Key Points
Question
Is the use of cimetropium bromide, an antispasmodic agent, associated with higher rates of detection of gastric neoplasms during esophagogastroduodenoscopy (EGD) screening?
Findings
In this cohort study of 67 683 participants who received EGD screening, the use of cimetropium bromide as premedication was associated with higher gastric neoplasm detection rates during EGD than nonuse. Lesions in the gastric body were detected more frequently in those who received cimetropium bromide compared with those who did not.
Meaning
This study’s findings suggest that cimetropium bromide may be considered as premedication for the detection of gastric neoplasms during EGD examination among individuals with no contraindications.
Abstract
Importance
Esophagogastroduodenoscopy (EGD) is a common procedure used to examine upper gastrointestinal diseases. Although cimetropium bromide and other antispasmodic agents are commonly administered as premedication to inhibit peristalsis during EGD examination, there are few data regarding the benefits of cimetropium bromide for the detection of gastric neoplasms.
Objective
To investigate the association between the use of cimetropium bromide as premedication and gastric neoplasm detection rates during EGD examination.
Design, Setting, and Participants
This propensity score–matched retrospective cohort study included 67 683 participants who received EGD screening at the Health Promotion Center of Seoul St. Mary’s Hospital, The Catholic University of Korea, from January 2, 2010, to June 30, 2017. Data were analyzed from April 1 to December 30, 2021.
Exposures
Participants were divided into 2 groups: those who received cimetropium bromide before EGD examination (intervention group) and those who did not (control group).
Main Outcomes and Measures
Gastric neoplasm detection rates.
Results
Among 67 683 participants, the mean (SD) age was 48.6 (10.8) years, and 36 517 participants (54.0%) were male; all participants were Asian (a racially homogenous population). Of those, 28 280 participants (41.8%; mean [SD] age, 50.3 [10.6] years; 57.8% male) received cimetropium bromide, and 39 403 participants (58.2%; mean [SD] age, 47.4 [10.8] years; 51.2% male) did not. Propensity score matching based on confounding variables yielded 41 670 matched participants (20 835 pairs). Detected lesions included 52 dysplasias (0.12%), 40 early cancers (0.10%), 7 advanced cancers (0.02%), and 3 lymphomas (0.01%). Gastric neoplasm detection rates were significantly higher in the intervention group (63 participants [0.30%]) vs the control group (39 participants [0.19%]; P = .02). A significant difference in the combined detection rate of dysplasia and early gastric cancer was found between those in the intervention group (57 participants [0.27%]) vs the control group (35 participants [0.17%]; P = .02). For small gastric lesions (<1 cm), those who received cimetropium bromide had higher detection rates (24 participants [0.12%]) than those who did not (11 participants [0.05%]; P = .03). Lesions in the gastric body were detected significantly more often in the intervention group (34 participants [0.16%]) vs the control group (15 participants [0.07%]; P = .007). In multivariate analyses involving all 67 683 participants, the use of cimetropium bromide was more likely to detect gastric neoplasms compared with nonuse (odds ratio, 1.42; 95% CI, 1.04-1.95; P = .03).
Conclusions and Relevance
In this study, the use of cimetropium bromide as premedication was significantly associated with increased gastric neoplasm detection rates during EGD screening, and lesions in the gastric body were detected more frequently among those who received cimetropium bromide compared with those who did not. These findings suggest that cimetropium bromide may be considered as premedication before EGD examination among individuals with no contraindications.
This cohort study assesses whether the use of an antispasmodic agent, cimetropium bromide, as premedication is associated with increases in gastric neoplasm detection rates during esophagogastroduodenoscopic examination.
Introduction
Esophagogastroduodenoscopy (EGD) is a common procedure used to examine upper gastrointestinal (GI) diseases, allowing for direct mucosal visualization and tissue acquisition.1 This procedure is also used as a screening tool to identify upper GI cancer in high-risk regions, allowing for detection of early-stage neoplasms, thereby reducing gastric cancer mortality.2,3 Because precancerous lesions and early cancers, even some advanced-stage cancers, often show subtle morphological changes on endoscopy, accurate diagnosis requires careful visualization of the mucosa.4
During EGD screening, gastric movement interferes with careful examination of the mucosal surface. Therefore, antispasmodic agents have been commonly administered as premedication to inhibit peristalsis during the examination.5,6,7,8,9 However, results regarding the benefits of the administration of antispasmodic agents before EGD examination have been inconsistent. Some studies10,11 have reported that antispasmodic agents suppress peristalsis and allow for better visualization of mucosal lesions. However, results of other studies12,13,14 have not supported these findings. Although there are few data confirming a change in diagnostic yield with the use of antispasmodic agents,11,15,16 a recent Asian consensus guideline recommended their use to improve the visual clarity of mucosal inspection before EGD examinations.17 In Korea, several antispasmodic agents, such as cimetropium bromide and hyoscine N-butylbromide, have been used as preendoscopic treatment. Cimetropium bromide, a quaternary ammonium compound that is chemically related to scopolamine, competes with acetylcholine to bind to the muscarinic receptors in the GI smooth muscle.18 This agent is frequently used to suppress gastric movement. Cimetropium bromide has few serious adverse effects but requires close monitoring when administered to patients with arrhythmia, benign prostatic hypertrophy, and glaucoma.19,20,21 The aim of this study was to investigate whether the use of cimetropium bromide as premedication was associated with higher gastric neoplasm detection rates during EGD examinations.
Methods
We conducted a propensity score–matched retrospective cohort study involving 132 352 participants who received comprehensive health screening at the Health Promotion Center of Seoul St. Mary’s Hospital, The Catholic University of Korea, from January 2, 2010, to June 30, 2017. Data were analyzed from April 1 to December 30, 2021. The study was approved by the institutional review board of Seoul St. Mary’s Hospital. The requirement for informed consent was waived because anonymous data were used. This study followed the principles of the Declaration of Helsinki22 and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
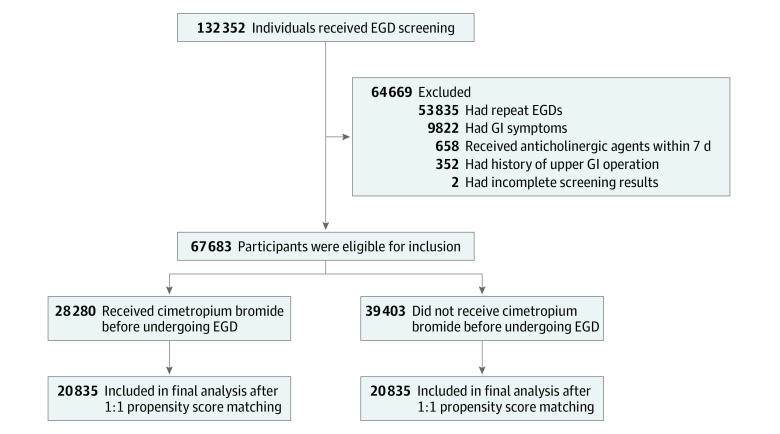
Each participant completed a structured questionnaire that included medical history; family history of cancer; and lifestyle information, such as smoking and alcohol consumption. Participants were included if they were aged 30 to 99 years. We excluded 64 669 participants who had repeat EGD screenings (n = 53 835), experienced GI symptoms that might have been associated with the endoscopic procedure or gastric cancer (n = 9822), received anticholinergic agents within 7 days before the EGD examination (n = 658), had a previous upper GI operation (n = 352), or had incomplete examinations (n = 2). Participant demographic characteristics, endoscopic reports, and histopathological results were retrieved from the hospital’s electronic database.
