Abstract
Background and aims
The clinical significance of Internet gaming disorder (IGD) is spreading worldwide, but its underlying neural mechanism still remains unclear. Moreover, the prevalence of IGD seems to be the highest in adolescents whose brains are in development. This study investigated the functional connectivity between large-scale intrinsic networks including default mode network, executive control network, and salience network. We hypothesized that adolescents with IGD would demonstrate different functional connectivity patterns among large-scale intrinsic networks, implying neurodevelopmental alterations, which might be associated with executive dysfunction.
Methods
This study included 17 male adolescents with Internet gaming disorder, and 18 age-matched male adolescents as healthy controls. Functional connectivity was examined using seed-to-voxel analysis and seed-to-seed analysis, with the nodes of large-scale intrinsic networks used as region of interests. Group independent component analysis was performed to investigate spatially independent network.
Results
We identified aberrant functional connectivity of salience network and default mode network with the left posterior superior temporal sulcus (pSTS) in adolescents with IGD. Furthermore, functional connectivity between salience network and pSTS correlated with proneness to Internet addiction and self-reported cognitive problems. Independent component analysis revealed that pSTS was involved in social brain network.
Discussion and conclusions
The results imply that aberrant functional connectivity of social brain network with default mode network and salience network was identified in IGD that may be associated with executive dysfunction. Our results suggest that inordinate social stimuli during excessive online gaming leads to altered connections among large-scale networks during neurodevelopment of adolescents.
Keywords: Internet gaming disorder, executive dysfunction, posterior superior temporal sulcus, social brain network, adolescence
Introduction
Internet gaming disorder (IGD) is a pattern of gaming behavior characterized by impaired control over gaming, increasing priority given to gaming over other activities, and continuation or escalation of gaming despite of the occurrence of negative consequences that result in personal, familial, social, occupational impairments for more than 12 months (WHO, 2018). Internet gaming disorder was first proposed as a condition for further study in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (Petry & O'Brien, 2013); With spread of the clinical significance of IGD worldwide, the World Health Organization recognized ‘gaming disorder’ as a mental health condition in the 11th Revision of the International Classification of Diseases (ICD-11). Diverse attempts have been made to unveil the neurobiology of IGD (Sugaya, Shirasaka, Takahashi, & Kanda, 2019). For example, IGD in some cases showed dysfunctional striatal circuits which is related to reward processing (Jin et al., 2016; Luijten, Schellekens, Kühn, Machielse, & Sescousse, 2017), others suggesting problems in emotion processing in IGD (Chun, Choi, Cho, Lee, & Kim, 2015; Lee et al., 2015), and cognitive control was also stressed out to be impaired in IGD (Goldstein & Volkow, 2011; Zhang et al., 2016). However, the neural mechanism of IGD and its comorbidity with other mental disorders still remain controversial (Petry et al., 2014).
As the prevalence of IGD seems highest in adolescence (Paulus, Ohmann, Von Gontard, & Popow, 2018), the development of adolescent brain should be considered in understanding the neural mechanism of IGD. Heterogeneous developmental trajectory of each brain region can explain why adolescents behave unlike adults, and why they are more vulnerable to several psychiatric illnesses (Gogtay et al., 2004; Powell, 2006; Shaw, Gogtay, & Rapoport, 2010). Especially, adolescents show some distinctive characteristics on decision-making (Blakemore & Robbins, 2012). Adolescents tend to make risky decisions despite of the predicted serious consequences, which are due to the slow development of prefrontal cortex responsible for cognitive control and subsuming response selection (Rubia et al., 2000). Similarly, the function of executive control network which supports decision making and inhibitory control might be insufficient in early adolescence as the network continues to mature during adolescence (Ordaz, Foran, Velanova, & Luna, 2013). “Dual system model” by Steinberg et al. (Steinberg et al., 2008) explains risk-taking behaviors of adolescents as linked to relatively strong socio-emotional system than cognitive control system for the reason of gap in developmental trajectory (Shulman et al., 2016), implying that socio-emotional brain networks other than executive control network also plays an important role in the decision-making of adolescents. Especially, networks related to social cognition and mentalization might be crucial in adolescents' decision making, because adolescents regard social contexts as salient clue in decision making than adults because of differences in mentalizing network between two groups (Blakemore & Robbins, 2012). Social brain network, a complex network responsible for social cognition, social processing and mentalization, is consisted of several brain regions including posterior superior temporal sulcus (pSTS), temporoparietal junction and medial prefrontal cortex (Blakemore, 2008; Mills, Lalonde, Clasen, Giedd, & Blakemore, 2014). Though pSTS is known to be involve in the perception of eye gaze and biological motions (Saxe, Whitfield-Gabrieli, Scholz, & Pelphrey, 2009), it is also one of component in mentalizing system (Burnett & Blakemore, 2009). Temporoparietal junction, anterior temporal cortex and medial prefrontal cortex are mainly activated in mentalizing situations. Unlike executive functions, social brain is known to develop during infancy and childhood. Two different systems suggested in “Dual system model” might be related to each other rather than working independently, as social dysfunction is known to be associated with default mode network, executive control network and salience network (Chen et al., 2020). As risky decision making and impulsivity followed by neurodevelopmental differences seems to underlie the addiction vulnerability in adolescence, it would also lead adolescents to fall easily into Internet gaming (Chambers, Taylor, & Potenza, 2003).
