Abstract
Background and aims
Growing concerns about the addictive nature of Internet and computer games led to the preliminary recognition of Internet Gaming Disorder (IGD) as an emerging disorder by the American Psychiatric Association (APA) and the official recognition of Gaming Disorder (GD) as a new diagnosis by the World Health Organization (WHO). While the definition of clear diagnostic criteria for (I)GD represents an important step for diagnosis and treatment of the disorder, potential neurobiological correlates of the criteria remain to be explored.
Methods
The present study employed a dimensional Magnetic Resonance Imaging (MRI) approach to determine associations between (I)GD symptom-load according to the APA and WHO diagnostic frameworks and brain structure in a comparably large sample of n = 82 healthy subjects.
Results
Higher symptom-load on both, the APA and WHO diagnostic frameworks convergently associated with lower volumes of the striatum.
Discussion
The results from this exploratory study provide the first initial evidence for a neurobiological foundation of the proposed diagnostic criteria for (I)GD according to both diagnostic classification systems and suggest that the transition from non-disordered to disordered gaming may be accompanied by progressive neuroplastic changes in the striatum, thus resembling progressive changes in other addictive disorders.
Conclusions
The proposed (I)GD criteria in both diagnostic systems were associated with neurostructural alterations in the striatum, suggesting an association with progressive changes in the motivational systems of the brain.
Keywords: internet gaming disorder, striatum, gray matter, DSM-5, ICD-11
Introduction
In 2019 the World Health Organization (WHO) formally recognized Gaming Disorder (GD) as official diagnosis to be included in the upcoming revision of the International Classification of Diseases (ICD, 11th version). Earlier in 2013, the American Psychiatric Association (APA) included Internet Gaming Disorder (IGD) as emerging disorder in the appendix of the DSM-5. Although the specific symptoms for disordered gaming differ across the two diagnostic frameworks, the proposed criteria in both frameworks strongly resemble diagnostic criteria for substance-based addictions, including preoccupation with gaming, loss of control over gaming and continued use despite negative consequences. For the categorical diagnosis of (I)GD both classification systems require a number of symptoms that must be exhibited over 12-months and lead to marked distress and functional impairments.
By proposing clear (I)GD symptom criteria, the classification systems have provided tremendous help for clinicians, researchers, and those in need of treatment for disordered gaming while accounting for growing public concerns about the detrimental effects of excessive gaming on mental health with the Chinese Government even releasing new policies in November 2019 to regulate Internet gaming time in adolescents (https://www.bbc.com/news/world-asia-50315960). It is likely that the inclusion of (I)GD as an addictive disorder may also pathologize and stigmatize normal gaming behavior as the neurobiological mechanisms underlying (I)GD symptoms and their resemblance to changes in other addictive disorders remain highly controversial (Zastrow, 2017). At the clinical and phenomenological levels (I)GD strongly resembles compulsive use and loss of control observed in substance-based addictions. Converging evidence from animal and human studies indicates that the transition from volitional to addictive and compulsive substance use is accompanied by progressive dysregulations in the motivational circuits of the brain. The striatum lies at the heart of these motivational circuits and plays a critical role in both the initial reinforcing effects of drugs as well as the development of compulsive use and progressive loss of control (Koob & Volkow, 2016). Neuroplastic changes in the striatum and associated circuitries engaged in reward processing and habit formation have been demonstrated extensively in animal models of substance use disorders (Everitt & Robbins, 2016). Moreover, concurring evidence from previous animal models and studies in individuals with substance use disorders suggests that different striatal regions control the transition from incentive-driven to compulsive substance use, corresponding to ventral and dorsal parts of the striatum respectively (Everitt & Robbins, 2016; Robbins, Ersche & Everitt, 2008; Vollstadt-Klein et al., 2010; Zhou et al., 2018, 2019). Human neuroimaging studies have also repeatedly reported striatal gray matter alterations in populations with substance use disorders, with the extent of volumetric reductions being associated with escalating substance use and clinical symptom severity (Becker et al., 2015).