Premedication Before EGD Examination
Among all participants, 10 mL of simethicone (Gasocol suspension; Taejoon Pharmaceutical Company) was used as an antifoaming agent. Topical pharyngeal anesthesia was administered using 10% lidocaine spray (Beracaine Spray; Firson Company) among participants without a history of allergy. Sedative endoscopy was performed among those who provided written informed consent for any additional costs, adverse effects, and benefits of sedative medications. For sedation, midazolam (Bukwang Pharmaceutical Company) was injected intravenously. The delivered dose of midazolam was decided by each endoscopist after consideration of the participant’s age and general physical condition.
For the antispasmodic agent, 5 mg of cimetropium bromide (Algiron; Boehringer Ingelheim) was administered intravenously 10 minutes before the EGD examination. In accordance with our institutional policy, all participants were asked about their history of glaucoma, prostatic hyperplasia, or drug allergy, all of which are possible contraindications for the use of anticholinergic agents6,23; patients with a history of any of these conditions did not receive cimetropium bromide. Cimetropium bromide was also not administered to patients who did not provide written informed consent for the additional costs, route of administration, and potential adverse events and benefits associated with the medication. No other factors were used to determine use or nonuse of cimetropium bromide, and no other antispasmodic agents were administered to participants who did not receive cimetropium bromide.
Endoscopic Examination
A total of 14 board-certified endoscopists participated in this study. All endoscopists used the same endoscopy unit (Olympus GIF-Q260 series; Olympus Medical Systems Corporation). Observation time was defined as the time between arrival into the duodenum and withdrawal of the scope. To avoid a possible prolongation of examination time due to biopsy sampling rather than assessment of possible lesions, observation time was assessed only for procedures that did not include biopsies.24 To calculate observation time, we used an image archiving system in which endoscopic images showed the time taken. We categorized the endoscopists into 2 groups (fast [≤2:51 minutes] and slow [>2:51 minutes]) using the median value of their observation times. The median observation time for each endoscopist was assessed during the first year after participation in this study. The biopsy rate for each endoscopist was also calculated during the first year after participation in this study and was defined as the proportion of participants with at least 1 biopsy performed during EGD. Endoscopists were again categorized into 2 groups (low [≤18.4%] and high [>18.4%]) based on the median value of their biopsy rates. Atrophic gastritis was evaluated according to the endoscopic atrophic grades described by Kimura and Takemoto.25
Groups and Outcomes
Participants were divided into 2 groups: those who received cimetropium bromide before EGD (intervention group) and those who did not (control group). All gastric lesions were confirmed by histopathological reports. Histopathological diagnosis was established in accordance with the Vienna classification for GI epithelial neoplasia.26 The primary outcome was the gastric neoplasm detection rate during EGD screening, with gastric neoplasm detection rate defined as the proportion of study participants with confirmed gastric neoplasms detected during EGD. Gastric neoplasm detection rates excluded the detection of esophageal and duodenal lesions. These types of lesions were excluded because esophageal movement resulting from a patient’s swallowing is not controllable with the use of antispasmodic agents, and the number of duodenal lesions was too small to accurately evaluate the impact of antispasmodic agents. The secondary outcome was the detection rate of small gastric lesions, with a small gastric lesion defined as a neoplasm smaller than 1 cm in diameter. The size of the gastric neoplasm was measured using biopsy forceps.
Covariates
Regular smoking was defined as smoking more than 1.5 pack-years during the past year. Alcohol drinking was defined as the consumption of more than 14 standard drinks per week for men and more than 7 standard drinks per week for women during the last 6 months.27 A standard drink was defined as any type of alcoholic drink that contained more than 12 g of pure alcohol.28 Helicobacter pylori infection was defined as a positive result on either a urea breath test or an immunoglobin G test for H pylori.
Statistical Analysis
Descriptive statistics for continuous variables were summarized as means with SDs and medians with IQRs. Because all continuous variables did not follow a normal distribution, significant differences between groups were assessed using the nonparametric Wilcoxon rank sum test. Categorical data were described using numbers with percentages and compared using χ2 or Fisher exact tests, as appropriate.
For propensity score matching, a 1:1 matching process without replacements was performed using a greedy algorithm with 0.05 caliper (0.25 multiplied by the SD of the logit of the propensity score), yielding 20 835 participants in the intervention group and 20 835 matched participants in the control group. Propensity scores were created using logistic regression modeling of the probability of a patient receiving cimetropium bromide based on age group (31-39 years, 40-59 years, 60-79 years, or ≥80 years), sex (female or male), body mass index (calculated as weight in kilograms divided by height in meters squared; <18.5, 18.5-24.9, 25.0-29.9, or ≥30.0), smoking (yes or no), alcohol drinking (yes or no), family history of gastric cancer (none, first-degree relatives, or other relatives), history of H pylori infection (yes or no), grade of atrophic gastritis (none, closed type, or open type), diabetes (yes or no), type of endoscopist by observation time (fast or slow), type of endoscopist by biopsy rate (low or high), and use of midazolam (yes or no). These variables were chosen because they were considered clinically relevant factors that may have been associated with gastric cancer development or neoplasm detection rates during EGD examination.2,24,29,30,31 The logistic model including the 12 variables had a C statistic of 0.74. Absolute standardized mean differences were estimated for all baseline covariates before and after matching to assess imbalance before matching and balance after matching. An absolute standardized mean difference of less than 0.1 for a given covariate indicated a relatively small imbalance. We classified unavailable data into a separate unknown category for each variable. This category was used to allow the creation of propensity scores without removing variables for which data were missing. Based on propensity score matching, outcomes were compared between groups using the McNemar test, and odds ratios (ORs) were calculated using generalized estimating equations with a log link and an unstructured correction matrix.
We conducted sensitivity analysis to assess whether unknown data produced any bias. Logistic regression analysis was used to identify independent factors associated with the detection of neoplasms in the unmatched cohorts. A multiple logistic regression model was constructed using stepwise selection with entry criteria of P ≤ .10 and stay criteria of P ≤ .05. Results were reported as ORs with 95% CIs.
For all analyses, 2-tailed P < .05 was considered statistically significant. All data were analyzed using SAS software, version 9.4 (SAS Institute Inc).
