Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected over 220 million individuals worldwide, and has been shown to cause increased disease severity and mortality in patients with active cancer versus healthy individuals. Vaccination is important in reducing COVID-19-associated morbidity and mortality. Thus, the aim of this article was to review the existing knowledge on effectiveness, immunogenicity and safety of COVID-19 vaccines in patients with cancer. Fifty-four articles were included following a search of PubMed and Google Scholar databases for studies published between January 2020 and September 2021 that investigated humoral and cell-mediated immune responses following COVID-19 vaccination in patients with cancer. Immunogenicity of vaccines was found to be lower in patients with cancer versus healthy individuals, and humoral immune responses were inferior in those with haematological versus solid cancers. Patient-, disease-, and treatment-related factors associated with poorer vaccine responses should be identified and corrected or mitigated when possible. Consideration should be given to offering patients with cancer second doses of COVID vaccine at shorter intervals than in healthy individuals. Patients with cancer warrant a third vaccine dose and must be prioritized in vaccination schedules. Vaccine adverse effect profiles are comparable between patients with cancer and healthy individuals.
Keywords: COVID-19, cancer, vaccines, solid malignancies, haematological malignancies, vaccine effectiveness, vaccine safety
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected more than 220 million individuals worldwide since it was first reported in December 2019. 1 In addition to its effects on the respiratory system, COVID-19 has been shown to cause a myriad of manifestations in other organ systems,2–5 and to cause increased disease severity and mortality in patients with active cancer.6–9 In addition to several proposed novel treatment strategies,10,11 vaccination is an important preventive strategy for reducing COVID-19-associated morbidity and mortality. 12 However, patients with cancer may have a poorer response to COVID-19 vaccination compared with healthy individuals. The emergence of SARS-CoV-2 variants has led to further changes in disease manifestations and the effectiveness of vaccines. 13 Shortened vaccination schedules and provision of a third dose of vaccines has been proposed for patients with cancer. In the present article, existing knowledge (as at the end of September 2021) on the effectiveness, immunogenicity and safety of COVID-19 vaccines in patients with cancer is discussed.
Immune responses following COVID-19 vaccines in healthy individuals
The currently available COVID-19 vaccines are efficacious at protecting against severe infection, hospitalization and death, but are less effective at providing complete protection against infection. 14 Fully vaccinated people are much less likely to suffer from severe SARS-CoV-2 infection. Following the first dose of a COVID-19 vaccine in a healthy individual, both antibody and cellular immune responses occur. 15 The emergence of SARS-CoV-2 variants with spike mutations that impact antibody recognition threatens the success of SARS-CoV-2 vaccine programs. 16 A single dose of the BNT162b2/Pfizer or ChAdOx1 nCoV-19 (Astra-Zeneca) vaccine provides around 30 percent effectiveness against the currently prevailing Delta variant. 17 However, following the second vaccine dose, an increase in both antibody and cellular immune responses are observed and the effectiveness of the vaccines increases to over 67–88% at two weeks post-second dose of the vaccine. 17
Immunogenicity and effectiveness of non-COVID-19 vaccines in patients with cancer
The immunogenicity and effectiveness of vaccines in patients with solid and haematological cancers have been assessed in different studies. The antibody response rate to the trivalent inactivated influenza vaccine in adult patients with lung cancer was found to be 78%, 4–6 weeks after a single dose, which is comparable to findings in healthy volunteers; 18 In another study, vaccine effectiveness was reported to be 21% and 20%, respectively, against laboratory-confirmed influenza infection or hospitalization, in patients with solid and haematological cancer. 19 Of note, vaccine effectiveness was significantly higher among patients with solid (25%) compared with haematological (8%) cancers, but no significant difference was seen in patients with solid tumours either receiving or not receiving active chemotherapy. 19 In a prospective study from the Roswell Park Cancer Institute among patients with colorectal cancer receiving the trivalent influenza vaccine, the immune response rate was 71%, with no significant difference between patients receiving or not receiving active chemotherapy. 20
A study involving paediatric patients with solid and haematological cancer, vaccinated with two doses of live-attenuated varicella vaccine, found a seroconversion rate of 19% and 94% after the first and second doses, respectively, with a significant rise in antibody titres following the second dose. 21 Among paediatric patients with solid and haematological (lymphoma) cancers, receiving a double dose of inactivated hepatitis A vaccine, the seroconversion rate was 60% and 74% following the first and second doses, with no significant difference in seropositivity between patients with solid tumours or lymphoma. 22 A randomized controlled trial of a 13-valent pneumococcal conjugate vaccine in patients with gastric and colorectal cancer, to assess immunogenicity at different time intervals between receiving the vaccine and initiation of chemotherapy, revealed no significant difference in antibody responses between those receiving the vaccine on day 1 of chemotherapy and those who received the vaccine 2 weeks prior to chemotherapy initiation. 23 These findings suggest that cancer patients can mount a reasonable response to vaccination despite being on chemotherapy. However, those with certain haematological malignancies may have a blunted and heterogenous vaccination response than those with solid malignancies.
Literature search
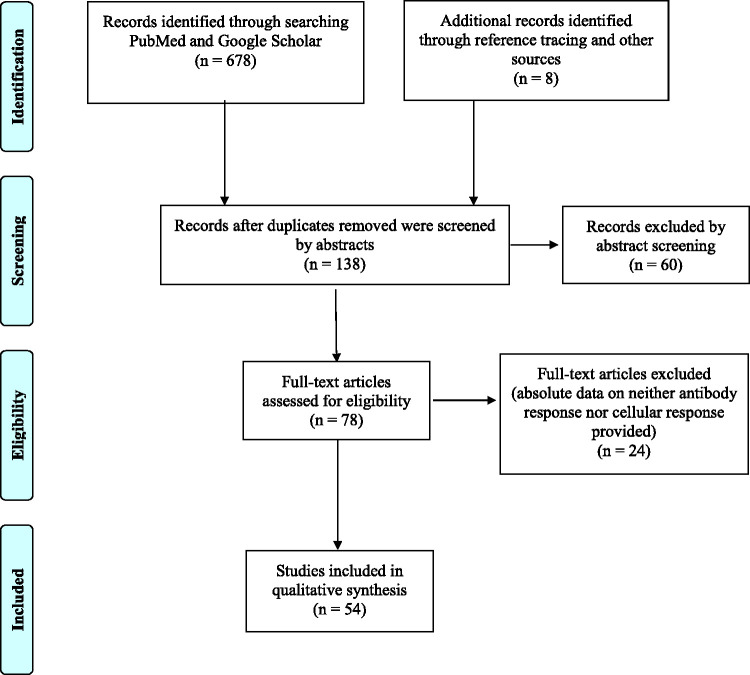
To review existing knowledge on the effectiveness, immunogenicity and safety of COVID-19 vaccines in patients with cancer, the PubMed and Google Scholar databases were searched for articles published between January 2020 and September 2021, using the search terms “(COVID-19[Title/Abstract]) AND (vaccine[Title/Abstract]) AND ((cancer[Title/Abstract]) OR (malignancy[Title/Abstract]) OR (neoplasm[Title/Abstract]))” and “((Vaccines[MeSH Major Topic]) AND (COVID-19[MeSH Major Topic])) AND (Neoplasms[MeSH Major Topic])” in PubMed and “COVID AND vaccine AND cancer” and “COVID AND vaccine AND haematological malignancy” in Google Scholar. Retrospective and prospective observational and cohort studies describing humoral and cell-mediated immune responses following COVID-19 vaccination in patients with cancer, with or without a control group comparison, were included following review of abstracts and full text after eliminating duplicates and non-English articles (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram specifying the number of studies screened and included in the present review.
Immunogenicity and effectiveness of COVID-19 vaccines in patients with cancer
A total of 54 studies on COVID-19 vaccines in patients with cancer were included in this review.24–77 Patients with cancer showed reduced immunogenicity in response to COVID-19 vaccines in terms of both humoral and cell-mediated immune responses. The important immune response findings, following the use of available COVID-19 vaccines in patients with solid and/or haematological cancer, are summarised in Table 1.
Table 1.
