Abstract
Background
Spinal ependymoma is the most common intramedullary tumor in adults. This study was performed to evaluate whether intraoperative yellow fluorescence use enhances our ability to identify the tumor margin and residual tumor tissue in intramedullary spinal cord ependymoma resection. We also evaluated patients’ clinical conditions at a 3-month follow-up.
Methods
We retrospectively evaluated 56 patients with intramedullary ependymoma. Thirty minutes before anesthesia, the patients received intravenous sodium fluorescein injections. Tumor resection was performed under two illumination modes, traditional white light and yellow fluorescence, and the residual tumor tissue was detected. Magnetic resonance imaging was performed 3 months postoperatively to observe the tumor resection outcome and residual tumor tissue. The McCormick spinal cord function grade was evaluated preoperatively and 3 months postoperatively.
Results
The total resection rate was 100.0% in all patients. Nine patients had no significant fluorescence imaging. After 3 months, patients with a spinal function grade of I to IV showed significant spinal function improvement. Magnetic resonance imaging showed no residual tumor tissue or recurrence.
Conclusion
Sodium fluorescein aids in total excision of intramedullary spinal cord ependymoma and intraoperative residual tumor tissue identification. At the 3-month follow-up, the patients’ functional outcome in the fluorescein group was good.
Keywords: Spinal cord tumor, ependymoma, yellow fluorescence, neurosurgery, residual tumor, functional outcome
Introduction
Spinal ependymoma is the most common intramedullary tumor in adults. It originates in the epithelial cells of the central canal ependyma of the spinal cord and tends to occur in the cervical spinal cord.1–3 The tumor grows slowly and expands longitudinally along the long axis of the spinal cord, with a clear boundary in most cases; thus, total resection has been the treatment of choice.4–6 However, it is often difficult to intraoperatively resolve the tumor’s absolute boundaries, especially when tumor apoplexy occurs: multiple intramedullary syringes are present within the spinal cord and severely adhere to the spinal cord, making it extremely difficult to resolve the tumor’s boundaries and determine the presence of residual tumor tissue.7,8 Intraoperative determination of the tumor’s boundaries and localization of residual tumor tissue are currently important challenges facing neurosurgeons.9–11 Sodium fluorescein is a chemical that can stain tumor tissue, thus enabling the tumor tissue to generate fluorescence under the irradiation of special excitation light. This distinguishes normal brain tissue, edema tissue, and tumor tissue, clearly guiding the surgeon to identify the boundary between normal brain tissue and tumor tissue.12,13 In the present study, 56 patients who underwent resection of intramedullary spinal cord ependymoma assisted by sodium fluorescein were retrospectively analyzed.
Materials and Methods
Clinical data
This study initially involved 83 patients with spinal cord tumors who were admitted to the Neurosurgical Department of Beijing Tsinghua Changgung Hospital from December 2014 to December 2015 and effectively followed up. Of these patients, 56 (67.5%) (39 men, 17 women; mean age, 43.5 ± 4.2 years; age range, 31–65 years) had pathologically confirmed spinal ependymomas. Among these 56 patients, 53 preoperatively exhibited single-limb/unilateral or double-limb/bilateral sensory dysfunction, 51 exhibited limb motor dysfunction, 46 manifested decreased muscle strength (grades II–IV), 5 had paraplegia (sudden paraplegia in 2), 4 had respiratory dysfunction, and 12 had urination and defecation dysfunction. Preoperative McCormick neurological function assessment showed grade I, II, III, and IV spinal cord function in 2 (3.6%), 31 (55.4%), 14 (25.0%), and 9 (16.1%) patients, respectively.
Imaging examination
All patients underwent a 3.0 T magnetic resonance imaging (MRI) examination. Among the 56 patients, 43 had lesions in the cervical spine, 9 had lesions in the thoracic spine, and 4 had lesions in the filum terminale. The lesion invaded 1 to 2 segments in 6 patients, 3 to 4 segments in 26 patients, 5 to 6 segments in 13 patients, and ≥7 segments in 11 patients. The lesions were complicated by syringomyelia in 45 patients. Forty-seven patients showed an enhanced solid tumor, seven showed intramedullary tumor apoplexy with uneven tumor enhancement, and two showed no enhancement.