Results
Participant Characteristics
A total of 67 683 participants who received EGD screening were enrolled in the study (Figure). The mean (SD) age was 48.6 (10.8) years; 36 517 participants (54.0%) were male and 31 166 (46.0%) were female (Table 1). All participants were Asian, representing a racially homogenous study population. In total, 28 280 participants (41.8%) received cimetropium bromide before EGD examination, and 39 403 (58.2%) did not. The mean (SD) age was 50.3 (10.6) years in the intervention group and 47.4 (10.8) years in the control group (P < .001), and most participants in both groups were male (57.8% in the intervention group and 51.2% in the control group; P < .001). Participants in the intervention vs control group had significantly higher body mass index (mean [SD], 23.7 [3.2] vs 23.4 [3.2]; P < .001), smoking rates (22.7% vs 15.3%; P < .001), alcohol drinking rates (46.5% vs 45.3%; P < .001), family history of gastric cancer (first-degree and other relatives: 9.2% vs 7.1%; P < .001), history of H pylori infection (33.6% vs 20.1%; P < .001), atrophic gastritis (closed and open type: 42.0% vs 35.9%; P < .001), and diabetes (4.4% vs 2.6%; P < .001). The proportion of endoscopists with longer median observation time (ie, >2:51 minutes) was significantly higher among those in the control vs intervention group (44.7% vs 42.1%; P < .001). The use of midazolam was also significantly higher in the control vs intervention group (51.9% vs 20.0%; P < .001).
Figure. Flow Diagram of Participant Inclusion.
EGD indicates esophagogastroduodenoscopy; GI, gastrointestinal.
Table 1. Participant Characteristics.
| Characteristic | Unmatched participants | Propensity score–matched (1:1) participants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total, No. (%) | Received cimetropium bromide, No. (%) | Did not receive cimetropium bromide, No. (%) | P valuea | Absolute SMD | Total, No. (%) | Received cimetropium bromide, No. (%) | Did not receive cimetropium bromide, No. (%) | P valuea | Absolute SMD | |
| Total participants, No. | 67 683 | 28 280 | 39 403 | NA | NA | 41 670 | 20 835 | 20 835 | NA | NA |
| Age, mean (SD), y | 48.6 (10.8) | 50.3 (10.6) | 47.4 (10.8) | <.001 | 0.26 | 49.4 (11.1) | 49.7 (11.2) | 49.0 (11.1) | <.001 | 0.07 |
| Age group, y | ||||||||||
| 31-39 | 15 886 (23.5) | 5083 (18.0) | 10 803 (27.4) | <.001 | 0.23 | 9377 (22.5) | 4718 (22.6) | 4659 (22.4) | <.001 | 0.01 |
| 40-59 | 40 806 (60.3) | 17 631 (62.3) | 23 175 (58.8) | 0.07 | 24 245 (58.2) | 11 716 (56.2) | 12 529 (60.1) | 0.08 | ||
| 60-79 | 10 729 (15.9) | 5488 (19.4) | 5241 (13.3) | 0.17 | 7897 (19.0) | 4323 (20.7) | 3574 (17.2) | 0.09 | ||
| ≥80 | 262 (0.4) | 78 (0.3) | 184 (0.5) | 0.03 | 151 (0.4) | 78 (0.4) | 73 (0.4) | 0 | ||
| Sex | ||||||||||
| Male | 36 517 (54.0) | 16 348 (57.8) | 20 169 (51.2) | <.001 | 0.13 | 24 492 (58.8) | 12 073 (57.9) | 12 419 (59.6) | <.001 | 0.03 |
| Female | 31 166 (46.0) | 11 932 (42.2) | 19 234 (48.8) | 0.13 | 17 178 (41.2) | 8762 (42.1) | 8416 (40.4) | 0.03 | ||
| BMI | ||||||||||
| Mean (SD) | 23.5 (3.2) | 23.7 (3.2) | 23.4 (3.2) | <.001 | 0.09 | 23.7 (3.2) | 23.7 (3.2) | 23.7 (3.2) | .49 | 0 |
| Range | ||||||||||
| <18.5 | 2766 (4.1) | 1008 (3.6) | 1758 (4.5) | <.001 | 0.05 | 1579 (3.8) | 805 (3.9) | 774 (3.7) | <.001 | 0.01 |
| 18.5-24.9 | 43 478 (64.2) | 17 688 (62.5) | 25 790 (65.5) | 0.06 | 26 596 (63.8) | 13 335 (64.0) | 13 261 (63.6) | 0.01 | ||
| 25.0-29.9 | 18 176 (26.9) | 7975 (28.2) | 10 201 (25.9) | 0.05 | 11 598 (27.8) | 5774 (27.7) | 5824 (28.0) | 0.01 | ||
| ≥30.0 | 2166 (3.2) | 935 (3.3) | 1231 (3.1) | 0.01 | 1416 (3.4) | 726 (3.5) | 690 (3.3) | 0.01 | ||
| Unknown | 1097 (1.6) | 674 (2.4) | 423 (1.1) | 0.10 | 481 (1.2) | 195 (0.9) | 286 (1.4) | 0.04 | ||
| Smoking | ||||||||||
| No | 26 584 (39.3) | 11 824 (41.8) | 14 760 (37.5) | <.001 | 0.09 | 15 201 (36.5) | 7625 (36.6) | 7576 (36.4) | <.001 | 0 |
| Yes | 12 471 (18.4) | 6428 (22.7) | 6043 (15.3) | 0.19 | 8157 (19.6) | 4275 (20.5) | 3882 (18.6) | 0.05 | ||
| Unknown | 28 628 (42.3) | 10 028 (35.5) | 18 600 (47.2) | 0.24 | 18 312 (43.9) | 8935 (42.9) | 9377 (45.0) | 0.04 | ||
| Alcohol drinking | ||||||||||
| No | 18 302 (27.0) | 7443 (26.3) | 10 859 (27.6) | .001 | 0.03 | 10 217 (24.5) | 5079 (24.4) | 5138 (24.7) | .17 | 0.01 |
| Yes | 31 015 (45.8) | 13 161 (46.5) | 17 854 (45.3) | 0.02 | 18 450 (44.3) | 9202 (44.2) | 9248 (44.4) | 0 | ||
| Unknown | 18 366 (27.1) | 7676 (27.1) | 10 690 (27.1) | 0 | 13 003 (31.2) | 6554 (31.5) | 6449 (31.0) | 0.01 | ||
| Family history of gastric cancer | ||||||||||
| No | 35 986 (53.2) | 16 557 (58.5) | 19 429 (49.3) | <.001 | 0.19 | 21 786 (52.3) | 10 872 (52.2) | 10 914 (52.4) | <.001 | 0 |
| First-degree relatives | 3491 (5.2) | 1717 (6.1) | 1774 (4.5) | 0.07 | 2230 (5.4) | 1192 (5.7) | 1038 (5.0) | 0.03 | ||
| Other relatives | 1912 (2.8) | 891 (3.2) | 1021 (2.6) | 0.03 | 1364 (3.3) | 741 (3.6) | 623 (3.0) | 0.03 | ||
| Unknown | 26 294 (38.8) | 9115 (32.2) | 17 179 (43.6) | 0.24 | 16 290 (39.1) | 8030 (38.5) | 8260 (39.6) | 0.02 | ||
| History of Helicobacter pylori infectionb | ||||||||||
| No | 17 997 (26.6) | 9166 (32.4) | 8831 (22.4) | <.001 | 0.23 | 11 028 (26.5) | 5399 (25.9) | 5629 (27.0) | <.001 | 0.03 |
| Yes | 17 416 (25.7) | 9498 (33.6) | 7918 (20.1) | 0.31 | 11 575 (27.8) | 6251 (30.0) | 5324 (25.6) | 0.10 | ||
| Unknown | 32 270 (47.7) | 9616 (34.0) | 22 654 (57.5) | 0.49 | 19 067 (45.8) | 9185 (44.1) | 9882 (47.4) | 0.07 | ||
