Key Points
Question
What is the long-term persistence of different biologic classes to treat psoriasis (PsO) and psoriatic arthritis (PsA)?
Findings
This cohort study of 16 892 patients with PsO and 6531 patients with PsA found an association between interleukin 17 inhibitors and higher treatment persistence compared with tumor necrosis factor inhibitors for PsO and PsA and interleukin 12 and interleukin 23 inhibitors for PsA. Interleukin 12 and 23 inhibitors were associated with higher treatment persistence than tumor necrosis factor inhibitors for PsO, and the persistence rates of all biologics remained globally low at 3 years.
Meaning
These findings suggest that for some patients, long-term control of PsO and PsA may require several courses of different agents.
This cohort study estimates the persistence of first-line biologics and compares this persistence among different therapeutic classes in a large national cohort of patients with psoriasis and psoriatic arthritis.
Abstract
Importance
Treatment options for psoriasis (PsO) and psoriatic arthritis (PsA) have evolved significantly throughout the era of biologics. Clinical trials are inadequate to assess the relative long-term efficacy of biologics and are often insufficient regarding safety.
Objectives
To assess the long-term persistence of different biologic classes to treat PsO and PsA.
Design, Setting, and Participants
This nationwide cohort study involved the administrative health care database of the French health insurance scheme linked to the hospital discharge database. All adults with PsO and PsA who were new users of biologics (not in the year before the index date) from January 1, 2015, to May 31, 2019, were included and followed up through December 31, 2019. Patients hospitalized for PsA in the PsO cohort and for PsO in the PsA cohort in the year before the index date were excluded. Data were analyzed from June 1 to October 31, 2021.
Main Outcomes and Measures
Persistence was defined as the time from biologic therapy initiation to discontinuation and was estimated using the Kaplan-Meier method. Comparison of persistence by biologic class involved using propensity score–weighted Cox proportional hazards regression models and adjustment on specific systemic nonbiologics (time-dependent variables).
Results
A total of 16 892 patients with PsO were included in the analysis (mean [SD] age, 48.5 [13.8] years; 9152 men [54.2%] men). Of these, 10 199 patients (60.4%) started therapy with a tumor necrosis factor (TNF) inhibitor; 3982 (23.6%), with an interleukin 12 and interleukin 23 (IL-12/23) inhibitor; and 2711 (16.0%), with an interleukin 17 (IL-17) inhibitor. An additional 6531 patients with PsA (mean [SD] age, 49.1 [12.8] years; 3565 [54.6%] women) were included; of these, 4974 (76.2%) started therapy with a TNF inhibitor; 803 (12.3%), with an IL-12/23 inhibitor; and 754 (11.5%), with an IL-17 inhibitor. Overall 3-year persistence rates were 40.9% and 36.2% for PsO and PsA, respectively. After inverse probability of treatment weighting and adjustment, the IL-17 inhibitor was associated with higher persistence compared with the TNF inhibitor for PsO (weighted hazard ratio [HR], 0.78 [95% CI, 0.73-0.83]) and PsA (weighted HR, 0.70 [95% CI, 0.58-0.85]) and compared with the IL-12/23 inhibitor for PsA (weighted HR, 0.69 [95% CI, 0.55-0.87]). No difference between the IL-17 inhibitor and IL-12/23 inhibitor for PsO was noted. The IL-12/23 inhibitor was associated with higher persistence than the TNF inhibitor for PsO (weighted HR, 0.76 [95% CI, 0.72-0.80]), with no difference observed for PsA.
Conclusions and Relevance
The findings of this cohort study suggest that IL-17 inhibitors are associated with higher treatment persistence than the TNF inhibitor for PsO and PsA. Interleukin 17 inhibitors were also associated with higher persistence than the IL-12/23 inhibitor for PsA, with no difference for PsO. However, the persistence rates of all biologics remained globally low at 3 years.
Introduction
Psoriasis (PsO) is a chronic, immune-mediated inflammatory skin disease affecting 1% to 5% of the world’s population and 4% of the French population.1,2,3 Psoriatic arthritis (PsA), which accounts for 14% to 23% of the population of PsO, presents as a heterogeneous chronic inflammatory arthritis with a combination of articular, periarticular, and extra-articular manifestations.4,5 These diseases can be severe, leading to impaired quality of life.6,7 With the fast emergence of biologics during the past 2 decades, treatments for PsO and PsA have changed greatly. Inhibitors of tumor necrosis factor (TNF) but also interleukin 12 and interleukin 23 (IL-12/23) and interleukin 17 (IL-17) are now recommended for moderate to severe PsO/PsA when nonbiologic treatments fail to control disease or are not tolerated.8,9 Currently, in Europe or Canada, biologic treatments may be positioned as second-line systematic therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure of, intolerance of, or contraindication for nonbiologic systemic agents). Such decisions were based on the lack of long-term safety knowledge but also took into account economic consideration. These therapies have been found effective and safe in various studies in the short term, but biologic therapy can be discontinued because of primary or secondary failure or adverse events.10 In addition, clinical trials—mainly placebo-controlled for 12 to 16 weeks—are insufficient to assess the relative long-term efficacy of biologics and are often incomplete regarding safety.11
Treatment persistence, defined as the time from initiation to discontinuation, is an important real-world outcome for assessing the total value of a drug and is a relevant indicator of a patient’s level of interest.12,13 This criterion can be considered a composite of efficacy (a treatment perceived as ineffective is likely to be discontinued) and safety (a poorly tolerated treatment is likely to be discontinued) but also of patient satisfaction or preference and adherence. In addition, patients with PsO/PsA have numerous comorbidities, which may also affect management as well as persistence of these treatments.14,15
Treatment persistence is of increasing interest in the fields of dermatology and rheumatology because it would allow for guiding treatment strategy on the basis of patient characteristics. However, existing studies are limited by selective recruitment or by the low number of patients included, and few have included the most recently marketed biologics.16,17,18,19,20 The present study aimed to estimate the persistence of first-line biologics for PsO and PsA and to compare this persistence among different therapeutic classes in a large national cohort of patients.
