Abstract
Chemoprevention strategies reduce malaria disease and death, but the efficacy of anti-malarial drugs used for chemoprevention is perennially threatened by drug resistance. This review examines the current impact of chemoprevention on the emergence and spread of drug resistant malaria, and the impact of drug resistance on the efficacy of each of the chemoprevention strategies currently recommended by the World Health Organization, namely, intermittent preventive treatment in pregnancy (IPTp); intermittent preventive treatment in infants (IPTi); seasonal malaria chemoprevention (SMC); and mass drug administration (MDA) for the reduction of disease burden in emergency situations. While the use of drugs to prevent malaria often results in increased prevalence of genetic mutations associated with resistance, malaria chemoprevention interventions do not inevitably lead to meaningful increases in resistance, and even high rates of resistance do not necessarily impair chemoprevention efficacy. At the same time, it can reasonably be anticipated that, over time, as drugs are widely used, resistance will generally increase and efficacy will eventually be lost. Decisions about whether, where and when chemoprevention strategies should be deployed or changed will continue to need to be made on the basis of imperfect evidence, but practical considerations such as prevalence patterns of resistance markers can help guide policy recommendations.
Background
After decades of dramatic reductions in malaria cases and deaths worldwide, progress toward malaria control and elimination had plateaued before the COVID-19 pandemic [1], and malaria cases and deaths rose in 2020 [2]. Further erosion of the recent gains in malaria control will lead to resurgences, at great cost to the health, lives, and economies of the world’s poorest countries [3]. Chemoprevention strategies, i.e., the use of anti-malarial medicines for prophylaxis and for preventive treatment, can be effective tools for malaria control and elimination, but the risks of resistance to anti-malarial drugs used for prevention and treatment must be mitigated and managed for momentum to be regained and sustained.
The chemoprevention strategies currently recommended by the World Health Organization (WHO) include intermittent preventive treatment in pregnancy (IPTp), intermittent preventive treatment in infants (IPTi), seasonal malaria chemoprevention (SMC), and mass drug administration (MDA) for the reduction of disease burden in emergency situations (Table 1). From the time that these chemoprevention strategies were first conceived, concerns have been raised both about their potential impact on the development and spread of drug resistance that might compromise the treatment efficacy of the drug classes used, and about the impact of drug resistance on the efficacy of different chemoprevention strategies.
Table 1.
Definitions of Malaria Chemoprevention Strategies*
| Intermittent preventive treatment in pregnancy (IPTp) | A full therapeutic course of anti-malarial medicine given to pregnant women at routine prenatal visits, regardless of whether the woman is infected with malaria |
| Intermittent preventive treatment in infants (IPTi) | A full therapeutic course of sulfadoxine-pyrimethamine delivered to infants in co-administration with DTP2/Penta2, DTP3/Penta3 and measles immunization, regardless of whether the infant is infected with malaria |
|
Seasonal malaria chemoprevention (SMC) |
Intermittent administration of full treatment courses of an anti-malarial medicine during the malaria season to prevent malarial illness. The objective is to maintain therapeutic concentrations of an anti-malarial drug in the blood throughout the period of greatest risk for malaria |
| Note: This intervention is recommended only for areas with highly seasonal malaria, where transmission occurs during a few months of the year | |
|
Mass drug administration (MDA) |
Administration of anti-malarial treatment to all age groups of a defined population or every person living in a defined geographical area (except those for whom the medicine is contraindicated) at approximately the same time and often at repeated intervals |
*Definitions from WHO Malaria Terminology, last updated 2019[4]
Measuring and monitoring resistance and efficacy
Clinical trials remain the gold standard for measuring and monitoring efficacy
As they have been developed and implemented, each chemoprevention strategy has been evaluated in complex prospective, controlled trials that typically randomly assign either individuals or clusters (e.g., villages or districts) to receive either the drug prevention regimen being tested, or an alternative regimen, or no preventive regimen. Because their primary outcome measures are affected by a number of host and parasite factors in addition to parasite resistance, efficacy trials do not directly measure drug resistance per se, but these prospective studies remain the gold standard for measuring the chemoprevention efficacy.
The efficacy of drugs used to treat malaria is monitored worldwide in single-arm clinical trials known as WHO Therapeutic Efficacy Studies (TES), which follow standardized protocols to provide direct evidence of drug efficacy to guide policy decisions [5]. Unlike these TES for routine monitoring of treatment efficacy, simplified protocols for routine monitoring of chemoprevention efficacy are not yet in use, although the WHO is presently developing such streamlined protocols.
Because anti-malarial drug resistance is considered paramount among the many host, parasite, pharmacological and other factors that affect the efficacy of anti-malarial drugs, methods for detecting the presence of resistant parasites have long been employed as a surrogate for efficacy trials.
In vitro susceptibility testing is of limited use for monitoring chemoprevention efficacy
In vitro assays for measuring drug resistance provide a direct measure of parasite response to drugs [6], but have proven to be even more limited in scope and suitability for surveillance than clinical trials. In vitro testing is particularly unreliable for antifolate drugs, especially the sulfas, because the tests are exquisitely sensitive to host folate blood levels, which are affected by diet and vary widely among different individuals [7]. For all these reasons, in vitro tests have not played a significant role in assessing resistance in relation to the currently recommended chemoprevention strategies. Nevertheless, in vitro methods are indispensable for confirming and characterizing newly emerging forms of resistance and for establishing and confirming the molecular mechanisms of resistance [8, 9].
Molecular resistance markers can be useful surrogates for efficacy
Elucidation of the molecular basis of in vitro Plasmodium falciparum resistance to the antifolates made it possible to define the determinants of in vivo resistance to these drugs, and to develop simple assays for molecular markers of antifolate resistance that can potentially serve as surrogate indicators of drug efficacy. Pyrimethamine and other antifolates such as proguanil (via its metabolite cycloguanil) and trimethoprim target P. falciparum dihydrofolate reductase (DHFR), while sulfadoxine and other sulfas target dihydropteroate synthase (DHPS). Resistance to DHFR inhibitors and sulfa drugs in vitro is conferred by single nucleotide polymorphisms (SNPs) in P. falciparum DHFR and DHPS, respectively [10–15]. Mutations in both genes tend to occur in a progressive, step-wise fashion, with higher levels of in vitro resistance occurring in the presence of multiple mutations.
Potential molecular markers have been identified for P. falciparum resistance to many but not all anti-malarial drugs [16, 17]), including currently used chemoprevention agents such as amodiaquine (SNPs in pfcrt and pfmdr1), lumefantrine, mefloquine (copy number variation in pfmdr1), piperaquine (copy number variation in plasmepsin2 and SNPs in pfcrt), and the artemisinins (SNPs in kelch13). Several of these putative markers are less well validated than those for SP and chloroquine resistance, and in some cases (e.g., lumefantrine), “resistance” markers are associated only with modest differences in susceptibility in vitro but not with clinical measures of resistance or treatment failure.
These resistance markers generally correlate very well with in vitro measures of resistance, but relationships between resistance mutations and chemoprevention outcomes are less straightforward, primarily because intrinsic drug resistance is only one of many factors that affect these outcomes, along with drug quality, intake, absorption, metabolism and clearance; nutritional and other health status indicators; and, especially, naturally acquired immunity to malaria, which can aid in clearing parasites, including drug-resistant parasites [18–22]. Because all these factors vary widely across individuals and populations, validating molecular markers as useful tools for measuring and monitoring drug treatment efficacy and chemoprevention outcomes has been challenging, and no marker or set of markers (haplotype) can reliably predict the outcome of a given drug regimen in an individual person.
Nevertheless, many clinical trials and epidemiological studies have demonstrated strong and consistent associations between the presence of specific mutations and outcomes of interest for both treatment and chemoprevention regimens, particularly those that rely on the antifolate drug sulfadoxine-pyrimethamine (SP). In most settings the presence of dhps K540E, which is highly prevalent in East African but scarce in West Africa, reliably signals the presence of four other key mutations, making it possible to use this single marker as a surrogate for the dhps “quintuple mutant” that is strongly associated with SP treatment failure [23], a strategy that has been recommended by the WHO for monitoring the efficacy of IPTi with SP [24].
Correlating parasite genetic markers with clinical outcomes can be even more challenging for chemoprevention than it is for treatment efficacy. This is because the relationships between parasite genotypes and efficacy outcomes are comparatively more straightforward in the case of drug treatment of clinical malaria. Drugs are administered and parasites are either cleared or not over the ensuing days. While factors other than resistance affect outcomes for both drug treatment and chemoprevention strategies, in the latter instance, the outcome is less immediate (e.g., birth outcomes following two or more doses of SP administered weeks apart), and the other factors influencing outcome are likely to play a more prominent role in chemoprevention outcomes.
In summary, drug resistance is but one of many factors that determine the efficacy of IPTp, IPTi, SMC and MDA. Clinical trials that measure health outcomes are the gold standard for measuring the efficacy of these chemoprevention strategies. Clinical trials of treatment efficacy cannot be used as a surrogate for chemoprevention efficacy. For antifolates and some other drugs, molecular markers accurately indicate the presence of drug resistant parasites, and are a useful but imperfect means of predicting the efficacy of chemoprevention strategies.
Impact of chemoprevention on resistance
Chemoprevention selects for drug resistant parasites
More than 60 years ago David Clyde showed that the prevalence of antifolate-resistant parasites increased rapidly and dramatically in Tanzanian villages whose residents received weekly pyrimethamine for malaria prophylaxis [25]. Parasitological evidence of resistance was also detected in nearby villages whose residents did not receive chemoprevention, with the highest rates of resistance found in villages closest to those whose residents received pyrimethamine. The molecular basis for this rapid emergence and spread of resistant parasites was demonstrated 45 years later when this field experiment was repeated in a village in Mali, where resistance-conferring mutations in P. falciparum dhfr rapidly and dramatically increased in prevalence in the village within just a few weeks of starting all consenting villagers on weekly pyrimethamine [26]. Please note that in this review, “prevalence” of a given mutation or haplotype is defined as the proportion of infected individuals in whom that marker or haplotype is detected, irrespective of whether other alleles or haplotypes (e.g., wild-type) are also present in the infection.
Many subsequent studies have confirmed that community use of SP, whether for treatment or chemoprevention, is often followed by increases in community prevalence of resistance mutations in both dhfr and dhps. In many of these studies, only very general temporal or ecological trends are reported that are consistent with, but not proof that, various chemoprevention strategies directly select for forms of resistance that affect clinical outcomes. For example, one report described trends of increasing prevalence of molecular markers for antifolate resistant P. falciparum in Kenya over a 20-year period when SP was in use, initially for treatment, and subsequently for IPTp [27]. While one novel dhps mutation, S436H, more than doubled in prevalence between 2010 and 2017/2018, most of “the usual suspects” of dhfr and dhps mutations that have been associated with reduced efficacy of SP treatment and chemoprevention were already at near-fixation in 2000, and their prevalence rose only marginally: dhfr N51I was prevalent at 90% in 2005, 99% in 2010, and 100% in 2017/2018, and dhps A437G was prevalent at 98% in 2000 and 2010 and 100% in 2017/2018. Even if the use of SP for treatment and IPTp was chiefly responsible for these increases, such small changes in prevalence would have minimal impact on SP efficacy. Another molecular survey pooled data from nearly 40,000 samples collected in 38 African countries between 1998 and 2018 [28], and found generally higher prevalences of dhps A581G in East compared to West Africa, with extensive heterogeneity including within countries.
While Clyde’s Tanzania study [25] and subsequent observations of the rapid selection of resistance mutations suggest that new forms of resistance can easily emerge locally under drug pressure, genomic epidemiology surveys have found that the most highly resistant forms of resistance to nearly all anti-malarial drugs do not tend to arise de novo wherever drugs are used; rather, parasites with levels of resistance sufficient to cause treatment failure have arisen just a few times, usually in Asia, before spreading to Africa [29–32] (reviewed in [22]). This means that before highly resistant parasites have arrived in an area, even heavy drug selection pressure may not lead to loss of efficacy, as may be the case for antifolate resistance in much of West Africa. However, once highly resistant parasites have been imported and are present even at low prevalence, they can increase in response to drug pressure, as appears to be the case with antifolate resistance in much of East Africa. As more genomic epidemiology studies are undertaken, they are uncovering exceptions to the general rule that clinically relevant forms of resistance tend to emerge in Asia and spread to Africa. It is also possible for new, highly-resistant variants to arise locally on existing genetic backgrounds, as has been reported for dhps A581G in East Africa [33].
One important consideration in evaluating the impact of chemoprevention strategies on resistance is that, if the strategy is effective at reducing malaria infections, the number of resistant infections in a population or setting may decrease even while the proportion of infections that are resistant increases as a result of selection pressure exerted by the chemoprevention drugs. Thus when a chemoprevention strategy is highly effective, such as MDA using an ACT, selection favouring resistant parasites may have minimal public health impact if there are so few post-MDA infections that the resistant parasites in a given individual are rarely if ever transmitted. This is why MDA has tended to work best when it is implemented in parallel with rigorous vector control strategies [34].
In contrast, chemoprevention strategies that are less effective at reducing infections, such as IPTp-SP, may be more likely to result in increasing not only the proportion but the number of resistant infections, since they exert their effect less by preventing infections than by reducing parasite densities. Thus, the impact of chemoprevention on resistance depends both on the probability that resistant parasites emerge in an individual infection, and the probability that such resistant infections occur and are successfully transmitted. Mathematical models that incorporate these factors can be helpful in assessing the impact of specific chemoprevention strategies on drug resistance and efficacy [35].
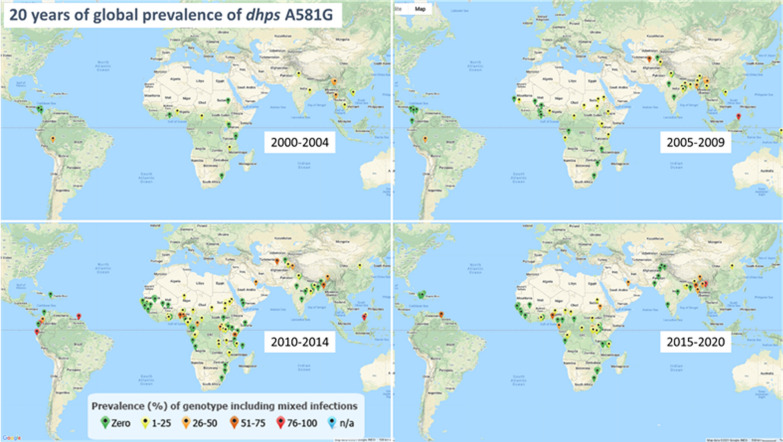
Published data on the prevalence of resistance markers including A581G have been compiled and made available online. For example, Fig. 1 shows global prevalence data for dhps A581G between 2000 and 2020. The geotemporal trends for this mutation are consistent with the generally observed pattern of clinically significant resistance mutations being found earlier and at higher prevalence in East as compared to West Africa [22]. The reasons for this pattern are unclear, but plausible potential explanations include: 1) earlier introduction of resistance as a result of more-frequent human migration between Asia, the most common site of origin of highly resistant parasites, and East Africa; 2) more-rapid spread of mutations as a result of higher and more perennial malaria transmission in East Africa; and/or 3) earlier introduction of next-line anti-malarial drugs (first SP, then ACT) in East Africa owing to the earlier emergence of chloroquine resistance there has resulted in earlier and more intense selection pressure favouring parasites resistant to the new drugs in East Africa before these drugs were widely introduced in West Africa.
