Abstract
Pedicled buccal fat pad (BFP) flaps have more recently been applied to primary cleft palate reconstruction, and yet the integrity of the flap and the long-term impact on the palate has not yet been studied. This case study uses magnetic resonance imaging (MRI) to evaluate the composition of the soft palate 5 years after the interpositional placement of bilateral pedicled BFP flaps during primary palatoplasty. Anatomical measures are used to quantify the flap and surrounding velopharynx using MRI and 3D computer technology, indicating that this surgical technique may have a lasting impact for children with cleft palate.
Keywords: cleft palate; magnetic resonance imaging; palatal muscles; speech; surgery, maxillofacial
Introduction
Cleft palate is one of the most common congenital craniofacial abnormalities, and its reconstruction remains a challenge. As evidenced by the recent publication of several larger case series, there is clearly a growing interest in the use of the pedicled BFP flap in primary cleft palate reconstruction to promote predictable healing. Numerous techniques have been employed in a wide range of ages including the use of the BFP to fill lateral soft tissue defects and cover exposed bone, to obliterate the space of Ernst, to reconstruct a purposeful nasal mucosal defect,1-3 and for placement at the junction of the hard and soft palate between the oral and nasal mucosa.4-6 Little is known about the long-term fate and effect of the flap on the velum when used at the time of primary palatoplasty, as neither long-term follow up nor objective instrumental evaluation of velopharyngeal anatomy and physiology have been reported.
This case report serves as a pilot study to (1) examine if the BFP flap is maintained within the velum at a 5-year post-operative time point and (2) quantify the structural changes in the velum as a result of BFP flap placement using MRI.
Materials and Methods
Case History
A single patient diagnosed with UCLP was recruited. The patient underwent primary palatoplasty at 12 months of age. At the time of the MRI (6.34 years of age), a perceptual speech assessment and oral mechanism examination were completed by a speech-language pathologist with 5 years of experience in treatment of craniofacial conditions. The patient’s hard and soft palate were visually intact, and muscle contraction within the velum was noted intraorally upon vowel phonation. Resonance was within normal limits based on perceptual assessment using a 5-point scale. No compensatory articulation errors were noted.
Surgical Technique
The patient underwent primary palatoplasty with an anterior 2-flap design in conjunction with a straight-line repair of the soft palate incorporating an intravelar veloplasty (IVV) and bilateral pedicled BFP flaps (Figure 1a). Specifically, the cleft margins were incised, dividing the oral and nasal mucosa from the uvula to the alveolar ridge. Scissors were used to open the margins of the soft palate. A lateral incision was made from the area of the maxillary tuberosity, carried anteriorly, and connected to the marginal incision. Subperiosteal elevation of the oral mucosa was carried beyond the posterior edge of the hard palate. The neurovascular bundle was circumferentially dissected. The nasal mucosa was elevated with a curved freer along the entire floor of the nose to the sidewall. The uvula was then reconstructed, and the nasal mucosa approximated with interrupted sutures along the soft palate and running suture along the hard palate. The oral mucosa was elevated in a submucosal plane. The muscular attachments were divided from the posterior edge of the hard palate, and a portion of the tensor tendon was divided. The tip of the divided tendon and palatopharyngeus were then swept posteriorly and dissected from the nasal mucosa until the nasal surface of the LVP bundle and beginning of the LVP tunnel was identified. Adequate mobilization was ensured to allow for retro-positioning across the midline. Once the identical procedure was completed on the contralateral side, the LVP was reconstructed with 5-0 PDS horizontal mattress sutures.
Figure 1.
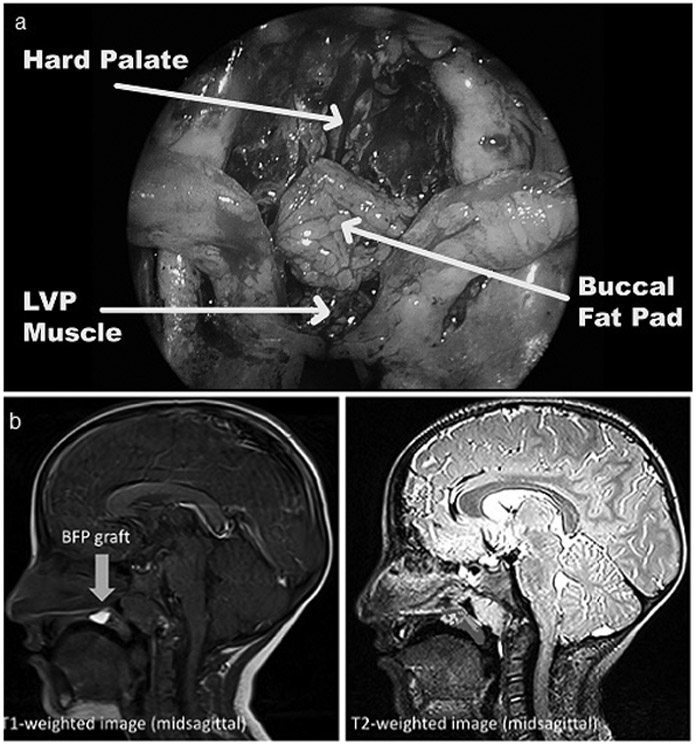
a. The pedicled buccal fat pad flap placed between the oral and nasal mucosa of the soft palate, anteriorly to the levator veli palatini muscle sling and posterior to the hard palate.