Emerging evidence from a growing number of human imaging studies in pathological gambling indicate that striatal circuits play an important role in the initial rewarding effects of gambling as well as the formation of compulsive gambling during later stages of the disorder (Clark, Boileau, & Zack, 2019). These findings suggest that these circuits may also mediate escalating and ultimately compulsive use in behavioral addictions. Furthermore, brain structural alterations in behavioral addictions such as pathological gambling might be less pronounced, probably due to the lack of neurotoxic effects (Clark et al., 2019), even though a growing body of evidence reported altered gray matter volume in individuals with (I)GD, including lower volume in striatal circuits, most consistently affecting the dorsal striatum, specifically the putamen (Qin et al., 2020; Yao et al., 2017). Initial studies reported associations between the extent of gaming or the level of “Internet/social media addiction” - symptoms and gray matter volume in reward processing regions, including the striatum, suggesting that progressive volumetric changes in this region may accompany the onset of (I)GD (Cai et al., 2016; Montag et al., 2017). Moreover, neuroplastic and functional changes of the striatum have already been observed during early stages of the addictive process, suggesting that this region may represent a promising marker to track early stages of the development of addiction (Brand et al., 2019; Everitt & Robbins, 2016; Vollstadt-Klein et al., 2010; Zhou et al., 2019).
To determine whether the proposed symptom-level criteria for (I)GD reflects the hypothesized neurobiological basis of the disorder and if the different symptomatic criteria proposed by the APA and WHO relate to the same neurobiological markers, the present study employed a dimensional neuroimaging approach with the goal to map subclinical symptoms of (I)GD and striatal morphology. This study employed non-parametric voxel-wise regression analyses on high-resolution T1-weighted MRI brain structural images with (I)GD symptom-load assessed by diagnostic-system specific scales (Internet Gaming Disorder Scale – Short-Form, IGDS9-SF, APA framework; Gaming Disorder Test, GDT, WHO framework) (Pontes & Griffiths, 2015; Pontes et al., 2019) as separate predictors in a large sample of healthy young male adults. Symptom load on dimensions such as depression and autism frequently associated with (I)GD were controlled for. Given the substantial associations between IGD and GD scores when assessed with the IGDS9-SF and the GDT (Montag et al., 2019), and the key role of the striatum in substance-based addictions, it was expected that higher symptom-load on both disordered gaming psychometric tests would be accompanied by stronger alterations in striatal morphology (i.e. lower striatal volume).
Method
Participants and procedures
For the present study N = 256 healthy male individuals from the Chengdu Gene Brain Behavior Project were re-contacted via telephone interviews (N = 256, at least 18 years old). All subjects underwent structural MRI assessment prior to the assessment of (I)GD symptoms (1.1 ± 0.764 years in the final sample). Following MRI data quality assessments and exclusion of duplicate datasets, subjects were contacted via telephone and underwent an interview to exclude those with current or previous history of psychiatric disorders and current use of medication or psychotropic substances. Among all eligible participants, N = 119 agreed to participate in the additional electronic data assessment. The severity of (I)GD symptoms was assessed in light of the APA and the WHO diagnostic frameworks. Given the existence of high levels of comorbidity between depressive disorder and autism spectrum disorder in disordered gaming (e.g. Li et al., 2019; Xu et al., 2020), the potential confounding effects for these two psychopathologies were controlled in the present study using the Beck Depression Inventory-II (BDI-II) and the Autism-Spectrum Quotient (ASQ) (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001; Beck, Steer, & Brown, 1996). Subjects meeting the clinical cut-off criteria were excluded and symptom severities in both dimensions were additionally included as covariates in the analyses. For the final sample, the following exclusion criteria were applied (1) history or current psychiatric disorders according to DSM-5 (validated by structured clinical interviews at the time of MRI assessment), (2) pathological levels of depressive (BDI-II ≥28) and autistic (ASQ ≥ 30) symptoms, (3) history of, or current medical disorder, including neurological and endocrinological disorders, (4) current or regular use of medication and other psychoactive substances, and (5) left-handedness. Of the eligible participants, a total of n = 82 individuals (age at MRI assessment = 21.8 ± 2.16 years) provided complete self-report and MRI data, and were thus included in the subsequent voxel-based morphometry (VBM) analysis (exclusion of subjects detailed in Fig. 1).
