Abstract
Mesenchymal stem cells derived from umbilical cord Wharton’s Jelly (WJ-MSCs) are emerging as promising therapeutics for a variety of diseases due to their ability of regeneration and immunomodulation, and their non-tumorigenic and non-immunogenic properties. Although multiple protocols have been developed for WJ-MSC isolation, insufficient cell numbers, heterogeneous cell population, and variations in procedures between different laboratories impede further clinical applications. Here, we compared six widely used WJ-MSC isolation methods regarding cell morphology, yield, purity, proliferation rate, and differentiation potential. Based on these analyses, we identified that the inefficiency of the extracellular matrix digestion results in low cell yield. Thus, we developed a new method called “Mince-Soak-Digest (MSD)” to isolate MSCs from WJ by incorporating a soaking step to facilitate the digestion of the extracellular matrix and release of the cells. Our newly developed method generates significantly higher cell yield (4- to 10-fold higher) than six widely used methods that we tested with high purity and consistency. Importantly, by transplantation of WJ-MSCs to the rat uterus, we repair the endometrial injury and restore the fertility of the rats. In conclusion, our results provide a robust and highly efficient approach for the isolation of WJ-MSCs to restore injured tissue. The higher efficiency of MSD assures the abundance of WJ-MSCs for clinical applications. Furthermore, the reliability of MSD contributes to the standardization of WJ-MSC isolation, which eliminates the discrepancies due to isolation procedures, thus facilitating the evaluation of the efficacy of WJ-MSCs across various human clinical applications.
Keywords: mesenchymal stem cells, Wharton’s jelly, transplantation, tissue repair, intrauterine adhesions
Introduction
The recent advance of stem cell technology has revolutionized the treatment of various diseases and laid the foundation for regenerative medicine, especially tissue repair1–3. Among many types of stem cells, mesenchymal stem cells (MSCs) have attracted much interest for preclinical/clinical studies as a potential treatment for a variety of diseases owing to their following properties. (1) Easy accessibility: MSCs exist in almost all tissues, including bone marrow, umbilical cord, endometrium, menstrual blood, and adipose 4 . (2) Self-renewal: MSCs are able to proliferate while maintaining the stem cell properties 5 . (3) Multipotency: MSCs exhibit the potential to differentiate into various lineages of mesoderm, ectoderm, and endoderm, such as bone, fat, chondrocyte, muscle, islet cells, and liver cells in vitro under appropriate stimuli6,7. (4) Immunomodulation: MSCs are shown to aid tissue regeneration by immune suppression of both innate and adaptive systems, primarily through paracrine effect8,9. (5) Low immunogenicity: MSCs are considered immune-privileged due to their limited expression of major histocompatibility complex class I (MHC I), lack of MHC II, and co-stimulatory antigen expression10,11. These properties make them an ideal candidate for allogeneic cell therapy. (6) Tropism: MSCs have the ability to migrate to damaged or diseased tissues or cells 12 .
Initially, MSCs were isolated from bone marrow and frequently used for autologous cell therapy13,14. However, the application has been hampered mainly due to their invasive and painful isolation procedure associated with significant morbidity and risk of infection 15 . A possible solution resides in the use of allogeneic MSCs from Wharton’s jelly (WJ), the connective tissue of the umbilical cord16,17. WJ is considered an advantageous source for MSCs in terms of accessibility, noninvasive isolation procedure, and abundance of cells18–20.
Isolation of MSCs from WJ is the first and critical step to assure optimal quantity and quality for the following applications in regenerative medicine. Currently, several protocols have been developed to isolate WJ-derived MSCs (WJ-MSCs), categorized into three primary techniques: enzymatic21–23, explant24–26, and enzymatic-explant27–29. In the enzymatic method, cells are dissociated from tissues by a combination of enzymes, such as collagenases, hyaluronidase, and trypsin, and then cultured in appropriate medium for proliferation. In the explant method, tissues are excised into a smaller size to allow attachment to the culture dish, and then cells migrate from tissues and adhere to the surface. In enzymatic-explant, the tissue is cut into small pieces and exposed to the enzymatic solution for incubation. Then, the enzymes are washed, and the tissue pieces are plated until the cells migrate out. Although these protocols are commonly used in a few laboratories, there is wide variation in procedures to harvest WJ-MSCs, resulting in different compositions of the resultant cells and inconsistent results between studies30,31. In addition, insufficient cells isolated from original tissues and limited proliferation ability of WJ-MSCs when cultured in vitro is one of the bottlenecks for application of WJ-MSCs to large-scale clinical trials32–34. Based on the published data, the average dose of MSCs treatment is 108/patient34–40. So, to complete the three phases of one clinical trial, we need approximately 1011 to 1012 MSCs. Although MSCs can be grown up to 20 passages, it has been reported that long-term cultured MSCs have shown upregulation of senescence genes and decreased regeneration and immunosuppressive ability compared with short-term cultured counterparts 6 . Thus, increasing the initial yield from the original tissue samples and generating a sufficient number of MSCs with fewer passage numbers (preferably below passage 10) by in vitro expansion is one of the urgent needs for large-scale clinical applications of MSCs. Furthermore, Thus, a robust and more efficient protocol that can be generalized as the standard approach for WJ-MSC isolation will be beneficial for the evaluation of the efficacy of WJ-MSCs across various clinical applications.
Here, we have compared six widely used WJ-MSC isolation methods with regard to cell morphology, yield, purity, and proliferation rate. Moreover, we develop a new isolation approach that significantly increases the yield of cells by 4- to 10-fold. We further investigate the therapeutic potential of these cells by transplantation of them to the uterus of a rat endometrium-injury model. Our results demonstrate that WJ-MSCs isolated by our approach could facilitate endometrium regeneration and fertility restoration. In summary, our study provides a robust and highly efficient approach for the isolation of WJ-MSCs to restore injured tissue, which would be beneficial to promote the large-scale application of WJ-MSCs to a broader field in regenerative medicine.
Materials and Methods
Collection of Umbilical Cord
Umbilical cord samples from women with healthy pregnancies were retrieved at the time of cesarean section from the Department of Obstetrics and Gynecology, The First Affiliated Hospital of University of Science and Technology of China. Umbilical cord samples were collected in phosphate-buffered saline (PBS) containing 500 IU heparin (Sigma–Aldrich, St. Louis, MO, USA), 300 U/ml penicillin, and 300 mg/ml streptomycin (Invitrogen, Grand Island, NY, USA) and were immediately transferred to the laboratory. Umbilical cord samples were processed within 12 h of collection.
Isolation of WJ From Umbilical Cord
Each human umbilical cord was transferred into a sterile laminar flow hood and washed twice in PBS to remove contaminating blood cells. The umbilical cord was cut into approximately 5-cm-long segments, which were subsequently cut longitudinally to expose the umbilical vein. Blood vessels (two arteries and one vein) were removed from each segment, and WJ was carefully separated from the amniotic membrane 41 .
Six Widely Used WJ-MSC Isolation Methods
M1 (explant method): 2 g of WJ was excised to about 1–2 mm size pieces, and they were transferred to 100 mm dishes (Corning) coated with 0.2% gelatin. In all, 2 g weight of tissues was plated per dish and covered entirely with the culture medium [DMEM/F12 with 20% fetal bovine serum (FBS), 300 U/ml penicillin, and 300 mg/ml streptomycin]. Half of the medium was changed on day 5 without disturbing the tissue pieces. On day 8, all the tissue pieces were removed, and the medium was completely replaced with fresh medium.
