Abstract
Catechins are a phytochemical present in plants such as tea leaves, beans, black grapes, cherries, and cacao, and have various physiological activities. It is reported that catechins have a health improvement effect and ameliorating effect against various diseases. In addition, antioxidant activity, liver damage prevention, cholesterol lowering effect, and anti-obesity activity were confirmed through in vivo animal and clinical studies. Although most diseases are reported as ones mediating various inflammations, the mechanism for improving inflammation remains unclear. Therefore, the current review article evaluates the physiological activity and various pharmacological actions of catechins and conclude by confirming an improvement effect on the inflammatory response.
Keywords: Catechins, Epigallocatechin gallate, Inflammation, Anti-inflammatory effect, NF-κB pathway
Introduction
Inflammation is a defense mechanism to protect the organs from external injury and infection (Lomax and Calder, 2009). The immune system increases the expression of immune cells and many other inflammatory mediators in response to changes that lead to tissue damage. However, the inflammatory response caused by excessive stimulation becomes chronic, contributing to the promotion and progression of disease in various tissues (Ferrucci and Fabbri, 2018). An immediate reaction to a microbial or virus infection can cause acute inflammation, while a slow and sustained response results in chronic inflammation (Krishnamoorthy and Honn, 2006). These inflammatory responses affect the whole body through blood and lymphatic vessels and exacerbate the onset and symptoms of various diseases (Schwager and Detmar, 2019). The increase in the chronic and systemic inflammatory response is known to be a hallmark of diseases such as cancer, diabetes, cardiotoxicity, metabolic syndrome, and respiratory disorders (Coussens and Werb, 2002; Halaris, 2013; Lontchi-Yimagou et al., 2013; Racanelli et al., 2018). Thus, it is very important to control the inflammatory response.
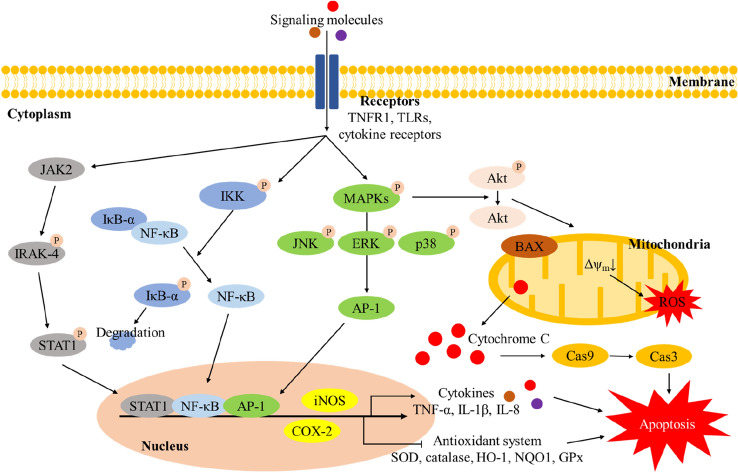
The inflammatory response appears as an interaction of various signaling pathways such as toll-like receptor (TLR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) with an increase in the content of nitric oxide (NO), inflammatory cytokines and chemokines (Kobayashi, 2010). When external pathogens such as lipopolysaccharide (LPS), lipopeptides, heavy metals and microbial or virus infections enter the body, they react with various TLRs in the cellular membrane to stimulate a signal in the cell (Bazzoni et al., 1991; Sochocka et al., 2019). The stimulation of TLRs activates Toll/IL-1 receptor (TIR) domain-containing adaptors, such as myeloid differentiation primary response 88 (MyD88), Toll/interleukin-1 receptor domain-containing adapter protein (TIRAP), and TIR-domain-containing adapter-inducing interferon-β (TRIF) (Piao et al., 2013). This activation continuously recruits the expression of IL-1 receptor-associated kinase-4 (IRAK-4), leading to activation of NF-κB, signal transducer and activator of transcription 1 (STAT1), activator protein 1 (AP-1), and mitogen-activated protein kinase (MAPK) containing c-Jun N-terminal kinases (JNK), p38 MAPK and extracellular signal-regulated kinase (ERK) (Li et al., 2002). Increased NF-κB, STAT1, and AP-1 enters the nucleus, and these proteins increase the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Lee et al., 2017). This downstream signaling ultimately inhibits the antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx), and stimulates the secretion of inflammatory cytokines and chemokines (Schulze-Osthoff et al., 1997) (Fig. 1). Therefore, to effectively eliminate the inflammatory reaction and prevent from various diseases, research on various natural products and compounds is being conducted (Keservani et al., 2010). In particular, the physiological activities of catechin, one of the phenolic compounds, are being continuously studied. However, a schematic pathway of the bioactivity of catechins is insufficient, and this paper was designed to effectively understand the contents.
Fig. 1.
A schematic pathway of inflammatory biomarkers regulated by catechins and their derivates in cell. TNFR1 TNF-α receptor 1, TLRs toll-like receptors, JAK2 Janus kinase 2, IRAK-4 IL-1 receptor-associated kinase-4, STAT1 Signal transducer and activator of transcription 1, IκB-α nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, IKK IκB kinase, MAPK mitogen-activated protein kinase, JNK c-Jun N-terminal kinases, ERK extracellular signal-regulated kinase, AP-1 activator protein 1, Akt protein kinase B, BAX BCL2 Associated X, ROS reactive oxygen species, Cas9 caspase-9, Cas3 caspase-3, iNOS inducible nitric oxide synthase, COX-2 cyclooxygenase-2, TNF-α tumor necrosis factor-α, IL-1β interleukin-1β, IL-8 interleukin-8, SOD superoxide dismutase, HO-1 heme oxygenase-1, NQO1 NAD(P)H quinone oxidoreductase 1, GPx glutathione peroxidase
Catechins
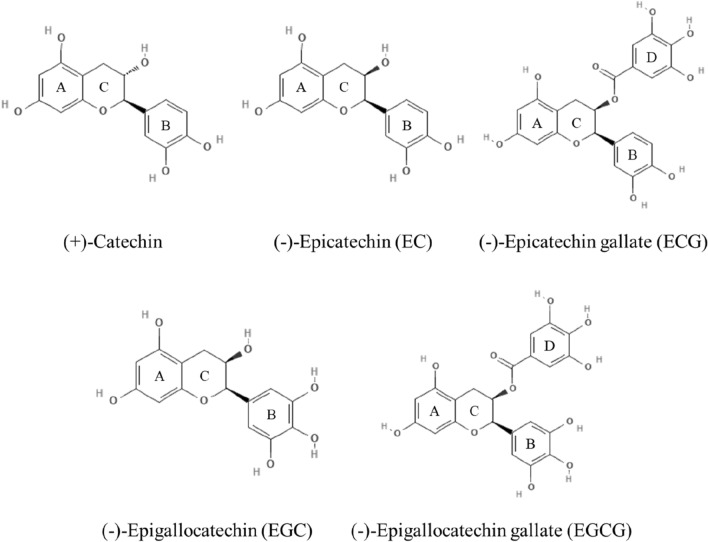
Catechins containing various isoforms are polyphenol compounds belonging to the flavonoid family and are present in various fruits and leaves of plants (Crespy et al., 2004). In general, catechins are not essential for human nutrition, but they can help prevent various diseases and improve health (Arts et al., 2001). Catechins are composed of two steric forms of (+)-catechin and its enantiomer, including the compounds such as epigallocatechin gallate (EGCG), epigallocatechin (EGC), and epicatechin gallate (ECG) (Fig. 2) (Tsuchiya, 2001). Catechin structure is composed of two or more aromatic A ring similar to resorcinol and B ring similar to catechol, each containing at least one aromatic hydroxyl connected by a carbon bridge and a dihydropyran heterocycle (C ring) having a hydroxyl group. The structure of C ring does not have double bond unlike flavonoid structure. EC and EGC are the epimer of a catechin containing 2 or 3 hydroxyl groups in the B ring and a hydroxyl group in the C-ring. ECG and EGCG are ester derivatives of EC and EGC, respectively, and have a structure bonding with gallate at the hydroxyl position of the C ring (Botten et al., 2015; Musial et al., 2020). These catechins are distributed in various plants including green tea, apples, persimmons, beans, peaches, black grapes, and berries, and various beverages such as cider and red wine (Andersen et al., 2005; Gadkari and Balaraman, 2015; Santos‐Buelga and Scalbert, 2000). Catechins are reported to have excellent antioxidant activity, antibacterial activity, and anti-diabetic effect (Iacopini et al., 2008; Kajiya et al., 2004; Kim et al., 2021). Catechins effectively scavenge oxidative stress and free radicals by binding proteins, lipids, nucleic acids and metals in tissues (Yang et al., 2014). These physiological activities are mainly caused by the presence of at least 5 hydroxyl groups included in the structure of the content of diphenylpropanoid skeleton (C6C3C6) of catechins, and these structural characteristics affect the antioxidant ability of catechins (Gadkari and Balaraman, 2015). In particular, catechins showed considerable antioxidant activities compared to glutathione (GSH), vitamin C and other flavonoids, which means that catechins might be functional material in ameliorating human health and improving cellular redox homeostasis (Grzesik et al., 2018).
Fig. 2.
