Abstract
Objective
Recent evidence suggests that patients suffering post-acute COVID syndrome frequently report cognitive complaints, but their characteristics and pathophysiology are unknown. This study aims to determine the characteristics of cognitive dysfunction in patients reporting cognitive complaints after COVID-19 and to evaluate the correlation between cognitive function and anxiety, depression, sleep, and olfactory function.
Methods
Cross-sectional study involving 50 patients with COVID-19 reporting cognitive complaints 9.12 ± 3.46 months after the acute infection. Patients were evaluated with a comprehensive neuropsychological protocol, and scales of fatigue, depression, anxiety, sleep and an olfactory test. Normative data and an age- and education matched healthy control group were used for comparison.
Results
COVID-19 patients showed a diminished performance on several tests evaluating attention and executive function, with alterations in processing speed, divided attention, selective attention, visual vigilance, intrinsic alertness, working memory, and inhibition; episodic memory; and visuospatial processing. Cognitive performance was correlated with olfactory dysfunction, and sleep quality and anxiety to a lesser extent, but not depression.
Conclusions
Patients with COVID-19 reporting cognitive symptoms showed a reduced cognitive performance, especially in the attention-concentration and executive functioning, episodic memory, and visuospatial processing domains. Future studies are necessary to disentangle the specific mechanisms associated with COVID-19 cognitive dysfunction.
Keywords: COVID-19, Cognitive, Neuropsychological assessment, Long-term COVID
1. Introduction
Recent investigations have found a novel syndrome called “long COVID” or post-COVID syndrome. This term is used to describe a constellation of symptoms that persist several weeks after the onset of COVID-19 and with an uncertain outcome. COVID-19 may damage several organs, including respiratory, renal, vascular, and neurological structures (Mehandru and Merad, 2022). Accordingly, long COVID is mainly characterized by chronic fatigue, dyspnea, pain, and cognitive symptoms. WHO has defined post-COVID condition as a series of symptoms that occur in individuals with a history of SARS-CoV-2 infection, usually three months from the onset of COVID-19 and lasting for at least two months and cannot be explained by an alternative diagnosis (WHO, 2021). Intriguingly, cognitive dysfunction was outlined by this consensus and a recent meta-analysis among one of the three most frequent symptoms (Premraj et al., 2022). In this regard, mounting evidence suggests that patients suffering post-acute COVID syndrome frequently report cognitive complaints, but their frequency, characteristics, and pathophysiology are unknown (Hewitt et al., 2021).
Previous research evaluating neuropsychological dysfunction after COVID-19 mainly used brief cognitive screening measures (e.g. Montreal Cognitive Assessment) and were performed in the setting of patients discharged from the hospital or in the first months after the disease onset (Vanderlind et al., 2021). Heterogeneous results have been observed, suggesting diminished attention, executive function, memory, visuospatial functioning, or language (Raman et al., 2021; Woo et al., 2020; Zhou et al., 2020). Studies performing comprehensive neuropsychological assessments are needed to characterize cognitive functioning in patients with COVID-19, evaluating the extent of cognitive dysfunction in these patients. Furthermore, the cause of cognitive deficits is unknown. Several factors, including hypoxia, vascular lesions, inflammatory dysfunction, sleep disorders, or neuropsychiatric comorbidities, have been suggested (Vanderlind et al., 2021). Identifying factors that contribute to neuropsychological dysfunction is needed to advance in the therapy of cognitive difficulties.
Our aim was two-fold: first, to determine the frequency and characteristics of cognitive dysfunction in patients reporting cognitive complaints after COVID-19; second, to evaluate the correlation between cognitive function and anxiety, depression, sleep, and olfactory function.
2. Methods
2.1. Study design
We conducted a cross-sectional study involving 50 patients with COVID-19 reporting cognitive complaints at least three months after the onset of the disease. The mean age of the patients was 51.06 ± 11.65 years, and 37 (74.0%) were women. Mean years of education were 13.58 ± 4.01, and the time since the onset was 9.12 ± 3.46 months. All participants were native Spanish speakers. Inclusion criteria were as follows: 1) Diagnosis of COVID-19 confirmed by RT-PCR at least three months before the inclusion in the study; 2) Cognitive complaints temporally related with the SARS-CoV-2 infection. Association between cognitive complaints and COVID-19 was reported by the patient, dating the onset of cognitive complaints during or immediately after the acute infection, and after excluding the presence of cognitive symptoms before the infection. Exclusion criteria included: 1) Any cognitive complaint before COVID-19; 2) History of stroke, traumatic brain injury or any neurological disorder potentially associated with cognitive impairment; 3) Active psychiatric disorder or previous psychiatric disease with a potential cognitive effect (e.g. schizophrenia); 4) History of abuse of alcohol or other toxics; 5) Drugs or uncontrolled medical conditions associated with cognitive impairment at the moment of the assessment 6) Sensory disorder potentially biasing cognitive assessments. The main clinical and demographic characteristics of COVID-19 patients are shown in Table 1 .