Methods
Data Source and Study Design
This nationwide cohort study was based on analyses of the French National Health Insurance database (Système National des Données de Santé), covering more than 66 million individuals (98.8% of the French population) identified by a unique, anonymous identifier. The French health care system provides universal and mandatory coverage: all citizens have free, equal, and universal access to health care for chronic diseases. As previously described,21,22 this database contains individualized outpatient health data (sociodemographic characteristics, including year of birth, sex, and vital status; number of units and date of reimbursed drug dispensation; date and nature of medical and paramedical interventions; and information on patient eligibility for fully reimbursed care related to severe, costly chronic diseases, such as moderate to severe PsO/PsA, encoded according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]) and public and private hospitalization data (dates of hospital admission and discharge; the ICD-10 code on discharge; the medical procedures performed in the hospital; and expensive drugs, such as biologics, administered in the hospital). This large database has been used for several pharmaco-epidemiological studies.23,24 The French data protection agency (Commission Nationale de L’informatique et des Libertés) provided specific approval to conduct this study and waived the obligation for informed consent for the use of pseudonymized data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
All adults (aged ≥18 years) with PsO or PsA registered in the Système National des Données de Santé were eligible for inclusion from January 1, 2015, to May 31, 2019. Adults with PsO were identified by the reimbursement of at least 2 topical vitamin D derivatives (Anatomical Therapeutic Chemical D05AX, the recommended first-line treatment for PsO in France) within a 2-year period, and adults with PsA were identified with a specific ICD-10 code (M07, except M07.4 and M07.5). Algorithms used to identify patients with PsO and PsA have been detailed and validated previously.25,26 Patients with at least 1 prescription of a biologic agent for PsO/PsA were identified. Next, we selected previously biologic-naive patients (ie, new users27), defined as those who had not filled a prescription for one of these drugs for 1 year. To ensure more homogeneous cohorts, we excluded patients hospitalized in the year before the index date for PsA from the PsO cohort and patients hospitalized in the year before the index date for PsO from the PsA cohort. Finally, we excluded patients who did not maintain treatment for at least 6 months. This protocol allowed us to take into account differences in the frequency of administration of biologics, which may lead to an overestimation of treatment persistence with the longest interval of administration (eg, for ustekinumab, the clinician assesses the efficacy after ≥6 months of treatment)13 but also to exclude early discontinuation due to change of patient opinion or injection experience (eg, pain, burning, and/or discomfort during injection, fear of injection or needles).28 The index date was the date of the first reimbursement of a biologic agent during the study period. Patients were followed up until December 31, 2019.
Outcome and Exposure Definition
The primary end point was persistence of biologic treatment, defined as the time from treatment initiation to discontinuation. The biologic agents (originator and biosimilar) included the TNF inhibitors etanercept, infliximab, adalimumab, certolizumab, and golimumab; the IL-12/23 inhibitor ustekinumab; and the IL-17 inhibitors secukinumab, ixekizumab, and brodalumab. Interleukin 23 inhibitors, which were marketed for PsO from 2019, were not analyzed in this study because of the limited time of use at the end of our inclusion period. Drugs were identified in outpatient and hospital discharge databases. The period covered by a prescription was 30 days for most TNF inhibitors and IL-17 inhibitors, 56 days for infliximab, and 84 days for the IL-12/23 inhibitor. We defined the discontinuation of treatment as a period of more than 60 days without filling a prescription for the same treatment after the period covered by the previous prescription.
Treatment initiation was defined as delivery of one of the study molecules in the database from January 1, 2015, to May 31, 2019, in a patient who had not received these treatments in the previous year. Only the first therapeutic sequence of biologics was considered in this analysis.
Other drugs used as add-on therapies to biologics were studied: methotrexate sodium, acitretin, and cyclosporine for the PsO cohort and methotrexate, leflunomide, and sulfasalazine for the PsA cohort. Exposure to drug combinations (biologic and nonbiologic drugs or prednisone) at baseline was defined as a period of 30 days or less from the prescription of the first to that of the second treatment.
Covariables
We collected data on basic demographics, including age, sex, complementary universal health coverage, French deprivation index (geographical indicator of social disadvantage specifically adapted to health studies of the French population29), inflammatory diseases associated with PsO/PsA (eg, inflammatory bowel disease, uveitis), variables used to calculate the Charlson Comorbidity Index adapted to the Système National des Données de Santé,30 and other comorbidities (eg, dyslipidemia; dispensing of nicotine replacement therapy, varenicline tartrate, or cytisine; other hospital discharge diagnoses related to tobacco, such as mental and behavioral disorders due to use of tobacco or problems related to tobacco use; or morbid or complicated obesity). These covariables are defined in eTable 1 in the Supplement. We also collected the number of consultations with a specialist (a dermatologist in the PsO cohort and a rheumatologist in the PsA cohort) and the number of patients hospitalized for the underlying disease (PsO/PsA) in the 2 years before the index date. During the follow-up, we compiled the vital status.
Statistical Analysis
Data were analyzed from June 1 to October 31, 2021. Categorical variables are reported as number (percentage). Quantitative variables are reported as median (IQR) or mean (SD).
The main analysis was conducted on a per-protocol basis. Patients were followed up until biologic therapy switch, biologic therapy discontinuation, death, or December 31, 2019, whichever came first.
Changes in treatment persistence over time were estimated for all biologics together, for each therapeutic class, and for each molecule involved by using the Kaplan-Meier method. Multivariate Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% CIs for the PsO and the PsA cohorts. The proportional hazards assumption was tested by using Schoenfeld residuals. To control for confounding by baseline covariables, weighted HR values were adjusted by using inverse probability of treatment weighting. Weights were based on the propensity score, which was estimated with logistic regression, including the covariables collected at the index date and related to the primary end point at P < .10 on univariate analysis but also covariables known to be risk factors for the primary end point, as drugs used as add-on therapies to biologics, and/or as drugs associated with initiation of a therapeutic class.31 Stabilized weights were calculated to preserve the sample size of the original data and produce an appropriate estimation of the main effect variance.32 The balance in baseline covariables was compared with standardized differences before and after weighting. The other drugs used as add-on therapies to biologics were also considered time-dependent variables and were used as adjustment variables in the weighted Cox proportional hazards regression model. We performed prespecified subgroup analyses of patients without associated inflammatory disease (no PsA in the PsO cohort, no active skin PsO requiring topical therapies in the PsA cohort, and no inflammatory bowel disease or uveitis) at the index date.
To assess the sensitivity of the estimated weighted HR with respect to several possible models, we performed the following additional analyses: (1) conventional multivariate Cox proportional hazards regression model computing adjusted HRs (the co-reimbursement of nonbiologic drugs or prednisone with biologics was considered a time-varying variable); (2) an intention-to-treat analysis, with follow-up censored at the time of treatment switch, death, or December 31, 2019, whichever came first; (3) defining treatment discontinuation as more than 30 days or more than 90 days without filling a prescription for the same treatment after the period covered by the previous prescription; (4) modifying the new-user definition as patients who had not filled a prescription for a biologic for 5 years before the index date (and not 1 year as in the main analysis); and (5) a model restricted to patients included in the cohort after July 2017 (to minimize any channeling bias related to the difference in reimbursement years for biologics in France; patients were included 1 year after all the therapeutic classes were marketed for both indications).
