Abstract
Dorsal root ganglion stimulation (DRGS) is a neuromodulation therapy for chronic pain that is refractory to conventional medical management. Currently, the mechanisms of action of DRGS-induced pain relief are unknown, precluding both our understanding of why DRGS fails to provide pain relief to some patients and the design of neurostimulation technologies that directly target these mechanisms to maximize pain relief in all patients. Due to the heterogeneity of sensory neurons in the dorsal root ganglion (DRG), the analgesic mechanisms could be attributed to the modulation of one or many cell types within the DRG and the numerous brain regions that process sensory information. Here, we summarize the leading hypotheses of the mechanisms of DRGS-induced analgesia, and propose areas of future study that will be vital to improving the clinical implementation of DRGS.
Keywords: dorsal root ganglion stimulation, electric stimulation, chronic pain, neuropathic pain, implanted neurostimulators
Introduction
Chronic pain remains one of the world’s largest public health challenges, affecting hundreds of millions of people throughout the world.66 Neurostimulation therapies are important tools in a pain physician’s clinical toolbox to manage chronic pain conditions that are refractory to conventional medical management. Spinal cord stimulation (SCS) has been a mainstay neurostimulation therapy for chronic pain for more than 50 years, and is an effective tool in managing intractable neuropathic pain in the lower limbs.73 Despite the overall success of SCS in treating many chronic pain conditions (e.g., failed back surgery syndrome), pain in specific areas, such as the groin, foot, low back, and knee, are difficult to accurately target with SCS. The complex anatomy of the spinal column, posture-related motion of the spinal cord in the thecal sac, and shunting of electrical current in the cerebrospinal fluid can limit the delivery of stimulation to the target fibers within the spinal cord.67 Therefore, patients with intractable pain in regions that are difficult to target with SCS are often left with few alternatives, presenting a large need for innovations in neurostimulation for pain management.
Dorsal root ganglion stimulation (DRGS) is a novel neurostimulation therapy for managing medically refractory chronic pain.25 As a single dorsal root ganglion (DRG) receives sensory information from a discrete region of the body, it was hypothesized that DRGS could be an effective strategy for managing pain in regions that are difficult to target with SCS. DRGS was approved by the United States Food and Drug Administration (FDA) in 2016 for the treatment of refractory complex regional pain syndrome (CRPS) in the lower limbs.27 Despite currently only having FDA approval for CRPS in the lower limbs, DRGS has shown promise in managing other pain etiologies, such as painful diabetic neuropathy,37 phantom limb pain,36 and groin pain.88 The ACCURATE clinical trial compared the safety and efficacy of SCS and DRGS in treating patients with intractable CRPS at 3 and 12 months post-implantation, and demonstrated that more patients were considered treatment successes (i.e., received ≥ 50% reduction in pain intensity) with DRGS than SCS.26 However, a 50% reduction in pain intensity (measured via the visual analog scale (VAS)) does not always lead to an improved quality of life, or facilitate a patient returning to their activities of daily living.57 Furthermore, more than a quarter of patients were not deemed treatment successes with DRGS, leaving them with few other pain management options because neurostimulation therapies are often a last-resort therapy in a clinician’s treatment algorithm.80 Presently, we do not have a clear scientific understanding of the physiological mechanisms of DRGS-induced pain relief. We believe that it is vital to elucidate these mechanisms, to: 1) understand why DRGS fails in some patients, to improve patient selection, and 2) innovate DRGS technologies to specifically target these mechanisms, to maximize pain relief in all patients.
Recently, there was an excellent review of human DRG anatomy;51 therefore, we will highlight only the anatomy essential to understanding DRGS. The DRG is a swelling in the dorsal spinal root, which houses the cell bodies of all primary sensory neurons (PSNs) (Fig. 1) innervating a specific dermatome (i.e., region of the body). There are bilateral pairs of DRG at each vertebral level (Fig. 1A), which receive information from roughly the same dermatome on opposite sides of the body. All DRG are encased by the meninges of the spinal cord as the meninges transition into the epineurium surrounding peripheral nerves.15 DRG reside in the neuroforamina (Fig. 1B), but the relative position of the DRG within the foramen may vary depending on the spinal level.54 During the implantation of a DRGS system, electrode lead bodies are percutaneously inserted using a Touhy needle, guided through the epidural space of the spinal column using X-ray fluoroscopy, and routed into the intraforaminal space where the array of electrode contacts are placed along the dorsal side of the DRG (Fig. 1A,B). The electrode lead(s) are connected to an implanted pulse generator, which resides in a body cavity usually around the posterior lateral flank.28
Figure 1:
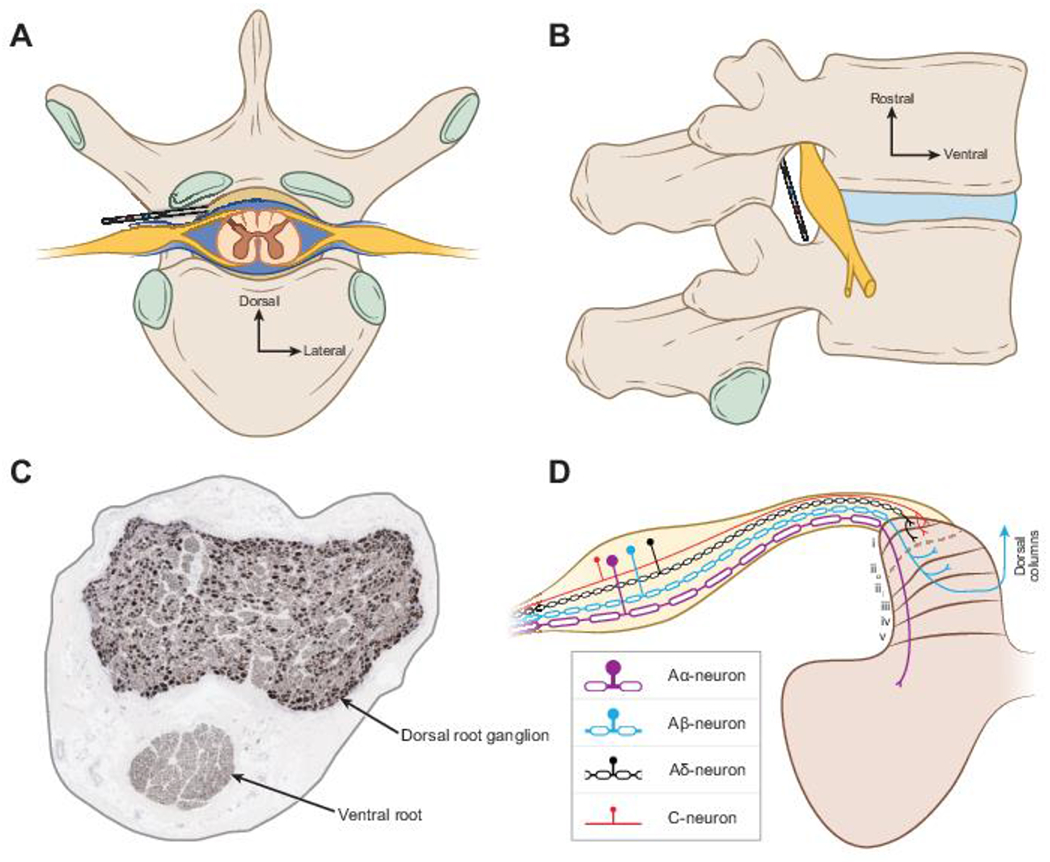
DRGS and surrounding anatomy. A) Axial view of a human spinal column with a DRGS electrode array in place. B) Sagittal view of a DRGS electrode array in the foramen. C) Histology image of a human lumbar DRG stained for 200 kDa neurofilament.115 Axons appear as miniscule dots; cell bodies appear as larger dark spots. D) Sensory neuron types in the DRG and their projections into the spinal cord. The first five lamina of the dorsal horn are labeled to indicate where different sensory neuron types send axon collaterals.
Extracellular electrical stimulation, like that utilized by DRGS, can affect different neural structures (e.g., cell bodies, axons, presynaptic terminals) depending on the parameters of the stimulus pulse (e.g., amplitude, frequency, pulse width, polarity).78 Therefore, the morphological characteristics of DRG neurons, and their location in the DRG relative to the stimulating electrodes, will greatly affect which cells are being modulated by DRGS. Clinically, DRGS is typically applied with a bipolar stimulation configuration and a tonic pulse train with a pulse frequency around 20 Hz, a pulse duration between 200 and 300 μs, and a pulse amplitude on the order of several hundred μA to a few mA.28 DRGS four-contact cylindrical electrode arrays are 1 mm in diameter with 1.25 mm long contacts, comparatively smaller than traditional SCS cylindrical electrode arrays.6 The electrode is typically placed within the foramen such that the second and third contacts span the pedicle, with the second and third contacts typically assigned as the cathode and anode, respectively.28 PSNs, the cells within the DRG, are pseudounipolar, i.e., their cell bodies have a single axon process called the stem axon, which bifurcates at a region called the T-junction into an axon that projects to the spinal cord (centrally projecting axon) and an axon that projects to the peripheral nervous system (peripherally projecting axon) (Fig. 1D).29 Recent histological results demonstrated that in human lumbar DRG, cell bodies typically organize around the dorsomedial region of the ganglion, while axons of passage are more homogeneously distributed throughout the rest of the ganglion.115 DRG cytoarchitecture is of great importance to the clinical implementation of DRGS (Fig. 1C). As distance from the stimulating electrode increases, extracellular potentials exponentially decrease, and therefore DRGS-induced activity may be focused on the most superficial neural elements. Such knowledge is crucial to designing stimulus waveforms that target the neural elements and PSN subtypes responsible for the pain-relieving effects of DRGS.