M2 (explant method): 2 g of WJ was excised to about 1–2 mm size pieces, then homogenized in DMEM/F12 + 20% FBS, and grinded crosswise for about 40 times using a homogenizer into a fine granular shape (1–2 mm in diameter). Then the sample was rinsed repeatedly with DMEM/F12 + 20% FBS and placed as tissue pieces on 100 mm dishes (Corning) coated with 0.2% gelatin. Half of the culture medium was replaced on day 5 without disturbing the tissue pieces. On day 8, all the tissue pieces were removed, and the medium was completely replaced with fresh medium. Every 2 days thereafter, the cells were observed and passaged according to their growth.
M3 (enzymatic-explant method): 2 g of WJ was excised to about 1–2 mm and incubated with 1 mg/ml of collagenase II (Sigma–Aldrich) at 4°C overnight. The next day, the tissue was filtered through a 100-mesh sieve to filter out large pieces of tissue and washed three times by PBS and one time by culture medium (DMEM/F12 + 20% FBS). Then, the tissue was plated to a 100-mm dish coated with 0.2% gelatin and covered by 5 ml of medium. The next day, cells started to dissociate from the tissue, and another 2–3 ml of medium was added to the plate. After 2 days, more cells were dissociated from the tissue, and another 2–3 ml of medium was added. The medium was changed every 3 days until day 8, or the cells grew to approximately 80% to 90% confluence.
M4 (enzymatic-explant method): WJ was excised to about 1–2 mm pieces, and about 2 g of tissue pieces was transferred to a 15-ml falcon tube to which 3 ml of enzyme mix (1 mg/ml collagenase II and 0.25 mg/ml trypsin) was added. The sample was incubated for 15 min in the shaker at 37°C, shaking at 200 × g. The reaction was neutralized by 7 ml of culture medium. The dissociated cells and tissue pieces were collected by centrifugation at 750 × g and transferred to a 10-cm dish coated with 0.2% gelatin at a density of 0.5–1.5 pieces/cm2. Five milliliters of culture medium was added to the plate, and the sample was placed in a 5% CO2 incubator for 4–5 days until the cells were attached. The culture medium was replaced on day 5 and changed every 2 days thereafter.
M5 (enzymatic method): 2 g of WJ was soaked in culture medium and excised to about 1–2 mm. The tissue pieces were washed three times by PBS to remove the culture medium completely. Then, the tissue pieces were transferred to a 15-ml falcon tube to which 1.6 ml of enzyme mix, 0.4 ml of collagenase I (10 mg/ml), 0.4 ml of collagenase IV (10 mg/ml), 0.4 ml of hyaluronidase (3 mg/ml), 0.4 ml of DNAse I (1 mg/ml), and 2.4 ml of trypsin (0.25%) were added. The sample was incubated for 2 h in the incubator shaker at 37°C, with agitation at 200 × g. The isolated cells were washed three times with PBS and the cell pellet was resuspended in the culture medium. The cells were cultured in 100 mm tissue culture plate and incubated in a 37°C incubator with 5% CO2, with a medium change every 3 days.
M6 (enzymatic method): 2 g of WJ was soaked in culture medium and excised to about 1–2 mm. The tissue pieces were washed three times by PBS to completely remove the culture medium. Then, the tissue pieces were transferred to a 15-ml falcon tube and subjected to two steps of digestion. In the first step, 0.5 ml of collagenase II (10 mg/ml), 4.5 ml of trypsin (0.25%), and 0.5% ethanol were used. In the second step, 0.5 ml of collagenase II (10 mg/ml), 0.5 ml of hyaluronidase (3 mg/ml), 0.5 ml of DNAse I (1 mg/ml), and 3.5 ml of culture medium were used. The sample was incubated in the incubator shaker at 37°C, with agitation at 200 × g for 90 min. The cells were collected by centrifugation at 700 × g and resuspended in 10 ml of culture medium. Then, the cells were cultured in 100-mm tissue culture plate and incubated in a 37°C incubator with 5% CO2, with a medium change every 3 days.
We used three umbilical cords for three independent sets of experiments. For each set of the experiment, one umbilical cord was cut into several 2 g pieces, and the cells were isolated by different methods by one researcher. Three independent researchers conducted three biological replicates.
Isolation of WJ-MSCs by “Mince-Soak-Digest (MSD)”
Two grams of WJ tissue sample was minced to about 1–2 mm and soaked in culture medium for 7 days on 100-mm tissue culture dish. Then, the tissue pieces were collected into a 15-ml falcon tube and subject to enzymatic digestion with a combination of collagenase II, III, and VI at 2 mg/ml and DNAse I (0.1 mg/ml) at 37°C for 1.5 h, under 200 × g agitation. The digestion mixture was filtered through a 40-µm filter to remove undigested tissue, and cells were washed three times by PBS to completely remove the digestion enzymes. Cells were then collected by centrifugation at 700 × g and resuspended in 10 ml of culture medium. Cells were cultured in 100-mm tissue culture dish coated with 0.2% gelatin and incubated in a 37°C incubator with 5% CO2, with a medium change every 3 days for additional 7 days.
Culture of WJ-MSCs
WJ-MSCs were maintained in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM-LG; Invitrogen), supplemented with 10% FBS (Sigma), 100 U/ml of penicillin, and 100 mg/ml of streptomycin. Cultures were grown at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed every 3 days until 80% confluence was reached. To passage the cells, 0.05% Trypsin-EDTA (Invitrogen) was used to dissociate the cells and neutralized them with the culture medium. The cells were then washed once with PBS and collected by centrifugation. The cells were seeded at 1 × 104 cells/cm2.
Cell Growth Curves
MSCs generated with different methods at passage 3 were seeded at 1,000 cells/well in 96-well plates in 100 μl of culture medium. Cell numbers with each method were counted for 14 days with each sample in triplicates, using a hemocytometer. Doubling time was estimated, and Trypan Blue staining assay (Invitrogen) was used to check the viability of the cells.
Flow Cytometry Analysis
Flow cytometry analysis was performed using Human MSC Analysis Kit (BD Biosciences; cat. no. 562245) based on the manufacturer’s protocol. Briefly, cells were dissociated using Accutase (BD Biosciences; cat. no. 561527) at 37 °C for 5 min and collected by centrifugation at 200 × g. Then, the cells were passed through 70 μm strainers to ensure they were digested as single cells before they were subject to flow cytometry analysis. Dissociated cells were incubated in 1% bovine serum albumin (BSA) in PBS containing primary antibodies on ice for 20 min. Cells were then analyzed using a BD FACSAriaII cell sorter. Analysis was performed using FlowJo software (Tree Star), and the gating was performed based on the instructions on doc.flowjo.com and published instructions 42 .
BCA (Bicinchoninic Acid) Protein Assays
BCA protein assays were performed to quantify the total protein concentration using Pierce BCA Protein Assay Kit (Prod #23225) based on the manufacturer’s protocol.
Differentiation Assays for WJ-MSCs
WJ-MSCs were differentiated into multiple mesenchymal lineages (adipogenic, osteogenic, and chondrogenic) using Human MSC Functional Identification kit (R&D Systems; cat. no. SC006) based on the manufacturer’s protocol.