All tests were 2-tailed, and results were considered statistically significant at P < .05. Analyses were performed with SAS Enterprise Guide, version 7.1 (SAS Institute Inc).
Results
Description of the Cohort Population
During our study period, we identified 20 749 patients with PsO and 9139 patients with PsA as new users of biologics. After excluding patients hospitalized in the year before the index date for PsA, 16 892 patients were included in the analysis in the PsO cohort (mean [SD] age, 48.5 [13.8] years; 9152 men [54.2%] and 7740 women [45.8%]; median follow-up, 1.3 [IQR, 0.9-2.4] years), with 10 199 (60.4%) initiating TNF inhibitor therapy, 3982 (23.6%) initiating IL-12/23 inhibitor therapy, and 2711 (16.0%) initiating IL-17 inhibitor therapy. An additional 6531 patients were included in the analysis in the PsA cohort (mean [SD] age, 49.1 [12.8] years; 2966 men [45.4%] men and 3565 women [54.6%]; median follow-up, 1.4 [IQR, 0.8-2.5] years), with 4974 (76.2%) initiating TNF inhibitor therapy, 803 (12.3%) initiating IL-12/23 inhibitor therapy, and 754 (11.5%) initiating IL-17 inhibitor therapy. Details for each biologic are provided in eTable 2 in the Supplement.
Table 1 presents characteristics for the PsO and PsA cohorts and by class for biologics. Overall, 7181 biologic-naive patients (42.5%) in the PsO cohort and 2220 (34.0%) in the PsA cohort had not received any nonbiologic drugs in the previous 2 years. At inclusion, nonbiologic drugs were prescribed for 4125 patients (24.4%) in the PsO cohort and 2582 (39.5%) in the PsA cohort; they were prescribed during follow-up for 3966 (23.5%) and 3085 (47.2%) of patients in each cohort, respectively.
Table 1. Characteristics of Psoriasis and Psoriatic Arthritis Patient Cohorts by Biologic Treatmenta.
| Characteristic | Psoriasis cohort | Psoriatic arthritis cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 16 892) | Biologic treatment | All (n = 6531) | Biologic treatment | |||||
| TNF inhibitors (n = 10 199) | IL-12/23 inhibitor (n = 3982) | IL-17 inhibitors (n = 2711) | TNF inhibitors (n = 4974) | IL-12/23 inhibitor (n = 803) | IL-17 inhibitors (n = 754) | |||
| Follow-up, median (IQR), y | 1.3 (0.9-2.4) | 1.2 (0.9-2.3) | 1.6 (0.9-2.6) | 1.4 (0.9-2.3) | 1.4 (0.8-2.5) | 1.4 (0.8-2.6) | 1.3 (0.8-2.5) | 1.2 (0.8-2.0) |
| Sociodemographic | ||||||||
| Age, mean (SD), y | 48.5 (13.8) | 48.8 (14.0) | 47.5 (13.6) | 49.0 (13.6) | 49.1 (12.8) | 48.7 (12.9) | 50.7 (12.9) | 50.1 (12.3) |
| Sex | ||||||||
| Women | 7740 (45.8) | 4976 (48.8) | 1654 (41.5) | 1110 (40.9) | 3565 (54.6) | 2708 (54.4) | 435 (54.2) | 422 (56.0) |
| Men | 9152 (54.2) | 5223 (51.2) | 2328 (58.5) | 1601 (59.1) | 2966 (45.4) | 2266 (45.5) | 368 (45.8) | 332 (44.0) |
| Complementary universal health coverage | 1513 (9.0) | 922 (9.0) | 366 (9.2) | 225 (8.3) | 767 (11.7) | 567 (11.4) | 96 (11.9) | 104 (13.8) |
| Associated inflammatory diseases | ||||||||
| IBD | 2497 (14.8) | 2237 (21.9) | 247 (6.2) | 13 (0.5) | 374 (5.7) | 325 (6.5) | 45 (5.6) | 4 (0.5) |
| Uveitis | 17 (0.1) | 16 (0.2) | 1 (0.03) | 0 | 16 (0.2) | 13 (0.3) | 1 (0.1) | 2 (0.3) |
| Charlson Comorbidity Index | ||||||||
| 1 | 12 178 (72.1) | 7101 (69.6) | 3037 (76.3) | 2040 (75.2) | 4359 (66.7) | 3374 (67.8) | 503 (62.6) | 482 (63.9) |
| 2 | 4476 (26.5) | 2950 (28.9) | 890 (22.3) | 636 (23.5) | 1908 (29.2) | 1423 (28.6) | 253 (31.5) | 232 (30.8) |
| 3 | 206 (1.2) | 131 (1.3) | 44 (1.1) | 31 (1.1) | 205 (3.1) | 139 (2.8) | 34 (4.2) | 32 (4.2) |
| 4 | 32 (0.2) | 17 (0.2) | 11 (0.3) | 4 (0.1) | 59 (0.9) | 38 (0.8) | 13 (1.6) | 8 (1.1) |
| Comorbidities | ||||||||
| Diabetes | 1451 (8.6) | 877 (8.6) | 316 (7.9) | 258 (9.5) | 632 (9.7) | 437 (8.8) | 98 (12.2) | 97 (12.9) |
| Dyslipidemia | 2416 (14.3) | 1487 (14.6) | 536 (13.5) | 393 (14.5) | 391 (6.0) | 282 (5.7) | 70 (8.7) | 39 (5.2) |
| COPD | 1828 (10.8) | 1151 (11.3) | 404 (10.1) | 273 (10.1) | 556 (8.5) | 395 (7.9) | 89 (11.1) | 72 (9.5) |
| Heart failure | 104 (0.6) | 57 (0.6) | 29 (0.7) | 18 (0.7) | 43 (0.7) | 23 (0.5) | 14 (1.7) | 6 (0.8) |
| Kidney failure | 130 (0.8) | 76 (0.7) | 38 (0.9) | 16 (0.6) | 47 (0.7) | 33 (0.7) | 10 (1.2) | 4 (0.5) |
| Liver disease | 221 (1.3) | 128 (1.3) | 57 (1.4) | 36 (1.3) | 128 (1.9) | 84 (1.7) | 25 (3.1) | 19 (2.5) |
| Therapies and/or codes related to tobacco | 1094 (6.5) | 673 (6.6) | 265 (6.7) | 156 (5.8) | 360 (5.5) | 258 (5.2) | 58 (7.2) | 44 (5.8) |
