), ∗∗(P < 0.01), ∗∗∗(P < 0.001); hr = hour. CTL = Control; PCTL = Positive control; ns (P ˃ 0.05). b. Shows the Western blot analysis on monocyte (THP1), macrophage (U937) and human PBMC. THP1, PBMC, and U937 cell lysates from treated or mock treated cells were immunoblotted with antibody against TGF-β. Actin was used as loading control. The band intensity was quantified using ImageJ Software and the relative fold increase in expression of TGF-β was plotted graphically (left panels). PHA = Phytohemagglutinin, Ex = Treatment concentration of the extract.
3.2. Effect of A. reticulata L. leaf extract on activation of macrophages
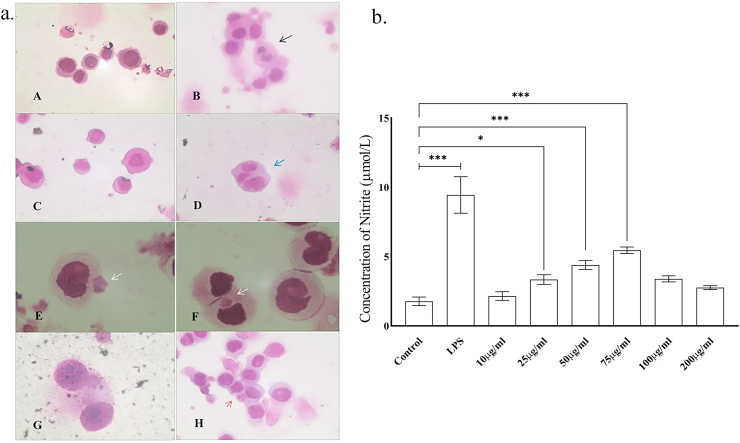
The extract caused activation of the macrophages (U937) both morphologically (Fig. 2a) and functionally (Fig. 2b) compared to the mock-treated cells. The basal nitrite level (control group) was 1.78 ± 0.31 μmol/L. The leaf extract significantly raised the nitrite levels with increasing concentrations and reached its peak 5.45 ± 0.24 μmol/L at the concentration of 75 μg/ml. LPS stimulated cells (positive control group), however, produced a higher amount of nitrite 9.50 ± 1.32 μmol/L.
Fig. 2.
a. Depicts H&E staining of U937 cells. (A) Normal macrophages of the control group. (B) Cluster of activated macrophages in syncytium with a dividing one (black arrow); positive control group, LPS treated. (C, D) Extract treated (25 μg/ml) macrophages exhibiting satellitism (blue arrow). (E, F) Extract treated (50 μg/ml) macrophages showing endocytosis (white arrow). With 75 μg/ml of extract (G) shows activated, dividing macrophages and (H) activated macrophages with satellitism and pinocytosis (red arrow). (For A, B, C, D, H – magnification 400×; scale bar-50 μm and for E, F, G – magnification is 1000×; scale bar-10 μm) b. Nitric oxide production (μmol/L) in media treated, LPS treated and extract treated (different concentrations) macrophages. Results expressed as mean ± S.D. of 3 independent experiments. (∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001).