Table 1.
Main demographic and clinical characteristics during the acute phase.
| Demographics | Age |
51.06 ± 11.65 |
|
|---|---|---|---|
| Sex (% women) |
37 (74%) |
||
| Years of education |
13.58 ± 4.01 |
||
| Handedness |
100% Right |
||
| Arterial hypertension |
14 (28%) |
||
| Diabetes mellitus |
8 (16%) |
||
| Dyslipidemia |
16 (32%) |
||
| Tobacco smoking | 4 (16%) | ||
| COVID history | Time from diagnosis of COVID-19 to assessment (months) | 9.42 ± 3.54 | |
| Anosmia or ageusia | 36 (72%) | ||
| Headache | 42 (84%) | ||
| Confusion | 23 (46%) | ||
| Hospitalization | 18 (36%) | ||
| Days of hospitalization | 19.06 ± 15.53 | ||
| ICU | 5 (10%) | ||
| Ventilatory assistance | 4 (8%) | ||
| MRI findings | Fazekas scale | Grade 0 | 47 (94%) |
| Grade 1 | 3 (6%) | ||
| Grade 2-3 | 0 (0%) | ||
| Presence of microbleeds | 2 (4%) | ||
Patients were recruited from patients consulting due to cognitive issues after COVID-19. Patients were first attended at the general neurology consultations (derived from primary physicians) or cognitive neurology (derived from post-COVID consultations of the Department of Internal Medicine). Patients with cognitive symptoms prior to the SARS-CoV-2 infection were excluded with the information provided by the patient, family or partner, and electronic records from primary care and the central database of medical records from our administrative region. After confirming the fulfilment of inclusion and exclusion criteria, 53 patients were consecutively invited to participate. The rate of acceptance to participate in the study was 94.3%.
2.2. Standard protocol approval and patient consents
The study was conducted with the approval of our hospital's Ethics Committee, and all participants gave written informed consent.
2.3. Neuropsychological and behavioural assessments
The neuropsychological protocol included the following standard paper and pencil tests that were administered in person by a trained neuropsychologist: forward and backward digit span, Corsi block-tapping test, Symbol Digit Modalities Test, Boston Naming Test (BNT), Judgment Line Orientation (JLO), Rey-Osterrieth Complex Figure (ROCF) (copy and recall at 3, 30 min, and recognition), Free and Cued Selective Reminding Test (FCSRT), verbal fluencies (animals and words beginning with “p” and “m" in 1 min each one), Stroop Color-Word Interference Test, and the Visual Object and Space Perception Battery (VOSP). These tests were co-normed and validated in our setting in several neurological disorders (Sanchez-Benavides et al., 2014; Matias-Guiu et al., 2020), and normative data are available in our country (Peña-Casanova et al., 2009, 2012). These normative data are represented using age- and education-adjusted scaled scores. Specifically, scaled-scores of 5 (equivalent to a percentile of ≤5 or z-score < −1.65) are considered as cognitively impaired.
Patients were also assessed using the computerized neuropsychological battery Vienna Test System® (Schuhfried GmbH; Mödling, Austria) with the following tests: the Trail Making Test (TMT, S1 form), Figural Memory Test (FGT, S11 form), Tower of London (TOL-F, S1 form) and Inhibition Response (INHIB, S13 form) (a variant of go-no go task), N-Back Verbal Test (S1 form), Cognitrone (S11 form), Reaction Test (RT, S3 form), Determination Test (DT, S1 form), and the WAF battery (S1 form) of perception and attention functions (Aschenbrenner et al., 2012). TMT, FGT, Tower of London, and Inhibition belong to the COGBAT battery. The computerized battery was self-administered at hospital under the supervision of a trained neuropsychologist.
These tests were selected to cover the main cognitive domains: 1) attention and processing speed (digit span forward, Corsi forward, Symbol Digit Modalities Test, TMT part A, Stroop Word reading and Color Naming, Reaction Test, WAF battery, Cognitrone); 2) executive function (digit span backwards, Corsi backwards, Stroop interference, TMT part B, INHIB, TOL-F, N-back verbal test); 3) episodic memory (FCSRT, ROCF memory at 3, 30 min, and recognition, FGT); 4) Visuospatial function (JLO, VOSP, ROCF copy); and 5) Language (BNT, Verbal fluencies) (McDonald et al., 2022).
Furthermore, we also administered the Brief Smell Identification Test (BSIT) (Doty et al., 1996), State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1983), Beck Depression Inventory-II (Beck et al., 1996), Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), and the Modified Fatigue Impact Scale (Kos et al., 2005). The following cutoffs were used according to the previous literature: BSIT ≤8 were categorized as having abnormal olfaction; STAI-S ≥40 was considered clinically significant anxiety; BDI-II ≥19 was regarded as moderate or severe depression (Beck et al., 1988); PSQI >5 defined poor sleep quality (Byusse et al., 2008); and MFIS ≥38 was considered as having fatigue (Strober et al., 2020). The FLEI questionnaire was administered to evaluate subjective mental ability (Beblo et al., 2010).