In this study, we aimed to identify the alteration of functional connectivity related to decision-making in adolescents with IGD by analyzing the functional connectivity between large-scale intrinsic networks. To attain our purpose, we selected default mode network, salience network, and executive central network. Previous studies have demonstrated that the interactions between these major large-scale networks underlie in wide range of psychopathologies including executive dysfunctions (Menon, 2011). We hypothesize that adolescents with IGD would demonstrate different functional connectivity pattern among these large-scale intrinsic networks which might be associated with executive dysfunction.
Materials and methods
Participants
Participants consisted of 17 male adolescents with Internet gaming disorder (age range: 12–15 years) and 18 age-matched male adolescents (age range: 12–15 years) as healthy controls (HC). Participants were recruited from the local community through announcements, flyers, or word of mouth. Only male adolescents whose main purpose of using Internet was playing multiplayer-online game called ‘League of Legend’ were included in study. Each participant was assessed through a structured interview, including the Korean Internet Addiction Proneness scale which is standardized in Korea with high reliability, a Cronbach's alpha of 0.838 (Sin, Kim, & Jeung, 2011). Participants were excluded if their main purpose of using Internet were other than playing online game such as social networking and watching videos, or in case they had history of current or past psychiatric disorders, neurological illness, traumatic brain injury, any radiological contraindications for MRI scanning. A psychiatrist confirmed the diagnosis of Internet gaming disorder based on the proposed criteria of Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).
Psychometric measures
Participants self-reported psychometric tests, including Barratt Impulsiveness Scale version 11 (Patton, Stanford, & Barratt, 1995), Conners-Wells Adolescent Self-report Scale (Conners et al., 1997), Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), and Beck Anxiety Inventory (Yook & Kim, 1997). Barratt Impulsiveness Scale version 11 measures impulsivity and possesses subscales of cognitive impulsiveness, motor impulsiveness, and non-planning impulsiveness (Patton et al., 1995). Conners-Wells Adolescent Self-report Scale is developed to screen adolescents with Attention-deficit hyperactivity disorder (ADHD), we used short-form of Conners-Wells Adolescent Self-report Scale with 27 items. Short-form of Conners-Wells Adolescent Self-report Scale contains cognitive problem (coefficient alpha≥ 0.80), hyperactivity problem (coefficient alpha≥ 0.80), and conduct problem (coefficient alpha = 0.73) as subscales (Conners et al., 1997; Steer, Kumar, & Beck, 2001).
Image acquisition and pre-processing
Functional magnetic resonance imaging (fMRI) was conducted on a 3T Siemens Magnetom MRI scanner equipped with an eight channel head coil. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo planar pulse sequence (echo time = 30 ms, repetition time = 2,200 ms, flip angle = 90°, field of view = 240 mm, matrix = 64 × 64, slice thickness = 4 mm) during passively viewing block scan. Participants were instructed to fixate on white cross on black background for 15 minutes to gain resting-state images. High resolution anatomical images were acquired with a T1 weighted spoiled gradient-echo sequence (echo time = 2.19 ms; repetition time = 1,780 ms, flip angle = 9°, field of view = 256 mm, matrix = 256 × 256, slice thickness = 1 mm) to serves as an anatomical underlay for the functional MRI data.
Pre-processing and statistical analysis of functional images were performed using SPM12 (Welcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm). Motion artifacts of each participants was monitored through visual inspection of realignment parameter estimations, to ensure that maximum head motion in each axis was <3 millimeters (mm) without any abrupt head motion. The anatomical volume was segmented into gray matter, white matter, and cerebrospinal fluid. Then gray matter image was used for determining the parameters of normalization onto the standard Montreal Neurological Institute template. The spatial parameters were applied into the realigned and unwarped functional volumes that were finally re-sampled to voxels of 2x2x2 millimeters. At the end of pre-processing, Images were smoothed with an 8-mm full-width at half-maximum kernel.