Surgical methods
The patients received an intravenous injection of sodium fluorescein (3–4 mg/kg) (Fluorescite; Akorn, Lake Forest, IL, USA) 30 minutes before induction of general anesthesia. The procedure was performed under an OPMI Pentero 900 microscope equipped with a 560-nm fluorescence yellow filter (Carl Zeiss Meditec, Jena, Germany). The surgical position was chosen based on the location of the tumor, and the patient’s limb somatosensory evoked potentials and surface electromyography (EMG) were monitored during the entire procedure. A posterior neck central approach was adopted based on the site and spinal cord segment of the tumor to expose the lamina of the upper and lower boundaries of the tumor, and the lamina of the corresponding segment was opened via piezosurgery. The dura was revealed, cut open, and suspended, and the arachnoid was then cut open. The tumor manifestations and boundary were observed under the microscopic white light mode. Intraoperatively, the microscope was switched to the 560-nm yellow fluorescence mode to visualize the tumor. The spinal cord was cut open in the posterior middle region but was not cut too deeply (to avoid affecting the tumor and causing bleeding and difficulty in determining the tumor boundaries). This action was taken to fully reveal the tumor boundaries, which were rather clear in most cases, and thus facilitate resection. Resection of tumors with apoplexy was difficult to perform; care was taken to avoid excessive traction and damage to the base of the spinal cord and to incise the tumor while stopping the bleeding, enabling complete and total resection. During the surgery, the tumor was visualized under the traditional white light mode and the sodium fluorescein mode, and these two modes were used alternatively to remove the tumor. After resection, the fluorescence mode was used again to determine whether residual tumor tissue was still present, especially at the upper and lower ends of the tumor and the syrinx wall. The pia mater and dura mater of the spinal cord were sutured after the resection. The suture line of the arachnoid membrane was determined according to the surgical situation, the two-hole connector of the craniofacial fixation system was used to fix the removed lamina and reset, and the incision was sutured. After the operation, special attention was paid to the patient’s breathing and motor function.
Efficacy evaluation
Each patient’s functional changes were clinically followed up at 7 days, 1 month, and 3 months postoperatively, and the McCormick grade 14 was determined preoperatively and 3 months postoperatively. MRI was also performed 3 months postoperatively to determine whether the tumor had relapsed or complications had developed.
Statistical analysis
The data were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Quantitative data are presented as mean ± standard deviation, and qualitative data are presented as ratio (%) or proportion. The differences in the distributions of the patients’ clinical functions at 7 days, 1 month, and 3 months postoperatively were compared using the row × column χ2 test, and multiple comparisons were performed using the χ2 division method. The differences in the patients’ McCormick grades before and after surgery were compared using the Mann–Whitney U test. The significance level was set at P = 0.05, and P < 0.05 was considered statistically significant.
The reporting of this study conforms to the STROBE guidelines. 15
Results
Intraoperative tumor visualization
Intraoperatively, 40 patients showed clear tumor boundaries without application of sodium fluorescein. Furthermore, 16 patients showed tumor adhesions to the spinal cord, making it difficult to remove the tumor; among these patients, seven showed intramedullary tumor apoplexy with severe adhesions. Under yellow fluorescence, the tumor was revealed in 47 (83.9%) patients, while the tumor was either not shown or not shown clearly in nine (26.1%) patients. Of these nine patients, seven exhibited intramedullary tumor apoplexy and demonstrated no obvious MRI enhancement, and the remaining two showed no MRI enhancement. Assisted by the white light and yellow fluorescence modes, total tumor resection was achieved in all 56 patients, with a total resection rate of 100.0% (56/56). Intraoperative neurophysiological monitoring revealed that after tumor resection, 44 patients showed decreased upper and/or lower limb somatosensory evoked potentials, 23 showed declined upper and/or lower limb EMG amplitudes, and 7 showed declined anal sphincter EMG amplitudes.
