Abstract
Measurable (minimal) residual disease (MRD) status in acute lymphoblastic leukemia (ALL) has largely superseded the importance of traditional risk factors for ALL, such as baseline white blood cell count, cytogenetics, and immunophenotype, and has emerged as the most powerful independent prognostic predictor. The development of sensitive MRD techniques, such as multicolor flow cytometry (MFC), quantitative polymerase chain reaction (PCR), and next-generation sequencing (NGS), may further improve risk stratification and expand its impact in therapy. Additionally, the availability of highly effective agents for MRD eradication, such as blinatumomab, inotuzumab ozogamicin, and chimeric antigen receptor (CAR) T-cell therapies, enabled the development of frontline regimens capable of eradicating MRD early in the treatment course. While long-term follow-up of this approach is lacking, it has the potential to significantly reduce the need for intensive post-remission treatments, including allogeneic bone marrow transplantation, in a significant proportion of patients with ALL.
Keywords: acute lymphoblastic leukemia, minimal residual disease, multicolor flow cytometry, polymerase chain reaction, next-generation sequencing
Introduction
The utilization of multi-agent chemotherapy has significantly improved outcomes in adult patients with acute lymphoblastic leukemia (ALL). Most patients achieve complete remission (CR) after standard induction chemotherapy; however, relapses are common. These relapses are caused by the persistent leukemic blasts resistant to cytotoxic chemotherapy and remain the primary cause of mortality in patients with ALL.1 Present at low levels during remission, residual leukemic blasts are usually undetected by morphological examination. However, with the use of multiparameter flow cytometry (MFC) and polymerase chain reaction (PCR), the residual disease can be detected in approximately 30–50% of cases that achieve CR.1–3 Persistent leukemic cells in the setting of remission are referred to as measurable (minimal) residual disease (MRD), which defines the remaining disease burden after therapy.1
MRD has become an essential prognostic tool for all ALL subtypes, including B-cell (Philadelphia chromosome-positive or negative) and T-cell lineages.4,5 Across all treatment regimens and methods of MRD evaluations, the detection of MRD after therapy correlates with worse disease-free survival (DFS) and overall survival (OS). In addition to its prognostic significance, MRD status can impact treatment decisions by guiding therapy to include MRD clearing regimens such as blinatumomab and suggesting or avoiding subsequent consolidation approaches without allogeneic stem cell transplantation (ASCT). Although MRD timing and evaluation are well established in pediatric ALL, the MRD status already guides treatment intensity and decision-making regarding (ASCT), MRD’s optimal timing and effect in adult ALL remain to be determined. This review will discuss the assessment, management, and prognostic role of MRD in adult patients with ALL.
Assessment of Measurable Residual Disease
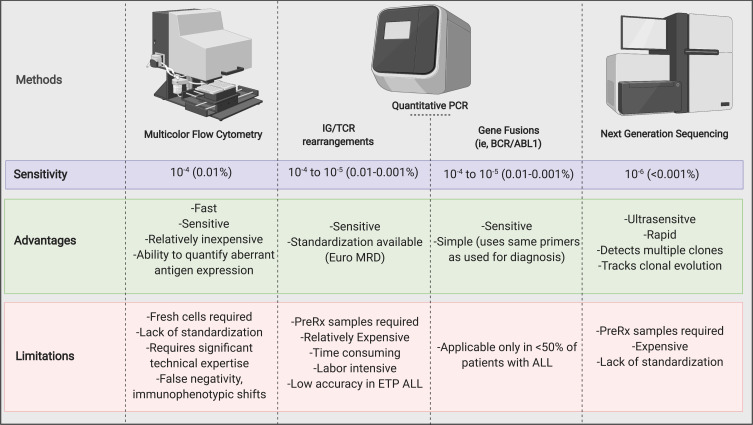
Several laboratory techniques have been validated to detect and quantify MRD in ALL. MFC and quantitative PCR by the analysis of recurrent gene fusions (eg, BCR/ABL1) or rearranged immunoglobulin (IG) or T-cell receptor (TCR) genes are the most used techniques.6 However, more sensitive, and specific MRD techniques have also been developed, such as high throughput next-generation sequencing (NGS) and droplet digital PCR. In Figure 1, we summarized the advantages and disadvantages of the commonly used MRD techniques.
Figure 1.
Measurable Residual Disease Assessment Methods.
Note: Created with https://BioRender.com.
Abbreviations: IG/TCR, immunoglobulin and T-cell receptor; Pre-Rx, before treatment.
Multiparametric Flow Cytometry
MFC is a fast and relatively cheaper technique that applies to most ALL cases. Test results are frequently finalized within 1–5 days, depending on the laboratory. Standard of care MFC assays can identify the persistent leukemic cells at the level of 10−4 (1 out of 10,000 nucleated cells) sensitivity by analyzing aberrant leukemia-associated immunophenotypes (LAIPs), including aberrant expression of myeloid antigens or altered density of antigens commonly expressed on benign lymphoid precursors.1 Use of ≥8-color flow cytometry assays allows for the analysis of the expression of more antigens.7–9 Compared with standard MFC (3–4 color assay) flow cytometry, ≥8-color flow cytometry increases the diagnostic accuracy.7–9
By one MFC-based approach, all LAIPs identified on leukemic cells at diagnosis are analyzed during treatment and count as MRD if they are still detected in the remission samples.1 However, the immunophenotypic shift can arise because of treatment which can compromise the accuracy of this method (comparing LAIPs between diagnostic and remission), potentially resulting in false-negative MRD assessments.