| Morbid or complicated obesity | 1528 (9.0) | 925 (9.1) | 371 (9.3) | 232 (8.5) | 621 (9.5) | 427 (8.6) | 113 (14.1) | 81 (10.7) |
| Therapies within 2 y | ||||||||
| Nonbiologic drugsb | 9711 (57.5) | 5283 (51.8) | 2869 (72.0) | 1559 (57.5) | 4311 (66.0) | 3307 (66.5) | 499 (62.1) | 505 (67.0) |
| Prednisone (on ≥3 occasions) | 3564 (21.1) | 2622 (25.7) | 545 (13.7) | 397 (14.6) | 1226 (18.8) | 969 (19.5) | 118 (14.7) | 139 (18.4) |
| Add-on therapies at baseline | ||||||||
| Nonbiologic drugsb | 4125 (24.4) | 2760 (27.1) | 864 (21.7) | 501 (18.5) | 2582 (39.5) | 2234 (44.9) | 166 (20.7) | 182 (24.1) |
| Prednisone | 1409 (8.3) | 1137 (11.1) | 147 (3.7) | 125 (4.6) | 1084 (16.6) | 899 (18.1) | 90 (11.2) | 95 (12.6) |
| Add-on therapies during follow-up | ||||||||
| Nonbiologic drugsb | 3966 (23.5) | 2606 (25.5) | 857 (21.5) | 503 (18.5) | 3085 (47.2) | 2509 (50.4) | 294 (36.6) | 282 (37.4) |
| Prednisone | 5682 (33.6) | 3655 (35.8) | 1195 (30.0) | 832 (30.7) | 1928 (29.5) | 1541 (31.0) | 196 (24.4) | 191 (25.3) |
| Care consumption | ||||||||
| Specialist consultation within 2 yc | ||||||||
| 0 | 4214 (24.9) | 3285 (32.2) | 477 (12.0) | 452 (16.7) | 1511 (23.1) | 971 (19.5) | 304 (37.9) | 236 (31.3) |
| 1 | 1170 (6.9) | 819 (8.0) | 183 (4.6) | 168 (6.2) | 774 (11.9) | 565 (11.4) | 92 (11.5) | 117 (15.5) |
| ≥2 | 11 508 (68.1) | 6095 (59.8) | 3322 (83.4) | 2091 (77.1) | 4223 (64.7) | 3420 (68.7) | 403 (50.2) | 400 (53.1) |
| Hospitalization for underlying disease within 2 y | 1943 (11.5) | 951 (9.3) | 651 (16.3) | 341 (12.6) | 1154 (17.7) | 914 (18.4) | 119 (14.8) | 121 (16.0) |
Abbreviations: COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; IL-12/23, interleukin 12 and interleukin 23; IL-17, interleukin 17; TNF, tumor necrosis factor.
Unless otherwise indicated, data are expressed as number (%) of patients. Percentages have been rounded and may not total 100.
Includes methotrexate sodium, acitretin, and cyclosporine for the psoriasis cohort and methotrexate, leflunomide, and sulfasalazine for the psoriatic arthritis cohort.
Defined as a dermatologist in the psoriasis cohort and a rheumatologist in the psoriatic arthritis cohort.
Persistence of Biologics Among Cohorts
During follow-up, 8185 (48.5%) biologic-naive patients discontinued their first treatment in the PsO cohort compared with 3744 (57.3%) in the PsA cohort. Kaplan-Meier survival analyses revealed a persistence rate of 76.6% for biologics in the PsO cohort and 72.7% for biologics in the PsA cohort in the first year of treatment (Table 2). The persistence rate decreased over time: 53.4% at the end of the second year and 40.9% at the end of the third year in the PsO cohort and 49.2% at the end of the second year and 36.2% at the end of the third year in the PsA cohort. One-year persistence rates for TNF inhibitors were 73.8% for PsO and 72.3% for PsA; for the IL-12/23 inhibitor, 81.1% for PsO and 72.2% for PsA; and for IL-17 inhibitors, 80.6% for PsO and 76.7% for PsA. The Figure summarizes the Kaplan-Meier analyses for each biologic class in the PsO and PsA cohorts.
Table 2. Persistence for Biologic Treatment at 1, 2, and 3 Years of Follow-up Among Psoriasis and Psoriatic Arthritis Patient Cohorts.
| Biologic treatment | Persistence rate, % | |||||
|---|---|---|---|---|---|---|
| Psoriasis cohort (n = 16 892) | Psoriatic arthritis cohort (n = 6531) | |||||
| 1 y | 2 y | 3 y | 1 y | 2 y | 3 y | |
| All | 76.6 | 53.4 | 40.9 | 72.7 | 49.2 | 36.2 |
| TNF inhibitor | 73.8 | 49.2 | 36.8 | 72.3 | 49.0 | 35.3 |
| Adalimumab | 72.9 | 49.6 | 37.3 | 72.4 | 47.7 | 33.2 |
| Certolizumab | 67.4 | 42.2 | 32.5 | 68.3 | 46.7 | 35.6 |
| Etanercept | 69.7 | 45.2 | 33.1 | 74.0 | 50.0 | 35.9 |
| Golimumab | NA | NA | NA | 70.6 | 53.3 | 41.4 |
| Infliximab | 82.2 | 49.8 | 35.2 | 57.7 | 49.1 | 38.6 |
| IL-12/23 inhibitor | ||||||
| Ustekinumab | 81.1 | 60.2 | 48.2 | 72.2 | 48.1 | 38.5 |
| IL-17 inhibitor | 80.6 | 58.8 | 45.1 | 76.7 | 51.3 | 41.7 |
| Secukinumab | 80.6 | 57.8 | 44.0 | 75.9 | 50.1 | 41.3 |
| Ixekizumab | 80.8 | 64.8 | 55.1 | 88.3 | 69.9 | 41.9 |
| Brodalumab | 80.2 | 76.2 | 76.2 | NA | NA | NA |
Abbreviations: IL-12/23, interleukin 12 and interleukin 23; IL-17, interleukin 17; NA, not applicable; TNF, tumor necrosis factor.
Figure. Kaplan-Meier Estimates of Biologic Treatment Persistence in Cohorts of Patients With Psoriasis and Psoriatic Arthritis.