These findings suggest that the use of sodium fluorescein can assist in the display of residual tumor tissue, can improve the tumor resection rate, and has teaching significance for surgeons who lack experience in resection of spinal cord tumors. In addition, 27 of the 83 patients had spinal astrocytoma/glioma. The analysis showed that yellow fluorescein did not develop in low-grade spinal astrocytoma/glioma and had little effect in assisting resection. When sodium fluorescein acts on high-level spinal astrocytoma/glioma, the tumor boundary is not clear, and sodium fluorescein-assisted excision may result in excessive resection and spinal cord injury. Therefore, sodium fluorescein has little effect on assisted resection of spinal astrocytoma/glioma, and the effect of sodium fluorescein on tumor development and localization needs further study and improvement.
Dysfunction improvement
Three months after surgery, patients with preoperative dysfunction showed significant improvement in sensory and motor function (P < 0.05); however, there was no significant change in urinary and defecation function. The patients’ respiratory function was fully recovered 7 days after surgery (Table 1). Three months after surgery, the sensory and motor functions improved significantly in patients with surgically induced dysfunction or aggravated original dysfunction (P < 0.05), whereas urination and defecation dysfunction improved, although not significantly (Table 2). The MRI follow-up at 3 months after surgery indicated no residual tumor or recurrence, no cerebrospinal fluid leakage or infection, no respiratory distress or death, and no other abnormal findings during the follow-up period.
Table 1.
Accumulated improvements in patients with spinal cord ependymoma exhibiting preoperative dysfunction.
| Preoperative dysfunction | Patients, n | Postoperative improvement |
χ2 value | P value | ||
|---|---|---|---|---|---|---|
| 7 days | 1 month | 3 months | ||||
| Sensory | 53 | 12 (22.6) | 27 (50.9)① | 44 (83.0)①,② | 38.767 | <0.001 |
| Motor | 51a | 23 (45.1)b | 28 (54.9)c | 42 (82.4)d,①,② | 15.958 | <0.001 |
| Respiratory | 4 | 4 (100.0) | 0 (0.0) | 0 (0.0) | / | / |
| Urinary and defecation | 12 | 5 (41.7) | 5 (41.7) | 8 (66.7) | 2.000 | 0.368 |
Data are presented as n (%).
aIncluding five patients with paralysis; bIncluding three patients with paralysis, with Grade I or II muscle strength; cIncluding four patients with paralysis, with Grade I or II muscle strength; dIncluding three patients with paralysis, with Grade I or II muscle strength. ①Significantly different compared with 7 days postoperatively (P < 0.05); ②Significantly different compared with 1 month postoperatively (P < 0.05).
Table 2.
Postoperative improvement of patients with aggravated or newly developed dysfunction.
| Aggravated or newly developed dysfunction | Patients, n | Postoperative improvement |
χ2 value | P value | ||
|---|---|---|---|---|---|---|
| 7 days | 1 month | 3 months | ||||
| Sensory | 44 | 16 (36.4) | 28 (63.6)① | 35 (79.5)① | 17.465 | <0.001 |
| Motor | 23 | 11 (47.8) | 17 (73.9)① | 20 (87.0)① | 7.375 | 0.025 |
| Urinary and defecation | 7 | 1 (14.3) | 4 (57.1) | 5 (71.4) | 4.964 | 0.084 |
Data are presented as n (%).
①Significantly different compared with 7 days postoperatively (P < 0.05).
Improvement in spinal cord function
Table 3 shows that at 3 months postoperatively, 32 (57.2%) patients had McCormick spinal function grade I, 12 (21.4%) had grade II, eight (14.3%) had grade III, and four (7.1%) had grade IV. These results were significantly different from those before the surgery (P < 0.05). Among the nine patients with preoperative grade IV spinal function, five improved in 3 months after surgery; however, among the five patients with preoperative bleeding paralysis, only one improved. Most surgically induced dysfunctions improved in the follow-up period, likely because the symptoms derived from the surgical procedures were induced and reversible primary injury reactions, or the results of secondary spinal cord edema gradually improved with recovery of the injury and disappearance of the edema.