Another MFC-based methodology called the “different from normal” (DfN) approach detects the difference in immunophenotypes observed in remission samples as compared with normal immunophenotype distribution.10 Thus, MRD may be analyzed without the need for an initial diagnostic sample or irrespective of immunophenotypic shift throughout therapy.6,10 However, there may be less diagnostic certainty with DfN approach, and therefore in most laboratories where this approach is practiced, baseline LAIPs are used for comparison (when baseline sample available). Some groups, such as European Leukemia Network, have advocated using an integrated “LAIP-based DfN” approach to evaluate MRD.11 Irrespective of the specific methods used, the lack of uniformity between laboratories and pathologists has been one of the important limitations of MFC for MRD assessment.1 Furthermore, analyzing antigen expression patterns and maturation of normal hematopoietic precursors in resting and regeneration states and interpreting the resultant data require significant expertise and knowledge, especially when the DfN approach used.
Real-Time Quantitative PCR
Real-time quantitative PCR (RQ-PCR) is a highly sensitive methodology that is used to detect and quantify MRD in ALL. In Ph-negative B-cell ALL and T-cell ALL, rearranged immunoglobulin IG or TCR genes, and in Ph-positive ALL, BCR-ABL1 mRNA transcripts are the predominantly chosen MRD targets for PCR.12,13 Other gene fusion involving CRLF2 or MLL might be utilized as targets in other ALL subtypes; however, there is limited clinical evidence to support their use as MRD indicators.1 Although methodologies are different, MFC and RQ-PCR assays result in highly concordant results.14,15 The choice between MFC and RQ PCR techniques depends on the availability and level of expertise of different laboratories.
RQ-PCR examines unique sequences of the junctional regions of rearranged IG, or TCR genes for which allele-specific oligonucleotides (ASO) are developed for each patient in Ph-negative B-cell ALL and T-cell ALL.14,16 Primers identified at baseline are then used for MRD quantification in subsequent post-therapy samples. Despite having a greater sensitivity than MFC (down to 10–5), designing ASO-PCR for each patient is a time-consuming, expensive, and complicated process that requires significant expertise.17 This method can be used in most patients with Philadelphia negative ALL.17 However, using ASO-PCR in early precursor T-ALL is challenging because the lymphoblasts are immature and usually have not completed TCR gene rearrangement.18
Reverse transcriptase PCR (RT-PCR) is a method that utilizes the BCR-ABL1 gene as PCR target in Philadelphia positive ALL. MRD is followed by quantifying BCR-ABL1 mRNA transcripts using the standard probes used for diagnostic.19 This is a simple, rapid, and broadly applicable MRD detection technique in Philadelphia positive ALL.
Next-Generation Sequencing
NGS is a novel technique for detecting MRD in ALL that can address some of the limitations of traditional MRD methods. NGS use multiplex PCR without patient-specific probes to amplify various combinations of altered IG and TCR genes concurrently. Therefore, it can detect and quantify numerous clones and subclones that can be monitored during the course of treatment.20,21 NGS is a relatively quick and reliable technique, with excellent concordance to traditional MFC or PCR procedures. An important advantage of NGS is the high level of sensitivity detecting 1 leukemic cell in 1,000,000 nucleated cells (sensitivity of 10–6).22 However, the clinical significance of very low levels of MRD is not well established. ClonoSEQ NGS technology (Adaptive Biotechnologies) has become the first NGS MRD assay in ALL authorized by the US Food and Drug Administration (FDA).23 Additional research examining the clinical impact of this extremely sensitive technique is eagerly awaited.
Prognostic Role of MRD in ALL
MRD information has surpassed the significance of conventional risk factors for ALL such as baseline white blood cell count, cytogenetics, and immunophenotype, and became the strongest independent prognostic factor.3,24–31 According to a large meta-analysis performed in 13,637 patients with ALL (including all ALL subtypes), those who achieved MRD negative response by MFC had superior event-free survival (EFS) and overall survival (OS). The 10-year EFS rate for MRD negative versus MRD positive patients was 77% vs 32% in children and 64% vs 21% in adults.32 A subsequent meta-analysis performed in adult patients with ALL confirmed these findings by demonstrating significant survival benefit for patients who achieved MRD negativity.33 Even though each contributing study differs in its design and patient demographics, these results support the use of MRD as a clinical tool for evaluating prognosis in ALL.
Depth of MRD
Among patients with detectable MRD, the level of MRD may also have prognostic implications. In one study, ALL patients with relatively lower MRD levels (10–4/10–3 versus 10–1) by either MFC or ASO-PCR had superior relapse free survival (RFS) and OS rates.34 Although the achievement of lower MRD levels was favorable, the best outcomes were achieved with the absence of MRD.
