Abstract
Objective: We assessed the efficacy and safety of transarterial chemoembolization (TACE) in combination with lenvatinib plus programmed death receptor-1 (PD-1) signaling inhibitors (camrelizumab or sintilimab) in unresectable hepatocellular carcinoma (uHCC). Methods: In this retrospective study, patients with uHCC were pretreated with lenvatinib for 1 to 2 weeks before TACE. Camrelizumab or sintilimab were initially administered intravenously in 1 week after TACE of a 21-day cycle. Primary objectives were objective response rate (ORR) and disease control rate (DCR) by modified Response Evaluation Criteria in Solid Tumors (mRECIST). The secondary endpoints included the progression-free survival (PFS), overall survival (OS), and toxicity. Results: Between March 5, 2019 and February 30, 2021, 53 patients were screened for eligibility. At data cutoff, 35.8% of patients remained on treatment. Median follow-up was 15.4 months. Confirmed ORR in the 51 evaluable patients was 54.9% (95% CI 41.4%-67.7%). DCR was 84.3% (95% CI 72.0%-91.8%). Median PFS was 8.5 months (95% CI 6.4 to 10.6 months). The median OS was not estimable. Grade ≥3 treatment-related adverse events occurred in 32.1% of patients. No new safety signals were identified. Conclusion: TACE in combination with lenvatinib plus anti-PD-1 inhibitors may have promising antitumor activity in uHCC. Toxicities were manageable, with no unexpected safety signals.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, lenvatinib, anti-PD-1inhibitors, efficacy
Introduction
Liver cancer is estimated to be the sixth most prevalent cancer worldwide and the third leading cause of cancer-related death. 1 Hepatocellular carcinoma (HCC) is the major histological subtype, accounting for 75% to 85% of liver cancers. Despite advances in early detection, most patients present with advanced unresectable disease, and have a poor prognosis.2,3
Transarterial chemoembolization (TACE) is the guideline-recommended therapy for intermediate and advanced stage HCC. 4 However, TACE is not beneficial for all of patients and also there is a high recurrence after TACE. 5 A preclinical study showed that TACE in combination with antiangiogenic therapy could reduce tumor volume and vessel density when compared with TACE alone. 6 TACTICS clinical trial demonstrated that TACE plus sorafenib, a multi-kinase inhibitor of angiogenesis, significantly improved progression-free survival (PFS) over TACE alone in patients with unresectable HCC (uHCC). 7
Lenvatinib, another multi-kinase inhibitor, was approved for first-line treatment of uHCC based on the REFLECT study. 8 Lenvatinib could also prolong the PFS of patients with intermediate-stage HCC refractory to TACE. 9 Immune checkpoint inhibitors have shown promising results in patients with advanced HCC. Anti-PD-1 inhibitors, pembrolizumab and nivolumab have been approved for the therapy of HCC.10,11 Basic research studies show that lenvatinib and anti-PD-1 antibodies have synergistic effects.12,13 A phase Ib trial of the combination of lenvatinib and pembrolizumab as first-line therapy in patients with uHCC demonstrated durable objective radiographic responses by modified Response Evaluation Criteria in Solid Tumors (mRECIST) in 46%. 14
On the basis of these researches, we presumed that TACE in combination with lenvatinib plus anti-PD-1 inhibitors may achieve greater therapeutic benefits for uHCC patients. However, the efficacy and safety of this combination strategy have not been evaluated. So we conducted the study to assess the efficacy and safety of TACE combined with lenvatinib plus anti-PD-1 inhibitors for uHCC.
Methods
Ethical Statement
The trial was carried out in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki, as well as local laws. Documented approval was obtained from the institutional review board of Beijing Ditan Hospital, Capital Medical University (Grant No. Jdlkz (2021) No. (003)-02). All participants provided written informed consent before therapy. We have de-identified all patient details to protect the privacy of patients.