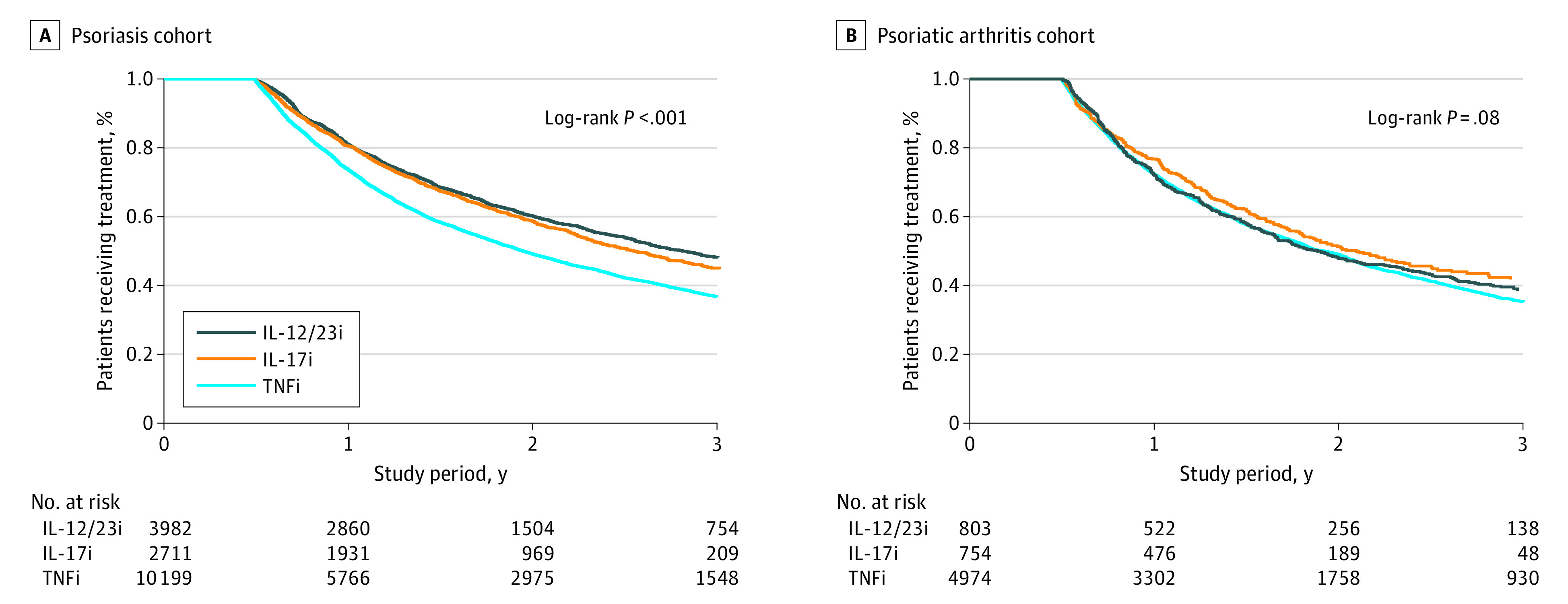
Persistence was defined as the time from biologic therapy initiation to discontinuation. IL-12/23i indicates interleukin 12/23 inhibitor; IL-17i, IL-17 inhibitor; TNFi, tumor necrosis factor inhibitor.
Comparison of Biologic Classes
After applying the stabilized propensity score, we obtained pseudocohorts in which the distribution of variables was similar, with a standardized difference of less than −0.1 or less than 0.1 between the different treatment classes. The results of the main analysis are presented in Table 3. After inverse probability of treatment weighting and adjustment for nonbiologic drugs and prednisone as time-dependent covariables, IL-17 inhibitors were associated with higher persistence compared with TNF inhibitors for PsO (weighted HR, 0.78 [95% CI, 0.73-0.83]) and PsA (weighted HR, 0.70 [95% CI, 0.58-0.85]) and compared with the IL-12/23 inhibitor for PsA (weighted HR, 0.69 [95% CI, 0.55-0.87]). We found no difference between IL-17 inhibitors and the IL12/23 inhibitor for PsO (weighted HR, 1.02 [95% CI, 0.94-1.10]). The IL-12/23 inhibitor was associated with higher persistence than TNF inhibitors for PsO (weighted HR, 0.76 [95% CI, 0.72-0.80]), with no difference for PsA (weighted HR, 1.03 [95% CI, 0.89-1.19]).
Table 3. Comparison of Treatment Classes Analyzed Using Inverse Probability of Treatment Weighting Cox Proportional Hazards Regression Models and Adjustment for Time-Dependent Covariablesa.
| Patient cohort | IL-17 vs TNF inhibitors | IL-12/23 vs TNF inhibitors | IL-17 vs IL-12/23 inhibitors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | IPTW | Unadjusted | IPTW | Unadjusted | IPTW | |||||||
| Crude HR (95% CI) | P value | Weighted HR (95% CI) | P value | Crude HR (95% CI) | P value | Weighted HR (95% CI) | P value | Crude HR (95% CI) | P value | Weighted HR (95% CI) | P value | |
| Psoriasis (n = 16 892) | 0.76 (0.72-0.82) | <.001 | 0.78 (0.73-0.83) | <.001 | 0.73 (0.69-0.77) | <.001 | 0.76 (0.72-0.80) | <.001 | 1.05 (0.98-1.14) | .18 | 1.02 (0.94-1.10) | .58 |
| Psoriatic arthritis (n = 6531) | 0.73 (0.61-0.88) | <.001 | 0.70 (0.58-0.85) | <.001 | 0.92 (0.79-1.07) | .29 | 1.03 (0.89-1.19) | .70 | 0.80 (0.63-1.00) | .05 | 0.69 (0.55-0.87) | <.001 |
Abbreviations: HR, hazard ratio; IL-12/23, interleukin 12 and interleukin 23; IL-17, interleukin 17; IPTW, inverse probability of treatment weighting; TNF, tumor necrosis factor.
Includes conventional, synthetic disease-modifying antirheumatic drugs and prednisone.
Subgroup and Sensitivity Analyses
The results did not differ for patients without associated inflammatory diseases (Table 4). The results of sensitivity analyses were consistent with those of the main analysis (eTables 3 and 4 in the Supplement). Male sex and older age were associated with increased therapeutic persistence; comorbidities and previous nonbiologic systemic agents were associated with decreased therapeutic persistence (eTable 5 in the Supplement).