Time to MRD Negativity
Along with the depth of the MRD response, time to MRD negativity is a clinically significant predictor of outcome, as demonstrated in multiple studies.25,35 For example, in a recent study, 215 patients with Philadelphia B-ALL were classified according to the length of time required to achieve MRD response (early vs late MRD negativity).35 Patients who achieved MRD negativity early (median time to MRD negativity 24 days) had significantly superior 3-year EFS and OS as compared with late MRD responders (median time to MRD negativity 110 days), 65% and 76% vs 42% and 58%, respectively. In a study by Bruggeman et al. PCR was used to track MRD in 196 patients for up to 9 months in the first year of treatment.27 The frequency of MRD positivity dropped from 88% at early induction to 13% at week 52.27 Identification of MRD predicted relapse at different time periods.27 For patients with fast MRD drop to 10−4 or below detection limit on days 11 and 24, the 3-year relapse rate was 0%.27 A high-risk group of 23% had an MRD of 10−4 or higher until week 16, with a 3-year relapse risk of 94%.27 These findings suggest that the impact of early MRD eradication is important and should be evaluated prospectively in future clinical trials in ALL.
Impact of Baseline Genomic Aberrations
Concurrent use of MRD information and baseline cytogenetic and molecular abnormalities in different ALL subtypes may enhance prediction of relapse. In one study, detection of IKZF1 gene deletion and MLL gene rearrangement in B-ALL, absence of NOTCH1/FBXW7 mutation and presence of KRAS/NRAS mutation or PTEN alterations in T-ALL were associated with poor outcomes.28 Both detectable MRD and unfavorable molecular alterations were independently associated with relapse and poor OS. In another study, baseline cytogenetic abnormalities such as low hypodiploidy/near triploidy and complex karyotype (defined as ≥5 chromosomal abnormalities) were associated with worse outcomes irrespective of MRD status.36 Hence, while achievement of undetectable MRD is a desirable outcome for all patients, it may not overcome the unfavorable impact of high-risk cytogenetic and molecular alterations. Prospective clinical trials are required to determine how to incorporate MRD status, cytogenetic, and molecular profiling into risk stratification schemes.37
Philadelphia-Chromosome Positive ALL
The presence of BCR/ABL1 fusion gene product provides an additional MRD monitoring tool for patients with Philadelphia positive ALL. Detection of MRD by RT-PCR of BCR/ABL1 transcripts is associated with worse outcomes.31,38,39 In a study, 85 patients with newly diagnosed Philadelphia positive ALL who received hyper-CVAD plus a tyrosine kinase inhibitor had MRD assessments at remission (CR) and at a 3-month time point.40 Achievement of complete molecular remission (undetectable BCR ABL 1 transcript by PCR) at the 3-month mark was significantly associated with longer OS (median 127 versus 38 months). Prospective clinical trials incorporating MRD-based treatment strategies for patients with Philadelphia positive ALL may elucidate the ideal post-remission therapy.
The Impact of Peritransplant MRD
Identification of MRD in both pre-and-post-ASCT settings predicts worse outcomes.41,42 In a study, adult ALL patients with pre-ASCT MRD a level ≥10−4 (measured by NGS) were more likely to relapse after ASCT (HR 7.7, 95% CI: 2.0–30, p < 0.01).43 Similarly, in pediatric patients, achieving MRD negativity prior to ASCT resulted in significantly better DFS and OS than those with positive MRD; 83% and 92% vs 41% and 64%, respectively.42 In a prospective study of pediatric patients with R/R ALL, pre-ASCT MRD was prognostic in the setting.41 MRD emergence after ASCT is also a sign of impending relapse. In a study of patients with ALL who were treated on Northern Italian leukemia group protocols, identification of MRD as measured by RQ-PCR at day +100 after ASCT was associated with a higher risk of relapse as compared with patients with undetectable MRD; 80% versus 7%, respectively.44 These data suggest that sequential MRD monitoring after ASCT has crucial importance as it may guide early post-ASCT therapy.
Role of MRD in Salvage Settings
While MRD status is significantly prognostic in the frontline setting, its impact is less clear in the salvage setting. The significance of MRD status seems to be more pronounced in earlier salvages than in later salvages. In a study of 130 patients with R/R ALL, achievement of MRD negativity (by MFC) at the time of best response was shown to be associated with superior EFS; median 18 versus 7 months, in the first salvage setting.45 However, achievement of MRD negative CR in the second and beyond salvages had no impact on survival. Patients who achieved undetectable MRD and underwent ASCT had the best outcome (2-year OS rate of 65%), irrespective of salvage status.
Role of MRD in Treatment Decisions
MRD status is used not only for risk stratification but also for post-induction treatment decision-making. By tailoring therapy based on MRD, patients with a high probability of relapse may receive risk-adapted treatments, such as the inotuzumab ozogamicin (anti-CD22 antibody-drug conjugate) or blinatumomab (CD3-CD19 bispecific T-cell engager), with or without subsequent ASCT. On the contrary, patients with a lower possibility of relapse (early MRD negativity, absence of adverse risk cytogenetic and molecular abnormalities) may benefit from less intensive treatment approaches and potentially avoid ASCT in CR1.