3.3. The extract stimulates lymphocyte proliferation and up regulates cytokine expression
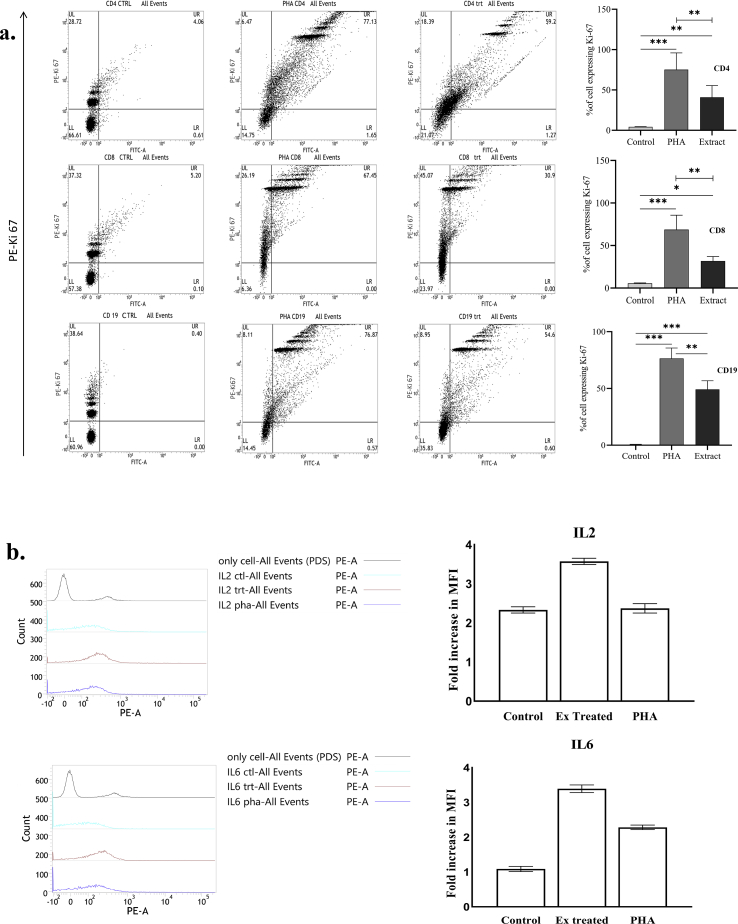
We observed the expression of Ki67 protein as a marker of cell proliferation. Flow cytometry analysis (Fig. 3a) on human PBMC, gated on lymphocytes, revealed significantly increased proliferation in the extract treated cells compared to the media treated cells, i.e., control group (40.9% ± 14.4% versus 4.3% ± 0.3% for CD4 cells, 31.8% ± 5.3% versus 5.51% ± 0.5% for CD8 cells, and 49.2% ± 7.6% versus 0.2% ± 0.3% for CD19 cells) (Supplement 4). Cytometry data also indicated a hike in the Mean Fluorescence Intesity (MFI) compared to both the untreated and PHA challenged cells implying an increased expression of intracellular IL-2 (MFI 364 ± 8) and IL-6 (MFI 346 ± 11) in the extract treated group. Fig. 3b shows the relative fold increase in MFI which is maximal in the extract treated group.
Fig. 3.
a. FACS analysis (dot plots) showing Ki67 expression in control, PHA challenged, and extract treated PBMC, gated on lymphocytes. Upper, middle, and lower panels represent CD4+, CD8+, and CD19+ lymphocytes respectively. Values in the upper right quadrant (UR) in each dot plot represent the percentage of cells expressing Ki67. The mean of that percentage value in different groups from 5 different experiments was plotted graphically to evaluate the difference between groups. (One way ANOVA; n = 5); ∗(P < 0.05), ∗∗(P < 0.01), ∗∗∗(P < 0.001). Data from one representative experiment out of five are shown. b. FACS profile showing expression of intracellular interleukins in control, PHA challenged, and extract treated PBMC. Panels on the right represent the fold shift in MFI signifying the expression of IL-2 and IL-6 respectively which has been plotted graphically (left panels). Data from one representative experiment out of five are shown. PE = phycoerythrin, FITC = fluorescein isothiocyanate, PHA = phytohemagglutinin, CTRL = Control, ctl = Control, trt = Extract (150 μg/ml) treated cells; MFI = mean fluorescence intensity.