Fig. 1.
Global map of the prevalence of sulfadoxine-pyrimethamine resistance marker dihydrofolate reductase A581G. Data are from published sources and available at http://wwwarn.org/dhfr-dhps-surveyor/#0 (accessed 12 April 2021)
These global and regional patterns of emergence and spread of drug resistance illustrate a key point about the impact of chemoprevention on resistance: while there are ample examples of malaria chemoprevention strategies being followed by increased drug resistance, it is clear that not every chemoprevention scheme in every setting and population leads to measurable increases in resistance that in turn lead to meaningful loss of drug efficacy in that setting and population. Moreover, it can be difficult to assess the impact of drug use on resistance, and vice-versa, in the many studies that report only prevalences of individual mutations, which by themselves are less reliable predictors of efficacy than full haplotypes. Other issues that commonly cloud interpretation of chemoprevention’s impact on resistance include neglecting to genotype mutations previously believed to be absent from an area, and failure to account for differences in exposure risk among comparator groups in non-randomized observational studies.
Impact of resistance on chemoprevention efficacy
Antifolate-resistant P. falciparum was already well established in Africa by the time IPTp, IPTi, and SMC were implemented there. Based on declining SP treatment efficacy in countries that were early adopters of SP following the rise of chloroquine resistance [23, 36], it was reasonable to expect SP-based chemoprevention strategies to follow a similar pattern. However, IPTp performed well even in settings where antifolate resistance led to SP treatment failure rates of 25% or higher in children [37] (discussed in more detail below).
About eight years after IPTp-SP was recommended by WHO in 1998, reports of increasing SP resistance led to renewed concern that, as one publication asserted, “In northern Tanzania, SP is a failed drug for treatment and its utility for prophylaxis is doubtful” (italics added) [38]. This assertion was based on the results of an open label single arm trial of SP efficacy for treating P. falciparum in symptomatic children and asymptomatic infants in Korogwe District, about 30 km north of Muheza, Tanzania. The trial had been stopped early owing to an early treatment failure rate of 39% and day 28 failure rate of 82% in the symptomatic children. The authors implicated dhps A581G as the culprit in these alarming failure rates, despite multivariate analyses showing that factors associated with treatment failure included young age, high parasite density, and presence of three dhfr mutations, but not the presence of dhps A581G, which was prevalent at 55%. Notably, the dhfr triple mutant had a prevalence of 96% in this study. The findings that this dhfr haplotype was at near-fixation in this setting and was nevertheless significantly associated with treatment failure, while dhps A581G was not, despite being present in roughly half of the infections, suggests that the lack of association of A581G with treatment failure was real, and not a result of low prevalence or insufficient study power.
This and other studies of SP efficacy for treating clinical malaria in Africa thus raised alarms about the potential impact of antifolate resistance on IPTp and other chemoprevention strategies, but their inconclusive results called for directly examining this question in chemoprevention efficacy trials.
Intermittent preventive treatment during pregnancy and resistance
Impact of IPTp on resistance
As IPTp-SP was being evaluated and implemented in the early 2000s, studies began to examine selection of resistant parasites by ITPp-SP. When dhfr and dhps mutations were compared in Malawian women from 2003–2006 before they started SP-IPTp and after delivery, the prevalence of the dhfr/dhps quintuple mutation increased significantly, from 81% before the intervention to 100% after delivery [39]. Around the same time, studies in other African countries compared marker prevalences in women receiving SP-IPTp and in those not receiving it. The prevalence of dhfr mutations was compared in pregnant Ghanaian women at early gestation who had not received IPTp, and in women at delivery, nearly all of whom had received at least one dose of ITPp-SP [40]. Prevalence of the dhfr triple mutant was similar between the two groups and did not increase with an increasing number of IPTp-SP doses. Thus, even though the overall prevalence of dhfr mutations in the study population doubled between 1998 and 2006 in parallel with the implementation of SP-IPTp, the authors suggested that SP-IPTp might not be responsible for this increase. Similarly, in a study of peripheral and placental samples obtained from pregnant women over a 13-year period in western Kenya, the prevalence of the dhfr/dhps quintuple mutant rose contemporaneously with the implementation of IPTp-SP [41]. However, presence of the quintuple mutant was not associated with IPTp-SP use in multivariate analyses, suggesting that other factors were chiefly responsible for its rising prevalence.
In Mozambique, the prevalence of the quintuple mutant was higher in placentas of women receiving IPTp-SP than those receiving a placebo [42]. This association was only significant in women who had received a dose of SP within the 2.5 months before delivery, reflecting the “selection window” [43, 44] during which blood concentrations of sulfadoxine and pyrimethamine remain sufficient to select resistant parasites. In an ITPp-SP study done in Burkina Faso in 2014–2015, dhfr and dhps triple mutants were more common at delivery than at first antenatal care visit, but the same mutations were even more common in the general population than in pregnant women at either encounter, and recent use of ITPp-SP was not associated with increased prevalence of mutations [45]. In this study, dhps K540E was very rare, and dhfr I164 and dhps A581G and A613S/T were not assessed. Another study in Burkina Faso reported a similar increase in lower-level dhfr mutations, but no increase in dhps mutations [46].
As dhps A581G began to rise in prevalence in Africa, more studies focused on this mutation, which typically occurred together with the other dhfr and dhps mutations comprising the quintuple mutant to form the so-called sextuple mutant. In a Tanzanian study discussed at length in the next section, dhps A581G prevalence was significantly higher in IPTp-SP recipients compared to pregnant women who had not received IPTp [47]. Surprisingly, a survey done ten years later found that A581G was rare or absent in all but one of seven sites in Tanzania [48].
The prevalence of resistance markers before and during IPTp with SP or dihydroartemisinin-piperaquine was compared in clinical efficacy trials conducted in two Ugandan districts, Tororo in 2014–2015, and Busia in 2016–2017 [49]. The dhfr/dhps quintuple mutant was already near fixation at both sites, while dhps A581G was absent in Tororo and prevalent at only 3% in Busia. Mutations associated with 4-aminoquinoline resistance, pfmdr N86Y and Y184F and pfcrt K76T, all appeared to be selected in the dihydroartemisinin-piperaquine arms of both trials. The dhfr/dhps quintuple mutations were all already prevalent at > 90% and did not increase significantly in the SP arms at either site. The prevalence of dhfr I164L remained less than 2% both before and during IPTp-SP in Tororo, but I164L rose in prevalence from 4% to 13.7% in Busia. This is consistent with selection by IPTp-SP at this site, but prevalence of this mutation also rose to 9% in women in the dihydroartemisinin-piperaquine arm who were unexposed to SP, so it is not possible to distinguish between selection by ITPp and community-wide trends in prevalence in Busia over the course of the study. The dhps A581G mutation remained absent in Tororo and did not increase in prevalence in either arm in Busia, decreasing from 3% at baseline to 0% in the dihydroartemisinin-piperaquine arm and to 1.9% in the SP arm. The authors speculated that the apparent lack of selection of A581G in Busia was due to its low baseline prevalence. This explanation is unconvincing, in that sharp increases in the prevalence of resistance, and resistance markers, is commonly seen under antifolate drug pressure for other antifolate mutations found at low baseline prevalence [25, 26]. Another study reported apparent selection favouring A581G in Uganda after a single dose of IPTp-SP, but this conclusion was based on very small numbers: A581G was found in two of 52 infected women at the first antenatal visit, compared with two of 12 at the second visit [50].
The dhps A581G and A613S/T mutations were reported to be selected by IPTp-SP in another study of antifolate resistance marker prevalence conducted in Ghana in 2015–2017. This cross-sectional study compared marker prevalence in pregnant women at their first antenatal visit and at delivery [51]. At delivery more than 70% of women had received at least two doses of IPTp-SP, so parasites were presumed to have been under selection pressure from SP. Unlike in the contemporaneous Ugandan study that found no increase in prevalence in dhps A581G, this West African study saw A581G increase from 9 to 16%. Statistical analyses were not presented, but this increase is not statistically significant (uncorrected X2 = 2.95, P = 0.09). While A613S/T had similar prevalence before and after delivery (15.2% to 17.5%, uncorrected X2 = 0.82, P = 0.37), the authors reported in the abstract that a septuple mutant with both A581G and A613S/T increased significantly from 6.1% at enrolment to 18.2% at delivery (P = 0.03). These results are difficult to compare with those of studies in East Africa, most of which have found that A581G is usually accompanied by K540E. In contrast, in this study A581G was always accompanied by the dhps triple mutant and dhps S436G, A437G and A613S/T, but never by K540E. Another recent West African survey, of asymptomatically infected pregnant women in Nigeria, similarly found that K540E was absent despite high prevalences of A581G (71%), S436A (55%) and A613S/T (36%) [52], and a survey in Ghana reported similar results [53]. This tendency for K540E to be uncoupled from A581G at some West African sites likely reflects the global patterns of spread of dhps haplotypes, with more highly resistant forms commonly found in East Africa having Asian origins, while less resistant homegrown dhps haplotypes predominate in West Africa [32].
The single study that provides the most convincing evidence that ITPp-SP does not strongly select dhps A581G comes from a well-designed randomized clinical trial done in Malawi in 2011–2014 [54]. Pregnant women were randomized to one of two intervention arms: standard IPTp-SP, or intermittent screening by rapid diagnostic test (RDT), and treatment of RDT-positive infections with dihydroartemisinin-piperaquine. No differences were found in the prevalence of dhps A581G in either the peripheral or placental blood among women in the IPTp group who had been exposed to SP, compared to women randomized to the screen-and-treat group who were not exposed to SP.
In summary, IPTp-SP appears to select for antifolate resistance mutations associated with low to moderate increases in drug resistance, but there is no convincing evidence of selection favouring the key mutations—especially dhps A581G—associated with higher level antifolate resistance and loss of ITPp-SP efficacy.
Impact of resistance on IPTp efficacy
The most recent WHO Guidelines for Malaria continue to recommend IPTp-SP for women living in areas of moderate-to-high transmission in Africa, including in areas with > 90% prevalence of the dhfr/dhps quintuple mutant [55]. The guidelines note that where infections with the quintuple mutant plus either dhfr I164L or dhps A581G are prevalent, “…the efficacy of IPTp-SP may be compromised. It is unclear by how much.” The following discussion considers whether currently available evidence can add clarity on this topic.
Many studies of widely varying quality have assessed the impact of SP resistance on IPTp efficacy. An influential systematic review and meta-analysis published in 2007 pooled data from seven clinical trials of IPTp-SP in relation to SP efficacy for treating symptomatic malaria in young children at or near the same times and locations of the IPTp trials [37]. The authors concluded that even in areas where SP had lost treatment efficacy in children (day 14 treatment failure rates of 19–26%), IPTp-SP continued to provide important health benefits to HIV-negative semi-immune pregnant women and their infants. Moreover, they found no evidence of a substantial loss of IPTp efficacy as SP treatment failure rose from 3 to 39% across sites. In women living with HIV, a group in which IPTp benefit is reduced, IPTp efficacy did decline with rising treatment failure.
The discordance between IPTp-SP benefit in HIV-uninfected pregnant women and SP treatment efficacy in children was attributed mainly to greater levels of acquired immunity in pregnant women. This systematic review did not directly address drug resistance as distinct from treatment failure, nor did it examine relationships between dhfr and dhps mutations and IPTp outcomes. Nevertheless, policymakers were reassured by the persistent benefit of IPTp-SP in the face of high rates of SP treatment failure in children, and the WHO recommended adopting IPTp-SP in Africa even where the prevalence of parasitological failure at Day 14 after SP treatment among children was as high as 50%, or even higher in areas where IPTp was already implemented [56].
A study done in the same region of Tanzania where earlier studies had found that rising prevalence of dhps A581G curtailed the efficacy of both SP treatment of children and IPTp-SP, appeared to support the concern that this mutation boded ill for ITPp-SP [47]. This study, which assessed clinical, parasitological, and histopathological outcomes of IPTp, was even more alarming than the report of very high SP treatment failure rates in Tanzanian children [38]. Based on a study in mice showing that resistant parasites grew to unexpectedly high densities when drug treatment eliminated sensitive parasites [57], the authors hypothesized that, with its compromised efficacy, SP might “select resistant parasites and exacerbate infections in the placenta”. SP resistance mutations, placental parasite densities, and placental inflammation were assessed in women enrolled at delivery between 2002 and 2005 who reported having received, or not having received, SP-IPTp. Those who reported receiving IPTp were classified as “recent IPTp” if they had measurable sulfa levels in their blood, and “early IPTp” if sulfa levels were undetectable.
The authors reported that IPTp was associated not only with higher prevalence of dhps A581G but with dramatically higher placental parasite density, and, most concerning, with increased placental inflammation. They reasoned that inflammation indicates chronic placental malaria infection; that inflammation should thus be absent in acute placental malaria; and that placental parasite density normally decreases as placental inflammation increases. Based on these expectations and the observation that inflammation was more common in women who received IPTp, they deduced that the high parasite densities could not be attributed to new acute infections and, therefore, must have resulted from the greater presence of resistant parasites carrying dhps A581G in the women who received IPTp. In a subsequent publication of data from the same observational study, the authors reported that IPTp did not reduce the odds of placental malaria, increase mean maternal haemoglobin, or increase birthweight, and IPTp was associated with lower cord haemoglobin and increased risk of foetal anaemia [58]. The implications for ITPp-SP seemed ominous.
This dire interpretation, however, depended on the inference that differences in outcomes were the result of IPTp and not other confounding factors in what was essentially a retrospective case–control study nested within a birth cohort. And while most baseline characteristics showed no significant differences between women who did or did not report receiving IPTp, there was one important, highly significant difference: only 29% of women who received no IPTp lived in rural areas, while 68% of women who received IPTp lived in rural villages. The paper did not discuss differences in malaria epidemiology or risk between the rural and urban sites. An earlier paper describing the parent birth cohort study [59] cited an annual entomological inoculation rate (EIR) of around 400 infected bites/person/year for the study area of Muheza District, but that paper in turn cited another paper from a decade earlier that reported heterogeneous transmission in Muheza, with EIRs ranging from 34 to 405 [60].
