Abstract
A total of 3,205 group A streptoccal isolates were collected in 1997 through a private laboratory which serves community physicians in southern Ontario and which represents a population base of 6 million people. Nonsusceptibility to erythromycin was detected for 67 (2.1%) isolates both by disk diffusion and by broth microdilution. Of these, 47 (70%) were susceptible to clindamycin and were found by PCR to possess the mef gene. Of the other 20 strains, 18 and 2 showed inducible and constitutive resistance, respectively, to clindamycin. Nineteen of these strains were shown by PCR to possess the ermTR gene, and a single constitutively resistant strain harbored an ermB gene. Sixteen (24%) erythromycin-resistant strains were also resistant to tetracycline. All were susceptible to penicillin and chloramphenicol.
Although there have been no confirmed reports of decreased susceptibility to penicillin in group A streptococci (GAS), resistance to erythromycin has emerged in some countries. Rates of resistance in Europe in the last decade have ranged from 10% in Sweden (13) up to 17% in Finland (15), 22% in the United Kingdom (23), and 47% in parts of Italy (3). Rates of resistance in excess of 50% have been reported in Taiwan and Japan (12, 19). Prevalence rates in some countries are still low, as in Argentina, where the current rate of erythromycin resistance is less than 1% (18).
Macrolides exert their action by binding to the bacterial ribosome and inhibiting protein synthesis. Resistance in streptococci has been shown to arise either through the presence of erm gene-encoded methyltransferases which induce ribosomal modification or through efflux. Macrolide efflux in GAS is effected by a membrane protein encoded by the mefA gene and is specific for 14- and 15-member macrolides such as erythromycin, clarithromycin, and azithromycin; the lincosamides and streptogramin antibiotics remain unaffected (M phenotype) (2). Erm methylases methylate 23S rRNA and induce ribosomal modification, which results in loss of binding not only to macrolides but also to lincosamides and the streptogramin B class of antimicrobials (MLSB phenotype) (29). Several methylases have been described for gram-positive bacteria (6); the ErmB (ErmAM) group is that most commonly found in streptococci (17). The ermTR gene in GAS has more homology to the ermA gene (83%) found in staphylococci than to the ermB gene (58%) from GAS (25). In a recent report, it was noted that in Finland, 60% of erythromycin-resistant strains had the M phenotype and all but one of the remaining strains with the MLSB phenotype had the ermTR gene (16).
Strains with a particular mechanism of resistance may have different prevalence rates depending on the country of origin. For example, with erythromycin-resistant Streptococcus pneumoniae, the M phenotype predominates in strains isolated in Canada and the United States (14, 28), whereas in South Africa, the MLSB phenotype is more prevalent (30). Although GAS strains with the M phenotype have been reported in the United States (4), there is little information about prevalence rates of mef and erm genes.
In this study we looked at the prevalence of erythromycin resistance in GAS clinical isolates collected in southern Ontario and examined their mechanisms of resistance.
MATERIALS AND METHODS
Strains.
From August to November 1997, all consecutive clinical isolates of GAS, 3,205 strains in total, were collected by a private laboratory serving community physicians throughout southern Ontario, Canada, representing a population base of 6 million people. Strains were identified by PathoDx (Diagnostic Products Corp., Los Angeles, Calif.).
Susceptibility testing.
Erythromycin-resistant strains were initially identified by disk diffusion on Mueller-Hinton agar supplemented with 5% sheep blood using 15-μg erythromycin disks (Becton Dickinson, Cockeysville, Md.) according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (21). MICs of penicillin, erythromycin, clindamycin, tetracycline, chloramphenicol (Sigma-Aldrich, Canada, Oakville, Ontario), and quinopristin-dalfopristin (Rhone-Poulenc Rorer, Collegeville, Pa.) were determined for erythromycin-resistant strains by using cation-supplemented Mueller-Hinton broth with 2% lysed horse blood in accordance with NCCLS guidelines (21). Incubation was performed at 37°C in O2, except for two strains which required CO2 for growth. S. pneumoniae ATCC 49619 and ATCC 6303 and Enterococcus faecalis ATCC 29212 were used as controls. In order to distinguish between M and MLSB phenotypes, erythromycin (15 μg) and clindamycin (2 μg) disks were placed on plates approximately 12 mm apart, and the plates were incubated overnight at 37°C in 5% CO2. Blunting of the growth inhibition zone around clindamycin in the area between the two disks was considered to indicate inducible MLSB resistance (MLSB phenotype); constitutive resistance was defined as zones of ≤15 mm around both the clindamycin and the erythromycin disk (21). The M phenotype was characterized by resistance to erythromycin and susceptibility to clindamycin.