3.4. Effect of the extract on cell mediated and humoral immunity
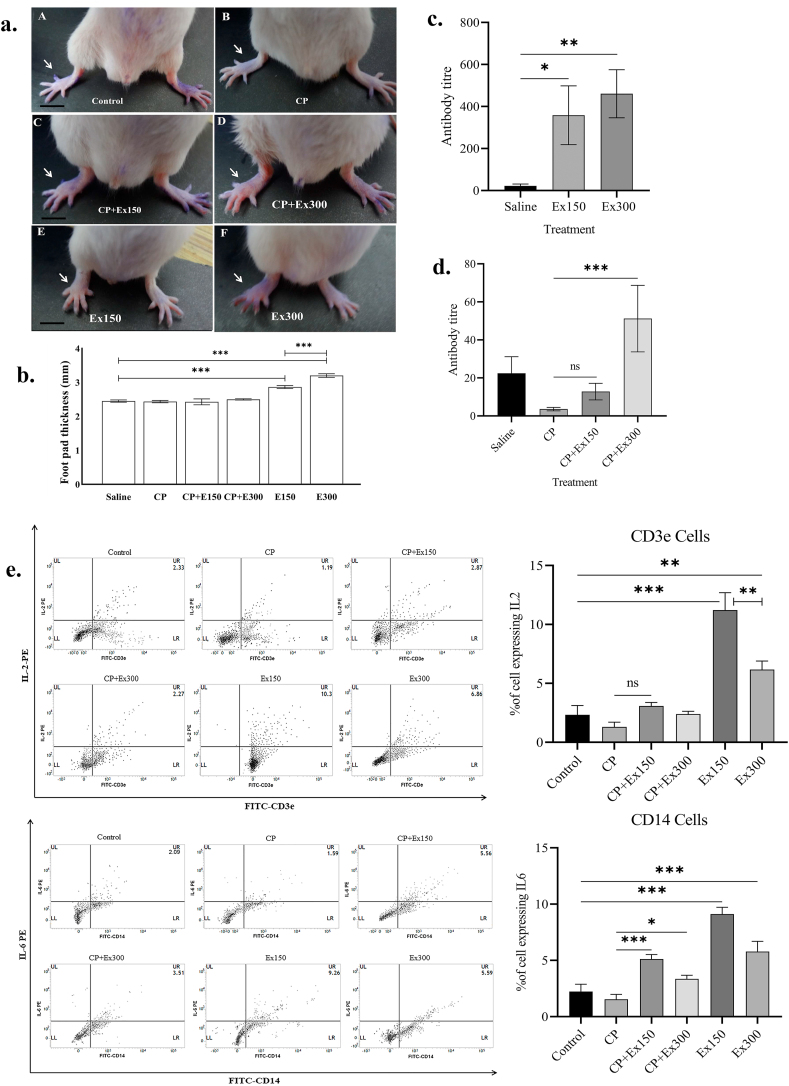
The extract induced a significant hike in the foot pad thickness of the treated animals compared to the control ones, in a dose dependent manner (Fig. 4a and b). Though not statistically significant, the extract could also strengthen the DTH response in immunosuppressed (CP treated) mice. We also noted that the extract prompted a significant rise in the antibody titer with both the doses when compared to the saline treated group (Fig. 4c). In addition, the immunosuppressed (CP treated) animals treated with plant extract exhibited significant recovery in the antibody titer (Fig. 4d).
Fig. 4.
a. Photographs of foot pad thickness (white arrow) observed in animals of different treatment groups. Scale bar = 5 mm; b. Graphical representation of foot pad thickness (mm) in different groups. Data expressed as mean ± S.D.; (one way ANOVA, n = 5 in each group); ∗(P < 0.05), ∗∗(P < 0.01), ∗∗∗(P < 0.001). c. Shows increase in antibody titer in extract (150 mg/kg, 300 mg/kg) treated mice compared to the saline treated. d. Shows the stimulatory effect of A. reticulata extract (150 mg/kg & 300 mg/kg) on antibody titer in immunosuppressed (CP treated) mice. Data expressed as mean rank difference ± SEM (Kruskal–Wallis test, n = 5 in each group). e. Dot plots (FACS) representing intracellular IL-2 and IL-6 expression in subpopulation of PBMC of different treatment groups. The upper panel shows the CD3e positive cells (lymphocytes) expressing IL-2 within the whole PBMC population; the lower panel shows the CD14 positive cells (monocytes & macrophages) expressing IL-6. Data from one representative experiment out of three are shown. Numbers in the upper right (UR) quadrants show percentages of positive cells expressing IL. The mean of that percentage value in different groups from 3 different experiments was plotted graphically to evaluate the difference between groups (left panels). (One way ANOVA; n = 3); ∗(P < 0.05), ∗∗(P < 0.01), ∗∗∗(P < 0.001); ns (P ˃ 0.05); PE = phycoerythrin, FITC = fluorescein isothiocyanate; CP = Cyclophosphamide; Ex150 = Extract 150 mg/kg; Ex300 = Extract 300 mg/kg.