Table 3.
McCormick spinal cord function grades of 56 patients with spinal cord ependymoma before and 3 months after surgery.
| McCormick grade | Preoperatively | 3 months postoperatively | U value | P value |
|---|---|---|---|---|
| Grade I | 2 (3.6) | 32 (57.2) | 768.000 | <0.001 |
| Grade II | 31 (55.4) | 12 (21.4) | ||
| Grade III | 14 (25.0) | 8 (14.3) | ||
| Grade IV | 9 (16.1) | 4 (7.1) |
Data are presented as n (%).
Consistency between imaging examination and intraoperative visualization
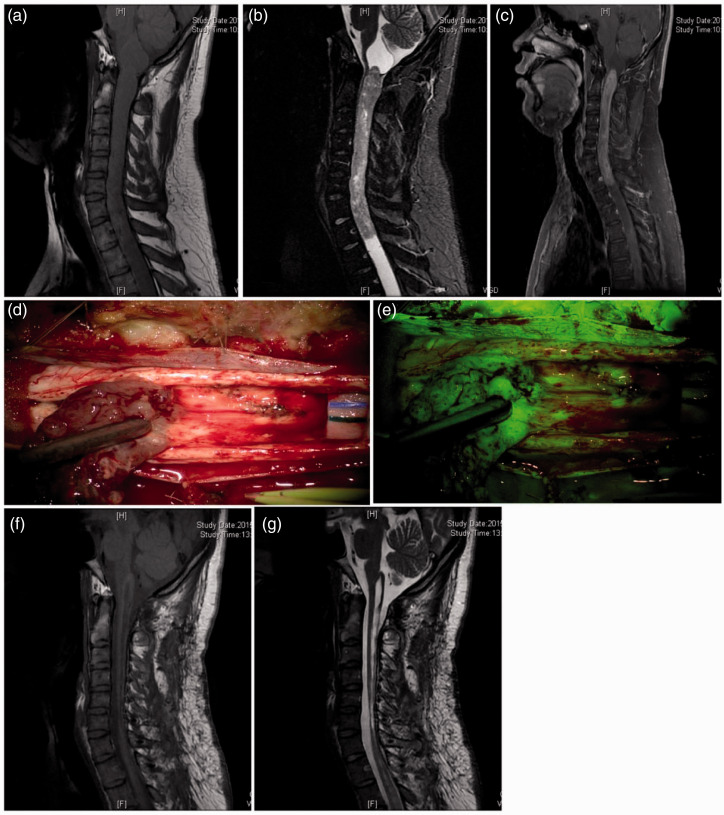
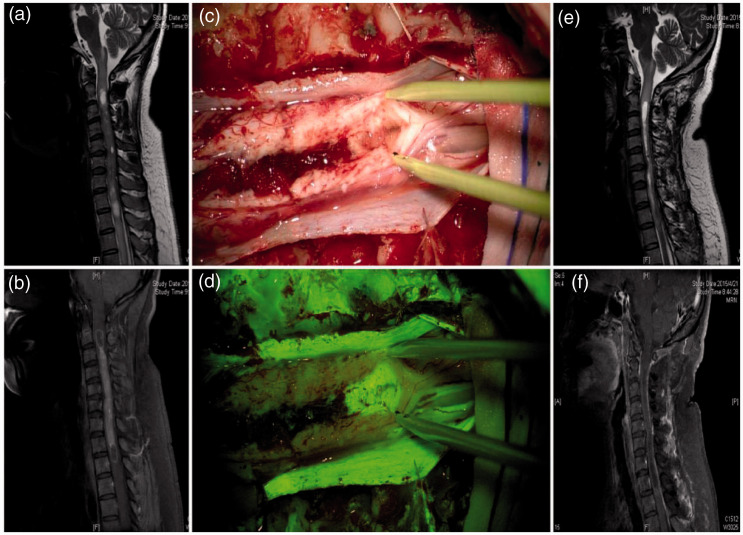
Among the 56 patients, 47 (83.9%) showed preoperative MRI enhancement, and intraoperative visualization via fluorescence significantly enhanced visualization in all cases. In the two patients without significant MRI contrast enhancement (Figure 1), intraoperative fluorescence visualization did not enhance the contrast. In the seven patients with tumor apoplexy showing uneven MRI contrast enhancement, intraoperative fluorescence visualization did not enhance the contrast. Forty-five patients exhibited syringomyelia, among whom 44 showed no significant MRI contrast enhancement in the wall of the syrinx, and intraoperative fluorescence visualization did not enhance the contrast of the syrinx wall (Figure 2). One patient showed enhanced MRI contrast in the syrinx wall at both ends of the tumor, but intraoperative fluorescence visualization showed enhanced contrast in the syrinx wall (Figure 3).