PSNs are a diverse class of neurons, with different types of neurons conveying different sensory modalities (e.g., touch, pain, itch). PSN axons are commonly classified as A-fibers (myelinated axons) and C-fibers (nonmyelinated axons). In this review, we will refer to DRG neurons with myelinated axons as A-neurons, and DRG neurons with nonmyelinated axons as C-neurons. C-neurons are traditionally thought to be nociceptors, though it is known that C-neurons consist of several molecularly defined subpopulations that convey a myriad of sensations, including innocuous or painful touch,70 innocuous and painful thermal sensations,55,127 chemical itch,108 pleasant touch,75 and some C-neurons code multiple types of sensations (i.e., polymodal C-neurons).70,127 A-neurons are further stratified into Aα-, Aβ-, and Aδ-neurons. Aα-neurons are thickly myelinated, large-diameter muscle afferents, and can be further classified as group Ia neurons, which innervate muscle spindles and code muscle stretch, and Ib neurons which innervate golgi tendon organs and code muscle tension.13 Aβ-neurons are also thickly myelinated, large-diameter afferents, though they are smaller in diameter than Aα-neurons. Aβ-neurons typically convey non-painful tactile stimuli,1 though there have been reports of nociceptive signals conducted in the Aβ-fiber conduction velocity range,8,32 and a subset of Aβ-neurons are important in transmitting mechanical itch.93 Aδ-neurons are thinly myelinated, medium-diameter afferents, that typically convey innocuous or painful touch31 or thermal sensations.55 The peripherally-projecting axon of a PSN terminates in a sensory end organ, depending on the type of sensations conveyed by that neuron.1 The centrally-projecting axon enters the spinal cord (Fig. 1D), where it can send collaterals into the grey matter to form excitatory synapses with spinal neurons (e.g., interneurons in the dorsal horn), enter a white matter tract and project caudally or rostrally (e.g., to the brain stem), or project to both spinal and supraspinal targets.14,119
Previous reviews have covered several aspects of DRGS, including in vivo and in vitro studies of DRGS,125 the DRG as a target for neurostimulation therapies,38,65 and clinical evidence of DRGS efficacy.53 However, to our knowledge, there has not been a review of the evidence supporting the current hypotheses of the physiological mechanisms of DRGS-induced pain relief. Therefore, our goal in this review is to summarize the evidence supporting the current hypotheses of DRGS mechanisms, and to provide an outlook on the scientific insight needed to facilitate technological innovations that will improve the efficacy of DRGS. We consider both the direct neural response to DRGS (i.e., the neuronal processes that are transiently modulated by DRGS-generated electric fields) and the indirect effects of DRGS (e.g., postsynaptic activation of neural circuits in the central nervous system, modulating the activity of non-neuronal targets). Due to the novelty of DRGS, there are few studies of the indirect effects of DRGS; therefore, much of our discussion will focus on the direct neural response to stimulation. We conclude by suggesting future research avenues towards painting the holistic picture of DRGS mechanisms.
Direct neural response to DRGS
Similar to other clinical neurostimulation therapies, DRGS interfaces with the body by generating spatiotemporally varying electric fields. These electric fields can perturb a cell’s transmembrane voltage, and can lead to the opening or closing of voltage-sensitive ion channels. Stimuli of sufficient intensity can induce an action potential (AP), while subthreshold stimuli would result in a transient change in membrane potential that does not induce an AP. However, it is possible that subthreshold stimuli have modulatory effects, such as integrating or disrupting ongoing neural activity, or by influencing voltage-sensitive channels whose dynamic ranges are below the AP threshold. In this paper, we define the direct neural response to DRGS as any voltage-sensitive neurophysiological process that is induced, prevented, or characteristically altered by the transient electrical stimuli generated by DRGS. This definition includes effects, such as generating APs in a particular cell type leading to neurotransmitter release from presynaptic terminals or intracellular second messenger systems that are triggered by voltage-sensitive processes (e.g., calcium influx through voltage-sensitive calcium channels). We believe that there are three primary hypotheses on the direct neural response to DRGS: 1) the driving of feed-forward pain-inhibition circuitry, 2) augmenting low-pass filtering mechanisms at the T-junction of PSNs, and 3) suppressing the hyperexcitability of PSNs generated by chronic pain states.
Driving input into pain-gating networks
In their seminal paper in 1965, Melzack and Wall proposed the gate control theory of pain,83 which states that activating large-diameter tactile afferents gates pain signals to the brain by driving inhibitory networks in the spinal cord, while also being modulated by top-down control from supraspinal structures. Only two years later, Wall and Sweet used this theory to demonstrate temporary analgesia in humans by electrically stimulating peripheral nerves, and Shealy et al. developed the first clinical usage of SCS.109,126 By stimulating the large diameter afferents in peripheral nerves and the dorsal columns of the spinal cord, respectively, these groups demonstrated the ability of exogenous electric fields to drive pain inhibition in humans, putatively by artificially driving feed-forward pain-inhibition networks in the spinal dorsal horn.84 As many of the Aβ-axons that constitute the dorsal columns originate in the DRG,90 and extracellular electrical stimulation preferentially activates large-diameter myelinated axons over small-diameter nonmyelinated axons,99,101 DRGS may share mechanisms of action with SCS.
Computational modeling is an important tool in uncovering the mechanisms of neurostimulation therapies.20 Bourbeau and colleagues used a combined biophysical and analytical model to study activation thresholds during microstimulation of the DRG with penetrating microelectrodes.12 Their results suggest myelinated afferent activation thresholds would be on the order of microamperes, and that microstimulation can preferentially activate small-diameter myelinated afferents over large-diameter myelinated afferents. This finding is contrary to conventional understanding that larger-diameter axons have lower activation thresholds than smaller-diameter axons.79 The authors suggested that this selectivity for small-diameter axons was due to smaller axons having more closely spaced nodes of Ranvier, increasing the probability that a node would be present near the penetrating microelectrode. However, their modeling framework was not designed to study clinical DRGS performed with non-penetrating macroelectrodes placed in the intraforaminal tissue, and it did not account for factors, such as the anatomy surrounding the DRG, which would affect the electric field delivered to DRG neurons. Furthermore, that study only modeled the axons in the DRG, assuming that cell bodies would not be excitable by extracellular stimulation, and it did not examine the effects on C-neurons. It is currently unclear if this selectivity is achievable with extragangliar electrodes, like those used in clinical DRGS.
Work from our laboratory implemented a field-cable modeling approach to study clinical DRGS, and compared the activation thresholds of Aβ-neurons with C-neurons.48 We showed that when applying DRGS with clinical leads and stimulation parameters, DRGS drives the activity of Aβ-neurons without affecting the activity of C-neurons (Fig. 2). Our follow-up study further examined the effect of DRGS on Aα- and Aδ-neurons.49 We showed that with clinical stimulation parameters, DRGS may activate Aδ-neurons that sense innocuous touch, without activating Aδ-neurons that sense mechanical pain. Furthermore, we showed that Aα-neurons may be widely activated during DRGS, but their low population within the DRG makes it difficult to determine their extent of activation in clinical scenarios.115 However, our models did not account for calcium channels or dynamics in DRG neurons, which may affect a neuron’s activation threshold, and likely affects the DRGS-influenced neural dynamics which operate on time-scales lasting seconds or longer,45 suggesting that additional mechanisms might be at play. Though computer models provide an excellent framework with which to probe the mechanisms of DRGS, there are several limitations to the computational approach, such as simplified cellular morphologies, and a lack of experimental data (e.g., spatiotemporal ion channel expression profiles) from which to build the models.
Figure 2:
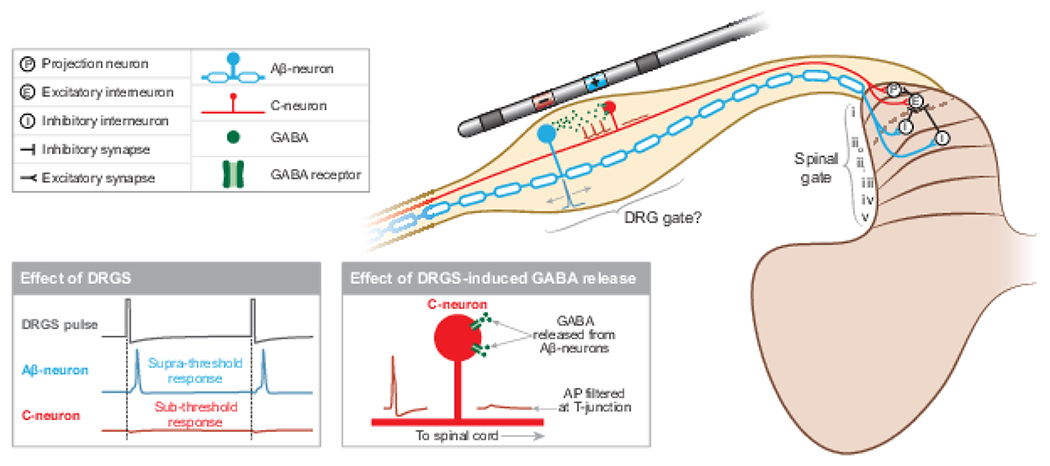
DRGS may drive pain-gating mechanisms in the spinal cord dorsal horn, the DRG, or both. DRGS applies trains of electrical pulses which induce APs in Aβ-neurons, which activate inhibitory interneurons in lamina iii and iii in the dorsal horn. Concurrently, Aβ-neurons may release GABA within the DRG, which can act on C-neurons and potentially prevent ectopic APs from propagating to the spinal cord.
Currently, there are a few in vivo studies of DRGS mechanisms, specifically in the context of neurostimulation for pain. However, these initial studies have provided interesting results. Using a rat model of DRGS, Chao and colleagues confirmed our modeling predictions that Aβ-neurons have the lowest activation thresholds during DRGS.22 Koetsier and colleagues showed that in rats, DRGS did not affect intracellular levels of gamma-aminobutyric acid (GABA) in the spinal cord dorsal horn, suggesting DRGS does not drive inhibitory circuitry in the spinal cord.62 Instead, the authors suggested that DRGS may drive GABAergic pain-gating circuits local to the DRG. Du and colleagues recently found that many DRG neurons possess the cellular machinery necessary to synthesize and release GABA, including large-diameter 200 kDa neurofilament-positive neurons, such as Aβ-neurons.33 They also demonstrated that optogenetically depolarizing these neurons caused behavioral changes indicative of reduced acute and chronic pain. Therefore, it is possible that clinical DRGS directly activates myelinated afferents, leading to a release of GABA within the DRG itself to inhibit pain perception (Fig. 2). However, these findings are preliminary, and more work is needed to understand the physiological consequences of in vivo GABA release in DRG, and whether clinical DRGS is capable of inducing somatic GABA release.