Fig. 1.
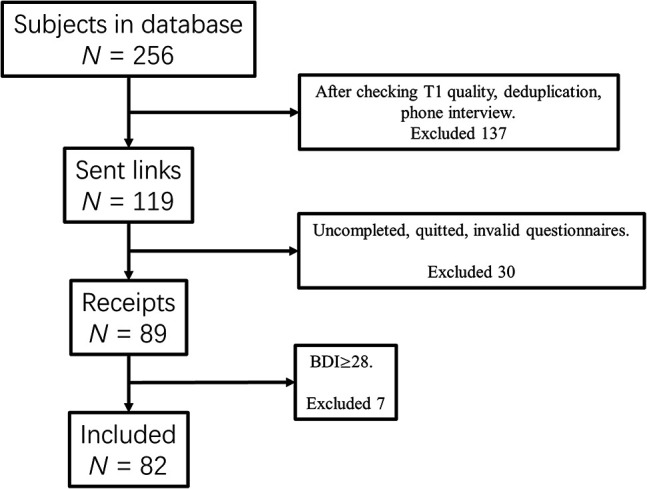
Flow diagram displaying screening and exclusion of participants.
Disordered Gaming symptom load assessment
In the present study Chinese versions of the IGDS9-SF (Yam et al., 2019) and the GDT (Pontes et al., 2019) were administered. The IGDS9-SF assesses IGD according to the APA framework using nine items answered on a five-point Likert scale (from 1 = never to 5 = very often). Moreover, the GDT consists of four items assessing GD according to the WHO framework on a five-point Likert scale (from 1 = never to 5 = very often). Answering with often or very often on the two tests indicates endorsement of the corresponding diagnostic criterion. Consequently, fulfilling five out of nine criteria on the IGDS9-SF and fulfilling all four criteria on the GDT indicates disordered gaming. Internal consistencies were satisfying for both tests (IGDS9-SF α = 0.845 and GDT α = 0.912) in the present sample.
MRI data acquisition
The data were acquired on a 3.0 T GE MR750 system (General Electric Medical Systems, Milwaukee, WI, USA). T1-weighted high-resolution anatomical images were acquired with a spoiled gradient echo pulse sequence, repetition time (TR) = 6 ms, echo time (TE) = 2 ms, flip angle = 9°, field of view (FOV) = 256 × 256 mm, acquisition matrix = 256 × 256, thickness = 1 mm, number of slices = 156.
Brain structural data – preprocessing
VBM analysis allows the voxel-wise estimation of the local gray matter volume (Ashburner & Friston, 2005). Structural MRI data were preprocessed with CAT12 implementing the computational anatomy approach (http://dbm.neuro.uni-jena.de/cat) based on SPM12 (Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12) running on MATLAB Version 8.3 (Math Works Inc., Natick, MA). The standard VBM preprocessing protocols of CAT12 (outlined in the CAT12 manual) were employed. Briefly, the T1-weight images were bias-corrected, segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) and spatially normalized to the standard Montreal Neurological Institute (MNI) space using the East Asian template. GM images were smoothed with a Gaussian kernel of 8 mm full-width at half maximum (FWHM) for subsequent statistical analysis and the total intracranial volume (TIV) was estimated to correct for individual differences in brain size. Default parameters were applied unless indicated otherwise. After preprocessing all images passed visual inspection artefacts and an automated quality protocol. Mean Image Quality Rating (IQR) of the final data was B- (84.37%) summarized by the quality assurance framework of CAT12, which allows the evaluation of essential image parameters such as noise, inhomogeneities, and image resolution, indicating excellent image quality in the present sample.