Animals
The animals were housed in an environment-controlled room at 22°C with a 12-h light and dark cycle. Animal handlers were blinded to the experimental group. Tissues obtained were examined in a blinded fashion.
Staging of the Estrous Cycle of the Rats
Sprague-Dawley female rats 8–10 weeks old were used for this study. Vaginal smear/cytology is applied to stage the estrous cycle of the rats as previously described 43 . Briefly, a cotton-tipped swab wetted with saline was introduced into the vagina of the rat. The swab was gently twisted against the vaginal wall to scratch the cells. The cells were collected onto a dry glass slide by gently rubbing the swab across the slide. The slide is air-dried, stained, and viewed under a microscope. The estrus phase is determined by the presence of abundant anucleated cornified epithelial cells. Rats at the estrus phase were used for establishing the endometrium-injury model. All uterine tissues were harvested at the same stage of the estrous cycle.
Establishment of the Rat Intrauterine Adhesions (IUA) Model
The rat model of endometrium-injury was established based on previously published protocols44–46 with a few major modifications. Essentially, to increase the survival rate and reduce the complication rate due to surgery, we exposed a relatively small incision (3–5 mm vs 3 cm in published protocols) and inserted a tweezer to scrape the inner uterine surface instead of a surgical scalpel blade. Briefly, 10% chloral hydrate (300 mg/kg) was injected in the lower left abdominal quadrant of the rat to induce anesthesia. After anesthesia, the left uterine endometrium was exposed by a 3- to 5-mm longitudinal incision on the uterine horns. A tweezer was inserted through the incision and the inner uterine surface was scratched by pulling the tweezer back and forth, left and right for 10 times at each direction. Then, wash the uterine surface with saline and close the uterine horn by three to four intermittent stitches using absorbable sutures.
Transplantation of WJ-MSCs (MSD) to the Rat Uterus
Twelve rats were randomly assigned to two groups: the control (IUA model) group and the transplantation group [transplanted with WJ-MSCs (MSD)]. The left uterine horns of all rats were incised open and then closed by three to four intermittent stitches using absorbable sutures as a sham control. Using the contralateral side as the sham control would avoid possible side effects on fertility due to surgery complications and cancel the inherent variations of fertility between animals. In addition, by testing the fertility ability on another 10 rats, we show that the ability of both uterine horns to conceive is symmetric (Supplementary Fig. S1A). Although the number of implanted embryos on each side is not completely the same, there is no statistical difference between two sides (Supplementary Fig. S1B). For the control group, the inner uterine surface on the right was injured as described above, and MSC culture media was injected as control. For the transplantation group, immediately after the uterine horn was exposed and injured, 2 × 106 WJ-MSCs (MSD) in 200 µl media were injected inside the uterine horn, and then the uterine horn was sutured. Two weeks after surgery, half of the rats in each group were used for fertility tests, and the other half were used for hematoxylin and eosin (H&E) staining and immunofluorescence analysis.
Fertility Test
Rats in estrus were mated with 10-week-old fertile male Sprague-Dawley rats. Day 0 of pregnancy was defined as the day a vaginal plug was found. Rats were then euthanized on gestation days 15–19, and uterine horns were examined to record the number of embryos.
H&E and Masson’s Trichrome Staining
Formalin-fixed rat uterine tissues were paraffin-embedded and sectioned into serial cross sections of 5–10 μm thickness each. Samples were stained as described previously (Animals 2020, 10(4), 683; https://doi.org/10.3390/ani10040683). From the images of H&E staining, the mean thickness of the endometrium was determined from four measurements at 0°, 90°, 180°, and 270°in horizontal sections, and the number of glands was counted from horizontal sections under a 40×magnification. Endometrium thickness was measured from luminal surface to beginning of circular smooth muscle layer on 40× microphotograph with ImageJ software.
Immunofluorescent Staining
Rat uterine sections were deparaffinized in xylene and rehydrated through an ethanol-graded series. For all samples, antigen retrieval was performed by boiling the sections in 0.01 M sodium citrate buffer (pH 6.0) for 20 min, followed by incubation at room temperature for 30 min. A 10% solution of Normal Donkey Serum (Jackson ImmunoResearch Laboratories, Inc., PA, USA) in PBS was used as a blocking buffer. Sections were incubated with the following primary antibodies diluted in blocking solution (1.0% Normal Donkey Serum, 0.1% Triton X-100, and sterile PBS) overnight at 4°C. The sections were washed and labeled with Alexa dye–conjugated secondary antibodies. Sections were mounted in ProLong Gold Antifade mounting media containing DAPI (Cat.#62248, Thermo Fisher Scientific Inc., MA, USA). Negative controls included incubation with rabbit immunoglobulin G antibodies and omission of the primary antibody for all samples.
RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA was extracted using TRIzol Reagent (Cat.#15596026, Thermo Fisher Scientific Inc., MA, USA) and purified with the RNeasy Mini Kit (Cat. # 74004, Qiagen, LLC., MD, USA). Reverse transcription was performed using the SuperScript II Kit (Cat.#18064022, Thermo Fisher Scientific Inc., MA, USA). Quantitative PCR analyses were performed in real time using an ABI PRISM 7900 sequence detection system and SYBR green master mix (Cat.#4309155, Thermo Fisher Scientific Inc., MA, USA). The primer information is provided in the Supplementary Table. Data are presented as mean ± SEM and derived from three independent experiments, which were performed by three independent researchers using one method from three different umbilical cord samples. The data were normalized to the geometric mean of three housekeeping genes: GAPDH, ACTB, and HPRT1.
Data Analysis
Data are presented as mean ± SD for at least three independent experiments (different patient samples) performed in triplicate unless stated otherwise. Statistical significance is determined by Student’s t-test and set at P < 0.05, and Kolmogorov–Smirnov test was used for normality test. Three independent researchers conducted three biological replicates. The quantification was performed blindly.