Studies on humoral and/or cellular immune response in patients with solid and/or haematological cancer, following vaccination with available COVID-19 vaccines.
| First author, publication month and year (country)a | Vaccine | Cancer type | Objective | Cancer cohort size | Methodology and study duration | Significant findings |
|---|---|---|---|---|---|---|
| Addeo, 24 Aug 2021 (USA and Switzerland) | P, M | S (81%), H (19%) | Seroconversion rates, anti-spike protein antibody titres | 131 | Prospective cohort study. Measurements following first and second doses of P and M vaccines. Treatment groups: clinical surveillance only (37%), cytotoxic chemotherapy (23%), endocrine therapy (15%), monoclonal antibody therapy (13%), kinase inhibitor therapy (11%), immunotherapy (11%) (Jan–Apr 2021) | Overall SCR – 81% after one dose, 94% after two doses (P: 83% and 93%; M: 80% and 95%). SCR and Ab titres lower with H (77%) versus S (98%). No Ab response in patients who received anti-CD20 Ab in last 6 months. Significant difference in Ab titres seen: highest in clinical surveillance, endocrine therapy groups and lowest in cytotoxic CTX, monoclonal Ab therapy. SCR higher in females versus males, no differences by age, race, vaccine type. |
| Agbarya, 25 Aug 2021 (Israel) | P | S | Seroconversion and antibody titres in S receiving anticancer therapy at vaccination | 140 | Cross-sectional study. 215 non-cancer controls. Ab levels measured 7 days after second dose. (Jan–Mar 2021) | Ab response significantly lower in cancer (14.3% seronegative) versus. non-cancer (1.4% seronegative) group. Median Ab levels significantly lower in cancer group. Lower response seen only in CTX group, not seen in cancer patients treated with non-CTX drugs. |
| Agha, 26 Apr 2021 (USA) | P, M | H | Antibody response | 67 | Ab levels assessed median 23 days after second dose. 44.8% under active therapy. Vaccine types: P (50.8%), M (41.8%) | No Ab response in 46.3% overall. Detectable Ab in only 23% of B-cell CLL patients. |
| Ariamanesh, 27 Sep 2021 (Iran) | SP | S plus H (6.6%) | Antibody level | 364 | Prospective study. Anti-spike and neutralizing Ab levels measured at first dose and 2 months after second dose. Treatment groups: CTX ± RT (72.7%), RT only (27.3%), follow-up only ± endocrine therapy (5.5%). Follow-up for 3 months. (Mar–Jun 2021) | Response to either/both anti-spike Ab and neutralizing Ab in 86.9% (77.1% anti-spike Ab, 80.7% neutralizing Ab). SCR lower with older age (79% in >60 years), H (61.9%) and with CTX (83.5%). Total five breakthrough infections. |
| Avivi, 28 Jul 2021 (Israel) | P | H (MM) | Antibody response after second dose | 171 | Prospective study with 64 healthy controls. Ab response assessed ≤30 days before first dose, and 14–21 days after second dose. Cancer types: 159 active MM, 12 SMM. (Dec 2020–Mar 2021) | Seropositivity: 76% in active MM, 100% in SMM, 98% in controls. Older age, ≥4 novel antimyeloma drugs, hypogammaglobulinemia associated with lower response. None of novel agents significantly decreased response. Adverse effects in 53% patients versus 55% controls. |
| Barrière, 29 Apr 2021 (France) | P, M | S | Spike protein receptor binding domain antibody response | 122 | Prospective cohort study with healthy controls, interim analysis. Assessed at first dose, second dose and 3–4 weeks after second dose. Treatment groups: CTX ± targeted therapy (86%). (Jan–Mar 2021) | SCR: 47.5% after first dose, 95.2% after second dose (versus 100% after first and second dose in control group). Lower SCR (42.9%) in patients under CTX after first dose versus patients without CTX or targeted therapy alone (76.5%). Median Ab levels significantly lower than control group after first dose and after second dose. But median Ab levels significantly increased after second dose in both cohorts. |
| Benjamini, 30 Jul 2021 (Israel) | P | H (CLL) | Antibody response after second dose | 373 | Prospective interventional study. Ab levels assessed 2–3 weeks after second dose. (Dec 2020–Feb 2021) | Seropositivity: 43% overall, 61% of treatment-naïve, 18% of ongoing CLL, 37% of previously treated with BTKi, 5% of recent anti-CD20 therapy, 62% of BCL2 monotherapy, 14% combined BCL2 + anti-CD20 therapy. Adverse effects in 50% (grade 1–2). Poor response in age >70, recent treatment with anti-CD20 therapy, active ibrutinib therapy, IgG <700, IgM <40. |
| Bird, 31 Apr 2021 (UK) | P, AZ | H (MM) | Serological response following first dose | 93 | Retrospective study. Anti-spike Ab level ≥21 days after first dose. Treatment groups: 71% on therapy at vaccination. 29% on stable/progressive disease. (All patients available on 16 Mar 2021) | Seropositivity: 54% after P versus 58% after AZ (not sig.); 48% on therapy versus 74% not on therapy (sig.), 30% stable/progressive disease versus 63% complete/very good response versus 75% partial response (sig.); 44% immunoparesis versus 66% no immunoparesis (sig.). No difference based on age, sex, time from vaccination to antibody test. Being on therapy reduced rate of positivity but no association with any specific treatment. |
| Chowdhury, 32 Jun 2021 (UK) | P, AZ | H (CML, ET, PV, MF, MDS) | Antibody level in chronic myeloid neoplasms | 59 | 232 controls. Anti-spike Ab levels measured. Treatment groups: active treatment (71%), no cytoreductive/tyrosine kinase inhibitors (29%) (Jan–Apr 2021) | SCR significantly lower in patients (58%) versus controls (97%) after first dose. No difference in SCR according to vaccine type. Reasonably high SCR following single dose in CML, and in MPN patients on interferon. Ab responses substantially impaired (versus controls) especially in patients receiving ruxolitinib and hydroxycarbamide. |
| Chung, 33 Sep 2021 (USA) | P, M | H | Antibody levels after first and second dose | 551 | Observational study with 69 healthy controls. Ab levels and neutralizing activity assessed at 1 and 3 months after first dose. (Dec 2020–Apr 2021) | SCR 51.5% at 1 month, 68.9% at 3 months. Neutralizing capacity 26.3% (versus 93.2% in controls) at 1 month, 43.6% (versus 100% in controls) at 3 months. Treatment with BKI, venetoclax, phosphoinositide 3-kinase inhibitors, anti-CD19/CD20-directed therapies, anti-CD38/BCMA-directed therapies significantly hindered responses, but single-agent immunomodulatory agents did not. |
| Ehmsen, 34 Jul 2021 (Denmark) | P, M | S (38%) plus H (62%) | Immune and T-cell responses | 524 | Prospective study. Anti-spike Ab levels and T-cell response assessed median 36 days and 3 months after second dose. Treatment groups: S on active therapy (100%), H on active therapy (39%), H post-HSCT (17%). | Seropositivity: 93% in S versus 66% in H. In H: significantly more seronegative patients had progressive disease and more seronegative patients were treated with anti-CD20, BTKi or CTX (versus seropositives). Significant association between steroid use and seronegativity. Anti-CD38 therapy not associated with seronegativity. Fewer seronegative patients had HSCT. T-cell response: 46% in S, 45% in H. Most seronegative patients (76%) did not elicit a T-cell response. Only lack of T-cell responses was significantly associated with steroid use. |
| Gavriatopoulou, 35 Jul 2021 (Greece) | P, AZ | H (WM, CLL, NHL) | Neutralizing antibody response after first dose | 58 | 213 controls. Neutralizing Ab levels assessed on days 1 and 22 after first dose. Vaccine types: P (76%), AZ (24%). | Median neutralizing Ab titre 17% in patients versus 32% in controls. Titres ≥30% in only 14% of patients versus 54% of controls. |
| Ghandili, 36 Jul 2021 (Germany) | P, M, AZ | H (MM, MGCS, systemic light chain amyloidosis) | Antibody levels | 78 | Observational study. Anti-spike Ab levels assessed ≥7 days after first dose. (Jan–May 2021) | Seropositivity in 23% of patients. Ab titre significantly higher with higher CD19+ B-cell counts and significantly lower with ongoing anti-CD38 Ab therapy. Higher age and insufficiently controlled disease negatively correlated with Ab titre. Treatment with immunomodulatory drugs did not harm the development of Ab titres. |
| Ghione, 37 Sep 2021 (USA) | P, M, J&J | H (lymphoma) | Antibody level in lymphoma patients receiving B cell-directed therapies after full vaccination | 105 | Prospective noninterventional study with 4 cohorts: BCL patients with treatment ≤9 months before, BCL patients with no treatment/treatment >9 months prior, BCL/TCL/MM with other treatments, HCW, nursing home residents >65 years. Ab levels assessed 2–8 weeks after final dose. | Ab response in 11% of BCL patients with treatment ≤9 months, 88% of BCL patients on no treatment/treatment >9 months, 61.5% of BCL/TCL/MM patients on other treatment, 100% of HCW, 91.5% of nursing home residents (variation among cohorts sig.). |