Study Design and Patients
This is a single-center, retrospective study of TACE in combination with lenvatinib plus anti-PD-1 inhibitors camrelizumab (Jiangsu Hengrui Pharmaceuticals Co., Ltd) or sintilimab (Innovent Biologics, Inc.) in patients with uHCC. Camrelizumab and sintilimab were used for clinical study. The diagnosis of HCC was predominantly based on the image analysis using dynamic enhanced CT or MRI. Eligible patients were aged ≥18 years, unresectable or metastatic advanced HCC, were classified as Barcelona Clinic Liver Cancer stage B or C, were treatment-naive or had progressed on or were intolerant to previous therapy, had at least one measurable lesion as defined by modified Response Evaluation Criteria in Solid Tumors (mRECIST), had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had a predicted life expectancy of greater than 12 weeks, had adequate organ function, were Child-Pugh class A or B7. Patients with chronic infections with hepatitis B virus (HBV) or hepatitis C virus (HCV) were allowed to enroll, but were required to start or continue a full course of standardized antiviral therapy. Patients were excluded if tumors with 70% or higher liver occupation, other active malignancies, symptomatic ascites, and gastrointestinal bleeding in the past 30 days. The patients were selected selectively according to above criteria.
Transarterial Chemoembolization (TACE)
TACE was performed on-demand according to the condition of the tumor. First, the portal vein patency and liver blood supply were confirmed. The participants underwent distal superselective 5-F catheterization of their tumor-feeding hepatic arteries with Embosphere microspheres, lipiodol, and epirubicin. A mixture of epirubicin (50 mg) (Pharmorubicin; Pfizer, Wuxi, China) and lipiodol (5-20 ml) (Lipiodol Ultra-Fluide; André Guerbet Laboratories, AulnaySous-Bois) was prepared for TACE. Absorbable embosphere microspheres (300-500 mm; Biosphere Medical Inc.) were used for embolization. TACE was repeated at an interval of 8 or 12 weeks for participants based on the condition of the patient and the tumor.
Lenvatinib and Anti-PD-1 Inhibitors
Patients received lenvatinib (bodyweight ≥60 kg, 12 mg; <60 kg, 8 mg) once daily for 1 to 2 weeks before TACE. Anti-PD-1 inhibitors (camrelizumab or sintilimab) 200 mg were initially administered intravenously in 1 week after TACE of a 21-day cycle based on the condition of the patient. Lenvatinib was interrupted on the day of TACE and several days after each session of TACE based on liver function and the judgment of the physician.
Treatment was continued until disease progression, presence unacceptable toxicity, or withdrawal of consent. According to the guidelines for administration of lenvatinib, when a patient develops grade ≥3 severe adverse events (AEs) or any unacceptable grade 2 drug related AEs, the drug dose should be reduced or the treatment interrupted until the symptom resolved to either grade 1 or 2.
Outcomes
The primary end points were objective response rate (ORR, the proportion of patients who achieved complete response [CR] or partial response [PR]) and disease control rate (DCR, the proportion of patients who achieved CR, PR, or stable disease as their best overall response). Secondary end points included PFS, overall survival (OS) and toxicity. Duration of response (DoR) was analyzed for patients with confirmed CR or PR. Tumor assessment scans were performed every 6 weeks until week 24 and then every 9 weeks. The treatment response was evaluated in accordance with mRECIST. Safety assessments consisted of the monitoring and recording of AEs according to Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v 4.03). The reporting of this study conforms to STROBE guidelines. 15
Statistical Analysis
The differences in the clinical characteristics between 2 groups were assessed by Student t-test and Chi-square test. PFS and OS were estimated using the Kaplan-Meier method and treatment groups were compared from the log-rank test (P < .05). All statistical analyses were performed using SPSS (version 25.0, SPSS Inc.).
Results
Patients’ Clinical Characteristics
Overall, 53 patients were enrolled in the study. At the data cutoff date (June 30, 2021), 19 patients (35.8%) were still receiving treatment; 34 patients (64.2%) had discontinued treatment; and 36 of the 53 patients remained in survival follow-up.