The participants were evaluated in 3 sessions lasting 75 min each to avoid fatigue. Patients were also examined using a 3.0 T MRI, to exclude other causes. Main MRI findings are shown in Table 1.
2.4. Normative data and healthy control group
Normative data for the standard neuropsychological tests administered in person were obtained from the Neuronorma studies (Peña-Casanova et al., 2009, 2012). These studies recruited a representative sample of cognitively healthy subjects from different parts of the country using strict inclusion and exclusion criteria. In addition, this battery was co-normed, a strategy that improves the reliability of between-tests comparisons (Peña-Casanova et al., 2009).
Regarding the tests administered using the computerized system, we selected a group of healthy individuals recruited in our setting. Healthy controls and patients with COVID-19 were matched 1:1 on the variables age (±5 years), sex, and level of education.
2.5. Statistical analysis
Statistical analysis was performed using SPSS Statistics 20.0. Case-control matching was performed using MedCalc v.20.0. Descriptive data are shown as mean ± standard deviation or median [interquartile range]. The Shapiro-Wilk's test was used to check normality.
We used the Mann-Whitney U test to compare scores between two groups (patients with COVID-19 and controls). The effect size was estimated with Cohen's d for two means comparison, considering the effect as small (d = 0.2), moderate (d = 0.5), or large (d = 0.8). Findings were considered to be statistically significant when the p-value was <0.05.
In addition, we calculated the percentage of impairment of each test. For standard tests with normative data available from the Neuronorma Project, we considered impaired those age- and education-adjusted scaled score ≤5 (percentile ≤5) (Peña-Casanova et al., 2009). For tests from the computerized battery, correlations between raw scores and age, sex, and years of education were estimated. If correlations were statistically non-significant, Z-scores were calculated, and a z-score < -1.5 was considered impaired. When significant, raw scores were adjusted for age and years of education using linear regression models and saving residuals. A “global cognitive” composite was derived as the mean of z-scores of all cognitive tests. This composite score was normally distributed. It was used to evaluate the association between cognitive status and clinical and demographic factors, and self-perceived cognitive function.
Partial correlations were used for the analysis of correlations between cognitive tests and neuropsychiatric scales, olfactory test, and sleep questionnaire, controlling by age and education. Correlations were regarded as low (<0.30), moderate (0.30–0.49), or high (>0.49).
3. Results
3.1. Comparison between cognitive COVID-19 group and healthy controls