Statistical analyses
Multiple linear regression models were employed in the Statistical non-Parametric Mapping toolbox (SnPM13, http://www.warwick.ac.uk/snpm) based on 10,000 random permutations, taking GM maps as dependent variables and IGDS9-SF and GDT scores respectively as independent variable (similar approach see Li et al., 2019). Although false positive rates in VBM are not strongly influenced by sample size, a non-parametric estimation has been demonstrated as robust against non-normal distribution of the underlying data and might be more robust independent of sample size (Scarpazza, Tognin, Frisciata, Sartori, & Mechelli, 2015; Silver, Montana, Nichols, & Alzheimer's Disease Neuroimaging, 2011).
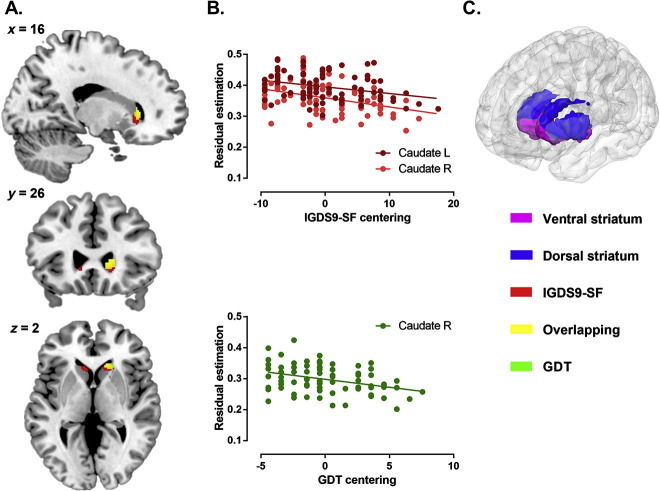
For both multiple linear regression models, age, education level, BDI-II, ASQ, and TIV were additionally included as covariates. In line with the regional-specific a priori hypothesis on striatal associations, voxel-wise analyses were restricted to the striatum (Fig. 2C) encompassing both bilateral ventral and dorsal striatal subregions (for a description of the masks see Zhou et al., 2019). Within the striatal mask, a voxel level threshold of p < 0.05 with FWE multiple comparison correction adjusted for the search volume was applied. To further control for the potential effects of time between the brain structural assessments and the assessment of (I)GD symptoms, the time between assessments was controlled for in additional control analyses.
Fig. 2.
Lower striatal volume with (I)GD symptom severity. A) Associations between symptom load and brain structure displayed at pFWE <0.05 within the striatum (IGDS9-SF = red, GDT = green, and overlapping = yellow). B) Extracted gray matter from the significant regions and associations with symptom severity. C) The striatal mask, including ventral and dorsal regions as used in the present study.
Ethics
The study and its procedures had full approval by the local ethics committee and adhered to the most recent version of the Declaration of Helsinki and all participants were required to provide informed consent.
Results
Demographic characteristic of participants
The present sample reported a mean (±SD) symptom severity of = 18.4 ± 6.53 for the IGDS9-SF and 8.43 ± 3.07 for the GDT. Moreover, the mean trait score for depression (BDI-II) was 6.26 ± 6.98, and for autism (ASQ) was 13.7 ± 3.77. Mean total intracranial volume was 1,563 ± 107, and education level ranged from high school graduate to master's degree student. Categorial analysis of the symptom-level data revealed that in terms of disordered gaming, n = 3 subjects fulfilled the diagnostic criteria for disordered gaming on the APA framework (IGDS9-SF) and n = 1 fulfilled all criteria in the WHO framework (GDT).