Median patient age was 56.9 years (range, 37-75 years). At baseline, 64.2% and 35.8% of patients had ECOG PS of 0 and 1, respectively. BCLC stage B was noted in 43.4% of patients and stage C in 56.6%. Child-Pugh scores of 5, 6, and 7 were reported for 35.8%, 28.3%, and 35.8% of patients, respectively. Proportions of patients with portal vein invasion and extrahepatic metastasis at baseline were 47.2% and 52.5%, respectively. Etiology of HCC included hepatitis B (84.9%), hepatitis C (9.4%), and alcohol (5.7%); and 34.0% of patients had an alpha fetoprotein level ≥400 ng/mL. About 24 patients (45.3%) had a history of treatment with other tyrosine kinase inhibitors, and 33 patients (62.3%) had accepted TACE treatment previously (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients.
| Characteristics | N (%) or median (range) |
|---|---|
| Total | 53 (100%) |
| Age (mean ± SD, years) | 56.9 years (range, 37-75 years) |
| Lower age (≤60 years) | 31 (58.5%) |
| Higher age (>60 years) | 22 (41.5%) |
| Gender | |
| Male | 45 (84.9%) |
| Female | 8 (15.1%) |
| Bodyweight, kg | |
| <60 | 20 (37.7%) |
| ≥60 | 33 (62.3%) |
| ECOG PS | |
| 0 | 34 (64.2%) |
| 1 | 19 (35.8%) |
| AFP (ng/ml) | |
| Low (<400) | 35 (66.0%) |
| High (≥400) | 18 (34.0%) |
| Liver cirrhosis | |
| No | 45 (84.9%) |
| Yes | 8 (15.1%) |
| Child-pugh score | |
| 5 | 19 (35.8%) |
| 6 | 15 (28.3%) |
| 7 | 19 (35.8%) |
| Etiology | |
| HBV | 45 (84.9%) |
| HCV | 5 (9.4%) |
| Alcohol | 3 (5.7%) |
| Vascular invasion | |
| No | 28 (52.8%) |
| Yes | 25 (47.2%) |
| Extrahepatic metastasis | |
| Lung | 10 (18.9%) |
| Lymph node | 21 (39.6%) |
| Bone | 6 (11.3%) |
| Other | 5 (9.4%) |
| BCLC stage | |
| B | 23 (43.4%) |
| C | 30 (56.6%) |
| Prior therapies | |
| Surgery | 9 (17.0%) |
| Ablation | 29 (54.7%) |
| TACE | 33 (62.3%) |
| TKIs | 24 (45.3%) |
| PD-1 inhibitors | |
| Camrelizumab | 25 (47.2%) |
| Sintilimab | 28 (52.8%) |
Study Drug and TACE Exposure
At the time of data cutoff, median duration of lenvatinib was 6.5 months (range, 0.7-22.4 months). Median number of anti-PD-1 inhibitors (camrelizumab or sintilimab) was 8 cycles (range, 2-21 cycles). The primary reason for treatment discontinuation was disease progression. During the study, 43 patients were treated with TACE for only once, 10 patients were treated with repeated TACE (8 patients for twice, 1 patient for 3 times and 1 patient for 5 times).
Clinical Efficacy
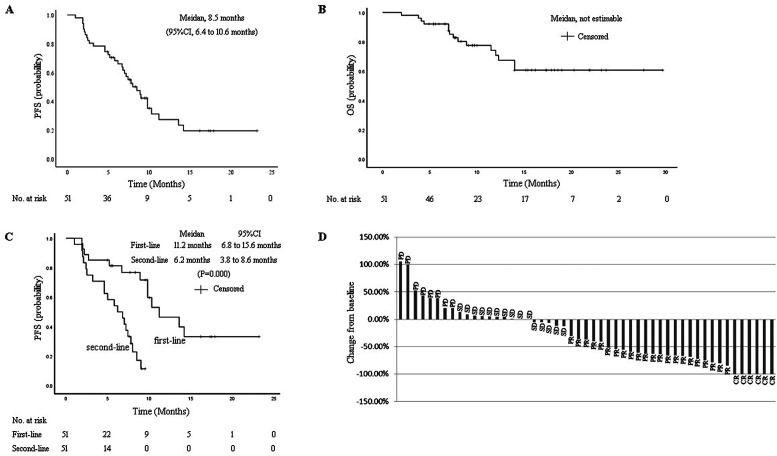
Efficacy data according to investigator assessment by mRECIST are summarized in Table 2. Median duration of follow-up was 15.4 months (95% CI, 9.5-17.9 months). In the 51 evaluable patients, CRs were observed in 6 patients (10.5%), while 22 (38.6%) had a PR. ORR was 54.9% (95% CI, 41.4%-67.7%). DCR was 84.3% (95% CI, 72.0%-91.8%). ORRs were consistent across various subgroups, including those with poor prognostic features, such as ECOG PS of 1, portal vein invasion, high alpha fetoprotein level, second-line treatment, and BCLC stage C. Median PFS was 8.5 months (95% CI, 6.4-10.6 months) (Figure 1A). Median OS was not estimable (NE) (Figure 1B). The median PFS for the patients of first-line therapy was 11.2 months (95% CI, 6.8-15.6 months), which is longer than that of second-line therapy (6.2 months, 95% CI, 3.8-8.6 months, P = .000) (Figure 1C). Reductions in tumor size were reported in 64.7% (33 of 51) of evaluable patients by mRECIST (Figure 1D), and the reductions seemed to be durable. Median DoR for confirmed responders was 6.5 months (95% CI, 5.2-13.1 months).