Patients with COVID-19 showed worst scores in the recall and recognition trials of the FGT, Inhibition test, NBV, TMT-A and TMT-B, and in several visual tasks of the WAF battery (intrinsic visual alertness, unimodal selective attention, visual vigilance, and smooth pursuit eye movements) (Table 2 ).
Table 2.
Computerized neuropsychological assessment. Comparison between patients with COVID-19 and controls.
| Raw scores |
Mann Whitney U (p-value) | Effect sizes | Percentage below z < -1.5 |
|||
|---|---|---|---|---|---|---|
| COVID-19 (n = 50) | Controls (n = 50) | COVID-19 | Controls | |||
| Cognitrone – Mean time correct rejectiona | 3.15 ± 1.04 | 3.08 ± 1.12 | 1041 (0.517) | 0.06 | 10.6% | 6.2% |
| Cognitrone – total correct rejection | 33.48 ± 3.18 | 34.04 ± 2.10 | 1082 (0.726) | 0.20 | 14.9% | 4.2% |
| Determination Test - correct reactions | 198.31 ± 48.63 | 215.83 ± 43.99 | 881 (0.066) | 0.37 | 14.9% | 10.4% |
| FGT Learning total | 24.60 ± 11.52 | 28.26 ± 11.11 | 1014 (0.103) | 0.32 | 20% | 0% |
| FGT Delayed Free Recognition I (5 min) | 5.70 ± 2.99 | 7.28 ± 2.38 | 820 (0.003) | 0.58 | 20% | 4% |
| FGT Delayed Free Recognition II (30 min) | 5.86 ± 2.74 | 6.96 ± 2.13 | 914 (0.019) | 0.44 | 16% | 4% |
| FGT Recognition | 14.40 ± 4.46 | 15.98 ± 3.33 | 860 (0.007) | 0.40 | 20% | 2% |
| NBV Incorrect responses a | 14.08 ± 37.00 | 5.04 ± 7.58 | 839 (0.004) | 0.33 | 14% | 6% |
| RT Motor speeda | 256.28 ± 95.34 | 239.58 ± 93.55 | 954 (0.329) | 0.17 | 8.9% | 4.2% |
| RT Reaction speeda | 509.63 ± 109.84 | 478.12 ± 98.13 | 877 (0.062) | 0.30 | 14.9% | 8.3% |
| TMT-Aa | 27.17 ± 13.59 | 23.18 ± 11.06 | 864 (0.008) | 0.32 | 8% | 6% |
| TMT-Ba | 46.31 ± 24.73 | 37.96 ± 25.59 | 855 (0.006) | 0.33 | 10% | 8% |
| Inhibition errorsa | 7.74 ± 3.91 | 5.84 ± 3.20 | 903 (0.016) | 0.53 | 32% | 10% |
| ToL planning capacity | 12.60 ± 4.66 | 13.73 ± 3.86 | 1036 (0.242) | 0.26 | 16.3% | 8.2% |
| WAF Intrinsic alertness (visual) a | 295.40 ± 121.32 | 248.85 ± 73.34 | 885 (0.017) | 0.46 | 22.4% | 6% |
| WAF crossmodal divided attention (visual – auditive) a | 561.37 ± 216.40 | 569.72 ± 191.29 | 1152 (0.863) | 0.04 | 8.3% | 6.1% |
| WAF unimodal selective attention (visual)a | 429.66 ± 124.86 | 391.73 ± 83.12 | 836 (0.043) | 0.35 | 21.7% | 6.2% |
| WAF Visual vigilancea | 504.63 ± 124.20 | 461.88 ± 107.38 | 717 (0.005) | 0.36 | 4.3% | 4.3% |
| WAF Smooth pursuit eye movementsa | 381.80 ± 118.34 | 342.49 ± 71.93 | 845 (0.050) | 0.40 | 21.7% | 6.2% |
Statistically significant p-values are shown in bold.
A higher value means a worst performance of this test.
3.2. Frequency of impairment
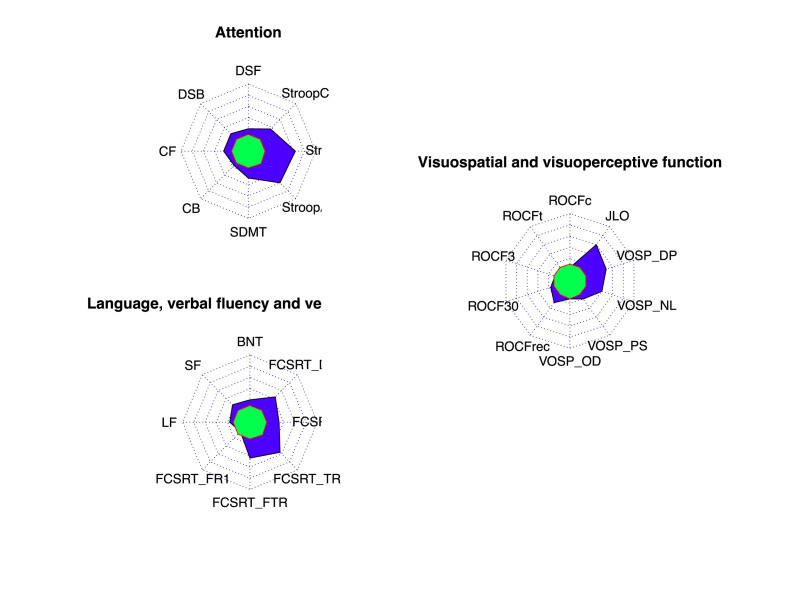
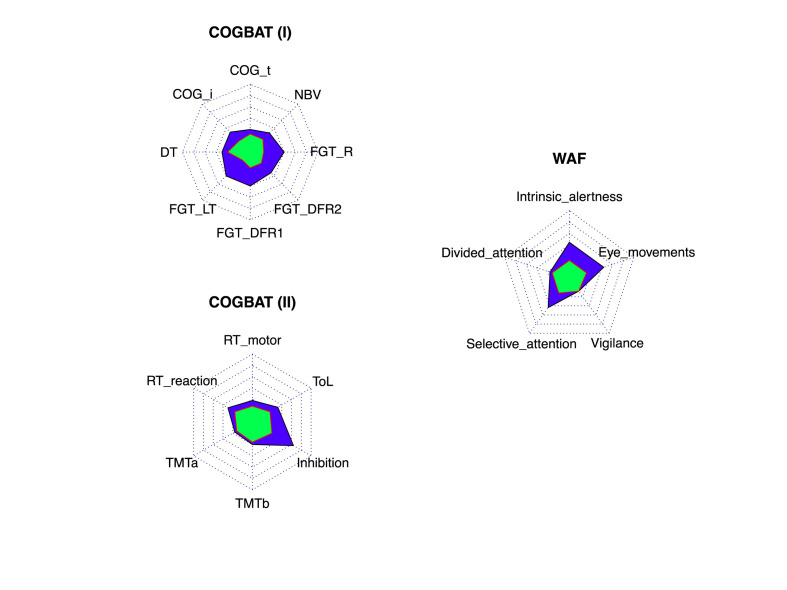
The frequency of impairment was at least three times more frequent in COVID-19 patients reporting cognitive symptoms than in the control group in Cognitrone (total correct rejection), all scores of the FGT, Inhibition, WAF visual intrinsic alertness, WAF unimodal selective attention, and WAF smooth pursuit eye movements. The frequency of impairment was two times more frequent in NBV and ToL (Fig. 1 ).