Furthermore, these data have not ruled out pain-gating inhibition in the dorsal horn as a mechanism of DRGS. Alternatively, DRGS may drive the release of other inhibitory neurotransmitters, such as glycine, in the dorsal horn. Aβ-neurons in the DRG have axonal projections to lamina iii and iii of the dorsal horn,14 where they form feed-forward circuits with glycinergic interneurons that gate mechanical allodynia, a common phenotype of neuropathic pain.76 It is possible that DRGS drives the activity of Aβ-projections to the dorsal horn, thereby increasing the glycinergic inhibitory tone in the spinal cord to gate neuropathic pain. Continued study of the complex pain-processing networks in the central nervous system is crucial to fully elucidating how DRGS-generated peripheral inputs reduce pain.
Additional studies have examined DRGS through the lens of other neurorehabilitation therapies, such as controlling bladder function,16 or as a target to provide somatosensory feedback and control in neuroprosthetic systems.9 Though these studies were not designed to provide evidence on the mechanisms of DRGS for pain relief, they still give insight into which types of DRG neurons respond to extracellular stimulation. For example, some studies found that low-amplitude intragangliar stimulation with penetrating microelectrodes elicits antidromic compound action potentials (CAPs) with conduction velocities in the Aβ- to Aα-range.41,43,60 Other studies showed similar findings with an electrode array placed on the surface of the ganglion.89 However, due to the small diameter of Aδ- and C-neuron axons, their APs can be difficult to resolve on CAP recordings, leaving the extent to which small-diameter axons are recruited during DRGS an open question.
Augmenting T-junction filtering
Several sources of experimental data have suggested that T-junction filtering could be a primary mechanism of DRGS-induced analgesia. Following frequency, the maximum frequency train of APs that can be conducted through an axonal branch point, is used as a measure of T-junction filtering in PSNs. Gemes and colleagues showed that in excised rat DRG, A-neurons have significantly larger following frequencies than C-neurons, i.e., A-neurons can transmit higher frequency trains of APs across the T-junction than C-neurons.44 Interestingly, peripheral nerve injury decreased the following frequency in A-neurons, but increased the following frequency in C-neurons, suggesting that in chronic pain states there is a coincident decrease of pain-gating signals and increase of painful signals entering the spinal cord. They also found that in C-neurons, increasing current through calcium-dependent ion channels (e.g., small conductance calcium-activated potassium (SK) channels) reduced the following frequency. This result suggests that a therapy designed to trigger calcium influx into C-neurons may enhance T-junction filtering and block pain signals from entering the central nervous system.
The T-junction, the region where the stem axon bifurcates into a spinally projecting axon and a peripherally projecting axon, is a morphological peculiarity nearly unique to DRG neurons (and the sensory neurons of the trigeminal ganglion).29 The T-junction is a large node of Ranvier,114 and the peripherally projecting axon is typically larger in diameter than the spinally projecting axon, though there may be differences in this diameter mismatch across cell types.50 Furthermore, the cell bodies of DRG neurons have active ion channels, which allow peripherally generated APs to invade the soma.2 These peculiarities can affect the transmission of afferent signals from the peripheral axon to the dorsal root axon. For example, in myelinated afferents, orthodromically propagating APs can generate ‘extra’ spikes in the initial segment, which rebound towards the T-junction and may occlude other orthodromic APs.3 This self-generated occlusion seems to increase the number of short and long inter-spike intervals, while decreasing the number of intermediate inter-spike intervals. In nonmyelinated afferents, combinations of morphological and electrophysiological features, such as stem axon length and slow hyperpolarizing conductances, can produce a low-pass filtering effect on orthodromically propagating APs.117 DRGS may provide analgesia by augmenting this filtering property in the DRG neurons responsible for pain pathophysiology.
The first computational study of clinical DRGS mechanisms examined the effect of DRGS on putatively nociceptive C-neurons. Kent and colleagues used a field-cable modeling approach to show that DRGS generates APs in C-neuron somata, which produces a net hyperpolarization by increasing the current through SK channels (Fig. 3B).58 Somatic hyperpolarization electrotonically hyperpolarized the stem axon and T-junction (Fig. 3C) to an extent that blocked AP propagation into the dorsal root axon (Fig. 3C), both painful APs from the periphery (Fig. 3A) and DRGS-generated APs from the soma. However, to produce the T-junction filtering effect, it was necessary to apply DRGS with a stimulation amplitude greater than 9 mA, which is far greater than typical clinical stimulation amplitudes (1 mA on average).28 It is important to note that anatomical simplifications and parameter selection (e.g., tissue conductivities, ion channel conductances) used in the field-cable model can dramatically affect the thresholds necessary to generate APs in modeled neurons.131 Therefore, future studies should examine which parameters affect the fidelity with which DRGS can augment T-junction filtering.
Figure 3:
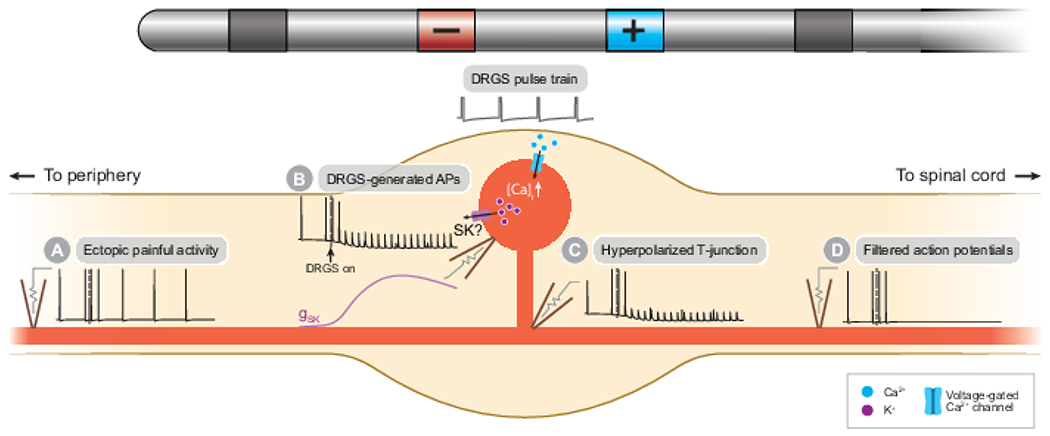
DRGS may augment the low-pass filtering properties of nociceptive C-neurons. A) Ectopic APs indicative of spontaneous pain propagate along the peripheral axons of C-neurons towards the central nervous system. B) The DRGS pulse train induces APs in or near C-neuron somata causing calcium influx through voltage-gated calcium channels, putatively triggering potassium efflux through calcium-activated SK channels. C) Potassium efflux hyperpolarizes the soma, which electrotonically hyperpolarizes the T-junction. D) Orthodromically propagating APs are unable to propagate passed the hyperpolarized T-junction into the spinal axon.
Koopmeiners and colleagues utilized an in vitro approach to study the effect of extracellular electrical stimulation on Aδ- and C-neurons in excised rat DRG.64 They demonstrated that field stimulation of excised DRG reduced the following frequency of putatively nociceptive neurons, while producing large transient increases in intracellular calcium concentration. Pan and colleagues then provided the first in vivo recordings of T-junction filtering in a rat model of DRGS for rheumatoid arthritis, though in this case, DRGS filtered antidromically propagating APs initiated via dorsal root stimulation and recorded via teased fiber recordings from the sural nerve.92 These results add further evidence that DRGS may provide pain relief by filtering the transmission of APs at the T-junction, and that this phenomenon is likely either calcium-dependent or calcium-sensitive.
Recently, Chao and colleagues used an in vivo rat model to study the mechanisms of action of DRGS.22 Using teased fiber dorsal root recordings, they demonstrated that when stimulating the sciatic nerve, the stimulation amplitude needed to activate nonmyelinated C-axons is more than 50 times greater than the amplitude needed to activate myelinated Aβ-axons, in agreement with conventional neurostimulation theory. However, when stimulating the DRG, the C-neuron activation threshold was only 1.5 times the Aβ-neuron threshold, contrary to previous modeling results.48 Furthermore, they found that within approximately 30 seconds of starting DRGS applied at 80% of motor threshold, the orthodromically propagating C-component of the teased fiber recording disappeared, suggesting that prolonged DRGS augments T-junction filtering in nociceptive neurons after a brief wash-in period. These experimental results are exciting, and imply that DRGS activates many types of neurons simultaneously, suggesting multiple pain-relieving mechanisms may be happening concurrently.
As with all animal models of human technology, it is critical to ensure that in vivo models accurately recapitulate clinical scenarios. Therefore, the electric fields generated by DRGS in preclinical studies must adequately approximate the electric fields generated by clinical DRGS systems to ensure the conclusions of experimental studies accurately inform our clinical understanding of the technology. In this way, there are three interrelated factors that we think are worth careful consideration when designing future experimental studies of DRGS: 1) the dimensions of the stimulating electrode contacts, 2) the dimensions of the animal model’s DRG and surrounding anatomy, and 3) the choice in stimulation parameters, particularly the pulse amplitude. Chao and colleagues used an in-house made electrode to apply bipolar DRGS that is necessarily smaller than the electrode leads used clinically, because the rat neuroforamina are considerably smaller than human neuroforamina. However, smaller electrode contacts will generate larger current densities in stimulated tissues compared to larger contacts for a given pulse amplitude, potentially lowering the activation threshold of small neurons (e.g., C-neurons). Furthermore, smaller neuroforamina, i.e., a more enclosed space surrounded by a bony structure, implies more current will enter the more conductive neural tissue, compared to the minimally conductive bone. A recent study of anatomical factors affecting the activation thresholds of neurons in the spinal cord dorsal columns emphasizes the importance of this latter point.131
Comparatively larger current densities resulting from smaller electrode contacts and neuroforamina make the already difficult task of choosing clinically relevant pulse amplitudes for in vivo DRGS studies even more challenging. Koetsier and colleagues applied DRGS using an intensity of 66.7% motor threshold (i.e., the minimum amplitude at which DRGS causes muscle activation in the patient’s or animal’s stimulated myotome), whereas Chao and colleagues suggested that 80% motor threshold was the optimal stimulating intensity.22,62 However, preliminary clinical data suggest that sensory thresholds (i.e., the minimum stimulation amplitude at which a human patient reports feeling paresthesias) can be between 33% and 70% of the motor threshold value.39 This finding, taken with the clinical reports that some DRGS patients utilize sub-perception DRGS (i.e., DRGS with amplitudes that do not produce paresthesias) and achieve successful pain relief,81,105 indicates further study is needed to determine how the ‘optimal’ pulse amplitude should be calculated in experimental studies to most accurately recapitulate clinical DRGS. Determining this relationship between experimental and clinical pulse amplitudes is a challenging problem because there are currently no clinically analogous methods for determining sensory thresholds in animal models.
