Abstract
The human population is generally subjected to diverse pollutants and contaminants in the environment like those in the air, soil, foodstuffs, and drinking water. Therefore, the development of novel purification techniques and efficient detection devices for pollutants is an important challenge. To date, experts in the field have designed distinctive analytical procedures for the detection of pollutants including gas chromatography/mass spectrometry and atomic absorption spectroscopy. While the mentioned procedures enjoy high sensitivity, they suffer from being laborious, expensive, require advanced skills for operation, and are inconvenient to deploy as a result of their massive size. Therefore, in response to the above-mentioned limitations, electrochemical sensors are being developed that enjoy robustness, selectivity, sensitivity, and real-time measurements. Considerable advancements in nanomaterials-based electrochemical sensor platforms have helped to generate new technologies to ensure environmental and human safety. Recently, investigators have expanded considerable effort to utilize polymer nanocomposites for building the electrochemical sensors in view of their promising features such as very good electrocatalytic activities, higher electrical conductivity, and effective surface area in comparison to the traditional polymers. Herein, the first section of this review briefly discusses the most important methods for polymer nanocomposites synthesis, such as in situ polymerization, direct mixing of polymer and nanofillers (melt-mixing and solution-mixing), sol–gel, and electrochemical methods. It then summarizes the current utilization of polymer nanocomposites for the preparation of electrochemical sensors as a novel approach for monitoring and detecting environmental pollutants which include heavy metal ions, pesticides, phenolic compounds, nitroaromatic compounds, nitrite, and hydrazine in different mediums. Finally, the current challenges and future directions for the polymer nanocomposites-based electrochemical sensing of environmental pollutants are outlined.
Graphical Abstract
1. INTRODUCTION
Over the past years, we have observed the increased global concern regarding the environmental contaminations and universal health influences caused by toxic and poisonous pollutants with heavy industrialization, agriculture activities, and the rapid urbanization. In addition, an acute global issue for half of the world population has been contaminated drinking water.1 In fact, people are constantly subjected to multiple adverse health impacts due to toxicity from these pollutants, fundamentally by ingesting the polluted drinking water and foodstuffs and inhaling the surrounding air with a higher concentration of contaminants.2 Exposure to toxic pollution may lead to numerous health consequences such as mental and physical dysfunctions, organ and neurological disorders, cancers, lower life expectancy, and the weakened body’s immune system, and in a many cases it results in mortality.3–8 The mentioned concerns have emphasized the prominence of devising reliable, highly sensitive, user-friendly, and inexpensive instruments to quantify pollutants at low concentration levels.
Some conventional analytical technologies such as gas chromatography/mass spectrometry (GC/MS),9 atomic absorption spectroscopy (AAS),10 high-performance liquid chromatography (HPLC),11 spectrofluorimetry,12 capillary electrophoresis,13 and flow injection chemiluminescence,14 etc. have been used to detect environmental pollutants. However, such analytical procedures have been laborious, expensive, needing advanced skills for their operation, and difficult to deploy due to the bulky size of the devices. In contrast, a quickly emerging class of electrochemical sensors shows promise as essential analytical detection devices because of their high sensitivity, selectivity, speed, flexibility, and cost-effectiveness.
Researchers describe a chemical sensor as one of the devices providing continual knowledge of its context. In an ideal situation, the chemical sensor would provide a specific kind of response with a direct relation to the amount of certain chemical samples. In fact, each chemical sensor comprises a transducer with the ability to transform a response into a visible signal on the contemporary instruments and a chemically selective layer, isolating the analyte response from its immediate context. These may be categorized based on their properties such as optical, electrical, thermal, or mass sensors. In addition, they have been devised for detecting and responding to an analyte in a liquid, gaseous, or solid state.15 In comparison to the mass, thermal, and optical sensors, the electrochemical sensors have been particularly attractive owing to the respective noticeable experimental simplification, detectability, and affordability.16–19 Moreover, the electrochemical sensors enjoy a prominent position among the contemporary sensors on the commercial level with major uses in industrial, clinical, agricultural, and environmental analysis.20–22
Electrochemical sensors typically function based on a redox reaction containing a target analyte in an electrolyte on a working electrode, resulting in the changes of an electrical signal; the signal generated is proportional to the concentration of the analyte. With regard to the signal that should be recorded, scientists classified the electrochemical techniques into amperometric, conductometric, impedimetric, potentiometric, and voltammetric methods.23 In recent times, the amperometric and voltammetric methods have played an important role in the field of electroanalysis.24–28
Amperometry is an electrochemical method in which the potential utilized to a sensing electrode is monitored instrumentally, and the current occurrence in oxidation/reduction on the surface of the electrode is measured as the analytical signal. The applied potential can be stepped to and held at a steady value, and the resulting current can be recorded as a function of time.
Voltammetry was identified by the Czech scientist Jaroslav Heyrovsky in 1920s. Contrary to the constant potential in the amperometric method, the voltammetric techniques utilize measuring current at different potentials in current voltage curves. Voltammetric procedures such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), square-wave voltammetry (SWV), linear sweep voltammetry (LSV), and stripping voltammetry, etc., have been regarded as a well-known device to determine the trace concentrations of critical environmental contaminants due to their high accuracy and sensitivity.
On the basis of the electrochemical sensors, the electrode surface alone has been considered as one of the robust tools to quantify an analyte. However, the bare electrodes suffer from several shortcomings such as nonreproducibility of the surface behaviors, slower kinetics of the electrochemical reactions for a number of compounds on the electrode surface and overpotential requirements. Thus, such variables restrict their capability and utilization to attain the analytical objectives. It is notable that the use of chemical and physical modification in the electrodes’ surfaces can eliminate such issues. In fact, the electrode modification has been viewed as one of the processes in which the bare electrodes’ surface would be covered by a chemical or biological compound included in the electrode matrix and altering its electrochemical attributes.29–33
The fusion of nanotechnology and electrochemical procedures would result in introducing robust and reliable electrical instruments for effectively processing and controlling the contamination. To develop highly sensitive electrochemical sensors, experts in the field have utilized many nanomaterials to modify the surfaces of the electrodes.34–36
In general, nanotechnology implies a field in the applied science and technology, which controls matter on the molecular or atomic levels in scales between 1 and 100 nm, and production of the tools in the mentioned range for dimension. Nanoparticles (NPs), with size features ranging from 1 to 100 nm, show distinctive physical and chemical properties such as unique electronic, magnetic, and optical properties, and chemical versatility.37 These distinctive features have rendered them as an excellent emerging sensing platform in the disciplines of analytical chemistry and electrocatalysis during the past two decades.38 While NPs contribute differently to diverse electrochemical sensors, the use of an NP-modified electrode enjoys many benefits based on electro-analysis outputs:
efficient catalysis
quick mass transfer
large sensor surface areas
reasonable control on the microenvironment of the electrode
Emerging polymers have been considered as a turning point in the contemporary analytical sciences. They are commonly pursued as signal-promoting elements in electro-analytical operations as a result of the respective prominent characteristics such as higher conductivity, lighter weight, more flexibility, simplified procurement, and greater active sites as well as strong adherence to the surface of the electrodes.39 Polymers do have limited applications, due to such factors as surface poisoning, low selectivity, low sensitivity due to the adsorbed interference, and intermediates from other species.40
Nanotechnology exposes inimitable opportunities to construct advanced combinations of nanoscale materials and polymeric materials to prepare polymer nanocomposites having unique properties. Toyota Research Group invented Nylon-6/clay nanocomposites in 1990 declaring a new horizon in the research area of polymer composites.41 Polymer composites comprising polymer and filler have been receiving significant attention lately. When a copolymer or polymer is utilized as a matrix or when nanofillers get dispersed in the polymer matrix,42–44 it constructs a polymer nanocomposite. The properties of these composites are often dominated by the filler. Polymers are generally reinforced by variable fillers to improve their limitations and expand their applications. The application of nanofillers to enhance physical and mechanical properties of polymers has provided an alternative fabrication route for the conventional polymer nanocomposites.45,46 This is because surface interactions with the adhesion, bonding, dispersion, matrix, particle motion, etc., depend to a large extent on the size of the filler particles. Some of these effects have profound impact on the polymer nanocomposite properties because they generally increase with a decrease in filler size. For instance, nanoscale fillers have very large surface to volume ratio and because properties such as adhesion, catalytic reactivity, chemical reactivity, electrical resistivity, and gas storage depend on the nature of the interface, these properties change dramatically. Therefore, polymer nanocomposites have many unique attributes, such as catalytical,47 electrochemical,48,49 mechanical,50 magnetic,51 optical,52 and biological properties,53 etc. It is notable that polymer nanocomposites have proven their prowess as an excellent transducer in electroanalytical sensors, because of their characteristics and synergic effects from the specific components in comparison with those obtained by their individual counterparts, such as morphological variations, facile synthesis, and smart responsibility.43 Their inimitable properties offer advantages, such as enhanced electrical conductivity, low cost, high specific surface area, rapid electrode kinetics, controllable chemical/electrochemical properties, high chemical stability in aqueous solutions, biocompatibility, and environmental stability. Therefore, recent development of polymer nanocomposites-based graphene (Gr), metal and metal oxide NPs, and carbon nanotubes (CNTs) have been attracting much interest for their use in electrochemical sensors.
This review focuses on the recent developments in polymer nanocomposite-based electrochemical sensors for the detection of environmental pollutants to protect the environment and human health. We first cover a brief description for the synthesis method of polymer nanocomposites. Then, variable electrochemical sensors based on polymer nanocomposites developed over the past decade are discussed for the analysis of diverse environmental pollutants (e.g., heavy metal ions, pesticides, phenolic compounds, nitroaromatic compounds, nitrite, and hydrazine). Finally, a summary of current challenges of polymer nanocomposites-based sensors, and an outlook of their future progress as emerging sensing materials, is explored.
2. METHODS FOR THE SYNTHESIS OF POLYMER NANOCOMPOSITES
The preparation of polymer nanocomposites using a proper processing technique is important to obtain their high efficiency. Several procedures have been developed for the synthesis of polymer nanocomposites. The most important methods are listed here.
2.1. In-Situ Polymerization.
The in situ polymerization method is the traditional technique for processing polymer nanocomposites.54–57 The principle of this synthesis method includes dispersion of the nanofiller in monomer solution followed by the polymerization of the resultant mixture in the presence of appropriate initiator such as heat, radiation, and oxidizing agent. The in situ polymerization aims to improve the properties of polymer nanocomposites due to good dispersion and distribution of the nanofillers in the polymer matrix. Furthermore, partially exfoliated structures are attainable via this method because of the homogeneous dispersion and intercalation of fillers in the polymer matrix. Despite the advancement of the in situ method in meliorating efficient dispersion of nanofillers in polymer matrices, this technique has several drawbacks namely complex procedures and processing steps and the requirement of expensive reactants. Further, the technique is not appropriate for all types of polymers, only those thermally unstable and insoluble in the solvent, and is suitable only for a limited number of elastomers.
A typical protocol was reported by Tamboli et al. for the synthesis of nanowires of silver–polyaniline (PANI) nanocomposite via in situ polymerization using a AgNO3 precursor and ammonium persulfate (APS) as an oxidizing agent; dodecylbenzenesulfonic acid (DBSA) was utilized as a protonic acid during the polymerization process.58 The synthesis of the Ag–PANI nanocomposite was carried out by direct addition of AgNO3 to double distilled water followed by the addition of DBSA under stirring. The precooled solution of the aniline monomer was added to the aforementioned solution at room temperature. Finally, APS was added dropwise to initiate the polymerization of aniline, and the reaction was continued with stirring at room temperature, to ensure complete polymerization of aniline. The ensuing greenish black precipitate was centrifuged and washed with distilled water to separate the impurities, such as excess acid, oxidant, and unreacted monomer, and eventually the product was dried.
2.2. Direct Mixing of Polymer and Nanofillers.
Direct mixing of nanofillers and a matrix polymer is a top-down method based on the breakdown of agglomerated fillers in the mixing process. This approach is appropriate for synthesizing polymer-based nanocomposites having submicrometer- or nanosized fillers with a dimension 1 or 2 orders of magnitude larger than the fillers dispersed in the hybrid materials. There are two main pathways for mixing the polymer and fillers:
2.2.1. Melt-Mixing.
Melt-mixing is a blending procedure of the nanofiller and the polymer melt to prepare the polymer nanocomposites. It has the advantage of being environmentally benign due to the lack of solvent utilization. Furthermore, it is compatible with industrial processes such as injection and extrusion molding, which makes it very convenient to apply the existing infrastructure and, thus, very economical. However, the elevated temperatures deployed during the process to melt the polymer can alter the surface modification of the filler.59
As an example, Al-Saleh et al. reported the preparation of Gr nanoplatelet-polystyrene (Gr NP/PS) nanocomposites which were prepared by melt-mixing of Gr NP and PS using a small batch mixer at 190 °C for 10 min at a mixing speed of 100 rpm.60 Then, the nanocomposites were placed in a compression molding machine after melt compounding to prepare 1.0 mm-thick rectangular plates; the molding being conducted under 25 MPa pressure at 220 °C for 10 min.
2.2.2. Solution-Mixing.
Solution-mixing is a very common method for preparing polymer nanocomposites on a laboratory scale.61 The polymer is dissolvable in a solvent such as an acetone, cyclohexane, dimethylformamide, tetrahydrofuran, toluene, etc. The process generally contains dispersion of nanofillers in an appropriate solvent and mixing them with the polymer solution. The resulting nanocomposites are recovered from solvent via evaporation of the solvent or by the solvent coagulation method; selection of the solvent is mostly governed by the solubility of the polymer matrix. The same solvent could be applied both, for the nanofiber and matrix.62 One of the advantages of this approach is the feasibility to attain good quality dispersion of the nanofillers in a suitable solvent. The obstacle is that this processing method cannot be used for insoluble polymers.63 Additionally, the elimination of organic solvent has environmental implications, which restricts the successful transition of this preparation technique from the laboratory to the industrial scale production.
In a typical example, Marroquin et al. reported the synthesis of a material based on chitosan (CHIT) in which the Fe3O4/multiwalled carbon nanotube (MWCNT)/CHIT nanocomposites were prepared by solution mixing.64 Fe3O4 and MWCNT were ultrasonicated for 1 h in distilled water before adding CHIT and acetic acid. The mixture was magnetically stirred followed by ultrasonication. The mixture was degassed and vacuum-dried to generate the nanocomposite.
2.3. Sol–Gel.
The sol–gel process (Figure 1) is a wet chemical technique used to synthesize polymer nanocomposites which are located on thin films.65,66 A relatively homogeneous distribution of NPs in the polymer matrix can be accomplished. In brief, sol–gel synthesis begins from the inorganic salt with the polymer monomers or highly reactive metal alkoxide which are used as precursors. Thereafter, the starting materials are mixed in the liquid phase, and it is important to control the molar ratio of the ingredients precisely; the subsequent hydrolysis reaction delivers the sol. After thermal treatment at low temperature, this sol would be changed to solid gel.67 As to the polymer nanocomposites which are prepared by this procedure, inorganic nanometer units, segments, and organic polymers are joined by hydrogen or covalent bonding.68 The advantages of this method include uniformity and high purity, easily controllable reaction, low sintering temperature, good forming ability, etc. However, there are several limitations in terms of actual application, for example, most of precursors are toxic and expensive. It is very demanding to prepare crystalline composites comprising polymers and inorganic oxide NPs, as the polymers are generally unaffordable at high temperature, which is the incumbent condition to make inorganic NPs form a crystal structure, and the process of sol–gel usually requires an extended period of a few days to weeks. In particular, the contraction brittle fracture due to the volatility of the solvents, small molecules, and water is the biggest problem for this method.
Figure 1.