(I)GD symptom load and striatal gray matter volumes across the APA and WHO diagnostic frameworks
In line with the regional a priori hypothesis the analysis focused on the bilateral striatum using a small-volume approach. Higher IGDS9-SF symptom severity was significantly associated with lower gray matter volume in the bilateral caudate (pFWE <0.05), whereas higher GDT symptom severity was associated with lower gray matter volume in the right caudate only (pFWE <0.05) (Table 1, Fig. 2A and B). Given the distinct contributions of the ventral and dorsal striatum to addiction (Zhou et al., 2019), significant effects were further mapped and overlapped (Fig. 2A), revealing that the IGDS9-SF and GDT shared 114 voxels in caudate volume. Additionally, for the IGDS9-SF 244 voxels mapped onto the ventral and 88 voxels mapped to the dorsal striatum. For the GDT, 53 voxels mapped onto the ventral and 65 on the dorsal striatum.
Table 1.
Significant negative associations between gray matter volume and the IGDS9-SF and GDT respectively
| Region | k (Cluster Size) | x | y | z | t Value |
| IGDS9-SF, negative associations | |||||
| Caudate R | 207 | 14 | 26 | 3 | 4.23 |
| 23 | 24 | 9 | 3.87 | ||
| Caudate L | 103 | −11 | 24 | −2 | 4.11 |
| −6 | 21 | 5 | 3.87 | ||
| Caudate L | 22 | −18 | 21 | 12 | 4.04 |
| GDT, negative associations | |||||
| Caudate R | 118 | 15 | 27 | 5 | 4.32 |
R = right, L = left.
Associations between (Internet) Gaming Disorder symptom load and striatal gray matter volumes across the APA and WHO diagnostic frameworks were additionally controlled for variations in the time between brain structural data acquisition and questionnaire assessment. To this end the identical statistical analysis pipeline was conducted with additionally including time between the assessments as additional covariate in the multiple regression model.
Findings revealed that higher IGDS9-SF symptom severity was significantly associated with lower gray matter volume in the bilateral caudate (pFWE <0.05, right caudate peak at [12, 26, 2], k = 205, left peak at [−9, 23, 0] and [−18, 21, 12], k = 128 and 15), whereas higher GDT symptom severity was associated with lower gray matter volume in the right caudate (pFWE <0.05, peak at [15, 27, 5], k = 76).
Discussion
In line with the study's hypothesis, the findings from this initial examination of the recently proposed (I)GD criteria revealed that stronger symptom-load across both diagnostic frameworks was associated with lower striatal gray matter volume, primarily in the caudate region. The caudate bridges the ventral and dorsal striatum and is strongly implicated in addiction-relevant domains, with ventral regions being implicated in the initial formation of reward-related salience and associative learning processes (Anderson et al., 2017) whereas dorsal parts mediate habit formation (Everitt & Robbins, 2016). Exaggerated cue-reactivity in the ventral striatum has not only been consistently observed in substance use disorders but also in individuals without substance use disorders (Vollstadt-Klein et al., 2010; Zhou et al., 2019). This finding provides initial and preliminary evidence for a neurobiological correlate of the proposed (I)GD criteria in the classification systems and further suggests that development of (I)GD may be accompanied by progressive neurobiological changes in brain motivational systems that have been suggested to mediate the development of established addictive disorders, including substance use disorders and pathological gambling (Clark et al., 2019; Koob & Volkow, 2016).
Although (I)GD criteria in both frameworks aligned with the respective core substance-based disorder diagnoses, the specific symptom criteria differ slightly. For scientists and practitioners alike, a key issue is related to diagnosing essentially the same condition using the two diagnostic frameworks (APA vs. WHO) and potential neurobiological discrepancies between them. The findings obtained suggest that the APA framework may capture more extensive striatal, particularly ventral striatal regions. The ventral striatum mediates dysregulations in reward-related processes during early, whereas dorsal regions involved in habit formation mediates compulsive use during later stages of the addictive process (Everitt & Robbins, 2016). The discrepancies in the neurobiological associations may reflect a higher sensitivity of the APA framework to detect early stages of disordered gaming, which aligns with the higher prevalence of IGD according to APA criteria in the present sample (see also similar results in a large-scale study Montag et al., 2019).
