Abstract
Pulmonary tumor embolism (PTE) and pulmonary tumor thrombotic microangiopathy (PTTM) are rare etiologies for rapidly progressive dyspnea in the setting of undiagnosed metastatic cancer. They occur most frequently in association with adenocarcinomas, with PTE being most frequently associated with hepatocellular carcinoma and PTTM being most commonly reported with gastric adenocarcinoma. Pulmonary tumor embolism and PTTM appear to be a disease spectrum where PTTM represents an advanced form of PTE. Pulmonary tumor embolism and PTTM are mostly identified postmortem during autopsy as the antemortem diagnosis remains a clinical challenge due to the rapidly progressive nature of these rare diseases. We report 2 cases of rapidly progressive respiratory failure leading to death, due to tumoral pulmonary hypertension resulting from PTE and PTTM, diagnosed postmortem. Both of the patients were middle-aged females, nonsmokers, and had a gastrointestinal source of their primary malignancy.
Keywords: pulmonary tumor thrombotic microangiopathy, pulmonary tumor embolism, tumoral pulmonary hypertension, metastatic cancer, cor pulmonale, PTE, PTTM, respiratory failure, autopsy
Introduction
Pulmonary tumor embolism (PTE) and pulmonary tumor thrombotic microangiopathy (PTTM) have been described as rare causes of exertional dyspnea that progress rapidly and are often fatal. 1 They represent a subtype of tumoral pulmonary hypertension (PH) in the presence of an underlying malignancy. 2 In 1897, PTE was first described by Schmidt in a patient with gastric carcinoma.3,4 It was in 1937 that Brill and Robertson identified subacute cor pulmonale arising due to numerous pulmonary microvasculature tumor emboli.5,6 Thereafter, PTTM was first described in 1990 by von Herbay et al in patients with metastatic carcinoma, after they identified intimal fibro cellular proliferation in their pulmonary arterial vessels. 7 Since then, PTE and PTTM have been reported in numerous malignancies. Pulmonary tumor embolism has been associated with hepatocellular carcinoma and renal cell carcinoma, as well as adenocarcinomas of the breast, stomach, lung, and colon that secrete extracellular mucin. 8 Meanwhile, PTTM occurs in the setting of gastric, breast, lung, cancer of unknown primary, bladder, ovarian clear cell, hepatocellular, gallbladder, and other malignancies, with adenocarcinoma being the most-reported histology.2,9 Pulmonary tumor thrombotic microangiopathy has been most frequently associated with gastric adenocarcinomas among these malignancies.1,7 Owing to the rapidly progressing course of PTE and PTTM and the challenge of diagnosing these rare clinical entities antemortem, it becomes important to consider these among the list of differentials and investigate the patient accordingly. Herein, we describe 2 patients in our case series who developed severe PH in the setting of advanced gastrointestinal malignancy and were found to have PTE and PTTM on the lung autopsy.
Case report: Written informed consent was obtained from the patient/legally authorized representative for their anonymized information to be published in this article.
Case 1: A 41-year-old woman with no significant past medical history presented with shortness of breath and dry cough with occasional hemoptysis for a few weeks. The patient was admitted to the hospital for acute hypoxic respiratory failure requiring 2 L of oxygen via nasal cannula. Testing for infectious respiratory viruses and bacteria was negative. A chest X-ray (CXR) revealed mild diffuse interstitial prominence (Figure 1A). Computed tomography (CT) angiography of the chest was negative for pulmonary embolism but revealed mild cardiomegaly, mild prominence of central pulmonary arteries, mild centrilobular nodular pattern, and interlobular septal thickening representing possible edema or inflammation. In addition, numerous small sclerotic bone lesions suspicious for metastatic disease with bilateral lower rib fractures were noted (Figure 1B). Computed tomography scan of the abdomen and pelvis was performed for abdominal pain which showed mural thickening of the stomach with omental inflammatory changes and sclerotic skeletal lesions. The patient underwent a needle biopsy of the pelvic bone lesion. Initial transthoracic echo (TTE) revealed an elevated estimated pulmonary arterial systolic pressure (ePASP) of 90 mm Hg, with severe right atrial and right ventricular enlargement, with a flattened interventricular septum and severely depressed right ventricular systolic function. The left ventricular ejection fraction (LVEF) was noted to be 60% to 65%.