PCR.
For the identification of ermABC and mef genes, multiplex PCR was performed with published primer sequences (27). Template DNA was prepared by mixing a loopful of bacteria grown overnight on blood agar with 100 μl of lysis buffer (100 mM NaCl, 10 mM Tris HCl [pH 8.3], 1 mM EDTA [pH 8.0], 1% Triton X-100) and boiling in a water bath for 10 min. After cooling, the suspensions were spun, and 5 μl of the supernatant was used as a template. Reactions were performed in a Perkin-Elmer 9600 thermocycler with a final reaction volume of 25 μl by using 4 mM MgCl2 and the following cycling conditions: denaturation at 94°C for 4 min, followed by 30 cycles of 94°C for 1 min, 57°C for 1 min, and 72°C for 1 min. A final elongation step was performed at 72°C for 5 min. The following primer sequences based on the sequence of the ermTR gene (GenBank accession no. AF002716) were used for detection of ermTR: GAAGTTTAGCTTTCCTAA (ermTRA, sense, 5′ to 3′) and GCTTCAGCACCTGTCTTAATTGAT (ermTRB, antisense, 5′ to 3′). Control strains were GAS 0261110 (ermTR) and S. pneumoniae 02J1175 (mefE), both kindly provided by J. Sutcliffe (Pfizer, Groton, Conn.). Reaction conditions were as described above except that 1.5 mM MgCl2 was used and amplification was carried out for 35 cycles with denaturation at 94°C for 30 s and annealing at 42°C for 30 s. PCR products were resolved by electrophoresis on 1% agarose gels. The expected sizes were 640 bp for ermA, ermB, or ermC; 348 bp for mef; and 400 bp for ermTR.
Southern hybridization.
Genomic DNA was isolated by lysis of a cell pellet derived from 1.5 ml of an overnight culture. Cells were washed with 1.0 ml of lysis buffer (50 mM glucose, 25 mM Tris [pH 8.0], 10 mM EDTA [pH 8.0], 150 mM NaCl) and incubated in 0.5 ml of lysis mixture consisting of lysis buffer plus 10 μl of DNase-free RNase (10 mg/ml), 50 μl of mutanolysin (1 mg/ml), and 50 μl of lysozyme (10 mg/ml) (all components were purchased from Sigma-Aldrich, Canada). Following lysis, DNA was extracted as described by Smith et al. (26) and resolved by electrophoresis on 1% agarose gels. Southern blotting was performed according to standard procedures (1). Hybridization was carried out with mefAE, ermB, and ermTR probes, derived by PCR as described above, from the following control strains. S. pneumoniae 02J1175 was used for mefE, and GAS 0261110 was used for ermTR. The ermB probe was amplified from S. pneumoniae BSP3585, which contains Tn1545 harboring ermB. Staphylococcus aureus RN4220, harboring plasmid pE194, was used for ermC, and ermA was amplified from Escherichia coli RN7951, containing an ermA gene from Tn554 cloned into pUC18 (both kindly provided by B. Kreiswirth, Public Health Research Institute, New York, N.Y.). Labeling and detection were carried out by using the ECL direct labeling enhanced chemiluminescence kit (Amersham Life Science, Oakville, Ontario, Canada) as outlined by the manufacturer.
PFGE.