| Goshen-Lago, 38 Jul 2021 (Israel) | P | S | Seroconversion and effectiveness against infection in S patients receiving active treatment | 232 | Prospective cohort study with 261 healthy controls. Serology assessed >10 days after first dose and 14 days after second dose plus additional sampling 4 weeks after second dose if seronegative. (Jan–Mar 2021) | SCR 29% after first dose (versus 84% controls) and 86% after second dose. Reduced immunogenicity (odds ratio 0.41). No infections throughout the study period following vaccination. Adverse events similar to existing trials in healthy individuals. |
| Greenberger, 39 Sep 2021 (USA) | P, M, J&J | H (B-cell derived haematological cancer) | Antibody levels after booster (third) dose | 49 | Observational study. Ab levels assessed 27 days before and 28 days after booster (third) dose. Cancer types: 51% CLL, 37% NHL, 8% MM, 4% others. Booster types: 67% heterologous and 33% homologous. (Jun–Jul 2021) | 78% seronegative before booster of which 55% seroconverted after booster. (Out of total cohort, 22% were already seropositive and seroelevated after booster while 35% were non-responders.) Immunogenicity of booster not affected by disease type, vaccine type, homologous vs. heterologous pairing, or other malignancy-target therapies. |
| Greenberger, 40 Aug 2021 (USA) | P, M | H | Antibody levels after second dose | 1495 | Prospective cohort study. Ab levels assessed 14 days after second dose. (Mar–May 2021) | 75% produced antibodies. Lowest seropositivity in most common B cell malignancies. Lymphoma: Seronegativity in almost all NHL. Seropositivity in almost all HL. Leukaemia: Seronegativity in AML (9%), ALL (12%), CML (2.9%). Vaccine type: seropositivity M > P. |
| Gurion, 41 Jul 2021 (Israel) | P | H (lymphoma) | Antibody levels after second dose | 162 | Cross-sectional study. Ab levels assessed 4 ± 2 weeks after second dose (Feb – Apr 2021) | Seropositivity in 51% patients. Anti-CD20 Ab therapy within 12 months before second dose and presence of active lymphoma associated with negative response. Seropositivity increased from 3% to 80% in patients vaccinated within 45 days of therapy to patients vaccinated >1 year after therapy (≈ to patients never exposed to antibody therapy). |
| Herishanu, 42 Jun 2021 (Israel) | P | H (CLL) | Antibody levels after the second dose | 167 | Prospective cohort with 52 controls. Ab levels assessed 2–3 weeks after second dose. | Ab response in 39.5% overall, significant reduction in CLL (52%) versus controls (100%). Highest response: patients in remission (79.2%), treatment-naïve patients (55.2%), patients on active treatment (16.0%). Low in patients on BKI (16.0%) and venetoclax ± anti-CD20 antibody (13.6%). 0% response in patients on anti-CD20 Ab <12 months prior. Independent predictors of response: younger age, female sex, lack of ongoing active treatment, high IgG levels, high IgM levels. |
| Heudel, 43 Aug 2021 (France) | P, M, AZ | S (80%) plus H (20%) | Effectiveness of vaccines and antibody levels in patients on active therapy after one and two doses | 1503 | COVID-19 RT-PCR positivity assessed for median follow-up of 44 months. Anti-spike Ab levels assessed at median 55 days after vaccination. (Jan–Apr 2021) | Overall 1.5% symptomatic plus PCR positive, 5% after single dose versus 0.4% after two doses. PCR positivity not correlated with age, comorbidities, cancer type. 12.5% of PCR positive patients died of COVID-19. 63% had detectable Ab. 80% of those who died had undetectable Ab after vaccination versus 34% of remaining patients. |
| Iacono, 44 Jun 2021 (Italy) | P | S (72%) plus H (28%) | Antibody prevalence in older patients (≥80 years) after second dose | 36 | 1:2 control group. Ab levels assessed one month after second dose. (Jan–Feb 2021) | Median Ab level significantly lower in patients versus controls. No difference with sex, CTX (versus other therapies), S versus H, early versus late-stage disease, continuative steroid use. |
| Jurgens, 45 Aug 2021 (USA) | P, M | H (lymphoma) | Antibody response | 67 | Observational cohort study with 35 controls. Ab levels assessed before and 11–70 days after second dose. Vaccine types: P (46%), M (54%). (Feb–Apr 2021) | Ab levels below cut-off: 41% of CLL, 40% of NHL, 0% of HL and 0% of controls. Median Ab titres lower in lymphoma versus controls (not sig.). No sig. difference between lymphoma types. Treatment‐naïve patients and patients without therapy for ≥2 years responded similar to controls. None in anti‐CD20 Ab therapy ≤6 months recipients responded to vaccination. |
| Karacin, 46 Aug 2021 (Turkey) | SV | S | Antibody levels in patients receiving active systemic therapy | 47 | Prospective observational study. Ab levels assessed 0–3 days before first dose and 4 weeks after second dose. Treatment groups: ≥1 cytotoxic drug (89%), monoclonal Ab or immunotherapy only (11%). | SCR 63.8% overall, 59.5% in patients receiving ≥1 cytotoxic drug, 100% in patients receiving monoclonal antibody/immunotherapy alone. Age independently predicted SCR. |
| Lasagna, 47 Aug 2021 (Italy) | P | S | Antibody level and T-cell response in patients on PD-1/PD-L1 inhibitors | 88 | Observational study. Ab levels and T-cell response assessed before first dose, before and 21 days after second dose. 14.8% of patients had prior SARS-CoV-2 exposure. (Mar–Apr 2021) | In patients without prior exposure: Ab titre rose significantly from 0–21 days after second dose. Vaccine able to elicit T-cell responses after two doses irrespective of previous exposure. No COVID-19 cases during study period. No significant association of PD-L1 status, TNM staging, histology, treatment type/setting/time gap with vaccination, previous exposure with T-cell response. |
| Ligumsky, 48 Aug 2021 (Israel) | P | S | Antibody levels in patients receiving anticancer therapy after two doses | 326 | Retrospective study with 164 healthy controls. Treatment groups: CTX ± additional therapy (63%), ICIs (17%), targeted therapy alone (12%), other combinations (9%). (Mar–Apr 2021) | Seronegativity: 12% of patients versus 3% of controls (sig.), higher with CTX (19%) versus ICI (9%) and targeted therapy groups (3%). Median Ab titres sig. lower in patients versus controls and sig. different among treatment types. |
| Lim, 49 Jul 2021 (UK) | P, AZ | H (lymphoma) | Antibody levels after first and second doses | 129 | Prospective cohort study with 150 healthy controls. Ab titres assessed before vaccination, 2 weeks after first dose and 2–4 weeks after second dose. 44% of patients on treatment ≤6 months before vaccination. (Jan–May 2021) | Seronegative: 72% after first dose, 61% after second dose (0% of controls). Ab titres: patients on treatment < those not on treatment. No association with age and disease remission status. Ab levels after both first & second doses of P > AZ. Patients with HL and aggressive B-cell NHL can develop robust serological responses as early as 6 months after treatment. |
| Mairhofer, 50 Aug 2021 (Austria) | P, M | S (45%) plus H (55%) | Antibody and T-cell response after second dose | 87 (83 included in analysis) | 44 controls (23 vaccinees, 15 non-vaccinated convalescents, 6 vaccinated after infection). Treatment groups: current/recent (<3 months anticancer or <6 months anti-CD20) therapy (80%), untreated (20%). (Mar 2021) | Ab response in 72% overall, 90% of S, 58% of H, 100% of controls. CD4 or CD8 T-cell response in 82% overall, 90% of S, 76% in H, 97% of controls. Triple-negative (absent Ab + CD4 + CD8 response) almost only with anti-CD20 Ab therapy, none in controls. No difference in cellular response in patients versus controls. T-cell positivity with Ab negativity seen more in patients on active therapy versus untreated. Ab and CD4 (but not CD8) responses significantly higher with M versus P vaccine. |
| Malard, 51 Aug 2021 (France) | P | H | Immunogenicity after first and second doses | 195 | Control group of health workers. Ab and T-cell responses assessed. (Jan–Mar 2021) | Ab titre rose from day 28 to 42, even at day 42 Ab titre lower versus controls. Lower Ab titre in males, older patients, ongoing CTX and anti-B-cell treatment ≤12 months prior. Seropositivity 1.5% after 42 days which rose to 46.7% after second dose (versus 87% in controls). Male sex and ongoing chemotherapy associated with seropositivity. Age, BMI, and CD19+ B-cell level had no impact on seropositivity. T-cell response in 53% after second dose. Low T-cell response in males. Absent T-cell response with ongoing treatment. |