| Atrophic gastritis | ||||||||||
| None | 41 688 (61.6) | 16 413 (58.0) | 25 275 (64.1) | <.001 | 0.13 | 25 108 (60.3) | 12 506 (60.0) | 12 602 (60.5) | <.001 | 0.01 |
| Closed type | 21 792 (32.2) | 9722 (34.4) | 12 070 (30.6) | 0.08 | 13 540 (32.5) | 6690 (32.1) | 6850 (32.9) | 0.02 | ||
| Open type | 4203 (6.2) | 2145 (7.6) | 2058 (5.2) | 0.10 | 3022 (7.3) | 1639 (7.9) | 1383 (6.6) | 0.05 | ||
| Diabetes | ||||||||||
| No | 38 914 (57.5) | 17 614 (62.3) | 21 300 (54.1) | <.001 | 0.17 | 23 200 (55.7) | 11 535 (55.4) | 11 665 (56.0) | <.001 | 0.01 |
| Yes | 2270 (3.4) | 1253 (4.4) | 1017 (2.6) | 0.10 | 1717 (4.1) | 977 (4.7) | 740 (3.6) | 0.06 | ||
| Unknown | 26 499 (39.2) | 9413 (33.3) | 17 086 (43.4) | 0.21 | 16 753 (40.2) | 8323 (39.9) | 8430 (40.5) | 0.01 | ||
| Type of endoscopist by observation timec | ||||||||||
| Fast (≤2:51 min) | 38 175 (56.4) | 16 387 (57.9) | 21 788 (55.3) | <.001 | 0.04 | 24 050 (57.7) | 12 330 (59.2) | 11 720 (56.3) | <.001 | 0.06 |
| Slow (>2:51 min) | 29 508 (43.6) | 11 893 (42.1) | 17 615 (44.7) | 0.04 | 17 620 (42.3) | 8505 (40.8) | 9115 (43.7) | 0.06 | ||
| Type of endoscopist by biopsy rated | ||||||||||
| Low (≤18.4%) | 29 454 (43.5) | 12 305 (43.5) | 17 149 (43.5) | .98 | 0 | 18 458 (44.3) | 9473 (45.5) | 8985 (43.1) | <.001 | 0.05 |
| High (>18.4%) | 38 229 (56.5) | 15 975 (56.5) | 22 254 (56.5) | 0 | 23 212 (55.7) | 11 362 (54.5) | 11 850 (56.9) | 0.05 | ||
| Use of midazolam | ||||||||||
| No | 41 586 (61.4) | 22 621 (80.0) | 18 965 (48.1) | <.001 | 0.70 | 30 577 (73.4) | 15 180 (72.9) | 15 397 (73.9) | <.001 | 0.02 |
| Yes | 26 097 (38.6) | 5659 (20.0) | 20 438 (51.9) | 0.70 | 11 093 (26.6) | 5655 (27.1) | 5438 (26.1) | 0.02 | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; SMD, standardized mean difference.
P values were determined using a χ2 or Wilcoxon rank sum test for unmatched data and a McNemar or Wilcoxon rank sum test for pair-matched data.
Positive results for Helicobacter pylori infection on either urea breath test or immunoglobulin G test.
Dichotomized using median value of observation time.
Dichotomized using median value of biopsy rate.
A total of 41 670 participants (20 835 pairs) who did and did not receive cimetropium bromide were propensity score matched (1:1) based on possible confounding variables. Among matched participants, the mean (SD) age was 49.4 (11.1) years; 24 492 participants (58.8%) were male, and 17 178 (41.2%) were female. Additional baseline characteristics of participants before and after propensity score matching are shown in Table 1.
Characteristics of Individual Endoscopists
Among 14 endoscopists, 8 were categorized as having fast observation times and 6 as having slow observation times using a median value of 2:51 minutes as the cutoff. The median observation time for a normal EGD examination without biopsy was 2:54 minutes (IQR, 2:24-3:40 minutes) for fast endoscopists and 3:23 minutes (IQR, 2:51-4:12 minutes) for slow endoscopists. A total of 7 endoscopists were categorized as having low biopsy rates and 7 as having high biopsy rates using a median value of 18.4% as the cutoff. The lowest biopsy rate was 188 of 2144 EGD examinations (8.8%), and the highest rate was 693 of 2244 EGD examinations (30.9%). Observation times, biopsy rates, use of midazolam and cimetropium bromide, and detection rate of gastric neoplasms for each endoscopist are shown in Table 2. Additional participant characteristics, including demographic information and smoking and alcohol consumption according to each endoscopist, are available in eTable 1 in the Supplement.
Table 2. Baseline Characteristics of Procedures by Individual Endoscopista.
| Endoscopist | EGD examinations performed, No. | Observation time without biopsy during first year, median (IQR) | No. (%) | |||
|---|---|---|---|---|---|---|
| Biopsy rate | Use of midazolam | Use of cimetropium bromide | Gastric neoplasm | |||
| A | 2586 | 2:52 (2:26-3:28) | 447 (17.3) | 776 (30.0) | 1032 (39.9) | 3 (0.1) |
| B | 7519 | 1:47 (1:29-2:12) | 1692 (22.5) | 2422 (32.2) | 3016 (40.1) | 17 (0.2) |
| C | 1682 | 2:37 (2:16-3:07) | 424 (25.2) | 1093 (65.0) | 726 (43.2) | 4 (0.2) |
| D | 2283 | 2:47 (2:27-3:16) | 269 (11.8) | 780 (34.2) | 989 (43.3) | 3 (0.1) |
| E | 2244 | 3:24 (2:43-4:19) | 693 (30.9) | 659 (29.4) | 860 (38.3) | 4 (0.2) |
| F | 8928 | 2:49 (2:28-3:19) | 1879 (21.0) | 3260 (36.5) | 4033 (45.2) | 24 (0.3) |
| G | 8990 | 2:25 (2:05-2:54) | 1154 (12.8) | 3414 (38.0) | 3746 (41.7) | 13 (0.1) |
| H | 6371 | 2:50 (2:28-3:24) | 1280 (20.1) | 2755 (43.2) | 2653 (41.6) | 14 (0.2) |
| I | 258 | 2:36 (2:10-3:07) | 23 (8.9) | 57 (22.1) | 180 (69.8) | 1 (0.4) |
| J | 6998 | 2:59 (2:31-3:35) | 1056 (15.1) | 3012 (43.0) | 2714 (38.8) | 18 (0.3) |
| K | 8676 | 3:37 (3:10-4:11) | 2225 (25.6) | 3306 (38.1) | 3589 (41.4) | 32 (0.4) |
| L | 2144 | 2:51 (2:28-3:22) | 188 (8.8) | 569 (26.5) | 1044 (48.7) | 2 (0.1) |
| M | 6195 | 3:30 (3:02-4:06) | 907 (14.6) | 2563 (41.4) | 2600 (42.0) | 16 (0.3) |
| N | 2809 | 2:56 (2:36-3:19) | 548 (19.5) | 1431 (50.9) | 1098 (39.1) | 9 (0.3) |
| Type of endoscopist by observation timeb | ||||||
| Fast (≤2:51 min) | 38 175 | 2:54 (2:24-3:40) | 6909 (18.1) | 14 350 (37.6) | 16 387 (42.9) | 78 (0.2) |
| Slow (>2:51 min) | 29 508 | 3:23 (2:51-4:12) | 5876 (19.9) | 11 747 (39.8) | 11 893 (40.3) | 82 (0.3) |
Abbreviation: EGD, esophagogastroduodenoscopy.