Eradicating MRD with ASCT
ASCT in CR1 is associated with a lower possibility of relapse and longer OS in patients with ALL who failed to achieve MRD negative response.46 The German multicenter study group for adult ALL (GMALL 07/03 trial) prospectively investigated the impact of MRD (≥10−4 measured by RQ-PCR) after induction/consultation therapy in patients with Philadelphia negative ALL.3 Overall, 47% of the patients with suboptimal MRD response received ASCT in CR1. Furthermore, the probability of continuous CR (after five years) was higher for patients with suboptimal MRD response and ASCT in CR1 than those without ASCT in CR1 (66% versus 12%; P <0.0001). The Landmark analysis also showed superior 5-year OS for patients who received ASCT in CR1 than those who received no ASCT (54% versus 33%; P=0.06). Conversely, patients who achieved MRD negative status had 5-year OS rates of 81% in the absence of ASCT. In the ALL-AR-03 clinical trial (PETHEMA, Treatment of High-Risk Adult Acute Lymphoblastic Leukemia [LAL-AR/2003]), patients with high risk (age 30–60 years, MLL rearrangement, or WBC > 30 × 109/l) Philadelphia-chromosome negative ALL were assigned to chemotherapy alone or chemotherapy followed by ASCT based on MRD response and early cytologic response (less than 10% leukemic blasts in bone marrow at day 14 of induction). Patients with the optimal response (MRD by MFC ≤10−4 and early cytologic response) continued to receive chemotherapy alone (N=108), and others with suboptimal response were assigned to receive ASCT (N=71). The 5-year DFS and OS in patients who have achieved optimal and suboptimal responses were 55% and 59%, and 32% and 37%, respectively. Both clinical trials suggest that MRD assessment at CR can be used to select patients who are likely to benefit from ASCT in CR1. They also highlight the relatively poor outcomes for patients who undergo ASCT with persistent MRD.
Total body irradiation (TBI) based conditioning regimens are the standard of care in the treatment of patients with ALL who requires ASCT. However, TBI is associated with late side effects, and therefore regimens without TBI have also been developed. In a retrospective study, NGS-based pre-ASCT MRD assessment was performed in 56 children and young adults with ALL.47 It was shown that patients with negative MRD by NGS before ASCT had very low risk of relapse irrespective of receiving TBI or non-TBI-based conditioning regimens. These findings suggest that TBI may be reserved for patients with positive pre-ASCT MRD.
Eradicating MRD with Therapies Other Than ASCT
Blinatumomab
Targeted therapies with alternative mechanisms of action may eradicate MRD and prevent relapse. Given its efficacy in patients with low disease burden, blinatumomab arises as a promising agent for the treatment of MRD.48 In a single-arm Phase 2 study (BLAST), adult patients with B-cell ALL in CR with detectable MRD (≥10−3 measured by RQ PCR) were treated with blinatumomab for up to 4 cycles.49 Of the 116 patients who received blinatumomab, 78% achieved MRD negative status after one cycle. Despite the inclusion of patients in second or later remission (35%), the median OS was 36.5 months. In a landmark analysis, complete MRD responders had significantly superior RFS (23.6 versus 5.7 months, P = 0.002) and OS (38.9 versus 12.5 months, P = 0.002) compared with MRD non-responders. Based on these results, the FDA approved blinatumomab for the treatment of B-cell ALL patients with detectable MRD.50
While the BLAST study was neither planned nor powered to evaluate the impact of ASCT after blinatumomab therapy, in a post-hoc analysis, investigators found no statistical difference in OS between transplanted (N=74) and non-transplanted patients (N=36) [p=0.24], in part because 27% of ASCT recipients died from ASCT-related complications. Interestingly, 33% of MRD responders did not receive any additional therapy after completing 4 cycles of blinatumomab, and 25% (9 of 36) of them remained in CR after a median 24 months of follow-up, suggesting that group of patients with positive MRD who respond to blinatumomab can achieve durable remission without the need of ASCT.
In the GMALL Trial 08/2013, 705 patients with newly diagnosed ALL (median age 35, range 18–55 years old) were treated on BFM-based pediatric inspired induction regimens.51 Patients with high-risk features (WBC>30,000, KMT2A rearrangements, ETP-ALL, Philadelphia positive) were considered for allogenic transplant in CR1.51 Patients with molecular failure after first cycle were candidate for targeted therapy (blinatumomab, nelarabine) followed by bone marrow transplant.51 Investigators compared the role of allogenic transplant versus standard risk therapy in high-risk patients with molecular remission after induction.51 Overall, molecular CR rates after cycle 1 was 61%.51 In total, 51 patients with molecular failure became candidates for targeted therapy and evaluable for assessment.51 The molecular response was achieved in 55% (n=40) and 18% (N=11) after 1 cycle of blinatumomab or nelarabine, respectively.51 The 3-year overall survival rate was 72% in patients with molecular failure.51 This large, prospective multicenter clinical trial is still ongoing.51 Preliminary results are promising for the combination of targeted therapy and allogenic transplant for patients with molecular failure.51
Inotuzumab Ozogamicin
Inotuzumab, an anti-CD22 antibody conjugated to calicheamicin, is a highly efficacious antibody-drug conjugate for patients with R/R B cell ALL. In INO-VATE trial, patients with R/R ALL were randomized to receive inotuzumab versus conventional chemotherapy. The CR rate was significantly higher in the inotuzumab group than in the conventional chemotherapy group (80.7% versus 29.4%, P<0.001). Compared with blinatumomab, inotuzumab appears to be more effective in inducing CR (80.7% versus 44% comparing across randomized Phase 3 trials) in R/R setting.52,53 However, the feasibility and role of inotuzumab in eradicating MRD are unknown. A clinical trial using inotuzumab for patients with B-cell ALL who have persistent or recurrent MRD is currently ongoing (NCT03441061).