3.5. The extract up regulated the expression of interleukins in mice PBMC
FACS data indicated increased percentage of cells expressing intracellular IL-2 (Fig. 4e, upper panel) in both the extract treated groups (11.22% ± 1.5% and 6.16% ± 0.7%) compared to the control group (2.33% ± 0.8%). Similarly IL-6 expression (Fig. 4e, lower panel) was also augmented in the extract treated groups (9.12% ± 0.6% and 5.78% ± 0.9%) in contrast to the control group (2.23% ± 0.6%) (Supplement 5). In both the occasions, the extract showed a stimulatory effect on the intracellular cytokine expression in the immunosuppressed (CP) mice.
3.6. Immunohistochemistry
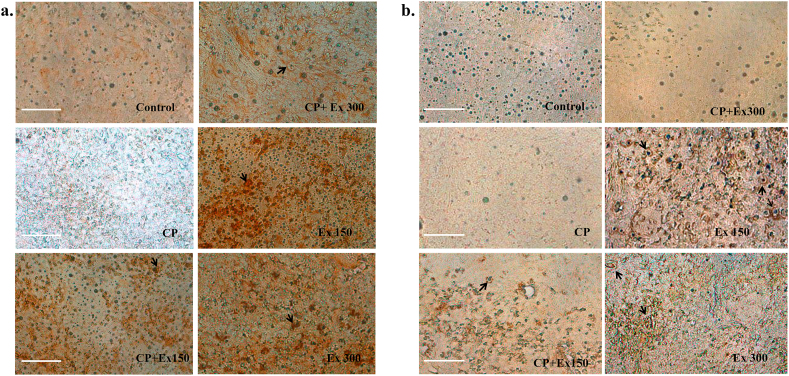
Mice spleen tissue stained strongly positive for IL-2 and IL-6 in the extract treated groups compared to the non-treated (control) animals which showed weak positivity (Fig. 5). We also observed that the immunoreactivity was more with the lower extract dose (150 mg/kg) than a higher dose (300 mg/kg). In the immunosuppressed (CP) animals, minimal immunoreactivity was observed; whereas, groups treated with both cyclophosphamide and plant extract showed moderate positivity.
Fig. 5.
Profile of interleukin expression in spleen tissue in different treatment groups. Immunostaining was performed with antibody against IL-2 and IL-6 in mouse spleen tissue. (a) Shows the immunoreactivity of spleen tissue against IL-2 and (b) shows the same against IL-6. In all the panels, immunoreactivity can be observed as brown deposits (black arrow). Magnification 400×; Scale bar = 100 μm. Each image in a single panel represents different treatment groups. CP = Cyclophosphamide; Ex150 = Extract 150 mg/kg; Ex300 = Extract 300 mg/kg.
4. Discussion
Plant bioactive compounds can either stimulate or suppress the immune system and hence can be utilized to treat various disorders involving the immune system. In this study, we found that 32% ethanol extract of A. reticulata leaf possesses immunomodulatory activity in both in vitro and in vivo experimental models of humoral and cell-mediated immunity. We investigated the ability of A. reticulata leaf extract to stimulate immune cell proliferation. A significant increment in the proliferation of cultured human monocytes (THP1), macrophages (U937), and human peripheral blood mononuclear cells (PBMC) indicated the possible immunomodulatory role of the extract.
TGF-β is a potent regulatory cytokine with diverse actions. It has both positive and negative regulatory influence on T-lymphocyte proliferation, differentiation, and function [15,24]. Likewise, B-lymphocytes have multiple high affinity receptors for TGF-β and studies have proven that the proliferation and differentiation of B-lymphocytes to plasma cells are finely controlled by various soluble factors including TGF-β [25]. The present study data indicate an increased expression of TGF-β in THP1 cells, U937 cells, and PBMC cells pre-treated with A. reticulata leaf extract, suggesting its possible immunomodulatory role. However further investigations are necessary to determine the exact mechanism.