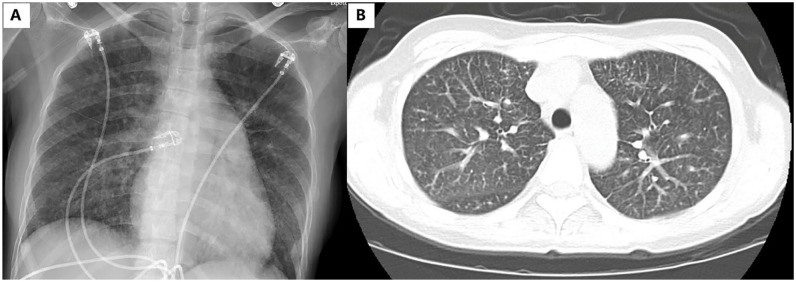
Figure 1.
(A) Chest radiograph revealing mild diffuse interstitial prominence and (B) CT angiography of chest revealing mild centrilobular nodular pattern and interlobular septal thickening.
Abbreviation: CT, computed tomography.
The patient underwent a right heart catheterization revealing severe precapillary PH with arterial pressure of 69/22 mm Hg and a mean arterial pressure of 43 mm Hg with pulmonary wedge pressure of 9 mm Hg. The estimated cardiac output was reduced at 2 L/min with a cardiac index of 1.4 L/m2/min. Pulmonary vascular resistance was elevated at 19.4 Wood units. On the fourth day of hospitalization, the patient’s condition rapidly deteriorated, and with the assistance of the advanced heart failure cardiology service, the patient was trialed on both milrinone, then dobutamine, with no clinical improvement with either. She subsequently became apneic and bradycardic before going into a pulseless electrical activity (PEA) arrest. After a prolonged course of advanced cardiac life support (ACLS) and unsuccessful extracorporeal membrane oxygenation (ECMO) cannulation attempt at the bedside, resuscitation efforts were stopped, and the patient was pronounced deceased.
Postmortem autopsy of her respiratory system revealed a poorly differentiated adenocarcinoma with extensive venous and lymphatic invasion, as well as tumor thrombosis within the pulmonary arteries leading to occlusion. Fibrocellular intimal and smooth muscle proliferation surrounding the tumor cells within the arteries was confirmed with special staining (Figure 2). The immunostaining performed on the pelvic bone biopsy suggested that the poorly differentiated adenocarcinoma likely originated from the upper gastrointestinal tract.
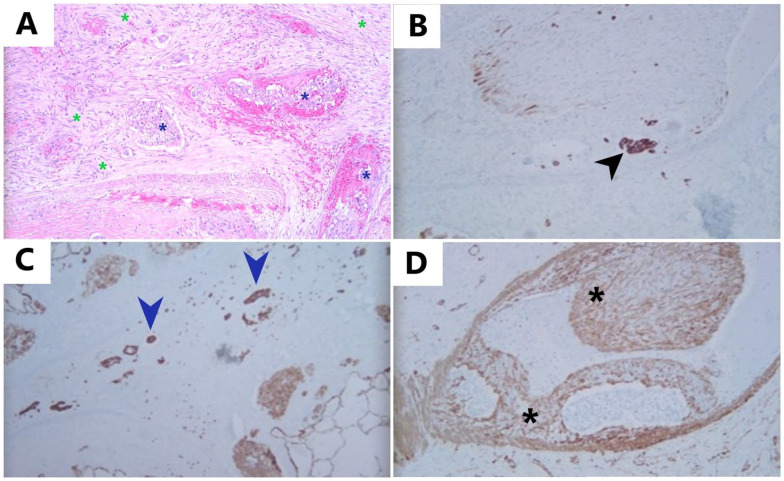
Figure 2.