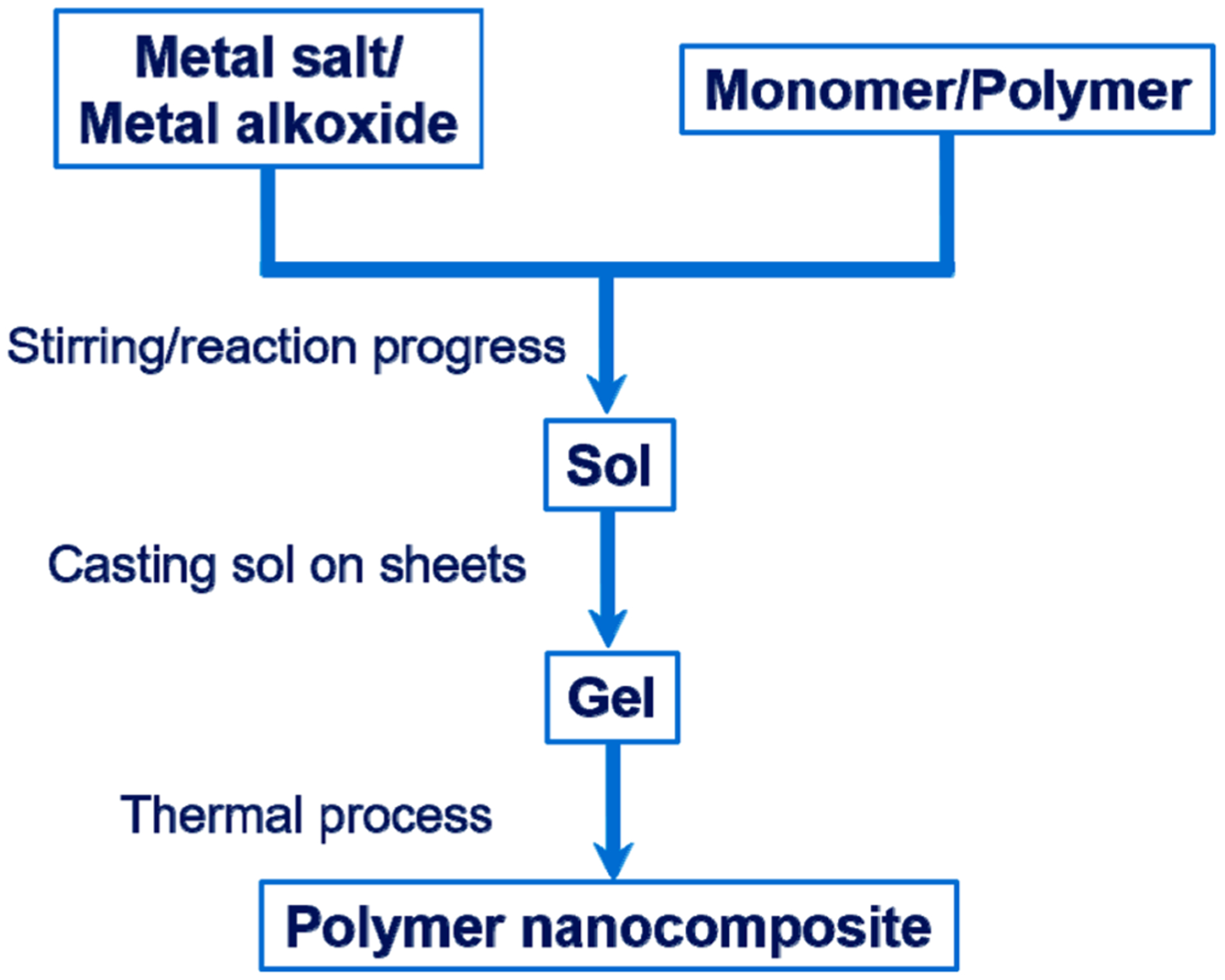
Preparation route for the sol–gel synthesis method.
As a typical example, Jaseela et al. prepared TiO2 poly(vinyl alcohol) (PVA) nanocomposite via the sol–gel route.69 Triethanolamine was added to the mixture followed by the dropwise addition of water to acquire the homogeneous sol. After the addition of PVA in the above sol and stirring, the solution at room temperature afforded the TiO2–PVA nanocomposite. After washing with a water–ethanol mixture, the product was dried in an oven. Then the TiO2–PVA nanocomposite was subjected to heat treatment in a muffle oven.
2.4. Electrochemical Method.
Electrochemical synthesis is a facile chemical preparation procedure which is achieved by electrochemical workstation.70,71 There are three electrodes including the counter electrode, working electrode, and reference electrode in the electrochemical process. As to the electrocatalytic applications, this synthesis procedure is the best way to prepare the polymer nanocomposite films which can be formed directly on the electrode surface.72 The electrochemical synthesis can be controlled simply by the applied potential or current density, and the amount of product can also be regulated by the integrated charges used. In general, this technique has many advantages, such as short reaction time and operational simplicity, and being easier to control and eco-friendly. In addition, the electrochemical method avoids application of the oxidant, thus achieving greater purity of the desired product. However, the use of electrochemical methods for large-scale production is limited because the working electrode has a limited surface area.
In a representative example, Gao et al. reported the dip-coating of reduced graphene oxide sheets (GNS) followed by electrodeposition of PANI nanofibers to prepare a layer-by-layer-GNS/PANI multilayer composite (Figure 2).73 Graphite oxide (GO) was synthesized using natural graphite by the Hummers method. Exfoliation of GO was accomplished by ultrasonication of the dispersion using an ultrasonic bath. Then, glucose was applied as reductant to prepare GNS. For the synthesis of GNS/PANI nanocomposite, stainless steel sheets were prepared as deposition substrates which were partially dipped into the GNS suspension and dried smoothly to form a GNS layer. The electro-polymerization was utilized to synthesize PANI nanofibers on the surface of the GNS film using a three-electrode electrochemical cell; a saturated calomel electrode (SCE) and platinum foil were applied. The stainless steel sheet coated with GNS film was directly utilized as working electrode for the deposition of PANI. Aniline (0.1M) and sulfuric acid (1 M) mixed solution was applied as electrolyte. Aniline monomers could be in situ electro-polymerized on GNS by cyclic voltammetry for two cycles at a scan rate of 5 mV s−1 with the applied potential of −0.2 to 1.2 V vs SCE. GNS/PANI nanocomposite could also be constructed via the electrostatic attraction between positively charged aniline monomer ions and negatively charged GNS nanosheets. Then the multilayer GNS/PANI composite could be assembled on the substrate by the deposition of the two components. The repeated electro-polymerization process (10 times) constructed the GNS/PANI multilayer composite. The coated samples were rinsed with ethanol and dried in an oven.
Figure 2.

(A) Schematic illustration for the fabrication of GNS/PANI nanocomposite: (i) preparation of GNS by glucose reduction of GO;(ii) GNS deposition on stainless steel substrate; (iii) electrodeposition of PANI nanofibers on GNS nanosheets. (B) Structure of PANI. Reproduced with permission from ref 73. Copyright 2013 Elsevier.
3. POLYMER NANOCOMPOSITES-BASED ELECTROCHEMICAL SENSORS FOR THE DETECTION OF ENVIRONMENTAL POLLUTANTS
Recently, environmental pollution is one of the most serious global challenges. Environmental pollution affects biodiversity, ecosystems, and human health worldwide by contaminating soil and water. This issue cannot generally be resolved through conventional tools and traditional strategies. Therefore, the development of simple, fast, and sensitive analytical methods to quantify pollutants in different media (e.g., food, soil, water, air, and biological samples) at trace level quantities has become a critical issue for both analytical chemists and environmental scientists. Chemical contaminants including heavy metal ions, pesticides, phenolic compounds, nitroaromatic compounds, nitrite, and hydrazine as major common pollutants are identified to be of great concern due to their toxicity, carcinogenicity, or endocrine disrupting effects. The appropriate applications of electrochemical methods have been proven to be very useful for resolving the environmental analysis issues due to high sensitivity, accuracy, and precision as well as a large linear dynamic range, with relatively simple and low-cost procedures. However, analysis by the unmodified electrodes is not generally feasible to acquire the quantitative results for the pollutant compounds due to the low level of environmental pollutants and the complex speciation in their sample matrices. Therefore, the modification of the electrode surface plays a crucial role in electrochemical sensor development. The efficient combination of different nanoscaled materials with proper conductive polymers opens a new avenue for utilizing novel polymer nanocomposites as applicable materials for constructing electrochemical sensing platforms for environmental pollutants detection with high performance. Indeed, this is a growing and demanding research area in analytical science. In this section, the application of polymer nanocomposites in electrochemical sensors for the detection of environmental pollutants namely heavy metal ions, pesticides, phenolic compounds, nitroaromatic compounds, nitrite and hydrazine in different mediums is briefly introduced.
3.1. Polymer Nanocomposite-Based Electrochemical Sensors for Heavy Metal Ions Detection.
According to the studies, heavy metal pollution could be of significant concern for environmental and human health due to its dramatic proliferation caused by both anthropogenic and natural sources, for example, automobile exhaust, industrial discharge, and mining.74 Moreover, some heavy metals are considered to be strongly carcinogenic even at insignificant amounts. Unlike organic pollutants, heavy metals are nonbiodegradable and have a tendency to aggregate in the food chain, which would severely impact people and environment health.75 Therefore, devising sensitive and selective procedures for initial identification of the trace level of heavy metals contamination in a variety of chemical systems such as the living system and the whole environment would be of paramount importance.
3.1.1. Hg2+.
Matlou et al. prepared the single-walled carbon nanotube poly(m-amino benzenesulfonic acid) (SWCNT-PABSA) modified gold (Au) electrode by the self-assembled monolayers (SAMs) procedure for the detection of Hg2+.76 A thiol comprising dimethyl amino ethanethiol (DMAET) was utilized for facilitating the SWCNT-PABSA molecules assembly over the surface of the electrode of Au. Moreover, Au-DMAET-(SWCNT-PABSA) has been utilized to detect Hg in water through the square wave anodic stripping voltammetry (SWASV) analyses. Furthermore, this sensor exhibited an acceptable sensitivity and a limit of detection (LOD) equal to 0.0634 μM. Also, the sensor showed a linear concentrations range between 20 and 250 ppb. It is notable that the analytical utility of the recommended technique with the sensor electrode was examined with real water samples; then, inductively coupled plasma mass spectrometry (ICP-MS) was used to validate the technique.
Zuo et al. recently devised an electrochemical sensor to detect Hg2+ at trace levels that was constructed using a poly(3,4-ethylene-dioxythiophene) nanorods/GO nanocomposite modified glassy carbon electrode (PEDOT/GO/GCE) (Figure 3).77 In addition, differential pulse stripping voltammetry (DPSV) has been utilized for detecting lower concentrations of Hg2+ on the PEDOT/GO/GCE. Furthermore, the experimental conditions such as the accumulation time (360 s), deposition potential (−0.2 V), and pH-values (0.1 M acetate buffer solution (ABS) in pH 5.0) have been optimized. On the basis of the optimized conditions, an acceptable linear association has been observed between the peak currents and Hg2+ concentration in the range between 10.0 nM and 3.0 mM. The LOD has been determined as 2.78 nM at the ratio of signal-to-noise of 3. Ultimately, the utility for detecting Hg2+ in the tap water samples was substantially approved.
Figure 3.
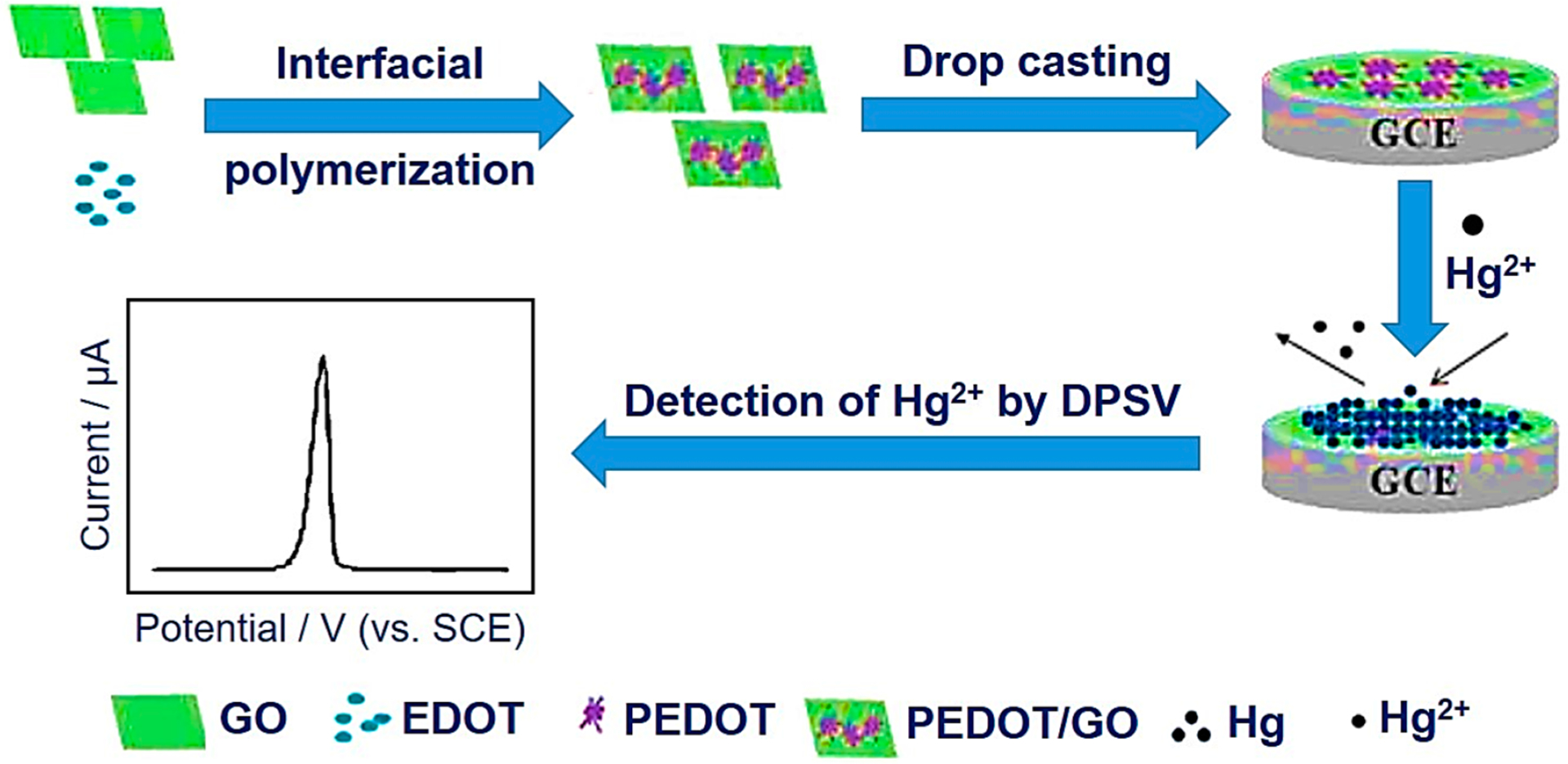
Preparation steps of PEDOT/GO/GCE and electrochemical detection of Hg2+. Reproduced with permission from ref 77. Copyright 2016 Elsevier.
3.1.2. Cd2+.
The preparation of a mulberry-shaped poly(2-methylaniline) (P2MA)-Ce2(WO4)3@CNT nanocomposite via sol–gel polymerization method was accomplished by Khan et al.78 This composite was characterized by various spectroscopic methods (scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), and thermogravimetric analysis). A very simple P2MA-Ce2(WO4)3@CNT nanocomposite was utilized for detection of cadmium ions using a Nafion(NF) bonded glassy carbon electrode (GCE). The sensitivity has been computed from the calibration curve; that is ~5.138 μA μM−1 cm−2. Additionally, the linear dynamic range of the P2MA-Ce2(WO4)3@CNT/NF/GCE sensor was utilized from 1.0 to 0.1 M (linearly, r2: 0.9917), in which LOD has been computed at about 0.11 nM (S/N) = 3. Also, the P2MA-Ce2(WO4)3@CNT/NF/GCE sensor was the most selective toward the Cd2+ ions compared to other ions.