Activation of macrophages followed by phagocytosis and killing comprises the body's first line of defense against the invading microorganisms [26]. Activated macrophages induce NO production as an effector molecule to destroy microbes [27]. In this study, we observed that the extract treated macrophages underwent morphological changes, became activated, and significantly increased NO (nitrite) production with increasing doses of the extract up to the dose of 75 μg/ml. The decrease in the NO production may be due to chemically induced cell death with higher concentrations (100 μg/ml and 200 μg/ml).
CD4+T cells and CD8+T cells constitute the key effectors of cell mediated immunity [28] and act via secretion of specific cytokines or by lysing cells directly [29,30]. Whereas, B-cells mediate humoral immune response by production of antibodies [31]. It is now established that IL-2 is important for activation-induced cell death, development of Treg cells, and development of cytotoxic T lymphocytes, as well as for secondary expansion of memory CD8+ T cells, modulating T helper cell differentiation and regulation of cytokine receptor expression [32]. IL-6 also plays an important role in the acquired immune response by stimulation of antibody production and effector T-cell development [33]. FACS analysis revealed an increased proliferation of CD4, CD8, B cells in the extract treated PBMC compared to the untreated; intracellular expression of IL-2 and IL-6 was obsreved to be augmented in the extract treated cells. We chose to perform flow cytometry instead of ELISA to detect intracellular expression of interleukins as it allows detection of a particular cell phenotype and the specific antigen expressed by it simultaneously [34] and to avoid some difficulties inherent to ELISA [35]. Altogether the data point towards the immunomodulatory prospect of the leaf extract.
The in vivo study revealed a significant increase in the delayed type of hypersensitivity reaction to SRBC with both the doses (150 mg/kg, 300 mg/kg) of A. reticulata leaf extract. A strong DTH response implies a coordinated interaction between immune cells (antigen presenting cell, T-cells) and several mediators (cytokines) in an effective way to protect the individual against numerous infectious organisms. The absence of DTH response to multiple antigens is termed as anergy and is indicative of increased risk of morbidity and mortality, more in the elderly [36]. In the present study, the plant extract showed its stimulatory effect on cell mediated immunity implying its beneficial role in enhancing host defence against cellular pathogens. Treated animals also showed increased antibody titer compared to the saline treated mice at both the doses. Furthermore, the immunosuppressed animals exhibited significant recovery in the antibody titer after being treated with the plant extract indicating its stimulatory effect on humoral immunity. Therefore we observe that the extract reinforces both humoral and cell mediated immunity. Yeap SK et al. demonstrated the immunomodulatory effect of Rhaphidophora korthalsii via increasing the expression of IL-2 [37]. In another isolated study, Abood WN et al. showed the immunomodulatory role of Tinospora crispa through enhancing the expression of IL-6 [38]. Therefore, we further investigated the possible effect of the extract on intracellular cytokine production. In this study, flow cytometry analysis on mice PBMC of different treatment groups showed an enhanced expression of IL-2 and IL-6 in the extract treated animals compared to the mock-treated as well as immune-compromised animals. However, it is interesting to note that the interleukin expression was significantly higher with a lower dose (150 mg/kg) of the extract and diminished with the higher dose (300 mg/kg). Crude plant extracts are usually a complex mixture of different constituents which could have antagonistic, allosteric, and synergistic effects. At a higher concentration, an active substance may lose its efficacy or can reduce the activity of a second bioactive constituent. However, further study is needed to understand the interplay of different phytochemicals at higher concentrations to determine the exact cause of this phenomenon. Similar results were obtained on immunohistochemical staining of mice spleen tissue. Here we would like to mention that we opted for immunohistochemical staining instead of ELISA (which otherwise would have been a better option) because we wanted to observe the intracellular expression of interleukins in spleen cells and visually represent it at the same time. So, we discerned that the ethanol extract of A. reticulata leaf enhanced the intracellular expression of IL-2 and IL-6 both in vitro and in vivo.