Chemical structures of catechins. Available from: https://pubchem.ncbi.nlm.nih.gov
Interest in the intake of various bioactive substances is increasing (Keservani et al., 2010). In particular, demand for the intake of catechins, which have considerable physiological activities, is continuously increasing. To reduce the inflammatory response caused by various diseases, the mechanism related to the anti-inflammatory effect of catechins with various physiological activities will be analyzed and presented.
Alzheimer's disease and inflammation
Alzheimer's disease (AD) is a typical neurodegenerative disease that includes continuous loss of memory and cognitive function (Kumar and Singh, 2015). Although the pathogenesis of AD has not been precisely elucidated, it is believed that various causes, such as the microglia-induced inflammatory response, oxidative stress, and neuroinflammation, affect the pathogenesis of AD (Kása et al., 1997; Martini et al., 2019; Selkoe, 1991; Tian et al., 2007). Especially, inflammatory cytokines such as TNF-α and interleukins activate the phosphorylation of MAPKs such as JNK, ERK and p38 (Lee et al., 2017). These phosphorylated kinases simulate the expression of NF-κB and STAT1 and reduced the activation of Akt (Patel et al., 2017). The inhibition of Akt activation induces apoptosis cascade, hyperphosphorylation of tau protein, amyloid beta (Aβ) plaque formation, and damage to the cholinergic system (Ksiezak-Reding et al., 2003; Petry et al., 2020; Zhang et al., 2015). In addition, these dysfunctions in brain tissue continuously stimulate the chronic inflammatory cascade resulting in the death of neuronal cells and dysfunction in cortical and hippocampal tissues, and ultimately initiates cognitive deficit, memory loss, abnormal behavior, and AD (Kumar and Singh, 2015; Tsai et al., 2019).
Most catechins are decomposed into (+)-catechin or (+)-epicatechin and gallic acid by intestinal microorganisms in the small intestine and are decomposed to various colonic microbial ring-fission metabolites in the large intestine and absorbed into the blood (Zhu et al., 2015). The catechins and these metabolites can cross brain tissue through the blood–brain barrier (BBB), and this suggests that catechins and their metabolites might play an important role in suppressing neurodegenerative diseases (Unno et al., 2017). Although compounds with various physiological activities in development show excellent bioactivity in eliminating inflammation, their utility as pharmaceuticals or health functional foods is less because they do not pass through the BBB (Shlosberg et al., 2010). On the other hand, catechins not only have excellent physiological activity, but can also easily pass through the BBB and affect brain neurons (Unno et al., 2017).
Intake of green tea catechins improved cholinergic dysfunction by regulating acetylcholine (ACh) content and acetylcholinesterase (AChE) activity in hippocampal tissue (Kim et al., 2021). In addition, it was reported that green tea catechins have an effect on cognitive function improvement by increasing ACh content and choline acyltransferase (ChAT) expression and inhibiting AChE activity in high-fat diet (HFD)-induced diabetic cognitive impairment mice (Kim et al., 2020b). In particular, EGCG suppressed recognition and memory dysfunction and synaptic damage by regulating synaptophysin and postsynaptic density protein 95 (PSD 95) in the frontal cortex and the hippocampus (Guo et al., 2017). ACh mainly plays a role in suppressing the expression of NF-κB in immune cells and macrophages, which inhibits the synthesis of pro-inflammatory cytokines and exhibits anti-inflammatory activity (Shenhar-Tsarfaty et al., 2014). Thus, inhibition of AChE and butyrylcholinesterase (BChE) by catechins might reduce the inflammatory response by inhibiting the degradation of ACh (Bertrand and Wallace, 2020). Chronic inflammation stimulates the production of tumor necrosis factor-α (TNF-α). Increased TNF-α is combined with TNF-α receptor (TNFR) and stimulates inflammatory response (Cheng et al., 2014). This signaling activates the phosphorylation of JNK, which is related to the initiation of apoptosis cascade, increasing caspase activation. An increase in apoptosis signaling causes neuronal inflammation and cell death (Li et al., 2018). However, administration of catechin and EGCG suppressed TNF-α release in primary glial cells, and expression of TLR4 in LPS-induced microglial BV-2 cells (Angeloni et al., 2012; Park and Chun, 2016). Persimmons, which are rich in catechins, suppressed mitochondrial damage by regulating mitochondrial function and apoptotic expression such as B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein (BAX), and cytochrome C in Aβ-induced mice (Kim et al., 2018; Lee et al., 2012). Kim et al. (2022) reported that EGCG improved cognitive dysfunction through an ameliorating effect against scopolamine-induced long-term potentiation (LTP) blockade of the CA1 region in the hippocampal tissue of SD mice. Administration of green tea rich in catechins inhibited tau and inflammatory signaling by suppressing the expression of p-JNK, phosphorylated protein kinase B p-(Akt) and p-tau, and stimulated the Aβ clearance pathway by regulating brain-derived neurotrophic factor (BDNF), insulin-degrading enzyme (IDE), and Aβ in HFD-induced diabetic mice (Kim et al., 2020b). Finally, the mechanism between Alzheimer’s disease and inflammatory effect of catechins were presented in Table 1.
Table 1.
Physiological studies of catechins on Alzheimer’s disease
| Materials | Dosesa | Species/organ (origin)b | Stressorsc | Biomarkersd | References |
|---|---|---|---|---|---|
| EGCG | 15 mg/kg (p.o.) | SAMP8 mice/FC, HIP | AD-transgenic |
↑Synaptophysin, PSD95 ↓p-tau, Aβ1–42, BACE-1 |
Guo et al. (2017) |
| EGCG | 2 μM/well | BV-2 cells/Microglia | LPS | ↓TLR4, nitric oxide, iNOS, TNF-α, IL-1β | Park and Chun (2016) |
| EGCG | 5 mg/kg/day (i.p.) | SD rats/HIP | Scopolamine |
↑AChE, SOD, LTP ↓MDA |
Kim et al. (2022) |
| EGCG |
50 mg/kg (p.o.) |
APP/PS1 mice/WB | AD-transgenic |
↑IL-10, IL-13 ↓IL-1β |
Bao et al. (2020) |
| Catechin hydrate |
50 mg/kg (p.o.) |
Wistar rats/HIP, CC | Streptozotocin |
↑GSH, GPx, GR, catalase ↓AChE, TNF-α, IL-1β |
Ahmed et al. (2013) |
| Green tea catechins | 40 mg/kg of b.w. (p.o.) | BALB/c mice/HIP | PM2.5 |
↑SOD, GSH, BCl-2, AChR-3α, ChAT ↓p-JNK, p-IκB-α, TNF-α, BAX, Aβ, p-tau, AChE |
Kim et al. (2021) |
| Green tea catechins | 50 mg/kg of b.w. (p.o.) | C57BL/6 mice/WB, HIP, CC | HFD |
↑p-Akt, BDNF, IDE ↓p-JNK, p-tau, Aβ, COX-2, IL-1β |
Kim et al. (2020b) |
| Persimmon catechin | 20 mg/kg of b.w. (p.o.) | ICR mice/WB | Trimethyltin chloride |
↑SOD, GSH, MMP, ATP, p-Akt ↓AChE, p-JNK, p-tau, cytochrome c, IRS-1pSer, TNF-α, p-NF-κB, BAX |
Kim et al. (2018) |
aMaximal dose of referred study. Body weight (b.w.)
bAbbreviation of organs. FC frontal cortex, HIP hippocampus, WB whole brain, CC cerebral cortex
cAbbreviation of stressors. AD Alzheimer’s disease, LPS lipopolysaccharide, PM particulate matter, HFD high-fat diet
dAbbreviation of biomarkers. PSD95 postsynaptic density protein 95, Aβ amyloid beta, BACE-1 beta-secretase 1, TLR4 toll-like receptor 4, iNOS inducible nitric oxide synthase, TNF-α tumor necrosis factor-α, IL interleukin, AChE acetylcholinesterase, SOD superoxide dismutase, LPT ling term potential, MDA malondialdehyde, GSH glutathione, GPx glutathione peroxidase, GR glutathione reductase, BCl-2 B-cell lymphoma 2, AChR-3α acetylcholine receptor α3, ChAT choline acyltransferase, p-JNK phosphorylated c-Jun N-terminal kinases, p-IκB-α phosphor-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, BAX BCl-2 associated X, BDNF brain derived neurotrophic factor, IDE insulin degrading enzyme, COX-2 cyclooxygenase-2, MMP mitochondrial membrane potential, IRS-1pSer phosphorylated insulin receptor substrate 1 (Ser), p-NF-κB phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells
Metabolic syndrome and inflammation
Metabolic syndrome is a disorder involving various diseases including glucose tolerance, obesity, dyslipidemia, and hypertension, and increases the incidence of cardiovascular disease, type 2 diabetes, and cancer (Kaur, 2014). In general, excessive intake of high fat and high sugar is the main cause of metabolic syndrome, and when these contents increase in the blood, lipid accumulation in the liver and adipose tissue is accelerated through dyslipidemia (Kumar et al., 2014). Non-alcoholic fatty liver disease (NAFLD) from the intake of HFD increases inflammatory cytokines by activating the TNF-a/receptor-interacting protein kinase 3 (RIPK3) axis (Xu et al., 2019). In addition, various saturated fatty acids and lipids stimulate the signaling of TLR by binding the fatty acid parts of ligands (Raetz, 1990). The activation of TLR increases the secretion of inflammatory cytokines such as TNF-α and interleukins by upregulating NF-κB and apoptotic pathways and increasing protein expression of TLR-mediated protein and gene signaling (Doğanyiğit et al., 2020). In particular, lipid accumulation in hepatic tissue stimulates the activation of immune cells that secrete inflammatory cytokines such as TNF-α and interleukin 1 beta (IL-1β), thereby stimulating gluconeogenesis and glycogenolysis, and it initiates insulin resistance and early diabetic symptoms through an increase in blood glucose (King, 2008; Ramnanan et al., 2010). Increased glucose and cytokines in serum abnormally phosphorylate the residue of insulin receptor substrate-1 (IRS-1) that regulates insulin signaling (Alipourfard et al., 2019). Phosphorylated IRS-1 increases cytokines by downregulating the expression level of Akt and accelerating apoptosis signaling and the NF-κB pathway in various organs such as the liver, heart, lung, brain, and kidney, and adipose tissues (Hussain et al., 2012; Zand et al., 2017).