Table 2.
Response Rates According to the Modified Response Evaluation Criteria in Solid Tumors.
| N (%) | |
|---|---|
| Best response | |
| Complete response (CR) | 6 (11.3%) |
| Partial response (PR) | 22 (41.5%) |
| Stable disease (SD) | 15 (28.3%) |
| Progressive disease (PD) | 8 (15.1%) |
| Not evaluable | 2 (3.8%) |
| ORR (CR + PR) | 54.9% (95% CI, 41.4%-67.7%) |
| DCR (CR + PR + SD) | 84.3% (95% CI, 72.0%-91.8%) |
| Median progression-free survival | 8.5 months (95% CI, 6.4-10.6 months). |
| Median duration of response | 6.5 months (95% CI, 5.2-13.1 months) |
Abbreviations: ORR, objective response rate; DCR, disease control rate.
Figure 1.
Kaplan-Meier estimates of (A) progression-free survival (PFS) by mRECIST, (B) overall survival (OS) by mRECIST, (C) progression-free survival (PFS) in the first-line and second-line therapy groups, (D) percentage change from baseline in sums of diameters of target lesions by mRECIST.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; mRECIST, modified Response Evaluation Criteria in Solid Tumors.
In the camrelizumb treatment cohort, the ORR assessed was 54.2% (95% CI 35.8%-72.1%), DCR was 72.1% (95% CI, 64.1%-93.3%). In the sintilimab cohort, the ORR was 55.6% (95% CI, 37.3%-72.4%), DCR was 72.4% (95% CI, 67.5%-94.1%). The median PFS was both 8.0 months in camrelizumab (95% CI, 5.0-10.3 months) and sintilimab (95% CI, 5.8-9.8 months) group.
Safety
Treatment-related AEs are summarized in Table 3. Most patients (96%) experienced a treatment-related AE. The most common any-grade treatment-related AEs were aspartate aminotransferase elevation (50.9%), hypothyroidism (49.1%), alanine aminotransferase elevation (47.2%), pyrexia (45.3%), proteinuria (34%), and hypertension (24.5%). Grade ≥3 treatment-related AEs occurred in 32.1% of patients. The most common were total bilirubin elevation (7.5%), increased aspartate aminotransferase (5.7%), and hypertension (5.7%). Lipase elevation was the only grade 4 treatment-related AE (2 of 53).
Table 3.
Treatment-Related Adverse Events (AEs) Occurring in ≥10% of Patients (N = 53).
| N (%) | ||||
|---|---|---|---|---|
| Preferred AE term | Any grade | Grade 1 | Grade 2 | Grade 3 |
| Hypertension | 13 (24.5%) | 4 (7.5%) | 6 (11.3%) | 3 (5.7%) |
| Proteinuria | 18 (34.0%) | 3 (5.7%) | 13 (24.5%) | 2 (3.8%) |
| Pyrexia | 24 (45.3%) | 16 (30.2%) | 8 (15.1%) | |
| ALT increase | 25 (47.2%) | 18 (34.0%) | 5 (9.4%) | 2 (3.8%) |
| AST increase | 27 (50.9%) | 17 (32.1%) | 7 (13.2%) | 3 (5.7%) |
| CK-MB increase | 15 (28.3%) | 12 (22.6%) | 3 (5.7%) | |
| TBIL increase | 22 (41.5%) | 11 (20.8%) | 7 (13.2%) | 4 (7.5%) |
| Hypothyroidism | 26 (49.1%) | 14 (26.4%) | 12 (22.6%) | |
| Diarrhea | 7 (13.2%) | 6 (11.3%) | 1 (1.9%) | |
| PPES | 6 (11.3%) | 4 (7.5%) | 2 (3.8%) | |
| Weight loss | 7 (13.2%) | 6 (11.3%) | 1 (1.9%) | |
| Decreased appetite | 10 (18.9%) | 9 (17.0%) | 1 (1.9%) | |
| Lipase increase | 6 (11.3%) | 2 (3.8%) | 2 (3.8%) | 2 (3.8%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; PPES, Palmar-plantar erythrodysesthesia syndrome.