Results
Comparison of Six Widely Used WJ-MSC Isolation Methods
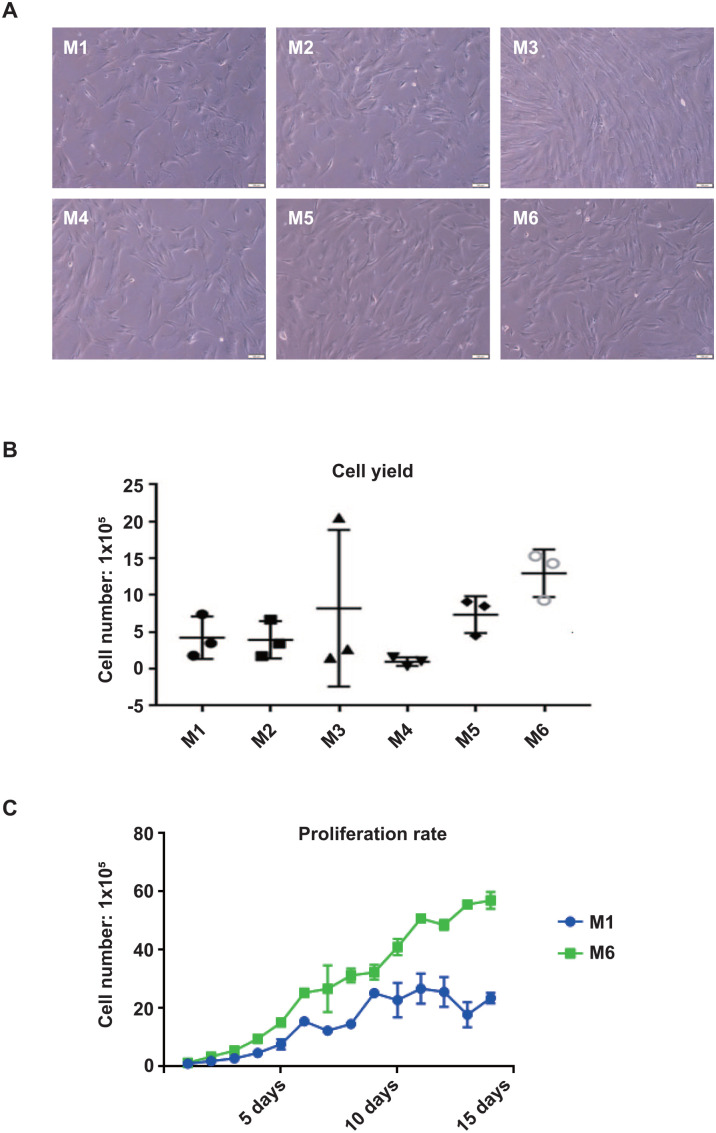
We started to compare the six widely used WJ-MSC isolation methods in terms of cell morphology, cell yields, purity, and proliferation rate. Two explant methods (M1 and M2), two enzymatic-explant methods (M3) and (M4), and two enzymatic methods (M5) and (M6) were tested for comparison. Two grams of umbilical cord tissue was used for each method, and the experiments were repeated three times using three different umbilical cord samples by three independent researchers for statistical analysis. In terms of cell morphology, all six methods generate spindle-like stromal cells with no obvious morphological difference after 14 days of tissue culture (Fig. 1A). However, cell yields vary significantly among different isolation methods. M1 and M2 (explant methods) generate similar cell numbers after 14 days of culture; approximately 5 × 105 cells were isolated from 2 g of WJ (Fig. 1B). This result suggests that additional homogenization procedure in M2 is not beneficial for releasing more cells from the WJ tissue in the explant/mechanical methods. M3, which digests the tissue by collagenase before explant, does not seem to be a reliable or robust isolation method, as the cell yield varies significantly between technical repeats. We noticed that the jelly-like structure remains after collagenase treatment, and the cells may be trapped within the jelly structure. This indicates that 4°C overnight collagenase treatment is not efficient enough to digest the WJ tissue completely. The other enzymatic-explant method, M4, which combines trypsin digestion and explant method, generates the least cell numbers. Two enzymatic methods, M5 and M6, yield more cells compared with the explant and enzymatic-explant methods. M6 generates the most cells, with an average number of 1.3 × 106 cells after 14 days of culture (Fig. 1B).
Figure 1.
Comparison of the six widely used isolation methods. (A) The morphology of cells isolated from M1 to M6; scale bars represent 100 µm; (B) counts of cell number using M1 to M6; (C) growth curve of isolated mesenchymal stem cells from M1 and M6.
Cell proliferation rate is another critical factor in determining the quality of the cells. M1 and M6 were selected to represent the explant and enzymatic methods for cell proliferation analysis. Explant-enzymatic methods were not chosen as their performance in cell yield is not as good as the other methods. The results showed that cells isolated by M6 have higher cell proliferation rate than M1, suggesting that enzymatic treatment does not compromise the ability of cell growth (Fig. 1C).
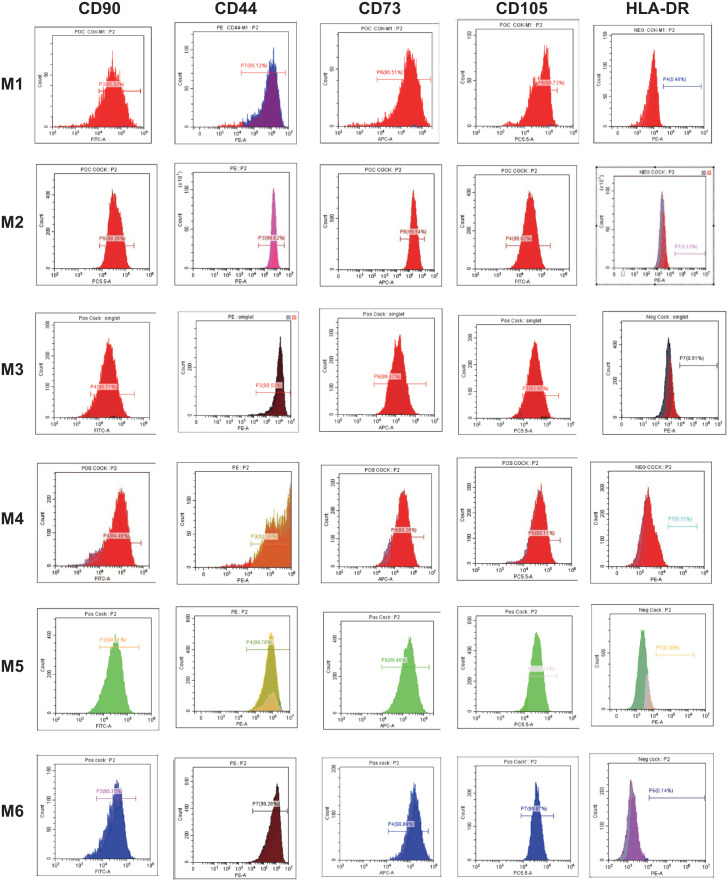
Furthermore, to compare the purity of MSCs generated by different methods, we performed flow cytometry analysis to profile the surface markers on the cells at passage 232. Based on the analysis, more than 90% of the cells generated by M2, M3, M5, and M6 are positive for the established MSC markers (CD44, CD90, CD73, and CD105), and less than 1% of the cells are positive for other lineage markers, such as CD34, CD11b, CD19, CD45, and HLA-DR (Fig. 2 and Table 1). However, the purity was lower in the cells generated by M1 and M4, with less than 90% of the cells being positive for MSC markers (Table 1, Fig. 2].
Figure 2.
Immunophenotype of isolated cells from M1 to M6. Isolated cells at passage 3 were used for flow cytometry analyses. Each histogram is a representative result of three biological repeats. Values represent the mean percentage of all assessed cells positively stained by the respective antibodies in the flow cytometry analyses.
Table 1.
The Percentage of Cells Positive for the Surface Proteins.*
| M1 (%) | M2 (%) | M3 (%) | M4 (%) | M5 (%) | M6 (%) | |
|---|---|---|---|---|---|---|
| CD44 | 95.78ns | 99.04ns | 96.43ns | 55.42*** | 99.75ns | 99.65 |
| CD73 | 96.33ns | 98.67ns | 99.12ns | 84.35*** | 99.58ns | 99.09 |
| CD90 | 86.73** | 99.05ns | 93.42ns | 87.51** | 93.94ns | 96.00 |
| CD105 | 62.23*** | 99.55ns | 92.75ns | 91.17* | 93.02ns | 99.68 |
| HLA-DR | 0.49*** | 0.12ns | 0.52*** | 0.40*** | 0.20* | 0.14 |
nsnot significant.* p < 0.1. **p < 0.01. ***p < 0.001.