Based on the HPLC–MS analysis from a previous study, [10] 32% ethanol extract of A. reticulata L. leaf contains quercetin and β-sitosterol as major bioactive compounds. Quercetin is a flavonoid naturally present in many plants, fruits, leaves, etc. It can both stimulate and suppress the immune system. Though quercetin inhibits macrophage activation and subsequent NO production [39] β-sitosterol may be responsible for the observed macrophage (U937) activation and the increased NO production [40]. β-sitosterol has also been shown to induce the expression and secretion of TGF-beta as well [41]. Leaf extract, rich in quercetin, has been reported to increase the proliferation of CD8 and CD4 T lymphocytes with increased expression of Th1 cytokines [42]. Similarly, β-sitosterol and β-sitosterolglucosidecan stimulate human peripheral blood lymphocyte proliferation as well as enhance IL2 secretion [43,44]. Recently Abood et al. have reported that quercetin containing T. crispa leaf exerts its immunomodulatory effect via increased expression of IL6 [38]. So, the role of quercetin [45,46] and β-sitosterol [47] in immunomodulation is well established. Therefore, in the present study, we observed that the putative mechanisms of action of immunomodulation by this plant extract occur via macrophage activation, augmentation of phagocytosis, proliferation of both cytotoxic and helper T-lymphocytes, enhancement of cellular immune function, enhancement of antigen specific antibody production, and increased expression of nonspecific immunity mediators like IL2, IL6. However, the ability of the extract to modulate multiple components of the immune system is noteworthy and needs further investigation to delineate the underlying molecular mechanisms.
5. Conclusion
Our data imply that the ethanol (32%) extract of A. reticulata leaf containing β-sitosterol and quercetin as major compounds can trigger various cellular events or release mediators that are critical to elicit an immune response and therefore can be a potential alternative in various diseases related to immune system. However, this is a preliminary study and is focused to establish the role of A. reticulata leaf extract in immunomodulation. Therefore, before extrapolating our results to human subjects further studies are needed to be implemented for elucidation of the underlying mechanisms, dosage in humans, side effect profile, etc. Nevertheless, to the best of our knowledge, this is the first study displaying the immunomodulatory effect of A. reticulata leaf.
Source of funding
This work was financially supported by DST Inspire fellowship program, Govt. of India and Department of Higher Education, Science & Technology and Biotechnology, Government of West Bengal (BT/ST/P/S&T/2G-13/2017).
Conflict of Interest
None.
Author Contributions
Swagata Mazumdar: Conceptualization, Methodology, Writing- Original draft preparation, Data curation. Amit Kumar Ghosh: Data curation, Writing- Original draft preparation, Software. Suman Purohit: Data curation Visualization, Investigation. Anjan Kumar Das: Supervision, Software, Validation. Arindam Bhattacharyya & Parimal Karmakar: Writing- Reviewing and Editing.
Acknowledgments
We thank Ananya Dutta and Sayantan Bose, Bose Institute, Kolkata for their technical and procedural assistance.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2022.100554.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Elkington L.J., Gleeson M., Pyne D.B., Callister R., Wood L.G. CRC Press; 2014. Inflammation and immune function; pp. 171–181. (Antioxidants Sport Nutr.). [DOI] [Google Scholar]
- 2.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12 doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nfambi J., Bbosa G.S., Sembajwe L.F., Gakunga J., Kasolo J.N. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J Basic Clin Physiol Pharmacol. 2015;26:603–611. doi: 10.1515/jbcpp-2014-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 5.Jamkhande P.G., Wattamwar A.S. Annona reticulata Linn. (Bullock's heart): plant profile, phytochemistry and pharmacological properties. J Tradit Complement Med. 2015;5:144–152. doi: 10.1016/j.jtcme.