DNA to be used for pulsed-field gel electrophoretic (PFGE) analysis was extracted essentially as described by Murray et al. (20) with modifications. A sample of 108 CFU from an overnight culture was washed in PIV buffer (1 M NaCl–10 mM Tris-HCl [pH 7.6]) and resuspended in 750 μl of the same buffer. An equal volume of 1.6% low-melting-point agarose (Bio-Rad Laboratories, Richmond, Calif.) at 65°C was added to the PIV-bacterial suspension; approximately 260 μl was aliquoted into PFGE plug molds (Bio-Rad Laboratories) and cooled to 4°C for 30 min. Plugs were incubated at 37°C in 1.5 ml of lysis mixture (100 mM Tris-HCl at pH 8.0–50 mM EDTA–1 M NaCl) containing 67 μg of RNase (Boehringer-Mannheim Canada, Laval, Quebec)/ml, 33 μg of mutanolysin/ml, and 300 μg of lysozyme (Sigma-Aldrich, Canada)/ml. After 5 h, 50 μl of 10-mg/ml proteinase K (Boehringer-Mannheim Canada) was added to the lysis mixture, and incubation was continued for 18 h. Plugs were washed three times with TE buffer (10 mM Tris-HCl–0.1 mM EDTA [pH 7.5]) and incubated with 10 U of SmaI (Boehringer-Mannheim Canada) at 25°C. DNA was resolved by using a contour-clamped homogeneous electric field (CHEF)-DRII apparatus (Bio-Rad Laboratories) in a 1% agarose gel at 175 V with an initial pulse time of 5 s and a final pulse time of 60 s for 24 h at 12°C in 0.5× Tris-borate-EDTA.
M typing.
Serotyping was carried out by the National Reference Center for Streptococci, Edmonton, Alberta, Canada, according to standard protocols (10).
RESULTS
Phenotypic characterization.
Of 3,205 isolates tested, 67 (2.1%) were found to be erythromycin resistant by disk diffusion. These isolates were also found to be erythromycin resistant by broth microdilution tests. Disk susceptibility results demonstrated that 47 (70%) were of the M phenotype and 20 (30%) were of the MLSB phenotype. Of the 20 MLSB resistant strains, 18 demonstrated blunting of the clindamycin inhibition zone when placed 12 mm from an erythromycin disk, indicating inducible resistance. Only two strains showed constitutive resistance to clindamycin.
Erythromycin MICs ranged from 1 to >16 μg/ml (Table 1). The MIC at which 90% of strains were inhibited (MIC90) of erythromycin for MLSB strains was 4 μg/ml, whereas that for strains demonstrating the M phenotype was 16 μg/ml. For all the erythromycin-resistant strains, including MLSB strains, the MIC of quinopristin-dalfopristin was 0.25 μg/ml or lower. Methylation of 23S rRNA results in resistance to streptogramin B-type compounds such as dalfopristin but not to streptogramin A-type compounds such as quinupristin, and synergy between the two compounds is conserved. All strains were susceptible to chloramphenicol and penicillin, but 16 (0.5%) were not susceptible to tetracycline; of these, 14 were of the MLSB phenotype.
TABLE 1.
In vitro susceptibility of 67 GAS strains to select antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)
|
||
|---|---|---|---|
| 50% | 90% | Range | |
| Erythromycin | 8.0 | 16.0 | 1.0–>16.0 |
| Clindamycin | 0.25 | 0.25 | 0.25–>16.0 |
| Quinupristin-dalfopristin | <0.12 | 0.12 | <0.12–1.0 |
| Tetracycline | 1.0 | 16.0 | ≤1.0–32.0 |
Genotypic characterization.