P values were <.001 for all comparisons with the exception of gastric neoplasm, for which the P value was .05. P values were calculated using a Wilcoxon rank sum test or χ2 test.
Dichotomized using median value of observation time. A total of 8 endoscopists were included in the fast category, and 6 endoscopists were included in the slow category.
Gastric Neoplasms Detected During EGDs
Among 41 670 participants in the propensity score–matched cohorts, gastric neoplasms were diagnosed in 102 individuals (0.24%) (Table 3). A total of 52 dysplasias (0.12%), 40 early cancers (0.10%), 7 advanced cancers (0.02%), and 3 lymphomas (0.01%) were detected. In the propensity score–matched cohorts, small gastric lesions (<1 cm) comprised 35 of the total 102 gastric neoplasms (34.3%), and most neoplasms were found in the gastric body (49 participants [48.0%]) and antrum (49 participants [48.0%]).
Table 3. Diagnostic Outcomes of Unmatched and Propensity Score–Matched Groups.
| Outcome | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unmatched participants | Propensity score–matched (1:1) participants | |||||||
| Total (n = 67 683), No. (%) | Received cimetropium bromide (n = 28 280), No. (%) | Did not receive cimetropium bromide (n = 39 403), No. (%) | P valuea | Total (n = 41 670), No. (%) | Received cimetropium bromide (n = 20 835), No. (%) | Did not receive cimetropium bromide (n = 20 835), No. (%) | P valuea | |
| Total gastric neoplasms | 160 (0.24) | 87 (0.31) | 73 (0.19) | .001 | 102 (0.24) | 63 (0.30) | 39 (0.19) | .02 |
| Dysplasia | 85 (0.13) | 44 (0.16) | 41 (0.10) | .06 | 52 (0.12) | 32 (0.15) | 20 (0.10) | .10 |
| Early cancer | 61 (0.09) | 36 (0.13) | 25 (0.06) | .006 | 40 (0.10) | 25 (0.12) | 15 (0.07) | .11 |
| Advanced cancer | 10 (0.01) | 6 (0.02) | 4 (0.01) | .34 | 7 (0.02) | 5 (0.02) | 2 (0.01) | .26 |
| Lymphoma | 3 (0.004) | 1 (0.004) | 2 (0.01) | >.99 | 3 (0.01) | 1 (0.005) | 2 (0.01) | .56 |
| Neuroendocrine tumor | 1 (0.001) | 0 | 1 (0.003) | >.99 | 0 | 0 | 0 | NA |
| Dysplasia and early cancer | 146 (0.22) | 80 (0.28) | 66 (0.17) | .002 | 92 (0.22) | 57 (0.27) | 35 (0.17) | .02 |
| Small gastric neoplasmsb | 56 (0.08) | 35 (0.12) | 21 (0.05) | .002 | 35 (0.08) | 24 (0.12) | 11 (0.05) | .03 |
| Dysplasia | 42 (0.06) | 26 (0.09) | 16 (0.04) | .008 | 26 (0.06) | 18 (0.09) | 8 (0.04) | .05 |
| Early cancer | 14 (0.02) | 9 (0.03) | 5 (0.01) | .09 | 9 (0.02) | 6 (0.03) | 3 (0.01) | .32 |
| Location | ||||||||
| Cardia and fundus | 5 (0.01) | 2 (0.01) | 3 (0.01) | >.99 | 4 (0.01) | 2 (0.01) | 2 (0.01) | >.99 |
| Body | 72 (0.11) | 44 (0.16) | 28 (0.07) | .001 | 49 (0.12) | 34 (0.16) | 15 (0.07) | .007 |
| Antrum | 82 (0.12) | 40 (0.14) | 42 (0.11) | .20 | 49 (0.12) | 27 (0.13) | 22 (0.11) | .48 |
Abbreviation: NA, not available.
P values were determined using an χ2 test or a Fisher exact test for unmatched data and a McNemar test for matched data.
Neoplasms <1 cm in diameter.
Gastric Neoplasm Detection Rates
In the propensity score–matched cohorts, neoplasm detection rates were significantly higher in the intervention group (63 participants [0.30%]) than the control group (39 participants [0.19%]; P = .02) (Table 3). When only accounting for dysplasia and early gastric cancer, which are potentially indicative of the need for future endoscopic resection, the combined detection rate remained significantly higher in the intervention group (57 participants [0.27%]) vs the control group (35 participants [0.17%]; P = .02). The detection rate of small gastric lesions was also significantly higher in the intervention group (24 participants [0.12%]) vs the control group (11 participants [0.05%]; P = .03), and neoplasms in the gastric body were more frequently found in the intervention group (34 participants [0.16%]) vs the control group (15 participants [ 0.07%]; P = .007) (Table 3). A sensitivity analysis using multiple imputations was performed to assess whether unknown data produced any bias. The use of cimetropium bromide was consistently associated with higher gastric neoplasm detection rates (pooled OR, 1.55; 95% CI, 1.03-2.33; P = .04) (eTable 2 in the Supplement).
Factors Associated With Gastric Neoplasm Detection Rates
In the univariate analysis of unmatched data, older age (≥80 years: OR, 62.63; 95% CI, 25.24-155.40; P < .001), male sex (OR, 2.11; 95% CI, 1.50-2.96; P < .001), smoking (OR, 1.55; 95% CI, 1.03-2.33; P = .04), history of H pylori infection (OR, 4.01; 95% CI, 2.44-6.61; P < .001), atrophic gastritis (OR, 4.39; 95% CI, 2.85-6.76; P < .001), high biopsy rate (OR, 1.43; 95% CI, 1.03-1.97; P = .03), and use of cimetropium bromide (OR, 1.66; 95% CI, 1.22-2.27; P = .001) were significantly associated with higher gastric neoplasm detection rates (Table 4). The multivariate analysis revealed that the use of cimetropium bromide was more likely to detect gastric neoplasms compared with nonuse (OR, 1.42; 95% CI, 1.04-1.95; P = .03) after adjusting for older age, male sex, low body mass index, H pylori infection, atrophic gastritis, endoscopists with slow observation times, and endoscopists with high biopsy rates. Even after including all variables, the multivariate analysis revealed a consistent association between the use of cimetropium bromide and higher gastric neoplasm detection rates (OR, 1.54; 95% CI, 1.11-2.13; P = .009) (eTable 3 in the Supplement).