Taken together, M6, which releases MSCs from WJ tissue by two steps of enzymatic digestion, has the best performance among all the methods tested in terms of cell morphology, cell yield, cell proliferation rate, and cell purity. This indicates that the synergy of different enzymes helps disrupt the WJ tissue matrix and release the cells. However, harsh treatment of multiple enzymes on the solid tissue could compromise the quality of the cells for use in subsequent cellular studies or clinical applications, especially stem cells that are more sensitive to enzymatic treatment. Besides, using multiple enzymes for isolation costs relatively more to recover the same number of cells.
Development of the MSD Method
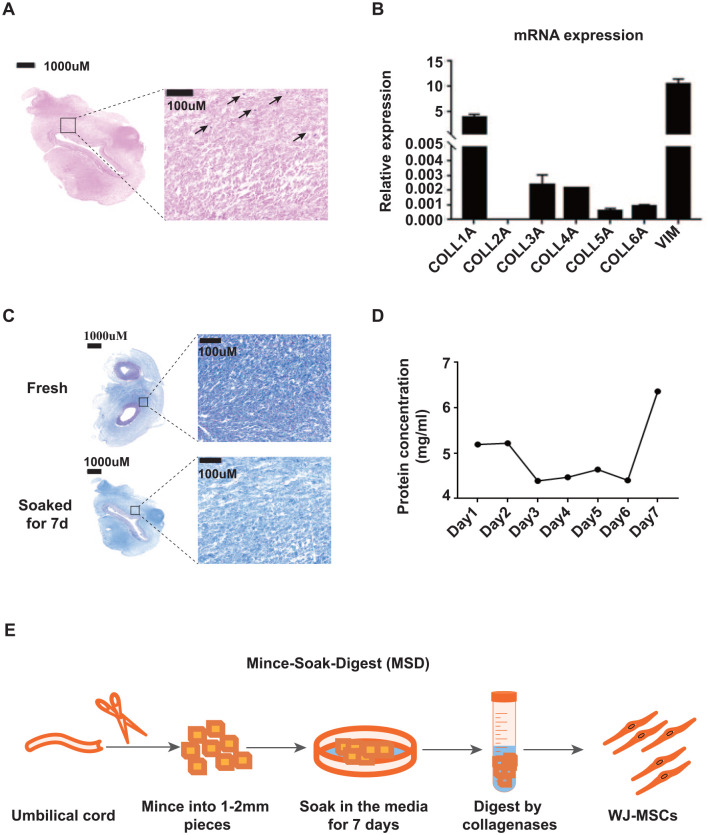
While using the existing methods to isolate WJ-MSCs, we noticed that many cells were trapped in the remaining tissue after migration in the explant methods (Fig. 3A). Similarly, big tissue pieces containing many cells were not digested completely even after long-time incubation with a combination of enzymes in the enzymatic methods. The thick collagen fibers seem to be the physical barrier for the cells to dissociate from the tissue. It is reported that collagen constitutes 95% wet weight of WJ 47 , and our mRNA expression analysis confirmed that COL1A1 (representing Collagen type I) is the predominant protein expressed in WJ (Fig. 3B) 48 .
Figure 3.
Development of the Mince-Soak-Digest (MSD) method for isolation of WJ-MSCs. (A) Hematoxylin and eosin staining of WJ tissue after explant isolation methods. Arrows indicate cells that are trapped in the tissue. Scale bar represents 1,000 µm; (B) mRNA expression of collagen genes in WJ. VIM (Vimentin) gene was used as a positive control. (C) Masson’s trichrome staining of collagen fibers in fresh and soaked WJ tissues. Scale bars on the left original tissue images represent 1,000 µm and on the right magnified images represent 100 µm. (D) BCA protein assays were used to quantify the total protein concentration of media used to soak WJ tissues. (E) Schematic diagram of the MSD method. WJ-MSCs: mesenchymal stem cells derived from umbilical cord Wharton’s Jelly; BCA: bicinchoninic acid.
We also noticed that the density of collagen fibers in WJ tissues is reduced significantly when soaked in the media for 7 days in the explant methods (Fig. 3C). The soaking media becomes viscous and the protein concentration increases, indicating that collagen proteins are released from the WJ tissue during the process of soaking (Fig. 3D). These observations prompt us to test whether a soaking procedure would facilitate the subsequent extracellular matrix digestion by collagenase, thus enhancing the resultant cell yield from WJ tissue.
Thus, we develop a new WJ-MSC isolation method that incorporates a soaking procedure in the enzymatic method, and we name it “Mince-Soak-Digest.” Briefly, we mince the WJ into small pieces and soak them in the MSC culture media for 7 days. After soaking, we digest the collagen-reduced WJ tissue with collagenase II and VI to collect the cells (Fig. 3E).
MSD Method Is Advantageous to the Six Widely Used Isolation Methods
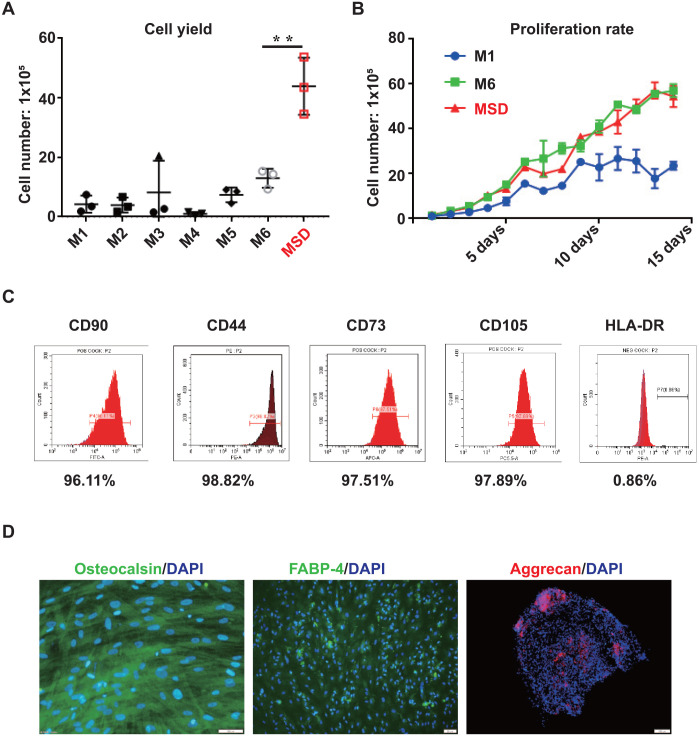
We went on to compare MSD with the other six methods in terms of cell yield, proliferation rate, and purity. We found that MSD generates significantly higher cell numbers consistently. An average of 4.7 × 106 cells were obtained from 2 g of tissues. This yield is 4- to 10-fold higher than the six widely used methods (Fig. 4A). Importantly, WJ-MSCs isolated by MSD [WJ-MSCs (MSD)] have comparable proliferation rate (Fig. 4B) and purity (>95% of the cells are positive for CD44, CD90, CD73, and CD105 and 0.86% of the cells are positive for CD34, CD11b, CD19, CD45, and HLA-DR) with M6, which has the best performance among the six tested methods (Fig. 4C).