Pulmonary tumor thrombotic microangiopathy: (A): H&E, 10×: H&E-stained slide showing fibrocellular intimal proliferation with spindle-shaped cells (green asterisks) surrounding the pulmonary tumor emboli (blue asterisks) and completely filling the lumen of this vessel, (B) Immunostaining for CK-7, 10×: CK-7 highlights tumor cells (black arrowhead) within the lumen of muscular arteries, adjacent to intimal proliferation, (C) Immunostaining for CK-7, 10×: CK-7-positive tumor cells (blue arrowheads) present in lymphatics, and (D) Immunostaining for SMA, 10×: smooth muscle actin (SMA) highlights proliferation of smooth muscle cells within tunica intima (intimal proliferation) (black asterisks).
Case 2: A 57-year-old woman with a past medical history of hypothyroidism presented to the clinic with cough and dysphagia. Chest X-ray showed diffuse reticular opacities and small bilateral pleural effusions, which could represent pulmonary edema, interstitial lung disease, or atypical infection (Figure 3A). She underwent a chest CT scan a week later, which demonstrated large bilateral pleural effusions with diffuse interstitial thickening, and scattered ground-glass opacities in association with prominent mediastinal lymphadenopathy (Figure 3B). The patient underwent left-sided thoracentesis, which returned positive for adenocarcinoma of unknown origin.
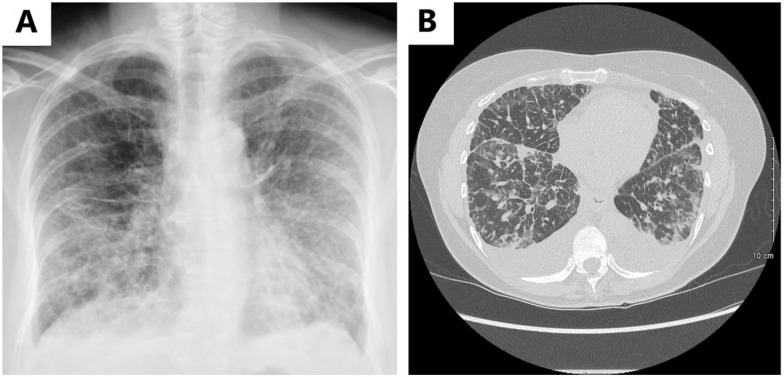
Figure 3.
(A) Chest radiograph at initial presentation revealing diffuse reticular opacities and tiny bilateral pleural effusions and (B) CT scan of chest without contrast revealing bilateral pleural effusions with diffuse interstitial septal thickening and scattered ground-glass opacities.
Abbreviation: CT, computed tomography.
The patient subsequently underwent positron emission tomography-computed tomography (PET-CT), which was significant for increased F-labeled 2-deoxyglucose (FDG) uptake in the appendix, read as possibly indicative of primary malignancy, as well as hypermetabolic adrenal thickening and extensive osseous metastasis (most prominently in the T12 vertebral body, right femoral greater trochanter, and left ilium). A biopsy of the left iliac lesion was performed, although nonspecific for the site of origin, was suggestive of the upper gastrointestinal tract or pancreaticobiliary cause based on immunohistochemical staining.
It was planned that the patient would begin outpatient radiation therapy for osseous metastasis; however, she was admitted to the hospital for worsening shortness of breath and worsening pleural effusion. During this admission, the patient had a tunneled pleural catheter placed, on the right side. She also underwent an upper endoscopy, which revealed congestion and erythema of the distal esophagus, with whitish exudates and friable mucosa of the gastric cardia, of which biopsies were taken. Subsequently, the patient was discharged with supplemental oxygen and received one dose of palliative radiation to her thoracic spine and left pelvis as an outpatient.
The patient developed worsening shortness of breath and hypoxia and was subsequently readmitted to the hospital. At this time, a chest CT was obtained, revealing a moderately sized left pleural effusion, stable small right effusion, severe diffuse interstitial thickening, and areas of irregular opacification concerning for pulmonary edema versus atypical pneumonia versus lymphangitic spread of potential pulmonary metastasis. Broad-spectrum antibiotics were started for coverage of community-acquired pneumonia.