| Malissen, 52 Aug 2021 (France) | P | S (95%) plus H (5%) | Antibody levels in patients undergoing oncology trials after one and two doses | 22 | Retrospective cohort study. Ab levels assessed before first dose, before and 1 month after second dose. All part of oncology trials: immunotherapy (64%), targeted therapy (27%), CTX (9%). (Jan–Apr 2021) | Seroconversion: 37% after first dose, 77% after second dose. No association with age, treatment type or lymphocyte count. |
| Maneikis, 53 Jul 2021 (Lithuania) | P | H | Immunogenicity after first and second doses | 857 (315 used for age-matched analysis) | Prospective cohort study with 67 healthy controls. Ab levels assessed 0–10 days before first dose, at second dose and 7–21 days after second dose. (Jan–Apr 2021) | Lower median Ab levels after two doses in H versus controls. Poorer Ab response in patients treated with BKI, ruxolitinib, venetoclax, and anti-CD20 therapy versus untreated patients. Highest responses: tyrosine kinase inhibitor treatment, autologous/allogenic HSCT. Ab levels following two doses correlated with age in H. |
| Massarweh, 54 Aug 2021 (Israel) | P | S | Seropositivity after second dose patients undergoing active treatment | 102 | Prospective cohort study with 78 healthy controls. (Feb–Mar 2021) | Seropositive: 90% after second dose (versus 100% controls). Median Ab titre lower in patients versus controls. Only treatment status with CTX + immunotherapy showed association with lower Ab titre. |
| Monin, 55 Apr 2021 (UK) | P | S (63%) plus H (37%) | Immunogenicity | 151 | Prospective cohort study with 54 healthy controls. Ab assessment before vaccination, 3 and 5 weeks after first vaccination. (Dec 2020–Feb 2021) | SCR: 38% in S, 18% in H (versus 94% in controls) after first dose; 95% in S, 60% in H (versus 100% in controls) after second dose. T-cell response: 71% in S, 50% in H (versus 82% in controls) after first dose; 88% in S, 75% in H (versus 100% in controls) after second dose. |
| Ollilla, 56 Aug 2021 (USA) | P, M, J&J | H | Antibody levels after first and second dose | 160 | Retrospective. Ab levels assessed. Disease status: active (51%), remission (39%), watchful waiting/never treated (9%). Vaccine types: P (60%), M (31%), J&J (7%), undetermined (1%). Treatment >12 months before vaccination in 25%. (Feb–Apr 2021) | SCR 39% overall. SCR sig. different for disease status: 27% with active disease, 49% with remission, 67% with watchful waiting/never treated. Higher SCR with longer time from last CTX to vaccination (69% if CTX >12 months before versus 24% if <12 months before vaccination). Ab titre lower in patients exposed to B/plasma-cell depleting Ab therapy and active disease. Three COVID-19 infections with one death in entire cohort. |
| Palich, 57 Apr 2021 (France) | P | S | Antibody response after first dose in patients on active treatment or treatment ≤2 years | 110 | Prospective cohort with 25 controls. Ab response assessed 4 weeks after the first dose. (Feb–Mar 2021) | SCR 55% in patients without prior COVID-19 exposure (versus 100% in controls). Ab titre lower in patients versus controls. Sex, cancer location, metastatic status similar in those who did and did not seroconvert. Age >65 years and CTX associated with lack of seroconversion. |
| Parry, 58 Jul 2021 (UK) | P, AZ | H (CLL) | Antibody response after first and second doses | 299 | Prospective cohort study with 93 healthy controls. Ab assessment 5–6 weeks after the first dose (in extended interval immunization) and 2 weeks after second dose. Vaccine types: 52% P, 48% AZ. | Seropositivity in 34% of CLL patients (versus 94% in controls) after first dose; 75% (versus 100% in controls) after second dose. Ab titres 104-fold lower in CLL versus controls after first dose; 74-fold lower after second dose. Current treatment with BKI and IgA deficiency associated with seronegativity following second dose. |
| Peeters, 59 Sep 2021 (Belgium) | P | S (79.5%) plus H (20.5%) | Antibody response in patients receiving anticancer treatment | 200 | Prospective interventional cohort study with healthy controls. Ab levels and neutralization titres assessed until 28 days after second dose (Feb–Mar 2021) | Ab titres lower in CTX group versus controls irrespective of timing of vaccination with CTX. Extremely low Ab response in rituximab recipients. Five cancer patients tested positive for infection during study. |
| Perry, 60 Aug 2021 (Israel) | P | H (B-cell NHL) | Antibody response after second dose | 149 | Prospective study with 65 healthy controls. Ab titre assessed 2–3 weeks after second dose. Treatment groups: treatment-naïve (19%), active treatment (37%), treated >6 months before vaccination. (Dec 2020–Mar 2021) | Seropositivity: 49% overall, 89% in treatment-naïve, 7.3% with active treatment, 67% with treatment >6 months, 98.5% in controls. Seropositivity with longer time since exposure to rituximab/obinutuzumab induction/maintenance, absolute lymphocyte count ≥900/μL. Median time-to-seropositivity longer with indolent versus aggressive B-cell NHL. |
| Pimpinelli, 61 May 2021 (Italy) | P | H (MM, MPS) | Immunogenicity in patients on active treatment | 92 | Prospective cohort study with 36 elderly controls. Titres of neutralizing Ab and seroprotection rates assessed at 3 and 5 weeks from first dose. (Mar–Apr 2021) | Seropositivity: 21.4% in MM, 52.0% in MPM (versus 52.8% in controls) after first dose; 78.6% in MM, 88% in MPM (versus 100% in controls) after second dose. Lower titres in patients versus controls. Lower titres associated with increasing age, but no effect by sex. Active treatment without daratumumab associated with higher probability of response in MM patients. |
| Pimpinelli, 62 Jul 2021 (Italy) | P | H (MPS: ET, PV, MF) | Immunogenicity in patients on active treatment | 42 | Prospective cohort study. Neutralizing Ab and seroprotection rates assessed at 3 and 5 weeks from first dose. | SCR: 10% in MF (versus 68.8% in ET plus PV) after first dose; 60% in MF (versus 93.8% in ET plus PV) after second dose. SCR and Ab response was lower in MF versus ET plus PV after both first and second doses. |
| Re, 63 Jul 2021 (France) | P, M | H | Antibody levels after second dose | 102 | Retrospective. Ab levels assessed 6–8 weeks after first dose. Vaccine type: P (93%), M (7%) (Jan–Mar 2021) | Seropositivity: 62% overall (50% in NHL/CLL, 74% in MM, 85% in MDS, 80% in MPS). SCR lower in patients vaccinated within <12 months of anti-CD20 therapy (6%) vs. patients vaccinated >12 months after anti-CD20 therapy (64%). No significant difference between patients vaccinated >12 months and patients who never received anti-CD20 therapy (78%). |
| Re, 64 Jul 2021 (France) | P | H (CLL, NHL, MM) | Antibody and T-cell response after third (booster) dose | 43 | Antibody and IFN-γ release responses assessed on day 1 and 3–4 weeks after third (booster) dose. | 41.8% seronegative before and all remained seronegative after third dose. All seropositives before third dose remained seropositive, 92% increased Ab titre. No increase in titre in CLL and/or previous anti-CD20 therapy within 12 months prior to third dose. Median IFN-γ secretion increased in all patients assessed. 36.4% double negative (Ab and cellular response) before and 22.7% remained double negative after third dose. Third dose stimulated Ab response in LM (particularly in MM) and in those without anti-CD20 treatment history ≤1 year. T-cell response increased with no active CTX. |
| Revon-Riviere, 65 Jun 2021 (France) | P | S | Immunogenicity in young patients (≥16 years) with solid tumour currently under treatment or within 6 months after treatment | 23 | Retrospective analysis. Antibody testing before second dose and one month after second dose. (Feb–Apr 2021) | Seropositivity 70% by second dose, 90% one month after second dose. All who seroconverted had a positive neutralisation test. None developed COVID infections |