Previous meta-analytic studies (Qin et al., 2020; Yao et al., 2017) revealed that hyperactivation of the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, caudate and precuneus and lower gray matter volume of the putamen, anterior cingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, and supplementary motor characterize (I)GD. A few previous studies that employed whole brain and region of interest analyses have also reported increased caudate volume in (I)GD (Cai et al., 2016; Seok & Sohn, 2018), however the small sample size and differences in the diagnostic criteria and potential confounders limit the comparability between studies. Moreover, these studies employed traditional case-control designs which are often hampered by limitations inherent to the design such as generally increased pathological symptom load, stress or alterations reflecting vulnerability factors that precede the onset of the disorder (Etkin, 2019). To this end, the present study employed the official diagnostic criteria for disordered gaming in combination with a dimensional neuroimaging approach (Li et al., 2019).
The observed association with volumes of the putamen resonate with overarching conceptualizations of the development of substance use disorder and IGD and may reflect the progression towards more clinical stages of (I)GD (Brand et al., 2019; Everitt & Robbins, 2016). On the one hand, the findings in healthy subjects will reflect changes during early stages of Internet gaming, which are still characterized by incentive-driven motives and probably an initial transfer to habitual behavior (specifically for the IGDS9-SF framework more items related to incentive aspects and may thus captured more ventral striatal alterations). On the other hand, the caudate and putamen are both also implicated in motor function (Ena, De Kerchove D'ExaErde, & Schiffmann, 2011), therefore the assumption that the association between symptom load and caudate volume may additionally reflect effects of gaming time cannot be ruled out. In summary, in the context of addictive behavior, the present findings may provide a preliminary approach to explore early stages of habitual control over behavior in IGD, yet long-term studies are needed to examine whether the striatal volume changes determine the transition to IGD.
In this context, it should be mentioned that the IGD criteria according to the DSM-5 seem to grasp disordered gaming earlier than the criteria for GD proposed in the ICD-11 (Montag et al., 2019). As aforementioned, first prevalence estimates of disordered gaming in a German sample were higher when applying the framework of APA compared to WHO. This might be due to the inherent diagnostic differences across the two disordered gaming frameworks as according to the APA five out of fine symptoms are needed for a disordered gaming diagnosis (every constellation is possible), whereas according to the WHO framework all criteria proposed need to be met (Table 2). Some symptoms such as lying about one's own gaming habits and using games to escape negative mood can only be found in the APA framework. An overlap can be seen for disordered gaming symptoms such as preoccupation/increased priority given to gaming, loss of control, engaging in gaming despite negative consequences, and significant impairments due to gaming in everyday life.
Table 2.
Comparison of (I)GD criteria between the APA and WHO framework
| APA framework | WHO framework | |
| Diagnosis | Five or more of these symptoms within a year | All symptoms for at least 12 months |
| Symptom |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
||
|
||
|
||
|
Original wording taken from APA: https://www.psychiatry.org/patients-families/internet-gaming.
Original wording taken from WHO: https://www.who.int/news-room/q-a-detail/gaming-disorder.
The findings of the present study need to be considered in the context of potential limitations. First, the present study applied a dimensional approach in a healthy sample and future studies need to extent to the findings reported to clinical samples with (I)GD according to the current official diagnostic criteria while long-term studies are needed to investigate the association between striatal volume and escalation of Internet gaming and subsequent transition to disordered gaming. Second, since disordered gaming is strongly intervened with the acquisition of new skills, particularly in the motor domain, future studies need to distinguish between neuroplastic changes related to motor learning or the addictive process (e.g. by inclusion of a group of healthy, yet non-disordered, gamers). Third, the study excluded subjects with pathological levels of depression. Together with the inclusion of depression levels as covariate, this allowed controlling at some extent for potential confounding effects. However, this approach may limit the generalizability of the findings.