Following admission, the patient developed significant hypotension and was transferred to the intensive care unit (ICU). The patient’s respiratory status continued to deteriorate, and she was started on noninvasive positive pressure therapy. Transthoracic echo (TTE) demonstrated moderately sized pericardial effusion without evidence of tamponade, as well as severe PH with a Doppler-derived PASP of 68 to 73 mm Hg and a hyperdynamic right ventricle. Left ventricular ejection fraction was noted to be 75% to 80%.
The patient continued to deteriorate, and on the second night of admission, became acutely unresponsive. The patient was found to be in cardiac arrest. Return of spontaneous circulation was achieved after advanced cardiac life support (ACLS). The patient was subsequently intubated and started on vasopressor therapy. Endoscopic biopsies of the gastroesophageal junction (GEJ) and stomach returned positive for adenocarcinoma. Given the concern for atypical infection versus lymphangitic spread of the tumor, a bronchoscopy with bronchoalveolar lavage (BAL) was performed. Transbronchial biopsy was considered but unable to be obtained in the setting of worsening thrombocytopenia. Bronchoalveolar lavage was negative for malignant cells or infection.
Despite antibiotic therapy and drainage of pleural effusions, the patient was unable to be liberated from the ventilator following several failed spontaneous breathing trials. Following extensive goals of care discussions with the patient’s family, and with assistance from Oncology and Palliative Care teams, the patient’s family decided to pursue comfort care only. The patient was compassionately extubated and died shortly thereafter. The patient succumbed to her illness within 2 months of her initial presentation.
An autopsy was performed, with pathology examination of the patient’s respiratory tissues significant for extensive pulmonary tumor emboli and metastatic adenocarcinoma, with an examination of the GEJ tissue significant for invasive moderately differentiated adenocarcinoma with signet ring cell features (Figure 4). Metastases were also identified in the right ventricle of the heart, liver, bilateral adrenal glands, bilateral kidneys, pituitary, dura mater, bone marrow, and multiple lymph nodes.
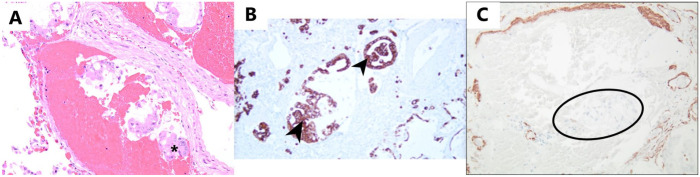
Figure 4.
Pulmonary tumor embolism: (A) H&E 10×: H&E-stained slide showing tumor cells (asterisk) within the lumen of a muscular artery, causing venous congestion without intimal proliferation, (B) Immunostaining for CK-7, 10×: CK-7 positive tumor cells (black arrowheads) in the lumen of blood vessels, and (C) Immunostaining for SMA, 10×: tumor cells (outlined in black) within the lumen of the artery without causing intimal proliferation. SMA staining is confined to the original wall of the artery and not extending into the lumen.
Abbreviation: SMA, smooth muscle actin.