CAR T-Cell Therapies
CD19-targeted chimeric antigen receptor (CAR) T-cell therapies are more effective in eradicating the disease in patients with low burden disease than in those with frank relapse. In a Phase 1 clinical trial, 53 patients with R/R B-cell ALL were treated with autologous CD19 CAR T-cells, and CR was observed in 83% of the patients.54 Patients with a low disease burden (defined as <5% bone marrow blasts) at baseline had longer remission duration and survival compared with patients with a higher disease burden (median EFS 11 versus 5 months, P = 0.01; median OS 20 versus 12 months, P = 0.02, respectively). These findings highlight that CAR T-cell therapy may play an essential role in the management of MRD, where such treatment may potentially cure a subset of patients.
Eradicating MRD in the Frontline Setting
Philadelphia-Chromosome Negative ALL
Expected outcomes in older ALL patients are poor, primarily due to adverse disease features and treatment-related toxicities, including prolonged myelosuppression and infections. In a study, 122 newly diagnosed older patients (age ≥ 60 years old) with Philadelphia negative B-cell ALL were treated with intensive chemotherapy (Hyper CVAD). The induction mortality rate, the death rate in CR, and the 5-year OS rate were 10%, 34%, and 20%, respectively.55 In the same study, 34 patients were treated with low-intensity chemotherapy (various pre-Hyper CVAD regimens). Although the death in CR rates were lower (15% versus 34%) in patients who received low-intensity chemotherapy, the risk of relapse was significantly higher (80% versus 40%). To increase efficacy and reduce toxicity in older patients with newly diagnosed Philadelphia negative B-cell ALL, a phase 2 clinical trial evaluated a combination of low-intensity chemotherapy (mini-Hyper CVD; a low-intensity version of the conventional Hyper CVAD) with inotuzumab.56 Of the 48 patients evaluable for morphological response, 47 (98%) achieved a response with induction mortality of 0%. Overall, 47 patients had MRD assessment (measured by MFC) within 3 cycles of therapy, 45 (96%) achieved MRD negativity (at any time point). The reported 3-year OS rate was 56%, which compares favorably with historical outcomes.57 In another phase 2 study, 29 older adults (median age 75, range 66–84) with newly diagnosed B-cell ALL were treated with blinatumomab (up to 3 cycles) and followed by 18 months of maintenance POMP therapy.58 Overall response rate and MRD negativity in responders were 66% and 92%, respectively. In this preliminary report, the 1-year OS was reported as 65%, with no induction mortality. Given its high MRD eradication potential, blinatumomab is currently being investigated in frontline therapy of younger adults with Philadelphia negative B-cell ALL. In a phase 2 study, 34 patients with newly diagnosed Philadelphia negative B-cell ALL (median age 36, range 17–59 years old) were treated with HyperCVAD and sequential blinatumomab.59 The CR and MRD negativity (by MFC) rates were 100% and a 97% MRD respectively.59 The anticipated 2-year OS of 86% compared well to the historical controls.59
Blinatumomab and inotuzumab are highly efficacious novel agents for eradicating MRD in ALL. Innovative clinical trials exploring various dose schedules and combinations of these agents with conventional chemotherapy for younger and older patients with Philadelphia-chromosome negative B-cell ALL are ongoing (NCT01371630, NCT02877303, NCT03150693).