b. T1- (left) and T2-weighted (right) MR images are shown from the midsagittal plane for the patient in this study. In the T1-weighted image, the bright buccal fat pad flap can be visualized as designated by the arrow. The T2-weighted image on the right shows the cross-sectional areas for the fat, muscle, and other velar tissue.
The posterior portion of the lateral releasing incisions were used to access the lateral buccal vestibule. Blunt dissection in the vestibule was used to identify the BFP, which was teased into the space of Ernst and then tunneled underneath the oral flap posteriorly to the neurovascular bundle. The pedicled BFP flaps were sutured together in the midline. The oral mucosal flaps were then closed, securing the fat with several horizontal mattress sutures at the junction of the hard and soft palate. The lateral edges of the oral mucosal flaps were loosely reapproximated to the palatal margin of the alveolar mucosa.
Magnetic Resonance Imaging Protocol
Magnetic resonance imaging was completed 5 years post-operatively since this is within the typical timeframe for receiving secondary surgical intervention for velopharyngeal insufficiency (if warranted). Imaging was performed with a 3 Tesla Siemens Magnetom Skyra (Erlangen, Germany) while the patient was lying in the supine position; no sedation was used. The patient was imaged using a T1 single slice true sagittal sequence and a high resolution, T2-weighted turbo-spin-echo (TSE) three-dimensional (3D) anatomical scan to acquire a large field of view covering the oropharyngeal anatomy (repetition time: 2500 ms, echo time: 268 ms, slice thickness: .80 mm, field of view: 256 mm, sequence scan time: 4:04). The MRI protocol, processing methods, and analysis are consistent with that used in previous literature.7
Results
The tissue boundary surrounding the BFP flaps was clearly delineated from surrounding tissues on all MR images, appearing as a bright white mass on the T1 image due to high density of adipose tissue (Figure 1b). Although bilateral BFP flaps were raised, there was no visible separation between the 2 flaps as seen on MRI. Measurements were taken from the T2 TSE 3D volumetric dataset. From the midsagittal image, the length and height of the BFP flap were 11.77 mm and 3.23 mm, respectively. The cross-sectional area of fat within the velum at midline was 25.919 mm2 (14.03%). The cross-sectional area of the LVP muscle was 34.295 mm2 (18.56%). The muscular mass present in the midsagittal slice appeared to consist of mainly LVP muscle fibers (no evidence of musculus uvulae). The patient presented with a cohesive LVP sling that could be visualized in its entirely without interruption from an oblique coronal slice. In the present study, the velar length (28.60 mm), thickness (7.80 mm), and distance from velar knee to posterior pharyngeal wall (4.21 mm) were well within the normative values by race and sex.8 The pharyngeal depth (13.87 mm) and effective velar length (17.37 mm) were observed to be greater in this patient compared to normative data.8 All patient measurements are listed in Supplemental Table 1.
Discussion
This report demonstrates the preservation and impact of the pedicled BFP flap in vivo after placement in the soft palate during primary palatoplasty. The bilateral pedicled BFP flaps are maintained within the velum 5 years post-operatively. The BFP continues to remain a viable adipose tissue that has maintained its position at the posterior margin of the hard palate.8 The authors anticipated these findings given successful clinical outcomes following the use of this technique. With the addition of approximately 8 minutes of operative time and without additional short- or long- term morbidity, pedicled BFP flaps may be considered for all cleft palate repairs with the goal of improving healing and eliminating the risk of oronasal fistulae. In addition, it is postulated that the addition of vascularized fat may improve anatomical and functional characteristics of the repaired palate, potentially allowing for improved velopharyngeal function and maxillary growth.