Discussion
Tumor cells may involve pulmonary vasculature, which can lead to tumoral PH and cor pulmonale. Tumor cells disseminate into the pulmonary vasculature primarily via the hematogenous route. Most of the tumor cells in the circulation are destroyed by the circulatory mechanical forces, shear stress or body’s immune system. Although the mechanism is poorly understood, some tumor cells are able to survive and reach the lungs. 4 Owing to the interactions between the signaling pathways for angiogenesis, apoptosis, and inflammation, the outcome of the surviving tumor cells in the lung varies. It ranges from metastasis, lymphatic invasion, PH, or eventual clearance of the tumor cells.8,10 Tumoral PH can result from 1 of the 4 ways: pulmonary microvascular diseases which include PTE and PTTM; generalized lymphangitic dissemination (lymphangitic carcinomatosis); proximal tumor macroembolism; or a combination of the above.8,11 Unlike proximal tumor macroembolism, which has a rapid onset of symptoms and presents similarly to massive thromboembolism, pulmonary microvascular disease and lymphangitic carcinomatosis have a more progressive course. There is a need to further explore the molecular and genetic influences, to better understand why a large number of patients have asymptomatic tumor emboli, yet only a few patients progress to PTE or PTTM. 2
Pulmonary tumor embolism and PTTM represent a spectrum of pulmonary microvascular diseases causing tumoral PH. In PTE, tumor cells cohesively occlude small pulmonary arteries and veins. Invasion of the surrounding interstitium by the tumor cells is not usually seen in PTE, which distinguishes it from pulmonary metastasis.8,12 Pulmonary tumor thrombotic microangiopathy represents an advanced form of PTE where the tumor cell nests in the pulmonary arteries lead to fibrointimal proliferation in their surroundings. These cell nests mediate the deposition of platelets and fibrin along arterial intima by inducing inflammation via cytokine release, activation of coagulation factors, and release of growth factors. Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and tissue factor play an important role in fibrointimal proliferation. Ultimately, this leads to narrowing and occlusion of small pulmonary arteries.2,13 The pulmonary vascular resistance increases progressively with the development of pulmonary arterial hypertension.14,15 Owing to the extensive involvement of pulmonary vasculature, patients with PTE and PTTM present with the clinical features of PH and right heart failure (cor pulmonale).2,16,17 The patients often develop rapidly progressive dyspnea, hypoxia, and respiratory failure with worsening right ventricular dysfunction.1,18
The antemortem diagnosis of PTE and PTTM is challenging, with only a few cases described in the literature that were diagnosed before death.19 -23 In a case described by Miyano et al, with resected gastric cancer, PTTM was suspected based on an elevation in serum VEGF and D-dimer levels. Even though there was an absence of cancer cells and inflammatory infiltrate, the presence of fibrocellular intimal proliferation in small pulmonary arteries on transbronchial lung biopsy hinted toward PTTM. They confirmed the diagnosis of PTTM in the setting of recurrent gastric adenocarcinoma with video-assisted thoracoscopic surgery. The patient was treated successfully with anticoagulation, corticosteroids, and chemotherapy due to a prompt diagnosis of PTTM. 19
As PTE and PTTM are frequently fatal and are caused by a malignancy, a high clinical suspicion is required for a timely diagnosis of PTE and PTTM in the setting of suggestive clinical features. These should be considered among the differential diagnoses of patients with deteriorating respiratory failure due to a newly diagnosed pulmonary arterial hypertension, even in cases without a prior diagnosis of cancer. 