Figure 1.
Preoperative and postoperative magnetic resonance imaging (MRI) examinations and intraoperative microscopic findings of patients with intramedullary spinal cord ependymoma at the T3–7 thoracic vertebrae. (a) Preoperative MRI in which a sagittal T2 image shows an intramedullary spinal cord ependymoma exhibiting a relatively regular contour signal at the T3–7 thoracic vertebrae, with a rather clear boundary. (b) Preoperative MRI in which a sagittal enhanced scan shows no contrast enhancement of the tumor. (c) Intraoperative microscopy indicates that the tumor is located at the dorsal region of the spinal cord (shown by gray color) with a clear boundary. (d) Under yellow fluorescence, the tumor does not show enhanced contrast. (e) T2 sagittal MRI 3 months postoperatively showing no residual tumor, but a visible tumor cavity in the surgical region. (f) Sagittal enhanced MRI 3 months postoperatively indicating no residual tumor or recurrence.
Figure 2.
Intramedullary spinal cord ependymoma at the C1–T1 segment. (a)–(c) Preoperative magnetic resonance imaging (MRI) in which a T1 image shows a thickened spinal cord; a T2 image shows the intramedullary contour signal of masses in the C1–T1 segment, with visible syringes at both ends; and an enhanced scan shows significant contrast enhancement of the tumor. (d) and (e) Intraoperative microscopy shows the intactness and clear boundary of the tumor; under the fluorescence mode, enhanced contrast of the tumor was achieved, especially at the base of the tumor. (f) and (g) MRI 3 months postoperatively in which the T1 and T2 images indicate no residual tumor or recurrence.
Figure 3.
Preoperative and postoperative magnetic resonance imaging (MRI) examinations and intraoperative findings of patients with ependymoma. (a) Preoperative MRI in which a sagittal T2 image shows intramedullary masses with an irregular contour signal in the C2–T2 segment, with syringes visible at both ends. (b) Preoperative MRI in which a sagittal enhanced scan shows significant contrast enhancement of the tumor mass and enhanced syrinx walls at both ends. (c) The syrinx of a tumor at the head end is shown under white light; it is difficult to determine the presence of the tumor. (d) The contrast of the syrinx wall is clear under the fluorescence mode, and the mass was removed as a suspected tumor. (e) MRI examination 3 months postoperatively; a sagittal T2 image shows the tumor cavity after tumor resection. (f) MRI examination 3 months postoperatively; a sagittal enhanced MRI scan indicates no residual tumor or recurrence.