Functional connectivity analysis
Functional connectivity maps of seed-to-voxel analysis were obtained using the CONN-fMRI functional connectivity toolbox version 18.b (http://www.nitrc.org/projects/conn). We used five ROIs as seed regions, posterior cingulate cortex (PCC) (Fox et al., 2005) for the default mode network, bilateral anterior insular cortex (AIC) (Mueller et al., 2018; Woodward, Rogers, & Heckers, 2011) for the salience network, and the bilateral dorsolateral prefrontal cortex (DLPFC) (Mueller et al., 2018; Woodward et al., 2011) for the executive control network. All seed regions were defined as a 5-mm radium sphere centered on previously reported coordinates (Fox et al., 2005; Mueller et al., 2018; Woodward et al., 2011). Using bandpass filter, the waveform of each brain voxel was temporally filtered (0.008 < f < 0.09 Hz) to exclude effects of low-frequency drift and high-frequency noise. Signals from white matter and ventricular regions were also eliminated through linear regression (Whitfield-Gabrieli & Nieto-Castanon, 2012). To reduce the artifacts by head motion, estimated subject-motion parameters (Friston, Williams, Howard, Frackowiak, & Turner, 1996) implemented in CONN's default denoising guideline were applied to the linear regression. The strength of functional connectivity, correlation coefficients were converted to z-values using Fisher's r-to-z transformation. In between-group analysis, independent-sample t-tests were performed using threshold of an uncorrected P-value <0.001, minimum cluster extent of 100 contiguous voxels. Afterward we made additional seed region, a 5-mm radium sphere centered on left pSTS from our seed-to-voxel result of right AIC (MNI coordinates −60 −54 6) and proceeded seed-to-seed analysis to identify within-group correlation of ROIs (PCC, bilateral AIC, bilateral DLPFC and an additional ROI from seed-to-voxel result). The within-group significance was determined using one-sample t tests with false discovery rate (FDR) correction (P-value<0.05). Furthermore, group independent component analysis (ICA) was done to clarify if the result of seed-to-voxel analysis can reflect interrelationship of functional connectivity networks not just informing relationship between clusters.
Group independent component analysis
Group independent component analysis (group ICA) was performed to investigate spatially independent network using group ICA of fMRI toolbox (GIFT ver.3.0b, http://mialab.mrn.org/software/gift). Preprocessed data were reduced through two stages of principal component analysis (Calhoun, Adali, Pearlson, & Pekar, 2001). Following the modified minimal description length criteria, thirty-eight ICA components were estimated with infomax algorithm (Bell & Sejnowski, 1995). Using ICASSO algorithm (Himberg, Hyvärinen, & Esposito, 2004), ICA analysis was repeated 20 times for stability of the result. As a result, 38 independent functional spatial maps for every participant were obtained.
For the selection of ICA components of interest, we performed stepwise estimations. First, each component was correlated with prior probabilistic maps of white matter and cerebrospinal fluid within a standardized brain space provided by MNI templates in SPM8. If components show spatial correlation greater than r2 = 0.025 in both white matter and cerebrospinal fluid, they were discarded from analysis because they can be considered as artifacts. Second, to sort out the candidates of large-scale intrinsic networks, components survived from first step were correlated with network atlas of default mode network, salience network, and left, right executive control network (Shirer, Ryali, Rykhlevskaia, Menon, & Greicius, 2012). Among candidates, we selected most suspected default mode network, salience network, and executive control network by inspecting conscientiously focusing on coactivated brain regions in components. Third, brain regions from seed-to-voxel analysis results were used as a mask to correlate with ICA components. Components with high spatial correlation with the mask were regarded as candidates of network of interest.
One-sample t tests and two-sample t tests of selected components were done to analyze within- and between-group differences. Analysis were completed using SPM 12 with the help of ‘SPM stat’ function in the GIFT toolbox. To compare correlations between selected components, participants' spatial maps of selected components were converted to Z values. Functional network connectivity was computed to evaluate correlations between components in the GIFT toolbox, FNC matrix and connectogram were generated.
Statistical analysis
Independent t-tests were performed to compare demographic and clinical variables between IGD group and HC group. To examine relationship between results of clinical assessments and functional connectivity, Pearson correlation analysis tested relations between the functional seed-target connectivity and behavioral performance. Statistical analyses were conducted by using SPSS (Chicago, IL) with P < 0.05 (two-tailed).
Ethics
The Institutional Review Board at Severance Hospital, Yonsei University approved all protocols for this study. Written informed consent was obtained from all subjects prior to participation.
Results
Demographic and clinical assessments
There were no differences between the two groups in age, verbal IQ, and performance IQ (Table 1). The Korean Internet Addiction Proneness Scale score was significantly higher in IGD group. Scores of the Conners-Wells Adolescent Self-report Scale and Barratt Impulsiveness Scale were significantly higher in the IGD group.
Table 1.