Table 4. Comparison of Treatment Classes Among Patients Without Associated Inflammatory Disease (Subgroup Analyses) Using Inverse Probability of Treatment Weighting Cox Proportional Hazards Regression Models and Adjustment for Time-Dependent Covariablesa.
| Patient cohort | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| IL-17 vs TNF inhibitors | IL-12/23 vs TNF inhibitors | IL-17 vs IL-12/23 inhibitors | ||||||
| Weighted HR (95% CI) | P value | Weighted HR (95% CI) | P value | Weighted HR (95% CI) | P value | |||
| Psoriasis (n = 12 972) | 0.73 (0.68-0.78) | <.001 | 0.71 (0.67-0.76) | <.001 | 1.02 (0.94-1.11) | .63 | ||
| Psoriatic arthritis (n = 3193) | 0.73 (0.58-0.92) | <.001 | 1.19 (0.98-1.44) | .07 | 0.61 (0.46-0.82) | <.001 | ||
Abbreviations: HR, hazard ratio; IL-12/23, interleukin 12 and interleukin 23; IL-17, interleukin 17; IPTW, inverse probability of treatment weighting; TNF, tumor necrosis factor.
Includes conventional synthetic disease-modifying antirheumatic drugs and prednisone.
Discussion
In this nationwide study involving 16 892 patients with PsO and 6531 with PsA, we sought to assess the long-term persistence of biologics in an unselected population of new users. In the first year, the overall persistence rate for biologics was 76.6% in the PsO cohort and 72.7% in the PsA cohort. Persistence decreased markedly over time, to 53.4% at the end of the second year and 40.9% at the end of the third year in the PsO cohort and to 49.2% and 36.2% at the end of the second and third years, respectively, in the PsA cohort. After inverse probability of treatment weighting and adjustments, IL-17 inhibitors were associated with higher persistence than TNF inhibitors for PsO and PsA and higher persistence than the IL-12/23 inhibitor for PsA. The IL-12/23 inhibitor had higher persistence than the TNF inhibitors for PsO.
Although persistence rates reported in the literature vary widely depending on the study and definition used, we found 1-year rates for biologics similar to those reported previously. Specifically, the previous 1-year rates of TNF inhibitor persistence ranged from 65% to 79% for PsO33 and from 56% to 90% for PsA.17,34,35,36 The corresponding values in the present study were 73.8% and 72.3%, respectively. During the first year, ustekinumab persistence rates were estimated at 80% to 89% for PsO18,19,37 and 41% to 74% for PsA38,39 compared with 81.1% and 72.2%, respectively, in our study. Finally, IL-17 inhibitor persistence rates had been reported from 78% to 86% at 1 year for PsO19 and 59% to 91% for PsA39,40,41,42,43; these rates were 80.6% and 76.7%, respectively, in our study. Longer-term persistence rates are less frequently reported and feature considerable heterogeneity.39,41,44,45 This situation may be explained by small sample sizes and potential selection bias. Similar to what was reported for randomized clinical trials, patients included in national cohorts and registries may not be representative of the general population of exposed individuals.46 Indeed, these studies have a number of inclusion and exclusion criteria that may lead to a particular selection of the study population.33,38 In addition, they are also subject to bias related to loss to follow-up (persistence is probably lower in patients lost to follow-up), the physician-patient relationship (especially if the physician wants the patient included in the study to continue with the prescribed treatment), and the country’s health care system (eg, if an alternative drug is not available in that country). In contrast, our study was based on an exhaustive and large-scale analysis of reimbursement data from an unselected population, with no loss to follow-up, thus avoiding these biases.
Our findings are important because to our knowledge, this is the largest study to date comparing persistence of biologics for PsO and PsA within real-world settings. The findings suggest a higher persistence of IL-17 inhibitors vs TNF inhibitors in both diseases but also vs the IL-12/23 inhibitor in PsA. This finding is consistent with previous findings from observational data18,39 but also with those from the head-to-head EXCEED (Efficacy of Secukinumab Compared to Adalimumab in Patients With Psoriatic Arthritis) trial, which found that secukinumab was associated with higher persistence than adalimumab in patients with PsA.47 A recent study of registers from the Nordic countries found no significant difference in treatment retention between secukinumab and adalimumab regardless of line of treatment in PsA. However, only 1-year persistence was assessed, and few patients were new users (579 for adalimumab and 165 for secukinumab).48 Our results are consistent with those from smaller studies reporting higher persistence rates for IL-12/23 inhibitors compared with TNF inhibitors44,49 but similar persistence rates to those of IL-17 inhibitors in biologic-naive patients with PsO,19 which may be associated with greater efficacy and higher tolerance of these therapeutic classes in our patients. Nevertheless, long-term persistence of all biologics remained low in our study.
Strengths and Limitations
This study has several strengths. Our cohort involved a large number of patients from a national database, with a data quality and consistency plan ensuring homogeneous data processing and with information captured during routine medical care.22 This framework minimizes selection bias. Furthermore, we used a new-user design27 and applied a propensity score method to more accurately estimate the persistence of the biologics and control channeling bias.50 It should be noted that TNF inhibitors, IL-12/23 inhibitors, and IL-17 inhibitors are recommended second-line therapies for moderate to severe disease.8 In France, each physician is free to choose the biologics labeled for PsO and/or PsA, and no discontinuation of biologic therapy is related to cost-sharing. Thus, in France, there is no discontinuation of biologic therapy due to loss or change of insurance. However, except for a few cases in which an extracutaneous or extra-articular manifestation (eg, active psoriasis, inflammatory bowel disease, or severe or repeated acute anterior uveitis) guides the choice of the prescriber, no factor is likely to influence this prescription at a population level. In this way, the different groups in our study were comparable with regard to several criteria (Table 1). Moreover, we controlled for the effect of corticosteroids and nonbiologic drugs—including methotrexate—at baseline and throughout follow-up (ie, time-dependent variables) to compare the different therapeutic classes adequately. Of note, Lindström et al36 reported a trend toward treatment retention for infliximab but not etanercept in comedication with methotrexate in PsA. We also limited classification bias by using a reproducible, well-accepted definition of drug persistence.13,51 Finally, several sensitivity analyses were performed and supported the integrity of our results.