Furthermore, neural activation generated when stimulating at a particular percent motor threshold in a preclinical model may not be directly analogous to the neural activation generated by stimulating at the same percent motor threshold in a human study, because of differences in DRG size across species. Muscle twitches resulting from DRGS are likely caused by: 1) direct activation of Ia afferents in the DRG causing postsynaptic activation of motor neurons in the ventral horn,21 or 2) direct activation of motor axons in the ventral root. Because Ia afferents have low populations in both rodent47 and human115 DRG, activation of sufficient numbers of Ia afferents to produce a muscle twitch could suggest widespread neural activation throughout the DRG. Similarly, assuming the stimulating electrode is placed on the dorsal side of the DRG, direct activation of the ventral root – a structure farther from the stimulating electrode than the DRG – would suggest that DRGS was applied with a large pulse amplitude, thereby likely causing widespread activation throughout the DRG.
However, rat DRG are much smaller than human DRG. In the dorsal-ventral axis, lumbar rat DRG are likely less than 1 mm wide30,98 while human lumbar DRG are approximately 6 mm wide,51 placing rodent ventral roots and Ia afferents much closer to the stimulating electrode contact than in clinical scenarios. Because the voltage at a given point in space is inversely proportional to its distance from a current source, a given percent of a motor threshold in a rat likely causes more neural activation in the DRG than the same percent motor threshold would cause in a DRGS patient. Future work should develop rigorous methodologies for selecting stimulation parameters in both clinical and preclinical DRGS experiments which allow for direct comparison between the two types of data.
Suppressing PSN hyperexcitability
Spontaneous firing of APs despite a lack of sensory input and increased firing of APs in response to peripheral stimuli, are thought to be two biomarkers of several chronic pain etiologies. For example, ongoing input into nociceptive C-neurons, generated perhaps from a peripheral neuroma, may lead to pain perception without an external noxious stimulus.107 Spontaneous activity in Aβ-neurons, which typically don’t convey pain signals, is thought to underly allodynia, a clinical symptom of neuropathic pain whereby previously non-painful stimuli cause pain.4,19 Current evidence suggests that the DRG can be responsible for the development of neuropathic pain through hyperexcitability and spontaneous ectopic firing of PSNs. One proposed mechanism of DRGS is that electrical stimulation rectifies this hyperexcitability and aberrant activity.
Koopmeiners and colleagues measured the number of APs generated in response to current-clamp stimulation in excised DRG from healthy rats.64 They demonstrated that putatively nociceptive neurons generate fewer APs in response to current clamp stimulation after exposure to 90 seconds of DRGS compared to before receiving DRGS, suggesting that DRGS reduces membrane excitability. Given that DRGS applied at an intensity that reduced membrane excitability also produced large increases in intracellular calcium, the authors hypothesized that the reduction of membrane excitability may be calcium-dependent or calcium-sensitive. It is currently unclear how different chronic pain etiologies affect neural dynamics and the response to DRGS, but these data provide evidence that justifies further study of DRGS’ ability to suppress neuronal excitability.
In a tibial nerve-injured (TNI) rat model of DRGS, Chao and colleagues showed that DRGS raised the mechanical threshold to elicit AP firing in both C- and Aδ-neurons, and significantly reduced the firing frequency of C- and Aδ-neurons in response to mechanical stimulation.22 As these data come from teased fiber recordings of dorsal root axons, it is difficult to ascertain whether these changes resulted from suppressing the excitability of the recorded cell, or from inducing T-junction filtering of orthodromically propagating APs (see previous section). However, the authors also showed that after DRGS, the average spontaneous firing frequency of C-neurons decreased, and other studies have shown that DRGS reduces spontaneous pain behavior in TNI rats,91,130 suggesting that excitability suppression is a possible culprit of the reduction in nociceptor activity.
To date, there are relatively few studies explicitly examining the hypothesis of hyperexcitability suppression, and the biophysical mechanisms through which excitability suppression is achieved are unclear. Extracellular electrical stimuli with durations lasting on the order of 1 ms or shorter, such as those utilized by DRGS, are conventionally believed to induce neural activity by transiently perturbing voltage-gated sodium channels to generate action potentials, counterintuitive to the idea of stimulation-induced suppression of excitability. Therefore, it is likely that DRGS-induced reductions in membrane excitability are due to voltage-sensitive intracellular secondary messenger cascades.46
Indirect effects of DRGS
Though the direct response to electrical stimulation is usually the generation of an AP in a target cell, the ultimate goal of neuromodulation therapies is to cause the release of neurotransmitters that modulate downstream neural activity which produces clinical benefit – so-called indirect effects of stimulation. Indirect effects are likely widely distributed throughout the neuraxis, particularly when stimulated tissue projects to many structures throughout the nervous system. Furthermore, the indirect effects of neuromodulation therapies may extend to non-neuronal targets, such as glial cells. Due to the novelty of DRGS, and the importance of first understanding which cells are directly affected by stimulation, there are as of yet few studies on the indirect effects DRGS. However, preliminary evidence suggests that DRGS may share indirect effects with conventional SCS, though continued study is vital to delineate the specific mechanisms unique to each therapy. Such knowledge may help improve patient selection and allow therapy assignment to be tuned to the pathophysiological differences across chronic pain etiologies.
Spinal/Segmental effects
The goal of DRGS – to stimulate a particular DRG to provide dermatome-specific pain relief – places fundamental importance on the effects of stimulation at the particular spinal segment receiving sensory information from a patient’s painful region. A wide range of experimental techniques exist to objectively characterize the segmental effects of neurostimulation. For example, quantitative sensory testing (QST) and standard clinical electrophysiological assessments (e.g., electromyography) have proven useful in characterizing the mechanisms of action of SCS (for review, see 106). Application of these techniques to patients receiving DRGS treatment could provide critical insight into the segmental effects of DRGS, and can be utilized in longitudinal studies to examine how the therapeutic effects of stimulation change over time, which are difficult to recapitulate using computational and preclinical models.
QST can assess a patient’s sensory capacity and has been used to characterize SCS and DRGS patients’ responses to both static stimuli (i.e., single transitory sensory stimuli) and dynamic stimuli (i.e., multiple stimuli). To date, there have been three studies using such experimental techniques to characterize the mechanisms of DRGS.23,59,105 All three studies found that DRGS increased patients’ pain thresholds in response to pressure stimuli localized to the patients’ painful regions, though Kinfe and colleagues noted that the increase they observed was not statistically significant. A localized increase in pressure pain threshold suggests that DRGS modulates patients’ perception of acute nociceptive pain, possibly through central mechanisms (e.g., segmental inhibition in the dorsal horn), or though peripheral mechanisms (e.g., suppression of nociceptive PSN activity), or both. DRGS may also affect patients’ abilities to detect non-painful mechanical stimuli by modulating the activity of large-diameter Aβ-neurons. Kinfe and colleagues found that DRGS lowered detection thresholds in response to non-painful punctate stimuli,59 while Chapman and colleagues found a similar, but non-statistically significant trend.23 However, work from our laboratory found that in patients receiving DRGS or SCS treatment, vibration detection thresholds did not change during stimulation compared to pre-treatment values,105 suggesting that continued study is needed to determine the effect of DRGS on patients’ perception of innocuous mechanical stimuli.
Furthermore, these early studies showed that DRGS reduces temporal summation (TS).59,105 TS is a dynamic QST metric that is typically measured by taking the difference between a patient’s reported pain intensity in response to a train of noxious stimuli and their reported pain intensity in response to a single noxious stimulus of equal magnitude. TS is a proxy to measure the “wind-up” phenomenon in humans. Wind-up is an increased firing rate of dorsal horn neurons in response to painful stimuli, believed to be mediated by activation of N-methyl-D-aspartic acid (NMDA) receptors on wide dynamic range neurons in the dorsal horn.35 Therefore, a reduction in TS by DRGS suggests stimulation provides pain relief in part by reducing the firing rate of pain-coding neurons in the central nervous system. It is reasonable to expect that DRGS may reduce TS by activating inhibitory circuits in the spinal cord dorsal horn, by causing nociceptive signals to be filtered out within the DRG, or a combination of the two. Interestingly, patients receiving SCS that demonstrate enhanced TS prior to implantation reported less overall pain after implantation.18 If SCS and DRGS both provide pain relief in part by driving the activity of Aβ-neurons, pre-implantation TS levels could similarly predict patients that will respond well to DRGS. Taken together, these preliminary studies indicate that DRGS may exert several concurrent effects on segmental pain processing, and demonstrate the utility of QST in studying the mechanisms of DRGS. Future work should include larger patient cohorts, examine the effects of pain etiology on changes in QST metrics, and determine if clinically quantifiable factors can be used as predictors for patient success with DRGS.
SCS has been shown to reduce the amplitude of some spinal cord reflex arcs, such as the H-reflex,7 a mono-synaptic reflex mediated by large-diameter muscle afferents, and the nociceptive flexor withdrawal reflex (also known as the RIII-reflex),7,11,42 a poly-synaptic reflex mediated by small-diameter nociceptors. Similar to TS, the nociceptive flexor withdrawal reflex is a proxy for assessing spinal excitability in humans. From these results, it is generally interpreted that SCS increases the inhibitory tone of the spinal cord, thereby decreasing the amplitude of the nociceptive reflex arc, and may reduce the amplitude of the H-reflex by causing AP collision in muscle afferents. Interestingly, the magnitude of attenuation of the nociceptive flexor withdrawal reflex correlates with SCS-induced pain relief, suggesting that the success of the therapy is at least partially attributable to facilitating segmental inhibition. We hypothesize one would see similar results using these measures to study DRGS, as computational and preclinical studies demonstrate that DRGS also generates APs in large-diameter afferents.22,48,49 However, it may be possible that DRGS exerts a larger attenuating effect on the nociceptive flexor withdrawal reflex, through the combined mechanisms of increasing inhibitory tone in the spinal cord, and reducing the net small-diameter input entering the spinal cord via T-junction filtering.