However, intake of powdered green tea, which is rich in catechins, reduced inflammatory cytokines such as IL-1β and TNF-α in adipose and hepatic tissues, and regulated lipid and cholesterol accumulation metabolism in HFD-induced C57BL/6 mice (Kim et al., 2020a, 2020b). Catechin-rich wine grape seed flour inhibited adipose tissue weight, and plasma lipid concentration in high fructose diet-induced mice (Seo et al., 2020). (+)-Catechin treatment inhibited the lipid degradation of adipocytes by reducing the expression of CCAAT-enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and regulating the cAMP/protein kinase A (PKA) signaling pathway in 3T3-L1 cells (Jiang et al., 2019). According to Raederstorff et al. (2003), the intake of EGCG has been reported to decrease the absorption of triglycerides and cholesterol. Catechins also help to excrete cholesterol and fat from the body, thereby lowering LDL cholesterol in the blood (Miura et al., 2001). EGCG inhibited the expression of monocyte chemoattractant protein-1 (MCP-1) and activation of NF-κB against TNF-α-induced human umbilical vein endothelial cells (HUVEC) (Relja et al., 2011). Finally, the mechanism between metabolic syndrome and inflammatory effect of catechins were presented in Table 2.
Table 2.
Physiological studies of catechins on metabolic disease related to high fat diet
| Materials | Dosesa | Species/organ (origin) | Stressorsb | Biomarkersc | References |
|---|---|---|---|---|---|
| EGCG | 50 μM/well |
QSG-7701 cells /Human liver cell |
OA |
↑SOD, catalase, GPx, LC3A/B, Beclin-1, p-ERK ↓P62, p-JNK, p-p38 |
Wu et al. (2021) |
| EGCG | 50 mg/kg/day | C57BL/6 J/liver | HFD | ↓Triglyceride, total cholesterol, ALT, AST, NEFA | Wu et al. (Physiological studies of catechins) |
| EGCG | 0.7 g/day/kg of b.w. (p.o.) | Wistar rats (female)/plasma, fecal | Semisynthetic diet high in cholesterol and fat |
↑HDLC ↓Total cholesterol, Triglyceride, free fatty acids, total lipid, cholesterol, fat |
Raederstorff et al. (2003) |
| Epicatechin | 200 mg/kg (p.o.) | C57BL/6 J/adipose tissue | HFD | ↓TNF-α, IL-6, Saa3, Ip-10, Ccl19, cd11c, Cidea | Sano et al. (2017) |
| ( +)-Catechin | 300 μmol/L | 3T3-L1 cells/adipocytes | IBMX, DEX |
↑cAMP, PKA, ATGL, PLIN ↓C/EBPβ, C/EBPδ, PPARγ, SREBP1C |
Jiang et al. (2019) |
| Green tea catechin | 1.7 mg/day (p.o.) | C57BL/6 J/plasma | ApoE-deficient transgenic mice |
↑HDLC ↓Total cholesterol, Triglyceride, VLDLC, MDA |
Miura et al. (2001) |
| Green tea catechin | 0.1% (w/v) (p.o.) | Lewis rats (female)/liver | Haemorrhage/resuscitation | ↓ALT, IL-6, PMNL, ICAM-1, p-IκB-α, CAE | Relja et al. (2011) |
| Green tea catechin | 50 mg/kg (p.o.) | C57BL/6/liver, adipose | HFD | ↓TNF-α, IL-1β, TNFR1, p-IRS-1, p-JNK, iNOS, COX-2, HMGCR, PPARγ, FAS | Kim et al. (2020b) |
| Wine grape seed flour catechin | 5% (w/v) (p.o.) | C57BL/6 J/plasma | HFD, HFrD |
↑HDLC ↓Triglyceride, Total cholesterol |
Seo et al. (2020) |
aMaximal dose of referred study. Body weight (b.w.)
bAbbreviation of stressors. OA oleic acid, HFD high-fat diet, IBMX isobutylmethylxanthine, DEX dexamethasone, HFrD high-fructose diet
cAbbreviation of biomarkers. SOD superoxide dismutase, GPx glutathione peroxidase, p-ERK phosphorylated extracellular signal-regulated kinase, p-JNK phosphorylated c-Jun N-terminal kinases, ALT alanine aminotransferase, AST aspartate aminotransferase, NEFA non-esterified fatty acids, HDLC high-density lipoprotein cholesterol, TNF-α tumor necrosis factor-α, IL interleukin, Saa3 serum amyloid A3, Ip-10C-X-C motif chemokine ligand 10, C–C motif chemokine, Ccl19 ligand 19, cd11c integrin αX subunit, Cidea cell death-inducing DNA fragmentation factor alpha-like effector A, cAMP cyclic adenosine monophosphate, PKA protein kinase A, ATGL adipose triglyceride lipase, PLIN perilipins, C/EBPβ CCAAT-enhancer-binding protein β, C/EBPδ CCAAT-enhancer-binding protein δ, PPARγ peroxisome proliferator activated receptor gamma, SREBP1C sterol regulatory element-binding protein 1C, VLDLC very-low-density lipoprotein cholesterol, MDA malondialdehyde, PMNL polymorphonuclear leukocyte, ICAM-1 intercellular adhesion molecule-1, p-IκB-α phosphor-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, CAE chloroacetate esterase, TNFR1TNF-α receptor 1, p-IRS-1 phosphorylated insulin receptor substrate-1, iNOS inducible nitric oxide synthase, COX-2 cyclooxygenase-2, HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase, FAS fatty acid synthase
Hepatic diseases and inflammation
Inflammation is the cause of diseases such as viral, alcoholic, fatty and autoimmune chronic liver dysfunction, which affects all stages of liver disease (Czaja, 2014). A prolonged inflammatory response affects the onset of liver fibrosis, cirrhosis, fatty liver, and cancer, and inhibits the detoxification of various toxins generated in the body, reducing the ability to maintain health in the body (Seki and Schwabe, 2015). Hepatic tissue damaged by chronic inflammation promotes apoptosis and activates hepatic stellate cells and Kupffer cells (Friedman et al., 2008). These cells induce inflammation and hepatic fibrosis, and also induce the transformation of hepatic stellate cells into myofibroblasts through the activation of transforming growth factor beta 1 (TGFβ1), and endothelial growth factor (Friedman and Arthur, 1989). This transformation produces inflammatory cytokines and chemokines, and increases the expression of antigens on T lymphocytes and natural killer T cells. Eventually, the chronic immune response leads to apoptosis and fibrosis (Uhal et al., 2007). In addition, activated Kupffer cells continuously stimulate inflammatory response by inducing the production of reactive oxygen stress (ROS) and NO, which causes DNA damage, apoptosis, and promotion of pro-inflammatory genes (Canbay et al., 2003).
Administration of green tea catechins suppressed hepatic fibrosis by reducing the expression of activator protein 1 (AP-1), α-smooth muscle actin (α‐SMA), TGF-β1, 4-hydroxynonenal (4-HNE), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) and inhibited oxidative stress by regulating the activation of stellate cells (Kobayashi et al., 2010). Catechins such as ECG, EGC and EGCG protected against liver fibrosis by inhibiting the expression of TGF-β and phosphorylation of ERK1/2 and Smad1/2 in CCl4-induced fibrotic rat (Wang et al., 2018). Green tea catechins also decreased the expression of procollagen-α1(I) and α-SMA, and inhibited pro-inflammatory cytokines, growth factor-β modification and accumulation of 4-HNE. This regulation resulted in the inhibition of liver fibrosis and bile duct adhesion-dependent changes by preventing the activation of astrocytes in the liver (Zhong et al., 2003). (+)-Catechin inhibited cirrhosis caused by chronic hepatitis C virus (HCV) infection by inhibiting HCV replication and inflammatory protein expression of COX-2 and NF-κB (Lee et al., 2011). Finally, the mechanism between hepatic diseases and inflammatory effect of catechins were presented in Table 3.
Table 3.