With the only available data on transmission intensity in the study area being a ten-year-old study that reported a more than tenfold range of EIRs, it is difficult to dismiss the baseline observation that significantly more rural women had received IPTp. A plausible alternative explanation for the higher parasite densities and placental inflammation in women who received IPTp would be that rural women may have been exposed to up to tenfold higher malaria transmission intensity than their peri-urban counterparts, increasing their risk of acute-on-chronic placental infections. Bed net use was also different at baseline: women who reported no IPTp also reported marginally and insignificantly higher use of bed nets (76.5% vs. 64.4%). However, while none of the (more urban) non-IPTp women reported using insecticide-treated nets (ITN), 16% of (more rural) women who had received IPTp reported using ITNs. The complete absence of ITN use among the non-IPTp women is consistent with alternative explanations, including the possibility that the parasitological and histopathological findings attributed to IPTp selection of A581G-carrying resistant parasites were actually a result of baseline differences in malaria risk between women who received IPTp and those who did not.
Subsequent studies failed to replicate the disquieting finding that IPTp-SP led to increased parasite growth in a setting with prevalent dhfr/dhps sextuple mutants. In another cohort of pregnant women in Korogwe District, less than 30 km from Muheza, dhfr and dhps were genotyped in samples from women who had P. falciparum-positive RDTs, and pregnancy outcomes were assessed [61]. During the study period of 2008–2010, the prevalence of the sextuple mutant with dhps A581G in Korogwe was 44%, slightly lower than in nearby Muheza several years earlier. The presence of the sextuple mutant was associated with substantially lower birthweights. However, in contrast to the Muheza cohort, the presence of the sextuple mutant was not associated with whether or not women had received IPTp-SP or with how many doses they received; peripheral parasite density tended to be lower, not higher, in women with the sextuple mutant; and there was no relationship between early or recent IPTp and the effect of dhfr/dhps haplotypes on birth weight. Studies in Mozambique [42] and Malawi [62] similarly failed to support the notion that IPTp was harmful in settings with high levels of antifolate resistance, although dhps A581G was rare (but present) in both studies.
Another systematic review was published in 2013 by the same group that conducted the 2007 review of IPTp efficacy. A meta-analysis of data from seven trials, one each in Kenya, Tanzania, Zambia, Burkina Faso, and Mali, and two in Malawi, found higher average birthweights and lower risk of low birthweight in women who had received three or more doses of IPTp-SP, compared with those who received only two doses [63]. This association was consistent across sites where the prevalence of the dhfr/dhps quintuple mutant—as indicated by the presence of dhps K540E—ranged from 0–96%. The dhps A581G mutant was not prevalent at any of the study sites. Based on these relatively encouraging findings, WHO recommended at least three doses of IPTp-SP irrespective of the presence of dhfr/dhps quintuple mutants [64].
To recap, as of 2013, two high quality systematic reviews [37, 63] and more recent clinical trials [42] and surveys [62] supported the WHO position that IPTp-SP was beneficial and should be used across a wide range of antifolate resistance and SP treatment efficacy. On the other hand, an open label SP efficacy study in children [38] and two observational studies in pregnant women [47, 58, 61] suggested that the sextuple mutant represented a dangerous threat to IPTp-SP efficacy, and might be causing IPTp to be not only ineffective but harmful in pregnancy. Each of these three studies portending bad news for IPTp had significant limitations in design and interpretation, and all three were conducted in two adjacent districts in Tanzania, limiting their generalizability to other sites in Africa, where the sextuple mutant remained mostly rare or absent.
Aiming to resolve these discrepant results, an observational study followed by a clinical trial in Malawi and a multi-country efficacy trial of IPTp-SP efficacy directly examined the relationship between SP resistance and IPTp outcomes. The effectiveness of IPTp-SP was assessed in 2009–2011 in Malawi, where the prevalence of the sextuple mutant was 8.4% [65]. The presence of A581G was associated with an approximately threefold increase in the occurrence of “patent” infections (both PCR and microscopy positive) in both peripheral and placental blood, and with higher parasite densities. However, A581G was not associated with any of the following: (1) histological evidence of active placental infection; (2) mean haemoglobin; (3) anaemia; (4) severe anaemia; (5) pre-term delivery; or (6) infants born small for gestational age. Furthermore, women infected with parasites carrying dhps A581G gave birth to infants with slightly higher birthweights and had a nearly twofold lower incidence of low birthweight, although these trends did not achieve statistical significance. And, the finding of higher parasite densities in A581G-carrying infections disappeared when the analysis was limited to women with “patent” infections, i.e., when infections that were PCR-positive but microscopy-negative were excluded from the analysis.
Some methodological issues cloud the interpretation of these results. For example, even though more than 90% of both patent (PCR and microscopy-positive) and “subpatent” (PCR-positive, microscopy-negative) infections were successfully genotyped, the prevalence of A581G was reported to be tenfold lower in subpatent infections, a surprising finding that is not explained by the authors. Further muddying the picture, most of the data are presented as pooled results from two study sites, one rural and one urban, even though the prevalence of A581G was more than twice as high at the rural site. Different microscopy staining and reading protocols were used at the two sites, with more rigorous standards at the urban site in Blantyre, and no quality control procedures were described, raising the possibility that rural–urban differences in both malaria epidemiology and the quality of microscopic diagnosis could account for some of the study findings. As with the Tanzanian study described above [47], it is possible that higher parasite densities attributed to resistant parasites were actually a reflection of higher transmission intensity or other epidemiological differences at the rural site where more A581G-carrying infections were found.
A subsequent trial, also done in Malawi by the same group, randomized pregnant women at three sites in 2011–2014 either to receive standard IPTp-SP or intermittent screening with RDTs and treatment of RDT-positive infections with dihydroartemisinin-piperaquine [54]. By the time of this study, the dhfr/dhps quintuple mutant was at near-fixation, and the dhfr I164L mutation was absent, while dhps A581G had a prevalence of 4% in infections found at enrolment in the IPTp-SP group, and 6% in placental infections at delivery. The presence of A581G in placental infections was associated with a significant decrease in gestational age and lower birthweights, but not with parasite placental density, placental inflammation, maternal haemoglobin level, or weight-for-age Z score. Overall, the timing of SP exposure had no impact on birth outcomes, but in the small group of women who had placental infections with A581G, more recent SP exposure was associated with significantly longer pregnancies and higher birthweights. However, a sensitivity analysis showed that this result was driven by a single premature birth of a very small infant to a woman who had received only a single dose of SP; when this outlier was accounted for, the association between recent SP and birth outcomes among women with A581G was not significant.
Taken together, the results of these two studies in Malawi confirmed a partial diminution of IPTp efficacy against A581G-containing placental malaria, but they did not support findings of the studies that had raised the alarm about ITPp-SP causing harm where A581G is prevalent.
None of the studies described so far that promoted the notion that antifolate resistance in the form of dhps A581G spelled doom for IPTp-SP in Africa were prospective, controlled trials designed specifically to address this question. In contrast, the relationship between antifolate resistance mutations and efficacy of IPTp-SP was prospectively assessed in a multi-country trial among asymptomatic, microscopy-confirmed P. falciparum-infected, HIV-uninfected, pregnant women. Prospective efficacy studies were undertaken between 2009 and 2013 at eight sites in six African countries spanning the continent and a range of prevalences of mutations in dhfr and dhps [66]. With weekly follow-up, treatment failure was defined as smear-positive P. falciparum on or after day 4, with both uncorrected and PCR-corrected efficacy estimates. Resistance genotyping was done using pooled sequencing, so any novel mutations should have been detected.
Study sites were characterized as having low, moderate, or high SP resistance, based on prevalence of dhps K540E of < 10%, 10–90% or > 90%, respectively. Defining SP resistance on the basis of this one mutation was reasonable, in that K540E serves as a good surrogate for the dhfr/dhps quintuple mutant in settings where dhps A581G is absent or rare, as it was in the study sites. A581G, the surrogate for the dhfr/dhps sextuple mutant, was absent in the moderate (Zambia) and low (Burkina Faso and two sites in Mali) resistance sites. A581G was found in each of the high resistance sites, with prevalence of less than 0.25% in Uganda, less than 2% in both Malawian sites, and just over 5% in Kenya. At these low levels, it was not possible to assess the relationship between the sextuple mutant and IPTp-SP outcomes in this study.
While SP resistance mutations in this multicentre study did appear to compromise parasite clearance and result in more reinfections as well as a shorter time to reinfection, resistance did not appear to affect birth outcomes. As the authors note, prevalence of resistance mutations was not the only difference across the sites. Although transmission intensity was not measured in this study, malaria transmission has historically been reported to be lower and more sharply seasonal in the West African low-resistance sites, and higher and more year-round in the moderate- and high-transmission sites in East and Southern Africa. This pattern makes it difficult to attribute outcomes solely to resistance. Moreover, lower resistance and transmission might lead us to expect better IPTp outcomes in West Africa, but this same lower transmission may also mean lower natural immunity contributing to poorer outcomes, making it challenging to sort out the relative contributions of these countervailing effects of resistance, exposure risk, and immune protection on IPTp birth outcomes. Finally, women who had been enrolled in the in vivo SP efficacy study were excluded from the birth outcome study. If women who had patent infections early in pregnancy were at higher risk of malaria, their exclusion from the birth outcome study might have reduced the study power to detect SP’s anti-malarial efficacy, lending more weight to non-malaria factors in determining outcomes. Nevertheless, this well-designed study further added to the growing body of evidence that the benefits of IPTp persist in the face of high rates of SP resistance as conferred by dhfr/dhps quintuple mutants. The impact of the sextuple mutant (the addition of dhps A581G) on IPTp outcomes remained unaddressed by this study, which finished data collection in 2013.
One potential factor contributing to the apparent lack of impact of antifolate resistance mutations on IPTp-SP efficacy is suggested by studies showing that IPTp with either dihydroartemisinin-piperaquine or SP results in comparable pregnancy outcomes despite dihydroartemisinin-piperaquine’s superior efficacy at clearing and preventing malaria infection [67–69]. Studies are underway to assess whether some of SP’s impact on pregnancy outcomes is mediated by non-malaria benefits, e.g., antibacterial activity.
Two additional systematic reviews have attempted to identify a threshold prevalence of dhps A581G above which IPTp-SP efficacy is lost. One pooled data from nine IPTp studies (five clinical trials and four observational studies completed at a total of 12 sites) to assess the impact of malaria transmission intensity on IPTp outcomes, as modulated by A581G prevalence [70]. Transmission intensity did not appear to influence the efficacy of IPTp on low birth weight. Data on A581G prevalence collected within two years and 250 miles of the IPTp studies were pooled. Two sites had > 50% prevalence of A581G, and the others had 10% prevalence or less, with four sites having no A581G. Among women who had received two or more doses, IPTp-SP efficacy was preserved at sites with 10% or lower prevalence of A581G. At the two sites with > 50% prevalence, efficacy was diminished but still significant among primigravid and secundigravid women, and absent in multigravid women. The authors concluded that the A581G prevalence threshold above which IPTp-SP should not be used was somewhere between 10–52%.
Another, larger, meta-analysis and systematic review was recently published by the same group that performed the two earlier meta-analyses discussed above, each of which pooled data from just seven studies [37, 63]. This meta-analysis pooled data from 57 studies done in 17 African countries between 1994 and 2014, and confirmed the relationship between prevalence of the quintuple mutant (signalled by dhps K540E) and diminished IPTp-SP efficacy [71]. The dhps A581G mutation, used as a surrogate for the dhfr/dhps sextuple mutant, was present in 16 of the studies, at prevalences ranging from 2.5% to 47%. The pooled analysis thus overcame the sample size limitations of the individual studies that had made it difficult to draw definitive conclusions about the impact of the sextuple mutant in IPTp-SP outcomes. However, the authors’ conclusion that IPTp-SP effectiveness is lost where dhps A531G prevalence exceeds 10% is based on just five of the 57 studies included in the meta-analysis.
Three of these five studies had small sample sizes in the reference group and a pooled prevalence of A581G of 21%, and together yielded a relative risk reduction (RRR) of 35%. This means that IPTp-SP retained good effectiveness comparable to that seen in low resistance sites, despite A581G prevalence greater than 20%. Of these three studies, two were from Tanzania and had limitations and discordant results that are discussed at length above [47, 58, 61]. Interpreting results from the third study, from Uganda, is confounded by the unusual finding that both dhps A581G and dhfr I164L had 36% prevalence, occurring together almost as often as not [72]. The dhfr I164L mutation, which has been largely absent or unreported in other studies of IPTp and resistance, is found in parasites with the highest measured pyrimethamine IC50s, approximately tenfold higher than IC50s of parasites carrying the dhfr triple mutant. The frequent occurrence of parasites carrying both of these mutations prevents clear attribution of IPTp outcomes in this study to A581G.
Of the five studies that provided the basis for concluding that IPTp-SP efficacy is lost above a 10% prevalence threshold for A581G, the remaining two were from the Democratic Republic of Congo [73] and Uganda [74]. Both of these studies had larger sample sizes and were conducted in areas with a pooled prevalence of A581G of 46%, much higher than that seen in the three smaller studies. Together, these two studies had an RRR of -2% for low birthweight (compared with 35% for the other three studies), signifying a complete loss of IPTp-SP efficacy. This means that among the 57 studies include in the meta-analysis, just two reported that IPTp-SP efficacy was lost where A581G prevalence exceeds 10%. Both studies were also done in areas where the prevalence of K540E was above 90%, making it difficult to attribute the loss of IPTp efficacy to the presence of A581G. Neither of these studies included molecular analyses of dhfr or dhps mutations—the meta-analysis relied on molecular data collected as close as possible in space and time to the field studies [75–77]. While some of these molecular studies did report prevalence of dhfr I164L, the meta-analysis only used data on dhps mutations.
In summary, despite some convincing evidence that the presence of dhps A581G at least partially compromises the efficacy of IPTp-SP, the worst-case scenario [47] was not borne out by subsequent trials [54, 65, 66]. The evidence supporting a recommendation to withhold ITPp-SP where the prevalence of dhps A581G exceeds a threshold of 10% is not strong.
Intermittent preventive treatment during infancy and resistance
As with IPTp, resistance has been of concern since IPTi was first conceived and evaluated. Most early studies incorporated molecular surveillance to assess the relationships between resistance markers and IPTi-SP efficacy, and to measure the impact of IPTi on the prevalence of resistance markers.
Impact of IPTi on resistance
In a 2003–2005 trial of IPTi-SP in aparasitaemic Ghanaian infants, the incidence of dhfr/dhps quintuple mutants during two months after the third dose of IPTi was twice as high in the treatment group compared a placebo group [78]. In contrast, the prevalence of dhfr triple and dhfr/dhps quadruple mutants (dhfr triple mutant plus dhps A437G in the same infection) remained stable over a one-year period as IPTi-SP was implemented in Mali in 2006–2007, with no differences in marker prevalences between 11 IPTi implementation zones and 11 control zones [79].