Philadelphia-Chromosome Positive ALL
Achievement of undetectable MRD affects outcomes favorably in patients with Philadelphia-chromosome positive ALL. Combined with intensive chemotherapy, ponatinib, a third-generation pan-BCR-ABL tyrosine kinase inhibitor, is associated with high complete molecular remission rates, resulting in superior OS compared with regimens incorporating earlier generation tyrosine kinase inhibitors such as dasatinib or imatinib. In a single-arm phase 2 study, patients with newly diagnosed Philadelphia-chromosome positive ALL were induced with Hyper CVAD + ponatinib, and all patients (100%) achieved a response with no early mortality.60 Of the 76 patients who had RT-PCR-based MRD assessment at any time point, 63 (83%) achieved complete molecular remission. The 3-year EFS and OS rates were 70% and 76%, respectively. In a propensity score analysis comparing outcomes from two phase 2 clinical trials, Hyper CVAD + ponatinib was associated with better complete molecular response rate and OS than hyper CVAD + dasatinib.61 All ASCT recipients for Philadelphia-positive ALL should receive post-ASCT tyrosine kinase inhibitor to reduce risk of relapse, particularly patients with detectable peritransplant MRD.62 Blinatumomab is also effective in Philadelphia chromosome-positive ALL in both R/R settings and for MRD clearance.49,63 In a phase two study of the combination of blinatumomab and dasatinib in adults with newly diagnosed Philadelphia chromosome positive ALL (median age 54), 98% of patients achieved complete remission, with 60% achieving molecular remission after two cycles.64 With a median follow-up of 18 months, the median OS and DFS were 95% and 88%, respectively.64 When blinatumomab is combined with a more potent tyrosine kinase inhibitor, such as ponatinib, even deeper molecular responses occur in a greater number of patients. In a preliminary report involving 28 patients with newly diagnosed or R/R Philadelphia chromosome positive ALL, the combination of blinatumomab and ponatinib achieved a response rate of 95%, with 86% achieving complete molecular remission.65 Several clinical trials are therefore investigating frontline regimens with blinatumomab and tyrosine kinase inhibitor combinations with the aim of superior MRD clearance with tolerable toxicity and minimizing the need for ASCT in these patients (NCT02143414, NCT 02744768, NCT03263572).
Conclusion
MRD status provides valuable information in the management of patients with ALL. Apart from its prognostic significance, MRD status guides post-remission treatment strategies, including utilization of novel chemotherapeutics, ASCT decision, conditioning regimen selection, maintenance therapies, and predicting/treating impending relapse. The development of highly sensitive MRD assays, such as NGS and RQ PCR, may provide even better risk stratification and increase its role in treatment decisions. In addition, the availability of agents highly effective in MRD settings, including inotuzumab, blinatumomab, and CAR T-cells, made it possible to develop frontline regimens with early MRD eradication potential. While long-term follow-up of this approach is still missing, it holds promise in minimizing the need for intensive post-remission chemotherapy, including ASCT for many patients with ALL.
Disclosure
Dr Nicholas Short reports Research Support from Takeda Oncology, Stemline Therapeutics, and Astellas, Consultant and Speaker’s Bureau from Amgen, being a Consultant for AstraZeneca, fNGMBio, Novartis, and Pfizer, and receiving a Grant from fNGMBio outside the submitted work. Dr Elias Jabbour reports grants, personal fees from Amgen, grants, personal fees from Adaptive biotechnologies, grants, personal fees from Pfizer, grants, personal fees from Takeda, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Abou Dalle I, Jabbour E, Short NJ. Evaluation and management of measurable residual disease in acute lymphoblastic leukemia. Ther Adv Hematol. 2020;11:204062072091002. doi: 10.1177/2040620720910023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–4162. doi: 10.1182/blood-2008-11-185132 [DOI] [PubMed] [Google Scholar]
- 3.Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868–1876. doi: 10.1182/blood-2011-09-377713 [DOI] [PubMed] [Google Scholar]
- 4.Jacquy C, Delepaut B, Van Daele S, et al. A prospective study of minimal residual disease in childhood B-lineage acute lymphoblastic leukaemia: MRD level at the end of induction is a strong predictive factor of relapse. Br J Haematol. 1997;98(1):140–146. doi: 10.1046/j.1365-2141.1997.1792996.x [DOI] [PubMed] [Google Scholar]
- 5.Cazzaniga G, Biondi A. Molecular monitoring of childhood acute lymphoblastic leukemia using antigen receptor gene rearrangements and quantitative polymerase chain reaction technology. Haematologica. 2005;90(3):382–390. [PubMed] [Google Scholar]
- 6.Chen X, Wood BL. How do we measure MRD in ALL and how should measurements affect decisions. Re: treatment and prognosis? Best Pract Res Clin Haematol. 2017;30(3):237–248. doi: 10.1016/j.beha.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Wood B. 9-Color and 10-color flow cytometry in the clinical laboratory. In: Archives of Pathology and Laboratory Medicine. Vol. 130. Allen Press;2006:680–690. doi: 10.5858/2006-130-680-cacfci [DOI] [PubMed] [Google Scholar]
- 8.Tembhare PR, Ghogale S. A High-Sensitivity 10-Color Flow Cytometric Minimal Residual Disease Assay in B-Lymphoblastic Leukemia/Lymphoma Can Easily Achieve the Sensitivity of 2-in-106 and Is Superior to Standard Minimal Residual Disease Assay: a Study of 622 Patients. Cytom Part B Clin Cytom. 2020;98(1):57–67. doi: 10.1002/CYTO.B.21831 [DOI] [PubMed] [Google Scholar]