Physiological studies of catechins on hepatic disease
| Materials | Dosesa | Species/organ (origin) | Stressorsb | Biomarkersc | References |
|---|---|---|---|---|---|
| EGCG | 6 g/kg/day | Wistar rats/liver | Ethanol |
↑Glycogen, ADH, ALDH, GST ↓AST, ALT, GGT, LDH, cytochrome P450, bilirubin |
Anuradha and Kaviarasan (2007) |
| Epicatechins | 300 mg/kg (p.o.) | Sprague Dawley rats/liver | CCl4 | ↓α‐SMA, TGF-β, p-ERK1/2, p-Smad1, TNF-α, MMP2, MMP9, IL-17 | Wang et al. (2018) |
| ( +)-Catechin | 16 μg/mL/well | Huh-7 cells/human hepatoma cells | HCV | ↓COX-2, p-NF-κB, NS5A-Myc | Lee et al. (2011) |
| Green tea catechin | 0.1% (w/v) (p.o.) | Sprague Dawley rats/Liver | BDL | ↓ALT, procollagen-α1(I), AP-1, α-SMA, 4-HNE, TGF-β1, TNF-α | Zhong et al. (2003) |
| Green tea catechin | 50 mg/kg/day (p.o.) | Wistar rats/liver | Sanfenon | ↓AST, ALT, 4-HNE, 8-OHdG, AP-1, TGF-β1, α‐SMA | Kobayashi et al. (2010) |
| Green tea catechin | 10% (w/v) (p.o.) | Hamsters/liver | CCl4 |
↑GSH, ADH, cytochrome P450 reductase, HDLC ↓MDA, total cholesterol, triglyceride, LDLC. p53 |
Elgawish et al. (2015) |
| Black tea catechin | 2% (w/v) (p.o.) | Sprague Dawley rats/liver | Aflatoxin |
↑SOD, catalase, GST, GR, GPx ↓AST, ALT, ALP |
Alm-Eldeen et al. (2015) |
aMaximal dose of referred study. Body weight (b.w.)
bAbbreviation of stressors. CCl4 carbon tetrachloride, HCV hepatitis C virus, BDL bile duct ligation
cAbbreviation of biomarkers. ADH alcohol dehydrogenase, ALDH aldehyde dehydrogenase, GST glutathione S-transferase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma glutamyl peptidase, LDH lactate dehydrogenase, α‐SMA α-smooth muscle actin, TGF-β transforming growth factor-β, p-ERK1/2 phosphorylated extracellular signal-regulated kinase ½, TNF-α tumor necrosis factor-α, MMP2 matrix metalloproteinase-2, MMP9 matrix metalloproteinase-9, IL-17 interleukin-17, COX-2 cyclooxygenase-2, p-NF-κB phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells, NS5A-Myc nonstructural protein 5A-Myc, AP-1 activator protein 1, 4-HNE 4-hydroxynonenal, 8-OHdG 8-hydroxy-2′-deoxyguanosine, GSH glutathione, HDLC high-density lipoprotein cholesterol, MDA malondialdehyde, LDLC low-density lipoprotein cholesterol, SOD superoxide dismutase, GR glutathione reductase, GPx glutathione peroxidase, ALP alkaline phosphatase
Respiratory disease and inflammation
Pulmonary inflammation is caused by the inhalation or invasion of external contaminants. Sources of external pollutants mainly include tobacco smoke, toxins, bacteria, viruses, and particulates including heavy metals (Adler and Li, 2001). The inflammatory response caused by cigarette smoke leads to chronic obstructive pulmonary disease (COPD), and air pollution containing particulate matter (PM), heavy metal, biomass fuels, carbon dioxide and ozone induce idiopathic pulmonary fibrosis (Johannson et al., 2014; Polosa et al., 2016). In addition, it has been reported that various pulmonary viruses such as influenza virus, respiratory syncytial virus (RSV), adenovirus, and coronavirus respond easily to the respiratory tract, and stimulate the inflammatory response of lung tissue, causing various symptoms such as tonsillitis, bronchitis, and pneumonia (Lessler et al., 2009). This viral lung injury causes secondary bacterial pneumonia, and inflammatory cytokines produced in the lung tissue have effects throughout the whole body (Conti et al., 2020). Lung tissue is involved in the expression of inflammation by interacting with various cells, including epithelial cells and immune cells surrounding the airways and alveoli. Airway epithelial cells secrete mucus to trap particles in the inhaled air as a physical system that repels external toxicants (Knudsen and Ochs, 2018). To suppress pulmonary damage by inducers, antimicrobial peptides, proteases, cytokines and chemokines are secreted in pulmonary epithelial cells (Wong et al., 2016). However, excessive chronic inflammation stimulates macrophages to secrete inflammatory mediators and various enzymes and increases the number of lymphocytes, resulting in the destruction of the alveoli (Ingersoll et al., 2011).
PM continuously increases toxicity in respiratory organs (Huang et al., 2017). Intake of green tea catechins ameliorated PM2.5-induced systemic inflammation in BALB/c mice by suppressing the deficits of the antioxidant system and mitochondrial function, and regulating the expression of TNF-α, p-JNK, p-NF-κB and IL-1β (Kim et al., 2021). PM contains heavy metals, carbon monoxide and polycyclic aromatic hydrocarbons (PAHs) (Shou et al., 2019). Administration of catechins hydrate modulated benzo(a)pyrene-induced apoptotic toxicity and inflammation by regulating the expression of TNF-α, NF-κB, COX-2, BAX and caspase-3 in mice lung tissue (Shahid et al., 2016). Wang et al. (2020) reported that EGCG helps the excretion of various heavy metals, including Hg, Cd, Cr, Ni, and Cu, absorbed into the body and reduces toxicity in tissues. Catechins have a high binding affinity with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins containing 3-chymotrypsin-like cysteine protease (3CL), RNA-dependent RNA polymerase (RdRp), and receptor-binding domain (RBD), so they have the potential to act as an excellent multi-targeting agent to regulate COVID-19 pandemic. (Mishra et al., 2021). In addition, catechins in green tea, coffee, and berries also act as a potent inhibitor of influenza A virus, preventing infection (Kaihatsu et al., 2014; Onishi et al., 2020; Sekizawa et al., 2013; You et al., 2018). Catechin and epicatechin inhibited damage of mitochondrial complex I, reduced ATP level, and NO production in amiodarone-induced human lung fibroblasts (Santos et al., 2017). Finally, the mechanism between respiratory disease and inflammatory effect of catechins were presented in Table 4.
Table 4.
Physiological studies of catechins on respiratory disease
| Materials | Dosesa | Species/organ (origin) | Stressorsb | Biomarkersc | References |
|---|---|---|---|---|---|
| EGCG | 200 mg/kg (p.o.) | Sprague Dawley rats/lung | Hg, Cd, Cr, Ni, Cu |
↑Excretion of heavy metals ↓Bilirubin, ALT, AST |
Wang et al. (2020) |
| Epicatechin | 500 μM/well | MRC-5 cells/human lung fibroblast cells | Amiodarone |
↑Mitochondrial complex I, ATP, SOD, catalase ↓MDA, PC, nitric oxide |
Santos et al. (2017) |
| Catechin hydrate | 40 mg/kg of b. w. (p.o.) | Swiss albino mice/lung | Benzo(a)pyrene |
↑GPx, GST, GR, GSH, catalase, SOD, QR, BCl-2, ↓LPO, LDH, CYPOR, mEH, NF-κB, IL-6, TNF-α, COX-2, p53, BAX, Caspase-3 |
Shahid et al. (2016) |
| Catechin | 100 μg/mL/well | A549 cells/pulmonary carcinoma | Influenza A (H1N1) virus | ↓Neuraminidase activity, plaque formation | You et al. (2018) |
| Catechin | 500 μM/well | MRC-5 cells/human lung fibroblast cells | Amiodarone |
↑Mitochondrial complex I, ATP, SOD, catalase ↓MDA, PC, nitric oxide |
Santos et al. (2017) |
| Green tea catechins | 10 μg/mL/well | A549 cells/pulmonary carcinoma | PM2.5 |
↑SOD, BCl-2 ↓ROS, apoptosis, MDA, caspase-3, BAX |
Zhang et al. (2018) |
| Green tea catechins | 40 mg/kg of b.w. (p.o.) | BALB/c mice/lung | PM2.5 |
↑SOD, GSH ↓MDA, TNF-α, p-JNK, p-IκB-α, p-NF-κB, BAX, caspase-1, COX-2, IL-1β |
Kim et al. (2021) |
aMaximal dose of referred study. Body weight (b.w.)