Ecological surveys accompanied IPTi-SP evaluations in Tanzania (2004–2007) [80] and Senegal (2006–2008) [81]. Two years after implementation of IPTi-SP, prevalence of the dhfr triple mutant in both countries was significantly higher in areas subjected to IPTi compared to nearby control areas. Prevalence of dhps A437G was also significantly higher in IPTi areas in Senegal, where dhps K540E was absent. While the prevalence of the dhps A437G/K540E double mutant was also higher in IPTi areas than in control areas in Tanzania, this difference was not significant.
Follow-up surveys in Senegal in 2009–2010 confirmed that dhps K540E remained absent both in zones where IPTi-SP (or IPTc-SP, as seasonal malaria chemoprevention was then called) had been implemented, as well as in control zones. The dhps A437G mutation decreased in prevalence in both IPT and control zones between 2009 and 2010. In the IPT zone both A581G and A613S declined from 3 to 0% and from 5 to 0%, respectively, while in the control zone A581G and A613S both rose from 0 to 3% in prevalence during the same two-year period.
In summary, while IPTi-SP has been accompanied by overall increases in the prevalence of some antifolate resistance markers, neither clinical trials of IPTi nor ecological surveys comparing IPTi implementation zones to control areas over time have shown evidence of significant selection of the dhfr/dhps haplotypes associated with reduced SP efficacy for treatment or chemoprevention. This conclusion is in agreement with that of an Institute of Medicine expert committee discussed in the next section [82], and with results of two independent modelling studies [83, 84].
Impact of resistance on IPTi efficacy
In 2008, the Institute of Medicine (now the National Academy of Medicine) in the USA convened an expert committee to evaluate the evidence concerning IPTi-SP efficacy, including an assessment of the impact of antifolate resistance [82]. The committee undertook a detailed review of published and unpublished data, including new meta-analyses of pooled data from pilot studies of IPTi-SP done at six sites in Tanzania, Ghana, Mozambique, and Gabon. They concluded that: (1) SP treatment efficacy for clinical malaria is not a reliable indicator of IPTi effectiveness; and (2) IPTi-SP retained 20–30% efficacy in the face of 40–80% prevalence of the dhfr triple mutant. Among the six sites where IPTi showed measurable efficacy were Ashanti, Ghana, where more than 60% of baseline infections had four or more dhfr/dhps mutations [85]; Tamale, Ghana, where the dhfr triple mutant plus dhps A437G (i.e., the quadruple mutant) was found in 44% of infected children aged less than five years [86]; and Manhica, Mozambique, where prevalence of the dhfr/dhps quintuple mutant in the placebo arm was 44% [87]. The committee was unable to evaluate the impact of dhfr I164L because it was absent or rare at African sites where studies had looked for this mutation. The committee did not consider the role of dhps mutations in IPTi efficacy in more detail, owing to the paucity of available data at a time when few studies had examined the role of dhps K540E in IPTi efficacy, and the A581G and A613S/T mutations were still rare in Africa.
Subsequent studies found that IPTi-SP efficacy diminished in parallel with rising prevalence of the dhfr/dhps quintuple mutant. While the sample sizes were small, all nine genotyped baseline infections had the quintuple mutant in an IPTi trial Korogwe, Tanzania, where four infections (44%) also carried the dhps A581G mutation [38]. Although marker prevalence was not reported for infants participating in a trial in Same, Tanzania, a 2001 survey of two sites nearby had found a 60–75% prevalence of the dhfr triple mutant and a 43–55% prevalence of the dhps double mutant [88]. Neither of these two trials demonstrated significant protective efficacy of IPTi-SP [89].
A pooled analysis of results from seven IPTi trials conducted between 1999 and 2008 found that the duration of protective efficacy was shortened by 50% in the presence of quintuple mutant parasites, from 42 days in Navrongo, Ghana (no dhfr/dhps quintuple mutant), to 21 days in Korogwe, Tanzania (89% prevalence of the quintuple mutant) [90]. This meta-analysis also found that protective efficacy in the 35-day period after the 9-month dose of IPTi-SP decreased with an increasing number of resistance markers, although there were not enough data points to determine the effects of specific markers. These data are consistent with a meta-analysis that found that the duration of post-treatment chemoprophylaxis for different artemisinin-based combinations was shorter when the prevalence of markers of resistance to the ACT partner drug was higher [91].
Based on the data available at the time, a 2009 WHO technical consultation recommended that IPTi-SP be implemented only “when parasite resistance to SP in the area is not high”, adding that “Precise cut-offs cannot be defined on the basis of available data.” [24] Just a few months later another technical consultation that included expertise in drug resistance reviewed essentially the same body of evidence and recommended that IPTi-SP not be implemented where prevalence of dhfr K540E exceeded 50% [24]. This recommendation was based on just two IPTi-SP trials, one showing 21% protective efficacy in Mozambique where baseline prevalence of dhfr K540E was 55%, and one in Tanzania showing no significant efficacy where K540E prevalence was 94%.
A subsequent analysis of molecular marker data collected across Africa from 2005–2011 found that, based on the 50% threshold for K540E prevalence, eight East African countries were classified as unsuitable for SP-IPTi; 14 Central and West African countries were classified as suitable; and seven countries could not be classified owning to a lack of available contemporary data [92]. A cost-effectiveness analysis concluded that IPTi-SP remained cost-effective across a range of SP resistance levels, but the analysis did not consider the high-level resistance conferred by dhps K540E, A581G and A613S/T, limiting relevance of the study for areas where these mutations are prevalent [93].
In the last decade, few new studies that inform the impact of resistance on IPTi efficacy have been published. When a cluster randomized trial of IPTi-SP in Tanzania found no survival benefit, the authors speculated that drug resistance was one of many possible factors that could account for this finding, along with operational deficits, decreasing malaria transmission, improving vector control, and better case management [94]. A recent Cochrane review of IPTi noted overall trends of declining IPTi efficacy in parallel with increasing antifolate resistance in Africa, but no new data on SP resistance markers underly this observation, so this meta-analysis does not help in more precisely defining a resistance threshold to guide IPTi implementation decisions [95].
As countries consider implementing IPTi or introducing new drug combinations where IPTi-SP has lost efficacy in the face of resistance, studies directly assessing not only efficacy but duration of protection against both asymptomatic infection and clinical malaria episodes in relation to the prevalence of resistance markers would be of value. As was shown for different ACT treatment regimens [91], the benefits of different chemoprevention regimens may be different in areas with different resistance patterns.
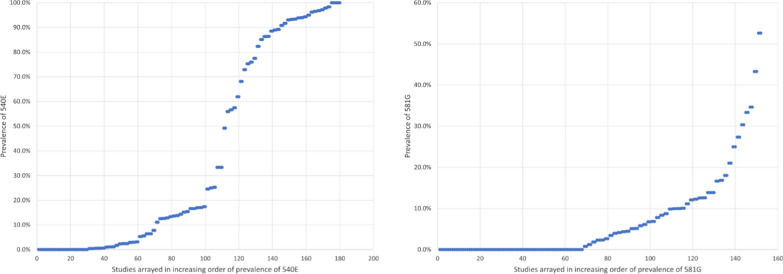
Given the continued paucity of data on the relationships between SP resistance markers and IPTi efficacy to justify a threshold of resistance above which IPTi should not be implemented or continued, more creative approaches may be needed. For example, Fig. 2 shows the frequency distribution of prevalence measures of dhps K540E and A581G for studies completed in sub-Saharan African countries between 2015 and 2021, arranged in increasing order of prevalence. The prevalence of the K540E mutation ranged from 0–100%, with a clear “break point” (sharp change in slope) at around 40% prevalence, providing a natural point for grouping sites with prevalences above and below that point. In contrast, the prevalence of A581G ranged from 0–53%, with a less obvious break point around 15%. When there is a wide range between prevalence levels at which IPTi efficacy persists or is lost, it might be reasonable to choose thresholds based on these break points in the data, to reflect naturally occurring clustering of prevalence levels. This approach might help policy makers avoid difficult decisions when measured prevalences lie very close to the thresholds.
Fig. 2.
Frequency distributions of prevalence estimates of dhps K540E (L) and A581G (R) mutations measured in studies completed in sub-Saharan Africa from 2015–2021. Data were downloaded from http://www.wwarn.org/dhfr-dhps-surveyor and studies completed before 2015 and outside of Africa were excluded. Recent measures of K540E prevalence tend to cluster below 20% and above 50%, while A581G prevalence estimates lack an obvious break point
In the case of IPTi efficacy and dhps K540E prevalence, based on the observation that IPTi retained 21% efficacy where K540E was prevalent at 55% but not where it was 94%, any threshold between 55 and 94% could have been selected to segregate sites where IPTi-SP might be expected to retain and lose efficacy. The WHO technical consultation recommended a 50% threshold based on the assumption that where prevalence was less than 50%, efficacy should be at least 21%. Based on this new analysis of more recent marker prevalence data as shown in Fig. 2, a case could be made for implementing IPTi where K540E has a prevalence of 40% or lower.
The prevalence of A581G is generally lower, with many studies having 0% prevalence and only two of 152 studies having more than 50% prevalence. Choosing a 10% threshold of A581G for implementing IPTi-SP would be problematic in that eight studies had measured prevalences between 9.9 and 10.1%. Choosing a prevalence threshold of 15% would make it easier for policy makers to segregate sites where IPTi should or should not be used, based on available recent data.
With additional analysis it might be possible to select thresholds based not only on clustering of prevalence estimates, but also geographical clustering. The intent would be to avoid having geographically adjacent areas with prevalence estimates just above and just below a given threshold. Having different IPTi policies in areas that are both geographically close and with similar malaria epidemiology could be confusing to policy makers. Selecting thresholds that would group countries or regions in a logical, understandable fashion could make recommendations easier to understand and follow. For example, choosing a threshold that results in IPTi being recommended in most of francophone West Africa. but not in anglophone East Africa, would be more palatable than one that results in different policies being recommended in coastal and western Kenya, or in northern and southern Tanzania.
These potential new approaches to setting guidelines for chemoprevention when data on resistance and efficacy are limited could be assessed in both field and modelling studies to gauge their utility and feasibility.
In summary, the evidence supporting a recommendation that IPTi-SP not be deployed where prevalence of dhps K540E exceeds 50% was thin when an expert group identified this threshold based essentially on just two trials ten years ago, and little new evidence is available to validate this threshold, or to set new criteria to guide IPTi policy (e.g., a prevalence threshold for dhfr I164L, dhps A581G, and/or dhps A613S/T). Efficacy studies of potential new IPTi drug regimens should include assessments of efficacy and duration of protection in relation to resistance markers. Until more evidence is available on the relationship between SP resistance and IPTi-SP efficacy, an alternative approach would be to select thresholds for implementing IPTi based in part on natural clustering of prevalence data in recent studies.
Seasonal malaria chemoprevention and resistance
In 2012 WHO recommended another chemoprevention strategy, seasonal malaria chemoprevention (SMC, formerly called Intermittent Preventive Treatment in Children or IPTc). SMC with SP and amodiaquine (SP-AQ) is recommended for children aged less than five years in regions of the West African Sahel with intense seasonal malaria transmission. As recommended by the WHO, SMC consists of a complete treatment course of SP-AQ administered to children aged 3–59 months at monthly intervals, beginning at the start of the transmission season, up to a maximum of four doses during the malaria transmission season. The relatively lower levels of antifolate resistance in West Africa, and the addition of amodiaquine to the regimen, gave rise to optimism that SMC might be less threatened by resistance than IPTp was in East Africa.
Impact of SMC on resistance.
In a 2008 trial in Burkina Faso, after three monthly rounds of SMC with SP-AQ the prevalence of infections with dhfr/dhps quadruple mutants (triple dhfr and dhps A437G mutants) was comparable in the treatment and placebo arms, with an overall increase over baseline prevalence in both groups [96]. In contrast, a contemporaneous trial in Mali appeared to show SMC selection of low- and mid-level antifolate resistance markers. While the dhfr/dhps quintuple mutant (quadruple plus dhps K540E) was absent, the prevalence of quadruple mutants was significantly higher in the SP-AQ group than in the placebo group, and prevalence increased from baseline in the SMC group but not in the placebo group [97]. In a trial of SMC with SP plus artesunate in Senegal, the post-intervention prevalence of quadruple mutants was also significantly higher in the intervention arm than the placebo arm, again with an increase in both groups from baseline [98]. Prevalence of resistance markers continued to rise in both groups, and no difference between the intervention and placebo arms was detected after the second year of follow up, possibly as a result of increased SP use in the general population following a change in national first-line treatment policy to SP-AQ [98]. A subsequent comparison of SMC with SP-AQ and dihydroartemisinin-piperaquine in Burkina Faso similarly found evidence of modest selection dhfr S108N and C59R and pfcrt K76T in the SP-AQ arm of the trial [99].
As noted above, the impact of SMC on resistance is related not only to the proportion of infections that carry resistant parasites, but on the proportion of people who become infected. Modelling studies may be useful in assessing whether SMC’s efficacy at reducing the prevalence of infection mitigates the risks posed by its effect of increasing the prevalence of resistance (defined here as the proportion of infections carrying resistant parasites).
Based on these early studies, it appeared that at least short-term selection of resistance markers may follow SMC implementation. Surveys of health districts that had or had not implemented SMC or IPTi in Senegal found significant selection of the dhfr triple mutant, but not for dhps mutations [100]. An ecological survey in Ghana that included areas where SMC had and had not been implemented reported similar increases in dhfr/dhps quintuple mutants, but this study did not test for the higher-level resistance mutations dhfr I164L and dhps A581G and A613S/T [101]. Another prospective SMC trial done in Mali in 2014 found that prevalence of the quintuple mutant remained similar and below 5% before and after IPTp-SP was implemented in two districts (this trial also did not assess higher-level SP resistance mutations) [102]. The Mali trial also reported no increases in the prevalence of pfcrt or pfmdr1 polymorphisms associated with diminished AQ susceptibility.
A large observational study of the scale-up of SMC with SP-AQ in seven Central and West African countries measured the prevalence of resistance markers in 2016 and 2018 among 10–30 year-olds to assess the overall trends in resistance markers in communities where under-fives were given SMC [103]. The dhfr triple mutant was already prevalent at more than 90% across the sites, and increased yet more; and dhps mutations were initially lower and increased proportionally more, with up to fourfold increases in prevalence over time. However, AQ resistance markers in pfmdr1 and pfcrt decreased modestly during the scale-up period. These results are consistent with SMC with SP-AQ selecting for antifolate resistance but not 4-aminoquinoline resistance. However, other plausible reasons for these changes in marker prevalence include reduced CQ use in the region resulting in reduced selection pressure for resistance to 4-aminoquinolines, and other sources of selection pressure favouring antifolate resistance by the use of SP or other antifolates such as trimethoprim-sulfamethoxazole (co-trimoxazole) for antibacterial treatment or chemoprevention. Notably, the fold-increases in the prevalence of dhps markers as well as various dhfr-dhps haplotypes associated with intermediate to high antifolate resistance were all lower (in many cases, 2–threefold lower) in the under-fives than in 10–30 year-olds, despite the younger group being subjected to direct selection for antifolates under SMC. This marked age difference further clouds the interpretation that SMC was solely responsible for the rise in antifolate markers over the study period.