Demographic and clinical characteristics of participants
| Internet Gaming Disorder | Healthy Control | T | P | |
| Age (years) | 13.7(0.9) | 13.4(1.0) | 0.647 | 0.522 |
| Verbal IQ | 8.7(2.6) | 10.3(3.3) | −1.592 | 0.121 |
| Performance IQ | 10.6(2.9) | 10.8(3.3) | −0.176 | 0.861 |
| KS* | 35.2(5.5) | 24.9(4.6) | 6.017 | <0.001 |
| CASS* | 30.6(16.0) | 14.7(8.6) | 3.637 | 0.001 |
| Cognitive problem* | 15.8(8.0) | 7.2(5.7) | 3.686 | 0.001 |
| Hyperactivity problem* | 10.1(6.3) | 4.9(2.4) | 3.208 | 0.004 |
| Conduct problem* | 4.8(3.0) | 2.6(2.7) | 2.213 | 0.034 |
| BIS* | 68.3(8.7) | 56.2(12.4) | 3.312 | 0.002 |
| Cognitive impulsiveness* | 19.0(3.0) | 14.8(3.7) | 3.618 | 0.001 |
| Motor impulsiveness* | 21.7(5.0) | 18.0(4.7) | 2.233 | 0.033 |
| Non-planning impulsiveness* | 27.6(4.1) | 23.4(5.9) | 2.377 | 0.024 |
| Beck Depression Inventory (BDI)* | 11.6(11.9) | 4.4(4.6) | 2.269 | 0.035 |
| Beck Anxiety Inventory (BAI) | 8.1(8.5) | 5.0(6.2) | 1.224 | 0.230 |
Note. Values are expressed as mean (SD). Verbal Intelligence Quotient (IQ) was assessed with Vocabulary in Wechsler Adult Intelligence scale, and Performance IQ was assessed with the Wechsler Adult Intelligence Scale (Block design). KS: Korean Internet Addiction Proneness Scale, CASS: Conners/Wells Adolescent Self-report Scale, BIS: Barratt Impulsiveness Scale, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory. *P-value < 0.05.
Functional connectivity analysis
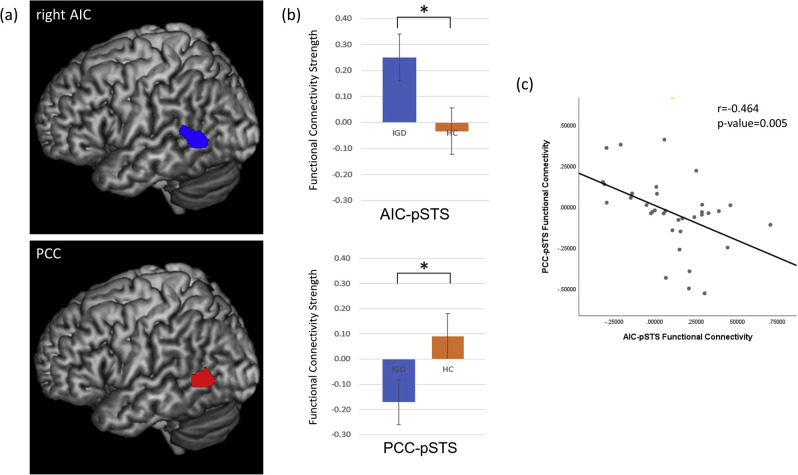
In seed-to-voxel analysis, the IGD group showed stronger positive functional connectivity between the right AIC and left pSTS than HC group. In contrast, HC group showed significant positive functional connectivity between PCC and left pSTS compared to IGD group (Fig. 1a, Supplementary material 1). Regarding that the PCC and AIC were selected to represent the default mode network and salience network respectively, the pSTS was positively correlated with salience network in IGD while presenting negative correlation with salience network in HC group (t: 4.219, P-value<0.001). On the other hand, pSTS was negatively correlated with default mode network in IGD opposite to HC group (t: −4.411, P-value<0.001) (Fig. 1b). There was a negative correlation between the PCC-pSTS functional connectivity strength and the AIC-pSTS functional connectivity strength (Pearson's r = −0.464, P-value = 0.005) (Fig. 1c).
Fig. 1.
Seed-to-voxel analysis and its functional connectivity strengths. The statistical inferences were thresholded using an uncorrected P value<0.001. Coordinates indicate the locations of the brain slices according to the Montreal Neurological Institute system. (a) Between group differences in seed-to-voxel analysis results. Compared to HC group, right AIC of IGD showed significantly positive functional connectivity with left STS (−60 −54 6) (kmax = 357, T = 5.35). PCC of HC group showed significant positive functional connectivity with left STS (−58 −58 6) (kmax = 175, T = 5.00) than IGD group. (b) Between group comparison of functional connectivity strength. AIC-pSTS functional connectivity showed significant difference, presenting positive functional connectivity in IGD and negative functional connectivity in HC (t:4.219, P-value<0.001). PCC-pSTS functional connectivity between two groups was also significantly different while IGD showing negative functional connectivity, HC showing positive functional connectivity (t:−4.411, P-value<0.001). (c) Pearson correlation analysis for AIC-pSTS functional connectivity and PCC-pSTS functional connectivity. Two functional connectivity showed significant anticorrelation (r = −0.464, P = 0.005)
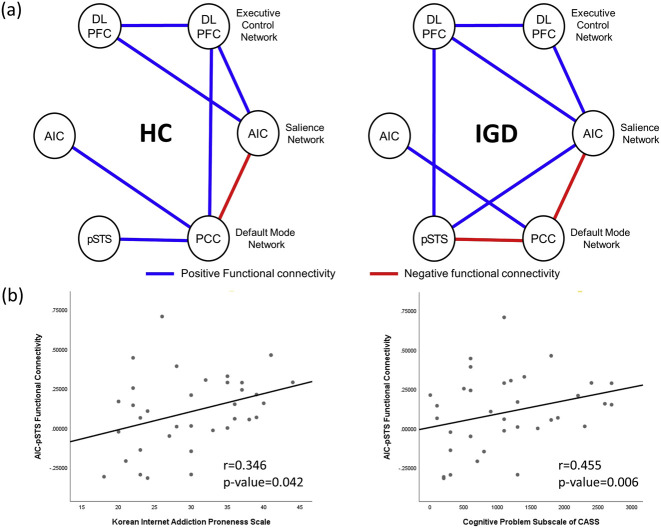
In seed-to-seed analysis, the pSTS functional showed different pattern in the IGD group. The pSTS showed additional functional connectivity with left DLPFC, right AIC only in IGD and altered functional connectivity with PCC in IGD compared to HC group. Moreover, functional connectivity between PCC and right DLPFC in healthy control was not observed in IGD group (Fig. 2a, Supplementary material 2).