bAbbreviation of stressors. PM particulate matter
cAbbreviation of biomarkers. ALT alanine aminotransferase, AST aspartate aminotransferase, SOD superoxide dismutase, MDA malonaldehyde, PC protein carbonyl groups, GPx glutathione peroxidase, GST glutathione-S-transferase, GR glutathione reductase, GSH glutathione, QR quinone reductase, BCl-2 B-cell lymphoma 2, LPO lipid peroxidation, LDH lactate dehydrogenase, CYPOR crystal structure of a NADPH-cytochrome P450, mEH microsomal epoxide hydrolase, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, IL-6 interleukin-6, TNF-α tumor necrosis factor-α, COX-2 cyclooxygenase-2, BAX BCl-2 associated X, PC protein carbonyl groups, ROS reactive oxygen species, p-JNK phosphorylated c-Jun N-terminal kinases, p-IκB-α phosphor-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, BAX BCl-2 associated X, IL-1β interleukin-1β
Gastrointestinal (GI) tract and inflammation
Inflammatory bowel disease (IBD) is a chronic immune disease of unknown etiology related to the uncontrolled mucosal immune response of the intestinal microflora in the host intestine (Takaishi et al., 2008). IBD damages tight junction (TJ) proteins, resulting in altered intestinal permeability and impaired epithelial barrier function, and increased immune response due to changes in intestinal flora (Lee, 2015). Alterations in the gut microbiota are responsible for influencing various diseases such as obesity, irritable bowel syndrome, tropical enteropathy, antibiotic-associated diarrhea, and vaginitis, and impair the digestion and absorption of nutrients, energy homeostasis, and maintenance of intestinal tissue of the host (Musso et al., 2010; Qin, 2002). Changes in the gut microbiota increase the inflammatory response by stimulating cytokine signaling pathways and indicate intestinal imbalances through changes in some microbial-derived metabolites such as short-chain fatty acids (SCFAs) (Huda-Faujan et al., 2010). Immune response eventually indicates damage to the intestinal tissue, causing nutritional abnormalities and an increase in inflammatory response (Musso et al., 2010). Symptoms of IBD are reported as Crohn's disease (CD) and ulcerative colitis (UC), and the immune pathology of IBD appears to be due to the overexpression of interferon-γ (IFN-γ) and TNF-α (Rafa et al., 2010). When the epithelial barrier is destroyed by an increase in the inflammatory response or infection of pathogenic bacteria, dendritic cells and macrophages are activated to react with antigens and present antigens to the surface through major histocompatibility complex (MHC) class II complexes (Bedford et al., 2006; Kelsall et al., 2005). This response promotes the differentiation of naive T cells into effector and regulatory T cells, and ultimately increases cytokines (Leon et al., 2006).
Catechins can regulate intestinal microbial balance by modulating components of intestinal metabolites. Catechins absorbed through the intestinal tract exhibit various physiological activities, but unabsorbed catechin also plays an important role in the intestine (Forester et al., 2012; Shabbir et al., 2021; Stalmach et al., 2010). This is reported to play the role of prebiotics by stimulating the growth of symbiotic bacteria such as Lactobacillus plantarum using phenolic compounds as substrates and perturbing the function of the cytoplasmic membrane of gram-negative pathogenic bacteria such as Stenotrophomonas maltophila (Liu et al., 2018; Taylor et al., 2005). Inflammation causes an imbalance of the Firmicutes and Bacteroidetes (F/B) ratio, leading to several pathologies including obesity, diabetes and IBD (Stojanov et al., 2020), whereas intake of catechins also increases microbial metabolic functions related to SCFAs biosynthesis by regulating the F/B ratio (Xue et al., 2016). In addition, catechin metabolites such as phenylvalerolactones, valerolactones, and phenylvaleric acids digested in the intestine promote the production of SCFAs by anaerobic fermentation to help improve intestinal health (Santangelo et al., 2019). EGCG reduced gut-derived endotoxin translocation and inhibited the loss of TJ proteins such as claudin-1, occludin, zonula occludens-1 (ZO1) and hypoxia-inducible factor 1-alpha (HIF-1α) in HFD-induced diabetic mice (Dey et al., 2020). In addition, catechins reduce the inflammatory response by regulating the expression of NF-κB, MAPK, and nuclear factor erythroid-2-related factor 2 (Nrf2) in the intestine and the infiltration and proliferation of immune-related cells including neutrophils, macrophages, and T lymphocytes (Fan et al., 2017; Brückner et al., 2012). Finally, the mechanism between gastrointestinal (GI) tract and inflammatory effect of catechins were presented in Table 5.
Table 5.
Physiological studies of catechins on gastrointestinal disease
| Materials | Dosesa | Species/organ (origin) | Stressorsb | Biomarkersc | References |
|---|---|---|---|---|---|
| EGCG | 100 mg/kg (i.g.) | CF-1 mice/Plasma | Non-stressor |
↑SGLT-1 ↓α-Amylase activity, GLUTs |
Forester et al. (2012) |
| EGCG | 1.5 mM (p.o.) | C57BL/6 mice (female)/colon | DSS |
↑Bowel length, SOD, GPx ↓MPO, MDA, |
Brückner et al. (2012) |
| EGCG | 0.3% (w/w) (p.o.) | C57BL/6 mice/colon, ileum | HFD |
↑claudin-1, occludin, ZO-1, JAMA, HIF-1α ↓TNF-α, calprotectin |
Dey et al. (2020) |
| EGCG | 0.05% (w/w) (p.o.) | ICR mice/colon | DSS |
↑HO-1, p-ERK 1/2 ↓p-PI3K, p-IκB-α, iNOS, COX-2, IL-6, IL-1β, TNF-α, p-p65, nitric oxide |
Chiou et al. (2012) |
| Epicatechin | 300 mg/kg (p.o.) | C57BL/6 J mice/colon | DSS |
↑SOD, GPx, catalase ↓TNF-α, IL-6, nitric oxide, MPO, NF-κB, MDA |
Zhang et al. (2016) |
| Cocoa catechin | 500 mg/kg (p.o.) | Balb/C mice (female)/colon | DSS |
↑Bowel length, ↓MPO, IL-6, nitric oxide, COX-2, pSTAT3, pSTAT1α, IL-1β, TNF-α, IFNγ |
Andújar et al. (2011) |
| Green tea catechin | 2% (w/w) (p.o.) | C57BL/6 J mice/Jejunal, ileal, colon | HFD | ↓TNF-α, iNOS, MCP-1, CD14, MD2, TLR4 | Dey et al. (2019) |
| Green tea catechin | 2% (w/w) (p.o.) | C57BL/6 mice/colon, ileum | HFD |
↑Claudin-1, occludin, ZO-1, JAMA, HIF-1α ↓TNF-α, calprotectin |
Dey et al. (2020) |
aMaximal dose of referred study. Body weight (b.w.)
bAbbreviation of Stressors. DSS dextran sulfate sodium, HFD high-fat diet
cAbbreviation of biomarkers. SGLT-1 sodium-glucose transport-1, GLUT glucose transporter, SOD superoxide dismutase, GPx glutathione peroxidase, MPO myeloperoxidase, MDA malondialdehyde, ZO-1 zonula occludens-1, JAMA junctional adhesion molecule A, HIF-1α hypoxia-inducible factor 1-alpha, TNF-α tumor necrosis factor-α, HO-1 heme oxygenase-1, p-ERK1/2 phosphorylated extracellular signal-regulated kinase 1/2, p-PI3K phosphorylated phosphatidylinositol 4,5-bisphosphate, p-IκB-α phosphor-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, iNOS inducible nitric oxide synthase, COX-2 cyclooxygenase-2, IL-6 interleukin-6, IL-1β interleukin-1β, pSTAT3 phosphorylated signal transducer and activator of transcription 3, pSTAT1α phosphorylated signal transducer and activator of transcription 1α, IFNγ interferon gamma, MCP-1 monocyte chemoattractant protein-1, CD14 cluster of differentiation-14, MD2 myeloid differentiation factor 2, TLR4 toll-like receptor 4
Safety concern of catechins
It is generally considered that safe for ingestion of low-dose catechins or green tea preparations that contain large amounts of catechins (Church et al., 2015; Lee et al., 2002; Mazzanti et al., 2015). In particular, administration of catechins has been reported to have a protective effect on liver tissue in various hepatic toxicity disease models such as HFD, carbon tetrachloride, acetaminophen, and D-galactosamine (Kim et al., 2020b; Liu et al., 2015; Park et al., 2015; Yao et al., 2015). However, recent studies have reported hepatic toxicity by intake of dietary supplements containing high doses of catechins or green tea. In a rodent model, ingestion of high concentration catechins increased serum alanine aminotransferase (ALT) and bilirubin content, and caused gastrointestinal (GI) tract toxicity (Galati et al., 2006; Isbrucker et al., 2006; Lambert et al., 2010). It has also been reported that administration of EGCG (500 mg/kg, i.g.) presented in liver and GI toxicity in beagle dogs (Isbrucker et al., 2006). According to Mazzanti et al. (2015), it was reported that intake of green tea containing high doses of catechins increased hepatotoxicity by increasing periportal and portal vein inflammation in patients. Although the numerous studies related to hepatic toxicity of high doses of catechins are reported, the mechanism of hepatotoxicity is unclear.
In conclusion, chronic inflammation is associated with various diseases, and the persistence of inflammation systemically indicates dysfunction and damage of various organs. Plant-derived catechins impart anti-inflammatory and inflammatory response stabilization based on excellent antioxidant activity. This review provides convincing evidence that catechins and plant materials rich in catechins are effective in suppressing inflammatory stress in the short and long term through an inflammatory mechanism in in vivo studies. Therefore, catechins themselves or nutraceutics with catechins can be used as strong anti-inflammatory agents or functional food materials with excellent physiological activity. However, some in vivo and clinical studies have continuously reported that high doses of catechins and green tea extract cause safety concerns and risks of hepatic damage and liver necrosis. Considering these reports, additional studies should be conducted to confirm the empirical evidence of hepatotoxicity pathway, or to make guidelines for stably ingesting catechins by limiting intake so that it does not induce toxicity.