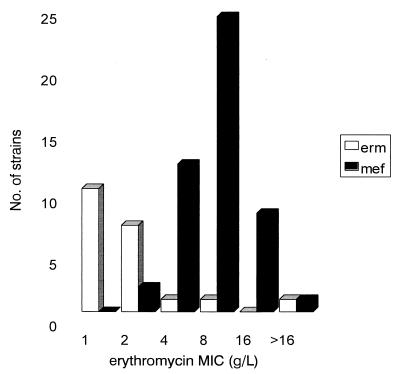
As predicted, all of the 47 (70%) strains bearing an M phenotype yielded an amplification product of 348 bp, which was consistent with the presence of the mef gene. The primers chosen in this study were based on conserved regions of the mefA gene from GAS and the mefE gene from S. pneumoniae, which have 90% homology. None of the strains with an M phenotype contained an erm determinant. Surprisingly, in the multiplex PCR assay using ermABC primers, only a single (constitutively resistant) MLSB strain yielded a product consistent with the presence of ermA, ermB, or ermC. This strain, for which the MIC was >16 μg/ml, was shown by Southern blotting to possess an ermB gene. However, when primers based on the ermTR gene were used, 19 strains were found to harbor ermTR (Table 2). Southern blotting of 12 representative strains confirmed these results. No hybridization with any of the probes was observed for five randomly selected erythromycin-sensitive strains. MICs for strains harboring ermTR genes generally were lower than those for strains possessing mef genes (Fig. 1).
TABLE 2.
Correlation of phenotype, M type, and genotype
| No. of strains with the indicated phenotypea | M type | T type | mef | ermA | ermB | ermC | ermTR |
|---|---|---|---|---|---|---|---|
| M (n = 46) | |||||||
| 18 | 4 | 4 | + | − | − | − | − |
| 1 | 11 | 11/12 | + | − | − | − | − |
| 2 | 12 | 12 | + | − | − | − | − |
| 1 | 28 | 28 | + | − | − | − | − |
| 21 | NTb | 4 | + | − | − | − | − |
| 2 | NT | 2/25 | + | − | − | − | − |
| 1 | NT | NT | + | − | − | − | − |
| MLSB (IR) (n = 19) | |||||||
| 1 | 2 | 2/28 | − | − | − | − | + |
| 1 | 4 | 4 | − | − | − | − | + |
| 1 | 12 | 12 | − | − | − | − | + |
| 1 | 22 | NT | − | − | − | − | + |
| 1 | 28 | 28 | − | − | − | − | + |
| 2 | 58 | 58 | − | − | − | − | + |
| 1 | 77 | 13 | − | − | − | − | + |
| 1 | 77 | 9/13 | − | − | − | − | + |
| 1 | 77 | 9/13/B3264 | − | − | − | − | + |
| 3 | NT | 12 | − | − | − | − | + |
| 1 | NT | 58 | − | − | − | − | + |
| 3 | NT | 3/13/B3264 | − | − | − | − | + |
| 1 | NT | 8/25/Imp19 | − | − | − | − | + |
| 1 | NT | NT | − | − | − | − | + |
| MLSB (CR) (n = 2) | |||||||
| 1 | 58 | 58 | − | − | − | − | + |
| 1 | 22 | 12/B3264 | − | + | − | − | − |
IR, inducible resistance; CR, constitutive resistance.
NT, nontypeable.
FIG. 1.
Correlation of the erythromycin MIC with the presence of mef and erm genes. For a single MLSB phenotype strain, the erythromycin MIC was >16 g/liter. This strain was constitutively resistant and was the only strain found to possess an ermB gene.
PFGE.
Of the 20 strains harboring ermTR, 12 showed different patterns, indicating that they were not clonal in origin. Despite several attempts, it was possible to resolve only 11 of 46 mef+ strains by PFGE, and among these, nine patterns were observed. No reason can be given for the lack of success in resolving M phenotype strains by PFGE, but this phenomenon has been noted by other groups (9).
Strain characteristics.
Of the 67 erythromycin-resistant strains, 64 were throat isolates and 3 were isolated from wounds. As shown in Table 2, 19 strains (28%) were M serotype, type 4; however, a large proportion of strains were nontypeable (34 of 67; 51%). The presence of mef or ermTR genes did not correlate with particular serotypes.