This study has some limitations. First, we defined drug exposure based on health care reimbursement data, which are not necessarily equivalent to days of use. However, in PsO, adherence rates for biologics are typically higher than for other treatment categories.52 Second, although we applied a propensity score and adjusted for time-dependent covariables, the presence of residual confounding outcomes cannot be ruled out. Our analyses are limited by the availability of data for some factors, in particular information on disease activity, and the absence of directly available data on smoking and obesity (although proxies for severe forms were used). New users would ideally be those using a treatment for the first time (ie, treatment-naive patients). To assess this criterion, lifetime treatment use data would be necessary; however, this framework is often unavailable, and in pharmaco-epidemiology, a washout window (ie, a period without delivery of the studied treatment) of 6 to 12 months is typical.53 To test the robustness of our main analysis, we conducted a sensitivity analysis by considering as new users those who had not filled a prescription for a biologic for 5 years before the index date, and the results were consistent. In addition, the drugs within each class were not separated out by analysis. Nevertheless, although there were fluctuations due to smaller numbers in some treatment groups (notably for certolizumab for the PsO cohort and infliximab for the PsA cohort), persistence was similar between the different molecules of each therapeutic class in the PsO and PsA cohorts (Table 2). In addition, some of the drugs studied came to the market years ago, whereas others came out more recently, which may account for a change in persistence. However, our study period was restricted to a recent time frame (2015-2019), and to avoid channeling bias (ie, a confounding outcome of assessing certain treatments in specific subgroups), we limited our analyses to biologic-naive patients and performed sensitivity analyses on patients included in the cohort after July 2017 (ie, 1 year after all therapeutic classes were commercialized for both indications). These sensitivity analyses and the main analysis yielded similar results, which support our main findings. Last, a previous study of 1866 patients54 showed that the drug persistence in PsO was not affected by the year of treatment initiation. Finally, the database analyzed in this study did not specify the reasons why a patient stopped filling prescriptions for a biologic, which could include loss of efficacy, occurrence of an adverse effect, presence of comorbidities, or extrinsic factors such as a wider range of treatment options or a lower price for biosimilar products.55 Of note, persistence also takes into account patient satisfaction or preference and adherence to treatment.
Conclusions
The findings of this cohort study suggest that IL-17 inhibitors were associated with higher therapeutic persistence than TNF inhibitors in new users of biologic drugs for PsO and PsA, with the possibility of excellent extrapolation of the results. Interleukin 17 inhibitors were also associated with higher persistence compared with the IL-12/23 inhibitor in PsA, with no observed difference in PsO. Given the many biologic treatment options available in the modern therapeutic environment, our results may help physicians optimize first-line treatment pathways. However, the persistence rates of the 3 therapeutic classes remained low at 3 years, which suggests that long-term control of these chronic diseases may require several therapeutic lines.
eTable 1. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) Codes for Covariates
eTable 2. New Biologic Agent Users According to Biologic Treatment for the Psoriasis (n = 16 892) and the Psoriatic Arthritis (n = 6531) Cohorts
eTable 3. Comparison of Treatment Classes in Sensitivity Analyses for the Psoriasis Cohort
eTable 4. Comparison of Treatment Classes in Sensitivity Analyses for the Psoriatic Arthritis Cohort
eTable 5. Results of the Conventional Multivariate Cox Proportional Hazards Model (Sensitivity Analyses)
References
- 1.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983-994. doi: 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 2.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM; Global Psoriasis Atlas . National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard MA, Corgibet F, Beylot-Barry M, et al. Sex- and age-adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the “OBJECTIFS PEAU” study. J Eur Acad Dermatol Venereol. 2018;32(11):1967-1971. doi: 10.1111/jdv.14959 [DOI] [PubMed] [Google Scholar]
- 4.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251-265.e19. doi: 10.1016/j.jaad.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 5.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi: 10.1016/j.rdc.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23. doi: 10.1136/ard.2004.033217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudu T, Kiltz U, de Wit M, Kvien TK, Gossec L. Mapping the effect of psoriatic arthritis using the International Classification of Functioning, Disability and Health. J Rheumatol. 2017;44(2):193-200. doi: 10.3899/jrheum.160180 [DOI] [PubMed] [Google Scholar]
- 8.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700-712. doi: 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 10.Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29-37. doi: 10.1016/j.semarthrit.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 11.Afach S, Chaimani A, Evrenoglou T, et al. Meta-analysis results do not reflect the real safety of biologics in psoriasis. Br J Dermatol. 2021;184(3):415-424. doi: 10.1111/bjd.19244 [DOI] [PubMed] [Google Scholar]
- 12.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44-47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 13.van den Reek JMPA, Kievit W, Gniadecki R, et al. Drug survival studies in dermatology: principles, purposes, and pitfalls. J Invest Dermatol. 2015;135(7):1-5. doi: 10.1038/jid.2015.171 [DOI] [PubMed] [Google Scholar]
- 14.Ogdie A, Schwartzman S, Eder L, et al. Comprehensive treatment of psoriatic arthritis: managing comorbidities and extraarticular manifestations. J Rheumatol. 2014;41(11):2315-2322. doi: 10.3899/jrheum.140882 [DOI] [PubMed] [Google Scholar]
- 15.Iannone F, Salaffi F, Fornaro M, et al. Influence of baseline modified Rheumatic Disease Comorbidity Index (mRDCI) on drug survival and effectiveness of biological treatment in patients affected with rheumatoid arthritis, spondyloarthritis and psoriatic arthritis in real-world settings. Eur J Clin Invest. 2018;48(11):e13013. doi: 10.1111/eci.13013 [DOI] [PubMed] [Google Scholar]
- 16.Haddad A, Gazitt T, Feldhamer I, et al. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res Ther. 2021;23(1):44. doi: 10.1186/s13075-021-02417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahe CH, Ørnbjerg LM, Jacobsson L, et al. Retention and response rates in 14 261 PsA patients starting TNF inhibitor treatment-results from 12 countries in EuroSpA. Rheumatology (Oxford). 2020;59(7):1640-1650. doi: 10.1093/rheumatology/kez427 [DOI] [PubMed] [Google Scholar]
- 18.Yiu ZZN, Mason KJ, Hampton PJ, et al. ; BADBIR Study Group . Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183(2):294-302. doi: 10.1111/bjd.18981 [DOI] [PubMed] [Google Scholar]
- 19.Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti–interleukin-17 antibodies: a time-period–adjusted registry analysis. Br J Dermatol. 2021;184(6):1094-1105. doi: 10.1111/bjd.19701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63(2):382-390. doi: 10.1002/art.30117 [DOI] [PubMed] [Google Scholar]