Table 4. Univariate and Multivariate Logistic Regression Analyses of Factors Associated With Detection of Gastric Neoplasm.
| Variable | No. (%) | Univariate analysis | Multivariate analysisa | |||
|---|---|---|---|---|---|---|
| Gastric neoplasm (n = 160) | Nongastric neoplasm (n = 67 523) | OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age group, y | ||||||
| 31-39 | 9 (5.6) | 15 877 (23.5) | 1 [Reference] | NA | 1 [Reference] | NA |
| 40-59 | 71 (44.4) | 40 735 (60.3) | 2.93 (1.49-5.77) | .002 | 1.80 (0.91-3.56) | .09 |
| 60-79 | 71 (44.4) | 10 658 (15.8) | 11.21 (5.70-22.07) | <.001 | 4.37 (2.14-8.91) | <.001 |
| ≥80 | 9 (5.6) | 253 (0.4) | 62.63 (25.24-155.40) | <.001 | 18.70 (7.15-48.90) | <.001 |
| Sex | ||||||
| Male | 114 (71.3) | 36 403 (53.9) | 2.11 (1.50-2.96) | <.001 | 2.04 (1.45-2.88) | <.001 |
| Female | 46 (28.8) | 31 120 (46.1) | 1 [Reference] | 1 [Reference] | ||
| BMI range | ||||||
| <18.5 | 11 (6.9) | 2755 (4.1) | 1.78 (0.97-3.29) | .06 | 2.74 (1.47-5.08) | .001 |
| 18.5-24.9 | 101 (63.1) | 43 377 (64.2) | 1 [Reference] | NA | 1 [Reference] | NA |
| 25.0-29.9 | 38 (23.8) | 18 138 (26.9) | 0.91 (0.63-1.32) | .61 | 0.73 (0.50-1.06) | .10 |
| ≥30.0 | 4 (2.5) | 2162 (3.2) | 0.89 (0.35-2.29) | .81 | 0.98 (0.39-2.50) | .97 |
| Unknown | 6 (3.8) | 1091 (1.6) | 2.55 (1.15-5.64) | .02 | 1.67 (0.73-3.80) | .22 |
| Smoking | ||||||
| No | 54 (33.8) | 26 530 (39.3) | 1 [Reference] | NA | [Reference] | NA |
| Yes | 39 (24.4) | 12 432 (18.4) | 1.55 (1.03-2.33) | .04 | NA | NA |
| Unknown | 67 (41.9) | 28 561 (42.3) | 1.15 (0.80-1.64) | .44 | NA | NA |
| Alcohol drinking | ||||||
| No | 48 (30.0) | 18 254 (27.0) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 67 (41.9) | 30 948 (45.8) | 0.82 (0.57-1.19) | .30 | NA | NA |
| Unknown | 45 (28.1) | 18 321 (27.1) | 0.93 (0.62-1.40) | .74 | NA | NA |
| Family history of gastric cancer | ||||||
| No | 84 (52.5) | 35 902 (53.2) | 1 [Reference] | NA | 1 [Reference] | NA |
| First-degree relatives | 10 (6.3) | 3481 (5.2) | 1.28 (0.67-2.44) | .45 | NA | NA |
| Other relatives | 4 (2.5) | 1908 (2.8) | 1.00 (0.39-2.59) | >.99 | NA | NA |
| Unknown | 62 (38.8) | 26 232 (38.8) | 1.01 (0.73-1.40) | .94 | NA | NA |
| History of Helicobacter pylori infection | ||||||
| No | 19 (11.9) | 17 978 (26.6) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 75 (46.9) | 17 341 (25.7) | 4.01 (2.44-6.61) | <.001 | 2.11 (1.27-3.49) | .004 |
| Unknown | 66 (41.3) | 32 204 (47.7) | 1.90 (1.15-3.15) | .01 | 2.00 (1.20-3.35) | .008 |
| Atrophic gastritis | ||||||
| No | 29 (18.1) | 41 659 (61.7) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 67 (41.9) | 21 725 (32.2) | 4.39 (2.85-6.76) | <.001 | 2.91 (1.87-4.55) | <.001 |
| Unknown | 64 (40.0) | 4139 (6.1) | 22.00 (14.22-34.06) | <.001 | 9.73 (6.03-15.70) | <.001 |
| Diabetes | ||||||
| No | 94 (58.8) | 38 820 (57.5) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 7 (4.4) | 2263 (3.4) | 1.36 (0.65-2.87) | .42 | NA | NA |
| Unknown | 59 (36.9) | 26 440 (39.2) | 0.92 (0.67-1.28) | .64 | NA | NA |
| Type of endoscopist by observation timeb | ||||||
| Fast (≤2:51 min) | 78 (48.8) | 38 097 (56.4) | 1 [Reference] | .05 | 1 [Reference] | .01 |
| Slow (>2:51 min) | 82 (51.3) | 29 426 (43.6) | 1.36 (1.00-1.85) | 1.49 (1.09-2.04) | ||
| Type of endoscopist by biopsy ratec | ||||||
| Low (≤18.4%) | 56 (35.0) | 29 398 (43.5) | 1 [Reference] | .03 | 1 [Reference] | .007 |
| High (>18.4%) | 104 (65.0) | 38 125 (56.5) | 1.43 (1.03-1.97) | 1.56 (1.13-2.15) | ||
| Use of midazolam | ||||||
| No | 102 (63.8) | 41 484 (61.4) | 1 [Reference] | .56 | 1 [Reference] | NA |
| Yes | 58 (36.3) | 26 039 (38.6) | 0.91 (0.66-1.25) | 1.31 (0.94-1.83) | ||
| Use of cimetropium bromide | ||||||
| No | 73 (45.6) | 39 330 (58.2) | 1 [Reference] | .001 | 1 [Reference] | .03 |
| Yes | 87 (54.4) | 28 193 (41.8) | 1.66 (1.22-2.27) | 1.42 (1.04-1.95) | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; OR, odds ratio.
Stepwise selection with entry criteria of P ≤ .10 and stay criteria of P ≤ .05.
Dichotomized using median value of observation time.
Dichotomized using median value of biopsy rate.
Discussion
This cohort study found that the use of an antispasmodic agent, cimetropium bromide, as premedication was associated with higher gastric neoplasm detection rates during EGD examinations. We also found that the use of cimetropium bromide before EGD screening was more likely to increase the detection rates of gastric dysplasia and early cancer, which are the ultimate targets of endoscopic screening. Furthermore, the detection rate of small gastric neoplasms was significantly higher among those who received cimetropium bromide before EGD screening than those who did not.