During the study, 2 patients (3.8%) died of upper gastrointestinal bleeding, which was of uncertain relationship to the study treatment. Treatment-related AEs led to treatment interruption, dose reduction, and treatment discontinuation of lenvatinib in 23 (3.4.0%), 19 (35.8%), and 8 patients (15.1%), respectively; treatment-related AEs led to treatment interruption and treatment discontinuation of PD-1 in 12 (22.6%) and 4 patients (7.5%), respectively.
Discussion
Locoregional therapies for uHCC were controversial. Trans-arterial radioembolization appears to be a viable experimental treatment option for patients with intermediate-advanced stage HCC, but there is no explicitly recommended. 16 TACE is the guideline-recommended local therapy for uHCC. 4 However, there is a high tumor recurrence rate after TACE. And also a meta-analysis demonstrated a nonsuperiority of bland embolization compared with TACE for HCC patients. 17
Local therapy combined with systemic therapy could improve the clinical outcome of uHCC. In our previous study, we found percutaneous radiofrequency ablation combined with TACE plus sorafenib demonstrated better efficacy than TACE plus sorafenib for large HCCs with types I/II portal vein tumor thrombosis. 18 But not all combination therapies are effective. A meta-analysis showed that the association of sorafenib did not seem to prolong survival nor delay disease progression in patients treated with radioembolization. 19 Recent evidence indicates that initial lenvatinib therapy followed by TACE markedly improves OS in TACE-refractory patients. 9 A clinical study confirmed lenvatinib-TACE sequential therapy prolonged the OS and PFS in patients with intermediate-stage HCC. 20 The pretreatment of lenvatinib could contribute to the vascular normalization and the consequent reduction in vascular permeability and tumor interstitial pressure, which improves the distribution of lipiodol-containing anticancer drugs within the tumor. Lenvatinib can also inhibit the release of hypoxia-inducible factors such as VEGF following TACE so as to prevent the recurrence and metastasis of the tumor. 21 Lenvatinib-TACE sequential therapy may become the standard therapy for patients who do not benefit from TACE alone. 22
Lenvatinib could also improve the clinical benefit of programmed death receptor-1 (PD-1) signaling inhibitor.12,13,23,24 A phase Ib trial of lenvatinib plus nivolumab in patients with uHCC indicated that the ORR of this combination is 76.7%. 14 KEYNOTE-524 showed that lenvatinib plus pembrolizumab yielded a ORR of 46% and a DCR of 85%. 25 The FDA granted lenvatinib plus pembrolizumab a breakthrough therapy for the first-line treatment of uHCC that is not amenable to locoregional therapy.
In this study, we assessed the efficacy and safety of TACE in combination with lenvatinib plus PD-1 signaling inhibitors (camrelizumab or sintilimab) in uHCC. In the evaluable 51 patients, ORR is 54.9% and DCR is 84.3% by mRECIST. Moreover, responses were durable (median DoR, 6.5 months). Despite improvements in ORR and DCR, the median PFS of this study is only 8.5 months, which is shorter than that of lenvatinib plus pembrolizumab (9.3 months). 25 This result may be attributed to the high proportion of patients with TACE failure. The other reasons are the later stage of the patients enrolled (56.6% for BCLC C stage), the high proportion of patients with previous tyrosine kinase inhibitors treatment failure (45.3%), and the inadequate follow-up. In this study, the median PFS for the patients of first-line treatment (11.2 months) was longer than that of lenvatinib plus pembrolizumab, which suggest that this combined therapy should be used for patients with uHCC as early as possible.