| Shroff, 66 May 2021 (USA) | P | S | Antibody levels and T-cell response in patients on active CTX | 53 | Observational study with 50 controls plus interventional study with third dose. Neutralizing and anti-spike Ab levels and T-cell response assessed at first dose, at second dose and 5–11 days after second dose, at third dose and 1 week after third dose. | Neutralizing Ab detected in 67% after first dose and 80% after second dose. Trends similar for anti-spike Ab and T-cell response. Lower responses vs. controls. Spike-specific plasma cell-biased memory B cell level similar in cancer patients after second dose versus controls after first dose. After third dose: Increased Ab response in 20 patients, three-fold rise in neutralizing titre, but no improvement in T-cell response. |
| Singer, 67 Aug 2021 (Austria) | P, M, AZ, J&J | S (61%) plus H (39%) | Antibody levels after first dose | 441 | Retrospective study with non-vaccinated patients as controls. Ab levels analysed. (Mar–May 2021) | Ab titres higher in S versus H. Titres equal among sexes. Age-dependent decrease in titres. |
| Stampfer, 68 Jul 2021 (USA) | P, M | H (MM) | Antibody response after first and second dose | 96 | Ab levels assessed 12–21 days after first dose and 12–21 days after second dose. Cancer types: 96 active MM. | 45% adequate response. 22% partial response. Lower titres associated with older age, impaired renal function, low lymphocyte counts, reduced uninvolved immunoglobulin levels, ≥2 lines of treatment, not being in complete remission. Higher titre with M versus P two doses. |
| Tadmor, 69 Aug 2021 (Israel) | P | H (CLL) | Antibody persistence after double dose vaccination | 84 | Prospective study. Ab levels assessed at median 22 and 100 days after second dose. | 48% seropositive at first test, of which 73% maintained it by second test. Of those seronegative at first test, 6% seroconverted by second test. Ab level decay among CLL patients is comparable to those in already published healthy controls ≥70 years. Higher responses in: therapy-naive, previously treated patients. Clear drop in titre across almost all therapy groups. Continuing therapy, adverse side effects to vaccine, being female, and low IgM Ab levels during first tested were associated with reduced IgG antibody persistence. |
| Terpos, 70 Sep 2021 (Greece) | P | H (CLL, NHL, HL) | Antibody levels after first and second dose | 132 | Prospective study with 214 healthy controls. Antibody levels assessed on day 1, 22 and 50 from first dose. Cancer types: NHL (43%), HL (17%), CLL (40%). Active treatment in 34%. (Jan–May 2021) | Seroconversion 50.8% in patients versus 98.1% in controls at day 50. Lower Ab response in patients versus controls at both day 22 and 50. Better response HL > NHL/CLL. Active treatment with rituximab, BKI or CTX independently predicted lower response. |
| Terpos, 71 Aug 2021 (Greece) | P, AZ | H (MM, SMM, MGUS) | Neutralizing antibodies after first and second dose | 276 | Prospective cohort study with 226 healthy controls. Neutralizing Ab assessed before first dose and days 22 and 50 after first dose. | Neutralizing Ab lower in MM (versus controls) on both days 22 and 50 with AZ single dose and P double dose. Lower neutralizing Ab response in MM versus MGUS on days 22 and 50. Active treatment with anti-CD39 monoclonal Ab/belantamab mafodotin/lymphopenia associated with suboptimal Ab response. |
| Terpos, 72 Jul 2021 (Greece) | P | H (MM, SMM) | Neutralizing antibodies after first dose | 48 | Prospective cohort study with 104 healthy controls. Neutralizing Ab assessed before first dose and day 22 after first dose. | Seropositivity in 25.0% of MM (versus 54.8% in controls) on day 22. Only 1 (11.1%) of 9 patients with SMM had seropositivity versus 11 (28.2%) of 39 patients with active MM. (Other 8 patients had immunoparesis in ≥1 uninvolved immunoglobulin.) |
| Terpos, 73 May 2021 (Greece) | P, M, AZ | S | Neutralizing antibodies patients receiving ICIs after first dose | 59 | Prospective cohort study with 283 healthy controls. Neutralizing Ab assessed before first dose and day 22 after first dose. Vaccine type: P (69.5%), Az (25.4%), M (5.1%). | Patients had lower neutralizing Ab titres versus controls. Seropositivity in 25% of patients (versus 65.7% in controls) on day 22. |
| Thakkar, 74 Jun 2021 (USA) | P, M, J&J | S (67%) plus H (33%) | SCR following vaccination | 200 | Cross-sectional cohort study. Antibody level measured. 75% with active malignancy. 67% on active cancer therapy at vaccination. 56% on CTX at vaccination, 19% on CTX within 48h of at least one doses. Vaccine types: P (54%), M (31%), J&J (10%), unknown (3 patients). | Seroconversion: 94% overall, 98% in S, 85% in H, 70% with anti-CD20 therapies, 73% with HSCT. High seroconversion rates with ICI therapy (97%) and endocrine therapies (100%). Higher Ab titres after vaccination in patients with prior infection. Lower titres with adenoviral (J&J) versus mRNA-based vaccines (P, M). |
| Tzarfati, 75 Jun 2021 (Israel) | P | H | Antibody levels after two doses | 315 | 108 comparator group without H. Ab levels assessed 30–60 days from second dose. Disease status: active (59%), remission (41%). (Feb–Apr 2021) | Seropositivity: 75% in patients versus 99% in controls; lowest in CLL & indolent lymphoma; lower with chemo-immunotherapy, anti-CD20 antibody, BCL2, BTK/JAK2 inhibitor therapy. Median Ab titres lower in patients versus controls. Older age, higher LDH, number of treatment lines correlated with lower Ab response. Absolute lymphocyte count, globulin level, time from last treatment to vaccination correlated with higher Ab response. |
| Van Oekelen, 76 Jun 2021 (USA) | P, M | H (MM) | Antibody levels after two doses | 320 | 67 controls. Ab levels assessed before first dose and ≥10 days after second dose. Vaccine types: P (69.1%), M (27.2%), unknown (3.8%). 18.8% had COVID-19 infection before vaccination. (Early 2021) | Seroconversion: 84.2% of patients (versus 100% in controls). Ab levels in fully vaccinated patients with prior infection 10-fold higher versus infection-naïve patients. MM treatment recipients had lower Ab levels versus non-recipients. Lower titres with anti-CD38 regimens plus BCMA-targeted therapy, but not for other treatments versus non-recipients. 10 COVID-19 infections in cohort after one/two doses with one death. |
| Waldhorn, 77 Sep 2021 (Israel) | P | S | Antibody levels 6 months after second dose in patients on treatment during vaccination and throughout 6 months after vaccination | 154 | Prospective study with 135 controls. Ab levels assessed after second dose and repeated 6 months after. Treatment groups: CTX (62%), biological agent (36%), immunotherapy (30%). This is a follow-up of a previous study (Goshen-Lago, 2021). (Jul–Aug 2021) | At 6 months: 79% seropositive in patients (versus 84% controls), 85% remained seropositive, 15% became seronegative, none of the initial seronegatives seroconverted. Ab titres decreased similarly in patients and controls. CTX associated with seronegativity versus other treatments. One COVID-19 infection among patients, none among controls. |
Ab, antibody; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; AZ, ChAdOx1 (Oxford-AstraZeneca); BCL, B-cell lymphoma; BCL2, B-cell lymphoma 2; BCMA, B-cell maturation antigen; BKI/BTK/BTKi, Bruton’s kinase inhibitors; BMI, body mass index; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; COVID-19, coronavirus disease 2019; CTX, chemotherapy; ET, essential thrombocythemia; H, haematological malignancy; HCW, healthcare worker; HL, Hodgkin lymphoma; HSCT, haematopoietic stem cell transplantation; ICI, immune checkpoint inhibitor; IFN-γ, interferon gamma; J&J, Janssen Ad26.COV2.S; JAK2, Janus Kinase 2; LDH, lactate dehydrogenase; LM, lymphoid malignancies; M, mRNA-1273/Moderna; MDS, myelodysplastic syndrome; MF, myelofibrosis; MGCS, monoclonal gammopathy of clinical significance; MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; MPM, myeloproliferative malignancies; MPN, myeloproliferative neoplasms; MPS, myeloproliferative syndrome; mRNA, messenger ribonucleic acid; NHL, non-Hodgkin lymphoma; P, BNT162b2/Pfizer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PV, polycythaemia vera; RT, radiotherapy; RT-PCR, reverse transcription–polymerase chain reaction; S, solid malignancy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCR, seroconversion rate; sig., statistically significant (P < 0.05); SMM, smouldering myeloma; SP, BBIBP CorV (Sinopharm); SV, CoronaVac (Sinovac Life Sciences); TCL, T-cell lymphoma; TNM, tumour, node, metastases; WM, Waldenstrom macroglobulinemia.
aOriginal publication year of preprint or Epub ahead of print.