- 9.Theunissen P, Mejstrikova E, Sedek L. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2017;129(3):347–357. doi: 10.1182/BLOOD-2016-07-726307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood BL. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytom Part B - Clin Cytom. 2016;90(1):47–53. doi: 10.1002/cyto.b.21239 [DOI] [PubMed] [Google Scholar]
- 11.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–1291. doi: 10.1182/blood-2017-09-801498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velden VHJ, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leuk. 2007;21(4):604–611. doi: 10.1038/sj.leu.2404586 [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer H, Cazzaniga G, van der Velden VHJ, et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leuk. 2019;33(8):1910–1922. doi: 10.1038/s41375-019-0413-0 [DOI] [PubMed] [Google Scholar]
- 14.Thörn I, Forestier E, Botling J, et al. Minimal residual disease assessment in childhood acute lymphoblastic leukaemia: a Swedish multi-centre study comparing real-time polymerase chain reaction and multicolour flow cytometry. Br J Haematol. 2011;152(6):743–753. doi: 10.1111/j.1365-2141.2010.08456.x [DOI] [PubMed] [Google Scholar]
- 15.Ryan J, Quinn F, Meunier A, et al. Minimal residual disease detection in childhood acute lymphoblastic leukaemia patients at multiple time-points reveals high levels of concordance between molecular and immunophenotypic approaches. Br J Haematol. 2009;144(1):107–115. doi: 10.1111/j.1365-2141.2008.07429.x [DOI] [PubMed] [Google Scholar]
- 16.Gaipa G, Cazzaniga G, Valsecchi MG, et al. Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica. 2012;97(10):1586–1593. doi: 10.3324/HAEMATOL.2011.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dongen JJM, Van Der Velden VHJ, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996–4009. doi: 10.1182/blood-2015-03-580027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Sherwood A, Fromm JR, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4:134. doi: 10.1126/scitranslmed.3003656 [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer H, Cazzaniga G, van der Velden VHJ, et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leukemia. 2019;33(8):1910–1922. doi: 10.1038/s41375-019-0413-0 [DOI] [PubMed] [Google Scholar]
- 20.Theunissen PMJ, Van Zessen D, Stubbs AP, et al. Antigen receptor sequencing of paired bone marrow samples shows homogeneous distribution of acute lymphoblastic leukemia subclones. Haematologica. 2017;102(11):1869–1877. doi: 10.3324/haematol.2017.171454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theunissen PMJ, de Bie M, van Zessen D, de Haas V, Stubbs AP. van der Velden VHJ. Next-generation antigen receptor sequencing of paired diagnosis and relapse samples of B-cell acute lymphoblastic leukemia: clonal evolution and implications for minimal residual disease target selection. Leuk Res. 2019;76:98–104. doi: 10.1016/j.leukres.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 22.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–5180. doi: 10.1182/blood-2012-07-444042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monter A, Nomdedéu JF. ClonoSEQ assay for the detection of lymphoid malignancies. Expert Rev Mol Diagn. 2019;19(7):571–578. doi: 10.1080/14737159.2019.1627877 [DOI] [PubMed] [Google Scholar]
- 24.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA. J Clin Oncol. 2014;32(15):1595–1604. doi: 10.1200/JCO.2013.52.2425 [DOI] [PubMed] [Google Scholar]
- 25.Brüggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120(23):4470–4481. doi: 10.1182/blood-2012-06-379040 [DOI] [PubMed] [Google Scholar]
- 26.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD study. Br J Haematol. 2008;142(2):227–237. doi: 10.1111/j.1365-2141.2008.07185.x [DOI] [PubMed] [Google Scholar]
- 27.Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–1123. doi: 10.1182/blood-2005-07-2708 [DOI] [PubMed] [Google Scholar]
- 28.Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749. doi: 10.1182/blood-2014-01-547695 [DOI] [PubMed] [Google Scholar]
- 29.Mortuza FY, Papaioannou M, Moreira IM, et al. Minimal Residual Disease Tests Provide an Independent Predictor of Clinical Outcome in Adult Acute Lymphoblastic Leukemia. J Clin Oncol. 2002;20(4):1094–1104. doi: 10.1200/jco.2002.20.4.1094 [DOI] [PubMed] [Google Scholar]
- 30.Vidriales MB, Pérez JJ, López-Berges MC, et al. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 2003;101(12):4695–4700. doi: 10.1182/blood-2002-08-2613 [DOI] [PubMed] [Google Scholar]
- 31.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172(3):392–400. doi: 10.1111/bjh.13834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580–e170580. doi: 10.1001/jamaoncol.2017.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassan R, Brüggemann M, Radcliffe HS, Hartfield E, Kreuzbauer G, Wetten S. A systematic literature review and metaanalysis of minimal residual disease as a prognostic indicator in adult B-cell acute lymphoblastic leukemia. Haematologica. 2019;104(10):2028–2039. doi: 10.3324/haematol.2018.201053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gökbuget N, Dombret H, Giebel S, et al. Minimal residual disease level predicts outcome in adults with Ph-negative B-precursor acute lymphoblastic leukemia. Hematol. 2019;24(1):337–348. doi: 10.1080/16078454.2019.1567654 [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz M, Kantarjian H, Wang X, et al. The early achievement of measurable residual disease negativity in the treatment of adults with Philadelphia-negative B-cell acute lymphoblastic leukemia is a strong predictor for survival. Am J Hematol. 2020;95(2):144–150. doi: 10.1002/ajh.25671 [DOI] [PubMed] [Google Scholar]