Acknowledgements
This research was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Export Promotion Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (617072-5). This study was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea (NRF 2018R1D1A3B07043398) funded by the Ministry of Education, Republic of Korea.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jong Min Kim, Email: myrock201@naver.com.
Ho Jin Heo, Email: hjher@gnu.ac.kr.
References
- Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. American Journal of Respiratory Cell and Molecular Biology. 2001;25:397–400. doi: 10.1165/ajrcmb.25.4.f214. [DOI] [PubMed] [Google Scholar]
- Ahmed ME, Khan MM, Javed H, Vaibhav K, Khan A, Tabassum R, Ashafaq M, Islam F, Safhi MM, Islam F. Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochemistry International. 2013;62:492–501. doi: 10.1016/j.neuint.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Alipourfard I, Datukishvili N, Mikeladze D. TNF-α downregulation modifies insulin receptor substrate 1 (IRS-1) in metabolic signaling of diabetic insulin-resistant hepatocytes. Mediators of Inflammation. 2019;2019:3560819. doi: 10.1155/2019/3560819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm-Eldeen AA, Mona MH, Shati AA, El-Mekkawy HI. Synergistic effect of black tea and curcumin in improving the hepatotoxicity induced by aflatoxin B1 in rats. Toxicology and Industrial Health. 2015;31:1269–1280. doi: 10.1177/0748233713491807. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Markham KR. Flavonoids: Chemistry, biochemistry and applications. Boca Raton: CRC Press; 2005. pp. 472–551. [Google Scholar]
- Andújar I, Recio MC, Giner RM, Cienfuegos-Jovellanos E, Laghi S, Muguerza B, Ríos JL. Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. Journal of Agricultural and Food Chemistry. 2011;59:6474–6483. doi: 10.1021/jf2008925. [DOI] [PubMed] [Google Scholar]
- Angeloni C, Pirola L, Vauzour D, Maraldi T. Dietary polyphenols and their effects on cell biochemistry and pathophysiology. Oxidative Medicine and Cellular Longevity. 2012;2012:583901. doi: 10.1155/2012/583901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha CV, Kaviarasan S. (-) Epigallocatechin gallate restores ethanol-induced alterations in hepatic detoxification system and prevents apoptosis. Oriental Pharmacy and Experimental Medicine. 2007;7:311–320. doi: 10.3742/OPEM.2007.7.3.311. [DOI] [Google Scholar]
- Arts ICW, Hollman PCH, Feskens EJM, de Mesquita HB, Kromhout D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. European Journal of Clinical Nutrition. 2001;55:76–81. doi: 10.1038/sj.ejcn.1601115. [DOI] [PubMed] [Google Scholar]
- Bao J, Liu W, Zhou HY, Gui YR, Yang YH, Wu MJ, Xiao Y, Shang J, Long G, Shu XJ. Epigallocatechin-3-gallate alleviates cognitive deficits in APP/PS1 mice. Current Medical Science. 2020;40:18–27. doi: 10.1007/s11596-020-2142-z. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E, Del Maschio A. Platelet-neutrophil interactions. Possible relevance in the pathogenesis of thrombosis and inflammation. Haematologica. 1991;76:491–499. [PubMed] [Google Scholar]
- Bedford PA, Todorovic V, Westcott ED, Windsor AC, English NR, Al-Hassi HO, Raju KS, Mills S, Knight SC. Adipose tissue of human omentum is a major source of dendritic cells, which lose MHC Class II and stimulatory function in Crohn's disease. Journal of Leukocyte Biology. 2006;80:546–554. doi: 10.1189/jlb.0905501. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Wallace TL. A review of the cholinergic system and therapeutic approaches to treat brain disorders. Behavioral Pharmacology of the Cholinergic System. 2020;45:1–28. doi: 10.1007/7854_2020_141. [DOI] [PubMed] [Google Scholar]
- Botten D, Fugallo G, Fraternali F, Molteni C. Structural properties of green tea catechins. The Journal of Physical Chemistry B. 2015;119:12860–12867. doi: 10.1021/acs.jpcb.5b08737. [DOI] [PubMed] [Google Scholar]
- Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. Journal of Crohn's and Colitis. 2012;6:226–235. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- Cheng X, Shen Y, Li R. Targeting TNF: A therapeutic strategy for Alzheimer's disease. Drug Discovery Today. 2014;19:1822–1827. doi: 10.1016/j.drudis.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Chiou YS, Ma NJL, Sang S, Ho CT, Wang YJ, Pan MH. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. Journal of Agricultural and Food Chemistry. 2012;60:3441–3451. doi: 10.1021/jf300441p. [DOI] [PubMed] [Google Scholar]
- Church RJ, Gatti DM, Urban TJ, Long N, Yang X, Shi Q, Eaddy S, Mosedale M, Ballard S, Churchill GA, Navarro V, Watkins PB, Threadgill DW, Harrill AH. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food and Chemical Toxicology. 2015;76:19–26. doi: 10.1016/j.fct.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P, Ronconi G, Caraffa AL, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. The Journal of Nutrition. 2004;134:3431–3440. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World Journal of Gastroenterology. 2014;20:2515. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P, Olmstead BD, Sasaki GY, Vodovotz Y, Yu Z, Bruno RS. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. Journal of Nutritional Biochemistry. 2020;84:108455. doi: 10.1016/j.jnutbio.2020.108455. [DOI] [PubMed] [Google Scholar]
- Dey P, Sasaki GY, Wei P, Li J, Zhu Wang L, J, Zhu J, McTigue D, Yu Z, Bruno RS, Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. Journal of Nutritional Biochemistry. 2019;67:78–89. doi: 10.1016/j.jnutbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Doğanyiğit Z, Okan A, Kaymak E, Pandır D, Silici S. Investigation of protective effects of apilarnil against lipopolysaccharide induced liver injury in rats via TLR 4/HMGB-1/NF-κB pathway. Biomedicine & Pharmacotherapy. 2020;125:109967. doi: 10.1016/j.biopha.2020.109967. [DOI] [PubMed] [Google Scholar]
- Elgawish RAR, Rahman HGA, Abdelrazek HM. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicology Reports. 2015;2:1149–1156. doi: 10.1016/j.toxrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan FY, Sang LX, Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22:484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester SC, Gu Y, Lambert JD. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Molecular Nutrition & Food Research. 2012;56:1647–1654. doi: 10.1002/mnfr.201200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. Journal of Clinical Investigation. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari PV, Balaraman M. Catechins: Sources, extraction and encapsulation: A review. Food and Bioproducts Processing. 2015;93:122–138. doi: 10.1016/j.fbp.2013.12.004. [DOI] [Google Scholar]
- Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radical Biology and Medicine. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chemistry. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhao Y, Nan Y, Wang X, Chen Y, Wang S. (−)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport. 2017;28:590–597. doi: 10.1097/WNR.0000000000000803. [DOI] [PubMed] [Google Scholar]
- Halaris A. Inflammation, heart disease, and depression. Current Psychiatry Reports. 2013;15:400. doi: 10.1007/s11920-013-0400-5. [DOI] [PubMed] [Google Scholar]
- Huang F, Pan B, Wu J, Chen E, Chen L. Relationship between exposure to PM2.5 and lung cancer incidence and mortality: A meta-analysis. Oncotarget. 2017;8:43322. doi: 10.18632/oncotarget.17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochemistry Journal. 2010;4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al AbdulMohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PloS ONE. 2012;7:39945. doi: 10.1371/journal.pone.0039945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopini P, Baldi M, Storchi P, Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. Journal of Food Composition and Analysis. 2008;21:589–598. doi: 10.1016/j.jfca.2008.03.011. [DOI] [Google Scholar]
- Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends in Immunology. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food and Chemical Toxicology. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ding S, Li F, Zhang C, Sun-Waterhouse D, Chen Y, Li D. Effects of (+)-catechin on the differentiation and lipid metabolism of 3T3-L1 adipocytes. Journal of Functional Foods. 2019;62:103558. doi: 10.1016/j.jff.2019.103558. [DOI] [Google Scholar]
- Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. European Respiratory Journal. 2014;43:1124–1131. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaihatsu K, Kawakami C, Kato N. Potential anti-influenza virus agents based on coffee ingredients and natural flavonols. Natural Products Chemistry & Research. 2014;2:2. doi: 10.4172/2329-6836.1000129. [DOI] [Google Scholar]
- Kajiya K, Hojo H, Suzuki M, Nanjo F, Kumazawa S, Nakayama T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. Journal of Agricultural and Food Chemistry. 2004;52:1514–1519. doi: 10.1021/jf0350111. [DOI] [PubMed] [Google Scholar]
- Kaur J. A comprehensive review on metabolic syndrome. Cardiology Research and Practice. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunological Reviews. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Keservani RK, Kesharwani RK, Vyas N, Jain S, Raghuvanshi R, Sharma AK. Nutraceutical and functional food as future food: A review. Der Pharmacia Lettre. 2010;2:106–116. [Google Scholar]