3.1.3. Pb2+.
In their study, procurement and utilization of the nanocomposite based on the ion imprinted polymer (IIP) (methacrylic acid)-1-(2-pyridylazo)-2-naphthol (PAN)/MWCNT was accomplished by Tarley et al. as one of the newly devised electrochemical selective sensing platforms of Pb2+ in the water specimens.79 On the basis of the outputs, the polymer applied synergistic impact of the imprinting effect, MWCNTs, and ligand PAN to enhance the selectively and sensitivity of detecting Pb2+. Moreover, the limit of quantitation (LOQ) and LOD were equal to 0.50 μg L−1 and 0.16 μg L−1. Furthermore, interday technique accuracy was 1.72 and 1.86% (relative standard deviation (RSD)) and intraday accuracy was 2.53 and 1.68% for 10.0 μg L−1 and 5.0 of Pb2+. Finally, the effective utilization of this sensor for the synthetic urine and water samples was observed with reasonable recovery values between 95 and 103%.
Zuo et al. fabricated one of the sensitive Pb(II) sensors-based on the Co3O4/reduced GO (RGO)/CHIT nanocomposite-modified GCE.80Figure 4 indicates the procurement procedure of Co3O4/RGO/CHIT/GCE and electrochemical determination of Pb(II). The calibration plot for Pb(II) has been linear in the concentration ranges between 1.0 and 200.0 nM. In addition, the combined reasonable conductivity and higher surface areas of RGO, powerful Pb(II) absorption capability of the Co3O4 NPs, and very good Pb(II) complex-forming capability of CHIT provided a sensitive Pb(II) sensor with the LOD equal to 0.35 nM. Then, a CHIT with positive charge could interact with the RGO with a negative charge to enhance the Co3O4/RGO nanocomposite stability. Finally, this hybrid nanocomposite Pb(II) sensor provided encouraging opportunities to analyze vegetables.
Figure 4.
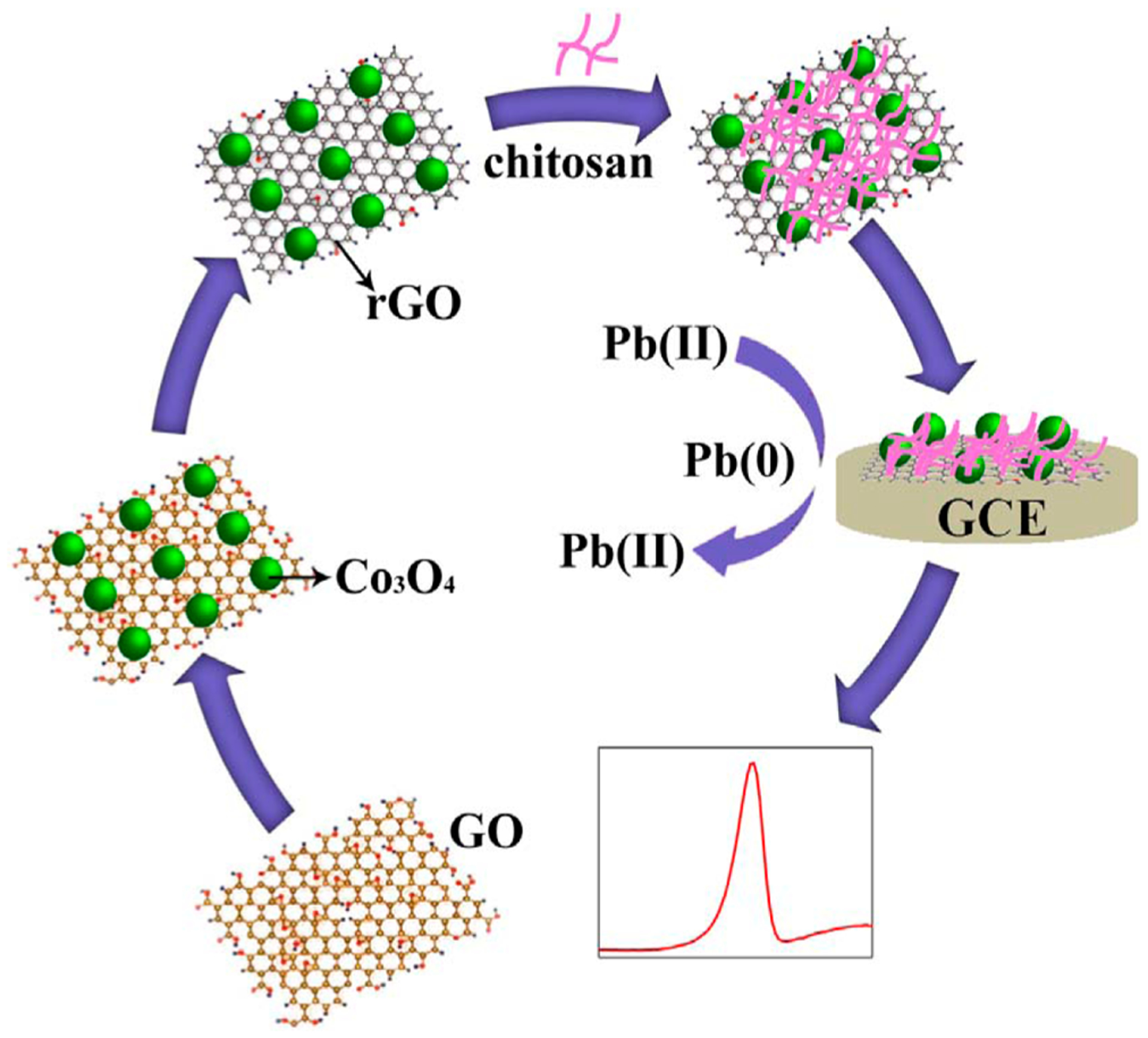
A schema of the production process of Co3O4/RGO/CHIT/GCE and electrochemical determination of Pb(II). Reproduced with permission from ref 80. Copyright 2017 Elsevier.
3.1.4. Cr(VI).
Hussein et al. fabricated poly(aniline-co-o-toluidine)/GO nanocomposite termed [PANI-co-PoT/GO] by a well-known in situ oxidative polymerization technique with ultrasonic assistance. However, one of the key objectives of their research was developing greater knowledge in electro-selectively applying the Au NPs as coatings.81 Thus, the Au NPs/PANI-co-PoT/GO nanocomposite modified Au electrode was effectively procured via an electroadsorption process as one of the electrochemical sensors to detect Cr(VI). Therefore, with the use of the SWV technique, the modified electrode linearly respond to Cr(VI) using a concentration range from 5–500 μM with the LOD equal to 0.0215 μM. This sensor had acceptable sensitivity, stability, selectivity and to detect Cr(VI) in the real samples.
3.1.5. As3+.
Dash and Munichandraiah devised the PEDOT modified Pd nanodendrite electrode, which was used for electro-analysis of As(III) in 1 M HCl solution.82 Moreover, the electrodeposition procedure was used to grow Pd nanodendrites on a porous thin film of PEDOT. According to CV investigations, the Pd-PEDOT/carbon paper electrode (CE) exhibited enhanced electrocatalytic activities toward the As(III)/As(0) redox reaction in comparison to Pd/CE. Then, researchers performed differential pulse anodic stripping voltammetry (DPASV) to analyze the As(III) ion at pH =1.0. It has been shown that the PEDOT-modified Pd electrode had higher sensitivity to detect As(III) (1482 μA cm−2μM−1). Furthermore, wider detection ranges to 10 μM and LOD = 7 nM (0.52 ppb) have been observed with a predeposition time equal to 120 s. Finally, Cu(II) ions demonstrated no interference when this test was performed.
3.1.6. Cd(II) and Pb(II).
Chamjangali et al. designed a voltammetric sensor based on GCEs coated with the MWCNT/poly(pyrocatechol violet) composite and bismuth film (GCE-MWCNT/poly(PCV)/Bi) to detect lead and cadmium as environmental contaminants.83 The presence of the Bi film improved the accumulation of analyte as a result of its capability for forming a fused alloy with lead and cadmium. This electrode linearly responded to Pb2+ and Cd2+ in an acceptable concentration ranging between 1.0 and 300.0 and 1.0–200.0 μg L−1. The LODs respectively were 0.20 μg L−1 and 0.40 μg L−1 for Cd2+ and Pb2+. As demonstrated by the findings, the sensor showed the lowest level of interferences from most of the common ions as a result of specific and selective interactions between modifier functionality and the cadmium and lead ions. However, the electrode response has been fixed for not less than 3 weeks of the consequent operations. Finally, this procedure has been utilized for simultaneously detecting the Cd2+ and Pb2+ content in the water samples.
Promphet et al. prepared one of the electrochemical sensors based on the Gr/PANI/PS nanoporous fiber modified screen printed electrode (SPE) for simultaneous detection of Cd2+ and Pb2+.84 To examine its application, the SWASV was utilized to simultaneously detect Cd2+ and Pb2+ in the presence of bismuth (Bi3+) on the Gr/PANI/PS nanoporous fiber-modified SPE. The anodic current linearly related to the metal ion concentrations within the ranges of 10 and 500 μg L−1 LOD (S/N = 3) equal to, respectively, 4.43 μg L−1 and 3.30 μg L−1 for Cd2+ and Pb2+. Additionally, researchers examined the impacts of common cation and anion interference that has been usually observed in environmental waters, accompanied by acceptable outputs. Ultimately, the designed electrode experienced a successful application to simultaneously detect Cd2+ and Pb2+ in real river water samples.
One of the electrochemical sensors for the simultaneous detection of the Cd(II) and Pb(II) ions, developed with a phytic acid (PA) functionalized polypyrrole (PPy)/GO modified electrode, was reported by Dai et al.85 The PA/PPy/GO modified GCE enjoyed higher electrochemical conductivity, notably increased peak current in comparison to PA/GO and PPy/GO modified electrodes. This sensor has been utilized to electrochemically analyze distinctive trace metal ions via DPV. Therefore, this modified electrode has been utilized for measuring Pb(II) and Cd(II) with the linear working ranges from 5 to 150 μg/L. The LOD (S/N = 3) was equal to 0.41 μg/L for Pb(II) and 2.13 μg/L for Cd(II). In addition, in order to evaluate the practical application of the PA/PPy/GO/GCE, it was used to determine Pb(II) and Cd(II) in tap water samples.
Baghayeri et al. introduced a GCE coated with poly(amidoamine) dendrimer functionalized magnetic GO (GO-Fe3O4–PAMAM) that has been utilized to simultaneously detect Pb(II) and Cd(II) in environmental water.86 The SWASV was utilized to detect the targeted metal ions. The electrochemical sensor was followed by a linear voltammetric response in the ranges between 0.4 and 120 μg L−1 for Pb(II) and 0.2 and 140 μg L−1 for Cd(II) with a coefficient of determination greater than 0.99. In addition, a lower LOD (130 ng L−1) was observed for Pb(II) and 70 ng L−1 for Cd(II). In addition, probable interferences from a number of metal ions such as Cu(II), Tl(I), Hg(II), Zn(II), Mg(II), Co(II), Mn(II), Ca(II), Fe(III), Ni(II), Cr(III), and In(III) have been investigated and it has been found that there has been no prominent interference. Ultimately, the current assay has been utilized to simultaneously detect the two cations in the real water samples; ICP-MS was used to verify the outputs.
Maleki et al. synthesized the second-generation (G2) PAMAM functionalized magnetic NPs (Fe3O4@G2-PAMAM) which has been utilized for concurrent electrochemical determination of Cd2+ and Pb2+ ions in the environmental water.87 The modified magnetic carbon paste electrode (MCPE) showed very good electrochemical features to simultaneously detect Cd2+ and Pb2+ with the use of SWASV. The linear range of 0.5–80 ng mL−1 for both metal ions was acquired at the Fe3O4@G2-PAMAM/MCPE. The observed LOD-values (0.17 ng mL−1 for Pb and 0.21 ng mL−1 for Cd) satisfied the threshold limit (10 ng mL−1 for Pb and 3 ng mL−1 for Cd) confirmed by the World Health Organization (WHO) for the normal volume of lead and cadmium in drinking water. Finally, they substantially dealt with the utility of Fe3O4@G2-PAMAM/MCPE to measure Cd2+ and Pb2+ ions in diverse real water samples (lake water, river water, and wastewater).
3.1.7. Hg(II) and Pb(II).
Wei et al. utilized PPy/carbonaceous nanospheres (PPy/CNSs) modified SPE in order to selectively detect the Hg(II) and Pb(II) ions.88 The optimization of the experimental conditions was performed using SWASV. Supporting Pb and Hg were deposited at the potentials of −1.0 V and −0.3 V for 120 s by the reduction of Hg(II) and Pb(II) in 0.1 M acetate buffer (pH 3.0). Considering the PPy and CNSs benefits in the electrochemical determination, they obtained very good sensitivity (0.113 μA nM−1 for Hg(II) and 0.501 μA nM−1 for Pb(II)). In addition, LODs (0.0041 nM for Pb(II) and 0.0214 nM for Hg(II) 3σ technique) have been very low in comparison to the guideline values for drinking water presented by the WHO. Finally, researchers substantially confirmed the analytical application of the designed electrode for detecting metal ions in a real sample.
3.1.8. Pb(II) and Cu(II).
Wang and co-workers described a new method for ultratrace detection of Pb(II) and Cu(II) with the use of Au nanocomposite electrode modified with NH2-MIL-53(Al)/PPy.89 The PPy nanowires were fabricated by a chemical polymerization method, and NH2-MIL-53(Al) was deposited on the PPy through an in situ electrochemical process. The electrochemical behavior of the modified electrode toward Pb(II) and Cu(II) was investigated by DPV. Because of the synergistic effect of NH2-MIL-53(Al) and PPy, the designed electrode displayed proper sensing performance to Pb(II) and Cu(II) in the range of 1–400 μg L−1 with an LOD of 0.315 μg L−1 and 0.244 μg L−1, respectively. NH2-MIL-53(Al)/PPy/Au also demonstrated strong anti-interference ability and good stability. In addition, this new sensor could be utilized for the determination of Pb(II) and Cu(II) in tap water samples.
3.1.9. Cd(II), Cu(II), and Pb(II).
Guo et al. used DPASV to devise the RGO–CHIT/poly-l-lysine nanocomposite modified GCE to simultaneously detect trace Pb(II), Cd(II), and Cu(II).90 The RGO and CHIT hybrid matrix were coated over the surface of GCE. Afterward, poly-l-lysine (PLL) films were procured by electro-polymerization with the CV technique for preparing the RGO–CHIT/PLL modified GCE (RGO–CHIT/PLL/GCE). It should be noted that three suitable sharp stripping peaks could be observed for Pb(II), Cu(II), and Cd(II). In addition, the stripping peak currents showed a suitable linear proportionality between 0.05 μg L−1 and 10.0 μg L−1 with the LOD equal to 0.01 μg L−1 for Cd(II), 0.02 μg L−1 for Cu(II), and 0.02 μg L−1 for Pb(II). Therefore, the LOD for Pb(II), Cu(II), and Cd(II) highly declined in comparison to the guideline values for drinking water presented by the WHO. Ultimately, researchers achieved substantial application for the analysis of tap water samples.
3.1.10. Cu(II), Hg(II), and Pb(II).
Deshmuket al. reported a ethylenediaminetetraacetic acid–PANI/SWCNTs (EDTA-PANI/SWCNTs) nanocomposite modified electrode to electrochemically detect lead(II), mercury(II), and copper(II) ions by DPV.91 In fact, a stainless steel electrode was rectified with the use of the PANI/SWCNTs nanocomposite followed by additional modification of the PANI/SWCNTs nanocomposite with the EDTA chelating ligand (Figure 5); the LOD for EDTA-PANI/SWCNTs/stainless steel toward the Pb(II), Hg(II), and Cu(II) was 0.08 ± 0.01 μM, 1.65 ± 0.01 μM, and 0.68 ± 0.01 μM, respectively. Furthermore, the simultaneous and selective detection of Cu(II), Pb(II), and Hg(II) ions was carried out in the presence 2 mM Co(II), Ni(II), and Cd(II) ions. The DPV voltammogram exhibited well-constructed and separated stripping peaks only for all Cu(II), Hg(II), and Pb(II); no rectified effect was observed after the addition of 2 mM of Cd(II), Co(II), and Ni(II).