Mucosal visualization during EGD examination is often limited by spasm or peristalsis of the GI tract, which hinders detailed observation and may result in missed detection of subtle gastric neoplasms.4,32 Improved visualization through the use of antispasmodic agents could theoretically enhance the detection of lesions during EGD examination. However, there is considerable variation in the routine use of antispasmodic agents based on the experiences and preferences of each country, institution, or endoscopist.33 In addition, there is no consensus on the use of antispasmodic agents.34,35,36
A recent Asian consensus guideline17 recommended the routine use of antispasmodic agents before EGD examination to increase the detection rate of precancerous neoplasms, even when there is a paucity of evidence to support this recommendation. However, a previous study37 reported that the use of scopolamine did not significantly increase the detection of upper GI neoplasms. The study enrolled 6625 pairs of participants who did and did not receive scopolamine before EGD examination, with propensity score matching performed based on possible covariates that may have been associated with neoplasm detection rates. A total of 54 lesions were found in the esophagus, stomach, and duodenum. The researchers concluded that the use of scopolamine was not associated with improvements in the detection rate of any upper GI neoplasm.37
Compared with the previous study,37 our study conducted an analysis among a sample that was 3 times larger. We focused on gastric neoplasm detection rates after excluding esophageal and duodenal lesions because esophageal movement cannot be controlled by antispasmodic agents, and the number of duodenal lesions was too small to accurately evaluate the impact of cimetropium bromide. Furthermore, we included other important confounders for gastric neoplasm detection rates, such as H pylori infection, grade of atrophic gastritis, and endoscopist observation time.24,38
Several antispasmodic agents, such as hyoscine butylbromide, glucagon, and cimetropium bromide, have been used clinically as premedication before GI endoscopic evaluations and have had good safety profiles. The Korea Institute of Drug Safety and Risk Management–Korea Adverse Event Reporting System reports cases of anaphylaxis and death associated with medication use. According to a study of case safety reports39 recorded in this system between January 2008 and December 2017, 4700 cases of drug-induced anaphylaxis and 8664 deaths associated with drugs were reported. However, there were no descriptions of anaphylaxis or death associated with antispasmodic agents, including cimetropium bromide. Consistent with this finding, no serious adverse events were reported in our study.
The present study found that cimetropium bromide was useful for detecting neoplasms located in the gastric body. During colonoscopy, antispasmodic agents also assist in flattening haustral folds and enhance visualization of the mucosa behind the folds to improve the detection rates of colon polyps and dysplasia.40 Because the presence of multiple folds in the gastric body presents obstacles during observation that are similar to those of haustral folds in the colon, the impact of antispasmodic agents may also be substantial. When peristalsis is increased by the stimulation of scope insertion during EGD screening, neoplasms located in the gastric body may not be easily detected in a contracted or incompletely dilated stomach. A previous systematic review41 reported that missed gastric cancers were mainly located in the gastric body. Therefore, the use of antispasmodic agents may reduce the contraction of the gastric body and decrease the rates of missed detection of gastric neoplasms. In the present study, the detection rates of neoplasms in the antrum were not significantly different between the 2 groups. This finding might be explained by the fact that the antrum is more easily exposed, even in cases of increased peristalsis, compared with other locations.42
Strengths and Limitations
This study has several strengths. First, we adjusted for many confounding factors that may have been associated with gastric neoplasm detection rates, including participant characteristics and endoscopist factors, such as biopsy rate and observation time. Second, we minimized the impact of endoscopists’ experience by including only board-certified endoscopists, thereby reducing variations associated with endoscopists’ differing levels of expertise. Third, we included more than 60 000 participants to provide adequate numbers for the analysis. Previous studies24,43 reported that the incidence of gastric neoplasms was less than 0.3% among participants who received screening. Therefore, a large number of participants were required to accurately assess the association of an antispasmodic agent with gastric neoplasm detection rates during EGD screening. Fourth, we analyzed the detection rate of small lesions as well as total gastric neoplasms to provide reliable results regarding the impact of cimetropium bromide, with both analyses yielding consistent findings.
The study also has limitations. First, the cohort included participants with unavailable data for some covariates, which might be a source of bias in a retrospective study. We dealt with these unavailable data by adding an unknown category for each variable. This method required approximation that may have produced variance in the data. Second, cimetropium bromide may not be available in all countries. However, it is worth noting that this drug has antiperistaltic benefits that are almost equivalent to those of hyoscine N-butylbromide, a widely used antispasmodic agent.8,9,10,23 Third, we could not fully investigate minor adverse events because no additional medical reports of minor events were recorded by our institution. Fourth, we could not directly evaluate the extent of peristalsis after the injection of cimetropium bromide during EGD examination. Different individuals may have different responses to cimetropium bromide. However, the measurement of peristalsis is difficult and not validated. Further research is needed to identify objective methods for combining the evaluations of antiperistaltic activity and gastric neoplasm detection rates.
Conclusions
This cohort study found a significant association between the use of cimetropium bromide as premedication and improvements in the detection rate of total gastric neoplasms, gastric dysplasia, and early gastric cancer during EGD screening. These findings suggest that cimetropium bromide may be considered for use as premedication before EGD examination among individuals with no contraindications.
eTable 1. Baseline Characteristics of Participants According to Endoscopists
eTable 2. Odds Ratios From Propensity Score Matching After Multiple Imputation
eTable 3. Univariate and Multivariate Logistic Regression Analyses for Factors Associated With Detection of Gastric Neoplasm
References
- 1.Veitch AM, Uedo N, Yao K, East JE. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12(11):660-667. doi: 10.1038/nrgastro.2015.128 [DOI] [PubMed] [Google Scholar]
- 2.Leung WK, Wu MS, Kakugawa Y, et al. ; Asia Pacific Working Group on Gastric Cancer . Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279-287. doi: 10.1016/S1470-2045(08)70072-X [DOI] [PubMed] [Google Scholar]
- 3.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152(6):1319-1328. doi: 10.1053/j.gastro.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 4.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26(1):11-22. [PMC free article] [PubMed] [Google Scholar]
- 5.Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005;27(5):655-665. doi: 10.1097/01.ftd.0000168293.48226.57 [DOI] [PubMed] [Google Scholar]
- 6.Bell GD. Premedication, preparation, and surveillance. Endoscopy. 2002;34(1):2-12. doi: 10.1055/s-2002-19389 [DOI] [PubMed] [Google Scholar]