Fig. 1.
Radar chart representing the percentage of patients showing age- and education-adjusted scaled score ≤5 in healthy controls (green) and COVID-19 (blue) in standard tests. Each concentric line represents a 10%. Percentage in healthy controls is an estimate according to normative data. Abbreviations: BNT: Boston Naming Test; DSF: Digit Span Forward; DSB: Digit span backwards; CF: Corsi forward; CB: Corsi Backwards; FCSRT: Free and Cued Selective Reminding Test (FR1: Free Recall Trial 1; FTR: Free Total Recall; TR: Total Recall; DFR: Delayed Free Recall; DTR: Delayed Total Recall); JLO: Judgment Line Orientation; LF: letter fluency; ROCF: Rey-Osterrieth Complex Figure (c: copy accuracy; t: copy time; 3: memory at 3 min; 30: memory at 30 min; rec: recognition); SDMT: Symbol Digit Modalities Test; SF: Semantic Fluency; Stroop A (word reading); Stroop B (color naming); Stroop C (interference); VOSP: Visual Object Space Perception Battery (DP: discrimination of position; NL: number location; OD: object decision; PS: progressive silhouettes).
The frequency of age- and education-adjusted scaled scores ≤5 for each test is shown in Fig. 2 . Frequency of impairment was at least three times more frequent than expected in a cognitively healthy population in the FCSRT (total free recall, total recall, delayed free recall, and delayed total recall), Stroop test, VOSP (discrimination of position and number location), and JLO. In the digit span forward and backwards, Corsi forward, ROCF (memory recognition), verbal fluency (animals and “p" words) and VOSP (progressive silhouettes), the frequency was at least two times more frequent than expected.
Fig. 2.
Radar chart representing the percentage of patients showing z-scores ≤1.5 (or ≥1.5 when appropriate) in healthy controls (green) and COVID-19 (blue) in the computerized battery. Each concentric line represents a 10%. Abbreviations: COG: Cognitrone (i: total correct rejection; t: mean time correct rejection); DT: determination test; NBV: N-back verbal; FGT (DFR1: Delayed Free Recognition at 5 min, DFR2: Delayed Free Recognition at 30 min; LT: Learning Total; R: Recognition); RT: Reaction Test; TMT: Trail Making Test; ToL: Tower of London.
Mean STAI-S (State) and STAI-T (Trait) was 41.08 ± 12.29 and 49.34 ± 11.47, respectively. The mean BDI score was 16.00 ± 8.86. Sleep quality according to PSQI was 10.10 ± 4.75, and BSIT was 9.00 ± 2.33. Mean MFIS was 55.15 ± 15.15. According to the specified cutoffs, 26 (52%) patients were regarded as having anxiety, 15 (30%) had depression (at least moderate), 40 (80%) had poor sleep quality, and 20 (40%) showed olfactory dysfunction at the moment of the assessment. According to MFIS, 43 (80%) had fatigue.
3.3. Correlations between cognitive tests with fatigue, sleep, olfaction, and neuropsychiatric scales
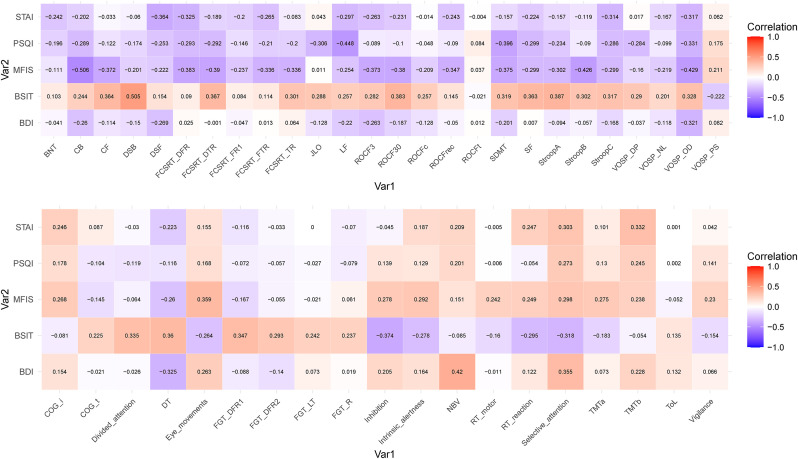
MFIS showed moderate correlations with Corsi test (backward), SDMT, FCSRT (delayed free and delayed total recall), ROCF (memory at 30 min), Stroop (part B), VOSP (object decision), and smooth pursuit eye movements. PSQI showed moderate correlations with SDMT and letter fluency.