Figure 5.
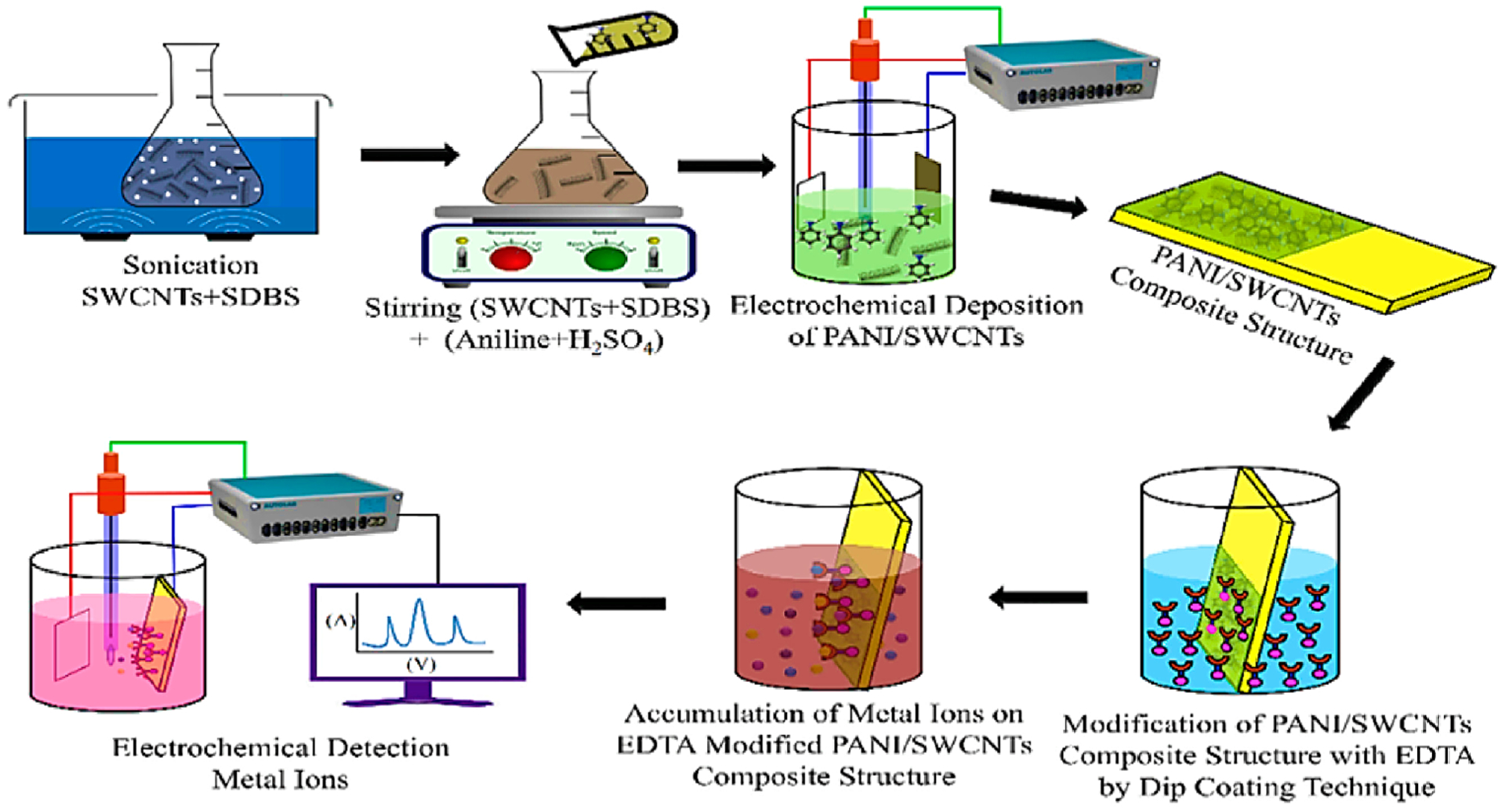
Diagram for the generation process of the EDTA-PANI/SWCNTs on a stainless steel electrode and utilization of the EDTA-PANI/SWCNTs/stainless steel electrode in detecting the heavy metal ions with DPV. Reproduced with permission from ref 91. Copyright 2018 Elsevier.
3.1.11. Cd(II), Pb(II), and Zn(II).
Ruecha et al. described a sensitive electrochemical sensor with the use of a Gr/PANI nanocomposite for simultaneously detecting the Cd(II), Zn(II), and Pb(II) ions.92 According to the analyses, the Gr/PANI-modified electrode had higher electrochemical conductivity and generated a 3-fold enhancement in the anodic peak current against the unmodified electrode. Moreover, the linear working range between 1 and 300 μg L−1 has been observed between the metal ion concentration and anodic current with the LOD equal to 1.0 μg L−1 for Zn(II), and 0.1 μg L−1 for both Pb(II) and Cd(II) (S/N = 3). Researchers found that the Gr/PANI-modified electrode selectively determined the targeted metals in the presence of common metal interference such as Cu(II), Fe(II), Fe(III), Mn(II), Ni(II), and Co(III) ions. Ultimately, their technique could be utilized to detect Cd(II), Zn(II), and Pb(II) ions in the serum of humans with the use of the standard addition procedure. Table 1 lists applications of polymer nanocomposites-based electrochemical sensors for detecting heavy metal ions in different environmental matrices.
Table 1.
Applications of Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Various Heavy Metal Ions in Environmental Samples
| electrochemical sensor | method | analyte | detection limit | linear range | ref |
|---|---|---|---|---|---|
| DMAET-SWCNT-PABSA/Au | SWASV | Hg2+ | 0.0634 μM | 20 ppb-250 ppb | 76 |
| PEDOT/GO/GCE | DPSV | Hg2+ | 2.78 nM | 10.0 nM-3.0 mM | 77 |
| P2MA-Ce2(WO4)3@CNT/NF-GCE | LSV | Cd2+ | 0.11 nM | 1.0 nM −0.1 M | 78 |
| IIP/PAN/MWCNT/GCE | DPASV | Pb2+ | 0.16 μg L−1 | 0.50−22.0 μg L−1 | 79 |
| Co3O4/RGO/CHIT/GCE | DPASV | Pb2+ | 0.35 nM | 1.0−200.0 nM | 80 |
| Au NPs/PANI-co-PoT/GO/Au | SWV | Cr(VI) | 0.0215 μM | 5−500 μM | 81 |
| Pd-PEDOT/CE | DPASV | As3+ | 7 nM (0.52 ppb) | 0.01 to 0.16 μM | 82 |
| GCE-MWCNT/poly(PCV)/Bi | DPASV | Cd2+ | 0.20 μg L−1 | 1.0−300.0 μg L−1 | 83 |
| Pb2+ | 0.40 μg L−1 | 1.0−200.0 μg L−1 | |||
| Gr/PANI/PS/SPE | SWASV | Pb2+ | 3.30 μg L−1 | 10−500 μg L−1 | 84 |
| Cd2+ | 4.43 μg L−1 | 10−500 μg L−1 | |||
| PA/PPy/GO/GCE | DPV | Cd(II) | 2.13 μg/L | 5−150 μg/L | 85 |
| Pb(II) | 0.41 μg/L | 5−150 μg/L | |||
| GO-Fe3O4−PAMAM/GCE | SWASV | Pb(II) | 130 ng L−1 | 0.4−120 μg L−1 | 86 |
| Cd(II) | 70 ng L−1 | 0.2−140 μg L−1 | |||
| Fe3O4@G2-PAMAM/MCPE | SWASV | Pb2+ | 0.17 ng mL−1 | 0.5−80 ng mL−1 | 87 |
| Cd2+ | 0.21 ng mL−1 | 0.5−80 ng mL−1 | |||
| PPy/CNSs/SPE | SWASV | Pb(II) | 0.0041 nM | 1−7 nM | 88 |
| Hg(II) | 0.0214 nM | 5−35 nM | |||
| NH2-MIL-53(Al)/PPy/Au | DPV | Pb(II) | 0.315 μg L−1 | 1−400 μg L−1 | 89 |
| Cu(II) | 0.244 μg L−1 | 1−400 μg L−1 | |||
| RGO−CHIT/PLL/GCE | DPASV | Cd(II) | 0.01 μg L−1 | 0.05−10.0 μg L−1 | 90 |
| Pb(II) | 0.02 μg L−1 | 0.05−10.0 μg L−1 | |||
| Cu(II) | 0.02 μg L−1 | 0.05−10.0 μg L−1 | |||
| EDTA-PANI/SWCNTs/stainless steel | DPV | Cu(II) | 0.08 ± 0.01 μM | 2 mM to 1.2 μM | 91 |
| Pb(II) | 1.65 ± 0.01 μM | 1.8 mM to 4 μM | |||
| Hg(II) | 0.68 ± 0.01 μM | 2 mM to 2 μM | |||
| Gr/PANI/SPE | SWASV | Zn(II) | 1.0 μg L−1 | 1−300 μg L−1 | 92 |
| Cd(II) | 0.1 μg L−1 | 1−300 μg L−1 | |||
| Pb(II) | 0.1 μg L−1 | 1−300 μg L−1 |
3.2. Polymer Nanocomposite-Based Electrochemical Sensors for the Detection of Pesticides.
As demonstrated by the relevant research, pesticides, including herbicides, fungicides, and insecticides are extensively applied in agriculture throughout the world to control pests in agricultural crops to enhance the agricultural production yield. Nonetheless, such a widespread utilization would lead to significant risks to the safety of the environment and human health. The precise assessment of their pollution status in the environment is needed for appropriate management and monitoring because of short-term health issues (i.e., eye and skin irritation, headache, nausea, and dizziness) and chronic diseases (i.e., asthma, diabetes, cancer, and neurological disorders) related to pesticide use.93 Consequently, simple, sensitive, and economical analyses of the trace amounts of the pesticides in environmental samples have been laborious tasks.
3.2.1. Fenitrothion (FNT).
Surucu et al. reported the electrochemical behavior and voltammetric determination of FNT on the basis of a pencil graphite electrode (PGE) modified with the RGO/poly(E)-1-(4-((4-(phenylamino)-phenyl)diazenyl)phenyl)ethanone (DPA) film.94 Analyses reflected the very good function of the modified PGE to detect FNT. Then, the SWV current response of RGO/DPA/PGE for FNT made a linear change with concentration ranges between 0.096 and 1.912 M with an LOD value of 3.480 × 10−9 M; analyses indicated suitable selectivity of RGO/DPA/PGE in the interference examinations and its application to detect FNT. Furthermore, the procedure resulted in the proper recovery of FNT from the spiked tomato specimens in a range between 96.4 and 106.9%, which reflected its utility to detect FNT in real environmental samples for the agricultural and industrial sectors.
3.2.2. Malathion (MLT).
Bolat and Abaci presented nonenzymatic electrochemical sensing of the MLT pesticide in apple and tomato samples on the basis of the Au NPs-CHIT-ionic liquid (IL) hybrid nanocomposite.95 PGEs have been adequately modified with an Au NPs-CHIT-IL hybrid for nonenzymatic electrochemical sensing of MLT directly. Thus, researchers demonstrated that the nanocomposite film expanded the electrodes’ surface area and created a very good ground to achieve the MLT electrochemical responses regardless of the acetylcholinesterase enzyme. Moreover, voltammetric current response displayed two linear dynamic ranges from 0.89 to 5.94 and 5.94 to 44.6 nM, which showed two binding sites with the LOD value of 0.68 nM. This technique has been exploited in analyzing real samples of tomato and apple, and outputs showed the possibility of the Au NP-CHIT-IL-modified electrodes to simply, rapidly, ultra-sensitively, and inexpensively detect MLT.
3.2.3. Paraquat.
Jeyapragasam and co-workers prepared poly(o-phenylenediamine) (PoPD)-MWCNT nanocomposite by the electropolymerization of o-phenylenediamine onto the functionalized MWCNT which acted as good modifiers for the electrochemical sensing of paraquat.96 The PoPD-MWCNT/GCE showed the highest cathodic current toward paraquat compared to GCE, MWCNT/GCE, and PoPD/GCE. The sensor calibration plot for paraquat was constructed using SWV in the linear range from 8.8 × 10−7 to 2.5 × 10−8 M. The developed sensor resulted in an LOD value of 1.4 × 10−9 M. Finally, this sensor has been substantially utilized for determining paraquat in cabbage samples.
3.2.4. Simazine (SMZ).
Zhang et al. presented their newly devised electrochemical sensor on the basis of the molecularly imprinted polymer membranes (MIPM) of poly(o-amino-thiophenol) with the Au NPs modified Au electrode to sensitively and selectively detect SMZ in the environmental samples.97 The nanoscaled MIPM with the increased specific surface area was procured by self-assembling the o-amino-thiophenol (ATP) and electrodeposition of ATP@Au NPs in the presence of the template SMZ. The linear dependency of the peak current on the SMZ concentration was in the range between 0.03 and 140 μM with an LOD of 0.013 μM; the SMZ imprinted sensor presented outstanding lengthy stability and reasonable repeatability. Additionally, it was successfully utilized for SMZ measurement in multiple real samples (tap and river water, and soil samples) so that the spiked recovery changed from 91.4% to 96.8%, which reflected an encouraging potential for functional applications.
3.2.5. Imidacloprid (IMI).
The IMI electrochemical detection with the use of the poly(carbazole)/chemically RGO modified GCE was devised by Lei et al.98 They used a simplified process to make polycarbazole (PCz) and PCz/chemically RGO (CRGO) modified GCEs and utilized them as the sensing electrodes for voltammetric exploration of the IMI insecticides. Moreover, CV and DPV have been utilized as the sensing procedures. According to the analyses, the two modified electrodes enjoyed acceptable electrocatalytic activities for reducing IMI. In addition, the presence of CRGO augmented the electrochemical signals of the modified GCE, increasing the sensor sensitivity. Furthermore, LOQ and LOD values for CV, respectively, have been 0.74 and 0.22 μM, whereas for DPV, they respectively equaled 1.52 and 0.44 μM.
3.2.6. Phoxim.
Wu et al. designed a newly fabricated nonenzyme amperometric platform on the basis of poly(3-methyl-thiophene)/nitrogen doped graphene rectified GCE (P3MT/NG/GCE) to detect the trace amounts of the pesticide phoxim.99 The linear variation of the reduction peak current of the CV curve was observed as the phoxim concentration changed over the two linear ranges between 0.02 and 0.2 μM and 0.2 and 2.0 μM so that lower LOD was 6.4 nM with regard to the declined linear range (S/N = 3). Moreover, the sensor had very good selectivity to phoxim in the wide range of probable interferences such as the ions, traditional environment contaminants, biomolecules, and the commonly applied organophosphatepesticides; environmental phoxim measurements were used to evaluate the reasonable functionality of the sensor.
3.2.7. Methyl Parathion (MP).
Xu et al. developed their newly devised poly(malachite green)/graphene nanosheets–NF (PMG/GrNSs–NF) composite film modified GCE for the electrochemical determination of MP.100 p-Nitrophenol, the alkaline hydrolysis product of MP, was substantially detected at a comparatively declined potential with the use of the proposed electrode as a result of very good electrocatalytic activities of the PMG/GrNSs–NF composite film. In a composite film, GrNSs promoted the PMG stability and the rate of the electron transfer. On the basis of the optimized conditions, chrono-amperometric response current was proportional to the MP concentration in ranges between 0.02 and 1.5 μM with the LOD of 2.0 nM. Consequently, the authors utilized their sensor to detect MP in real samples (cabbage, river water, tap water) wherein reasonable outputs with recovery have ranged between 97.20 and 104.53%.