In summary, while some prospective trials and ecological studies of SMC with SP-AQ in West Africa have reported increased prevalence of the dhfr/dhps quadruple and quintuple mutants, other studies found no such evidence of selection. No evidence has been reported of SMC being followed by increased prevalence of the higher-level resistance mutations that most severely impair SP efficacy, nor does SMC appear to select for parasites carrying mutations associated with diminished AQ susceptibility.
Impact of resistance on SMC efficacy.
While the dhfr/dhps quadruple mutant was already prevalent in West Africa as SMC was being tested and implemented, dhps K540E was still rare in the region [104]. SMC efficacy using SP combined with either amodiaquine or artesunate ranged from 70–87% at sites in Senegal, Mali, and Burkina Faso with baseline prevalences of 32–58% of the dhfr triple mutant and 22–29% for dhps A437G [96–98], suggesting that SMC benefit persists in the face of moderate levels of the quadruple mutant. A meta-analysis of SMC trials was conducted [105], but because baseline prevalence of resistance markers prior to implementation was generally not reported, marker prevalence could not be associated with efficacy, nor could selection be measured. Putative molecular markers for amodiaquine resistance, including mutations in pfcrt and pfmdr1, have generally not proven reliable predictors of SMC efficacy. For example, a clinical trial of SP, AQ and SP-AQ for treatment of clinical malaria in Cameroon found that prevalence of pfcrt and pfmdr1 mutations thought to be associated with reduced susceptibility to AQ was higher at sites where AQ and SP-AQ treatment failures were lower [106].
In summary, unless and until high-level resistance mutations become more prevalent in areas where SMC is used, it will not be possible to draw conclusions about the impact of resistance on SMC efficacy.
Mass drug administration and resistance
Mass drug administration (MDA) refers to mass drug treatment of an entire population, irrespective of the presence of symptoms and without individual testing for malaria [34, 107]. During the last century MDA schemes often led to declines in malaria rates, but gains were usually temporary [108]. Exceptions to this pattern include instances of MDA being deployed in combination with aggressive vector control and rigorous surveillance in low-transmission areas, and in geographically conscribed areas, such as islands [34, 109]. MDA was blamed for driving drug resistance, most notably after introduction of anti-malarial drugs in table salt in the 1950s [110], and the WHO stopped recommending it. However, in response to the renewed call for malaria eradication and the emergence of artemisinin resistance, MDA has been re-examined [107, 109, 111]. Trials and implementation projects have been undertaken both in low burden settings slated for elimination such as the Greater Mekong Subregion [112] as well as in Africa [113]. These more recent experiences with MDA have provided the opportunity to gain a better understanding of the impact of MDA on the emergence and spread of resistance, and the impact of drug resistance on MDA efficacy.
Impact of MDA on resistance
In MDA, every consenting member of a malaria-exposed population is administered curative doses of anti-malarial drugs, irrespective of infection status. This is often repeated at intervals, e.g., two monthly cycles repeated annually for two years. It would seem obvious that such a massive drug exposure would exert powerful selection pressure favouring resistant parasites—and, indeed, MDA has been indicted for hastening resistance throughout the history of malaria control. Malaria icon Walther Wernsdorfer (who literally wrote the book on malaria) asserted 40 years ago that “Mass drug administration in its various forms, and insufficient treatment are obviously the most important motors of selection.” [114] While this is an oft-repeated notion, the evidence is less clear cut.
Theoretical arguments have been made that MDA prevents rather than fosters resistance, based on calculating probabilities of emergence and spread of resistance in relation to parasite density [115]. This prediction appears to be supported by recent well-executed MDA schemes in low-transmission elimination zones with highly efficacious drugs that found no evidence of selection for drug resistant parasites. For example, mutations in P. falciparum kelch13 associated with artemisinin resistance were already prevalent when MDA with dihydroartemisinin-piperaquine and low-dose primaquine was evaluated in eastern Myanmar, where a piperaquine resistance marker (multiple copies of the P. falciparum genes plasmepsin2/3, or pfpm2/3) was absent at baseline. There was no evidence of selection of resistance by MDA: after MDA, the piperaquine resistance marker was still absent, and kelch13 mutations had decreased in prevalence from 86 to 57% [116].
A cluster-randomized trial of MDA with dihydroartemisinin-piperaquine included Southeast Asian sites with varying levels of resistance. MDA was randomly either initiated or delayed in 16 villages with about 500 residents each [117]. A highly resistant parasite lineage with both the kelch13 artemisinin-resistance mutation C580Y and the piperaquine resistance marker, multiple copies of pfpm2/3, was absent at baseline in Myanmar and Lao PDR, but present in Vietnam and Cambodia at prevalences of 4% and 63% of genotyped infections, respectively. Only 14 of the 258 individuals who were infected with P. falciparum at baseline and completed three rounds of MDA were persistently infected a month later, 13 in Vietnam and one in Cambodia. Only the single persistent infection in Cambodia carried the highly resistant haplotype.
In Mozambique, where malaria transmission and parasite densities are much higher than in Southeast Asia, the prevalence of resistance markers was compared before and after two annual cycles of two monthly rounds of MDA with dihydroartemisinin-piperaquine [118]. No evidence of selection was found for markers of resistance to artemisinins (k13) or piperaquine (pfpm2 and pfcrt).
Modelling studies have both supported and undermined the notion that MDA is a potent force driving resistance. One study concluded that the “windows of selection” for drugs used in chemoprevention were longer than estimated based on clinical data, leading the authors to assert that MDA and other chemoprevention strategies using full treatment regimens “will be far more potent drivers of resistance than previously thought” [119]. However, another modelling study that also incorporated pharmacodynamic properties as well as resistance mechanisms of MDA drugs came to different conclusions. This study found that while MDA using drugs to which parasites can become highly resistant with a single mutation, such as atovaquone, would result in high levels of resistance even after a single round, MDA with artemisinin-based combinations would retain efficacy because of the lower grade of resistance generated by more complex and therefore less frequently occurring genetic mechanisms [120]. The latter model appears to align better with the results of recent MDA experiences with ACT in both low and high malaria transmission settings.
In summary, there is no evidence that MDA in the modern era using highly effective artemisinin-based combination results in increased drug resistance, although studies addressing this topic are limited.
Impact of resistance on MDA efficacy.
In early experiences with MDA using sub-curative drug regimens, MDA quickly selected for resistance, which in turn compromised efficacy [25, 34]. However, in more recent MDA schemes in Southeast Asia, the high efficacy of ACT has been preserved, even in areas with more than 60% prevalence of artemisinin resistance, and efficacy has been stable across sites with low and high rates of resistance to both artemisinins and ACT partner drugs [112, 117]. MDA with ACT has been less efficacious in Africa, not because of drug resistance but because of epidemiological and parasitological factors that differ from low-transmission areas slated for elimination. For example, MDA has either failed or been followed by rebounding malaria incidence when it has been attempted in limited areas adjacent to non-MDA areas that serve as a source for rapid re-introduction of malaria to the populations subjected to MDA [34, 109]. Even in lower transmission settings, MDA’s effects are short-lived if it is applied with less-than-ideal rigor in the absence of effective vector control methods [121]. The near-complete absence of clinically relevant levels of resistance to ACT drugs in Africa precludes any assessment of the impact of resistance on MDA efficacy there.
In summary, in the past drug resistance diminished the efficacy of MDA when drugs were used in sub-curative formulations and dosing regimens (e.g., single drugs used at doses that fail to clear infection). However, in the twenty-first century, MDA with highly effective combination drugs has proven efficacious even in the face of high levels of resistance. Nevertheless, policy makers continue to express worries about MDA promoting resistance [122].
Other potential uses of chemoprevention and resistance
While other potential uses of chemoprevention for malaria control and elimination are not presently recommended by the WHO, evidence from evaluations of new chemoprevention strategies can shed light on the relationships between drug resistance and the WHO-recommended strategies reviewed here. For example, several studies have explored the benefits of preventive drug treatment for malaria among school-age children in East and Southern Africa, where malaria transmission tends to be more perennial than in the West African countries where SMC has been tested and implemented.
A recent systematic review and meta-analysis of preventive treatment among school-age children in Africa that pooled data from 13 studies [123] noted that “…the only study to measure directly the effect of school-based treatment on drug resistance showed that recent treatment with dihydroartemisinin–piperaquine was associated with higher prevalence of molecular markers of drug resistance.” The study in question, from Uganda [124], measured the proportion of P. falciparum infections carrying only the “pure mutant” forms of known resistance markers in relation to the time of the most recent dose of dihydroartemisinin–piperaquine given as monthly chemoprevention to school-age children. This analysis thus combined mixed infections (containing both resistant mutant parasites and sensitive wild-type parasites) with pure wild-type infections in the reference (ostensibly non-resistant) group. This analytical approach limited the “resistant” outcome to those infections in which only “pure mutant” forms were detected. For the purpose of assessing selection of resistance and risk of treatment failure, arguably the more appropriate analysis would have been to compare the proportion of infections containing any resistant parasites, whether or not wild-type parasites were also present in the infection. This is because it is the presence of resistant parasites (irrespective of the presence or absence of sensitive parasites) that signals the risk of treatment failure—the additional presence of wild-type sensitive parasites should have no effect on whether or not the resistant parasites are cleared by drug treatment.
As shown in Fig. 3, when the data in the Uganda paper [124] are re-analysed using this approach of assessing the presence or absence of resistant parasite genotypes (irrespective of presence of wild-type genotypes), there is no suggestion of increased prevalence of any resistance markers in infections occurring further in time from dihydroartemisinin-piperaquine administration. In fact, one of the resistance markers, pfmdr1 N86Y, appears to be significantly less prevalent in infections that occurred sooner after drug treatment, consistent with selection favouring wild-type parasites. The other marker that had appeared in the original analysis to be selected by chemoprevention in this setting, pfcrt K76T, was prevalent at near-fixation levels in all infections, irrespective of temporal proximity to drug treatment, as shown in Fig. 3.
Fig. 3.
Re-analysis of data purportedly showing selection of resistance markers by monthly seasonal malaria chemoprevention in school-age Ugandan children. For each resistance marker, the three bars represent proportion of infections containing mutant genotypes at increasingly distant times from last drug treatment with Dihydroartemisinin-piperaquine. Panel A shows the original analysis, depicted here in graph form, and showing apparent selection of “pure mutant” genotypes of pfmdr1 N86Y and pfcrt K76T based on their increasing in prevalence after drug treatment. Panel B depicts a re-analysis of the same data showing no evidence of positive selection for mutant genotypes when all infections containing the mutation in question are considered to have resistant parasites. Data from Nankabirwa et al. Antimicrob Agents Chemother 2016, 60:5649–54
In summary, the evidence that malaria chemoprevention in school-age children increases drug resistance does not stand up to careful scrutiny. This example illustrates the importance of rigorous study design and analysis in assessing the relationships between drug resistance and malaria chemoprevention strategies and lends further support to the idea that selection of clinically relevant forms of resistance by chemoprevention is not inevitable.
Potential approaches to manage and mitigate the risk of resistance
The history of antimicrobial use is rife with examples of drugs being used in inappropriate ways that hasten the emergence and spread of resistance, such as overprescribing antibacterial drugs for viral illnesses, or adding antibiotics to livestock feed to enhance animal growth. In the case of malaria, it is hard to dispute the inadvisability of practices like adding anti-malarial drugs to table salt [110] and the unfettered sale and use of drugs of questionable quality in the private sector [125]. Concerns about resistance can trigger policymakers to resist new or expanded uses of valuable drugs. While this protective urge is understandable, and can lead to useful initiatives such as expanding diagnostic capacity to reduce empiric malaria treatment for all fever cases, it comes with a risk of restricting access to beneficial drugs that could be deployed in ways that do not appreciably shorten their useful lifespans. Understanding of resistance mechanisms may offer potential approaches for finding the optimal balance between treating and preventing malaria and preserving drug efficacy.
Can countervailing resistance mechanisms be exploited to preserve efficacy?
The WHO and others have recommended that the risk of chemoprevention hastening the demise of treatment drugs should be mitigated by using different drugs for chemoprevention and first-line treatment. IPTp, IPTi and SMC programmes generally follow this recommendation, as SP and SP-AQ are not recommended first-line treatments in countries where these strategies are deployed. Recent MDA programs have been less compliant with this advice, in that MDA with ACT has been used in areas where ACT is also the first-line malaria treatment. This means that the same class of drug—the artemisinins—are subjected to potential selection pressure for resistance in both treatment and chemoprevention regimens, in the same areas if not in the same populations. ACT is likely to remain the first choice for MDA until other equally highly efficacious and well-tolerated regimens are available.
In the meantime, one approach for reducing the potential for MDA to select forms of resistance that impair ACT efficacy is to use different regimens for MDA and first-line treatment, with ACT partner drugs that have antagonistic resistance mechanisms. For example, resistance to mefloquine has been associated with increased copy number of the pfmdr1 gene [126–128] and piperaquine resistance is associated with increased copy number of the pfpm2 and pfpm3 genes [129, 130]. Parasites with increased copy numbers of pfpm2/3 signalling piperaquine resistance usually occur together with the wild-type single-copy pfmdr1 associated with mefloquine sensitivity. These antagonistic resistance mechanisms could potentially be exploited to preserve efficacy by deploying artemisinin-based combinations with countervailing resistance selection pressure, e.g., using dihydroartemisinin-piperaquine for MDA and artesunate-mefloquine or artemether-lumefantrine for treatment. A recent trial of ITPp with mefloquine reported apparent selection against the pfmdr1 N86Y mutation that is associated with chloroquine resistance, raising the possibility that IPTp-mefloquine could drive selection of mefloquine-resistant but chloroquine-sensitive parasites [131].
The two anti-malarial drugs for which counter-resistance is best documented, chloroquine and mefloquine, have recovered efficacy after being withdrawn in some areas and are being evaluated for reintroduction into use. When chloroquine was withdrawn and replaced with SP as the first-line drug in Malawi, chloroquine resistance disappeared over a period of about eight years [132]. Chloroquine was shown to be highly efficacious once again for malaria treatment [133], and weekly and intermittent chloroquine chemoprevention had similar efficacy to IPTp-SP in pregnant women [134]. Chloroquine resistance also declined dramatically after chloroquine was no longer recommended in Tanzania [135] and Zambia [136]. Similarly, after six years as first-line treatment in Thailand, mefloquine efficacy declined from 98% to up to 50% [137]. When dihydroartemisinin-piperaquine was used in the region, mefloquine efficacy recovered, and it is now being studied in the region as a component of a triple ACT [138].