- Kim MJ, Hwang ES, Kim KJ, Maeng S, Heo HJ, Park JH, Kim DO. Anti-amnesic effects of epigallocatechin gallate on scopolamine-induced learning and memory dysfunction in Sprague-Dawley rats. Antioxidants. 2022;11:1. doi: 10.3390/antiox11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Kang JY, Park SK, Han HJ, Lee KY, Kim AN, Kim JC, Choi SG, Heo HJ. Effect of storage temperature on the antioxidant activity and catechins stability of Matcha (Camellia sinensis) Food Science and Biotechnology. 2020;29:1261–1271. doi: 10.1007/s10068-020-00772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Lee U, Kang JY, Park SK, Kim JC, Heo HJ. Matcha improves metabolic imbalance-induced cognitive dysfunction. Oxidative Medicine and Cellular Longevity. 2020;2020:8882763. doi: 10.1155/2020/8882763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Kang JY, Park SK, Moon JH, Kim MJ, Lee HL, Jeong HR, Kim JC, Heo HJ. Powdered Green Tea (Matcha) Attenuates the cognitive dysfunction via the regulation of systemic inflammation in chronic PM2.5-exposed BALB/c mice. Antioxidants. 2021;10:1932. doi: 10.3390/antiox10121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Park SK, Kang JY, Park SB, Yoo SK, Han HJ, Kim CW, Lee U, Kim SH, Heo HJ. Ethyl acetate fraction from persimmon (Diospyros kaki) ameliorates cerebral neuronal loss and cognitive deficit via the JNK/Akt pathway in TMT-induced mice. International Journal of Molecular Sciences. 2018;19:1499. doi: 10.3390/ijms19051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GL. The role of inflammatory cytokines in diabetes and its complications. Journal of Periodontology. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochemistry and Cell Biology. 2018;150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. Journal of Leukocyte Biology. 2010;88:1157–1162. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Asagiri K, Asakawa T, Tanikawa K, Kage M, Yagi M. The antioxidant effect of green tea catechin ameliorates experimental liver injury. Phytomedicine. 2010;17:197–202. doi: 10.1016/j.phymed.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Honn KV. Inflammation and disease progression. Cancer and Metastasis Reviews. 2006;25:481–491. doi: 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Pyo HK, Feinstein B, Pasinetti GM. Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochimica Et Biophysica Acta. 2003;1639:159–168. doi: 10.1016/j.bbadis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar P, Bhandari U, Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. BioMed Research International. 2014;2014:606021. doi: 10.1155/2014/606021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh A. A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacological Reports. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Kása P, Rakonczay Z, Gulya K. The cholinergic system in Alzheimer's disease. Progress in Neurobiology. 1997;52:511–535. doi: 10.1016/S0301-0082(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food and Chemical Toxicology. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intestinal Research. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee YB, Seo WD, Kang ST, Lim JW, Cho KM. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok) Preventive Nutrition and Food Science. 2012;17:141–151. doi: 10.3746/pnf.2012.17.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Lee WS, Shin JS, Jang DS, Lee KT. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. International Immunopharmacology. 2017;49:21–29. doi: 10.1016/j.intimp.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiology and Prevention Biomarkers. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Lee JC, Tseng CK, Wu SF, Chang FR, Chiu CC, Wu YC. San-Huang-Xie-Xin-Tang extract suppresses hepatitis C virus replication and virus-induced cyclooxygenase-2 expression. Journal of Viral Hepatitis. 2011;18:315–324. doi: 10.1111/j.1365-2893.2010.01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon F, Smythies LE, Smith PD, Kelsall BL. Immune mechanisms in inflammatory bowel disease. New York: Springer; 2006. Involvement of dendritic cells in the pathogenesis of inflammatory bowel disease; pp. 117–132. [DOI] [PubMed] [Google Scholar]
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: A systematic review. Lancet Infectious Diseases. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proceedings of the National Academy of Sciences. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xu B, Chen Z, Zhou C, Liao L, Qin Y, Yang C, Zhang X, Hu Z, Sun L, Zhu D, Xie P. PI3K/AKT/JNK/p38 signalling pathway-mediated neural apoptosis in the prefrontal cortex of mice is involved in the antidepressant-like effect of pioglitazone. Clinical and Experimental Pharmacology and Physiology. 2018;45:525–535. doi: 10.1111/1440-1681.12918. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bruins ME, Ni L, Vincken JP. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. Journal of Agricultural and Food Chemistry. 2018;66:8469–8477. doi: 10.1021/acs.jafc.8b02233. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu JF, Wen XY, Kan J, Jin CH. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. International Journal of Biological Macromolecules. 2015;72:1479–1484. doi: 10.1016/j.ijbiomac.2014.09.066. [DOI] [PubMed] [Google Scholar]
- Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: A review of the evidence from studies conducted in humans. Current Pharmaceutical Design. 2009;15:1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Current Diabetes Reports. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- Martini F, Rosa SG, Klann IP, Fulco BCW, Carvalho FB, Rahmeier FL, Fernandes MC, Nogueira CW. A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer's disease. Journal of Psychiatric Research. 2019;109:107–117. doi: 10.1016/j.jpsychires.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: An update. Archives of Toxicology. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- Mishra CB, Pandey P, Sharma RD, Malik MZ, Mongre RK, Lynn AM, Prasad R, Jeon R, Prakash A. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: an integrated computational approach. Briefings in Bioinformatics. 2021;22:1346–1360. doi: 10.1093/bib/bbaa378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M. Tea catechins prevent the development of atherosclerosis in apoprotein E–deficient mice. Journal of Nutrition. 2001;131:27–32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. International Journal of Molecular Sciences. 2020;21:1744. doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Current Opinion in Lipidology. 2010;21:76–83. doi: 10.1097/MOL.0b013e3283347ebb. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, PubChem. https://pubchem.ncbi.nlm.nih.gov. Accessed Jan 20, 2022.
- Onishi S, Mori T, Kanbara H, Habe T, Ota N, Kurebayashi Y, Suzuki T. Green tea catechins adsorbed on the murine pharyngeal mucosa reduce influenza A virus infection. Journal of Functional Foods. 2020;68:103894. doi: 10.1016/j.jff.2020.103894. [DOI] [Google Scholar]
- Park E, Chun HS. Green tea polyphenol Epigallocatechine gallate (EGCG) prevented LPS-induced BV-2 micoglial cell activation. Journal of Life Science. 2016;26:640–645. doi: 10.5352/JLS.2016.26.6.640. [DOI] [Google Scholar]
- Park YM, Lim JH, Lee JE, Seo EW. Protective effect of Semisulcospira libertina extract on induced hepatitis in rats. Journal of Life Science. 2015;25:539–547. doi: 10.5352/JLS.2015.25.5.539. [DOI] [Google Scholar]
- Patel H, Zaghloul N, Lin KI, Liu SF, Miller EJ, Ahmed M. Hypoxia-induced activation of specific members of the NF-kB family and its relevance to pulmonary vascular remodeling. International Journal of Biochemistry & Cell Biology. 2017;92:141–147. doi: 10.1016/j.biocel.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Petry FDS, Coelho BP, Gaelzer MM, Kreutz F, Guma FTCR, Salbego CG, Trindade VMT. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytotherapy Research. 2020;34:796–807. doi: 10.1002/ptr.6560. [DOI] [PubMed] [Google Scholar]
- Piao W, Ru LW, Piepenbrink KH, Sundberg EJ, Vogel SN, Toshchakov VY. Recruitment of TLR adapter TRIF to TLR4 signaling complex is mediated by the second helical region of TRIF TIR domain. Proceedings of the National Academy of Sciences USA. 2013;110:19036–19041. doi: 10.1073/pnas.1313575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, Bruno CM. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respiratory Research. 2016;17:1–10. doi: 10.1186/s12931-016-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XF. Impaired inactivation of digestive proteases by deconjugated bilirubin: the possible mechanism for inflammatory bowel disease. Medical Hypotheses. 2002;59:159–163. doi: 10.1016/S0306-9877(02)00243-8. [DOI] [PubMed] [Google Scholar]
- Racanelli AC, Kikkers SA, Choi AM, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14:221–232. doi: 10.1080/15548627.2017.1389823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. Journal of Nutritional Biochemistry. 2003;14:326–332. doi: 10.1016/S0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Raetz CR. Biochemistry of endotoxins. Annual Review of Biochemistry. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rafa H, Amri M, Saoula H, Belkhelfa M, Medjeber O, Boutaleb A, Aftis S, Nakmouche M, Touil-Boukoffa C. Involvement of interferon-γ in bowel disease pathogenesis by nitric oxide pathway: a study in Algerian patients. Journal of Interferon & Cytokine Research. 2010;30:691–697. doi: 10.1089/jir.2010.0012. [DOI] [PubMed] [Google Scholar]