Zeng et al. developed a voltammetric sensor to determine MP using the MWCNTs–poly(acrylamide) (MWCNTs–PAAM) nanocomposite film modified GCE.101 Considering the experimental outputs, MWCNTs–PAAM/GCE displayed increased absorption capacity and stronger affinity toward the MP in comparison to a number of metal ions and nitroaromatic compositions present in the environmental samples. Notably, the absorbed content of the MP on the MWCNTs–PAAM/GCE approximated the equilibrium value during a 5 min absorption time. Afterward, a linear calibration curve for MP was discerned in a concentration range between 5.0 × 10−9 and 1.0 × 10−5 M with an LOD of 2.0 × 10−9 M. Finally, researchers demonstrated that MWCNTs–PAAM/GCE is one of the appropriate sensing tools for quickly, sensitively, and selectively detecting MP in the samples of environmental water.
Zhao et al. presented a molecularly imprinted polymer–IL–Gr composite film coated GCE (MIP–IL–Gr/GCE) for electrochemical determination of MP.102 The researchers produced the electrode by coating a GCE with the IL–GO mix, accompanied by MIP suspension. In the next stage, the obtained electrode was conditioned at −1.3 V (versus SCE) in the Na2SO4 solution for making the GO turn to G. Then, MIP was procured via free radical polymerization of methacrylic acid as a functional monomer, MP as the template, ethylene-glycol dimethacrylate as the cross-linking reagent, and 2,2’-azobis(isobutyronitrile) as an initiator. The MP peak current linearly ranged between 0.010 and 7.0 μM with a sensitivity equal to 12.5 μA/μM and LOD of 6 nM (S/N = 3). This method displayed higher stability and selectivity and was utilized for detecting MP in the samples (cabbage, apple peel) so that the recovery ranged between 97 and 110%. Table 2 lists the applications of polymer nanocomposites-based electrochemical sensors for detection of pesticides in different environmental matrices.
Table 2.
Applications of Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Various Pesticides in Environmental Samples
| electrochemical sensor | method | analyte | detection limit | linear range | ref |
|---|---|---|---|---|---|
| RGO/DPA/PGE | SWV | fenitrothion | 0.096−1.912 μM | 3.480 × 10−9 M | 94 |
| Au NPs-CHIT-IL/PGE | SWV | malathion | 0.68 nM | 0.89−44.6 nM | 95 |
| PoPD-MWCNT/GCE | SWV | paraquat | 1.4 × 10−9 M | 8.8 × 10−7 to 2.5 × 10−8 M | 96 |
| MIP/ATP@Au NPs/ATP/Au | CV | simazine | 0.013 μM | 0.03 to 140 μM | 97 |
| PCz/CRGO/GCE | CV | imidacloprid | 0.22 μM | 3 to 10 μM | 98 |
| P3MT/NG/GCE | CV | phoxim | 6.4 nM | 0.02−2.0 μM | 99 |
| PMG/GrNSs−NF/GCE | chronoamperometry | methyl parathion | 2.0 nM | 0.02 to 1.5 μM | 100 |
| MWCNTs−PAAM/GCE | DPV | methyl parathion | 2.0 × 10−9 M | 5.0 × 10−9 to 1.0 × 10−5 M | 101 |
| MIP-IL-Gr/GCE | DPV | methyl parathion | 6 nM | 0.010−7.0 μM | 102 |
3.3. Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Phenolic Compounds.
The detection of phenolic compounds in food, medical, environmental ground, and surface water has been a key issue because of the respective toxicity and persistency in the environment.103 Some phenol derivatives are from industrial waste due to their use as intermediates in the production drugs, explosives, wood preservatives, and in leather treatments. These polluting chemicals are readily adsorbed by the skin and mucous membrane of human and animal. Moreover, their toxicity has a direct influence on multiple tissues and limbs, mainly the lung, liver, kidney, and the genitourinary system.104 The permissible level established by the United States Environmental Protection Agency (EPA) has been 1 mg/L for most of the common phenolic compounds.105 Therefore, numerous researchers have strived to address the simplified and efficacious detection of the phenolic compounds.
3.3.1. Phenol.
Yang et al. reported phenol electrochemical oxidation at a polydiphenylamine (PDPA)-MWCNT composite film modified GCE.106 The researchers used CV to polymerize PDPA on the MWCNT modified GCE in H2SO4(0.1 M). The composite film of PDPA-MWCNT exhibited suitable electrocatalytic behavior for oxidizing phenol at pH 7 where it displayed clear anodic peak at 0.65 V (versus the Ag/AgCl electrode). Results showed increase in the peak current by 2.36 and 8.48 times over that of the PDPA/GCE and MWCNT/GCE electrode with the use of MWCNT in the PDPA-MWCNT film. Peak current experienced a linear enhancement as the phenol concentration increased. Furthermore, linear ranges from 9.8 and 80 μM have been observed for the amperometric detection of phenol at the composite film modified electrode.
3.3.2. 2-Aminophenol (2-AP) and 4-Aminophenol (4-AP).
Yin et al. devised and utilized a Gr–CHIT composite film modified GCE for the voltammetric detection of 4-AP.107 According to the results obtained from the 0.1 M pH 6.3 phosphate buffer solution (PBS), the 4-AP redox peak currents notably increased, oxidation overpotential lowered, and a great decline was observed in the peak to peak separation at the Gr–CHIT composite film modified GCE in comparison to the bare GCE and CHIT modified GCE, which reflected electrocatalytic activities of graphene toward 4-AP. The current of the oxidation peaks corresponded to the range of 4-AP concentration between 0.2 and 550 μM with the correlation coefficient equal to 0.9930 and LOD of 0.057 μM (S/N = 3). According to this newly designed procedure, 4-AP could be readily detected in water specimens and paracetamol pills via the standard addition, which reflected the devised sensor utilization for detecting 4-AP in the environment and drug.
Fan et al. devised one of the electrochemical sensors on the basis of the Gr–PANI nanocomposite for voltammetric detection of 4-AP;108 CV was used to investigate the electrochemical behaviors of the 4-AP at the Gr–PANI composite film modified GCE where the analyses showed the increase of voltammetric response at Gr–PANI modified GCE via 4-AP. Moreover, it exhibited an acceptable analytical function to detect 4-AP with an LOD of 6.5 × 10−8 M and higher sensitivity of 604.2 μA mM−1. In addition, it could be possible to simultaneously detect the 4-AP and paracetamol with no interferences from them in the larger dynamic range. Finally, this Gr–PANI nanocomposite could present a novel ground to devise the graphene-based electrochemical sensors and biosensors.
In another study, one of the electrochemical sensors based on the RGO/poly-l-glutathione nanocomposite (RGO/P-L-GSH), developed for the determination of 4-AP, was reported by Ezhil Vilian et al.109 Researchers electrodeposited the P-LGSH on the RGO to generate the RGO/P-L-GSH composite matrix on a GCE (RGO/P-L-GSH/GCE). In addition, their electrochemical sensor superbly responded to 4-AP over a wide broad range between 0.4 and 200 μM (R2 = 0.9934) with the decreased LOD = 0.03 μM (S/N = 3) and sensitivity = 27.2 μA μM−1 cm−2. However, the sensor demonstrated suitable anti-interference capability in the presence of hydrogen peroxide, glucose, paracetamol, fructose, l-isoluecine, ethanol, l-cysteine, l-histidine, l-serine, dopamine, l-tyrosine, urea, phenylalanine, K+, Ca2+, Na+, F−, SO42−, Cu2+, Fe3+, Zn2+, SO42−, SO32−, Cl−, ascorbic acid, and folic acid. Finally, researchers experienced successful utilization of their procedure for producing a sensor to detect 4-AP in water and juice samples.
In their research, Yi et al. reported a strongly sensitive and simultaneous electrochemical detection of 2-AP and 4-AP.110 By simple electro-polymerization of l-arginine (l-Arg) and β-cyclodextrin (β-CD) on the CNTs@graphene nanoribbons (CNTs@GNRs) surface, the P-β-CD-l-Arg/CNTs@GNRs composites-modified GCE was achieved, which subsequently was applied for electrochemical sensing of the 4-AP and 2-AP (Figure 6). However, P-β-CD-l-Arg/CNTs@GNRs/GCE showed synergistic impacts from β-CD (that has a high host–guest recognition and enrichment ability), l-Arg (very good electrocatalytic activities), and CNTs@GNRs (major electrochemical features and larger surface areas). The P-β-CD-l-Arg/CNTs@GNRs/GCE was employed for the simultaneous determination of 2-AP and 4-AP with the linear response in the ranges between 25.0 and 1300.0 nM.
Figure 6.
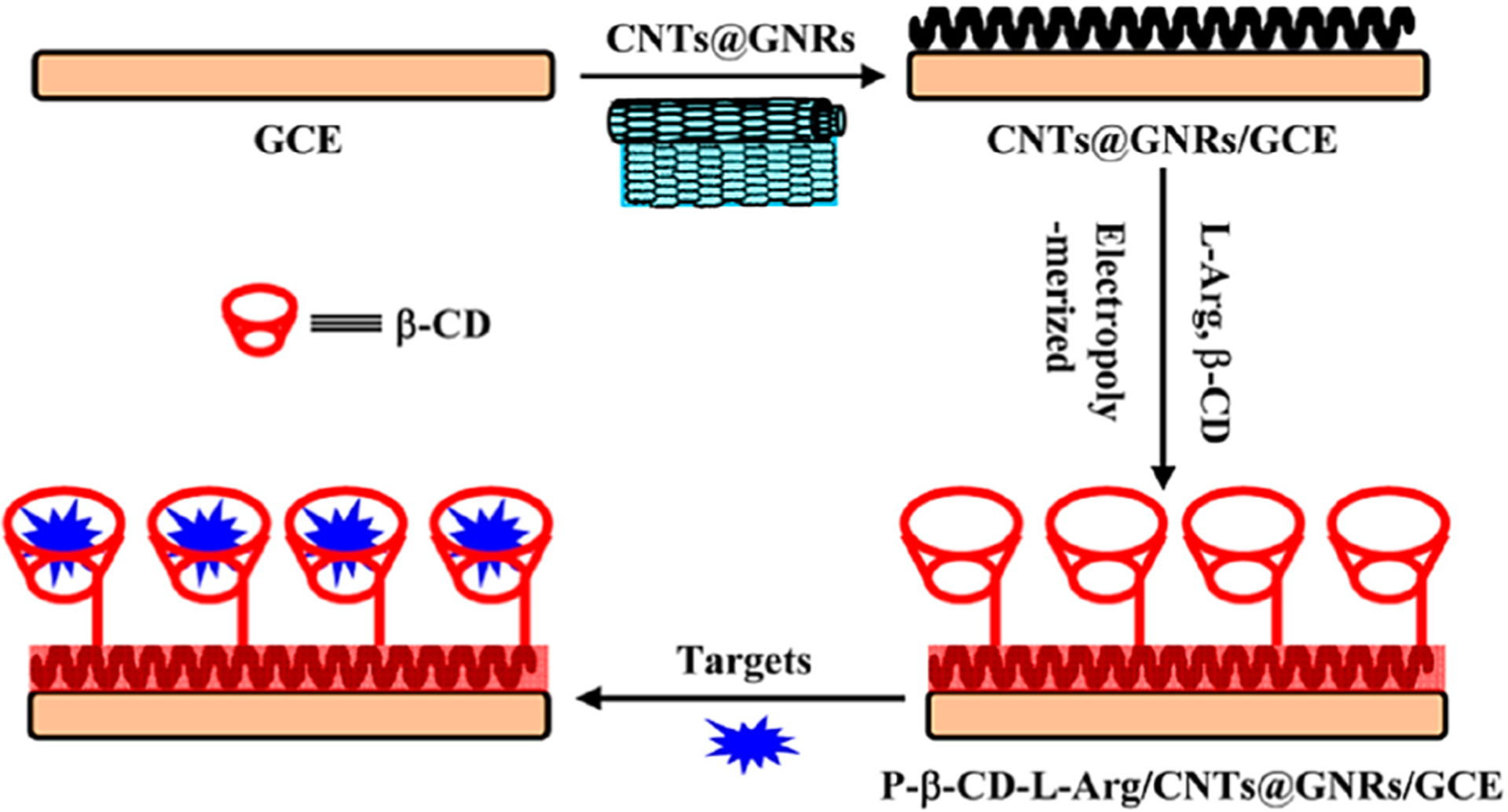
Procurement procedure for P-β-CD-l-Arg/CNTs@GNRs/GCE and sensing 2- and 4-AP with electrochemical technique. Reproduced with permission from ref 110. Copyright 2016 Elsevier.
3.3.3. Nitrophenol (NP).
Ndlovu et al. reported the electrochemical detection of ortho-nitrophenol (o-NP) by SWV with the use of a nanocomposite of the G2 poly(propyleneimine) dendrimer and the Au NPs modified GCE (PPI-Au NPs/GCE).111 Therefore, a simplified and reproducible one-step electrocodeposition of PPI dendrimer and Au NPs on the GCE was carried out with a typical reduction peak at ca-650 mV for o-NP with the greater currents. Then, they utilized the nanocomposite electrode as a sensor to detect the organic pollutant of the model with highly acceptable reproducible and stable features. It was found that the reduction peak current had a direct relationship with o-NP concentrations in a range from 6.1 × 10−7 M to 6.25 × 10−4 M with the LOD of 4.5 × 10−7 M.
Tang et al. procured the Gr-CHIT composite film-modified GCE (Gr-CHIT/GCE) for the simultaneous detection of o-NP and para-nitrophenol p-NP)112 and investigated the electrochemical behavior of p-NP and o-NP by CV; the modified GCE displayed very good electrocatalytic activities to reduce p-NP and o-NP. Using LSV method, p-NP exhibited the linear determination ranges from 0.1–140 μM with an LOD of 0.09 μM (S/N = 3) at G-CHIT/GCE, while 1–240 μM for o-NP with the LOD equal to 0.1 μM. Finally, Gr-CHIT was demonstrated as an encouraging sensor material for simultaneously detecting the nitrophenol isomers. The excellent recovery of the spiked samples indicated that the as-prepared electrode was practical for the detection of nitrophenol isomers in real samples.
An electrochemical sensor for sensitively detecting the o-NP was constructed by Arfin et al. using the GO-poly-(ethylenimine) dendrimer (GO–PEI)-modified GCE which showed very good electrochemical behaviors toward o-NP redox via a combination of the PEI and GO features.113 The peak currents linearly related to o-NP concentration in a range between 5 and 155 μM. According to the analyses, the LOD of o-NP has been as low as 0.10 μM characterized by the signal-to-noise (S/N = 3) properties. In addition, the RSD for three times the detection of o-NP has been 0.12%, revealing acceptable features of the sensor such as its ability to be reproducible, selective, and stable. Finally, researchers reported successful utilization for the sensor to treat the industrially polluted waters in the course of removing the heavy metals such as Ni2+, Fe3+, Pb2+, Cu2+, and Zn2+ ions.
Wei and co-workers developed ZnFe2O4/PANI@RGO aerogel as a convenient electrochemical sensor for the detection of p-NP.114 The synthesized ZnFe2O4/PANI@RGO aerogel exhibited excellent electrochemical sensing for p-NP and efficient catalytic reduction. In addition, ZnFe2O4/PANI@RGO modified GCE demonstrated a linear concentration range between 1 and 100 μM, excellent sensitivity of 36.898 mA mM−1 cm−2, and an LOD equal to 0.083 μM for p-NP. The ZnFe2O4/PANI@RGO/GCE offered long-term stability and excellent anti-interference ability for p-NP detection. In addition, the detection reliability of tap water was estimated, and an average recovery of 101.16% was maintained.
3.3.4. Chlorophenols (CPs).
Wang et al. constructed a strongly selective electrochemical sensor to detect 4-chlorophenol (4-CP). Therefore, to attain higher selectivity, they dealt with the synthesis of the MIP of 4-CP and blended it with poly(diallyldimethylammonium chloride) (PDDA)-functionalized Gr(PDDA-Gr).115 This MIP/PDDA-Gr/GCE exhibited a higher sensing function toward 4-CP. On the basis of the optimum conditions, their sensor linearly responded to 4-CP concentration in the wider ranges between 0.8 to100 μM with an LOD of (S/N=3) of 0.3 μM. Furthermore, the imprinted sensor had very good specific recognition capability for 4-CP, avoiding the interference of the phenolic compounds with the same structure. Finally, its successful utilization for detecting 4-CP in tap and lake water samples was illustrated with satisfactory analytical results.