In this study, Treatment-related AEs with the combination were consistent with the known AEs of each individual agent. The most frequent any-grade treatment-related AEs were abnormal hepatic function, hypothyroidism, pyrexia, proteinuria and hypertension; however, grade ≥3 AEs occurred in 32.1% of patients. Upper gastrointestinal bleeding commonly occurred in patients with HCC. In the IMbrave 150 trial, 26 the incidence of upper gastrointestinal bleeding observed in the atezolizumab–bevacizumab group was 7%. In our study, upper gastrointestinal hemorrhage was reported in 2 patients (3.8%). We found the incidence of increased aminotransferase was high, and this might have resulted from TACE and a higher proportion of patients with HBV infection included in this study. Overall, the toxicity profile of this combination is manageable with appropriate monitoring.
The present study had several limitations. First, the study design was retrospective, and the sample size was small. Second, median follow-up period was relatively short which made impossible to fully evaluate PFS and OS. Third, the impact on OS was not evaluated due to the limited observational period. Fourth, the major limitation of the present study is a lack of pathological data. Therefore, future study using larger, multicenter cohorts with sufficient observational period is needed to validate the present outcomes, also the predictive markers for this combined therapy should be analyzed using serum or tissue specimens in further study.
Conclusion
TACE in combination with lenvatinib plus PD-1 signaling inhibitors displayed promising efficacy in patients with uHCC. Furthermore, this combined therapy was well tolerated. This combination strategy might be suitable as a treatment option for this patient population.
Abbreviations
- AFP
alpha fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- TACE
transarterial chemoembolization
- TKIs
tyrosine kinase inhibitors
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- ORR
objective response rate
- DCR
disease control rate
Footnotes
Authors’ Note: The data sets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Foundation of Capital Distinctive Clinical Application Research (grant number Z181100001718131).
Ethical Statement: The trial was carried out in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki, as well as local laws. Documented approval from the institutional review board of Beijing Ditan Hospital, Capital Medical University, was obtained before commencing the study (Grant No. 2020-044). All participants provided written informed consent before therapy.
ORCID iD: Ying Teng https://orcid.org/0000-0002-1527-2856
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555-e567. doi: 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429-442. doi: 10.1053/jhep.2003.50047 [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014; 87(Suppl 1):22-31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Meng Q, Tan H, et al. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121(2):416-424. doi: 10.1002/ijc.22655 [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492-1501. doi: 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173. doi:S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Shimose S, Kawaguchi T, Tanaka M, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020;20(3):2257-2265. doi: 10.3892/ol.2020.11758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. doi: S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 12.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325-340. doi: 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8 + T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One. 2019;14(2):e0212513. doi: 10.1371/journal.pone.0212513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo M, Ikeda M, Motomura K, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38(4_suppl):513. doi: 10.1200/JCO.2020.38.4suppl.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 16.Rognoni C, Ciani O, Sommariva S, et al. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: systematic review and meta-analyses. Oncotarget. 2016;7(44):72343-72355. doi: 10.18632/oncotarget.11644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facciorusso A, Bellanti F, Villani R, et al. Transarterial chemoembolization versus bland embolization in hepatocellular carcinoma: a meta-analysis of randomized trials. United Eur Gastroenterol J. 2017;5(4):511-518. doi: 10.1177/2050640616673516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding X, Sun W, Chen J, et al. Percutaneous radiofrequency ablation combined with transarterial chemoembolization plus sorafenib for large hepatocellular carcinoma invading the portal venous system: a prospective randomized study. Front Oncol. 2020;10:578633. doi: 10.3389/fonc.2020.578633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facciorusso A, Paolillo R, Tartaglia N, et al. Efficacy of combined transarterial radioembolization and sorafenib in the treatment of hepatocarcinoma: a meta-analysis. Dig Liver Dis. 2021;S1590–8658(21):00318-2. doi: 10.1016/j.dld.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond Up-To-seven criteria and child-pugh A liver function: a proof-Of-concept study. Cancers (Basel). 2019;11(8):1084. doi: 10.3390/cancers11081084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58-62. doi: 10.1126/science.1104819 [DOI] [PubMed] [Google Scholar]
- 22.Kudo M. A New treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299-311. doi: 10.1159/000502905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500-506. doi: 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1–6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993-4002. doi: 10.1111/cas.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib Plus pembrolizumab in patients With unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960-2970. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn RS. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021; 39(3_suppl):267-267. doi: 10.1200/JCO.2021.39.3_suppl.267 [DOI] [Google Scholar]