- 7.Cattau EL Jr, Artnak EJ, Castell DO, Meyer GW. Efficacy of atropine as an endoscopic premedication. Gastrointest Endosc. 1983;29(4):285-288. doi: 10.1016/S0016-5107(83)72634-9 [DOI] [PubMed] [Google Scholar]
- 8.Steger AC, Galland RB, Murray JK, Spencer J. The use of hyoscine in upper gastrointestinal tract endoscopy. Am J Gastroenterol. 1986;81(7):615. [PubMed] [Google Scholar]
- 9.Imagawa A, Hata H, Nakatsu M, et al. Peppermint oil solution is useful as an antispasmodic drug for esophagogastroduodenoscopy, especially for elderly patients. Dig Dis Sci. 2012;57(9):2379-2384. doi: 10.1007/s10620-012-2194-4 [DOI] [PubMed] [Google Scholar]
- 10.Bianchi Porro G, Lazzaroni M, Parente F. The use of antimuscarinic drugs in upper gastrointestinal tract endoscopy. Am J Gastroenterol. 1987;82(9):916. [PubMed] [Google Scholar]
- 11.Hiki N, Kaminishi M, Yasuda K, et al. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Dig Endosc. 2012;24(2):79-86. doi: 10.1111/j.1443-1661.2011.01163.x [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Adachi K, Ishimura N, et al. Safety and efficacy of glucagon as a premedication for upper gastrointestinal endoscopy—a comparative study with butyl scopolamine bromide. Aliment Pharmacol Ther. 2002;16(1):111-118. doi: 10.1046/j.1365-2036.2002.01148.x [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Kim SJ, Choi CS, et al. The efficacy of cimetropium bromide as a premedication before esophagogastroduodenoscopy. Clin Endosc. 2008;37(6):403-408. [Google Scholar]
- 14.Katoh K, Nomura M, Iga A, et al. Comparison of gastric peristalsis inhibition by scopolamine butylbromide and glucagon: evaluation by electrogastrography and analysis of heart rate variability. J Gastroenterol. 2003;38(7):629-635. doi: 10.1007/s00535-003-1114-y [DOI] [PubMed] [Google Scholar]
- 15.Fujishiro M, Kaminishi M, Hiki N, et al. Efficacy of spraying l-menthol solution during endoscopic treatment of early gastric cancer: a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Gastroenterol. 2014;49(3):446-454. doi: 10.1007/s00535-013-0856-4 [DOI] [PubMed] [Google Scholar]
- 16.Hiki N, Kaminishi M, Yasuda K, et al. Antiperistaltic effect and safety of L-menthol sprayed on the gastric mucosa for upper GI endoscopy: a phase III, multicenter, randomized, double-blind, placebo-controlled study. Gastrointest Endosc. 2011;73(5):932-941. doi: 10.1016/j.gie.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 17.Chiu PWY, Uedo N, Singh R, et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. 2019;68(2):186-197. doi: 10.1136/gutjnl-2018-317111 [DOI] [PubMed] [Google Scholar]
- 18.Imbimbo BP, Daniotti S, Vidi A, Foschi D, Saporiti F, Ferrante L. Discontinuous oral absorption of cimetropium bromide, a new antispasmodic drug. J Pharm Sci. 1986;75(7):680-684. doi: 10.1002/jps.2600750713 [DOI] [PubMed] [Google Scholar]
- 19.Goei R, Nix M, Kessels AH, Ten Tusscher MP. Use of antispasmodic drugs in double contrast barium enema examination: glucagon or buscopan? Clin Radiol. 1995;50(8):553-557. doi: 10.1016/S0009-9260(05)83191-5 [DOI] [PubMed] [Google Scholar]
- 20.Treweeke P, Barrett NK. Allergic reaction to buscopan. Br J Radiol. 1987;60(712):417-418. doi: 10.1259/0007-1285-60-712-417-b [DOI] [PubMed] [Google Scholar]
- 21.Hasselkus W. Inappropriate rise in heart rate caused by intravenous administration of trospium chloride during upper gastrointestinal endoscopy. Endoscopy. 1998;30(6):580. doi: 10.1055/s-2007-1001350 [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Parente F, Lazzaroni M, Imbimbo BP, Panza E, Ardizzone S, Bianchi Porro G. The use of cimetropii bromidum as premedication for endoscopy of the upper gastro-intestinal tract: a double-blind controlled clinical trial. J Int Med Res. 1985;13(6):332-337. doi: 10.1177/030006058501300606 [DOI] [PubMed] [Google Scholar]
- 24.Park JM, Huo SM, Lee HH, Lee BI, Song HJ, Choi MG. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017;153(2):460-469. doi: 10.1053/j.gastro.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1(3):87-97. doi: 10.1055/s-0028-1098086 [DOI] [Google Scholar]
- 26.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51(1):130-131. doi: 10.1136/gut.51.1.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen JP, Columbus M, eds. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. Diane Publishing; 1997. [Google Scholar]
- 28.Brick J. Standardization of alcohol calculations in research. Alcohol Clin Exp Res. 2006;30(8):1276-1287. doi: 10.1111/j.1530-0277.2006.00155.x [DOI] [PubMed] [Google Scholar]
- 29.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48(13):3554-3560. [PubMed] [Google Scholar]
- 30.You WC, Zhang L, Gail MH, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92(19):1607-1612. doi: 10.1093/jnci/92.19.1607 [DOI] [PubMed] [Google Scholar]
- 31.Cai Q, Zhu C, Yuan Y, et al. ; Gastrointestinal Early Cancer Prevention & Treatment Alliance of China (GECA) . Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68(9):1576-1587. doi: 10.1136/gutjnl-2018-317556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faigel DO, Eisen GM, Baron TH, et al. ; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy . Preparation of patients for GI endoscopy. Gastrointest Endosc. 2003;57(4):446-450. doi: 10.1016/S0016-5107(03)80006-8 [DOI] [PubMed] [Google Scholar]
- 33.Uedo N, Gotoda T, Yoshinaga S, et al. Differences in routine esophagogastroduodenoscopy between Japanese and international facilities: a questionnaire survey. Dig Endosc. 2016;28(suppl 1):16-24. doi: 10.1111/den.12629 [DOI] [PubMed] [Google Scholar]
- 34.Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. 2017;66(11):1886-1899. doi: 10.1136/gutjnl-2017-314109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisschops R, Areia M, Coron E, et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2016;48(9):843-864. doi: 10.1055/s-0042-113128 [DOI] [PubMed] [Google Scholar]
- 36.Park WG, Shaheen NJ, Cohen J, et al. Quality indicators for EGD. Gastrointest Endosc. 2015;81(1):17-30. doi: 10.1016/j.gie.2014.07.057 [DOI] [PubMed] [Google Scholar]
- 37.Omata F, Kumakura Y, Ishii N, et al. Noneffectiveness of scopolamine for facilitating detection of upper gastrointestinal neoplasia during screening esophagogastroduodenoscopy: propensity score–matched study. Endoscopy. 2020;52(7):556-562. doi: 10.1055/a-1130-6127 [DOI] [PubMed] [Google Scholar]
- 38.Januszewicz W, Wieszczy P, Bialek A, et al. Endoscopist biopsy rate as a quality indicator for outpatient gastroscopy: a multicenter cohort study with validation. Gastrointest Endosc. 2019;89(6):1141-1149. doi: 10.1016/j.gie.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 39.Cho MK, Moon M, Kim HH, et al. Analysis of individual case safety reports of drug-induced anaphylaxis to the Korea Adverse Event Reporting System. Article in Korean. Allergy Asthma Respir Dis. 2020;8(1):30-35. doi: 10.4168/aard.2020.8.1.30 [DOI] [Google Scholar]
- 40.Corte C, Dahlenburg L, Selby W, et al. Hyoscine butylbromide administered at the cecum increases polyp detection: a randomized double-blind placebo-controlled trial. Endoscopy. 2012;44(10):917-922. doi: 10.1055/s-0032-1310009 [DOI] [PubMed] [Google Scholar]
- 41.Pimenta-Melo AR, Monteiro-Soares M, Libânio D, Dinis-Ribeiro M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28(9):1041-1049. doi: 10.1097/MEG.0000000000000657 [DOI] [PubMed] [Google Scholar]
- 42.Ren W, Yu J, Zhang ZM, Song YK, Li YH, Wang L. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol. 2013;19(13):2092-2096. doi: 10.3748/wjg.v19.i13.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park CH, Kim B, Chung H, et al. Endoscopic quality indicators for esophagogastroduodenoscopy in gastric cancer screening. Dig Dis Sci. 2015;60(1):38-46. doi: 10.1007/s10620-014-3288-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Participants According to Endoscopists
eTable 2. Odds Ratios From Propensity Score Matching After Multiple Imputation
eTable 3. Univariate and Multivariate Logistic Regression Analyses for Factors Associated With Detection of Gastric Neoplasm