Zhu et al. constructed a novel electrochemical sensing platform to quantitatively determine and assess the toxicity of of 2,4-dichlorophenol (2,4-DCP) with the use of poly(eosinY)/hydroxylated MWCNT modified electrode (PEY/MWCNTs–OH/GCE).116 Researchers observed linear enhancement of the oxidation peak current as the concentration was enhanced from 0.005 to 0.1 μM and 0.2 to 40.0 μM with an LOD of 1.5 nM. In addition, PEY/MWNTs–OH/GCE displayed very good electrocatalytic activities onto the intracellular electro-active samples. They attributed two sensitive electrochemical signals to adenine/hypoxanthine and guanine/xanthine in the human hepatoma (HepG2) cells, which were determined concurrently, and successful application of the sensor was demonstrated for the evaluation of 2,4-DCP toxicity to the HepG2 cells. According to the analyses, the IC50-values based on two electrochemical signals were 252.83 and 201.07 μM.
3.3.5. Dihydroxybenzenes Isomers.
Yin et al. prepared Gr–CHIT composite film modified GCE (Gr–CHIT/GCE).117 Their fabricated electrode had very good electrochemical catalytic activity toward oxidation of resorcinol (RS), catechol (CC), and hydroquinone (HQ) and their simultaneous detection in the water specimens. The oxidation overpotentials of RS, CC, and HQ notably declined and accordingly the oxidation current enhanced significantly in comparison to currents observed at the bare GCE and CHIT modified GCE. They utilized DPV to simultaneously detect the RS, CC, and HQ in their ternary mix. Moreover, the peak to peak potential separation between RS and HQ, HQ and CC, and CC and RS, respectively, was 0.484, 0.096, and 0.388 V. Furthermore, calibration curves for RS, HQ, and CC, respectively, ranged from 1 × 10−6 to 5.5 × 10−4 M, 1 × 10−6 to 3 × 10−4 M, and 1×10−6 to 4 × 10−4 M with an LOD of 7.5 × 10−7 M(S/N = 3).
Xu et al. developed a sensitive amperometric sensor for the selective detection and electrocatalytic oxidation of HQ in cosmetics on the basis of superb electrocatalytic properties of CNT-doped PEDOT conducting polymer.118 The electrochemical oxidization of HQ at the PEDOT/CNT modified CPE was investigated using CV and DPV which reflected the highly declined oxidation potential of HQ on the PEDOT/CNT/CPE in comparison to the bare electrode. The DPV current of the sensor was linear with the HQ concentration in the ranges between 1.1 and 125 μM with an LOD of 0.3 μM. Thus, this sensor could readily quantify HQ in the presence of conventional interference and could be used in real cosmetics specimens with reasonable accuracy.
Nambiar et al. designed the specific Au atomic cluster–PEDOT nanocomposite modified Au electrode (AuAC/PEDOT/Au) for ultrasensitive voltammetric determination of CC.119 The addition of copper(II) resulted in the enhancement of the CC electrocatalytic oxidation by forming copper(I). Figure 7 presents a schematic illustration of the preparative procedure for the AuAC/PEDOT/Au electrode which offers a broader calibration range between 1 × 10−4 and 10 μM with the minimum LOD of 6.3 pM for CC. The electrochemical sensor displayed acceptable selectivity reproducibility (1.23% for 1 nM of CC), and it has been applied for routinely detecting and quantifying catechol in natural water specimens.
Figure 7.

Schematic representation for the fabricated catechol electrochemical sensor. Reproduced with permission from ref 119. Copyright 2013 Royal Society of Chemistry.
Tehrani et al. devised a voltammetric sensor to simultaneously detect CC and HQ using Gr and poly(4-vinylpyridine) modified GCE (Gr-P4VP/GCE)120 where the electrode applied a synergistic impact of Gr and P4VP on the electrocatalytic oxidation of HQ and CC in sodium PBS at 2.5 pH. Then, CV and DPV were deployed to resolve the anodic peak potential of Epa, providing simultaneous detection of the two compounds. Thus, Gr-P4VP/GCE had very good sensitivity for measuring CC and HQ with an LOD of 26 and 8.1 nM, respectively, and had no impact on the conventional interference by nitrophenol, phenol, chloro-phenols, and aminophenols. Finally, it has been used to simultaneously detect the CC and HQ spikes in tap and lake waters with reasonable outputs.
Li et al. prepared a GCE modified with poly(sulfosalicylic acid) (PSA) and PDDA-Gr via a convenient self-assembly procedure for the simultaneous determination of CC and HQ.121 Under optimal conditions, the modified GCE exhibited two complete redox waves for HQ and CC in CV with a peak potential separation equal to 111 mV, ensuring the anti-interference potential of the electrochemical sensor and providing the simultaneous detection of the dihydroxybenzene isomers in real specimens. The results showed a linear anodic peak current of CC within a concentration range between 1 × 10−6 and 4 × 10−4 M in the presence of 3 × 10−5 M HQ and an LOD of 2.2 × 10−7 M (S/N = 3). Additionally, the HQ anodic peak current was found to be linear in a concentration range between 2 × 10−6 and 4 × 10−4 M in the presence of 2 × 10−5 M CC with an LOD of 3.9 × 10−7 M (S/N = 3) and it was utilized for concurrently detecting HQ and CC in tap water with reasonable outputs.
Tian et al. synthesized one-dimensional (1D) PEDOT-Gr composites with the help of in situ electro-polymerization of 3,4 ethylene-dioxythiophene on the Gr.122 The 1D PEDOT-Gr composite showed outstanding electrocatalytic activity toward dihydroxybenzene isomers and NO2– ions. Simultaneous detection of the four analytes namely CC, HQ, nitrite, and RS, was recorded by DPV using the PEDOT-Gr/Ta electrode which exhibited the peak to peak separation of 392 mV between CC and RS, 108 mV between HQ and CC, as well as 188 mV between RS and nitrite, while the LOD respectively was 0.08, 0.06, 7, and 0.16 mM for CC, HQ, nitrite, and RS. Furthermore, 1D PEDOT-Gr composite displayed higher electrocatalytic activities and selectivity, and faster response ability.
Liu et al. prepared poly(N-methylthionine) (PNMTh) at RGO electrode in one of the strong acid solutions with the use of electrochemical polymerization; PNMTh/RGO nanocomposite served as electrochemical sensing materials for sensitively detecting HQ.123 As a result of the synergistic effects between RGO (large specific surface area and higher conductivity) and PNMTh (redox properties), the PNMTh/RGO nanocomposite efficiently enhanced the electron transfer between the electrode and HQ, thus reducing the overpotential of the HQ oxidation. In addition, the linear regression area ranged between 1.0 and 1000.0 μM, the LOD was 0.75 μM, and importantly catechol had no interference with HQ detection.
Cheng et al. constructed an electrochemical sensor by electrodeposition of dendritic Au nanostructures (DGNs) and electropolymerization of PEDOT consecutively on GCE (PEDOT/DGNs/GCE). The constructed sensor with a dendritic structure, large surface area, and good conductivity, displayed high sensitivity for the detection of dihydroxybenzene isomers124 with a lower LOD of 9.5 × 10−7 M for HQ, 1.6 × 10−6 M for CC, and 1.3 × 10−6 M for RS. Finally, their sensor showed acceptable results for a successful application to determine dihydroxybenzene in cosmetic samples with satisfactory results.
3.3.6. Naphthol Isomers.
Li et al. fabricated a polymer film incorporated with the Au NPs modified electrode125 which entailed the eletrodeposition of Au NPs and electro-polymerization of 3-methylthiophene (3MT) over the GCE; semi-derivative voltammetry was used for simultaneously detecting naphthol isomers at the P3MT nano-Au/GCE in the presence of surfactant, cetyltrimethylammonium bromide (CTAB). Additionally, the researchers evaluated the contribution of assorted surfactants, and the respective mechanisms were comprehensively illustrated. Under the optimized conditions, the linear calibration range for detecting naphthols was between 7.0 × 10 and 7 M to 1.5 × 10 and 4 M for 1 naphthol and 1.0 × 10−6 and 1.5 × 10−4 M for 2-naphthol with an LOD of 1.0 × 10−7 and 3.0 × 10−7 M (S/N = 3), respectively.
3.3.7. Bisphenol A (BPA).
Yin et al. proposed a GCE, which was modified with the PAMAM dendrimers and composite Au NPs as well as the silk fibroin (PAMAM-Au NPs-SF/GCE)126 which showed a differentiated electrochemical response to BPA. The electrochemical impedance spectroscopy (EIS) was utilized to characterize the surface where the electrode showed improvement in the absorption capacity and increase in the response to BPA in comparison to a surface with no modification; a linear response in the concentration ranges between 1 nM and 1.3 μM with a correlation coefficient of 0.9991 and LOD of 0.5 nM at an S/N of 3 was discerned. Thus, the procedure could be used for detecting BPA in water samples with the recovery ranging between 97% and 105%.
Yin et al. presented a simplified and sensitive electro-analytical technique to detect BPA with the use of PAMAM and Fe3O4 magnetic NPs modified GCE.127 However, in comparison to the bare electrode, the PAMAM–Fe3O4 modified electrode notably improved the BPA oxidation peak current and declined the oxidation overpotential, thus reflecting on the remarkable improvement in the detection of BPA sensitivity via the modified electrode. A linear increase of the oxidation current was observed as the BPA concentration range was enhanced from 1 × 10−8 to 3.07 × 10−6 M with a correlation coefficient of 0.9996 and LOD of 5 × 10−9 M. Then, the current approximated 95% of the steady-state current in ~6 s. Finally, the procedure was utilized with encouraging outputs for detecting BPA in milk samples.
Zhang et al. devised a ratiometric electrochemical sensor for strong sensitive and selective detection of BPA (Figure 8).128 Their assay strategy was established on the competitive host–guest interactions between poly-β-cyclodextrin/electro-reduced graphene (Pβ-CD/ERGr) and Rhodamine B (RhB) probe or BPA target molecule. In fact, RhB is capable of entering the β-CD hydrophobic inner cavity and displayed a clear oxidation peak on the Pβ-CD/ERGr modified GCE. Consequently, in the presence of BPA, RhB molecules were displaced by BPA as the host–guest interaction between BPA and β-CD was more powerful in comparison to the interaction between RhB and β-CD. Hence, RhB (IRhB) oxidation peak current decreased with the increase in the oxidation BPA (IBPA) peak current. Furthermore, the logarithmic value of IBPA/IRhB was linear with the logarithm of BPA concentration in a range between 1 and 6000 nM and LOD of 52 pM (S/N = 3). Finally, the developed electrode was used to detect BPA in real-water samples.
Figure 8.

A schematic of the ratiometric electrochemical sensor to detect BPA. Reproduced with permission from ref 128. Copyright 2015 Elsevier.
Peng et al. described an electrochemical sensor to simultaneously detect BPA and HQ.129 This electrochemical sensor was devised on a CPE that was doped with Gr and coated with poly(melamine) (PME/Gr-CPE). The modified electrode exhibited very good conductivity, electro-oxidative activities, and absorptive abilities with DPV peaks separated for BPA and HQ by ~300 mV. It was found that the amperometric response (at 0.5 V for HQ and 0.8 V for BPA both versus SCE) was linear for BPA in a concentration ranging between 9.0 μM and 1.0 mM, and for HQ between 7.0 μM and 1.0 mM with LOD values of 10.5 and 74.0 nM, respectively. Finally, the sensor was reproducible with high stability and sensitivity, and could be utilized for the simultaneous detection of HQ and BPA in spiked tap water and wastewater.
Li et al. proposed a simplified electrochemical sensor with the RGO-Ag/PLL modified GCE for BPA detection,130 where the synthesized RGO-Ag/PLL nanocomposites displayed higher electrocatalytic activities toward BPA electrochemical oxidation. Additionally, DPV was applied as an analytical procedure to quantitatively detect BPA as the electrochemical sensor linearly responded to BPA in ranges between 1 and 80 μM with an LOD of 0.54 μM at the signal-to-noise ratio of 3. The BPA sensor exhibited excellent selectivity, stability, and reproducibility and was finally utilized for detecting BPA in the drinking water satisfactorily.
Bolat et al. presented an electrochemical for the detection of BPA based on a convenient procedure131 where CTAB and MWCNTs were blended via electro-polymerization on the PGE surface through CV to produce the poly(CTAB)-MWCNTs composite. Analysis showed a significant increase of the BPA electrochemical signal at poly(CTAB)-MWCNTs modified PGE with an LOD value of 134 pM with an acceptable regression between the BPA peak current (R2 = 0.993) and the concentration with the use of the SWV in ranges between 2 and 808 nM. The technique was used to detect BPA in the bottled water, a baby’s bottle, and a teether from local markets with the greatest level of recovery.
Ramu and colleagues synthesized the Fe3O4–PEI nanocomposite for the sensitive electrochemical detection of BPA in real samples.132 The Fe3O4–PEI/carbon electrode showed high electrochemical sensitivity and good catalytic activity toward BPA. According to the analyses, the linear ranges of BPA were equivalent to 0.01 to 50 μM. The LOD was 3.2 nM. The fabricated electrode successfully detected BPA in milk and water samples with high recovery percentages of 100.9% and 100.5%, respectively. Table S1 in the Supporting Information lists the recent applications of polymer nanocomposites-based electrochemical sensors for the detection of phenolic compounds in different environmental matrices.106–132
3.4. Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of the Nitroaromatics.
Nitroaromatic compounds have been associated with contaminants, toxicity, carcinogenesis, mutagenesis, and treatment actions, have appeared as intermediates in the syntheses of complicated organic molecules, and are found to be responsible for major environmental pollution issues. Therefore, the development of specific and convenient analytical methods for nitroaromatic compounds is very significant.
3.4.1. Nitrobenzene (NBz).
Xu et al. developed a selective and sensitive electrochemical sensor for detection on a CPE modified nanocomposite procured from the conducting polymer PEDOT and MWCNT133 which displayed excellent catalytic activity toward the electrochemical reduction of NBz; the NBz reduction potential was significantly lowered with an increase in the reduction current. The modified CPE could determine NBz in a concentration range between 0.25 and 43 μM with an LOD of 83 nM. The highly stable sensor endowed with reapplicable potential had no interference by other widely found nitrocompounds and could be utilized for the detection of NBz in wastewater specimens.
Ren et al. devised a sensitive electrochemical sensor to detect nitrobenzene on immobilized silver NPs (AgNP)-PME-MWCNT on a GCE.134 It has been found that the modified electrode had an effective electrocatalytic contribution to reduce NBz and remarkably declined the nitrobenzene overpotential; therefore, the peak current enhanced greatly compared with the bare GCE or the other modified electrodes. The sensor had a wider linear calibration range between 20 and 1000 μM and between 1000 and 6000 μM, with lower LOD of 0.55 μM to detect NBz. The modified electrode enjoyed acceptable stability, reproducibility, and selectivity and was successful applied as a sensor for detecting NBz in the river water and tap water samples with a recovery between 97.2 to 104.6%.
Kariuki et al. developed an electrochemical sensing platform for NBz with the use of the silver NPs (Ag NPs) inserted in a poly(amic) acid (PAA) polymer matrix (PAA-Ag NPs)135 and the PAA-Ag NPs-modified GCE exhibited encouraging potential as an electrochemical sensor. It exhibited wider linear dynamic ranges between 10 and 600 μM with a correlation coefficient of 0.9735, LOD of 1.6 at 8 μM, and sensitivity of 7.88 μA/μM. This sensor showed the least interference impacts from structurally similar nitroaromatic compositions and metal samples namely 2-nitrophenol (2 NP), 4-nitroaniline (4-NA), dinitrobenzene (DNBz), Pb2+, and Cd2+.
3.4.2. 2,4,6-Trinitrotoluene (TNT).