Supraspinal effects of DRGS
The original gate control theory of pain emphasized the importance of ‘central control’ – descending efferent fibers which modulate the gate control system – to the experience of pain, and stimulation-induced neural activity likely modulates brain regions involved in descending control. For example, SCS-induced activation of Aβ-axons in the dorsal columns activates neurons in brainstem regions, such as the rostroventral medulla and locus coeruleus,110,111 which in turn drive descending inhibition via serotonergic and noradrenergic efferents.112,113 As DRGS likely also drives the activation of Aβ-neurons,22,48,49 it may modulate similar supraspinal regions to SCS. However, as experimental data also suggest DRGS may directly affect the activity of small-diameter PSNs,22 there may be considerable differences in the brain regions engaged by SCS and DRGS.
In the first study of the supraspinal regions involved in DRGS, Pawela and colleagues performed functional magnetic resonance imaging (fMRI) in a rat model of DRGS to examine the effect of DRGS on the blood oxygen-level dependent (BOLD) signal evoked by noxious electrical stimulation of the hindpaw.95 They found that DRGS reduced the magnitude of the BOLD response in regions associated with the sensory-discriminative component of pain, such as the somatosensory cortices and the ventral posterolateral and ventral posteromedial nuclei of the thalamus, similar to findings from studies performing fMRI during SOS.61,86,116 Interestingly, they also found that DRGS reduced the magnitude of the BOLD response in the nucleus accumbens – a limbic structure that may play a role in the motivational aspect of chronic pain – which was recently implicated as a potential supraspinal target of SCS in a rat fMRI study.85 These results suggest that DRGS may provide pain relief through similar mechanisms to tonic SCS, though additional supraspinal mechanisms may also be at work. Continued study of the supraspinal mechanisms of DRGS will be critical, particularly studies performed in humans, as anesthesia used in non-human functional imaging studies adds additional confounds to interpreting results.
Parker and colleagues recently used magnetoencephalography (MEG) to study the effect of DRGS-induced pain relief on cognitive performance.94 They found that patients receiving pain relief from DRGS displayed a reduction in gamma-band (30-Hz) activity in the somatosensory and anterior cingulate cortices (ACC) – brain structures that have been shown to be implicated in the pain-relieving effects of conventional tonic SCS and burst SCS, respectively.103 It is hypothesized that burst SCS – a type of SCS that applies stimulation in high-frequency bursts – provides pain relief in part by modulating the medial spinothalamic pain pathway. This pathway flows from C-neurons in the DRG which project to lamina i of the dorsal horn,14 to several brain regions including the ACC, and is associated with the affective and attentional components of pain. Therefore, Parker and colleagues’ observation that DRGS-induced pain relief is accompanied by modulation of the ACC adds additional evidence that DRGS may directly act upon C-neurons, as suggested by previous computational and animal studies.22,58 Interestingly, Parker and colleagues found a reduction in gamma-band activity in the ACC, while De Ridder and colleagues found an increase in alpha-band activity (8-10 Hz) in the ACC,102 but neither study considered the effects of stimulation across the full power spectrum of neural activity. More work is needed to understand how different patterns of peripheral input affect the activity of different functional brain networks. This understanding could lead to the design of DRGS stimulation patterns that directly target different components of the pain matrix (i.e., sensory, affective, cognitive), allowing for the personalized design of a patient’s stimulation parameters based on their individual needs.
Studying the effect of DRGS on somatosensory evoked potentials (SSEPs) may also give insight into the supraspinal effects of the therapy, and SSEPs have been used for several decades to characterize the effects of SCS. SSEPs consist of positive and negative voltage deflections as a sensory stimulus travels through the nervous system. SSEPs are often obtained by recording electroencephalogram signals from the scalp in response to an external stimulus (e.g., percutaneous nerve stimulation), and can provide insight into how a therapy affects sensory processing. Generally, SCS-induced activation of Aβ-axons in the dorsal columns decreases the amplitude of SSEPs,17,68,69,96 and as DRGS likely acts in part through similar mechanisms, we may expect similar findings. A recent study of the effect of DRGS on laser evoked potentials (LEPs) – an SSEP measured in response to noxious laser stimulation of the skin – showed that DRGS increases the N2-P2 amplitude of LEPs, and that larger N2-P2 amplitudes may be correlated with lower pain ratings.87 Given that LEPs are designed to only study the cortical response to noxious stimulation, and that there are possible coincident effects of DRGS on small-diameter afferents which may produce different effects on SSEPs, continued investigation into the cortical effects of DRGS is necessary. A multi-modal approach to studying the supraspinal effects of DRGS will be necessary not only to understand the mechanisms of action of the therapy, but to also understand its effects on patients across pain etiologies and comorbidities.
Effects on glia
Historically, the non-neuronal effects of neurostimulation technologies have largely been ignored, except in the context of the foreign body response to implanted materials.77 An area of basic research that remains underexplored is the effect of DRGS on satellite glial cells (SGCs), the glial cell present in the DRG.52 SGCs are known to be important in the development and maintenance of chronic pain.24 Though they do not generate APs, SGCs possess voltage-sensitive ion channels, and communicate directly with neurons in the DRG,56 suggesting that they may be directly influenced by the DRGS-generated electric fields, or indirectly by DRGS-induced activity in PSNs. Though the effects of stimulation on glial cells have not yet been studied in the context of DRGS, early studies in SCS suggest it is an avenue well worth exploring.
SCS is most commonly applied to the lower thoracic spinal cord, where there are approximately 20 glial cells for every neuron.104 A high density of glial cells around common SCS targets indicates that SCS-generated electric fields could modulate glial activity, depending on the electrophysiological characteristics of glial cells and how those characteristics are affected by SCS stimulus waveforms.122 Differential targeted multiplexed programming-SCS (DTMP-SCS) was developed to concurrently drive neuronal and glial mechanisms of pain relief, by concurrently delivering a low-frequency SCS waveform (~50 Hz) and a high-frequency SCS waveform (~1200 Hz). Vallejo and colleagues demonstrated that in rats with spared nerve injuries, DTMP-SCS provided a greater reversal in chronic pain behavioral metrics, such as mechanical and thermal hypersensitivity, relative to low-frequency or high-frequency SCS alone.123 Furthermore, spared nerve injury affected the expression levels of many glia-related genes, and DTMP-SCS drove the expression level of many of those genes back towards naive levels. These results suggest that simultaneously modulating the neuronal and glial components of pain may lead to greater pain relief than modulating the neuronal component alone.
It is currently unclear how DRGS modulates the SGC component of pain. However, SGCs being the singular glial cell type in the DRG may make DRGS an ideal use case to study the differential effects of neurostimulation on neurons versus glia, and how communication between neurons and glia are modulated by extracellular stimulation. It is worth investigating the extent to which current clinical DRGS stimulation waveforms affect SGC physiology, and if further innovation is necessary to modulate the SGC component of pain (e.g., by multiplexing DRGS, similar to DTMP-SCS). Finally, in addition to the importance of glia in stimulation-induced pain relief, these studies also highlight the nascent but important study of omics, both to understand the mechanisms of neurostimulation therapies and as potential biomarkers of various chronic pain states.118,124
Looking forward
Several decades after helping construct the gate control theory of pain, Melzack synthesized a wide body of work across the medical and biological sciences into a new theory, the so-called neuromatrix theory. The neuromatrix theory places greater emphasis on not only the sensory component of pain, but also on the role of cognitive and affective components.82 Much as the foundation of this new theory drew from evidence across several disciplines, so too should our approach towards elucidating the mechanisms of action of neurostimulation therapies to manage pain. We believe that it is unlikely that these therapies provide pain relief through a single mechanism, and that a holistic understanding of electrical stimulation induced pain relief is vital towards successful and consistent implementation of these therapies. Therefore, much work remains to be done through the combined efforts of basic neuroscience, engineering, and clinical pain research.
Presently, there is considerable disagreement between the conceptual understanding of neurostimulation biophysics and the available experimental data of DRGS mechanisms. One of the most striking results of the work of Chao and colleagues is that the ratio of C-neuron to Aβ-neuron activation threshold was dramatically lower than the corresponding ratios when stimulating the sciatic and saphenous nerves.22 This result contrasts greatly with modeling studies comparing Aβ- and C-neuron activation thresholds.48,49 These conflicting results suggest several potential explanations that warrant further study. Firstly, the recent discovery of GABAergic communication within the DRG, suggests possible synaptic action within the DRG itself.33 Because GABA depolarizes C-neurons due to the atypical higher concentration of chloride within C-neurons than the extracellular space,97 it is possible that DRGS drives GABA release from Aβ-neurons, which then depolarizes C-neurons to induce T-junction filtering as described above. However, it is unclear if GABAergic activation alone is sufficient to induce filtering. Secondly, there could be one or more features of the soma and stem axon complex of C-neurons that are missing from existing computer models that significantly reduce the activation thresholds of these neurons.
For example, much of our intuition of extracellular stimulation-induced neural activation comes from modeling studies of the peripheral nerve, characterized by long, straight axons.79,99,100 In reality, DRG neurons have complex, winding stem axon trajectories5,71,114,129 that haven’t been accounted for in previous models. These trajectories could produce complex spatiotemporal profiles of depolarization and hyperpolarization in response to DRGS, making prediction of neural activation difficult. In simplified models of retinal ganglion cells, the presence of a 90-degree bend in an axon was sufficient to produce complex spatial distributions of activation thresholds,40 suggesting that more complex trajectories may require a new conceptual framework to predict neural activation. Furthermore, the effects of ephaptic coupling (i.e., transmembrane currents contributing to the extracellular potential at other parts of the cell) are typically ignored, but could be on the order of several millivolts.121 Because DRG neurons have tightly coiled stem axons closely apposed to large cell bodies (which would produce large transmembrane currents), intra- and inter-cellular ephaptic effects in the DRG could be significant. Future modeling work should aim to develop new intuitions of neural activation when stimulating cells with complex morphologies and axon trajectories, and use these novel intuitions to assist in interpreting experimental data of DRGS. Such intuitions will be critical to understanding not only the mechanisms of DRGS, but the mechanisms of other clinical neuromodulation therapies, including SCS.