18 A plain chest radiograph can be normal or show diffuse reticulonodular opacities, Kerley B lines, and pleural effusions. Meanwhile, a parenchymal CT can reveal centrilobular nodules, peri-broncho-vascular ground-glass opacities, interlobular septal thickening, and consolidation. There can be signs of PH on the CT, including central pulmonary artery enlargement, enlargement of the right heart chamber, and flattening of the intraventricular septum.1,2,24,25 The presence of dilated, beaded, or tree-in-bud appearance of pulmonary arteries on CT pulmonary angiography is suggestive of PTE and PTTM. 26 An elevated blood D-dimer level and serum VEGF level, PH on right heart catheterization, multiple small subsegmental perfusion defects (mottled/beaded appearance) on ventilation/perfusion study can suggest PTE and PTTM, even in situations where CXR, high-resolution CT chest, and CT angiography are unrevealing.2,18,19 It is imperative for these patients to be screened for an underlying malignancy. Cytological examination of blood aspirated from wedged pulmonary artery catheter can be useful in identifying tumor cells, although the diagnostic accuracy is uncertain and does not distinguish between PTE and PTTM. This technique has a sensitivity of 80% to 88% and a specificity of 82% to 94% in identifying PTE and PTTM.2,12 It can be considered in patients who are too sick to undergo a lung biopsy or those who decline it.2,8 Lung biopsy, either CT-guided, bronchoscopic, or using video-assisted thoracoscopic surgery, can be used to diagnose PTTM antemortem. Lung biopsy should be performed early in the disease course for patients who are stable and can tolerate invasive procedures. Of all the methods enlisted, surgical biopsy has the highest procedural risk but the best diagnostic yield and can be considered in patients who could not be diagnosed using other techniques. 2
Although there is no definitive therapy for PTE and PTTM, various treatment options have been explored that target PTE and PTTM along with underlying cancer. Supportive therapies like oxygen administration, mechanical ventilation, and inotropic support can be administered, while the diagnostic workup and definitive therapies are ongoing. Pulmonary vasodilators, endothelin receptor antagonists, glucocorticoids, warfarin and aspirin, antiproliferative therapies like PDGF inhibitor (imatinib), and VEGF inhibitor (bevacizumab) have also been utilized to target PTTM, especially in patients with PH leading to decompensated right heart failure or shock. Administering hydrocortisone has historically led to a subjective improvement in breathlessness. Anticoagulants and antibiotics should be used only in cases where another indication warranting their use is present. In patients with right-sided heart failure with fluid retention secondary to PH, diuretics can be used for symptomatic benefits as they prevent a dilated right ventricle from impacting left ventricular filling. Although, caution must be exercised with diuretics, to prevent excessive preload reduction and hypovolemia, and thereby a decreased cardiac output. A simultaneous reduction of tumor burden with chemotherapy, radiation therapy, or surgical resection is important to address the underlying cause of PTE and PTTM. Therefore, a combined treatment approach may be more beneficial.2,27,28
Conclusion
Pulmonary tumor embolism and PTTM are uncommon but important causes of tumoral PH, which can lead to rapidly progressive heart failure and death. Diagnosis of these conditions antemortem is challenging, and therefore, physicians should consider them in the list of differentials for respiratory failure. Although, a deeper understanding is required to comprehend why certain patients with pulmonary tumor emboli remain asymptomatic, in contrast to other patients who develop extensive vascular remodeling and manifest a progressive course leading up to cor pulmonale. With a targeted clinical approach, judicious use of radiologic and microscopic tissue diagnostic modalities, a timely diagnosis can lead to a more targeted therapeutic approach that can improve outcomes. Advances in treatment options with newer therapies targeting the growth factors need to be investigated further to improve the survival of patients with PTE and PTTM.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
ORCID iD: Kurt Fisher  https://orcid.org/0000-0002-8391-390X
https://orcid.org/0000-0002-8391-390X
References
- 1. Uruga H, Fujii T, Kurosaki A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med. 2013;52(12):1317-1323. [DOI] [PubMed] [Google Scholar]
- 2. Price LC, Seckl MJ, Dorfmüller P, et al. Tumoral pulmonary hypertension. Eur Respir Rev. 2019;28(151):180065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt MB. Die Verbreitungswege der Karzinome und die Beziehung generalisierter Sarcome zu den leukaemischen Neubildungen. Fischer; 1903. [Google Scholar]
- 4. Bassiri AG, Haghighi B, Doyle RL, et al. Pulmonary tumor embolism. Am J Respir Crit Care Med. 1997;155(6):2089-2095. [DOI] [PubMed] [Google Scholar]
- 5. Brill IC, Robertson TD. SUBACUTE COR PULMONALE. Arch Intern Med (Chic). 1937;60(6):1043-1057. [Google Scholar]