- 21.Moulis G, Lapeyre-Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36(6):411-417. doi: 10.1016/j.revmed.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 22.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the Système National d’information Interrégimes de l’Assurance Maladie (SNIIRAM) to the Système National des Données de Santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149-S167. doi: 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55(7):1953-1962. doi: 10.1007/s00125-012-2538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer A, Rudant J, Drouin J, Weill A, Carbonnel F, Coste J. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: a French equivalence study. Ann Intern Med. 2019;170(2):99-107. doi: 10.7326/M18-1512 [DOI] [PubMed] [Google Scholar]
- 25.Penso L, Dray-Spira R, Weill A, Pina Vegas L, Zureik M, Sbidian E. Association between biologics use and risk of serious infection in patients with psoriasis. JAMA Dermatol. 2021;157(9):1056-1065. doi: 10.1001/jamadermatol.2021.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pina Vegas L, Sbidian E, Penso L, Claudepierre P. Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database. Rheumatology (Oxford). 2021;60(3):1243-1251. doi: 10.1093/rheumatology/keaa448 [DOI] [PubMed] [Google Scholar]
- 27.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 28.Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121-131. doi: 10.2147/PPA.S70834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997-2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannay A, Chaignot C, Blotière P-O, et al. The best use of the Charlson Comorbidity Index with electronic health care database to predict mortality. Med Care. 2016;54(2):188-194. doi: 10.1097/MLR.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 31.Wyss R, Stürmer T. Commentary: balancing automated procedures for confounding control with background knowledge. Epidemiology. 2014;25(2):279-281. doi: 10.1097/EDE.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277. doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632-2640. doi: 10.1038/jid.2015.208 [DOI] [PubMed] [Google Scholar]
- 34.Belhassen M, Tubach F, Hudry C, et al. Impact of persistence with tumour necrosis factor inhibitors on healthcare resource utilization and costs in chronic inflammatory joint diseases. Br J Clin Pharmacol. 2021;87(1):163-177. doi: 10.1111/bcp.14387 [DOI] [PubMed] [Google Scholar]
- 35.Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP; British Society for Rheumatology Biologics Register . Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009;11(2):R52. doi: 10.1186/ar2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindström U, Di Giuseppe D, Delcoigne B, et al. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis: data from the EuroSpA collaboration. Ann Rheum Dis. 2021;80(11):1410-1418. doi: 10.1136/annrheumdis-2021-220097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalom G, Cohen AD, Ziv M, et al. Biologic drug survival in Israeli psoriasis patients. J Am Acad Dermatol. 2017;76(4):662-669.e1. doi: 10.1016/j.jaad.2016.10.033 [DOI] [PubMed] [Google Scholar]
- 38.Iannone F, Santo L, Bucci R, et al. Drug survival and effectiveness of ustekinumab in patients with psoriatic arthritis: real-life data from the Biologic Apulian Registry (BIOPURE). Clin Rheumatol. 2018;37(3):667-675. doi: 10.1007/s10067-018-3989-2 [DOI] [PubMed] [Google Scholar]
- 39.Letarouilly JG, Flachaire B, Labadie C, et al. Secukinumab and ustekinumab treatment in psoriatic arthritis: results of a direct comparison. Rheumatology (Oxford). 2021;60(6):2773-2782. doi: 10.1093/rheumatology/keaa710 [DOI] [PubMed] [Google Scholar]
- 40.Chimenti MS, Fonti GL, Conigliaro P, et al. One-year effectiveness, retention rate, and safety of secukinumab in ankylosing spondylitis and psoriatic arthritis: a real-life multicenter study. Expert Opin Biol Ther. 2020;20(7):813-821. doi: 10.1080/14712598.2020.1761957 [DOI] [PubMed] [Google Scholar]
- 41.Ramonda R, Lorenzin M, Carriero A, et al. ; on behalf Spondyloartritis and Psoriatic Arthritis SIR Study Group “Antonio Spadaro” . Effectiveness and safety of secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study. RMD Open. 2021;7(1):e001519. doi: 10.1136/rmdopen-2020-001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto Tasende JA, Maceiras Pan FJ, Mosquera Martínez JA, Fernández Dominguez L, Correa Rey B, García Porrúa C. Secukinumab as biological treatment for psoriatic arthritis in real clinical practice. Article in Spanish. Reumatol Clin (Engl Ed). 2021;17(4):203-206. doi: 10.1016/j.reuma.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 43.Michelsen B, Georgiadis S, Di Giuseppe D, et al. Real-world 6 and 12-month drug retention, remission and response rates of secukinumab in 2,017 psoriatic arthritis patients in 13 European countries. Arthritis Care Res (Hoboken). Published online January 18, 2021. doi: 10.1002/acr.24560 [DOI] [PubMed] [Google Scholar]
- 44.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30(7):1148-1158. doi: 10.1111/jdv.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray K, Turk M, Alammari Y, et al. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res Ther. 2021;23(1):25. doi: 10.1186/s13075-020-02380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Doval I, Carretero G, Vanaclocha F, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol. 2012;148(4):463-470. doi: 10.1001/archdermatol.2011.2768 [DOI] [PubMed] [Google Scholar]
- 47.McInnes IB, Behrens F, Mease PJ, et al. ; EXCEED Study Group . Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496-1505. doi: 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 48.Lindström U, Glintborg B, Di Giuseppe D, et al. Comparison of treatment retention and response to secukinumab versus tumour necrosis factor inhibitors in psoriatic arthritis. Rheumatology (Oxford). 2021;60(8):3635-3645. doi: 10.1093/rheumatology/keaa825 [DOI] [PubMed] [Google Scholar]
- 49.Sbidian E, Mezzarobba M, Weill A, Coste J, Rudant J. Persistence of treatment with biologics for patients with psoriasis: a real-world analysis of 16 545 biologic-naïve patients from the French National Health Insurance database (SNIIRAM). Br J Dermatol. 2019;180(1):86-93. doi: 10.1111/bjd.16809 [DOI] [PubMed] [Google Scholar]
- 50.Lobo FS, Wagner S, Gross CR, Schommer JC. Addressing the issue of channeling bias in observational studies with propensity scores analysis. Res Social Adm Pharm. 2006;2(1):143-151. doi: 10.1016/j.sapharm.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 51.Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65(5):1213-1223. doi: 10.1002/art.37876 [DOI] [PubMed] [Google Scholar]
- 52.Aleshaki JS, Cardwell LA, Muse ME, Feldman SR. Adherence and resource use among psoriasis patients treated with biologics. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):609-617. doi: 10.1080/14737167.2018.1512408 [DOI] [PubMed] [Google Scholar]
- 53.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221-228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bettuzzi T, Bachelez H, Beylot-Barry M, et al. ; PsoBioTeq study group . Evolution of drug survival with biological agents and apremilast between 2012 and 2018 in psoriasis patients from the PsoBioTeq Cohort. Acta Derm Venereol. 2021. Published online November 22, 2021. doi: 10.2340/actadv.v101.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) Codes for Covariates
eTable 2. New Biologic Agent Users According to Biologic Treatment for the Psoriasis (n = 16 892) and the Psoriatic Arthritis (n = 6531) Cohorts
eTable 3. Comparison of Treatment Classes in Sensitivity Analyses for the Psoriasis Cohort
eTable 4. Comparison of Treatment Classes in Sensitivity Analyses for the Psoriatic Arthritis Cohort
eTable 5. Results of the Conventional Multivariate Cox Proportional Hazards Model (Sensitivity Analyses)