BSIT showed moderate correlations with digit span (backwards), ROCF (memory at 30 min), and Stroop A. In the computerized battery, BSIT showed moderate correlations with the Inhibition test, Determination Test, divided attention, selective attention, and FGT (Delayed Free Recognition I).
STAI showed moderate correlations with digit span forward, FCSRT (delayed free recall), VOSP (object decision), TMT part B, and WAF (unimodal visual selective attention). BDI only correlated with N-Back, Determination Test, and selective attention in the computerized battery. All correlations are shown in Fig. 3 .
Fig. 3.
Heatmap of Pearson correlations between STAI, PSQI, MFIS, BSIT, and BDI with neuropsychological tests (A: Standard tests; B: Computerized battery).
3.4. Association between global cognitive composite and self-perceived cognition
Global cognitive composite showed moderate correlations with FLEI subscores (attention r = −0.344, p = 0.015; executive functioning r = −0.431, p = 0.002; memory r = 0.349, p = 0.014; and mental capacity r = −0.408, p = 0.004). Composite score also correlated with olfactory function (r = 0.448, p = 0.001), sleep quality (r = −0.328, p = 0.022) and anxiety (r = −0.342, p = 0.016). It was not correlated with depression (r = −0.234, p = 0.109) or time since acute COVID-19 (r = 0.020, p = 0.889).
BDI correlated with FLEI executive function (r = 0.553, p < 0.001) and mental capacity (r = 0.416, p = 0.004), but not with the other subscales (p > 0.05). Sleep questionnaire only correlated with FLEI executive function (r = 0.363, p = 0.011). STAI-S correlated with FLEI executive function (r = 0.485, p < 0.001), attention (r = 0.375, p = 0.009), and mental capacity (r = 0.408, p = 0.004).
4. Discussion
In this study, we evaluated the presence of cognitive dysfunction in patients with COVID-19 reporting cognitive complaints that persisted after the acute phase. We found a lower than expected performance in several cognitive tests, which is consistent with the existence of cognitive dysfunction in this subgroup of COVID-19 patients. Two different procedures support these findings. On the one hand, a battery of standard neuropsychological tests administered by a trained neuropsychologist in person and using normative data from a large multicentre normative available in our country. On the other hand, a computerized battery by comparison with a matched healthy control group recruited in our centre. In this regard, several cognitive tasks were impaired two, three or four times more than expected in healthy controls. Similarly, statistically significant differences were observed in many tests in comparison with healthy controls. These findings confirm that patients reporting cognitive complaints after COVID-19 actually showed lower performance on cognitive testing. Importantly, our findings are restricted to patients reporting cognitive symptoms after COVID-19, but they are not generalizable to all patients affected by COVID-19. Furthermore, although we only enrolled patients with no known factors associated with cognitive impairment, we cannot exclude the role of other reasons explaining cognitive dysfunction beyond COVID-19, including vulnerabilities (psychological, medical or environmental) before or during the infection.
Another remarkable finding was the analysis of the specific cognitive tests impaired. COVID-19 patients showed a diminished performance on several tests evaluating the following cognitive functions. First, attention and executive function, with alterations in processing speed (SDMT, TMT-A), divided attention (TMT-B), selective attention (WAF and Stroop), visual vigilance, intrinsic alertness, working memory (span, N-back), and inhibition (Inhibition test, Stroop). Second, episodic memory (FCSRT, FGT). And third, visuospatial processing (JLO, VOSP, visual tasks of WAF battery).
Notably, effect sizes were generally small for most cognitive tests. This finding suggests that, on average, the magnitude of cognitive deficits was generally small. However, considering that this performance is detected in young patients, it could have a high socio-economic impact.
One of the most interesting findings of our study is the low correlation of cognitive tests with neuropsychiatric scales. Although according to previous studies anxiety and depression in our sample was present in a subgroup of patients (Vanderlind et al., 2021), scores in depression and anxiety questionnaires did not significantly correlate with most cognitive performance. In tests showing statistically significant correlations, the percentage of the variance of the cognitive test explained by depression or anxiety scales was low (<15%). This suggests that depression and anxiety do not explain cognitive findings in these patients and supports that the cognitive disorder is not secondary to psychological aspects. As expected, depression was weakly correlated with some attentional tasks, especially the N-back, which has been previously suggested as a cognitive signature in depressed patients (Nikolin et al., 2021). Most of the patients included in our study did not require hospitalization, and ICU admission was performed in 10% of cases, suggesting that cognitive complaints also occurred in patients with mild forms of acute COVID-19.