In addition to complex stem axon trajectories, DRG neurons have active ion channels in their somata. Typically, APs are thought to be rarely generated in the soma,78 but the presence of active ion channels in DRG somata, and the computer modeling predictions that some APs may initiate in the somata of DRG neurons,48,58 suggest that this intuition may not apply to DRGS. There is a myriad of sodium10 and potassium120 channel isoforms important to physiological and pathological pain processing, many of which are expressed in DRG. In a recent study from our lab, we implemented two models of Aδ-neurons that were morphologically and electrically identical, except for the voltage-gated sodium channels that they expressed.49 Aδ-neurons that expressed Nav1.6 had lower activation thresholds than Aδ-neurons that expressed Nav1.7 and Nav1.8, suggesting that in addition to morphological properties, the electrophysiological properties of a given neuron are crucial in determining its response to extracellular stimulation. Future experimental data summarizing the types, densities, and spatial distributions of ion channels in DRG neurons will be critical to developing accurate computer models with which to study the mechanisms of DRGS.
In addition to accurately representing the electrophysiological characteristics of the neural systems under study, computational and experimental studies of neuromodulation therapies must utilize electric fields that are representative of the fields generated during clinical implementation of the therapies. Patient-specific modeling is a powerful technique in understanding and developing neurostimulation therapies, and for ensuring computer models mimic clinical implementation of the therapy.72 This approach has already proven useful in investigating the mechanisms of SCS for pain.74 Patient-specific DRGS models could provide evidence for why DRGS fails in some patients but not others, or serve as the basis for a clinical decision-support system to accelerate the time-consuming process of titrating an individual patient’s stimulation parameters.
There is good agreement between experimental22 and clinical28 studies of preferred DRGS parameters, particularly that the preferred stimulation frequency is 20 Hz in both human patients and rats. The work of Gemes and colleagues showed that when stimulating ex vivo rat DRG, Aδ- and C-neurons typically produced the largest calcium transients in response to frequencies between 3-7 Hz, while Aβ-neurons produced the largest calcium transients in response to 20-50 Hz stimulation.45 Furthermore, the calcium transients in putative nociceptors (i.e., Aδ- and C-neurons) had larger amplitudes and slower decay time constants than the Aβ-neurons. These results may suggest different calcium-dependent mechanisms of stimulation on different functional groups of DRG neurons. Augmented T-junction filtering of nociceptive signals is believed to be achieved through triggering of calcium-activated slow hyperpolarizing currents, which may suggest that larger amplitude, slowly decaying levels of internal calcium may be more conducive to sustained filtering in nociceptors (Fig. 3). It is also possible that the release of GABA within the DRG from Aβ-neuron somata (Fig. 2) requires comparatively smaller increases in intracellular calcium and benefits from faster decay times to allow somatic neurotransmitter resources to quickly reprime for the next release event. Could 20 Hz DRGS then be a ‘happy medium’ between the optimal frequencies to produce the necessary diversity of somatic calcium transients in both nociceptive and non-nociceptive DRG neurons? Such a finding would further suggest DRGS provides pain relief by directly activating multiple types of PSNs.
In the more than five decades since Melzack and Wall proposed the gate control theory, a superb amount of effort has gone in to elucidating the specific neural circuits which govern pain processing and transmission in the dorsal horn (for review, see 14,34,128). Much of the currently available data on DRGS mechanisms give evidence for the direct effects of stimulation, but it remains an open question as to how DRGS-induced peripheral input is integrated by neural networks in the central nervous system. With the advent of preclinical techniques to dissect neural circuits, such as optogenetics, calcium imaging in awake behaving animals, and transsynaptic tracing, it is now possible to elucidate the network dynamics that lead to chronic pain phenotypes. Applying these techniques to preclinical studies of DRGS could provide mechanistic knowledge into the systems-level effects of stimulation, and inform the design of novel DRGS technologies to specifically target these mechanisms.
To fully elucidate the mechanisms of DRGS, we must also understand how DRGS affects an individual patient’s pain experience (e.g., time course of pain relief, pain diagnosis, sensory profile). There is a dearth of clinical data on the temporal features of DRGS-induced analgesia, such as how long it takes for analgesia to onset (i.e., wash-in time) and offset (i.e., wash-out time). Preliminary clinical studies suggest that wash-in and wash-out times for DRGS are on the order of minutes,22 with DRGS possibly having faster wash-out times than SCS.63 Studying these phenomena in humans and comparing these temporal characteristics with clinical outcomes could provide insight into whether the analgesic effects of DRGS predominantly rely on faster mechanisms (e.g., transient induction or interruption of neural firing patterns), or slower mechanisms (e.g., inducing changes in synaptic plasticity).
Finally, using a mechanistic understanding of DRGS to design prospective clinical studies examining DRGS outcomes in patients with specific pain etiologies will be critical to determine which patient populations are best managed with DRGS. Determining if there are clinically measurable sensory features (e.g., mechanical allodynia, cold hypersensitivity) that correlate with DRGS-induced pain relief (e.g., through clinical studies performing QST), could not only provide further insights into the mechanisms of action of DRGS, but also improve patient selection. Furthermore, functional neuroimaging (e.g., fMRI, MEG) and clinical electrophysiology (e.g., electroencephalography) studies will be critical to investigating how DRGS modulates brain regions associated not just with the sensory component of pain, but also with the affective and cognitive components of pain. As DRGS has been shown to provide greater improvement in depression- and mood-related metrics than tonic SCS,26 improving our understanding of how DRGS modulates the non-sensory components of the pain neuromatrix may further assist in patient stratification between neurostimulation therapies. Continued study of the clinical manifestations of the effect of DRGS on the nervous system will be crucial to bridging the gap between our mechanistic understanding of DRGS-induced analgesia and improvements in patients’ quality of life.
Conclusion
Dorsal root ganglion stimulation is an important tool in a pain physician’s toolbox for managing intractable chronic pain. It is unlikely that any neuromodulation therapy acts through a single mechanism alone, and the growing body of evidence suggests that DRGS is no exception. Current evidence suggests that DRGS may provide pain relief by post-synaptic activation of pain-gating circuitry in the dorsal horn and possibly the DRG itself, by augmenting the low-pass filtering of painful signals at the T-junction of nociceptive neurons, and by reducing the intrinsic excitability of DRG neurons. Continued study of the mechanisms of action of DRGS, particularly into the supraspinal effects of DRGS, as well as the role of cognitive and affective networks in the patient response to DRGS, is warranted. DRGS is an excellent use case to develop new intuitions on the morphological and electrophysiological factors contributing to neural activation, which will be invaluable in innovating current, and developing novel, neurostimulation therapies for neurological disorders. Such innovation will be crucial to reduce the world-wide impact of chronic pain.
Perspective.
This article synthesizes the evidence supporting the current hypotheses of the mechanisms of action of DRGS for chronic pain and suggests avenues for future interdisciplinary research which will be critical to fully elucidate the analgesic mechanisms of the therapy.
Research funding:
This work was supported by the National Institutes of Health [F31 NS113407].
Conflicts of interest:
SFL has equity in Hologram Consultants, LLC, is a member of the scientific advisory board for Abbott Neuromodulation, and receives research support from Medtronic, Inc. SFL also holds stock options, received past research support, and serves on the scientific advisory board of Presidio Medical, Inc. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraira VE, Ginty DD: The sensory neurons of touch. Neuron 79:618–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir R, Devor M: Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys J 84:2181–91,2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir R, Devor M: Extra spike formation in sensory neurons and the disruption of afferent spike patterning. Biophys J 84:2700–8, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amir R, Liu CN, Kocsis JDK, Devor M: Oscillatory mechanism in primary sensory neurones. Brain 125:421–35, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Amir R, Michaelis M, Devor M: Membrane potential oscillations in dorsal root ganglion neurons: Role in normal electrogenesis and neuropathic pain. J Neurosci 19:8589–96, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirdelfan K, Kramer J, Cusack WF, Burton AW: Advanced neuromodulation techniques: Dorsal root ganglion stimulation. Adv. Proced. Pain Manag. A Step-By-Step Atlas. 2018. [Google Scholar]
- 7.de Andrade DC, Bendib B, Hattou M, Keravel Y, Nguyen JP, Lefaucheur JP: Neurophysiological assessment of spinal cord stimulation in failed back surgery syndrome. Pain 150:485–91, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG: Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 93:179–93, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Ayers CA, Fisher LE, Gaunt RA, Weber DJ: Microstimulation of the lumbar DRG recruits primary afferent neurons in localized regions of lower limb. J Neurophysiol 116:51–60, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD: The role of voltage-gated sodium channels in pain signaling. Physiol Rev 99:1079–151, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Biurrun Manresa JA, Sörensen J, Andersen OK, Arendt-Nielsen L, Gerdle B: Dynamic changes in nociception and pain perception after spinal cord stimulation in chronic neuropathic pain patients. Clin J Pain 31:1046–53, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Bourbeau DJ, Hokanson JA, Rubin JE, Weber DJ: A computational model for estimating recruitment of primary afferent fibers by intraneural stimulation in the dorsal root ganglia. J Neural Eng 8:056009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd IA, Kalu KU: Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol 289:277–97, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braz J, Solorzano C, Wang X, Basbaum A: Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron 82:522–36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brierley JB: The penetration of particulate matter from the cerebrospinal fluid into the spinal ganglia, peripheral nerves, and the perivascular spaces of the central nervous system. J Neurol Neurosurg Psychiatry 13:203–15, 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns TM, Weber DJ, Gaunt RA: Microstimulation of afferents in the sacral dorsal root ganglia can evoke reflex bladder activity. Neurourol Urodyn 34:65–71, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buonocore M, Bodini A, Demartini L, Bonezzi C: Inhibition of somatosensory evoked potentials during spinal cord stimulation and its possible role in the comprehension of antalgic mechanisms of neurostimulation for neuropathic pain. Minerva Anestesiol 78:297–302, 2012. [PubMed] [Google Scholar]