Whether recovery of efficacy results from counter-resistance favouring drugs lost to resistance, or simply resurgence of sensitive parasites in the absence of drug pressure [139], rotating or alternating anti-malarial drugs could be a useful approach for managing resistance. Alternatively, drugs with countervailing resistance profiles could be deployed in parallel: a strategy of “multiple first line therapies” has been proposed to preserve efficacy of treatment drugs [140], and the rationale for “triple therapy” ACT includes the possibility of using drugs with antagonistic resistance profiles [138, 141].
Triple therapy in the form of dihydroartemisinin combined with piperaquine and mefloquine has been proposed as a way to protect ACT partner drug efficacy [142] and is being evaluated for malaria treatment in western Cambodia [138]. Where ACT efficacy is severely compromised triple drug therapy offers a valuable option for malaria treatment, but the added expense and safety considerations make triple therapy less viable for chemoprevention strategies. A recent systematic review of mefloquine for preventing malaria in pregnancy found that while it had superior efficacy to ITPp-SP, high rates of mefloquine-related adverse events limit its potential effectiveness [143]. Other proposed approaches for mitigating or overcoming the impact of resistance on chemoprevention include using antibacterial drugs that have modest anti-malarial efficacy and are thought to be refractory to resistance, such as azithromycin [144, 145], doxycycline [146], or trimethoprim-sulfamethoxazole [147]; increasing the dosage or changing the dosing interval to protect against resistant parasites [148]; and adopting screen-and-treat instead of intermittent treatment [54, 67, 149–151]. None of these approaches has gained acceptance as a viable alternative to IPTp-SP.
Another potential approach for deterring resistance is matching pharmacokinetic properties of drugs used in combination, so that longer-acting partner drugs are not left “unprotected” by persisting at levels that select for resistance after the shorter-acting partner drug has been eliminated [152–154]. Matching half-lives and elimination curves is an attractive approach that should ideally be incorporated into the design of future anti-malarial drug combinations. In the meantime, with the limited number of effective drugs currently available, most drug combinations in use now, and all artemisinin-based combinations, include partner drugs with grossly mis-matched pharmacokinetic profiles. Compared to artemisinin-based combinations, which all pair longer-acting partners with extremely rapidly cleared artemisinins, SP and SP-AQ are reasonably well-matched combinations.
Each of these approaches to mitigating and deterring resistance comes with significant challenges. In discussions about multiple first-line therapies, National Malaria Control Programme managers have explained to researchers and modellers that implementing changes in first-line malaria treatment drugs is not simply a matter of issuing recommendations—doing so effectively requires major investment of resources, time, and effort in training health providers, educating the public, and establishing new procurement and distribution systems. With mathematical models yielding divergent predictions about the benefits of multiple first-line therapies [155], policy makers remained understandably sceptical about this approach.
Proposed chemoprevention strategies that rely on drugs with adverse effects that are tolerable when treating ill patients (e.g., doxycycline, mefloquine) may not be acceptable to the healthy people who are the target population for chemoprevention strategies. Increasing drug dosages to overcome resistance likewise increases safety concerns, especially for use in infants, children, and pregnant women. Screen-and-treat strategies are appealingly efficient, in that they avoid treating uninfected individuals, but they also miss the large reservoir of sub-patent infections and miss out on the post-treatment prophylaxis benefit for people infected shortly after treatment that accounts for much of the benefit of IPT and SMC.
In summary, standardized protocols for measuring and monitoring chemoprevention efficacy are needed. With imperfect evidence, practical considerations such as known prevalence patterns can help guide recommendations on when and where to deploy chemoprevention strategies. Using different drugs for chemoprevention and treatment and combining drugs with countervailing resistance mechanisms may help to preserve efficacy. The best approach for mitigating and managing drug resistance to protect the efficacy of chemoprevention strategies is to ensure that there is a pipeline of safe and effective new malaria drugs, ideally with diverse mechanisms of action and resistance, to replace those lost to resistance.
Summary and final perspectives
The evidence reviewed here about the relationships between drug resistance and malaria chemoprevention strategies comes from a patchwork of studies of diverse designs and varying quality that sometimes yield conflicting results. Studying the relationships between resistance and efficacy is only possible where there is both a high enough prevalence of resistance and high enough level of efficacy to measure associations with adequate statistical power. The heterogeneous settings, populations, and malaria epidemiologies where chemoprevention strategies are tested and used limit the generalizability of individual studies.
Meta-analyses of pooled data have been helpful in guiding policy recommendations, but even large meta-analyses are limited by the small numbers of well-designed studies in which data on resistance were directly collected. This can mean that seemingly robust analyses that draw conclusions based on pooled data from dozens of studies may in fact base those conclusions as few as one or two studies. Most of the meta-analyses reviewed here also pooled molecular marker data from separate surveys that were done as close as possible in time and space to chemoprevention trials. Conclusions thus rely on the suspect assumption that the prevalence of molecular markers is stable across time, space, and populations.
These limitations in the quality and comparability of the available data mean that it is much easier to draw conclusions about what is not known than to develop clear evidence-based guidance based on what we do know. For this reason, many of the key findings summarized in Table 2 are conclusions that the evidence justifying various recommendations is insufficient or weak. Ultimately, health policy makers must make decisions in the face of substantial uncertainty. For example, the WHO has recommended specific molecular marker prevalence thresholds above which certain chemoprevention strategies should not be implemented. The available evidence may support only wide ranges—if the data tell us that a given strategy is likely to retain efficacy if the prevalence of a given marker is somewhere between 10–50%, do we recommend a threshold of 10%, or 50%, or something in between?
Table 2.
Summary of key findings
| Measuring and monitoring resistance |
Drug resistance is but one of many factors that determine the efficacy of IPTp, IPTi, SMC and MDA Clinical trials that measure health outcomes are the gold standard for measuring chemoprevention efficacy Drug treatment efficacy is not a reliable surrogate for chemoprevention efficacy Molecular markers accurately indicate the presence of drug resistant parasites, and can serve as useful but imperfect means of predicting chemoprevention efficacy Specific resistance markers must be validated independently as predictors of efficacy for each different chemoprevention regimen |
| Impact of IPTp on resistance | IPTp-SP appears to select for antifolate resistance mutations associated with low to moderate increases in drug resistance, but there is no convincing evidence of selection favouring the key mutations associated with higher level antifolate resistance and loss of ITPp-SP efficacy |
| Impact of resistance on IPTp |
Despite some evidence that high level antifolate resistance at least partially compromises IPTp-SP efficacy, a worst-case scenario of harmful effects in the presence of SP resistance was not borne out by subsequent studies The evidence supporting a recommendation to withhold ITPp-SP where the prevalence of dhps A581G exceeds a threshold of 10% is not strong |
| Impact of IPTi on resistance | While IPTi-SP has been accompanied by overall increases in the prevalence of some antifolate resistance markers, there is little evidence of significant selection of the forms of resistance known to compromise SP efficacy for treatment or chemoprevention |
| Impact of resistance on IPTi | The evidence supporting a recommendation that IPTi-SP should not be deployed where prevalence of dhps K540E exceeds 50% remains limited |
| Impact of SMC on resistance |
While some studies have reported that SMC is followed by increased prevalence of resistance markers, other studies found no such evidence of selection There is no evidence that SMC results in increased prevalence of the higher-level resistance mutations that most severely impair SP efficacy, nor does SMC appear to select for parasites carrying mutations associated with amodiaquine resistance |
| Impact of resistance on SMC | Unless and until high-level resistance mutations become more prevalent in areas where SMC is used, it will not be possible to draw conclusions about the impact of resistance on SMC efficacy |
| Impact of MDA on resistance | There is no evidence that MDA in the modern era using highly effective ACTs results in increased drug resistance |
| Impact of resistance on MDA |
In the past, drug resistance has diminished the efficacy of MDA when drugs have been used in sub-curative formulations and dosing regimens However, in the twenty-first century, MDA with highly effective combination drugs has proven efficacious even in the face of high levels of resistance |
| Other chemoprevention strategies |
Evidence that seasonal malaria chemoprevention in school-age children increases drug resistance does not stand up to careful scrutiny Selection of clinically relevant forms of resistance by chemoprevention is not inevitable |
| Managing and mitigating resistance |
Standardized protocols for measuring and monitoring chemoprevention efficacy are needed With imperfect evidence, practical considerations can help guide recommendations on when and where to deploy chemoprevention strategies Using different drugs for chemoprevention and treatment and combining drugs with countervailing resistance mechanisms may help to preserve efficacy The best approach for mitigating and managing drug resistance to protect the efficacy of chemoprevention strategies is to ensure a pipeline of safe and effective new malaria drugs with diverse mechanisms of action and resistance |
Recommendations may need to be tempered to offer broader guidelines than precise prevalence thresholds for resistance markers. For example, guidelines may include statements along the lines of: “IPTx has been shown to be efficacious in settings with a prevalence of [resistance marker] up to XX% but not in a setting with a [resistance marker] of YY%. The relationship between efficacy and mutation frequencies between XX% and YY% remains unknown. Resistance may not have been the only factor influencing efficacy in these settings.”
When selecting thresholds for recommending where and when chemoprevention strategies should be used, practical factors unrelated to evidence about resistance and efficacy should also be considered, such as whether or not current or future treatment drugs share resistance mechanisms with chemoprevention drugs, or whether a given threshold might result in confusing situations such as different recommendations in adjacent areas with similar malaria epidemiologies that happen to have resistance prevalences just above and below the threshold.
These limitations can be mitigated to some extent by standardizing study designs and coordinating multi-centre trials and pooled analyses, as has been done by consortia that have formed to test and implement some chemoprevention strategies. The WHO recommendations on research priorities can also guide researchers to conduct studies that will yield data useful to policy makers, to the extent that researchers are made aware of and follow such recommendations. For example, a standardized protocol for “Preventive Efficacy Studies (PES)” akin to TES studies is currently being developed by the WHO Global Malaria Programme.
It is somewhat encouraging that malaria chemoprevention does not inevitably lead to meaningful increases in resistance, and even high rates of resistance do not necessarily impair chemoprevention efficacy. At the same time, it can reasonably be anticipated that, over time, as drugs are widely used, resistance will generally increase, and sooner or later efficacy will be lost. Decisions about whether, where and when chemoprevention strategies should be deployed will continue to need to be made on the basis of imperfect evidence. It is hoped that this assessment of what is known about the relationships between resistance and chemoprevention will be useful as the WHO evaluates and updates its chemoprevention recommendations.
Acknowledgements
The author thanks Philip Rosenthal and colleagues at the WHO Global Malaria Programme for reading drafts of the review and providing helpful feedback.
Abbreviations
- ACT
Artemisinin-based combination therapy
- AQ
Amodiaquine
- DHA
Dihydroartemisinin
- DHFR
Plasmodium falciparum Dihydrofolate reductase enzyme
- dhfr
P. falciparum Gene that encodes DHFR
- DHPS
P. falciparum Dihydropteroate synthase enzyme
- dhps
P. falciparum Gene that encodes DHPS
- EIR
Entomological inoculation rate
- IC50
50% Inhibitory concentration (measure of in vitro drug resistance)
- IPTi
Intermittent preventive treatment in infants
- IPTp
Intermittent preventive treatment in pregnancy
- ITN
Insecticide-treated net
- k13
P. falciparum kelch 13 Gene
- MDA
Mass drug administration
- pfcrt
P. falciparum Chloroquine resistance transporter gene
- pfmdr1
P. falciparum Multi-drug resistance gene
- pfpm2/3
P. falciparum Plasmepsin 2/3 gene(s)
- RDT
Rapid diagnostic test
- RRR
Relative risk reduction
- SMC
Seasonal malaria chemoprevention
- SNP
Single nucleotide polymorphism (also known as point mutation)
- SP
Sulfadoxine-pyrimethamine
- WHO
World Health Organization
- Chemoprevention
The use of anti-malarial medicines for prophylaxis and for preventive treatment. This review focuses on preventive treatment, and not on prophylaxis for visitors to endemic areas
- Prevalence
Prevalence of a given mutation or haplotype is defined here as the proportion of infected individuals in whom that marker or haplotype is detected, irrespective of whether other alleles or haplotypes (e.g., wild-type) are also present in the infection
- Quadruple mutant
Dhfr triple mutant plus dhps A437G in the same infection
- Quintuple mutant
Dhfr N51I/C59R/S108N and dhps A437G/K540E2 in the same P. falciparum infection. Some publications use “quintuple mutant” (or sextuple or septuple mutant) to refer to any dhfr/dhps haplotype containing any combination of five (or six or seven) mutations, but most use the definition applied here
- Sextuple mutant
Dhfr/dhps quintuple mutant plus dhps A581G in the same infection
- Triple mutant
Dhfr mutations N51I/C59R/S108N in the same infection
Authors' contributions
The author reviewed the literature and wrote the review.
Funding
This work was supported by the WHO Global Malaria Programme.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The author has no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World malaria report 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.WHO . World malaria report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 3.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO malaria terminology. Geneva: World Health Organization; 2019.