Fig. 2.
Between-network connectivity and functional connectivity correlation with clinical variables. (a) Seed-to-seed within-group analysis (blue line: positive functional connectivity; red line: negative functional connectivity). The statistical inferences were thresholded using an FDR-corrected P value<0.05. In IGD group, functional connectivity of pSTS to left DLPFC and right AIC was newly identified which were not observed in HC group, while alteration in pSTS-PCC functional connectivity (positive to negative) was also found in IGD. Functional connectivity of PCC with right DLPFC shown in HC was not significant in IGD. (b) Pearson correlation analysis for clinical correlation of functional connectivity. We identified positive correlation between AIC-pSTS functional connectivity and total score of Korean Internet Addiction Proneness Scale (r = 0.346, P = 0.042), and positive correlation between AIC-pSTS functional connectivity and cognitive problem subscale of Conners-Wells Adolescents Self-report Scale (r = 0.455, P-value = 0.006)
Correlation between functional connectivity strength and psychometric measures
The AIC-pSTS functional connectivity strength correlated with higher scores of Korean Internet Addiction Proneness Scales (Pearson's r = 0.346, P-value = 0.042). The AIC-pSTS functional connectivity strength also correlated with higher scores of the cognitive problem subscale of the Conners-Wells Adolescent Self-report Scale (Pearson's r = 0.455, P-value = 0.006) (Fig. 2b). After Bonferroni correction, the AIC-pSTS functional connectivity strength was significantly correlated only with high scores of the cognitive problem subscale of the Conners-Wells Adolescent Self-report Scale (Adjusted P-value = 0.0125). When analysis was restricted to IGD group, the correlations between functional connectivity in AIC-pSTS and psychometric measures were not significant. There was no significant correlation between the PCC-pSTS functional connectivity strength and any psychometric measures.
Group independent component analysis
Six out of 38 independent component (IC; IC 15, IC 17, IC 24, IC 26, IC 29, IC 34) passed our selection criteria. Four among six components were highly correlated with grey matter and Stanford templates respectively: default mode network: IC 34, salience network: IC 17, left executive control network: IC 15, right executive network: IC 24 (Supplemental material 3). Though both IC 26 and IC 29 seemed to be related to the activation of left pSTS, IC 29 was excluded from our analysis because it showed restricted BOLD signal activation only in both pSTS. The IC 26 was composed of left pSTS, bilateral DLPFC, and right temporoparietal junction in within-group analysis of component (FWE-adjusted P-value = 0.05, extended voxel >100) (Supplementary material 4). Based on these regions, we identified IC 26 as the social brain network (Blakemore, 2008; McCormick, van Hoorn, Cohen, & Telzer, 2018). In two-sample t-test of social brain network, the left pSTS was hyperactivated in IGD group compared to HC group (Supplementary material 5).
Discussion
In line with our hypothesis, we identified aberrant functional connectivity of salience network and default mode network with the pSTS in adolescents with IGD. Moreover, functional connectivity between the salience network and pSTS correlated with proneness to Internet addiction and self-reported cognitive problems. Regarding that the pSTS is an essential part of the social brain network, our findings imply that excessive exposure to game-related social stimuli during adolescence might affect the dynamic interaction between the salience network and social brain network, which seems to be associated with executive dysfunction and cognitive problems.