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suresh H.M., Shivakumar B., Hemalatha K., Heroor S.S., Hugar D.S., Sambasiva Rao K.R.S. In vitro antiproliferative activity of Annona reticulata roots on human cancer cell lines. Pharmacognosy Res. 2011;3:9–12. doi: 10.4103/0974-8490.79109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamthe L., Fokou P., Mbouna C., Keumoe R., Ndjakou B., Djouonzo P., et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines. 2015;2:55–66. doi: 10.3390/medicines2020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen W., Lin Y., Ti Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflammatory activities of ethanolic seed extract of Annona reticulata L. in streptozotocin induced diabetic rats. Front Endocrinol (Lausanne) 2019;10 doi: 10.3389/fendo.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavan M.J., Wakte P.S., Shinde D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat Prod Res. 2012;26:1515–1518. doi: 10.1080/14786419.2011.564583. [DOI] [PubMed] [Google Scholar]
- 10.Mazumdar S., Ghosh A.K., Dinda M., Das A.K., Das S., Jana K., et al. Evaluation of wound healing activity of ethanol extract of Annona reticulata L. leaf both in vitro and in diabetic mice model. J Tradit Complement Med. 2019;11(1):27–37. doi: 10.1016/j.jtcme.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim G.T., Tran N.K.S., Choi E.H., Song Y.J., Song J.H., Shim S.M., et al. Immunomodulatory efficacy of standardized annona muricata (Graviola) leaf extract via activation of mitogen-activated protein kinase pathways in RAW 264.7 macrophages. Evid Based Complement Altern Med. 2016;2016:1–10. doi: 10.1155/2016/2905127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh S.H., Chang F.R., Wu Y.C., Yang Y.L., Zhuo S.K., Hwang T.L. An anti-inflammatory ent-kaurane from the stems of Annona squamosa that inhibits various human neutrophil functions. Planta Med. 2005;71:904–909. doi: 10.1055/s-2005-871234. [DOI] [PubMed] [Google Scholar]
- 13.Justiz Vaillant A.A., Qurie A. StatPearls Publishing; 2021. Interleukin. [PubMed] [Google Scholar]
- 14.Yoshimura A., Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2010;350:127–147. doi: 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 15.Lee H.M., Rich S. Differential activation of CD8+ T cells by transforming growth factor-beta 1. J Immunol. 1993;151:668–677. [PubMed] [Google Scholar]
- 16.Migliorini P., Corradin G., Corradin S.B. Macrophage NO2− production as a sensitive and rapid assay for the quantitation of murine IFN-γ. J Immunol Methods. 1991;139:107–114. doi: 10.1016/0022-1759(91)90357-L. [DOI] [PubMed] [Google Scholar]
- 17.Jaatinen T., Laine J. Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc02a01s1. Chapter 2. [DOI] [PubMed] [Google Scholar]
- 18.Soares A., Govender L., Hughes J., Mavakla W., de Kock M., Barnard C., et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscella A., Vetrugno C., Cossa L.G., Antonaci G., De Nuccio F., De Pascali S.A., et al. In vitro and in vivo antitumor activity of [Pt (O,O’ -acac)(gama-acac)(DMS)] in malignant pleural mesothelioma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka S., Hashimoto M., Tobe M., Kobayashi K., Sekizawa J., Nishimura M. A simple method for screening assessment of acute toxicity of chemicals. Arch Toxicol. 1990;64:262–268. doi: 10.1007/BF01972985. [DOI] [PubMed] [Google Scholar]
- 22.Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 23.Bin-Hafeez B., Ahmad I., Haque R., Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–18. doi: 10.1016/S0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 24.Gray J.D., Liu T., Huynh N., Horwitz D.A. Transforming growth factor beta enhances the expression of CD154 (CD40L) and production of tumor necrosis factor alpha by human T lymphocytes. Immunol Lett. 2001;78:83–88. doi: 10.1016/s0165-2478(01)00233-4. [DOI] [PubMed] [Google Scholar]