Our study has some limitations. First, we only enrolled patients reporting cognitive complaints after COVID-19 without any previous potential cause of cognitive dysfunction. Hence, our findings are limited to these patients and not to all patients with COVID-19. Second, we did not have previous neuropsychological assessments of patients enrolled in this study. Consequently, it is not possible to draw definitive conclusions about a causal relationship between COVID-19 and cognitive dysfunction. However, we tried to reduce the impact of this limitation using strict inclusion and exclusion criteria. Accordingly, the prevalence of risk factors (e.g. arterial hypertension, diabetes) is low, comorbidities with a potential cognitive impact were specifically excluded, and the visual analysis of MRI did not reveal significant findings. In addition, we used two methods of analysis from two independent control groups (comparison with a healthy control group and use of normative data at country level) with consistent findings. Third, we did not examine potential associations between cognitive deficits and clinical, demographic, or neuroimaging characteristics. Future studies with larger samples are necessary to evaluate these features, which are essential to understand the pathophysiology of cognitive dysfunction in COVID-19 patients (Matias-Guiu et al., 2020; Balcom et al., 2021). Patients in our study were evaluated 9.42 ± 3.54 months after COVID-19 onset of symptoms, which excluded patients in an acute confusional state and it hints that cognitive dysfunction may be detected several months after the acute stage.
In conclusion, our study shows that patients with COVID-19 reporting cognitive symptoms showed a reduced cognitive performance, especially in the attention-concentration and executive functioning, episodic memory, and visuospatial processing domains. Cognitive performance was correlated with olfactory dysfunction and sleep quality and anxiety to a lesser extent, but not depression. Self-perceived cognitive functions were correlated with both cognitive performance and mood. Our study is a first step characterizing cognitive dysfunction in patients reporting cognitive complaints after COVID-19. Future studies combining cognitive assessment with a multimodal evaluation (such as neuroimaging, immunological measurements, serum or CSF biomarkers) and longitudinal follow-up are necessary to disentangle the specific mechanisms associated with COVID-19 cognitive dysfunction. In addition, population-based epidemiological studies are necessary to define the frequency and consequences of cognitive deficits in patients with COVID-19.
Author contributions (CREDIT roles)
Cristina Delgado-Alonso: data curation; formal analysis; investigation; writing original draft;
Maria Valles-Salgado: data curation; investigation; writing review and editing.
Alfonso Delgado-Álvarez: data curation; formal analysis; writing review and editing.
Miguel Yus: data curation; project administration; writing review and editing.
Natividad Gómez-Ruiz: data curation; writing review and editing.
Manuela Jorquera: investigation; writing review and editing.
Carmen Polidura: data curation; investigation; writing review and editing.
María José Gil: data curation; investigation; writing review and editing.
Alberto Marcos: data curation; investigation; writing review and editing.
Jorge Matías-Guiu: funding acquisition; methodology; project administration; writing review and editing.
Jordi A Matías-Guiu: data curation; formal analysis; funding acquisition; methodology; project administration; writing original draft.
Funding
This research has received funding from the Nominative Grant FIBHCSC 2020 COVID-19 (Department of Health, Community of Madrid). Jordi A Matias-Guiu is supported by Instituto de Salud Carlos III through the project INT20/00079 (co-funded by European Regional Development Fund “A way to make Europe”). María Valles-Salgado is supported by the Instituto de Salud Carlos III through a predoctoral contract (FI20/000145) (co-funded by European Regional Development Fund “A way to make Europe”).
Availability of data and material
The datasets generated and analysed are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the Ethics and Research Committee from our centre (code 20/633-E) and was performed according to the Declaration of Helsinki and its later amendments.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
We acknowledge Dr Andrea Valcárcel, Dr Mariam Farid, and Dr Ernesto Botella, from the Department of Internal Medicine of our centre, and Dr Maria Romeral, Dr José Luis González, Dr Patricia Simal, and Dr Jesús Porta-Etessam, from the Department of Neurology, for the help in the recruitment.