Adolescents with IGD showed different functional connectivity patterns among large-scale intrinsic networks. The PCC and right AIC, which are the hubs of the default mode network and salience network, both showed significant interactions with the left pSTS only in adolescents with IGD. In addition, there was a negative correlation between the PCC-pSTS functional connectivity strength and the AIC-pSTS functional connectivity strength. These findings suggest that the left pSTS was involved in the interaction between these two large-scale intrinsic networks. The pSTS plays an important role in detection of face and eye gaze in humans (Allison, Puce, & McCarthy, 2000; Johansson, 1973) and is known as a key node of the social brain network including mentalization (Blakemore, 2008). To achieve successful theory of mind (higher-level subsystem), it is necessary to recognize socially relevant face and motions (lower-level subsystem), and to prepare cohesive response such as empathy that links preprocessed sensory input to mentalizing response (intermediate-level subsystem). Nodes of social brain network such as medial prefrontal cortex and temporoparietal junction (higher-level subsystem), inferior frontal gyrus (intermediate-level subsystem), and fusiform gyrus (lower-level subsystem) are activated in one or more subsystems in social brain network. As pSTS plays a crucial role in lower- and intermediate-level subsystem in social brain network (Alcalá-López et al., 2018), it is directly affected by external social stimuli. Independent Component Analyses confirmed that the pSTS activation in this study was a part of a larger network (IC 26), the social brain network, which was composed of social brain nodes such as the pSTS, bilateral DLPFC, and right temporoparietal junction. DLPFC can be explained as coactivated region of social brain network due to correlation between social brain network and fronto-striatal connectivity (McCormick et al., 2018). Besides the pSTS, the AIC also is known to take part in social processing. The AIC helps recognizing social stimuli as salient events and makes individuals to remain attentive to social situations by coactivaiton with other social brain nodes (Menon & Uddin, 2010). Furthermore, the functional connectivity of AIC and pSTS is increased when individuals share emotional content such as embarrassment of social target (Paulus, Müller-Pinzler, Jansen, Gazzola, & Krach, 2015), and decreased in autism spectrum disorder facing social stimuli (Odriozola et al., 2016). These findings suggest that the AIC-pSTS functional connectivity is sensitive to social situations.
Our finding provide evidence that excessive online gaming behavior might affect social brain functioning in adolescent with IGD. As online games require real-time communication with peers and other players, adolescents can easily be exposed to enormous social stimuli which cannot be ignored. Moreover, adolescents are sensitive to social judgments (Blakemore & Robbins, 2012), and tend to regard social interaction in online gaming as salient stimuli. Considering that the influence of online social situations is similar to that of face-to-face experience on brain activation (Crone & Konijn, 2018), we assume that the stronger functional connectivity between the social brain network and salience network in adolescents with IGD might be related to the increased time and heavy loading of social interactions while playing online games.
It is noteworthy that the AIC-pSTS functional connectivity correlated with higher scores of the cognitive problem subscale of the Conners-Wells Adolescent Self-report Scale. Social functions such as social cognition and mentalization are reported to be closely related to executive dysfunction in adolescents (Crone, 2009) as the default mode network and salient network are closely related to social cognition and affect each other (Chen et al., 2020; Mary et al., 2016). As the cognitive problem subscale in Conners-Wells Adolescent Self-report Scales implies executive functioning such as inhibitory control, organizing work, finishing task (Steer et al., 2001), our findings, which demonstrated aberrant functional connectivity between the default mode network, salience network and social brain network, might provide further understanding about the neural basis of executive dysfunction and cognitive problems in IGD.
Differences in developmental trajectory of the neural networks are one of the developmental characteristics in adolescence, and the adolescent brain is sensitive to experiential input with synaptic reorganization (Blakemore & Choudhury, 2006). The underlying neurodevelopmental vulnerability of adolescent brain, such as functional gaps between networks and insufficient segregation of networks (Stevens, 2016), might also play an important role in the neurobiological changes induced by excessive social stimuli. Considering that the social brain network is early-developed compared to other functional networks (Dunbar & Shultz, 2007; McCormick et al., 2018), we suppose that the functional gap between the social network and cognitive network is related to sensitive social perception to excessive social stimuli resulting in increased functional connectivity between salience network and social brain network in IGD. Aberrant functional connectivity of social brain network to other intrinsic networks might also be associated with insufficient segregation during brain maturation. Segregation is one of the key process of brain maturation which is associated with pruning of brain architectures (Supekar, Musen, & Menon, 2009). Compared to adults, children can show distinctive functional connectivity linking two independent large-scale networks due to insufficient segregation process (Fair et al., 2007). As our participants are in age of early adolescence (mean age 13.7 years in IGD group, 13.4 years in HC group), segregation of functional networks might be insufficient and changes in patterns of functional connectivity would be possible depending on individual's experience.
There are several limitations to our study. First, as our study was performed in cross-sectional design, we could not conclude whether the aberrant functional connectivity patterns were the result of IGD or a predisposition factor of IGD. To explore this limitation, longitudinal study involving early childhood should be followed. Second, significant difference was observed in Beck Depression Inventory (BDI). Although there was no statistically significant influence on our result as a covariate, the effects of depression still cannot be completely excluded. Third, the size of sample was relatively small. Accordingly, the correlations between AIC-pSTS functional connectivity and psychometric measures in IGD group was not significant. Studies with large sample size should be followed. Fourth, executive functions are evaluated only with self-report scales. Further studies with objective neuropsychological tests evaluating executive function may clarify our results.