Cotchim et al. used a PME/GO layer modified GCE for the adsorption and electrochemical determination of 2,4,6-TNT.136 Then, the electrochemical behavior and surface morphology of the PME/GO/GCE were established by FT-IR, SEM, CV, and adsorptive cathodic stripping voltammetry (AdCSV). The produced PME/GO/GCE exhibited magnificent adsorption and electrochemical reduction of TNT via the AdCSV method with two linear ranges, 1–90 mg L−1 and 100–1000 mg L−1, an LOD of 0.34 mg L−1 and LOQ of 1.14 mg L−1, respectively; good repeatability, high sensitivity, and selectivity were discernible which prompted its successful application for detecting TNT in soil samples with reasonable recovery that ranged from 93 to 99%.
Mahmoud et al. developed a flexible electrochemical sensor on poly(styrene-co-acrylic acid) PSA/SiO2/Fe3O4/Au NPs/lignin modified GCE for selectively detecting traces of TNT in an aqueous medium with enhanced sensitivity.137 Figure 9 depicts the fabrication steps of lignin-modified magnetic microspheres (L-MMS). The LOD was equal to that of TNT detected by amperometric i–t curve, ~35 pM. Notably, higher sensitivity and very good catalytic activities of the modified electrode may be ascribed to the lignin layer, and the strongly packed Fe3O4/Au NPs on the surface of the electrode. Furthermore, total recovery of TNT from tap water and seawater matrices was found to be 98% and 96%, respectively. Moreover, the electrode film was highly stable following five iterated absorption/desorption cycles, and the newly devised electrochemical sensing provided a very sensitive, selective, and flexible assay for in situ determination of TNT in the complicated water media.
Figure 9.
Synthesis of PSA/SiO2/Fe3O4/Au NPs/L-MMS.
Shamsipur et al. used the electrochemically reduced graphene oxide-PAMAM hybrid (ErGO-PAMAM)-modified GCE (ErGO-PAMAM/GCE) for the fabrication of a TNT electrochemical sensor138 which had high sensitivity to TNT relative to those of GCE, GO/GCE, GO-PAAM/GCE, and ErGO/GCE. As demonstrated by the outputs, ErGO-PAMAM/GCE had higher response to the TNT in comparison to the remaining graphene-based modified electrodes and the sensor displayed acceptable response to the TNT with a linear range between 0.05 and 1.2 ppm and LOD of0.0015 ppm.
Sağlam et al. developed a electrochemical sensor to detect the nitroaromatic explosive substances on Au NP-modified GCE coated with the poly(o-phenylenediamine-aniline film) (GCE/P(O-PDA-CO-ANI)-Au nano electrode).139 The calibration curve of the current intensity vs concentration linearly ranged at 2.5 to 40 mg L−1 for 2,4,6-TNT with an LOD of 2.1 mg L−1, 2 to 40 mg L−1 for 2,4-dinitrotoluene (DNT) with an LOD of 1.28 mg L−1, and 5 to 100 mg L−1 for tetryl. The coefficients of variation of intra-assay and inter-assay measurements, respectively, were 0.6% and 1.2% (N = 5), which denoted higher reproducibility of the assay. The technique experienced a successful application for analyzing nitro-aromatics in military explosives, namely, comp B, octol, and tetrytol.
Yang et al. prepared molybdenum disulfide–PABSA (MoS2–PABSA) nanocomposite with the help of a simplified electro-polymerization process to detect TNT.140 The nanocomposite possesses a very good electrochemical detection function for aromatic ring compositions such as TNT with robust π–π interactions with MoS2–PABSA sensing platform; a significantly sensitive determination for TNT, LOD of 0.043 ppb (0.187 nM), and a linear range between 0.1 and 200 ppb was observed. Their study revealed that the MoS2–PABSA was a worthy electrochemical sensing platform for additional simplified and sensitive determination of several other aromatic ring compositions. Table 3 lists applications of polymer nanocomposite-based electrochemical sensors for the detection of nitroaromatic compounds in various environmental matrices.
Table 3.
Applications of Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Various Nitroaromatic Compounds in Environmental Samples
| electrochemical sensor | method | analyte | detection limit | linear range | ref |
|---|---|---|---|---|---|
| PEDOT/MWCNT/CPE | amperometry | nitrobenzene | 83 nM | 0.25−43 μM | 133 |
| AgNP/PME/MWCNT/GCE | DPV | nitrobenzene | 0.55 μM | 20−6000 μM | 134 |
| PAA-Ag NPs/GCE | DPV | nitrobenzene | 1.68 μM | 10−600 μM | 135 |
| PME/GO/GCE | AdCSV | 2,4,6-trinitrotoluene | 0.34 mg L−1 | 1−1000 mg L−1 | 136 |
| PSA/SiO2/Fe3O4/Au NPs/lignin/GCE | amperometry | 2,4,6-trinitrotoluene | 35 pM | 0.05−10 nM | 137 |
| ErGO-PAMAM/GCE | DPV | 2,4,6-trinitrotoluene | 0.0015 ppm | 0.05 to 1.2 ppm | 138 |
| 2,4,6-trinitrotoluene | 2.1 mg L−1 | 2.5−40 mg L−1 | |||
| P(O-PDA-CO-ANI)-Au NPs/GCE | CV | 2,4-dinitrotoluene | 1.28 mg L−1 | 2−40 mg L−1 | 139 |
| tetryl | 3.8 mg L−1 | 5−100 mg L−1 | |||
| MoS2−PABSA/GCE | DPV | 2,4,6-trinitrotoluene | 0.043 ppb | 0.1−200 ppb | 140 |
3.5. Polymer Nanocomposites-Based Electrochemical Sensors for Detection of Nitrite.
The nitrite ions (NO2–) are major prevalent poisonous inorganic contaminants found in soil, food, physiological systems, and water. Further, the increased concentration of nitrite ions may be derived from caustic radioactive wastes.141 Excessive nitrite ions are harmful to humans, and the increased concentration of nitrite in drinking water would be a severe hazard for the health of infants. Additionally, nitrite can combine with hemoglobin in the blood of humans forming methemoglobin, causing a phenomenon that is usually called “blue baby syndrome”. Further, it is possible to convert nitrite into nitrosamine that has been implicated in cancer and high blood pressure.142 Due to increasingly stringent environmental protection to protect public health, the quantitative monitoring of nitrite in food and drinking water has become an important task.
Ye et al. fabricated a nitrite sensor on a Gr/PPy/CHIT nanocomposite film-modified GCE (Gr/PPy/CHIT/GCE).143 It was found that −NH3+ in the structure of CHIT attracted the ions with negative charge such as NO2–so that it could accumulate the nitrite ions on the surface of electrode; CV and EIS were used to examine the behavior of the electron transfer of the modified electrode in [Fe(CN)6]3–/4– redox probe. Moreover, DPV and amperometry were utilized to study the electrochemical features of the sensor which displayed reasonable stability and reproducibility to detect nitrite. The linear response ranged between 0.5 and 722 μM with an LOD of 0.1 μM (S/N = 3) to detect nitrite, and this sensor was utilized for the determination of nitrite in lake water and river water samples with satisfactory results.
Wang et al. examined the oxidative electro-chemistry of nitrite on the GCEs modified with cobalt NPs, Gr, and PEDOT (Co NPs-PEDOT-Gr/GCE)144 because of the synergistic effect of the nanoscale properties of Co NPs and the promoted electron transferrance by PEDOT-Gr composite, the overpotential for nitrite electro-oxidation was reduced at the Co NPs-PEDOT-Gr/GCE (~500 mV lower than unmodified GCE); the peak current linearly related to the nitrite concentration in the range from 0.5 μM to 240 μM, with an LOD of 0.15 μM. The modified electrodes enabled the determination of nitrite at low potentials where the noise level and interferences by other electro-oxidizable compounds are weak.
Ning et al. used a simplified chemical reduction technique to synthesize silver NPs stabilized by PAMAM dendrimer.145 The newly devised electrochemical sensor detected nitrite on the basis of PAMAM nanocomposite. Figure 10 depicts the construction process of a nitrite electrochemical sensor based on Ag–PAMAM nanocomposite which displayed very good electrocatalytic function to oxidize nitrite; the sensor showed a broad linear range between 4.0 μM and 1.44 mM at 6.0 pH of the PBS. Additionally, this sensor enjoyed attributes such as higher sensitivity (265 μAmM−1 cm−2) and a shorter response time of three seconds. Moreover, an LOD of 0.4 μM and the sensor ability to detect nitrite have been observed in tap water and milk samples.
Figure 10.
A schematic for the procurement procedure of Ag-PAMAM/GCE. Reproduced with permission from ref 145. Copyright 2014 Elsevier.
Xu et al. constructed a sensitive electrochemical nitrite sensor based on poly(diallyldimethylammonium chloride) (PDDA) functionalized RGO (PDDA-RGO).146 Modification with PDDA endows a positive surface charge and RGO with good dispersity facilitating adsorption of nitrite as investigated by an amperometric method. The PDDA-RGO modified electrode exhibits lower overpotential equal to 0.75 V, with a wider linear range from 0.5 to 2 mM and a lower LOD of 0.2 μM for nitrite sensing. Additionally, the recoveries for NO2−(10 μM) spiked into drinking water is in the range of 95.4% to 106%, thus illustrating the possibility of the proposed sensor to sense nitrite in real samples.
Lin et al. described the synthesis of a nanocomposite with PEDOT doped with the NPs (Au NPs) by electropolymerizing the EDOT in a solution comprising colloidal Au NPs.147 According to the outputs, nanocomposite exhibited reasonable conductivity as well as a three-dimensional microporous network structure as a result of the presence of the Au NPs; they function as the conductive dopant and as a template to grow the microporous polymer. The GCE modified with nanocomposite exhibited very good electrocatalytic activities to oxidize nitrite. Finally, in the case of the best operation at a voltage of 0.8 V (vs Ag/AgCl), it could determine nitrite in a concentration ranging between 0.2 and 1400 μM with the LOD of 60 nM.
Liu et al. reported a sensitive nitrite sensor on the basis of the PDDA-coated Fe1.833(OH)0.5O2.5-decorated NG ternary hierarchical nanocomposite (Fe1.833(OH)0.5O2.5/NG@PDDA.148 The sensor exhibited very good analytical functions to detect nitrite linearly within two distinct concentration ranges between 0.1 and 347 μM as well as 347 and 1275 μM with an LOD of 0.027 μM (S/N = 3). Additionally, the electrochemical sensor showed acceptable stability, reproducibility, and very good antiinterference capability versus the metal ions and electro-active samples and exhibited reasonable recovery to detect nitrite in drinking water.
Madhuvilakku et al. designed a GCE modified with RGO/molybdenum disulfide/PEDOT nanocomposite (RGO/MoS2/PEDOT/GCE) for the detection of nitrite in water and milk.149 Electrochemical results indicated that the RGO-MoS2–PEDOT modified GCE exhibited improved electrochemical activity in 0.1 M PBS (pH = 4) compared to bare GCE, RGO-MoS2/GCE, and PEDOT/GCE. The modified electrode demonstrated good sensitivity (874.19 μA Mm−1cm−2), low LOD (0.059 μM, S/N = 3), and a wide linear range(0.001–1 mM) of nitrite with high selectivity, reproducibility, and long-term stability. The practical usability of the sensor is verified by the successful detection of nitrite ion in water and milk samples with outstanding correlation.
Sahoo and co-workers synthesized RGO/MnFe2O4/PANI nanocomposite for the detection of nitrite in a neutral medium.150 The prepared RGO/MnFe2O4/PANI nanocomposite provided enhanced surface area for excellent preconcentration of nitrites and promoted fast charge transfer between the electrode surface and nitrite. It also demonstrated good catalytic activity toward the oxidation of nitrite. Moreover, a linear calibration plot was observed within the concentration ranges between 0.05 and 12000 μM of nitrite with an LOD of 0.015 μM. The presented sensing platform was found to have very good reproducibility and selectivity and was applied to detect nitrite in rainwater samples.
Table 4 lists applications of polymer nanocomposites-based electrochemical sensors for the detection of nitrite in various environmental matrices.
Table 4.
Applications of Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Nitrite in Environmental Specimens
| electrochemical sensor | method | analyte | detection limit | linear range | ref |
|---|---|---|---|---|---|
| Gr/PPy/CHIT/GCE | amperometry | nitrite | 0.1 μM | 0.5−722 μM | 143 |
| Co NPs-PEDOT-Gr/GCE | amperometry | nitrite | 0.15 μM | 0.5 μM to 240 μM | 144 |
| Ag−PAMAM/GCE | amperometry | nitrite | 0.4 μM | 4.0 μM to 1.44 mM | 145 |
| PDDA-RGO/GCE | amperometry | nitrite | 0.2 μM | 0.5 μM to 2 mM | 146 |
| PEDOT/AuNP/GCE | amperometry | nitrite | 60 nM | 0.2 to 1400 μM | 147 |
| Fe1.833(OH)0.5O2.5/NG@PDDA/GCE | amperometry | nitrite | 0.027 μM | 0.1−1275 μM | 148 |
| RGO/MoS2/PEDOT/GCE | DPV | nitrite | 0.059 μM | 0.001−1.0 mM | 149 |
| RGO/MnFe2O4/PANI/GCE | DPV | nitrite | 0.015 μM | 0.05−12000 μM | 150 |
3.6. Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Hydrazine.
Hydrazine (N2H4) has extensively been applied in various fields, for example, pharmaceuticals, industrial factories, as corrosive inhibitor, and in photography chemicals.151–153 However, it is well-known that N2H4 is a very toxic substance, identified as a carcinogen and mutagenic by the EPA;154 exposure causes damage to lungs, the liver, nervous system, and the kidney. In addition, N2H4 is absorbed easily when acute exposure to N2H4 occurs, which can lead to death.155 Consequently, a technique for the easy, accurate, and sensitive determination of N2H4 is very significant for both environmental safety and the health of humans.
Peng et al. conducted an electrochemical detection of hydrazine based on synthesized sulfur-poly(pyrrole-Co-aniline) (S/PPyA) nanofiber.156 A copolymer poly(pyrrole-co-aniline) PPyA was synthesized via the copolymerization reaction between aniline and pyrrole. In the next stage, an S/PPyA composite was obtained via the treatment of a mixture of PPyA and sublimed sulfur at 160 °C for 24 h. Afterward, S/PPyA was applied to modify GCE, and electrochemical activities of the as-synthesized S/PPyA/GCE to oxidize hydrazine were addressed; a lower LOD of 0.18 μM (S/N =3) and faster response time of three seconds were discerned.
Rahman et al. reported an interesting hydrazine sensor based on the silver NPs decorated PANI tungstophosphate nanocomposite157 accumulated on a GCE causing the improvement in the electrochemical sensor to dissolve hydrazine with the help of I–V (current–voltage). The operation of the sensor at +0.5 V displayed a rapid response time (10 s), good sensitivity (~12.5 μA·mM−1·cm−2), and extended stability. Its response to hydrazine was found to be linear in the concentration ranging from 10 nM to 10 mM with an LOD of 2.8 nM at the signal-to-noise ratio equal to 3.
Kaur et al. illustrated nanomolar concurrent detection of hydrazine and phenylhydrazine based on Cu NPs-decorated PANI-zeolite nanocomposite-modified GCE (Cu NPs(5%)-PANI-Nano-ZSM-5/GCE);158 higher electrocatalytic activities, greater stability, sensitivity, and selectivity were observed with wider linear ranges between 4 and 800 μM with the LOD of 1 nM for phenyl-hydrazine and hydrazine. The analytical function of the devised sensor was established for the determination of phenyl-hydrazine and hydrazine in distinctive water bodies with reasonable outputs.
Peng et al. synthesized polydopamine-RGO (PDA-RGO) nanocomposites in which the GO sheets have been functionalized with PDA via self-polymerization.159 Hydrazine could be detected by the electrochemical feature of the PDA-RGO, and hydrazine electrochemical oxidation was notably enhanced by the PDA-RGO nanocomposite as a result of the greater accessible surface areas of the electrode. Finally, a rapid amperometric response was noticed for the electrochemical sensor nanocomposite to measure hydrazine in a wider linear range from 0.03 to100 μM in which the LOD value was 0.01 μM.