Antibody responses
A large study in Lithuania found significantly lower anti-S1 IgG antibody titres in 315 adult patients with haematological malignancies compared with 67 age-matched healthy controls after full (double-dose) vaccination with BNT162b2/Pfizer vaccine. 53 Seroconversion rates and neutralization capacity are reduced in cancer patients compared to healthy controls. 33 In a US study of 551 patients with haematological cancer, seroconversion rates after first and second doses of the BNT162b2/Pfizer and mRNA-1273/Moderna vaccines were 52% and 69%, respectively. The neutralizing capacity in these patients was 26% and 43% after the first and second doses, which was considerably lower than in healthy controls (93% and 100%, respectively). 33 Low seroconversion rates in patients with cancer have been reported after the ChAdOx1 (Oxford-AstraZeneca),35,36,58,71,73 Janssen Ad26.COV2.S,37,56,74 BBIBP CorV (Sinopharm), 27 and CoronaVac (Sinovac Life Sciences, Beijing, China) vaccines. 46
In a study of 1 495 patients with haematological cancer, there was a significantly higher likelihood of an immune response following full (double-dose) vaccination with mRNA-1273/Moderna compared with the BNT162b2/Pfizer vaccine. 40 The authors speculated that this may be due to the larger dose of spike protein encoding mRNA (100 µg in the mRNA-1273/Moderna vaccine versus 30 µg in the BNT162b2/Pfizer vaccine), or differences in mRNA sequence or composition, host cell penetrance, and dosing schedules. 40 A higher antibody titre following double-dose vaccination with mRNA-1273/Moderna compared with BNT162b2/Pfizer vaccines was also seen among 96 patients with multiple myeloma. 68 Among 551 patients with haematological malignancies, significantly higher antibody titres were observed after double-dose vaccination with mRNA-1273/Moderna compared with the BNT162b2/Pfizer vaccine, however, seropositivity rates were similar. 33 In 200 patients with solid and haematological malignancies, the Janssen Ad26.COV2.S (viral vector) vaccine was associated with lower antibody titres compared with the BNT162b2/Pfizer and mRNA-1273/Moderna mRNA vaccines. 74 Among 129 patients with lymphoma, significantly higher antibody titres were observed following the BNT162b2/Pfizer compared with ChAdOx1 (Oxford-AstraZeneca) vaccine, after both the first and second doses. 49 However, other studies have reported no differences in seroconversion rates between the BNT162b2/Pfizer and mRNA-1273/Moderna vaccines, 76 or in the magnitude of antibody response between the BNT162b2/Pfizer and ChAdOx1 (Oxford-AstraZeneca) vaccines.58,71
The magnitude of the antibody response following vaccination has been shown to differ between solid and haematological cancers. Antibody titres, seroconversion rates and neutralization capacity are lower in patients with haematological malignancy versus those with solid cancers.24,27,34,55,67,74 This is observed with the mRNA-based BNT162b2/Pfizer and mRNA-1273/Moderna vaccines,24,34,55,67,74 as well as the ChAdOx1 (Oxford-AstraZeneca), 67 Janssen Ad26.COV2.S,67,74 and BBIBP CorV (Sinopharm) vaccines. 27 A small study of 36 patients from Italy with solid and haematological malignancies found that cancer type (solid versus haematological) was not associated with median antibody levels. However, the study focused on older patients with cancer (aged ≥80 years), and this may have accounted for the finding. 44
The antibody response appears to be associated with treatment-, patient-, and disease-related factors. Among patients with haematological malignancies, treatment with anti-CD20 therapy (rituximab, ofatumumab, obinutuzumab, ocrelizumab, and veltuzumab), anti-CD38 therapy (daratumumab and isatuximab) and Bruton’s tyrosine kinase inhibitors (BTKi; e.g., ibrutinib, acalabrutinib and zanubrutinib), B-cell maturation antigen (BCMA) targeted therapy, including bispecific T-cell engager and chimeric receptor antigen T-cell (CAR-T) therapy, kinase inhibitors (ruxolitinib), B-cell lymphoma-2 (BCL2) inhibitors (venetoclax) and hydroxycarbamide, have all been shown to be associated with poorer antibody responses, hence leaving the patient largely unprotected.32–34,36,37,42,53,56,58,59,70,75,76 The timing of vaccination in relation to these therapies may be important, as vaccination at a shorter interval from anti-CD20 antibody therapy has been associated with poorer responses. The interval varied among the different studies, ranging between 9 months, 37 12 months,41,42,56,63 and up to 2 years. 45 Older age,42,75 higher lactate dehydrogenase values, 75 IgA deficiency, 58 lymphopenia, 71 male sex, 42 and presence of immunoparesis, 31 have been associated with lower antibody response. Among those with solid cancers, cytotoxic chemotherapy is reported to be associated with poorer antibody response compared with immune checkpoint inhibitor (pembrolizumab, ipilimumab, nivolumab and atezolizumab) and endocrine therapy.24,25,29,46,48,54,57,74,77 Patients on tyrosine kinase inhibitors (TKIs) (imatinib, dasatinib, nilotinib, ponatinib, and bosutinib) and those who underwent autologous or allogeneic hematopoietic stem cell transplantation (HSCT) showed high numerical humoral responses to vaccination. 53 However, significantly lower seroconversion rates have also been noted in patients who received HSCT and CAR-T therapy. 74
Cellular responses
There are fewer studies on T-cell responses following COVID-19 vaccination in patients with cancer. A UK prospective study of 151 patients with solid and haematological cancer found that a lower proportion of patients elicited a T-cell response following one dose of the BNT162b2/Pfizer vaccine: 71% and 50% of patients with solid or haematological cancer, respectively, compared with 82% of healthy controls. 55 A low T-cell response was also found in a study from Denmark, where 46% and 45% of patients with solid or haematological cancer, respectively, elicited a T-cell response. Most (76%) of the seronegative patients did not elicit a T-cell response, and low T-cell responses were significantly associated with steroid use for immunosuppression in cancer. 34 In contrast to the antibody responses, these two studies found no significant difference between solid and haematological cancers regarding T-cell response to vaccination.
An Austrian study that compared humoral and cellular immune responses to mRNA vaccines between 86 patients with cancer and 44 control participants found that although humoral responses were significantly lower in patients with cancer versus controls, there was no statistically significant difference between the two groups regarding cellular immune response. 50 Furthermore, cancer patients were found to have discordant humoral and cellular immune responses, where those who were on active treatment showed no humoral response but a positive cellular immune response, or, conversely, absence of both facets of the immune response. A higher proportion of patients with cancer were found to generate a specific CD4+ or CD8+ T cell immunity in response to the mRNA-1273/Moderna vaccine (73.2%) than to the BNT162b2/Pfizer vaccine (54.8%).
Of note, T-cell-mediated vaccine approaches against SARS-CoV-2 are under clinical investigation for patients with B-cell immune deficiencies (e.g., NCT04954469). 78
Breakthrough infections and vaccine effectiveness
Few studies have investigated vaccine effectiveness and breakthrough infections in patients with cancer. In a large study of 1 503 patients with solid and haematological cancer, followed-up for a median of 44 months, 1.5% of all patients with cancer developed symptoms and/or reverse transcription–polymerase chain reaction-positive COVID-19; with rates of 5% after a single dose and 0.4% after two doses of the vaccine. 43 The study included participants who received the BNT162b2/Pfizer, mRNA-1273/Moderna and ChAdOx1 (Oxford-AstraZeneca) vaccines, and found a significantly higher overall death rate in the first two months post-first dose in those who received only a single dose. 43 A study of 364 patients with solid and haematological cancers who received the BBIBP CorV (Sinopharm) vaccine found five break through infections, four of which occurred in the first month, with no hospitalizations or deaths during a three-month follow-up period. 27 Other study findings include: an infection rate of 10 out of 320 patients with multiple myeloma vaccinated with the BNT162b2/Pfizer and mRNA-1273/Moderna vaccines, 76 none out of 232 patients with solid cancer vaccinated with the BNT162b2/Pfizer vaccine, 38 five out of 200 patients with solid and haematological cancer vaccinated with the BNT162b2/Pfizer vaccine, 59 none out of 88 patients with solid cancer vaccinated with the BNT162b2/Pfizer vaccine, 47 none out of 23 young patients with solid cancer vaccinated with the BNT162b2/Pfizer vaccine, 65 one out of 154 patients with haematological malignancies vaccinated with BNT162b2/Pfizer vaccine, 77 and nine out of 885 patients with haematological malignancies vaccinated with BNT162b2/Pfizer vaccine, including three deaths due to COVID-19 pneumonia. 53
Persistence of immune response and effect of third or booster dose
Among 154 Israeli patients with solid cancer given two doses of the BNT162b2/Pfizer vaccine, antibody responses were seen to wane after a few months. 77 Of those who seroconverted after one and/or two doses of the vaccine, 15% became seronegative by 6 months. The overall seropositivity at 6 months was 79%, and at the end of the 6-month follow-up period, none of the initially seronegative patients became seropositive. The decrease in seropositivity rates and antibody titres were similar between patients and healthy controls. 77 A third vaccine dose has been proposed as a solution to increase seroconversion rates in patients who did not seroconvert following full (double-dose) vaccination and to combat waning immunity. A study investigating antibody persistence in patients with chronic lymphocytic leukaemia (CLL), at a median of 100 days following second dose of BNT162b2/Pfizer vaccine, found antibody decay to be comparable to healthy controls. 69
On 12 August 2021, the US Food and Drug Administration modified the emergency use authorizations granted for the BNT162b2/Pfizer and mRNA-1273/Moderna COVID-19 vaccines to allow for administration of a third dose for certain immunocompromised individuals (e.g., people who have undergone solid organ transplantation or have been diagnosed with conditions that are considered to have an equivalent level of immunocompromise). 79 On 1 September 2021, the Joint Committee on Vaccines and Immunization in the UK recommended a third vaccine dose for individuals (aged 12 years and above) with severely weakened immune systems, including conditions such as acute or chronic leukaemia, aggressive lymphomas, advanced HIV, recent organ transplants, those on immunosuppressive therapy, those with chronic immune-mediated inflammatory disorders, and persons who have received high-dose steroids in the month before vaccination. 80
A few studies have assessed the immunogenicity of an additional dose of vaccine in patients with cancer. Among a cohort of 52 US patients with solid cancer on active cytotoxic cancer therapy who received the BNT162b2/Pfizer vaccine, a three-fold rise in neutralizing antibody titre was seen following a third vaccine dose, without an improvement in the T-cell response. 66 In 49 US patients with haematological cancer, who were initially vaccinated with the BNT162b2/Pfizer, mRNA-1273/Moderna or Janssen Ad26.COV2.S vaccines, the use of a homologous (33%) or heterologous (67%) additional booster dose resulted in 55% of those who were seronegative after two doses to seroconvert. 39 Importantly, 35% did not respond to initial full vaccination, and remained unresponsive to the additional third dose; no significant associations were found between immunogenicity of the additional dose and type of haematological cancer, type of vaccine pairing (i.e., homologous versus heterologous), or the use of malignancy-target therapies. 39
Similar observations were made in a study of 43 French patients with haematological cancer who were vaccinated with the BNT162b2/Pfizer vaccine; 42% were seronegative before administration of a third dose and all of these patients remained unresponsive following the third dose. 64 Of the patients already seropositive before the third dose, 92% had an increased antibody titre following the third dose. Thirty-six percent of the patients were double-negative (i.e., seronegative and no demonstrable cellular immune response) before the third dose and 23% remained double-negative following the third dose. The increased antibody response following the third dose was seen in patients who had not received anti-CD20 antibody treatment in the past year, whilst the increase in T-cell response was seen in those who were not on active chemotherapy. 64
Immunogenicity and effectiveness of COVID-19 vaccines in specific haematological malignancies
Disease-associated immune dysfunction and treatment-related immunosuppression are assumed to cause poor immunogenicity with COVID-19 vaccination, particularly in B lymphoid malignancies.