Discussion
Sodium fluorescein helps diagnose malignant tumors and vascular lesions. 16 It has mainly been applied in ophthalmic diseases; it has not been used in localizing and removing intracranial tumors since 1948 because its use has been limited by the high requirements of visualization techniques and the nonspecific visualizing outcome.17–21 Normally, sodium fluorescein does not readily enter the circulation of the brain tissue because of the blood–brain barrier; however, in patients with neurovascular tumors and associated endothelial functional lesions, fluorescein sodium accumulates in the vascular tissue of the tumor and can be observed under a yellow filter (560-nm wavelength). 17 Fluorescence visualization factors include the presence of a malignant tumor, vascular permeability defects, the blood–brain barrier, and neovascular defects.20,21 Because sodium fluorescein molecules are small and can reversibly bind to hemoglobin and erythrocytes, these molecules can also penetrate the outsides of blood vessels to stain a tumor’s peripheral tissue.22,23 Recent studies have shown that fluorescence-coupled hemoglobin technology can be used to enhance the contrast between tumor tissue and normal tissue. 18 Sodium fluorescein can be used to improve tumor visibility and resection; however, the reliability of fluorescence visualization must be improved, particularly in resolving tumor boundaries and their effect on patients’ prognosis. 24
Most spinal ependymomas have clear boundaries and a high total resection rate that does not require yellow fluorescence-assisted resection. However, in a previous report, we summarized the findings of 210 patients who underwent surgical treatment of spinal ependymoma from 1999 to 2008, and the resection rate among all patients was 81.9% under naked-eye white light mode. 25 Longer and larger tumors and tumor stroke (i.e., tumor apoplexy or hemorrhage) may easily lead to intraoperative hemorrhage and residual tumor tissue. Therefore, we applied fluorescence imaging technology to spinal ependymoma resection in an effort to improve the total resection rate and reduce the incidence of residual tumor tissue, providing implications for future clinical reference.
The 56 patients with intramedullary spinal cord ependymoma were treated with tumor resection under the aid of sodium fluorescein. Of these 56 patients, 47 (83.9%) showed remarkable fluorescence visualization and guiding significance in tumor resection, whereas nine (16.1%) did not show remarkable fluorescence visualization; in those patients, the resections were performed under white light. The intraoperative applications of yellow fluorescence with intramedullary tumors have been rarely reported; however, a few cases have focused on brain tumors. Koc et al. 17 reported that the total tumor resection rate of brain glioblastoma with the aid of sodium fluorescein was 83%, which was higher than that in the control group (55%); however, it had no significant effect on survival. Shinoda et al. 21 showed that the total tumor resection rates of brain glioblastomas under sodium fluorescein and conventional microscopic white light modes were 84.4% and 30.1%, respectively. The patients in the present study had intramedullary spinal cord ependymoma, and the yellow fluorescence visualization rate was 83.9%. A total resection rate of 100% was achieved by combining the yellow fluorescence mode with the white light mode. During the follow-up period, the patients’ postoperative sensory and motor dysfunctions gradually improved; however, urination and defecation dysfunctions did not significantly improve, likely because recovery from urination and defecation dysfunctions is intrinsically slow and effective rehabilitation measures are lacking. Three months after surgery, the patients’ McCormick spinal cord function grades gradually improved. Although the surgeries caused short-term sensory dysfunction (44 patients), motor dysfunction (23 patients), and bowel and bladder disorders in these patients, the improvement rates were 79.5% (35/44), 87.0% (20/23), and 71.4% (5/7), respectively, during the 3-month follow-up period. Most of these surgically induced dysfunctions improved during follow-up, likely because the symptoms derived from the surgical procedures were induced and reversible primary injury reactions, or the results of secondary spinal cord edema gradually improved with recovery of the injury and disappearance of the edema. Patients with preoperative respiratory dysfunctions also improved to varying degrees, which was likely associated with the relief of pressure on the spinal cord, decreased tension, and improved blood supply after tumor resection. The five patients with preoperative tumor apoplexy paralysis had poor muscle strength and functional improvement. In such cases, patients and clinicians should choose the timing of surgery to avoid the occurrence of severe functional impairments and paralysis and choose the surgical treatment even if paralysis has already occurred in the patient, although the short-term efficacy of the surgery may not be significant. MRI reviews during the follow-up period showed no residual tumor or recurrence, indicating that total tumor removal can be achieved under the fluorescence microscopy and white light modes. Furthermore, although rapid relapse in the short-term follow-up was not observed, long-term follow-up will be conducted to further determine whether the tumors relapse.