Despite the potential limitations, the present study is to the best of the authors' knowledge, the first to examine disordered gaming within a comparative framework between the APA and WHO diagnostic framework in the context of MRI. Additionally, the findings reported provide initial evidence for a neurobiological basis of the official disordered gaming diagnostic criteria in accordance with both current diagnostic systems and suggest that the proposed criteria may reflect dysregulations in the motivational systems of the brain.
Funding sources
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0701400), National Natural Science Foundation of China (NSFC, No. 91632117, 31530032); Fundamental Research Funds for Central Universities (ZYGX2015Z002), Science, Innovation and Technology Department of the Sichuan Province (2018JY0001). The position of CM was funded by a Heisenberg grant awarded to him by the German Research Foundation (DFG, MO2363/3-2).
Authors' contribution
XZ, BB, and CM conceptualized, designed, and wrote the initial protocol and draft. XZ conducted and validated the formal analysis. RW, CL, JK, and YC carried out the investigation and MRI acquisition. HMP, DY, KK, BB, and CM reviewed and revised the manuscript. BB and CM obtained funding and administrated the project. All authors contributed to and approved the final version of the manuscript, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors report no conflict of interest.
Contributor Information
Benjamin Becker, Email: ben_becker@gmx.de.
Christian Montag, Email: christian.montag@uni-ulm.de.
References
- Anderson, B. A., Kuwabara, H., Wong, D. F., Roberts, J., Rahmim, A., Brasic, J. R., et al. (2017). Linking dopaminergic reward signals to the development of attentional bias: A positron emission tomographic study. Neuroimage, 157, 27–33. 10.1016/j.neuroimage.2017.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Becker, B., Wagner, D., Koester, P., Tittgemeyer, M., Mercer-Chalmers-Bender, K., Hurlemann, R., et al. (2015). Smaller amygdala and medial prefrontal cortex predict escalating stimulant use. Brain, 138(Pt 7), 2074–2086. 10.1093/brain/awv113. [DOI] [PubMed] [Google Scholar]
- Brand, M., Wegmann, E., Stark, R., Müller, A., Wölfling, K., Robbins, T. W., et al. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience & Biobehavioral Reviews, 104, 1–10. [DOI] [PubMed] [Google Scholar]
- Cai, C., Yuan, K., Yin, J., Feng, D., Bi, Y., Li, Y., et al. (2016). Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging and Behavior, 10(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Clark, L., Boileau, I., & Zack, M. (2019). Neuroimaging of reward mechanisms in gambling disorder: An integrative review. Molecular Psychiatry, 24(5), 674–693. 10.1038/s41380-018-0230-2. [DOI] [PubMed] [Google Scholar]
- Ena, S., De Kerchove D'ExaErde, A., & Schiffmann, S. N. (2011). Unraveling the differential functions and regulation of striatal neuron sub-populations in motor control, reward, and motivational processes. Frontiers in Behavioral Neuroscience, 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. (2019). A reckoning and research agenda for neuroimaging in psychiatry. American Journal of Psychiatry, 176(7), 507–511. [DOI] [PubMed] [Google Scholar]
- Everitt, B. J., & Robbins, T. W. (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annual Review of Psychology, 67, 23–50. 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry, 3(8), 760–773. 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Xu, L., Zheng, X., Fu, M., Zhou, F., Xu, X., et al. (2019). Common and dissociable contributions of alexithymia and autism to domain-specific interoceptive dysregulations: A dimensional neuroimaging approach. Psychotherapy and Psychosomatics, 88(3), 187–189. 10.1159/000495122. [DOI] [PubMed] [Google Scholar]
- Montag, C., Markowetz, A., Blaszkiewicz, K., Andone, I., Lachmann, B., Sariyska, R., et al. (2017). Facebook usage on smartphones and gray matter volume of the nucleus accumbens. Behavioural Brain Research, 329, 221–228. [DOI] [PubMed] [Google Scholar]