- 5.WHO . Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009. [Google Scholar]
- 6.Nguyen-Dinh P, Payne D. Pyrimethamine sensitivity in Plasmodium falciparum: determination in vitro by a modified 48-hour test. Bull World Health Organ. 1980;58:909–912. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Brobey RK, Horii T, Sims PF, Hyde JE. Utilization of exogenous folate in the human malaria parasite Plasmodium falciparum and its critical role in antifolate drug synergy. Mol Microbiol. 1999;32:1254–1262. doi: 10.1046/j.1365-2958.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 8.Plowe CV. Monitoring antimalarial drug resistance: making the most of the tools at hand. J Exp Biol. 2003;206:3745–3752. doi: 10.1242/jeb.00658. [DOI] [PubMed] [Google Scholar]
- 9.Laufer MK, Djimde AA, Plowe CV. Monitoring and deterring drug resistant malaria in the era of combination therapy. Am J Trop Med Hyg. 2007;77(Suppl 6):160–169. [PubMed] [Google Scholar]
- 10.Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowman AF, Morry MJ, Biggs BA, Cross GA, Foote SJ. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci USA. 1988;85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snewin VA, England SM, Sims PF, Hyde JE. Characterisation of the dihydrofolate reductase-thymidylate synthetase gene from human malaria parasites highly resistant to pyrimethamine. Gene. 1989;76:41–52. doi: 10.1016/0378-1119(89)90006-1. [DOI] [PubMed] [Google Scholar]
- 13.Zolg JW, Plitt JR, Chen G-X, Palmer S. Point mutations in the dihydrofolate reductase-thymidylate synthase gene as the molecular basis for pyrimethamine resistance in Plasmodium falciparum. Mol Biochem Parasitol. 1989;36:253–262. doi: 10.1016/0166-6851(89)90173-4. [DOI] [PubMed] [Google Scholar]
- 14.Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foote SJ, Galatis D, Cowman AF. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc Natl Acad Sci USA. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ippolito MM, Moser KA, Kabuya JB, Cunningham C, Juliano JJ. Antimalarial drug resistance and implications for the WHO Global Technical Strategy. Curr Epidemiol Rep. 2021;8:46–62. doi: 10.1007/s40471-021-00266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad MD, Rosenthal PJ. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis. 2019;19:e338–e351. doi: 10.1016/S1473-3099(19)30261-0. [DOI] [PubMed] [Google Scholar]
- 18.Plowe CV, Kublin JG, Doumbo OKP. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: epidemiology and role in clinical resistance to antifolates. Drug Resist Updat. 1998;1:389–396. doi: 10.1016/s1368-7646(98)80014-9. [DOI] [PubMed] [Google Scholar]
- 19.Djimde AA, Doumbo OK, Traore O, Guindo AB, Kayentao K, Diourte Y, et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–563. [PubMed] [Google Scholar]
- 20.Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Laufer MK, Djimde AA, Plowe CV. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am J Trop Med Hyg. 2007;77:160–169. [PubMed] [Google Scholar]
- 22.Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009;103(Suppl 1):S11–S14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Report of the Technical Consultation on Intermittent Preventive Treatment in Infants (IPTi) Geneva: World Health Organization; 2009. [Google Scholar]
- 25.Clyde DF, Shute GT. Resistance of Plasmodium falciparum in Tanganyika to pyrimethamine administered at weekly intervals. Trans R Soc Trop Med Hyg. 1957;51:505–513. doi: 10.1016/0035-9203(57)90039-1. [DOI] [PubMed] [Google Scholar]
- 26.Doumbo OK, Kayentao K, Djimde A, Cortese JF, Diourte Y, Konare A, et al. Rapid selection of Plasmodium falciparum dihydrofolate reductase mutants by pyrimethamine prophylaxis. J Infect Dis. 2000;182:993–996. doi: 10.1086/315787. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco MA, Schneider KA, Cheng Q, Munde EO, Ndege C, Onyango C, et al. Changes in the frequencies of Plasmodium falciparum dhps and dhfr drug-resistant mutations in children from Western Kenya from 2005 to 2018: the rise of Pfdhps S436H. Malar J. 2020;19:378. doi: 10.1186/s12936-020-03454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amimo F, Lambert B, Magit A, Sacarlal J, Hashizume M, Shibuya K. Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: a systematic analysis of national trends. BMJ Glob Health. 2020;5:e003217. doi: 10.1136/bmjgh-2020-003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 30.Cortese JF, Caraballo A, Contreras CE, Plowe CV. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J Infect Dis. 2002;186:999–1006. doi: 10.1086/342946. [DOI] [PubMed] [Google Scholar]
- 31.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 32.Mita T, Venkatesan M, Ohashi J, Culleton R, Takahashi N, Tsukahara T, et al. Limited geographical origin and global spread of sulfadoxine-resistant dhps alleles in Plasmodium falciparum populations. J Infect Dis. 2011;204:1980–1988. doi: 10.1093/infdis/jir664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alifrangis M, Nag S, Schousboe ML, Ishengoma D, Lusingu J, Pota H, et al. Independent origin of Plasmodium falciparum antifolate super-resistance, Uganda, Tanzania, and Ethiopia. Emerg Infect Dis. 2014;20:1280–1286. doi: 10.3201/eid2008.131897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Seidlein L, Greenwood BM. Mass administrations of antimalarial drugs. Trends Parasitol. 2003;19:452–460. doi: 10.1016/j.pt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Pongtavornpinyo W, Yeung S, Hastings IM, Dondorp AM, Day NP, White NJ. Spread of anti-malarial drug resistance: mathematical model with implications for ACT drug policies. Malar J. 2008;7:229. doi: 10.1186/1475-2875-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 37.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 38.Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, Madebe R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS ONE. 2009;4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JT, Mbewe B, Taylor SM, Luntamo M, Meshnick SR, Ashorn P. Increased prevalence of dhfr and dhps mutants at delivery in Malawian pregnant women receiving intermittent preventive treatment for malaria. Trop Med Int Health. 2013;18:175–178. doi: 10.1111/tmi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Hommerich L, Holmberg V, Von OC, et al. Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis. 2008;198:1545–1549. doi: 10.1086/592455. [DOI] [PubMed] [Google Scholar]
- 41.Iriemenam NC, Shah M, Gatei W, van Eijk AM, Ayisi J, Kariuki S, et al. Temporal trends of sulphadoxine-pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malar J. 2012;11:134. doi: 10.1186/1475-2875-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez C, Serra-Casas E, Scahill MD, Sanz S, Nhabomba A, Bardaji A, et al. HIV and placental infection modulate the appearance of drug-resistant Plasmodium falciparum in pregnant women who receive intermittent preventive treatment. Clin Infect Dis. 2011;52:41–48. doi: 10.1093/cid/ciq049. [DOI] [PubMed] [Google Scholar]
- 43.Hastings IM, Watkins WM. Tolerance is the key to understanding antimalarial drug resistance. Trends Parasitol. 2006;22:71–77. doi: 10.1016/j.pt.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruizendaal E, Tahita MC, Geskus RB, Versteeg I, Scott S, d'Alessandro U, et al. Increase in the prevalence of mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates collected from early to late pregnancy in Nanoro, Burkina Faso. Malar J. 2017;16:179. doi: 10.1186/s12936-017-1831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cisse M, Awandare GA, Soulama A, Tinto H, Hayette MP, Guiguemde RT. Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar J. 2017;16:38. doi: 10.1186/s12936-017-1695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington WE, Mutabingwa TK, Muehlenbachs A, Sorensen B, Bolla MC, Fried M, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci USA. 2009;106:9027–9032. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngondi JM, Ishengoma DS, Doctor SM, Thwai KL, Keeler C, Mkude S, et al. Surveillance for sulfadoxine-pyrimethamine resistant malaria parasites in the Lake and Southern Zones, Tanzania, using pooling and next-generation sequencing. Malar J. 2017;16:236. doi: 10.1186/s12936-017-1886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayebare P, Asua V, Conrad MD, Kajubi R, Kakuru A, Nankabirwa JI, et al. Associations between malaria-preventive regimens and Plasmodium falciparum drug resistance-mediating polymorphisms in Ugandan pregnant women. Antimicrob Agents Chemother. 2020;64:e01047–e1120. doi: 10.1128/AAC.01047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mbonye AK, Birungi J, Yanow SK, Shokoples S, Malamba S, Alifrangis M, et al. Prevalence of Plasmodium falciparum resistance markers to sulfadoxine-pyrimethamine among pregnant women receiving intermittent preventive treatment for malaria in Uganda. Antimicrob Agents Chemother. 2015;59:5475–5482. doi: 10.1128/AAC.00507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tornyigah B, Coppee R, Houze P, Kusi KA, Adu B, Quakyi I, et al. Effect of drug pressure on promoting the emergence of antimalarial-resistant parasites among pregnant women in Ghana. Antimicrob Agents Chemother. 2020;64:e02029–e2119. doi: 10.1128/AAC.02029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fagbemi KA, Adebusuyi SA, Nderu D, Adedokun SA, Pallerla SR, Amoo AOJ, et al. Analysis of sulphadoxine-pyrimethamine resistance-associated mutations in Plasmodium falciparum isolates obtained from asymptomatic pregnant women in Ogun State, Southwest Nigeria. Infect Genet Evol. 2020;85:104503. doi: 10.1016/j.meegid.2020.104503. [DOI] [PubMed] [Google Scholar]
- 53.Osarfo J, Tagbor H, Magnussen P, Alifrangis M. Molecular markers of Plasmodium falciparum drug resistance in parasitemic pregnant women in the middle forest belt of Ghana. Am J Trop Med Hyg. 2018;98:1714–1717. doi: 10.4269/ajtmh.18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor SM, Levitt B, Freedman B, Madanitsa M, Thwai KL, Kalilani-Phiri L, et al. Interactions between antenatal sulfadoxine-pyrimethamine, drug-resistant Plasmodium falciparum parasites, and delivery outcomes in Malawi. J Infect Dis. 2020;222:661–669. doi: 10.1093/infdis/jiaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO. Guidelines for malaria 2021. Geneva, World Health Organization, 2021. https://www.who.int/publications/i/item/guidelines-for-malaria
- 56.WHO. Recommendations on the use of sulfadoxine-pyrimethamine for intermittent preventive treatment during pregnancy in areas of moderate to high resistance to sulfadoxinepyrimethamine in the African region. Geneva, World Health Organization, 2005.
- 57.Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc Natl Acad Sci USA. 2007;104:19914–19919. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis. 2011;53:224–230. doi: 10.1093/cid/cir376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellman R, Maxwell C, Finch R, Shayo D. Malaria and anaemia at different altitudes in the Muheza district of Tanzania: childhood morbidity in relation to level of exposure to infection. Ann Trop Med Parasitol. 1998;92:741–753. doi: 10.1080/00034989858989. [DOI] [PubMed] [Google Scholar]
- 61.Minja DT, Schmiegelow C, Mmbando B, Bostrom S, Oesterholt M, Magistrado P, et al. Plasmodium falciparum mutant haplotype infection during pregnancy associated with reduced birthweight. Tanzania Emerg Infect Dis. 2013;19:1446–1454. doi: 10.3201/eid1909.130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor SM, Antonia AL, Chaluluka E, Mwapasa V, Feng G, Molyneux ME, et al. Antenatal receipt of sulfadoxine-pyrimethamine does not exacerbate pregnancy-associated malaria despite the expansion of drug-resistant Plasmodium falciparum: clinical outcomes from the QuEERPAM study. Clin Infect Dis. 2012;55:42–50. doi: 10.1093/cid/cis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. Policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva, World Health Organization, 2013.
- 65.Gutman J, Kalilani L, Taylor S, Zhou Z, Wiegand RE, Thwai KL, et al. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine-pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis. 2015;211:1997–2005. doi: 10.1093/infdis/jiu836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desai M, Gutman J, Taylor SM, Wiegand RE, Khairallah C, Kayentao K, et al. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis. 2016;2:323–333. doi: 10.1093/cid/civ881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desai M, Gutman J, L'Lanziva A, Otieno K, Juma E, Kariuki S, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kajubi R, Ochieng T, Kakuru A, Jagannathan P, Nakalembe M, Ruel T, et al. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet. 2019;393:1428–1439. doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 69.Kakuru A, Jagannathan P, Kajubi R, Ochieng T, Ochokoru H, Nakalembe M, et al. Impact of intermittent preventive treatment of malaria in pregnancy with dihydroartemisinin-piperaquine versus sulfadoxine-pyrimethamine on the incidence of malaria in infancy: a randomized controlled trial. BMC Med. 2020;18:207. doi: 10.1186/s12916-020-01675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chico RM, Cano J, Ariti C, Collier TJ, Chandramohan D, Roper C, et al. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health. 2015;20:1621–1633. doi: 10.1111/tmi.12595. [DOI] [PubMed] [Google Scholar]
- 71.van Eijk AM, Larsen DA, Kayentao K, Koshy G, Slaughter DEC, Roper C, et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:546–556. doi: 10.1016/S1473-3099(18)30732-1. [DOI] [PubMed] [Google Scholar]
- 72.Braun V, Rempis E, Schnack A, Decker S, Rubaihayo J, Tumwesigye NM, et al. Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J. 2015;14:372. doi: 10.1186/s12936-015-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Likwela JL, D'Alessandro U, Lokwa BL, Meuris S, Dramaix MW. Sulfadoxine-pyrimethamine resistance and intermittent preventive treatment during pregnancy: a retrospective analysis of birth weight data in the Democratic Republic of Congo (DRC) Trop Med Int Health. 2012;17:322–329. doi: 10.1111/j.1365-3156.2011.02935.x. [DOI] [PubMed] [Google Scholar]
- 74.Ndyomugyenyi R, Clarke SE, Hutchison CL, Hansen KS, Magnussen P. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually-randomised placebo-controlled trial using intermittent preventive treatment and insecticide-treated nets in the Kabale Highlands, southwestern Uganda. Trans R Soc Trop Med Hyg. 2011;105:607–616. doi: 10.1016/j.trstmh.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okell LC, Griffin JT, Roper C. Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa. Sci Rep. 2017;7:7389. doi: 10.1038/s41598-017-06708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor SM, Antonia AL, Parobek CM, Juliano JJ, Janko M, Emch M, et al. Plasmodium falciparum sulfadoxine resistance is geographically and genetically clustered within the DR Congo. Sci Rep. 2013;3:1165. doi: 10.1038/srep01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch C, Pearce R, Pota H, Cox J, Abeku TA, Rwakimari J, et al. Emergence of a dhfr mutation conferring high-level drug resistance in Plasmodium falciparum populations from southwest Uganda. J Infect Dis. 2008;197:1598–1604. doi: 10.1086/587845. [DOI] [PubMed] [Google Scholar]
- 78.Marks F, von Kalckreuth V, Kobbe R, Adjei S, Adjei O, Horstmann RD, et al. Parasitological rebound effect and emergence of pyrimethamine resistance in Plasmodium falciparum after single-dose sulfadoxine-pyrimethamine. J Infect Dis. 2005;192:1962–1965. doi: 10.1086/497698. [DOI] [PubMed] [Google Scholar]
- 79.Dicko A, Sagara I, Djimde AA, Toure SO, Traore M, Dama S, et al. Molecular markers of resistance to sulphadoxine-pyrimethamine one year after implementation of intermittent preventive treatment of malaria in infants in Mali. Malar J. 2010;9:9. doi: 10.1186/1475-2875-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearce RJ, Ord R, Kaur H, Lupala C, Schellenberg J, Shirima K, et al. A community-randomized evaluation of the effect of intermittent preventive treatment in infants on antimalarial drug resistance in southern Tanzania. J Infect Dis. 2013;207:848–859. doi: 10.1093/infdis/jis742. [DOI] [PubMed] [Google Scholar]
- 81.Faye B, Ndiaye M, Ndiaye JL, Annie A, Tine RC, Lo AC, et al. Prevalence of molecular markers of Plasmodium falciparum resistance to sulfadoxine-pyrimethamine during the intermittent preventive treatment in infants coupled with the expanded program immunization in Senegal. Parasitol Res. 2011;109:133–138. doi: 10.1007/s00436-010-2236-9. [DOI] [PubMed] [Google Scholar]