- 25.Tamayo E., Alvarez P., Merino R. TGFβ superfamily members as regulators of B cell development and function—implications for autoimmunity. Int J Mol Sci. 2018;19:3928. doi: 10.3390/ijms19123928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirayama D., Iida T., Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2018;19:92. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son C.G., Shin J.W., Cho J.H., Cho C.K., Yun C.H., Chung W., et al. Macrophage activation and nitric oxide production by water soluble components of Hericium erinaceum. Int Immunopharmacol. 2006;6:1363–1369. doi: 10.1016/j.intimp.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Kumari J., Selvan S.R., Becart S., Chattopadhyay S., Dalmo R.A. Cell-mediated immunity and vaccines. J Immunol Res. 2014;2014:1–2. doi: 10.1155/2014/632632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4 +T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:1–12. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg R.E., Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18:338–343. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Lebien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao W., Lin J.-X., Leonard W.J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freer G., Rindi L. Intracellular cytokine detection by fluorescence-activated flow cytometry: basic principles and recent advances. Methods. 2013;61:30–38. doi: 10.1016/j.ymeth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 35.O'Mahony L., Holland J., Jackson J., Feighery C., Hennessy T.P.J., Mealy K. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol. 1998;113:213–219. doi: 10.1046/j.1365-2249.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorn M., Hudson A.W., Kreeger J., Kawabe T.T., Bowman C.J., Collinge M. Evaluation of a novel delayed-type hypersensitivity assay to Candida albicans in adult and neonatal rats. J Immunotoxicol. 2015;12:350–360. doi: 10.3109/1547691X.2014.980925. [DOI] [PubMed] [Google Scholar]
- 37.Yeap S.K., Omar A.R., Ali A.M., Ho W.Y., Beh B.K., Alitheen N.B. Immunomodulatory effect of Rhaphidophora korthalsii on natural killer cell cytotoxicity. Evid Based Complement Altern Med. 2012;2012:786487. doi: 10.1155/2012/786487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abood W.N., Fahmi I., Abdulla M.A., Ismail S. Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement Altern Med. 2014;14:205. doi: 10.1186/1472-6882-14-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui S., Wu Q., Wang J., Li M., Qian J., Li S. Quercetin inhibits LPS-induced macrophage migration by suppressing the iNOS/FAK/paxillin pathway and modulating the cytoskeleton. Cell Adhes Migr. 2019;13:1–12. doi: 10.1080/19336918.2018.1486142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boubaker J., Ben Toumia I., Sassi A., Bzouich-Mokded I., Ghoul Mazgar S., Sioud F., et al. Antitumoral potency by immunomodulation of chloroform extract from leaves of Nitraria retusa, Tunisian medicinal plant, via its major compounds β-sitosterol and palmitic acid in BALB/c mice bearing induced tumor. Nutr Cancer. 2018;70:650–662. doi: 10.1080/01635581.2018.1460683. [DOI] [PubMed] [Google Scholar]
- 41.Kassen A., Berges R., Senge T. Effect of beta-sitosterol on transforming growth factor-beta-1 expression and translocation protein kinase C alpha in human prostate stromal cells in vitro. Eur Urol. 2000;37:735–741. doi: 10.1159/000020227. [DOI] [PubMed] [Google Scholar]
- 42.Okoye F.B.C., Odimegwu D.C., Nworu C.S., Agbo M.O., Esimone C.O., Osadebe P.O., et al. Modulation of intracellular expression of IFNγ and IL-2 in culture of splenic T lymphocytes by some flavonoid glycosides of Alchornea floribunda. Pharm Biol. 2016;54:1873–1880. doi: 10.3109/13880209.2015.1133659. [DOI] [PubMed] [Google Scholar]
- 43.Bouic P.J.D., Etsebeth S., Liebenberg R.W., Albrecht C.F., Pegel K., Van Jaarsveld P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol. 1996;18:693–700. doi: 10.1016/S0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.H., Lee J.Y., Park J.H., Jung H.S., Kim J.S., Kang S.S., et al. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine. 2007;25:3834–3840. doi: 10.1016/j.vaccine.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzade A., Sadeghi O., Biregani A.N., Soukhtehzari S., Brandt G.S., Esmaillzadeh A. Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol. 2019;10:51. doi: 10.3389/fimmu.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh D., Tanwar H., Jayashankar B., Sharma J., Murthy S., Chanda S., et al. Quercetin exhibits adjuvant activity by enhancing Th2 immune response in ovalbumin immunized mice. Biomed Pharmacother. 2017;90:354–360. doi: 10.1016/j.biopha.2017.03.067. [DOI] [PubMed] [Google Scholar]
- 47.Alappat L., Valerio M., Awad A.B. Effect of vitamin D and β-sitosterol on immune function of macrophages. Int Immunopharmacol. 2010;10:1390–1396. doi: 10.1016/j.intimp.2010.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.