- 18.Campbell CM, Buenaver LF, Raja SN, Kiley KB, Swedberg LJ, Wacnik PW, Cohen SP, Erdek MA, Williams KA, Christo PJ: Dynamic pain phenotypes are associated with spinal cord stimulation-induced reduction in pain: A repeated measures observational pilot study. Pain Med 16:1349–60, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell JN, Raja SN, Meyer RA, Mackinnon SE: Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32:89–94, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Capogrosso M, Lempka SF: A computational outlook on neurostimulation. Bioelectron Med 6:1–7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Luciani LB, Courtine G, Micera S: A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33:19326–40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B: Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain 161:2872–86, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman KB, van Roosendaal BK, Yousef TA, Vissers KC, van Helmond N: Dorsal root ganglion stimulation normalizes measures of pain processing in patients with chronic low-back pain: A prospective pilot study using quantitative sensory testing. Pain Pract 21:1–10, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Costa FAL, Neto FLM: Satellite glial cells in sensory ganglia: Its role in pain. Brazilian J Anesthesiol 65:73–81, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM: A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation 16:67–72, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail NA: Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain 158:669–81, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deer TR, Pope JE: Dorsal root ganglion stimulation approval by the Food and Drug Administration: Advice on evolving the process. Expert Rev Neurother 16:1123–5, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Deer TR, Pope JE, Lamer TJ, Grider JS, Provenzano D, Lubenow TR, FitzGerald JJ, Hunter C, Falowski S, Sayed D, Baranidharan G, Patel NK, Davis T, Green A, Pajuelo A, Epstein LJ, Harned M, Liem L, Christo PJ, Chakravarthy K, Gilmore C, Huygen F, Lee E, Metha P, Nijhuis H, Patterson DG, Petersen E, Pilitsis JG, Rowe JJ, Rupert MP, Skaribas I, Sweet J, Verrills P, Wilson D, Levy RM, Mekhail NA: The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromodulation 22:1–35, 2019. [DOI] [PubMed] [Google Scholar]
- 29.Devor M: Unexplained peculiarities of the dorsal root ganglion. Pain Suppl 6 :S27–35, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Devor M, Govrin-Lippmann R: Neurogenesis in adult rat dorsal root ganglia. Neurosci Lett 61:189–94, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Djouhri L: Aδ-fiber low threshold mechanoreceptors innervating mammalian hairy skin: A review of their receptive, electrophysiological and cytochemical properties in relation to Aδ-fiber high threshold mechanoreceptors. Neurosci Biobehav Rev 61:225–38, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Djouhri L, Lawson SN: Aβ-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev 46:131–45, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Du X, Hao H, Yang Y, Huang S, Wang C, Gigout S, Ramli R, Li X, Jaworska E, Edwards I, Deuchars J, Yanagawa Y, Qi J, Guan B, Jaffe DB, Zhang H, Gamper N: Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 127:1741–56, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan B, Cheng L, Ma Q: Spinal circuits transmitting mechanical pain and itch. Neurosci Bull 34:186–93, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eide PK: Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain 4:5–15, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Eldabe S, Burger K, Moser H, Klase D, Schu S, Wahlstedt A, Vanderick B, Francois E, Kramer J, Subbaroyan J: Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation 18:610–7, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Eldabe S, Espinet A, Wahlstedt A, Kang P, Liem L, Patel NK, Vesper J, Kimber A, Cusack W, Kramer J: Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation 21:787–92, 2018. [DOI] [PubMed] [Google Scholar]
- 38.Esposito MF, Malayil R, Hanes M, Deer T: Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med 20:S23–30, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falowski SM, Dianna A: A prospective analysis of neuromonitoring for confirmation of lead placement in dorsal root ganglion stimulation. Oper Neurosurg 14:654–9, 2018. [DOI] [PubMed] [Google Scholar]
- 40.Finn KE, Zander HJ, Graham RD, Lempka SF, Weiland JD: A patient-specific computational framework for the Argus II implant. IEEE Open J Eng Med Biol 1:190–6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher LE, Ayers CA, Ciollaro M, Ventura V, Weber DJ, Gaunt RA: Chronic recruitment of primary afferent neurons by microstimulation in the feline dorsal root ganglia. J Neural Eng 11:036007, 2014. [DOI] [PubMed] [Google Scholar]
- 42.García-Larrea L, Sindou M, Mauguière F: Nociceptive flexion reflexes during analgesic neurostimulation in man. Pain 39:145–56, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Gaunt RA, Hokanson JA, Weber DJ: Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: Thresholds and recruitment properties. J Neural Eng 6:055009, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gemes G, Koopmeiners A, Rigaud M, Lirk P, Sapunar D, Bangaru ML, Vilceanu D, Garrison SR, Ljubkovic M, Mueller SJ, Stucky CL, Hogan QH: Failure of action potential propagation in sensory neurons: Mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol 591:1111–31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gemes G, Rigaud M, Koopmeiners AS, Poroli MJ, Zoga V, Hogan QH: Calcium signaling in intact dorsal root ganglia: New observations and the effect of injury. Anesthesiology 113:134–46, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh A, Greenberg ME: Calcium signaling in neurons: Molecular mechanisms and cellular consequences. Science 268:239–47, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Giacobassi MJ, Leavitt LS, Raghuraman S, Alluri R, Chase K, Finol-Urdaneta RK, Terlau H, Teichert RW, Olivera BM: An integrative approach to the facile functional classification of dorsal root ganglion neuronal subclasses. Proc Natl Acad Sci 117:1–8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham RD, Bruns TM, Duan B, Lempka SF: Dorsal root ganglion stimulation for chronic pain modulates Aβ-fiber activity but not C-fiber activity: A computational modeling study. Clin Neurophysiol 130:941–51, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham RD, Bruns TM, Duan B, Lempka SF: The effect of clinically controllable factors on neural activation during dorsal root ganglion stimulation. Neuromodulation 24:655–71, 2021. [DOI] [PubMed] [Google Scholar]
- 50.Ha H: Axonal bifurcation in the dorsal root ganglion of the cat: A light and electron microscopic study. J Comp Neurol 140:227–40, 1970. [DOI] [PubMed] [Google Scholar]
- 51.Haberberger RV, Barry C, Dominguez N, Matusica D: Human dorsal root ganglia. Front Cell Neurosci 13:1–17, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanani M, Spray DC: Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 21:485–98, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison C, Epton S, Bojanic S, Green AL, FitzGerald JJ: The efficacy and safety of dorsal root ganglion stimulation as a treatment for neuropathic pain: A literature review. Neuromodulation 21:225–33, 2018. [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa T, Mikawa Y, Watanabe R, An HS: Morphometric analysis of the lumbrosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine 21:1005–9, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Hu L, Cai MM, Xiao P, Luo F, lannetti GD: Human brain responses to concomitant stimulation of Aδ and C nociceptors. J Neurosci 34:11439–51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L-YM, Gu Y, Chen Y: Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 61:1571–81, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen MP, Chodroff MJ, Dworkin RH: The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 68:1178–82, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Kent AR, Min X, Hogan QH, Kramer JM: Mechanisms of dorsal root ganglion stimulation in pain suppression: A computational modeling analysis. Neuromodulation 21:234–46, 2018. [DOI] [PubMed] [Google Scholar]
- 59.Kinfe T, von Willebrand N, Stadlbauer A, Buchfelder M, Yearwood TL, Muhammad S, Chaudhry SR, Gravius S, Randau T, Winder K, Maihofner C, Gravius N, Magerl W: Quantitative sensory phenotyping in chronic neuropathic pain patients treated with unilateral L4-dorsal root ganglion stimulation. J Transl Med 18:1–14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King KW, Cusack WF, Nanivadekar AC, Ayers CA, Urbin MA, Gaunt RA, Fisher LE, Weber DJ: DRG microstimulation evokes postural responses in awake, standing felines. J Neural Eng 17:016014, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiriakopoulos ET, Tasker RR, Nicosia S, Wood ML, Mikulis DJ: Functional magnetic resonance imaging: A potential tool for the evaluation of spinal cord stimulation: Technical case report. Neurosurgery 41:501–4, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Koetsier E, Franken G, Debets J, Heijmans L, van Kuijk SMJ, Linderoth B, Joosten EA, Maino P: Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther 26:136–43, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koetsier E, Franken G, Debets J, van Kuijk SMJ, Perez RSGM, Linderoth B, Joosten EAJ, Maino P: Effectiveness of dorsal root ganglion stimulation and dorsal column spinal cord stimulation in a model of experimental painful diabetic polyneuropathy. CNS Neurosci Ther 25:367–74, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koopmeiners AS, Mueller S, Kramer J, Hogan QH: Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 16:304–11, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Krames ES: The dorsal root ganglion in chronic pain and as a target for neuromodulation: A review. Neuromodulation 18:24–32, 2015. [DOI] [PubMed] [Google Scholar]
- 66.Kuehn BM: Chronic pain prevalence. JAMA 319:1632, 2018. [DOI] [PubMed] [Google Scholar]
- 67.Kumar K, Caraway DL, Rizvi S, Bishop S: Current challenges in spinal cord stimulation. Neuromodulation 17:22–35, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Lang E, Krainick JU, Gerbershagen HU: Spinal cord transmission of impulses during high spinal anesthesia as measured by cortical evoked potentials. Anesth Analg 69:15–20, 1989. [PubMed] [Google Scholar]
- 69.Larson SJ, Sances A, Riegel DH, Meyer GA, Dallmann DE, Swiontek T: Neurophysiological effects of dorsal column stimulation in man and monkey. J Neurosurg 41:217–23, 1974. [DOI] [PubMed] [Google Scholar]
- 70.Lawson SN, Fang X, Djouhri L: Nociceptor subtypes and their incidence in rat lumbar dorsal root ganglia (DRGs): focussing on C-polymodal nociceptors, Aβ-nociceptors, moderate pressure receptors and their receptive field depths. Curr Opin Physiol 11:125–46, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KH, Chung K, Chung JM, Coggeshall RE: Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol 243:335–46, 1986. [DOI] [PubMed] [Google Scholar]