- 82.Institute of Medicine . Assessment of the role of intermittent preventive treatment for malaria in infants: letter report. Washington: DC. National Academy of Sciences; 2008. [Google Scholar]
- 83.O'Meara WP, Smith DL, McKenzie FE. Potential impact of intermittent preventive treatment (IPT) on spread of drug-resistant malaria. PLoS Med. 2006;3:e141. doi: 10.1371/journal.pmed.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexander N, Sutherland C, Roper C, Cissé B, Schellenberg D. Modelling the impact of intermittent preventive treatment for malaria on selection pressure for drug resistance. Malar J. 2007;6:9. doi: 10.1186/1475-2875-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobbe R, Kreuzberg C, Adjei S, Thompson B, Langefeld I, Thompson PA, et al. A randomized controlled trial of extended intermittent preventive antimalarial treatment in infants. Clin Infect Dis. 2007;45:16–25. doi: 10.1086/518575. [DOI] [PubMed] [Google Scholar]
- 86.Mockenhaupt FP, Teun BJ, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, et al. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop Med Int Health. 2005;10:901–908. doi: 10.1111/j.1365-3156.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 87.Mayor A, Serra-Casas E, Sanz S, Aponte JJ, Macete E, Mandomando I, et al. Molecular markers of resistance to sulfadoxine-pyrimethamine during intermittent preventive treatment for malaria in Mozambican infants. J Infect Dis. 2008;197:1737–1742. doi: 10.1086/588144. [DOI] [PubMed] [Google Scholar]
- 88.Pearce RJ, Drakeley C, Chandramohan D, Mosha F, Roper C. Molecular determination of point mutation haplotypes in the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum in three districts of northern Tanzania. Antimicrob Agents Chemother. 2003;47:1347–1354. doi: 10.1128/AAC.47.4.1347-1354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gosling RD, Gesase S, Mosha JF, Carneiro I, Hashim R, Lemnge M, et al. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1521–1532. doi: 10.1016/S0140-6736(09)60997-1. [DOI] [PubMed] [Google Scholar]
- 90.Griffin JT, Cairns M, Ghani AC, Roper C, Schellenberg D, Carneiro I, et al. Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. PLoS ONE. 2010;5:e12618. doi: 10.1371/journal.pone.0012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bretscher MT, Dahal P, Griffin J, Stepniewska K, Bassat Q, Baudin E, et al. The duration of chemoprophylaxis against malaria after treatment with artesunate-amodiaquine and artemether-lumefantrine and the effects of pfmdr1 86Y and pfcrt 76T: a meta-analysis of individual patient data. BMC Med. 2020;18:47. doi: 10.1186/s12916-020-1494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naidoo I, Roper C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology. 2011;138:1469–1479. doi: 10.1017/S0031182011000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ross A, Maire N, Sicuri E, Smith T, Conteh L. Determinants of the cost-effectiveness of intermittent preventive treatment for malaria in infants and children. PLoS ONE. 2011;6:e18391. doi: 10.1371/journal.pone.0018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schellenberg JR, Maokola W, Shirima K, Manzi F, Mrisho M, Mushi A, et al. Cluster-randomized study of intermittent preventive treatment for malaria in infants (IPTi) in southern Tanzania: evaluation of impact on survival. Malar J. 2011;10:387. doi: 10.1186/1475-2875-10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esu EB, Oringanje C, Meremikwu MM. Intermittent preventive treatment for malaria in infants. Cochrane Database Syst Rev. 2019;12:CD011525. doi: 10.1002/14651858.CD011525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Konate AT, Yaro JB, Ouedraogo AZ, Diarra A, Gansane A, Soulama I, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8:e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8:e1000407. doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cissé B, Sokhna C, Boulanger D, Milet J, el Bâ H, Richardson K, et al. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 99.Somé AF, Zongo I, Compaoré YD, Sakandé S, Nosten F, Ouédraogo JB, et al. Selection of drug resistance-mediating Plasmodium falciparum genetic polymorphisms by seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother. 2014;58:3660–3665. doi: 10.1128/AAC.02406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ndiaye M, Tine R, Faye B, Ndiaye JL, Diouf I, Lo AC, et al. Selection of antimalarial drug resistance after intermittent preventive treatment of infants and children (IPTi/c) in Senegal. C R Biol. 2013;336:295–300. doi: 10.1016/j.crvi.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Dieng CC, Gonzalez L, Pestana K, Dhikrullahi SB, Amoah LE, Afrane YA, et al. Contrasting asymptomatic and drug resistance gene prevalence of Plasmodium falciparum in Ghana. Implications on seasonal malaria chemoprevention. Genes (Basel). 2019;10:538. doi: 10.3390/genes10070538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diawara F, Steinhardt LC, Mahamar A, Traore T, Kone DT, Diawara H, et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita, Mali. Malar J. 2017;16:325. doi: 10.1186/s12936-017-1974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Partnership A-S. Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. Lancet. 2020;396:1829–1840. doi: 10.1016/S0140-6736(20)32227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naidoo I, Roper C. Spatial distribution of dhfr and dhps mutations in Africa. Trends Parasitol. 2013;12:249. [Google Scholar]
- 105.Wilson AL. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS ONE. 2011;6:e16976. doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mbacham WF, Evehe MS, Netongo PM, Ateh IA, Mimche PN, Ajua A, et al. Efficacy of amodiaquine, sulphadoxine-pyrimethamine and their combination for the treatment of uncomplicated Plasmodium falciparum malaria in children in Cameroon at the time of policy change to artemisinin-based combination therapy. Malar J. 2010;9:34. doi: 10.1186/1475-2875-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Newby G, Hwang J, Koita K, Chen I, Greenwood B, von Seidlein L, et al. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg. 2015;93:125–134. doi: 10.4269/ajtmh.14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Molineaux L, Gramiccia G. The Garki Project. Research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. World Health Organization, Geneva; 1980.
- 109.malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLoS Med. 2011;8:e1000402. [DOI] [PMC free article] [PubMed]
- 110.Pinotti M. A new method of malaria prophylaxis: the addition of an antimalarial drug to cooking salt used in daily preparation of food. Rev Brasil Malariol. 1953;10:241–246. [Google Scholar]
- 111.Eisele TP. Mass drug administration can be a valuable addition to the malaria elimination toolbox. Malar J. 2019;18:281. doi: 10.1186/s12936-019-2906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lwin KM, Imwong M, Suangkanarat P, Jeeyapant A, Vihokhern B, Wongsaen K, et al. Elimination of Plasmodium falciparum in an area of multi-drug resistance. Malar J. 2015;14:319. doi: 10.1186/s12936-015-0838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eisele TP, Bennett A, Silumbe K, Finn TP, Chalwe V, Kamuliwo M, et al. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in southern province Zambia: a cluster-randomized controlled trial. J Infect Dis. 2016;214:1831–1839. doi: 10.1093/infdis/jiw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wernsdorfer WH, Payne D. The dynamics of drug resistance in Plasmodium falciparum. Pharmacol Ther. 1991;50:95–121. doi: 10.1016/0163-7258(91)90074-v. [DOI] [PubMed] [Google Scholar]
- 115.White NJ. Does antimalarial mass drug administration increase or decrease the risk of resistance? Lancet Infect Dis. 2017;17:e15–e20. doi: 10.1016/S1473-3099(16)30269-9. [DOI] [PubMed] [Google Scholar]
- 116.Landier J, Kajeechiwa L, Thwin MM, Parker DM, Chaumeau V, Wiladphaingern J, et al. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of Eastern Myanmar. Wellcome Open Res. 2017;2:81. doi: 10.12688/wellcomeopenres.12240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.von Seidlein L, Peto TJ, Landier J, Nguyen TN, Tripura R, Phommasone KP, et al. The impact of targeted malaria elimination with mass drug administrations on falciparum malaria in Southeast Asia: a cluster randomised trial. PLoS Med. 2019;16:e1002745. doi: 10.1371/journal.pmed.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta H, Galatas B, Chidimatembue A, Huijben S, Cistero P, Matambisso G, et al. Effect of mass dihydroartemisinin-piperaquine administration in southern Mozambique on the carriage of molecular markers of antimalarial resistance. PLoS ONE. 2020;15:e0240174. doi: 10.1371/journal.pone.0240174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kay K, Hastings IM. Measuring windows of selection for anti-malarial drug treatments. Malar J. 2015;14:292. doi: 10.1186/s12936-015-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maude RJ, Nguon C, Dondorp AM, White LJ, White NJ. The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J. 2014;13:380. doi: 10.1186/1475-2875-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garfield RM, Vermund SH. Changes in malaria incidence after mass drug administration in Nicaragua. Lancet. 1983;2:500–503. doi: 10.1016/s0140-6736(83)90523-8. [DOI] [PubMed] [Google Scholar]
- 122.Kaehler N, Adhikari B, Cheah PY, Day NPJ, Paris DH, Tanner M, et al. The promise, problems and pitfalls of mass drug administration for malaria elimination: a qualitative study with scientists and policymakers. Int Health. 2019;11:166–176. doi: 10.1093/inthealth/ihy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohee LM, Opondo C, Clarke SE, Halliday KE, Cano J, Shipper AG, et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Health. 2020;8:e1499–e1511. doi: 10.1016/S2214-109X(20)30325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nankabirwa JI, Conrad MD, Legac J, Tukwasibwe S, Tumwebaze P, Wandera B, et al. Intermittent preventive treatment with dihydroartemisinin-piperaquine in Ugandan schoolchildren selects for Plasmodium falciparum transporter polymorphisms that modify drug sensitivity. Antimicrob Agents Chemother. 2016;60:5649–5654. doi: 10.1128/AAC.00920-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Djimde A, Plowe CV, Diop S, Dicko A, Wellems TE, Doumbo O. Use of antimalarial drugs in Mali: policy versus reality. Am J Trop Med Hyg. 1998;59:376–379. doi: 10.4269/ajtmh.1998.59.376. [DOI] [PubMed] [Google Scholar]
- 126.Peel SA, Bright P, Yount B, Handy J, Baric RS. A strong association between mefloquine and halofantrine resistance and amplification, overexpression, and mutation in the P-glycoprotein gene homolog (pfmdr) of Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1994;51:648–658. doi: 10.4269/ajtmh.1994.51.648. [DOI] [PubMed] [Google Scholar]
- 127.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sidhu ABS, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huijben S, Macete E, Mombo-Ngoma G, Ramharter M, Kariuki S, Desai M, et al. Counter-selection of antimalarial resistance polymorphisms by intermittent preventive treatment in pregnancy. J Infect Dis. 2020;221:293–303. doi: 10.1093/infdis/jiz451. [DOI] [PubMed] [Google Scholar]
- 132.Kublin JG, Cortese JF, Njunju EM, RA GM, Wirima JJ, Kazembe PN, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–5. [DOI] [PubMed]
- 133.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 134.Divala TH, Mungwira RG, Mawindo PM, Nyirenda OM, Kanjala M, Ndaferankhande M, et al. Chloroquine as weekly chemoprophylaxis or intermittent treatment to prevent malaria in pregnancy in Malawi: a randomised controlled trial. Lancet Infect Dis. 2018;18:1097–1107. doi: 10.1016/S1473-3099(18)30415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mohammed A, Ndaro A, Kalinga A, Manjurano A, Mosha JF, Mosha DF, et al. Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malar J. 2013;2:415. doi: 10.1186/1475-2875-12-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mwanza S, Joshi S, Nambozi M, Chileshe J, Malunga P, Kabuya JB, et al. The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J. 2016;15:584. doi: 10.1186/s12936-016-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nosten F, ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster HK, Edstein M, et al. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991;337:1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- 138.van der Pluijm RW, Tripura R, Hoglund RM, Pyae Phyo A, Lek D, Islam AU, et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395:1345–1360. doi: 10.1016/S0140-6736(20)30552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–808. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci USA. 2008;105:14216–14221. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dini S, Zaloumis S, Cao P, Price RN, Fowkes FJI, van der Pluijm RW, et al. Investigating the efficacy of triple artemisinin-based combination therapies for treating Plasmodium falciparum malaria patients using mathematical modeling. Antimicrob Agents Chemother. 2018;62:e01068–e1118. doi: 10.1128/AAC.01068-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Pluijm RW, Amaratunga C, Dhorda M, Dondorp AM. Triple Artemisinin-based combination therapies for malaria - a new paradigm? Trends Parasitol. 2021;37:15–24. doi: 10.1016/j.pt.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 143.Gonzalez R, Pons-Duran C, Piqueras M, Aponte JJ, ter Kuile FO, Menendez C. Mefloquine for preventing malaria in pregnant women. Cochrane Database Syst Rev. 2018;3:CD011444. doi: 10.1002/14651858.CD011444.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chico RM, Pittrof R, Greenwood B, Chandramohan D. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar J. 2008;7:255. doi: 10.1186/1475-2875-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kimani J, Phiri K, Kamiza S, Duparc S, Ayoub A, Rojo R, et al. Efficacy and safety of azithromycin-chloroquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of Plasmodium falciparum malaria infection in pregnant women in Africa: an open-label, randomized trial. PLoS ONE. 2016;11:e0157045. doi: 10.1371/journal.pone.0157045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gaillard T, Boxberger M, Madamet M, Pradines B. Has doxycycline, in combination with anti-malarial drugs, a role to play in intermittent preventive treatment of Plasmodium falciparum malaria infection in pregnant women in Africa? Malar J. 2018;17:469. doi: 10.1186/s12936-018-2621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Denoeud-Ndam L, Zannou DM, Fourcade C, Taron-Brocard C, Porcher R, Atadokpede F, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. J Acquir Immune Defic Syndr. 2014;65:198–206. doi: 10.1097/QAI.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 148.Wallender E, Zhang N, Conrad M, Kakuru A, Muhindo M, Tumwebaze P, et al. Modeling prevention of malaria and selection of drug resistance with different dosing schedules of dihydroartemisinin-piperaquine preventive therapy during pregnancy in Uganda. Antimicrob Agents Chemother. 2019;63:e01393–e1418. doi: 10.1128/AAC.01393-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS ONE. 2010;5:e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tagbor H, Cairns M, Bojang K, Coulibaly SO, Kayentao K, Williams J, et al. A non-inferiority, individually randomized trial of intermittent screening and treatment versus intermittent preventive treatment in the control of malaria in pregnancy. PLoS ONE. 2015;10:e0132247. doi: 10.1371/journal.pone.0132247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Esu E, Berens-Riha N, Pritsch M, Nwachuku N, Loescher T, Meremikwu M. Intermittent screening and treatment with artemether-lumefantrine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in pregnancy: a facility-based, open-label, non-inferiority trial in Nigeria. Malar J. 2018;17:251. doi: 10.1186/s12936-018-2394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peters W. The problem of drug resistance in malaria. Parasitology. 1985;90:705–715. doi: 10.1017/s003118200005232x. [DOI] [PubMed] [Google Scholar]
- 153.Nyunt MM, Plowe CV. Pharmacologic advances in the global control and treatment of malaria. Combination therapy and resistance. Clin Pharmacol Ther. 2007;82:601–605. doi: 10.1038/sj.clpt.6100361. [DOI] [PubMed] [Google Scholar]
- 154.Hastings I. How artemisinin-containing combination therapies slow the spread of antimalarial drug resistance. Trends Parasitol. 2011;27:67–72. doi: 10.1016/j.pt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 155.WHO . Minutes of the Drug Resistance and Containment Technical Expert Group (TEG) Geneva: World Health Organization; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.