References
- Aschenbrenner S., Kaiser S., Pfüller U., Roesch-Ely D., Weisbrod M. Schuhfried GmbH; Mödling: 2012. Testset COGBAT. [Google Scholar]
- Balcom E.F., Nath A., Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain. 2021;144:3576–3588. doi: 10.1093/brain/awab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T., Kunz M., Brokate B., Scheurich A., Weber B., Albert A., Richter P., Lautenbacher S. Entwicklung eines Fragebogens zur subjektiven Einschaätzung der geistigen Leistungsfahigkeit (FLei) bei Patienten mit psychischen Störungen. Z. für Neuropsychol. 2010;21:143–151. [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. second ed. Manual. The Psychological Corporation; San Antonio, TX: 1996. BDI-II. Beck Depression Inventory. [Google Scholar]
- Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Hall M.L., Strollo P.J., Kamarck T.W., Owens J., Lee L., Reis S.E., Matthews K.A. Relationships between the Pittsburgh sleep quality Index (PSQI), epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J. Clin. Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- Doty R.L., Marcus A., Lee W.W. Development of the 12-item cross-cultural Smell identification test (CC-sit) Laryngoscope. 1996;6:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Hewitt K.C., Marra D.E., Block C., Cysique L.A., Drane D.L., Haddad M.M., Lojek E., McDonald C.R., Reyes A., Eversole K., Bowers D. Central nervous system manifestations of COVID-19: a critical review and proposed research agenda. J. Int. Neuropsychol. Soc. 2021;1:15. doi: 10.1017/S1355617721000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos D., Kerckhofs E., Carrea I., Verza R., Ramos M., Jansa J. Evaluation of the modified fatigue impact scale in four different European countries. Mult. Scler. 2005;11:76–80. doi: 10.1191/1352458505ms1117oa. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu J.A., Sánchez-Benavides G., Rivera-Àvila N., Cortés-Martínez A., Delgado-Alonso C., Delgado-Álvarez A., Montero P., Pytel V., Matías-Guiu J., Peña-Casanova J. Validation of the Neuronorma battery for neuropsychological assessment in multiple sclerosis. Mult. Scler. Relat. Disor. 2020;42:102070. doi: 10.1016/j.msard.2020.102070. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu J., Gómez-Pinedo U., Montero-Escribano P., Gómez-Iglesias P., Porta-Etessam J., Matías-Guiu J.A. Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia. 2020;35:170–175. doi: 10.1016/j.nrl.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C.R., Busch R.M., Reyes A., Arotta K., Barr W., Block C., Hessen E., Loring D.W., Drane D.L., Hamberger M.J., Wilson S.J., Baxendale S., Hermann B. For the IC-CoDE task force. Neuropsychology. 2022 doi: 10.1037/neur0000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolin S., Tan Y.Y., Schwaab A., Moffa A., Loo C.K., Martin D. An investigation of working memory deficits in depression using the n-back task: a systematic review and meta-analysis. J. Affect. Disord. 2021;284:1–8. doi: 10.1016/j.jad.2021.01.084. [DOI] [PubMed] [Google Scholar]
- Peña-Casanova J., Blesa R., Aguilar M., Gramunt-Fombuena N., Gómez-Ansón B., Oliva R., Molinuevo J.L., Robles A., Barquero M.S., Antúnez C., Martínez Parra C., Frank-García A., Fernández M., Alfonso V., Sol J.M., NEURONORMA Study Team Spanish multicenter normative studies (NEURONORMA Project): methods and sample characteristics. Arch. Clin. Neuropsychol. 2009;24:307–319. doi: 10.1093/arclin/acp027. [DOI] [PubMed] [Google Scholar]
- Peña-Casanova J., Casals-Coll M., Quintana M., Sánchez-Benavides G., Rognoni T., Calvo L., Palomo R., Aranciva F., Tamayo F., Manero R.M. Spanish normative studies in a young adult population (NEURONORMA young adults Project): methods and characteristics of the sample. Neurologia. 2012;27:253–260. doi: 10.1016/j.nrl.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Premraj L., Kannapadi N.V., Briggs J., Seal S.M., Battaglini D., Fanning J., Suen J., Robba C., Fraser J., Cho S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., Okell T., Sheerin F. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, posthospital discharge. EClinicalMed. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Benavides G., Peña-Casanova J., Casals-Coll M., Gramunt N., Molinuevo J.L., Gómez-Ansón B., Aguilar M., Robles A., Antúnez C., Martínez-Parra C., Frank-García A., Fernández-Martínez M., Blesa R., NEURONORMA Study Team Cognitive and neuroimaging profiles in mild cognitive impairment and Alzheimer's disease: data from the Spanish Multicenter Normative Studies (NEURONORMA Project) J. Alzheimers Dis. 2014;41:887–901. doi: 10.3233/JAD-132186. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Strober L.B., Bruce J.M., Arnett P.A., Alschulet K.N., DeLuca J., Chiaravalloti N., Lebkuechr A., Di Benedetto M., Cozart J., Thelen J., Guty E., Román C.A.F. Tired of not knowing what fatigue score means? Normative data of the Modified Fatigue Impact Scale (MFIS) Mult. Scler. Relat. Disord. 2020;46:102576. doi: 10.1016/j.msard.2020.102576. [DOI] [PubMed] [Google Scholar]
- Vanderlind W.M., Rabinovitz B.B., Miao I.Y., Oberlin L.E., Bueno-Castellano C., Fridman C., Jaywant A., Kanellopoulos D. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr. Opin. Psychiatr. 2021;34:420–433. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., Schmiedel S., Addo M.M., Gerloff C., Heesen C., Schulze Zur Wiesch J., Friese M.A. Frequent neurocognitive deficits after recovery from COVID-19. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa205. fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 6 October 2021. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. WHO/2019-nCoV/Post_COVID-19_condition/Clinical_case_definition/2021.1. [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed are available from the corresponding author on reasonable request.