Figure 4.
Comparison of the MSD method with the six widely used methods. (A) Counts of cell numbers of MSD and M1–M6. (B) Growth curve of cell number of M1, M6, and MSD. (C) Flow cytometry analysis on MSD-isolated cells. (D) Immunofluorescence staining of MSD-isolated cells after directed differentiation. Scale bars represent 100 µm. MSD: Mince-Soak-Digest.
To further confirm the stem cell potential of WJ-MSCs isolated by MSD, we tested the differentiation abilities of these cells to become osteogenic, chondrogenic, and adipogenic cells. To our expectation, these cells are able to differentiate into all three lineages, as demonstrated by the immunofluorescent staining of FABP-4 as adipocyte marker, aggrecan as chondrocyte marker, and osteocalcin as osteocyte marker (Fig. 4D).
Collectively, we have developed a robust and highly efficient method to isolate MSCs from WJ with significantly higher cell yield (4- to 10-fold higher) than any of the widely used methods that we tested.
Transplantation of WJ-MSCs (MSD) Promotes Endometrium Regeneration in IUA Rats
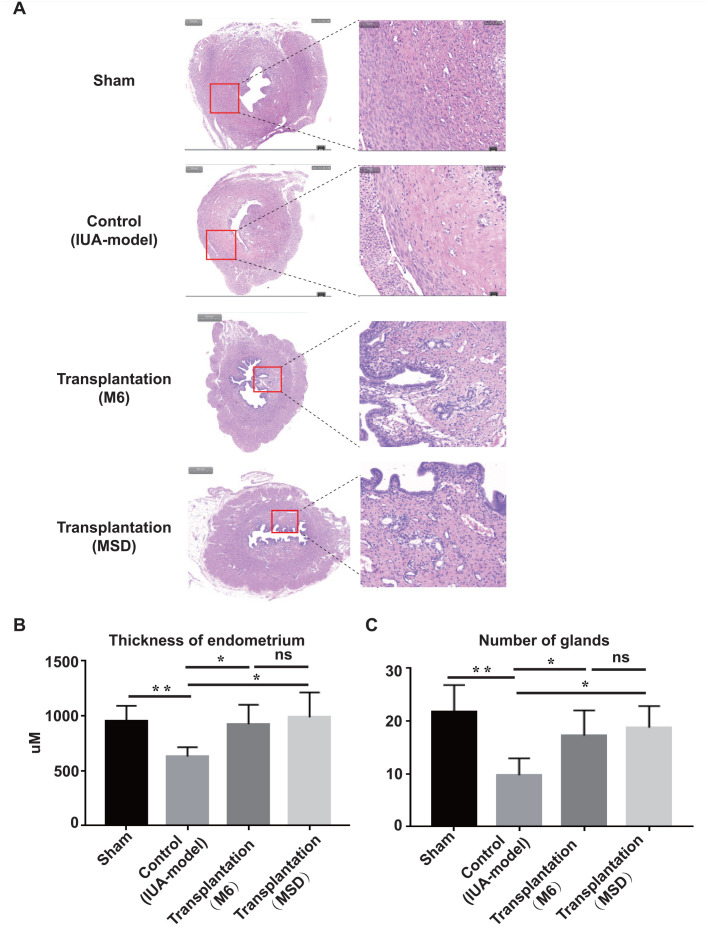
MSCs from multiple sources, including WJ-MSCs, have been shown to facilitate endometrial regeneration and fertility restoration in rat and rhesus monkey IUA models, primarily due to their paracrine effects at the lesion site36,49–52. To further test the therapeutic potential of WJ-MSCs (MSD) for tissue regeneration, we established a rat IUA model and transplanted WJ-MSCs generated by M6 and MSD into the uterus of the rats. The rat IUA model is developed by mechanical injury based on previously published protocols with some modifications. We confirmed by H&E staining that the thickness of the uterine in the IUA model was thinner, and gland numbers were decreased compared with the uterine in the sham group (Fig. 5A–C). After transplantation of WJ-MSCs, generated either by MSD or by M6, into the injured uterine of the IUA model, the uterine thickness, as well as the number of the uterine glands, was recovered to the level comparable to the sham group (Fig. 5A–C). Note that although there is no significant difference between MSD- and M6-generated WJ-MSCs in terms of the recovery effect, MSD has the advantage of generating higher cell yield compared with M6 and other methods.
Figure 5.
Endometrial regeneration by Wharton’s jelly-mesenchymal stem cells (Mince-Soak-Digest). (A) Hematoxylin and eosin staining of rat uteri under different treatments. Scale bars on the left original tissue images represent 1,000 µm and on the right magnified images represent 100 µm. Statistical analysis of endometrial thickness (B) and the number of endometrial glands (C) under different treatments.
Transplantation of WJ-MSCs (MSD) Restores the Fertility of IUA Rats
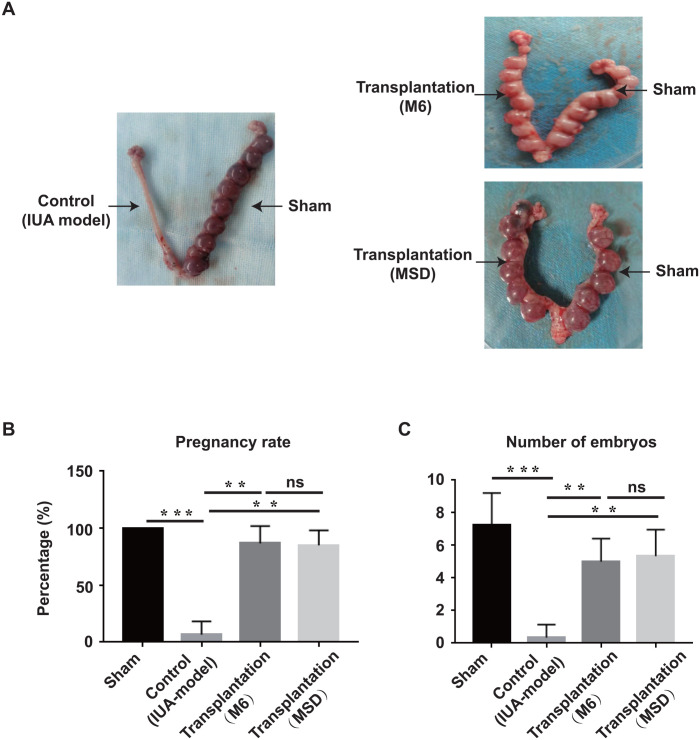
Fertility assessment is one of the “gold standards” to test the function of the regenerated endometrium. Two weeks after surgery, we went on to perform a fertility test for sham, control (IUA model), and transplantation groups. The pregnancy rate for the sham side is 100%, as all the left uterine horns are able to receive embryos. However, due to the injury, the pregnancy rate for the control (IUA model) group is only about 7%, which verified the reliability of our IUA model. Significantly, after transplantation of WJ-MSCs, generated either from MSD or from M6, the pregnancy rate has increased to more than 80% (Fig. 6B). Furthermore, we examined the implantation efficiency by quantification of the well-developed embryos, which have comparable weights and dimensions as normal embryos developed in the sham group (Fig. 6A). The results showed that each uterine horn from the sham group received seven embryos on average, while only one out of the six uterine horns in the control (IUA model) group received two embryos. Significantly, each uterine horn from the transplantation group received five well-developed embryos on average.