DISCUSSION
In this study, the overall rate of erythromycin resistance in GAS strains was low, at 2.1%, compared with the overall rates of 10 to 47% reported in European countries (3, 13, 15, 23). In an earlier study of Canadian strains, resistant strains were detected at a rate of 0.24% in 1971, increasing to 1.4% in 1972 (7). It is encouraging that the prevalence has remained low in Canada. In some countries, such as Spain, it increased steadily from 1.2% before 1990 to 35% in 1995, falling to 18% in 1996, possibly as a result of decreased macrolide use (22). The prevalence of erythromycin-resistant strains with the M phenotype (70%) in this study is lower than that reported in Spain and Sweden (90%) but is similar to that recently reported in Finland (13, 16, 22). In Sweden, the M phenotype has been highly prevalent since the early 1980s (13), and it was also present in Spanish isolates collected in the late 1980s (22). In the study of Canadian isolates collected in 1971 to 1972 in which 145 erythromycin-resistant strains were examined, no M phenotype isolates were identified (7). It is interesting that this phenotype has become so prevalent in Canada in a relatively short time, not only in GAS but also in S. pneumoniae (14). Kataja et al. (16) have shown that the mefA gene can be transferred from GAS to E. faecalis by conjugation, which may explain the spread.
All but 1 of the 20 strains showing the MLSB phenotype possessed the ermTR gene. The MICs of erythromycin for these strains, in general, were lower than those for strains harboring mef genes. This is unusual, since it has been noted previously in GAS, and also in S. pneumoniae, that MICs for MLSB (ermB+) strains tend to be higher than those for strains with the M phenotype (14, 22). It was also interesting that 14 of 16 tetracycline-resistant strains were of the MLSB phenotype. In S. pneumoniae, Tn1545, which confers MLSB resistance through the presence of the ermB gene, also confers tetracycline resistance through the tetM gene (5). Future studies should examine whether MLSB resistance in GAS might also be transposon mediated. The fact that the ermTR strains in this study had 12 different PFGE patterns suggests that the gene may have spread by horizontal transfer to strains of different genetic backgrounds.
It is encouraging that the GAS strains in this study were uniformly susceptible to penicillin and indeed that resistance to most antimicrobials was nonexistent or extremely low. Resistance to penicillin has not emerged in GAS despite extensive use of β-lactam drugs in many countries, and the reason for this is unknown (11). On the other hand, erythromycin resistance has correlated with antibiotic usage both in Finland and in Japan, and resistance was shown to decline when the use of macrolides was restricted (8, 24). It is, therefore, important to continue to monitor macrolide resistance rates on a national level so that if prevalence rates begin to increase significantly, attempts to control their use can be made.
ACKNOWLEDGMENTS
This work was funded in part by the Canadian Bacterial Diseases Network.
We thank the MDS Laboratory Service for providing the clinical isolates.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 2.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 3.Cocuzza C E, Mattina R, Mazzariol A, Orefici G, Rescaldani R, Primavera A, Bramati S, Masera G, Parizzi F, Cornaglia G, Fontana R. High incidence of erythromycin-resistant Streptococcus pyogenes in Monza (North Italy) in untreated children with symptoms of acute pharyngo-tonsillitis: an epidemiological and molecular study. Microb Drug Resist. 1997;3:371–378. doi: 10.1089/mdr.1997.3.371. [DOI] [PubMed] [Google Scholar]
- 4.Coonan K M, Kaplan E L. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr Infect Dis J. 1994;13:630–635. doi: 10.1097/00006454-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Courvalin P, Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986;205:291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- 6.Courvalin P, Ounissi H, Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985;16(Suppl. A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- 7.Dixon J M, Lipinski A E. Infections with beta-hemolytic Streptococcus resistant to lincomycin and erythromycin and observations on zonal-pattern resistance to lincomycin. J Infect Dis. 1974;130:351–356. doi: 10.1093/infdis/130.4.351. [DOI] [PubMed] [Google Scholar]