- Montag, C., Schivinski, B., Sariyska, R., Kannen, C., Demetrovics, Z., & Pontes, H. M. (2019). Psychopathological symptoms and gaming motives in disordered gaming-A psychometric comparison between the WHO and APA diagnostic frameworks. Journal of Clinical Medicine, 8(10), 1691. 10.3390/jcm8101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes, H. M., & Griffiths, M. D. (2015). Measuring DSM-5 internet gaming disorder: Development and validation of a short psychometric scale. Computers in Human Behavior, 45, 137–143. 10.1016/j.chb.2014.12.006. [DOI] [Google Scholar]
- Pontes, H. M., Schivinski, B., Sindermann, C., Li, M., Becker, B., Zhou, M., et al. (2019). Measurement and conceptualization of gaming disorder according to the world health organization framework: The development of the gaming disorder test. International Journal of Mental Health and Addiction. 10.1007/s11469-019-00088-z. [DOI] [Google Scholar]
- Qin, K., Zhang, F., Chen, T., Li, L., Li, W., Suo, X., et al. (2020). Shared gray matter alterations in individuals with diverse behavioral addictions: A voxel-wise meta-analysis. Journal of Behavioral Addictions, 9(1), 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, T. W., Ersche, K. D., & Everitt, B. J. (2008). Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences, 1141, 1–21. [DOI] [PubMed] [Google Scholar]
- Scarpazza, C., Tognin, S., Frisciata, S., Sartori, G., & Mechelli, A. (2015). False positive rates in voxel-based morphometry studies of the human brain: Should we be worried? Neuroscience & Biobehavioral Reviews, 52, 49–55. 10.1016/j.neubiorev.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Seok, J. W., & Sohn, J. H. (2018). Altered gray matter volume and resting-state connectivity in individuals with Internet gaming disorder: A voxel-based morphometry and resting-state functional magnetic resonance imaging study. Frontiers in Psychiatry, 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M., Montana, G., Nichols, T. E., & Alzheimer's Disease Neuroimaging, I. (2011). False positives in neuroimaging genetics using voxel-based morphometry data. NeuroImage, 54(2), 992–1000. 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstadt-Klein, S., Wichert, S., Rabinstein, J., Buhler, M., Klein, O., Ende, G., et al. (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105(10), 1741–1749. 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Xu, X., Dai, J., Liu, C., Chen, Y., Xin, F., Zhou, F., et al. (2020). Common and disorder-specific neurofunctional markers of dysregulated empathic reactivity in major depression and generalized anxiety disorder. Psychotherapy and Psychosomatics, 89(2), 114–116. 10.1159/000504180. [DOI] [PubMed] [Google Scholar]
- Yam, C. W., Pakpour, A. H., Griffiths, M. D., Yau, W. Y., Lo, C. L. M., Ng, J. M., et al. (2019). Psychometric testing of three Chinese online-related addictive behavior instruments among Hong Kong university students. Psychiatric Quarterly, 90(1), 117–128. 10.1007/s11126-018-9610-7. [DOI] [PubMed] [Google Scholar]
- Yao, Y. W., Liu, L., Ma, S. S., Shi, X. H., Zhou, N., Zhang, J. T., et al. (2017). Functional and structural neural alterations in Internet gaming disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 83, 313–324. 10.1016/j.neubiorev.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Zastrow, M. (2017). Correction for Zastrow, News Feature: Is video game addiction really an addiction? Proceedings of the National Academy of Sciences of the U S A, 114(21), E4316. 10.1073/pnas.1707226114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F., Zimmermann, K., Xin, F., Scheele, D., Dau, W., Banger, M., et al. (2018). Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis‐dependent males. Human Brain Mapping, 39(12), 5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., Zimmermann, K., Xin, F., Zhao, W., Derckx, R. T., Sassmannshausen, A., et al. (2019). Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(8), 751–762. 10.1016/j.bpsc.2019.04.006. [DOI] [PubMed] [Google Scholar]