For cases in which tumor apoplexy occurs, tumor identification and resection need to be performed by intraoperatively combining the fluorescence and white light modes.26,27 However, because hemosiderin accumulation leads to severe adhesion of tumor tissue to normal spinal cord tissue, it is very difficult to resolve the tumor boundaries and residual tissue even under the white light and fluorescence modes. 28 Additionally, small-vessel angiogenesis increases the blood supply of the tumor, which greatly increases the difficulty of surgical resection.29,30 Our preliminary results show that the two are consistent to a certain extent. The present study shows that yellow fluorescein can help to find the tumor; increase tumor identification; and improve the resection rate for residual tumors, spinal ependymoma with cavity wall enhancement, and tumor hemorrhage.
In short, these observations indicated that the fluorescence visualization and the preoperative MRI enhanced image were consistent. Although intraoperative fluorescence visualization could reveal the invasion or planting of a tumor, this visualization mode offered little help to patients with tumor apoplexy. We will increase the sample size in follow-up studies to further investigate the correlations between MRI and intraoperative fluorescence visualization and its reliability.
Conclusion
Fluorescein sodium can be used to assist in the resection of intramedullary spinal cord ependymoma. Combining the microscopic white light mode with the fluorescence mode can help to identify tumor tissues and residual tumors and can improve the resection rate. However, for tumors that have no intraoperative fluorescence visibility, identification under normal white light and the benefits of the surgeon’s experience are still required. In future studies, the fluorescein sodium-assisted visualization of intramedullary spinal cord ependymomas with different pathological conditions and their resection rates will be investigated, while the sensitivity and specificity of tumor visualization will be further assessed to mitigate the visualization interference of peritumoral edema.
Acknowledgement
We would like to acknowledge the reviewers for their helpful comments on this paper.
Footnotes
Compliance with ethical standards: In this study, we followed the ethical standards of biomedical research involving humans in Tsinghua Changgeng Hospital.
Conflicts of interest: The authors declare that there are no competing interests regarding the publication of this paper.
Ethics approval and informed consent: All procedures involving humans were carried out in accordance with the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Human Subjects (2002). All patients voluntarily provided written informed consent for participation in this clinical trial at Beijing Tsinghua Changgung Hospital (No. 161256).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This project was supported by both the Beijing Municipal Science & Technology Commission (Z171100001017199) and the Neuro-oncology Project from the Chinese Anti-Cancer Association (CSNO-2016-MSD05).
ORCID iD: Guihuai Wang https://orcid.org/0000-0001-8053-0711
References
- 1.Ehrhardt A, Stepp H, Irion K, et al. Fluorescence detection of human malignancies using incoherent light systems. Med La App 2003; 18: 27–35. [Google Scholar]
- 2.Ewelt C, Nemes A, Senner V, et al. Fluorescence in neurosurgery: its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B-Biol 2015; 148: 302–309. [DOI] [PubMed] [Google Scholar]
- 3.Keil VC, Schmitt AJ, Martin S, et al. Optimising treatment strategies in spinal ependymoma based on 20 years of experience at a single centre. J Clin Neurosci 2016; 29: 52–58. [DOI] [PubMed] [Google Scholar]
- 4.Kim D, Kim J, Choi SH, et al. Differentiation between Intramedullary spinal ependymoma and astrocytoma: comparative MRI analysis. Clin Radiol 2014; 69: 29–35. [DOI] [PubMed] [Google Scholar]
- 5.Kukreja S, Ambekar S, Sharma M, et al. Outcome predictors in the management of spinal myxopapillary ependymoma: an integrative survival analysis. World Neurosurg 2015; 83: 852–859. [DOI] [PubMed] [Google Scholar]