- 8.Fujita K, Murono K, Yoshikawa M, Murai T. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr Infect Dis J. 1994;13:1075–1078. doi: 10.1097/00006454-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Goossens H, Chapelle S, Hauchecorne M, Wijdooghe M, Descheemaeker P. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Characterization of macrolide resistance among group A Streptococcus in Belgium, abstr. C-14; p. 72. [Google Scholar]
- 10.Griffith F. Serological classification of Streptococcus pyogenes. J Hyg (London) 1934;34:542–584. doi: 10.1017/s0022172400043308. . (Abstract.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn D L, Zabriskie J B, Austrian R, Cleary P P, Ferretti J J, Fischetti V A, Gotschlich E, Kaplan E L, McCarty M, Opal S M, Roberts R B, Tomasz A, Wachtfogel Y. Why have group A streptococci remained susceptible to penicillin? Report on a symposium. Clin Infect Dis. 1998;26:1341–1345. doi: 10.1086/516375. [DOI] [PubMed] [Google Scholar]
- 12.Hsueh P R, Chen H M, Huang A H, Wu J J. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239–2242. doi: 10.1128/aac.39.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasir A, Schalen C. Survey of macrolide resistance phenotypes in Swedish clinical isolates of Streptococcus pyogenes. J Antimicrob Chemother. 1998;41:135–137. doi: 10.1093/jac/41.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Johnston N J, De Azavedo J C, Kellner J D, Low D E. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2425–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataja J, Huovinen P, Muotiala A, Vuopio-Varkila J, Efstratiou A, Hallas G, Seppala H the Finnish Study Group for Antimicrobial Resistance. Clonal spread of group A streptococcus with the new type of erythromycin resistance. J Infect Dis. 1998;177:786–789. doi: 10.1086/517809. [DOI] [PubMed] [Google Scholar]
- 16.Kataja J, Huovinen P, Skurnik M, Seppala H the Finnish Study Group for Antimicrobial Resistance. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopardo H A, Venuta M E, Vidal P, Rosaenz L, Corthey C, Farinati A, Couto E, Sarachian B, Sparo M, Kaufman S, De Mier C A, Gubbay L, Scilingo V, Villaverde P. Argentinian collaborative study on prevalence of erythromycin and penicillin susceptibility in Streptococcus pyogenes. The Argentinian Streptococcus Study Group. Diagn. Microbiol Infect Dis. 1997;29:29–32. doi: 10.1016/s0732-8893(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama S, Yoshioka H, Fujita K, Takimoto M, Satake Y. Sensitivity of group A streptococci to antibiotics. Prevalence of resistance to erythromycin in Japan. Am J Dis Child. 1979;133:1143–1145. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- 20.Murray B E, Singh K V, Heath J D, Sharma B R, Weinstock G M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 22.Perez-Trallero E, Urbieta M, Montes M, Ayestaran I, Marimon J M. Emergence of Streptococcus pyogenes strains resistant to erythromycin in Gipuzkoa, Spain. Eur J Clin Microbiol Infect Dis. 1998;17:25–31. doi: 10.1007/BF01584359. [DOI] [PubMed] [Google Scholar]
- 23.Phillips G, Parratt D, Orange G V, Harper I, McEwan H, Young N. Erythromycin-resistant Streptococcus pyogenes. J Antimicrob Chemother. 1990;25:723–724. doi: 10.1093/jac/25.4.723. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 24.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 25.Seppala H, Skurnik M, Soini H, Roberts M C, Huovinen P. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob Agents Chemother. 1998;42:257–262. doi: 10.1128/aac.42.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A M, Klugman K P, Coffey T J, Spratt B G. Genetic diversity of penicillin-binding protein 2B and 2X genes from Streptococcus pneumoniae in South Africa. Antimicrob Agents Chemother. 1993;37:1938–1944. doi: 10.1128/aac.37.9.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression—a review. J Antimicrob Chemother. 1985;16(Suppl. A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- 30.Widdowson C A, Klugman K P. Emergence of the M phenotype of erythromycin-resistant pneumococci in South Africa. Emerg Infect Dis. 1998;4:277–281. doi: 10.3201/eid0402.980216. [DOI] [PMC free article] [PubMed] [Google Scholar]