- Ramnanan CJ, Edgerton DS, Rivera N, Irimia-Dominguez J, Farmer B, Lautz M, Donahue EP, Meyer CM, Roach PJ, Neal DW, Cherrington AD. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes. 2010;59:1302–1311. doi: 10.2337/db09-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relja B, Töttel E, Breig L, Henrich D, Schneider H, Marzi I, Lehnert M. Effects of green tea catechins on the pro-inflammatory response after haemorrhage/resuscitation in rats. British Journal of Nutrition. 2011;105:1791–1797. doi: 10.1017/S000711451000560X. [DOI] [PubMed] [Google Scholar]
- Sano T, Nagayasu S, Suzuki S, Iwashita M, Yamashita A, Shinjo T, Kushiyama A, Kanematsu T, Nishimura F. Epicatechin downregulates adipose tissue CCL19 expression and thereby ameliorates diet-induced obesity and insulin resistance. Nutrition, Metabolism and Cardiovascular Diseases. 2017;27:249–259. doi: 10.1016/j.numecd.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Santangelo R, Silvestrini A, Mancuso C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food and Chemical Toxicology. 2019;123:42–49. doi: 10.1016/j.fct.2018.10.042. [DOI] [PubMed] [Google Scholar]
- Santos LFS, Stolfo A, Calloni C, Salvador M. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. Journal of Arrhythmia. 2017;33:220–225. doi: 10.1016/j.joa.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. Journal of the Science of Food and Agriculture. 2000;80:1094–1117. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1. [DOI] [Google Scholar]
- Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S. Regulation of NF-κB activation by MAP kinase cascades. Immunobiology. 1997;198:35–49. doi: 10.1016/S0171-2985(97)80025-3. [DOI] [PubMed] [Google Scholar]
- Schwager S, Detmar M. Inflammation and lymphatic function. Frontiers in Immunology. 2019;10:308. doi: 10.3389/fimmu.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa H, Ikuta K, Mizuta K, Takechi S, Suzutani T. Relationship between polyphenol content and anti-influenza viral effects of berries. Journal of the Science of Food and Agriculture. 2013;93:2239–2241. doi: 10.1002/jsfa.6031. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Seo KH, Yokoyama W, Kim H. Comparison of polyphenol-rich wine grape seed flour-regulated fecal and blood microRNAs in high-fat, high-fructose diet-induced obese mice. Journal of Functional Foods. 2020;73:104147. doi: 10.1016/j.jff.2020.104147. [DOI] [Google Scholar]
- Shabbir U, Rubab M, Daliri EBM, Chelliah R, Javed A, Oh DH. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients. 2021;13:206. doi: 10.3390/nu13010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid A, Ali R, Ali N, Hasan SK, Bernwal P, Afzal SM, Vafa A, Sultana S. Modulatory effects of catechin hydrate against genotoxicity, oxidative stress, inflammation and apoptosis induced by benzo(a)pyrene in mice. Food and Chemical Toxicology. 2016;92:64–74. doi: 10.1016/j.fct.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Shenhar-Tsarfaty S, Berliner S, Bornstein NM, Soreq H. Cholinesterases as biomarkers for parasympathetic dysfunction and inflammation-related disease. Journal of Molecular Neuroscience. 2014;53:298–305. doi: 10.1007/s12031-013-0176-4. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature Reviews Neurology. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Huang Y, Zhu X, Liu C, Hu Y, Wang H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer's disease. Ecotoxicology and Environmental Safety. 2019;174:344–352. doi: 10.1016/j.ecoenv.2019.02.086. [DOI] [PubMed] [Google Scholar]
- Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Molecular Neurobiology. 2019;56:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmach A, Mullen W, Steiling H, Williamson G, Lean ME, Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Molecular Nutrition & Food Research. 2010;54:323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, Inoue N, Ogata H, Iwao Y, Nomoto K, Tanaka R, Hibi T. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. International Journal of Medical Microbiology. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Taylor PW, Hamilton-Miller JM, Stapleton PD. Antimicrobial properties of green tea catechins. Food Science and Technology Bulletin. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian DS, Xie MJ, Yu ZY, Zhang Q, Wang YH, Chen B, Chen C, Wang W. Cell cycle inhibition attenuates microglia induced inflammatory response and alleviates neuronal cell death after spinal cord injury in rats. Brain Research. 2007;1135:177–185. doi: 10.1016/j.brainres.2006.11.085. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Gildengers AG, Hsu JL, Chung KH, Chen PH, Huang YJ. Inflammation associated with volume reduction in the gray matter and hippocampus of older patients with bipolar disorder. Journal of Affective Disorders. 2019;244:60–66. doi: 10.1016/j.jad.2018.10.093. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H. Stereospecificity in membrane effects of catechins. Chemico-Biological Interactions. 2001;134:41–54. doi: 10.1016/S0009-2797(00)00308-2. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-β1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Current Pharmaceutical Design. 2007;13:1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- Unno K, Pervin M, Nakagawa A, Iguchi K, Hara A, Takagaki A, Nanjo F, Minami A, Nakamura Y. Blood–brain barrier permeability of green tea catechin metabolites and their neuritogenic activity in human neuroblastoma SH-SY5Y cells. Molecular Nutrition & Food Research. 2017;61:1700294. doi: 10.1002/mnfr.201700294. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Li Z, Hua Q, Wang L, Song X, Zou B, Ding M, Zhao J, Tang C. Joint toxicity of a multi-heavy metal mixture and chemoprevention in Sprague Dawley rats. International Journal of Environmental Research and Public Health. 2020;17:1451. doi: 10.3390/ijerph17041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang G, Yuan L, Yang Y, Zhao H, Ho CT, Li S. Green tea catechins effectively altered hepatic fibrogenesis in rats by inhibiting ERK and Smad1/2 phosphorylation. Journal of Agricultural and Food Chemistry. 2018;67:5437–5445. doi: 10.1021/acs.jafc.8b05179. [DOI] [PubMed] [Google Scholar]
- Wong J, Magun BE, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. International Journal of Chronic Obstructive Pulmonary Disease. 2016;11:1391–1401. doi: 10.2147/COPD.S106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Liu Z, Wang Y, Zhang Q, Li J, Zhong P, Xie Z, Ji A, Li Y (2021) Epigallocatechin-3-gallate alleviates high-fat diet-induced nonalcoholic fatty liver disease via inhibition of apoptosis and promotion of autophagy through the ROS/MAPK signaling pathway. Oxidative Med Cell Longevity. 2021:5599997 [DOI] [PMC free article] [PubMed]
- Xu M, Ge C, Qin Y, Gu T, Lv J, Wang S, Ma Y, Lou D, Li Q, Hu L, Wang M, Huang P, Tan J. Activated TNF-α/RIPK3 signaling is involved in prolonged high fat diet-stimulated hepatic inflammation and lipid accumulation: inhibition by dietary fisetin intervention. Food & Function. 2019;10:1302–1316. doi: 10.1039/C8FO01615A. [DOI] [PubMed] [Google Scholar]
- Xue B, Xie J, Huang J, Chen L, Gao L, Ou S, Wang Y, Peng X. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal bacteroidetes and firmicutes in vitro. Food & Function. 2016;7:1501–1507. doi: 10.1039/C5FO01438G. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen G, Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. Journal of Traditional and Complementary Medicine. 2014;4:17–23. doi: 10.4103/2225-4110.124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HT, Yang YC, Chang CH, Yang HT, Yin MC. Protective effects of (-)-epigallocatechin-3-gallate against acetaminophen-induced liver injury in rats. Biomedicine. 2015;5:1–6. doi: 10.7603/s40681-015-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HL, Huang CC, Chen CJ, Chang CC, Liao PL, Huang ST. Anti-pandemic influenza A (H1N1) virus potential of catechin and gallic acid. Journal of the Chinese Medical Association. 2018;81:458–468. doi: 10.1016/j.jcma.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand H, Morshedzadeh N, Naghashian F. Signaling pathways linking inflammation to insulin resistance. Diabetes & Metabolic Syndrome. 2017;11:307–309. doi: 10.1016/j.dsx.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Darland D, He Y, Yang L, Dong X, Chang Y. Reduction of PM2.5 toxicity on human alveolar epithelial cells A549 by tea polyphenols. Journal of Food Biochemistry. 2018;42:12496. doi: 10.1111/jfbc.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng A, Zhang Z, Yu Z, Liu Y, Peng S, Wu L, Qin H, Wang W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacological Reports. 2016;68:514–520. doi: 10.1016/j.pharep.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang Z, Sheng C, Peng W, Hui S, Gong W, Chen S. Icariin prevents amyloid beta-induced apoptosis via the PI3K/Akt pathway in PC-12 cells. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1. doi: 10.1155/2015/235265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Froh M, Lehnert M, Schoonhoven R, Yang L, Lind H, Lemasters JJ, Thurman RG. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;285:1004–1013. doi: 10.1152/ajpgi.00008.2003. [DOI] [PubMed] [Google Scholar]
- Zhu YF, Chen JJ, Ji XM, Hu X, Ling TJ, Zhang ZZ, Bao GH, Wan XC. Changes of major tea polyphenols and production of four new B-ring fission metabolites of catechins from post-fermented Jing-Wei Fu brick tea. Food Chemistry. 2015;170:110–117. doi: 10.1016/j.foodchem.2014.08.075. [DOI] [PubMed] [Google Scholar]