- 72.Lempka SF: Can patient-specific computer models help in pain clinics using spinal cord stimulation? Bioelectron Med 3:1–4, 2020. [Google Scholar]
- 73.Lempka SF, Patil PG: Innovations in spinal cord stimulation for pain. Curr Opin Biomed Eng 8:51–60, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lempka SF, Zander HJ, Anaya CJ, Wyant A, Ozinga JG, Machado AG: Patient-specific analysis of neural activation during spinal cord stimulation for pain. Neuromodulation 23:572–81, 2020. [DOI] [PubMed] [Google Scholar]
- 75.Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H: Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12:547–8, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun Y-Y, Ji R-R, Xiong L: A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123:4050–62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malaga KA, Schroeder KE, Patel PR, Irwin ZT, Thompson DE, Nicole Bentley J, Lempka SF, Chestek CA, Patil PG: Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates. J Neural Eng 13:016010, 2015. [DOI] [PubMed] [Google Scholar]
- 78.McIntyre CC, Grill WM: Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 76:878–88, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNeal DR: Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng BME- 23:329–37, 1976. [DOI] [PubMed] [Google Scholar]
- 80.Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T: Clinical applications of neurostimulation: Forty years later. Pain Pract 10:103–12, 2010. [DOI] [PubMed] [Google Scholar]
- 81.Mekhail NA, Deer TR, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Scowcroft J, Golovac S, Kapural L, Paicius R, Pope J, Samuel S, McRoberts WP, Schaufele M, Kent AR, Raza A, Levy RM: Paresthesia-free dorsal root ganglion stimulation: An ACCURATE study sub-analysis. Neuromodulation 23:185–95, 2020. [DOI] [PubMed] [Google Scholar]
- 82.Melzack R: From the gate to the neuromatrix. Pain Suppl 6 :S121–6, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Melzack R, Wall PD: Pain mechanisms: A new theory. Science 150:971–9, 1965. [DOI] [PubMed] [Google Scholar]
- 84.Mendell LM: Constructing and deconstructing the gate theory of pain. Pain 155:210–6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meuwissen KPV, Toorn A Van Der, Gu JW, Zhang TC, Dijkhuizen RM, Joosten EAJ: Active recharge burst and tonic spinal cord stimulation engage different supraspinal mechanisms: A functional magnetic resonance imaging study in peripherally injured chronic neuropathic rats. Pain Pract 20:510521, 2020. [DOI] [PubMed] [Google Scholar]
- 86.Moens M, Sunaert S, Mariën P, Brouns R, De Smedt A, Droogmans S, Van Schuerbeek P, Peeters R, Poelaert J, Nuttin B: Spinal cord stimulation modulates cerebral function: An fMRI study. Neuroradiology 54:1399–407, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Morgalla MH, de Barros Filho MF, Chander BS, Soekadar SR, Tatagiba M, Lepski G: Neurophysiological effects of dorsal root ganglion stimulation (DRGS) in pain processing at the cortical level. Neuromodulation 22:36–43, 2019. [DOI] [PubMed] [Google Scholar]
- 88.Morgalla MH, Bolat A, Fortunato M, Lepski G, Chander BS: Dorsal root ganglion stimulation used for the treatment of chronic neuropathic pain in the groin: A single-center study with long-term prospective results in 34 cases. Neuromodulation 20:753–60, 2017. [DOI] [PubMed] [Google Scholar]
- 89.Nanivadekar AC, Ayers CA, Gaunt RA, Weber DJ, Fisher LE: Selectivity of afferent microstimulation at the DRG using epineural and penetrating electrode arrays. J Neural Eng 17:016011, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, Luo W: Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci 33:17691–709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH: Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain 17:1349–58, 2016. [DOI] [PubMed] [Google Scholar]
- 92.Pan B, Zhang Z, Chao D, Hogan QH: Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation 21:247–53, 2018. [DOI] [PubMed] [Google Scholar]
- 93.Pan H, Fatima M, Li A, Lee H, Cai W, Horwitz L, Hor CC, Zaher N, Cin M, Slade H, Huang T, Xu XZS, Duan B: Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103:1135–1149.e6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker T, Huang Y, Raghu ALB, Fitzgerald JJ, Green AL, Aziz TZ: Dorsal root ganglion stimulation modulates cortical gamma activity in the cognitive dimension of chronic pain. Brain Sci 10:1–13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pawela CP, Kramer JM, Hogan QH: Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: Insights into a possible mechanism for analgesia. Neuroimage 147:10–8, 2017. [DOI] [PubMed] [Google Scholar]
- 96.Poláček H, Kozák J, Vrba I, Vrána J, Stančák A: Effects of spinal cord stimulation on the cortical somatosensory evoked potentials in failed back surgery syndrome patients. Clin Neurophysiol 118:1291–302, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA: Chloride regulation in the pain pathway. Brain Res Rev 60:149–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puigdellívol-Sánchez A, Prats-Galino A, Ruano-Gil D, Molander C: Sciatic and femoral nerve sensory neurones occupy different regions of the L4 dorsal root ganglion in the adult rat. Neurosci Lett 251:169–72, 1998. [DOI] [PubMed] [Google Scholar]
- 99.Rattay F: Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng BME-33:974–7, 1986. [DOI] [PubMed] [Google Scholar]
- 100.Rattay F: Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng 36:676–82, 1989. [DOI] [PubMed] [Google Scholar]
- 101.Rattay F: The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 89:335–46, 1999. [DOI] [PubMed] [Google Scholar]
- 102.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S: Burst spinal cord stimulation for limb and back pain. World Neurosurg 80:642–649.e1, 2013. [DOI] [PubMed] [Google Scholar]
- 103.De Ridder D, Vanneste S: Burst and tonic spinal cord stimulation: Different and common brain mechanisms. Neuromodulation 19:47–59, 2016. [DOI] [PubMed] [Google Scholar]
- 104.Ruiz-Sauri A, Orduña-Valls JM, Blasco-Serra A, Tornero-Tornero C, Cedeño DL, Bejarano-Quisoboni D, Valverde-Navarro AA, Benyamin R, Vallejo R: Glia to neuron ratio in the posterior aspect of the human spinal cord at thoracic segments relevant to spinal cord stimulation. J Anat 235:997–1006, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sankarasubramanian V, Chiravuri S, Mirzakhalili E, Anaya CJ, Scott JR, Brummett CM, Clauw DJ, Patil PG, Harte SE, Lempka SF: Quantitative sensory testing of spinal cord and dorsal root ganglion stimulation in chronic pain patients. Neuromodulation 24:672–84, 2021. [DOI] [PubMed] [Google Scholar]
- 106.Sankarasubramanian V, Harte SE, Chiravuri S, Harris RE, Brummett CM, Patil PG, Clauw DJ, Lempka SF: Objective measures to characterize the physiological effects of spinal cord stimulation in neuropathic pain: A literature review. Neuromodulation 22:127–48, 2019. [DOI] [PubMed] [Google Scholar]
- 107.Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, Navarro X, Quiles C: Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain 153:42–55, 2012. [DOI] [PubMed] [Google Scholar]
- 108.Sharif B, Ase AR, Ribeiro-da-Silva A, Séguéla P: Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 106:940–951.e4, 2020. [DOI] [PubMed] [Google Scholar]
- 109.Shealy CN, Mortimer JT, Reswick JB: Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg 46:489–91, 1967. [PubMed] [Google Scholar]
- 110.Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B: Exploration of supraspinal mechanisms in effects of spinal cord stimulation: Role of the locus coeruleus. Neuroscience 253:426–34, 2013. [DOI] [PubMed] [Google Scholar]
- 111.Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B: The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience 247:134–44, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Song Z, Meyerson BA, Linderoth B: Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 152:1666–73, 2011. [DOI] [PubMed] [Google Scholar]
- 113.Song Z, Ultenius C, Meyerson BA, Linderoth B: Pain relief by spinal cord stimulation involves serotonergic mechanisms: An experimental study in a rat model of mononeuropathy. Pain 147:241–8, 2009. [DOI] [PubMed] [Google Scholar]
- 114.Spencer PS, Raine CS, WiśNiewski H: Axon diameter and myelin thickness - unusual relationships in dorsal root ganglia. Anat Rec 176:225–43, 1973. [DOI] [PubMed] [Google Scholar]
- 115.Sperry ZJ, Graham RD, Peck-Dimit N, Lempka SF, Bruns TM: Spatial models of cell distribution in human lumbar dorsal root ganglia. J Comp Neurol 528:1644–59, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stančák A, Kozák J, Vrba I, Tintěra J, Vrána J, Poláček H, Stančák M: Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur J Pain 12:137–48, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Sundt D, Gamper N, Jaffe DB: Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: A modeling study. J Neurophysiol 114:3140–53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tilley DM, Lietz CB, Cedeno DL, Kelley CA, Li L, Vallejo R: Proteomic modulation in the dorsal spinal cord following spinal cord stimulation therapy in an in vivo neuropathic pain model. Neuromodulation 24:22–32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Todd AJ: Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–36, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsantoulas C, McMahon SB: Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci 37:146–58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tveito A, Jæger KH, Lines GT, Paszkowski Ł, Sundnes J, Edwards AG, Māki-Marttunen T, Haines G, Einevoll GT: An evaluation of the accuracy of classical models for computing the membrane potential and extracellular potential for neurons. Front Comput Neurosci 11:1–18, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vallejo R, Bradley K, Kapural L: Spinal cord stimulation in chronic pain. Spine 42:S53–60, 2017. [DOI] [PubMed] [Google Scholar]
- 123.Vallejo R, Kelley CA, Gupta A, Smith WJ, Vallejo A, Cedeño DL: Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain 16:, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vallejo R, Tilley DM, Cedeño DL, Kelley CA, DeMaegd M, Benyamin R: Genomics of the effect of spinal cord stimulation on an animal model of neuropathic pain. Neuromodulation 19:576–86, 2016. [DOI] [PubMed] [Google Scholar]
- 125.Vuka I, Vučić K, Repić T, Ferhatović Hamzić L, Sapunar D, Puljak L: Electrical stimulation of dorsal root ganglion in the context of pain: A systematic review of in vitro and in vivo animal model studies. Neuromodulation 21:213–24, 2018. [DOI] [PubMed] [Google Scholar]
- 126.Wall PD, Sweet WH: Temporary abolition of pain in man. Science 155:108–9, 1967. [DOI] [PubMed] [Google Scholar]
- 127.Wang F, Bélanger E, Côté SL, Desrosiers P, Prescott SA, Côté DC, De Koninck Y: Sensory afferents use different coding strategies for heat and cold. Cell Rep 23:2001–13, 2018. [DOI] [PubMed] [Google Scholar]
- 128.Wercberger R, Basbaum AI: Spinal cord projection neurons: A superficial, and also deep analysis. Curr Opin Physiol 11:109–15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoshida S, Matsuda Y: Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol 42:1134–45, 1979. [DOI] [PubMed] [Google Scholar]
- 130.Yu G, Segel I, Zhang Z, Hogan QH, Pan B: Dorsal root ganglion stimulation alleviates pain-related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology 133:408–25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zander HJ, Graham RD, Anaya CJ, Lempka SF: Anatomical and technical factors affecting the neural response to epidural spinal cord stimulation. J Neural Eng 17:036019, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