Conclusion
In conclusion, aberrant functional connectivity of the social brain network with default mode network and salience network was identified in IGD, stronger functional connectivity with salience network and weaker functional connectivity with default mode network in resting state. Altered interaction among networks might be associated with executive dysfunction in Internet gaming disorder. Our results suggest that inordinate social stimuli during excessive online gaming leads to altered connections among large-scale networks during neurodevelopment in adolescence. Future studies on characteristics of brain maturational trajectory in Internet gaming disorder might help with the understanding of the neurobiological mechanism.
Funding sources
This study was supported by a grant from the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HM14C2578).
Authors' contribution
JL and Y-C J conceived and designed the study. JL and DL recruited participants. JL analyzed data and drafted the manuscript. KN provided critical revision of the manuscript and important intellectual content. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically reviewed and approved the final version of this manuscript for publication.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Contributor Information
Junghan Lee, Email: humanjhl@gmail.com.
Deokjong Lee, Email: pangelt@yuhs.ac.
Kee Namkoong, Email: keen@yuhs.ac.
Young-Chul Jung, Email: eugenejung@yuhs.ac.
References
- Alcalá-López, D., Smallwood, J., Jefferies, E., Van Overwalle, F., Vogeley, K., Mars, R. B., et al. (2018). Computing the social brain connectome across systems and states. Cerebral Cortex, 28(7), 2207–2232. [DOI] [PubMed] [Google Scholar]
- Allison, T., Puce, A., & McCarthy, G. (2000). Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences, 4(7), 267–278. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. [DOI] [PubMed] [Google Scholar]
- Bell, A. J., & Sejnowski, T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. & Choudhury, S. (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47(3‐4), 296–312. [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. & Robbins, T. W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15(9), 1184. [DOI] [PubMed] [Google Scholar]
- Burnett, S., & Blakemore, S. J. (2009). Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience, 29(6), 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D., Adali, T., Pearlson, G. D., & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, R. A., Taylor, J. R., & Potenza, M. N. (2003). Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry, 160(6), 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M.-H., Chen, Y.-L., Bai, Y.-M., Huang, K.-L., Wu, H.-J., Hsu, J.-W., et al. (2020). Functional connectivity of specific brain networks related to social and communication dysfunction in adolescents with attention-deficit hyperactivity disorder. Psychiatry Research, 112785. [DOI] [PubMed] [Google Scholar]
- Chun, J., Choi, J., Cho, H., Lee, S., & Kim, D. (2015). Dysfunction of the frontolimbic region during swear word processing in young adolescents with Internet gaming disorder. Translational Psychiatry, 5(8), e624–e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners, C. K., Wells, K. C., Parker, J. D., Sitarenios, G., Diamond, J. M., & Powell, J. W. (1997). A new self-report scale for assessment of adolescent psychopathology: Factor structure, reliability, validity, and diagnostic sensitivity. Journal of Abnormal Child Psychology, 25(6), 487–497. [DOI] [PubMed] [Google Scholar]
- Crone, E.(2009). Executive functions in adolescence: Inferences from brain and behavior. Developmental Science, 12(6), 825–830. 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Crone, E. A., & Konijn, E. A. (2018). Media use and brain development during adolescence. Nature Communications, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, R. I., & Shultz, S. (2007). Evolution in the social brain. Science, 317(5843), 1344–1347. [DOI] [PubMed] [Google Scholar]
- Fair, D. A., Dosenbach, N. U., Church, J. A., Cohen, A. L., Brahmbhatt, S., Miezin, F. M., et al. (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences, 104(33), 13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D., Snyder, A. Z., Vincent, J. L., Corbetta, M., Van Essen, D. C., & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg, J., Hyvärinen, A., & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22(3), 1214–1222. [DOI] [PubMed] [Google Scholar]
- Jin, C., Zhang, T., Cai, C., Bi, Y., Li, Y., Yu, D., et al. (2016). Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging and Behavior, 10(3), 719–729. [DOI] [PubMed] [Google Scholar]
- Johansson, G. (1973). Visual perception of biological motion and a model for its analysis. Perception & Psychophysics, 14(2), 201–211. [Google Scholar]
- Lee, J., Lee, S., Chun, J. W., Cho, H., Kim, D.-J., & Jung, Y.-C. (2015). Compromised prefrontal cognitive control over emotional interference in adolescents with internet gaming disorder. Cyberpsychology, Behavior, and Social Networking, 18(11), 661–668. [DOI] [PubMed] [Google Scholar]
- Luijten, M., Schellekens, A. F., Kühn, S., Machielse, M. W., & Sescousse, G. (2017). Disruption of reward processing in addiction: An image-based meta-analysis of functional magnetic resonance imaging studies. Jama Psychiatry, 74(4), 387–398. [DOI] [PubMed] [Google Scholar]