- 6. Balasubramanian V, Mathur J, Aparnath M. Non-thrombotic pulmonary embolism. In: Çobanoğlu U eds. Pulmonary Embolism. INTECH Open Access Publisher; 2012: 75-118. [Google Scholar]
- 7. von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66(3):587-592. [DOI] [PubMed] [Google Scholar]
- 8. Roberts KE, Hamele-Bena D, Saqi A, et al. Pulmonary tumor embolism: a review of the literature. Am J Med. 2003;115(3):228-232. [DOI] [PubMed] [Google Scholar]
- 9. Ho AL, Szulakowski P, Szulakowsi P, et al. The diagnostic challenge of pulmonary tumour thrombotic microangiopathy as a presentation for metastatic gastric cancer: a case report and review of the literature. BMC Cancer. 2015;15:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bast RC. Holland Frei Cancer Medicine. 5th ed. Hamilton, ON, Canada: B.C. Decker; 2000. [Google Scholar]
- 11. Kane RD, Hawkins HK, Miller JA, Noce PS. Microscopic pulmonary tumor emboli associated with dyspnea. Cancer. 1975;36(4):1473-1482. [DOI] [PubMed] [Google Scholar]
- 12. Winterbauer RH, Elfenbein IB, Ball WC., Jr. Incidence and clinical significance of tumor embolization to the lungs. Am J Med. 1968;45(2):271-290. [DOI] [PubMed] [Google Scholar]
- 13. Kuwabara H, Yoshida S, Takasu T, et al. Pulmonary tumor thrombotic microangiopathy caused by gastric cancer. Ann Thorac Med. 2012;7(3):168-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinckard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4(3):154-157. [DOI] [PubMed] [Google Scholar]
- 15. Buser M, Felizeter-Kessler M, Lenggenhager D, et al. Rapidly progressive pulmonary hypertension in a patient with pulmonary tumor thrombotic microangiopathy. Am J Respir Crit Care Med. 2015;191(6):711-712. [DOI] [PubMed] [Google Scholar]
- 16. Pulmonary tumor embolism and lymphangitic carcinomatosis in adults: epidemiology, etiology, and pathogenesis. UpToDate. https://www.uptodate.com/contents/pulmonary-tumor-embolism-and-lymphangitic-carcinomatosis-in-adults-epidemiology-etiology-and-pathogenesis?search=pulmonary+tumor+embolism+and+lymphangitic+carcinomatosis+in+adults&source=search_result&selectedTitle=1~40&usage_type=default&display_rank=1. Accessed February 10, 2022.
- 17. Jorens PG, Van Marck E, Snoeckx A, et al. Nonthrombotic pulmonary embolism. Eur Respir J. 2009;34(2):452-474. [DOI] [PubMed] [Google Scholar]
- 18. Endicott-Yazdani T, Ghazi A, Armstrong D, Guileyardo J, Schuller D. Fatal pulmonary tumor thrombotic microangiopathy caused by undiagnosed metastatic gastric adenocarcinoma. Proc (Bayl Univ Med Cent). 2015;28(4):482-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyano S, Izumi S, Takeda Y, et al. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25(5):597-599. [DOI] [PubMed] [Google Scholar]
- 20. Kitamura A, Nishimura N, Jinta T, et al. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol. 2013;2013:259080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kayatani H, Matsuo K, Ueda Y, et al. Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med. 2012;51(19):2767-2770. [DOI] [PubMed] [Google Scholar]
- 22. Mandaliya R, Farhat S, Uprety D, et al. Occult gastric cancer presenting as hypoxia from pulmonary tumor thrombotic microangiopathy. J Gastric Cancer. 2014;14(2):142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujishiro T, Shuto K, Shiratori T, et al. A case report of pulmonary tumor thrombotic microangiopathy (PTTM) caused by esophageal squamous cell carcinoma. Esophagus. 2013;10:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Godbole R, Ghatol A, Betancourt J, Sacoolidge J, Kamangar N. Pulmonary tumor thrombotic microangiopathy: clinical, radiologic, and histologic correlation. J Clin Imaging Sci. 2015;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar N, Price LC, Montero MA, Dimopoulos K, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy: unclassifiable pulmonary hypertension. Eur Respir J. 2015;46(4):1214-1217. [DOI] [PubMed] [Google Scholar]
- 26. Peixoto LS, Valiante PM, Rodrigues RS, Barreto MM, Zanetti G, Marchiori E. An unusual cause of tree-in-bud pattern: pulmonary intravascular tumor embolism caused by chondrosarcoma. Lung. 2015;193(1):151-153. [DOI] [PubMed] [Google Scholar]
- 27. Murugan LT, Saha A, Mabourakh D, et al. Tumoral pulmonary hypertension: a rare but deadly complication of metastatic cancer. Chest. 2020;158(4):2101-2102. [Google Scholar]
- 28. Fuso L, Baldi F, Di Perna A. Therapeutic strategies in pulmonary hypertension. Front Pharmacol. 2011;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]