Chronic lymphocytic leukaemia
Among patients with CLL, efficacy of the BNT162b2/Pfizer vaccine was found to be markedly reduced. 42 In 167 patients with CLL, who were fully vaccinated with two BNT162b2/Pfizer doses, an antibody response was elicited in only 39.5% of patients. 42 The humoral response rate was 80% in those who had completed treatment and were in remission, 55% in those who were treatment-naive for CLL, and 16% in those on active treatment. Being female, relatively young, having serum IgG levels >550 mg/dL and IgM levels >40 mg/dL, and currently not receiving active treatment, were associated with better antibody responses to COVID-19 vaccination. 42 Another study found poor persistence of antibody titres at 100 days in patients with CLL, particularly among those who were female, were receiving ongoing therapy, had adverse effects to vaccination, and had low IgM antibody levels following the first dose. 69 Treatment with BTKi or venetoclax, with or without anti-CD20 antibodies, significantly impaired antibody responses. 42 Thus, administering the second dose at an earlier interval than 10–12 weeks, providing a booster third dose, and continuing non-pharmacological methods, have been recommended. 42 A subsequent study of 299 patients with CLL reported a detectable antibody response after the first dose of BNT162b2/Pfizer and ChAdOx1 (Oxford-AstraZeneca) vaccines in 34% of patients (compared with 94% of controls). 58 This response rate rose to 75% of patients versus 100% of controls following the second dose, however, antibody titres were 74-fold lower in patients with CLL. IgA deficiency was shown to be associated with poor antibody response, even after the second dose. 58
Multiple myeloma
In patients with multiple myeloma (MM), the seroconversion rate following a first dose of the BNT162b2/Pfizer or ChAdOx1 (Oxford-AstraZeneca) vaccines was 56%, and lower seropositivity rates were found if the MM was active, there was immunoparesis, or if the patients were on active treatment. 31 An interim analysis of 48 elderly patients (median age, 83 years) in a similar study showed poor immune response compared with controls. 72 In a subsequent analysis of the same trial, including 276 patients with MM, smouldering myeloma and monoclonal gammopathy of undetermined significance, neutralizing antibody production was found to be lower in patients with MM compared with 226 healthy controls. 71 Another study found 55% of patients with MM to have no or partial antibody response to vaccination, 68 whilst in a separate cohort, the response rate was 76% among patients with MM versus 98% in healthy adults. 28 A large-scale study of 320 patients with MM found a seroconversion rate of 84% versus 100% in the control group, with significantly lower antibody titres in those who had received MM treatment compared with those who had not. 76 In this same study, 15.8% of myeloma patients failed to develop any detectable SARS-CoV-2 spike IgG antibodies. In a further study of elderly patients that included 42 patients with MM and 50 patients with myeloproliferative neoplasms (including 30 with chronic myeloid leukaemia), antibody titres were assessed 5 weeks after BNT162b2/Pfizer vaccine and showed 78.6% versus 88% antibody response, respectively. 61 In addition, anti-CD38 antibody and BCMA-targeted therapy was associated with significantly poorer antibody responses. Current evidence suggests that serological response to COVID vaccination in patients with MM can be highly variable. Of note, fully vaccinated patients with MM and prior reported COVID-19 infections were found to have 10 times higher antibody levels than patients with MM who were COVID-19-naïve at the time of vaccination. 76
Lymphoma
Among 162 patients with lymphoma, the seroconversion rate following double-dose BNT162b2/Pfizer vaccination was 51%. 41 A similar rate of serological response was demonstrated in another study that included 149 patients with B-cell (B)-non-Hodgkin lymphoma (NHL). 60 A study from Greece that included 132 patients with CLL, 53 with NHL and 22 with Hodgkin lymphoma (HL) also showed poor antibody response following BNT162b2/Pfizer vaccination compared with 214 matched healthy controls. 70 Poor responses were associated with active lymphoma, being on active treatment and anti-CD20 antibody therapy in the 12 months preceding vaccination.41,60 The seropositivity rates increased with increasing interval from the anti-CD20 antibody therapy: 3% when administered within 45 days, rising to 80% if administered ≥1 year prior to vaccination. 41 A similar association with interval between the last anti-CD20 treatment and vaccination has been found in subsequent studies.37,63 Further, lymphocyte count at the time of vaccination ≥0.9 × 103/μL was also a predictor of positive serological response on multivariate analysis. 60 Among 67 patients with lymphoma, no significant difference was seen in antibody response between lymphoma types. 45 In contrast, a few studies have shown differences in humoral response based on lymphoma type. For example, patients with HL showed superior humoral responses versus CLL/NHL subgroups in a study from Greece. 70 The median time between last exposure to anti-CD20 antibody treatment and the attainment of positive serology was significantly longer in patients with indolent B-NHL than with aggressive B-NHL (36 versus 19.8 months). Response rates over time since last anti-CD20 antibody treatment exposure remained lower in patients with indolent B-NHL compared with those in patients with aggressive B-NHL. 60
Potential side-effect profile of COVID-19 vaccines in patients with cancer
The side-effect profile of the currently authorized vaccines is very similar between patients with haematological and solid cancer and the general public. A reported exception is significantly higher rate of pain at the injection site, stated in one study to be more severe in patients with B-NHL compared with healthy controls. 60 The majority of patients with cancer only experience mild to moderate adverse effects. Among patients with cancer, the most common local adverse effect following BNT162b2/Pfizer vaccination is pain at the injection site (reported in 19–69% of patients with cancer),30,38,47,48 whilst the most common systemic adverse effects are fatigue (24%) and myalgia/arthralgia/headache (7–18%).38,47,48 A similar side-effect profile has been found in patients with cancer who received the BBIBP CorV (Sinopharm) vaccine (with the exception that fever (32%) was the most common systemic adverse effect), 27 and the CoronaVac (Sinovac Life Sciences) vaccine. 46 In a study of patients with B-NHL, the rate of side effects reported with the BNT162b2/Pfizer vaccination was 51%, 60 and 2.5% of patients reported transient lymphadenopathy.
According to the US Centers for Disease Control and Prevention, lymphadenopathy following administration of the BNT162b2/Pfizer vaccine may occur within 2 to 4 days and last for an average of 10 days. 81 New onset lymphadenopathy in the cervical and/or axillary regions, detected clinically or radiologically (by mammography, ultrasound, or fluorodeoxyglucose–positron emission tomography), have been reported following COVID-19 vaccination.38,82 Lymphadenopathy may occur 2–13 days following vaccination, on the same side as the arm on which the vaccine was given. In most cases, no additional imaging is needed, but should be considered if the swelling persists or there are other symptoms. Understandably, for patients with lymphoma who developed lymph node enlargement as a sign of their cancer, any enlargement may cause concern. In addition, subclinical, radiologically detected lymphadenopathy may also be a cause of concern as a potential sign of metastasis in patients undergoing routine screening following previously treated cancer. 83
Other considerations in fully vaccinated patients with cancer
Due to the high risk of serious breakthrough SARS-CoV-2 infections, it is imperative that household contacts and caregivers of cancer patients are fully vaccinated and non-pharmacological protective measures, such as wearing face masks, social distancing and thorough hand washing, are strictly adhered to by patients, caregivers, and family members.