- 6.Xie T, Qian J, Wu X, et al. Unilateral, multilevel, interlaminar fenestration in the removal of a multisegment cervical intramedullary ependymoma. Sp J 2013; 13: 747–753. [DOI] [PubMed] [Google Scholar]
- 7.Butte P, Mamelak AN, Nuno M, et al. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. NeuroImage 2011; 54: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da W. Microneurosurgical management of multisegmental intramedullary spinal cord ependymoma in cervico-thoracic junction. Chin J Con Neu 2009; 9: 149–152. [Google Scholar]
- 9.Lee JYK, Thawani JP, Pierce J, et al. Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery 2016; 79: 856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sastry R, Bi WL, Pieper S, et al. Applications of ultrasound in the resection of brain tumors. J Neuroimaging 2017; 27: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munkvold BKR, Jakola AS, Reinertsen I, et al. The diagnostic properties of intraoperative ultrasound in glioma surgery and factors associated with gross total tumor resection. World Neurosurg 2018; 115: e129–e36. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, Wang H, Ge P, et al. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci 2012; 9: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neira JA, Ung TH, Sims JS, et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J Neurosurg 2017; 127: 111–122. [DOI] [PubMed] [Google Scholar]
- 14.Mccormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990; 72: 523–532. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 16.Scorletti F, Hammill A, Patel M, et al. Malignant tumors misdiagnosed as benign vascular anomalies. Pediatr Blood Cancer 2018; 65: e27051. [DOI] [PubMed] [Google Scholar]
- 17.Koc K, Anik I, Cabuk B, et al. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Brit J Neurosurg 2008; 22: 99–103. [DOI] [PubMed] [Google Scholar]
- 18.Kremer P, Fardanesh M, Ding R, et al . Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 2009; 64: 53–60. [DOI] [PubMed] [Google Scholar]
- 19.Okuda T, Kataoka K, Yabuuchi T, et al. Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J Clin Neurosci 2010; 17: 118–121. [DOI] [PubMed] [Google Scholar]
- 20.Schebesch K, Proescholdt M, Hohne J, et al. Sodium fluorescein–guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir 2013; 155: 693–699. [DOI] [PubMed] [Google Scholar]
- 21.Shinoda J, Yano H, Yoshimura S, et al. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 2003; 99: 597–603. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Powe AM, Baker GA, et al. Molecular fluorescence, phosphorescence, and chemiluminescence spectrometry. Anal Chem 2012; 84: 597–625. [DOI] [PubMed] [Google Scholar]
- 23.Liu J-N, Bu W, Shi J. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem Rev 2017; 117: 6160–6224. [DOI] [PubMed] [Google Scholar]
- 24.Diaz RJ, Dios RR, Hattab EM, et al. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg 2015; 122: 1360–1369. [DOI] [PubMed] [Google Scholar]
- 25.Li TY, Chu JS, Xu YL, et al. Surgical strategies and outcomes of spinal ependymomas of different lengths: analysis of 210 patients: clinical article. J Neurosurg Spine 2014; 21: 249–259. [DOI] [PubMed] [Google Scholar]
- 26.Widhalm G, Traub-Weidinger T, Hainfellner JA, et al. Chapter 33 – Bioimaging and surgery of brain tumors [M]//KOVACS G G, ALAFUZOFF I. Handbook of Clinical Neurology. Elsevier . 2018: 535–545. [DOI] [PubMed] [Google Scholar]
- 27.Riley CA, Soneru CP, Tabaee A, et al. Technological and ideological innovations in endoscopic skull base surgery. World Neurosurg 2019; 124: 513–521. [DOI] [PubMed] [Google Scholar]
- 28.Boddupalli A. Study of collagen organization in cell-laden hydrogels and animal tissue samples for effective tissue engineering scaffolds [D]. Ames, Iowa; Iowa State University, 2018. [Google Scholar]
- 29.Abdalla AME, Xiao L, Ullah MW, et al. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018; 8: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudvarski Stanković N, Bicker F, Keller S, et al. EGFL7 enhances surface expression of integrin α5β1 to promote angiogenesis in malignant brain tumors. Embo Mol Med 2018; 10: e8420. [DOI] [PMC free article] [PubMed] [Google Scholar]