Figure 6.
Fertility restoration by Wharton’s jelly-mesenchymal stem cells (Mince-Soak-Digest). (A) Embryo implantation images of rat uteri under different treatment. Black arrows indicate treatment received for each side of uteri horn. Statistical analysis of pregnancy rate (B) and the number of received well-developed embryos (C).
Taken together, these results demonstrate that WJ-MSCs have therapeutic potential to restore endometrium tissue injury and fertility capability. Again, although there is no significant difference between MSD- and M6-generated WJ-MSCs in terms of the therapeutic effect, MSD increases the quantity of the cell yield without compromising the quality. These results suggest that MSD could be generalized as an isolation method to obtain WJ-MSCs for large-scale clinical trials for tissue repair.
Discussion
WJ-MSCs show promising efficacy in animal models for a variety of degenerative diseases. However, in most studies, they do not produce the same results when translated to the human system53,54. One possible problem is the heterogeneity of the cells, which largely results from the current isolation methods. Besides, the limited sources and in vitro proliferation ability of WJ-MSCs make the cell number a bottleneck for large-scale clinical application. Based on carefully analyzing multiple widely used isolation protocols, we find that the biggest obstacle for WJ-MSC isolation is the inefficiency in the digestion of the extracellular matrix. Thus, we have developed MSD, which disrupts the extracellular matrix of WJ by soaking before enzymatic digestion. This results in a significant increase in cell yield by 4- to 10-fold with high purity and consistency. Importantly, we show that the isolated MSCs have the powerful regenerative potential for tissue repair and could be generalized for large-scale clinical trials.
Explant culture and enzymatic digestion are the two prevalent methods to isolate WJ-MSCs. The absence of proteolytic stress and the presence of tissue origin are supposed to increase the viability of the cells in the explant culture method. However, the structure of the extracellular matrix within the tissue creates a big physical barrier for cells to migrate out, which results in only a small percentage of total cells managing to grow out of the entire tissue piece. The enzymatic method, on the other hand, is more efficient to obtain a single cell population from the tissue, but the concentration of the enzymes and the incubation time for digestion are difficult to optimize to achieve high cell viability. Moreover, dense collagen fibers are difficult to be digested completely, leaving many of the cells to remain in the collagen fibers. In many cases, the two methods are combined (explant-enzymatic) to complement each other. However, in our test, the combination of explant and enzymatic M3 and M4 are not as good as the other methods in terms of cell yield, reproducibility, and purity. Essentially, among all the tested methods, we find that there are considerable variations in the technical details from published protocols, including the type and concentration of collagenase used, as well as the temperature and duration of enzymatic digestion or explant incubation, which leads to variable sizes of undigested tissues. These variations in different laboratories produce inconsistent results between studies.
An important step that we integrate into our method is the soaking step, which has been proven to facilitate the disruption of the extracellular matrix in the WJ tissue. While soaking, the collagen fibers leach out to the media and leave the remaining loose matrix easier to be digested. This step makes the dissociation of cells much simpler and more controllable. Thus, we propose that the combination of a soaking step with enzymatic digestion contributes to the elimination of the variations between studies due to the cell heterogeneity.
IUA, also called Asherman’s syndrome 55 , is characterized by partial or complete bonding of the uterine cavity due to the formation of scar tissue inside the uterus caused by uterine surgery 56 . Severe recurrent IUA hinders embryos implantation and leads to miscarriage 57 . The primary goal of IUA treatment is to restore endometrial regeneration, and stem cell–based therapy has become one of the promising adjuvant therapies for IUA treatment 58 . Substantial progress has been made in preclinical and clinical studies of using umbilical cord–derived- SCs for the treatment of IUA. However, large-scale, double-blind, randomized trials are urgently needed to evaluate the safety and efficacy of the treatment. Our results show that our WJ-MSCs (MSD) is capable of recovering the regeneration of the endometrium and restoring fertility. We propose that sufficient clinical-grade WJ-MSCs could be generated by MSD and apply to large-scale clinical trials for degenerative diseases.
Conclusion
In summary, we have established a robust and highly efficient approach, “Mince-Soak-Digest,” to isolate WJ-MSCs. Compared with the current widely used approaches, MSD could significantly increase the cell yield by 4- to 10-fold with high purity and consistency. Importantly, we show that the isolated MSCs have the powerful regenerative potential for endometrial tissue repair. Taken together, our study contributes to the standardization of WJ-MSC isolation, which assures the abundance of WJ-MSCs and eliminates the discrepancies due to isolation procedures, thus facilitating the evaluation of the efficacy of WJ-MSCs across various human clinical applications.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897221084354 for A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton’s Jelly for Tissue Repair by Shengxia Zheng, Yanyan Gao, Kai Chen, Yusheng Liu, Ninuo Xia and Fang Fang in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897221084354 for A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton’s Jelly for Tissue Repair by Shengxia Zheng, Yanyan Gao, Kai Chen, Yusheng Liu, Ninuo Xia and Fang Fang in Cell Transplantation
Footnotes
Authors’ Contributions: SZ: Conception and design, provision of study material or patients, final approval of the manuscript.
YG: Provision of study material or patients, collection and assembly of data, data analysis and interpretation.
KC: Collection and/or assembly of data, data analysis and interpretation.
YL: Conception and design, provision of study material or patients.
NX: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript.
FF: Conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript.
Availability of Data and Material: The original data are available from the corresponding author on request.
Ethical Approval: All procedures involving the human umbilical cord were conducted in accordance with the Institutional Review Board (IRB) guidelines for the University of Science and Technology of China (USTC). Collection of the umbilical cord was approved under IRB code no. 1603-IGX-016-CS. All animal experiments were compliant with all relevant ethical regulations regarding animal research approved by the ethics committee of USTC under IRB code no. USTACUC202101023.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the University of Science and Technology of China Institutional Review Board’s (code no. 1603-IGX-016-CS and USTACUC202101023) approved protocols.
Statement of Informed Consent: Written informed consent for the use of the tissue was obtained from each woman.
Consent for Publication: All authors agree to publish this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the National Natural Science Foundation of China (Grant No. 32070830 and Grant No. 81971339), the Natural Science Foundation of Anhui Province (Grant No. 1808085MH242), and the research funds from the University of Science and Technology of China (WK9110000141 and YD9100002007).