Conclusions
Coronavirus disease 2019 (COVID-19) has significant adverse clinical, laboratory and general effects among patients with cancers. Vaccination is an important preventive measure for patients with solid and haematological malignancies against developing severe COVID-19. Immunogenicity (both humoral and cellular immune responses) of vaccines in patients with cancer is lower compared with healthy individuals. Furthermore, humoral immune responses are inferior in those with haematological versus solid cancers. Patient-, disease-, and treatment-related factors that are associated with poorer vaccine responses should be identified and corrected or mitigated when possible. Consideration should be given to offering patients with cancer a second dose of the COVID-19 vaccine at a shorter interval than in healthy individuals. Patients with cancer warrant a third vaccine dose and must be prioritized in vaccination schedules. The adverse-effect profile of vaccines in patients with cancer is comparable to that in healthy individuals.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Suranjith L Seneviratne https://orcid.org/0000-0002-6548-5673
References
- 1.World Health Organization. Weekly epidemiological update on COVID-19 – 21 September 2021: Edition 58, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-september-2021 (2021, accessed 29 September 2021).
- 2.Jayasekara D, Seneviratne SL, Jayasekara A, et al. Atypical presentations of COVID-19. Advances in Infectious Diseases 2020; 10: 136–142. [Google Scholar]
- 3.Kariyawasam JC, Jayarajah U, Riza R, et al. Gastrointestinal manifestations in COVID-19. Trans R Soc Trop Med Hyg 2021; 115: 1362–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman A, Niloofa R, De Zoysa IM, et al. Neurological manifestations in COVID-19: a narrative review. SAGE Open Med 2020; 8: 2050312120957925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman A, Niloofa R, Jayarajah U, et al. Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg 2021; 104: 1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020; 10: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol 2021; 32: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov 2020; 10: 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020; 21: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seneviratne SL, Abeysuriya V, De Mel S, et al. Favipiravir in Covid-19. International Journal of Progressive Sciences and Technologies 2020; 19: 143–145. [Google Scholar]
- 11.Seneviratne SL, Niloofa R, De Zoysa I, et al. Remdesivir and Covid-19. Int J of Adv Res 2020; 8: 565–567. [Google Scholar]
- 12.Seneviratne S, Jayarajah U, Abeysuriya V, et al. COVID-19 vaccine landscape. Journal of the Ceylon College of Physicians 2020; 51: 120–131. [Google Scholar]
- 13.Seneviratne S, Yasawardene P, Hettiarachchi D, et al. The delta variant of SARS-CoV-2: the current global scourge. Sri Lankan Family Physician 2021; 36: 17–25. [Google Scholar]
- 14.Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing 23, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018547/Technical_Briefing_23_21_09_16.pdf (17 September 2021. London: Public Health England).
- 15.Angyal A, Longet S, Moore SC, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe 2022; 3: e21–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaway E. Fast-spreading COVID variant can elude immune responses. Nature 2021; 589: 500–501. [DOI] [PubMed] [Google Scholar]
- 17.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021; 385: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson H, Petrie K, Berrisford C, et al. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer 1999; 80: 219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchette PS, Chung H, Pritchard KI, et al. Influenza vaccine effectiveness among patients with cancer: a population-based study using health administrative and laboratory testing data from Ontario, Canada. J Clin Oncol 2019; 37: 2795–2804. [DOI] [PubMed] [Google Scholar]
- 20.Puthillath A, Trump DL, Andrews C, et al. Serological immune responses to influenza vaccine in patients with colorectal cancer. Cancer Chemother Pharmacol 2011; 67: 111–115. [DOI] [PubMed] [Google Scholar]
- 21.Leung T-F, Li C-K, Hung ECW, et al. Immunogenicity of a two-dose regime of varicella vaccine in children with cancers. Eur J Haematol 2004; 72: 353–357. [DOI] [PubMed] [Google Scholar]
- 22.Köksal Y, Yalçin B, Aydın GB, et al. Immunogenicity of hepatitis A vaccine in children with cancer. Pediatr Hematol Oncol 2006; 23: 619–624. [DOI] [PubMed] [Google Scholar]
- 23.Choi W, Kim JG, Beom SH, et al. Immunogenicity and optimal timing of 13-valent pneumococcal conjugate vaccination during adjuvant chemotherapy in gastric and colorectal cancer: a randomized controlled trial. Cancer Res Treat 2020; 52: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021; 39: 1091–1098.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agbarya A, Sarel I, Ziv-Baran T, et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers (Basel) 2021; 13: 4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agha M, Blake M, Chilleo C, et al. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. Preprint 7 April 2021: 2021.04.06.21254949. [Google Scholar]
- 27.Ariamanesh M, Porouhan P, PeyroShabany B, et al. Immunogenicity and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest 2022; 40: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol 2021; 195: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrière J, Chamorey E, Adjtoutah Z, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol 2021; 32: 1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of the BNT162b mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Haematologica 2022; 107: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol 2021; 8: e389–e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury O, Bruguier H, Mallett G, et al. Impaired antibody response to COVID-19 vaccination in patients with chronic myeloid neoplasms. Br J Haematol 2021; 194: 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung DJ, Shah GL, Devlin SM, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2021; 2: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehmsen S, Asmussen A, Jeppesen SS, et al . Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021; 39: 1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavriatopoulou M, Terpos E, Kastritis E, et al . Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med 2021; 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghandili S, Schönlein M, Lütgehetmann M, et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers (Basel) 2021; 13: 3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell–directed therapies. Blood 2021; 138: 811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goshen-Lago T, Waldhorn I, Holland R, et al . Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021; 7: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenberger LM, Saltzman LA, Senefeld JW, et al. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell 2021; 39: 1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberger LM, Saltzman LA, Senefeld JW, et al . Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021; 39: 1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurion R, Rozovski U, Itchaki G, et al . Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2022; 107: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021; 137: 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heudel P, Favier B, Assaad S, et al. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol 2021; 32: 1443–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iacono D, Cerbone L, Palombi L, et al . Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol 2021; 12: 1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am J Hematol 2021; 96: E410–E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karacin C, Eren T, Zeynelgil E, et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol 2021; 17: 4447–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasagna A, Agustoni F, Percivalle E, et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open 2021; 6: 100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ligumsky H, Safadi E, Etan T, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst 2022; 114: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol 2021; 8: e542–e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mairhofer M, Kausche L, Kaltenbrunner S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell 2021; 39: 1171–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J 2021; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malissen N, Ninove L, De Lamballerie X, et al. Safety and immunogenicity after 2 doses of the BNT162b2 COVID-19 vaccine in an early-phase oncology trial centre population. Eur J Cancer 2021; 156: 125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol 2021; 8: e583–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021; 7: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021; 22: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ollila TA, Lu S, Masel R, et al . Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol 2021; 7: 1714–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol 2021; 32: 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021; 11: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peeters M, Verbruggen L, Teuwen L, et al . Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 2021; 6: 100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 2021; 5: 3053–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol 2021; 14: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pimpinelli F, Marchesi F, Piaggio G, et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J Hematol Oncol 2021; 14: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Re D, Barrière J, Chamorey E, et al. Low rate of seroconversion after mRNA anti-SARS-CoV-2 vaccination in patients with hematological malignancies. Leuk Lymphoma 2021; 62: 3308–3310. [DOI] [PubMed] [Google Scholar]
- 64.Re D, Seitz-Polski B, Carles M, et al. Humoral and cellular responses after a third dose of BNT162b2 vaccine in patients treated for lymphoid malignancies. medRxiv. Preprint 22 July 2021: 2021.07.18.21260669. [Google Scholar]
- 65.Revon-Riviere G, Ninove L, Min V, et al. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer 2021; 154: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shroff RT, Chalasani P, Wei R, et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. medRxiv. Preprint 25 August 2021: 2021.05.13.21257129. [Google Scholar]
- 67.Singer J, Le N-S, Mattes D, et al. Evaluation of antibody responses to COVID-19 vaccines among solid tumor and hematologic patients. Cancers (Basel) 2021; 13: 4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stampfer SD, Goldwater M-S, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021; 35: 3534–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tadmor T, Benjamini O, Braester A, et al . Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia 2021; 35: 2727–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terpos E, Gavriatopoulou M, Fotiou D, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers (Basel) 2021; 13: 4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J 2021; 11: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood 2021; 137: 3674–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terpos E, Zagouri F, Liontos M, et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol 2021; 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021; 39: 1081–1090.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tzarfati KH, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol 2021; 96: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 2021; 39: 1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waldhorn I, Holland R, Goshen-Lago T, et al. Six month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov 2021; 11: 2430–2435. [DOI] [PubMed] [Google Scholar]
- 78.B-pVAC-SARS-CoV-2: Study to Prevent COVID-19 Infection in Adults With Bcell/Antibody Deficiency, https://clinicaltrials.gov/ct2/show/NCT04954469.
- 79.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (2021, accessed 28 September 2021).
- 80.Department of Health and Social Care. Independent report: Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination, https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (2021, accessed 7 September 2021).
- 81.Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events, https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (2021, accessed 28 September 2021).
- 82.Mortazavi S. COVID-19 vaccination–associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol 2021; 217: 857–858. [DOI] [PubMed] [Google Scholar]
- 83.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med 2021; 46: 353–354. [DOI] [PubMed] [Google Scholar]