- Mary, A., Slama, H., Mousty, P., Massat, I., Capiau, T., & Drabs, V., et al. (2016). Executive and attentional contributions to Theory of Mind deficit in attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology, 22(3), 345–365. 10.1080/09297049.2015.1012491. [DOI] [PubMed] [Google Scholar]
- McCormick, E. M., van Hoorn, J., Cohen, J. R., & Telzer, E. H. (2018). Functional connectivity in the social brain across childhood and adolescence. Social Cognitive and Affective Neuroscience, 13(8), 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V.(2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. L., Lalonde, F., Clasen, L. S., Giedd, J. N., & Blakemore, S.-J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, F., Musso, F., London, M., de Boer, P., Zacharias, N., & Winterer, G. (2018). Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. NeuroImage: Clinical, 19, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola, P., Uddin, L. Q., Lynch, C. J., Kochalka, J., Chen, T., & Menon, V. (2016). Insula response and connectivity during social and non-social attention in children with autism. Social Cognitive and Affective Neuroscience, 11(3), 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz, S. J., Foran, W., Velanova, K., & Luna, B. (2013). Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience, 33(46), 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- Paulus, F. W., Müller-Pinzler, L., Jansen, A., Gazzola, V., & Krach, S. (2015). Mentalizing and the role of the posterior superior temporal sulcus in sharing others' embarrassment. Cerebral Cortex, 25(8), 2065–2075. [DOI] [PubMed] [Google Scholar]
- Paulus, F. W., Ohmann, S., Von Gontard, A., & Popow, C. (2018). Internet gaming disorder in children and adolescents: A systematic review. Developmental Medicine and Child Neurology, 60(7), 645–659. [DOI] [PubMed] [Google Scholar]
- Petry, N. M., & O'Brien, C. P. (2013). Internet gaming disorder and the DSM‐5. Addiction, 108(7), 1186–1187. [DOI] [PubMed] [Google Scholar]
- Petry, N. M., Rehbein, F., Gentile, D. A., Lemmens, J. S., Rumpf, H. J., Mößle, T., et al. (2014). An international consensus for assessing internet gaming disorder using the new DSM‐5 approach. Addiction, 109(9), 1399–1406. [DOI] [PubMed] [Google Scholar]
- Powell, K. (2006). How does the teenage brain work? In: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Rubia, K., Overmeyer, S., Taylor, E., Brammer, M., Williams, S., Simmons, A., et al. (2000). Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience & Biobehavioral Reviews, 24(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Saxe, R. R., Whitfield‐Gabrieli, S., Scholz, J., & Pelphrey, K. A. (2009). Brain regions for perceiving and reasoning about other people in school‐aged children. Child Development, 80(4), 1197–1209. [DOI] [PubMed] [Google Scholar]
- Shaw, P., Gogtay, N., & Rapoport, J. (2010). Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping, 31(6), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, E. P., Smith, A. R., Silva, K., Icenogle, G., Duell, N., Chein, J., et al. (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin, G., Kim, D., & Jeung, Y. (2011). Third standardization of Korean internet addiction proneness scale. Seoul: National Information Society Agency. [Google Scholar]
- Steer, R. A., Kumar, G., & Beck, A. T. (2001). Use of the conners-wells' adolescent self-report scale: Short form with psychiatric outpatients. Journal of Psychopathology and Behavioral Assessment, 23(4), 231–239. [Google Scholar]
- Steinberg, L., Albert, D., Cauffman, E., Banich, M., Graham, S., & Woolard, J. (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 44(6), 1764. [DOI] [PubMed] [Google Scholar]
- Stevens, M. C. (2016). The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neuroscience & Biobehavioral Reviews, 70, 13–32. [DOI] [PubMed] [Google Scholar]
- Sugaya, N., Shirasaka, T., Takahashi, K., & Kanda, H. (2019). Bio-psychosocial factors of children and adolescents with internet gaming disorder: A systematic review. BioPsychoSocial Medicine, 13(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar, K., Musen, M., & Menon, V. (2009). Development of large-scale functional brain networks in children. PLoS Biology, 7(7), e1000157. 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- WHO . (2018). Gaming disorder. Retrieved from http://www.who.int/features/qa/gaming-disorder/en/. [Google Scholar]
- Woodward, N. D., Rogers, B., & Heckers, S. (2011). Functional resting-state networks are differentially affected in schizophrenia. Schizophrenia Research, 130(1–3), 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook, S., & Kim, Z. (1997). A clinical study on the Korean version of Beck anxiety inventory: Comparative study of patient and non-patient. Korean Journal of Clinical Psychology, 16(1), 185–197. [Google Scholar]
- Zhang, J. T., Yao, Y. W., Li, C. S. R., Zang, Y. F., Shen, Z. J., Liu, L., et al. (2016). Altered resting‐state functional connectivity of the insula in young adults with I nternet gaming disorder. Addiction Biology, 21(3), 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]