ORCID iD: Fang Fang  https://orcid.org/0000-0002-5272-302X
https://orcid.org/0000-0002-5272-302X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–11. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007; 25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 3. Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 5. Lakshmipathy U, Verfaillie C. Stem cell plasticity. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 7. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–89. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 10. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005; 2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13. Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 14. Li H, Ghazanfari R, Zacharaki D, Lim HC, Scheding S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 2016;1370:109–18. doi: 10.1111/nyas.13102. [DOI] [PubMed] [Google Scholar]
- 15. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–10. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 16. Simoes IN, Boura JS, dos Santos F, Andrade PZ, Cardoso CM, Gimble JM, da Silva CL, Cabral JM. Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnol J. 2013;8:448–58. doi: 10.1002/biot.201200340. [DOI] [PubMed] [Google Scholar]
- 17. Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655–67. doi: 10.3727/096368910X536473. [DOI] [PubMed] [Google Scholar]
- 18. Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15:708–18. doi: 10.1016/s1472-6483(10)60539-1. [DOI] [PubMed] [Google Scholar]
- 19. Fong CY, Subramanian A, Biswas A, Gauthaman K, Srikanth P, Hande MP, Bongso A. Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton’s jelly stem cells. Reprod Biomed Online. 2010;21:391–401. doi: 10.1016/j.rbmo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 20. Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7:1–16. doi: 10.1007/s12015-010-9166-x. [DOI] [PubMed] [Google Scholar]
- 21. Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–37. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 22. Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton’s jelly of umbilical cord. Methods Cell Biol. 2008;86:101–19. doi: 10.1016/S0091-679X(08)00006-X. [DOI] [PubMed] [Google Scholar]
- 23. Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253–61. doi: 10.1111/j.1365-3148.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- 24. Hassan G, Kasem I, Soukkarieh C, Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: using explant method and umbilical cord blood serum. Int J Stem Cells. 2017;10:184–92. doi: 10.15283/ijsc17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendijani F. Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50:e12334. doi: 10.1111/cpr.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marmotti A, Mattia S, Bruzzone M, Buttiglieri S, Risso A, Bonasia DE, Blonna D, Castoldi F, Rossi R, Zanini C, Ercole E, et al. Minced umbilical cord fragments as a source of cells for orthopaedic tissue engineering: an in vitro study. Stem Cells Int. 2012;2012:326813. doi: 10.1155/2012/326813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varaa N, Azandeh S, Khodabandeh Z, Gharravi AM. Wharton’s jelly mesenchymal stem cell: various protocols for isolation and differentiation of hepatocyte-like cells; narrative review. Iran J Med Sci. 2019;44:437–48. doi: 10.30476/ijms.2019.44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JR, Cromer A, Weiss ML. Human umbilical cord mesenchymal stromal cell isolation, expansion, cryopreservation, and characterization. Curr Protoc Stem Cell Biol. 2017;41:1F.18.1–23. doi: 10.1002/cpsc.24. [DOI] [PubMed] [Google Scholar]
- 29. Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, Chaudhry GR. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;122:55224. doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bharti D, Shivakumar SB, Park JK, Ullah I, Subbarao RB, Park JS, Lee SL, Park BW, Rho GJ. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018;372:51–65. doi: 10.1007/s00441-017-2699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yi X, Chen F, Liu F, Peng Q, Li Y, Li S, Du J, Gao Y, Wang Y. Comparative separation methods and biological characteristics of human placental and umbilical cord mesenchymal stem cells in serum-free culture conditions. Stem Cell Res Ther. 2020;11:183. doi: 10.1186/s13287-020-01690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 33. Nekanti U, Mohanty L, Venugopal P, Balasubramanian S, Totey S, Ta M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5:244–54. doi: 10.1016/j.scr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Shi L, Lin X, Zhou F, Xin L, Xu W, Yu H, Li J, Pan M, Pan Y, Dai Y, et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12:420. doi: 10.1186/s13287-021-02499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087–96. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 36. Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, Wang J, Bai D, Wang J, Wang L, Zhou Q, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9:192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, Paterson K, Boyd R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14:213–30. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 38. Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Huang S, Li S, Li M, Shi J, Bai W, Wang Q, Zheng L, Liu Y. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Devel Ther. 2019;13:4331–40. doi: 10.2147/DDDT.S225613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–99. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verschoor CP, Lelic A, Bramson JL, Bowdish DM. An introduction to automated flow cytometry gating tools and their implementation. Front Immunol. 2015;6:380. doi: 10.3389/fimmu.2015.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract. 2020;6:5. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuramoto G, Takagi S, Ishitani K, Shimizu T, Okano T, Matsui H. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum Reprod. 2015;30:406–16. doi: 10.1093/humrep/deu326. [DOI] [PubMed] [Google Scholar]
- 45. Feng Q, Gao B, Zhao X, Huang H, Yi S, Zou L, Liu X, Xue M, Xu D. Establishment of an animal model of intrauterine adhesions after surgical abortion and curettage in pregnant rats. Ann Transl Med. 2020;8:56. doi: 10.21037/atm.2020.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang H, Wu S, Feng R, Huang J, Liu L, Liu F, Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res Ther. 2017;8:267. doi: 10.1186/s13287-017-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davies JE, Walker JT, Keating A. Concise review: Wharton’s jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6:1620–30. doi: 10.1002/sctm.16-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bankowski E, Romanowicz L, Jaworski S. Collagen of umbilical cord arteries and its alterations in EPH-gestosis. J Perinat Med. 1993;21:491–98. doi: 10.1515/jpme.1993.21.6.491. [DOI] [PubMed] [Google Scholar]
- 49. Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, Ma L, Gao C, Zhang S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92: 160–71. doi: 10.1016/j.actbio.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 50. Benor A, Gay S, DeCherney A. An update on stem cell therapy for Asherman syndrome. J Assist Reprod Genet. 2020;37: 1511–29. doi: 10.1007/s10815-020-01801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keyhanvar N, Zarghami N, Bleisinger N, Hajipour H, Fattahi A, Nouri M, Dittrich R. Cell-based endometrial regeneration: current status and future perspectives. Cell Tissue Res. 2021;384:241–54. doi: 10.1007/s00441-021-03419-6. [DOI] [PubMed] [Google Scholar]
- 52. Wang L, Yu C, Chang T, Zhang M, Song S, Xiong C, Su P, Xiang W. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6:eaba6357. doi: 10.1126/sciadv.aba6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamal MM, Kassem DH. Therapeutic potential of Wharton’s jelly mesenchymal stem cells for diabetes: achievements and challenges. Front Cell Dev Biol. 2020;8:16. doi: 10.3389/fcell.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Asherman JG. Traumatic intra-uterine adhesions. J Obstet Gynaecol Br Emp. 1950;57:892–96. doi: 10.1111/j.1471-0528.1950.tb06053.x. [DOI] [PubMed] [Google Scholar]
- 56. Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil Steril. 1982;37:593–610. doi: 10.1016/s0015-0282(16)46268-0. [DOI] [PubMed] [Google Scholar]
- 57. Di Guardo F, Della Corte L, Vilos GA, Carugno J, Török P, Giampaolino P, Manchanda R, Vitale SG. Evaluation and treatment of infertile women with Asherman syndrome: an updated review focusing on the role of hysteroscopy. Reprod Biomed Online. 2020;41:55–61. doi: 10.1016/j.rbmo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 58. Zhao Y, Luo Q, Zhang X, Qin Y, Hao J, Kong D, Wang H, Li G, Gu X, Wang H. Clinical efficacy and safety of stem cell-based therapy in treating Asherman syndrome: a system review and meta-analysis. Stem Cells Int. 2020;2020:8820538. doi: 10.1155/2020/8820538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897221084354 for A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton’s Jelly for Tissue Repair by Shengxia Zheng, Yanyan Gao, Kai Chen, Yusheng Liu, Ninuo Xia and Fang Fang in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897221084354 for A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells From Wharton’s Jelly for Tissue Repair by Shengxia Zheng, Yanyan Gao, Kai Chen, Yusheng Liu, Ninuo Xia and Fang Fang in Cell Transplantation