- 36.Issa GC, Kantarjian HM, Yin CC, et al. Prognostic impact of pretreatment cytogenetics in adult Philadelphia chromosome-negative acute lymphoblastic leukemia in the era of minimal residual disease. Cancer. 2017;123(3):459–467. doi: 10.1002/cncr.30376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor D, Enshaei A, Bartram J, et al. Genotype-Specific minimal residual disease interpretation improves stratification in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2018;36(1):34–43. doi: 10.1200/JCO.2017.74.0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue Y, Cheng Y, Lu A, et al. Allogeneic Hematopoietic Stem Cell Transplantation, Especially Haploidentical, May Improve Long-Term Survival for High-Risk Pediatric Patients with Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia in the Tyrosine Kinase Inhibitor Era. Biol Blood Marrow Transplant. 2019;25(8):1611–1620. doi: 10.1016/j.bbmt.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Du J, Huang A, et al. Chemotherapy vs. allogeneic transplantation as post molecular remission therapy in patients aged less than 60 years with Philadelphia-positive ALL. Bone Marrow Transplant. 2020;55(1):245–248. doi: 10.1038/s41409-019-0514-4 [DOI] [PubMed] [Google Scholar]
- 40.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507. doi: 10.1182/blood-2016-03-707562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bader P, Kreyenberg H, Henze GHR, et al. Prognostic value of Minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–384. doi: 10.1200/JCO.2008.17.6065 [DOI] [PubMed] [Google Scholar]
- 42.Sutton R, Shaw PJ, Venn NC, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. 2015;168(3):395–404. doi: 10.1111/bjh.13142 [DOI] [PubMed] [Google Scholar]
- 43.Logan AC, Vashi N, Faham M, et al. Immunoglobulin and t cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant. 2014;20(9):1307–1313. doi: 10.1016/j.bbmt.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612–618. doi: 10.3324/haematol.10965 [DOI] [PubMed] [Google Scholar]
- 45.Jabbour E, Short NJ, Jorgensen JL, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. 2017;123(2):294–302. doi: 10.1002/cncr.30264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhédin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486–2496. doi: 10.1182/blood-2014-09-599894 [DOI] [PubMed] [Google Scholar]
- 47.Friend BD, Bailey-Olson M, Melton A, et al. The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2020;67(2):e28079. doi: 10.1002/pbc.28079 [DOI] [PubMed] [Google Scholar]
- 48.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell - Engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270 [DOI] [PubMed] [Google Scholar]
- 49.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–1531. doi: 10.1182/blood-2017-08-798322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hilal T, Prasad V. Eliminating MRD — FDA approval of blinatumomab for B-ALL in complete remission. Nat Rev Clin Oncol. 2018;15(12):727–728. doi: 10.1038/s41571-018-0087-y [DOI] [PubMed] [Google Scholar]
- 51.Goekbuget N, Stelljes M, Viardot A, et al. First Results of the Risk-Adapted, MRD-Stratified GMALL Trial 08/2013 in 705 Adults with Newly Diagnosed Acute Lymphoblastic Leukemia/Lymphoma (ALL/LBL). Blood. 2021;138(Supplement 1):362. doi: 10.1182/BLOOD-2021-146306 [DOI] [Google Scholar]
- 52.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/nejmoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/nejmoa1509277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JH, Rivière I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/nejmoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097–2101. doi: 10.1002/cncr.23819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–248. doi: 10.1016/S1470-2045(18)30011-1 [DOI] [PubMed] [Google Scholar]
- 57.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186–3191. doi: 10.1002/cncr.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Advani AS, Moseley A, O’Dwyer KM, et al. Results of SWOG 1318: a Phase 2 Trial of Blinatumomab Followed By Pomp (Prednisone, Vincristine, Methotrexate, 6-Mercaptopurine) Maintenance in Elderly Patients with Newly Diagnosed Philadelphia Chromosome Negative B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):33. doi: 10.1182/blood-2018-99-111992 [DOI] [Google Scholar]
- 59.Short NJ, Kantarjian HM, Ravandi F, et al. Hyper-CVAD and Sequential Blinatumomab in Adults with Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukemia: results from a Phase II Study. Blood. 2020;136(Supplement 1):9–11. doi: 10.1182/BLOOD-2020-138565 [DOI] [Google Scholar]
- 60.Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618–e627. doi: 10.1016/S2352-3026(18)30176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2016;122(23):3650–3656. doi: 10.1002/CNCR.30231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giebel S, Czyz A, Ottmann O, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the acute leukemia working party of the. Cancer. 2016;122(19):2941–2951. doi: 10.1002/cncr.30130 [DOI] [PubMed] [Google Scholar]
- 63.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a Phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531 [DOI] [PubMed] [Google Scholar]
- 64.Foà R, Bassan R, Vitale A, et al. Dasatinib–Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613–1623. doi: 10.1056/NEJMOA2016272/SUPPL_FILE/NEJMOA2016272_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 65.Short NJ, Kantarjian HM, Konopleva M, et al. Combination of ponatinib and blinatumomab in Philadelphia chromosome-positive acute lymphoblastic leukemia: early results from a phase II study. Vaccine. 2021;39(15_suppl):7001. doi: 10.1200/JCO.2021.39.15_SUPPL.700134750014 [DOI] [Google Scholar]