The majority of prior publications regarding the use of pedicled BFP flaps at the time of primary palatoplasty focus on short-term palatal healing and reducing post-operative fistulae, as evaluated by intra-oral examination. Several case reports and small case series report the intentional creation of a nasal defect to help lengthen the soft palate and subsequent utilization of the pedicled BFP flap to reconstruct the nasal layer, presumably to improve velar function yet no speech results are reported.1-3 Horswell and Chou 4 utilized interpositional BFP flaps in a large series of patients undergoing primary palatoplasty but focused on the impact of variations in staging and double opposing z-plasty technique on post-operative healing. Grobe et al 6 reported on the selective interpositional use of BFP flaps in 5 primary straight-line IVV repairs in patients with wide cleft, confirming excellent healing and minimal donor morbidity. Most recently, Kim et al5 published the use of interpositional BFP flaps in 36 primary repairs over a range of ages employing multiple techniques and a post-operative palatal splint to target short-term healing and the reduction of fistulae. Until this study, the only long-term results were reported by Zhang et al9, who employed the BFP in a lateral position to cover denuded bone, demonstrating an increase in maxillary growth in the BFP group. Additionally, no immediate complications have been reported in the literature associated with the use of pedicled BFP flaps in children, and the impact on facial growth and symmetry have been shown to be negligible.10
Surgical techniques, such as the IVV and double opposing z-plasty, aim to restore the muscular sling and retroposition the LVP to a more favorable location in the velum. It has been hypothesized that healing of the oral mucosa post-palatoplasty may yield anterior migration of the LVP bundles back to their original position. It may be possible that the presence of the BFP flap at the junction between the hard and soft palate prevents the restored LVP sling from migrating anteriorly. The BFP flap in the present study comprised 50.7% of the total effective velar length region (anterior to the LVP muscle). This supports the notion that the BFP flap may prevent anterior migration of the LVP after surgery. Future research should be employed using longitudinal data to monitor any changes in shape and position of the BFP flap over time in a larger cohort of patients.
Conclusion
The present study serves as preliminary support that this surgical technique may create a favorable velopharyngeal system by adding tissue to the velum that appears to remain in place up to 5 years post-palatoplasty. Upon further investigation, this may prove to create a more advantageous system for speech production over traditional cleft palate repair techniques.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research under award number F31DE027878 and the Oral and Maxillofacial Surgery Foundation under a research support grant. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Oral and Maxillofacial Surgery Foundation. We have no additional conflicts of interest to disclose. This study was approved by the East Carolina University and New Hanover Regional Medical Center Institutional Review Boards. The participant’s legal guardian gave informed consent to this work. Authors have read the Helsinki Declaration and have followed the guidelines in this investigation.
References
- 1.Pappachan B, Vasant R: Application of bilateral pedicled buccal fat pad in wide primary cleft palate. Brit J Oral Maxillofac Surg 46:310, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Pinto P, Debnath S: Use of pedicled graft of buccal fat pad to line a nasal defect in releasing pushback palatoplasty. Brit J Oral Maxillofac Surg 45:249, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Li L, Tan W, Chen L, Gao N, Bao C: Application of unilateral pedicled buccal fat pad for nasal membrane closure in the bilateral complete cleft palate. J Oral Maxillofac Surg 68:2029, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Horswell BB, Chou J: Does the CHOP modification improve fistula rate in Furlow double-opposing a-plasty? J Oral Maxiollofac Surg: 2019 [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Kim S, Park Y, Hwang DS, Paeng J, Seok H: The effect of buccal fat pad graft in the palatoplasty and the risk factor of postoperative palatal fistula. J Craniofac Surg 31:658, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Grobe A, Eichhorn W, Hanken H, Precht C, Schmelzle R, Heiland M, Blessman M: The use of buccal fat pad (BFP) as a pedicled graft in cleft palate surgery. Int J Oral Maxillofac Surg 40:685, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kollara L, Perry J: Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging. Cleft Palate Craniofac J 51:669, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Perry JL, Kollara L, Kuehn DP, Sutton BP, Fang X: Examining age, sex, and race characteristics of velopharyngeal structures in 4- to 9- year old children using magnetic resonance imaging. Cleft Palate Craniofac J 55:21, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Zhang X, Zheng C: Application of buccal fat pads in pack palate relaxing incisions on maxillary growth: A clinical study. Int J Clin Exp Med 8:2689, 2015 [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett KG, Thurston TE, Vercler CJ, Kasten SJ, Buchman SR: Harvesting the buccal fat pad does not result in aesthetic deformity in cleft patients: A retrospective analysis. Plast Reconstr Surg 140:362, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