Faisal et al. developed an electrochemical method for the effective and rapid determination of hydrazine using polythiophene (PTh)/ZnO nanocomposite modified GCE;160 the synthesis of PTh/ZnO nanocomposite for the detection of hydrazine via amperometry is depicted in Figure 11. Because of the synergistic catalytic effect of metal oxide and conducting polymer, the PTh/ZnO-modified GCE based sensor presented improved performance toward the detection of hydrazine when compared to the bare GCE or pure ZnO. Hydrazine was detected over the concentration range of 0.5–48 μM, with high sensitivity (1.22 μA μM−1 cm−2) and lower detection limit (0.207 μM). The amperometric analysis clarified that this proposed hydrazine sensor presented rapid (<5 s) response with appropriate operational and long-term stability, repeatability, and outstanding selectivity.
Figure 11.
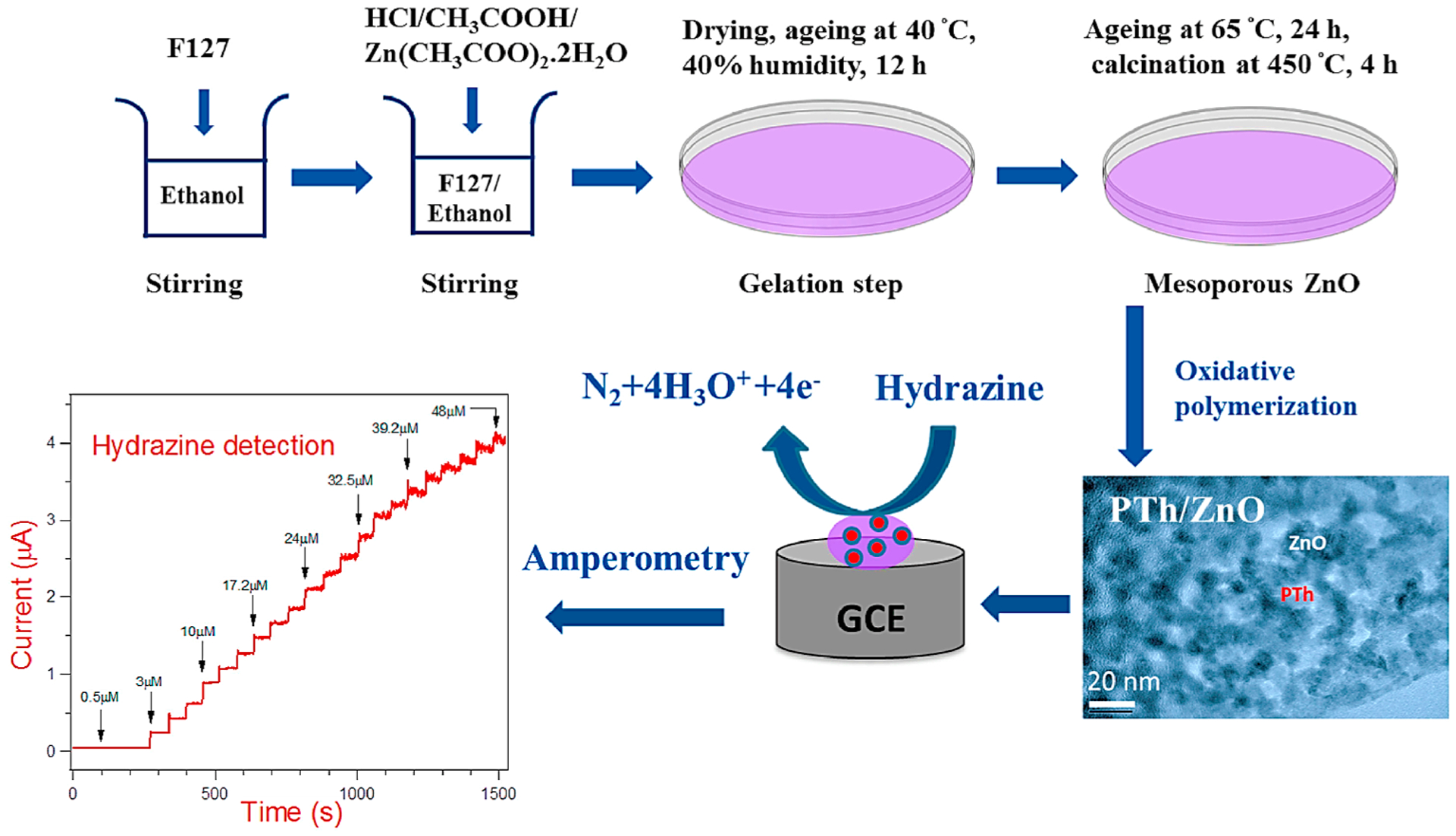
Sol–gel synthesis of PTh/ZnO nanocomposite with the amperometric detection of hydrazine on modifed GCE. Reproduced with permission from ref 160. Copyright 2018 Elsevier.
Afshari et al. prepared graphitic carbon nitride/PANI/silver nanocomposites (PANI/g-C3N4/Ag NPs) by electrochemical polymerization and followed their performance by potentiometric methods.161 Then, the fabricated PANI/g-C3N4/Ag NPs electrode was utilized for the electrochemical oxidation of hydrazine in the alkaline media; it displayed a wider linear range from 5–300 mM and an LOD of 300 μM (S/N = 3). Moreover, the amperometric currents are highly stable in the presence of interfering species. The PANI/g-C3N4/Ag NPs electrode could selectively detect hydrazine in the presence of interfering compounds, for example, fructose, galactose, glucose, and hydroxylamine.
Kaladevi and co-workers reported the synthesis of silver NPs-decorated PANI/RGO nanocomposite by interfacial polymerization under static conditions. The electrocatalytic oxidation of hydrazine was investigated at the surface of the modified electrode (AgNPs@PANI-RGO/GCE).162 The electrochemical behavior of AgNP@PANI-RGO was studied by CV and EIS. The amperometry method was applied for the quantitative detection of hydrazine. Furthermore, hydrazine detection linearly ranged between 0.4 to 2.2 μM with the LOD equal to 70 nM. The results showed that the proposed sensor possesses good accuracy toward the trace travel detection of hydrazine in tap water, lake water, and drinking water.
Saeb and colleagues provided a sensitive electrochemical sensor for hydrazine oxidation, by titanium oxide@PANI@Au NPs (TiO2@PANI@Au nanocomposite) on the GCE surface.163 The fabricated sensor displayed high electrocatalytic activity to hydrazine oxidation. The DPV technique was used to construct the calibration plot for hydrazine. According to the results, it was possible to detect hydrazine in the linear ranges of 0.9 × 10−6 to 1.2 × 10−3 M with an LOD to 0.15 ±0.02 μM by this sensor. Additionally, the TiO2@PANI@Au/GCE presented good stability and repeatability in hydrazine detection. Finally, the designed electrochemical sensor was successfully applied to detect hydrazine in various water samples (e.g., power plant sewage water and hookah water).
Table 5 lists the applications of polymer nanocomposites-based electrochemical sensors for the detection of hydrazine in various environmental matrices.
Table 5.
Applications of Polymer Nanocomposites-Based Electrochemical Sensors for the Detection of Hydrazine in Environmental Samples
| electrochemical sensor | method | analyte | detection limit | linear range | ref |
|---|---|---|---|---|---|
| S/PPyA/GCE | amperometry | hydrazine | 0.18 μM | 1 μM to 1 mM | 156 |
| tungstophosphate-PANI/Ag/GCE | amperometry | hydrazine | 2.8 nM | 10 nM to 10 mM | 157 |
| Cu NPs(5%)-PANI-Nano-ZSM-5/GCE | DPV | hydrazine | 1 nM | 4 nM-800 μM | 158 |
| phenylhydrazine | 1 nM | 4 nM-800 μM | |||
| PDA-RGO/GCE | SWV | hydrazine | 0.01 μM | 0.03 to 100 μM | 159 |
| PTh/ZnO/GCE | amperometry | hydrazine | 0.207 μM | 0.5−48 μM | 160 |
| PANI/g-C3N4/Ag NPs/GCE | CV | hydrazine | 300 μM | 5−300 mM | 161 |
| AgNPs@PANI-rGO/GCE | amperometry | hydrazine | 70 nM | 0.4−2.2 μM | 162 |
| TiO2@PANI@Au/GCE | DPV | hydrazine | 0.15 ± 0.02 μM | 0.9 × 10−6-1.2 × 10−3 M | 163 |
4. CHALLENGES AND PERSPECTIVES
Although polymeric nanocomposite-based electrochemical sensors offer great potential toward the sensitive detection of environmental pollutants, they also pose significant challenges. It is often difficult to achieve a uniform dispersion of nanofillers in the polymer matrix, and to enable a strong interaction at the interface between the nanofiller and the polymer matrix. The large scale production of polymer nanocomposites is an important issue because of the complicated fabrication steps involved. Besides the construction complexity, one should consider the suitable selection of fabrication methods and appropriate operating conditions. The realization of advanced, highly sensitive, selective, accurate, portable, and integrative detection equipment is in the preliminary stages and still remains between laboratory prototypes and their widespread commercial applications. It is necessary to make extensive collaborative effort between multidisciplinary science and engineering disciplines to design and fabricate easy to use sensors especially for the environment.
In future development, researchers should focus on novel synthetic methods and an efficient combination of novel NPs and nanostructures with good conductive polymers that can produce polymer nanocomposites for appliances in electrochemical devices as elements to improve the selectivity and sensitivity for the electrochemical sensing of environmental pollutants. The improvement in the uniform dispersion of nanofillers in the polymer matrix by physical treatment, surfactants, and chemical functionalization of the surface of nanofillers may open new avenues for applying novel polymer nanocomposites in fabricating novel electrochemical sensing platform. Moreover, the biopolymer nanocomposites based electrochemical sensors and biosensors are expected to be a promising platform for the detection of environmental pollutants due to their nontoxicity, biocompatibility, biodegradability, multiple functional groups, pH-dependent solubility in aqueous media, good adhesion, and broad chemical modification capacity.
5. CONCLUSIONS
The unique properties of polymer nanocomposites such as high electrical and thermal conductivity, chemical stability, and mechanical strength with the tunable characteristics of polymers in terms of their chemical structure, lightweight, simplified procurement, high active sites, and strong adherence to the surface of the electrodes expose them as ideal materials for the development of practical chemical sensors. In this review article, we have summarized the common synthesis methods of polymer nanocomposites and their recent progressing applications for the electrochemical detection of a wide range of environmental pollutants. As demonstrated in Tables 1–5 and in the Supporting Information, polymer nanocomposites as a sensing platform have exhibited high performance in the construction of electrochemical sensors for the detection of environmental pollutants including heavy metal ions, pesticides, phenolic compounds, nitroaromatics, nitrites, and hydrazine. Although the tables also provide a comparison study for the environmental pollutants detection by the various electrochemical sensors, they are not an accurate source to verify which sensor functions more efficient than another on the basis of LOD, wide linear range, and the type of polymer nanocomposite used for analysis. Indeed, there are several influential factors that should be considered for a true comparison besides those shown in the tables, including ease of modification procedure, response time, total cost of modification, simplicity of sample pretreatment before analysis, selectivity, stability, repeatability, and reproducibility of electrochemical sensor, etc. The present review provides a comprehensive overview on the electrochemical sensing applications of polymer nanocomposites for environmental pollutants detection and aims to open new horizons for the practical use of these nanostructured materials toward fabrication of novel sensing platforms with improved properties.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020M2D8A206983011). Furthermore, the financial supports of the Basic Science Research Program (2017R1A2B3009135) through the National Research Foundation of Korea is appreciated. This research was also supported by Kerman University of Medical Sciences, Kerman, Iran.
The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the U.S. EPA.
ABBREVIATIONS
- GC/MS
gas chromatography/mass spectrometry
- AAS
atomic absorption spectroscopy
- HPLC
high-performance liquid chromatography
- CV
cyclic voltammetry
- SWV
square-wave voltammetry
- DPV
differential pulse voltammetry
- LSV
linear sweep voltammetry
- NPs
nanoparticles
- Gr
grapheme
- CNTs
carbon nanotubes
- PANI
polyaniline
- APS
ammonium persulfate
- DBSA
dodecylbenzene sulfonic acid
- NP
nanoplatelet
- PS
polystyrene
- MWCNT
multi-walled carbon nanotube
- PVA
poly(vinyl alcohol)
- GNS
reduced graphene oxide sheets
- GO
graphene oxide
- SCE
saturated calomel electrode
- SWCNT
single-walled carbon nanotube
- PABSA
poly(m-amino benzene sulfonic acid)
- SAMs
self-assembled mono-layers
- DMAET
dimethyl amino ethane thiol
- SWASV
square wave anodic stripping voltammetry
- LOD
limit of detection
- ICP-MS
inductively coupled plasma mass spectrometry
- PEDOT
poly(3,4-ethylene-dioxythiophene
- GCE
glassy carbon electrode
- DPSV
differential pulse stripping voltammetry
- P2MA
poly(2-methylaniline)
- SEM
scanning electron microscopy
- XRD
X-ray diffraction
- FT-IR
Fourier transform-infrared spectroscopy
- IIP
ion imprinted polymer
- PAN
1-(2-pyridylazo)-2-naphthol
- LOQ
limit of quantitation
- RSD
relative standard deviation
- RGO
reduced GO
- PANI-co-PoT
poly(aniline-co-o-toluidine)
- CE
carbon paper electrode
- DPASV
differential pulse anodic striping voltammetry
- poly(PCV)
poly(pyrocatechol violet)
- SPE
screen printed electrode
- PA
phytic acid
- PPy
poly-pyrrole
- PAMAM
poly(amidoamine)
- G2
second-generation
- MCPE
magnetic carbon paste electrode
- WHO
World Health Organization
- CNSs
carbonaceous nanospheres
- PLL
poly-l-lysine
- EDTA
ethylenediaminetetraacetic acid
- SS
stainless steel
- FNT
Fenitrothion
- PGE
pencil graphite electrode
- DPA
poly(E)-1-(4-((4-(phenylamino)phenyl)diazenyl)-phenyl)ethanone
- MLT
malathion
- IL
ionic liquid
- PoPD
poly(o-phenylenediamine)
- SMZ
simazine
- MIPM
molecularly imprinted polymer membranes
- ATP
o-aminothiophenol
- IMI
imidacloprid
- PCz
polycarbazole
- CRGO
chemically rgo
- P3MT
poly(3-methyl-thiophene)
- NG
nitrogen doped grapheme
- MP
methyl parathion
- PMG
poly(malachite green)
- GrNSs
graphene nano-sheets
- PAAM
poly(acrylamide)
- MIP
molecularly imprinted polymer
- PDPA
polydiphenylamine
- 2-AP
2-aminophenol
- 4-AP
4-aminophenol
- PBS
phosphate buffer solution
- P-l-GSH
poly-l-glutathione
- l-Arg
l-arginine
- β-CD
β-cyclodextrin
- GNRs
graphene nano-ribbons
- NP
nitrophenol
- o-NP
ortho-nitrophenol
- PPI
poly(propyleneimine)
- p-NP
para-nitrophenol
- PEI
poly(ethyleneimine)
- 4-CP
4-chlorophenol
- PDDA
poly(diallyldimethylammonium chloride)
- 2,4-DCP
2,4-dichlorophenol
- PEY
poly(eosin Y)
- MWCNTs–OH
hydroxylated MWCNT
- RS
resorcinol
- CC
catechol
- HQ
hydroquinone
- AuAC
gold atomic cluster
- P4VP
poly(4-vinylpyridine)
- PSA
poly(sulfosalicylic acid)
- 1D
one-dimensional
- PNMTh
poly(n-methylthionine)
- DGNs
dendritic gold nanostructures
- 3MT
3-methylthiophene
- CTAB
cetyl-trimethyl ammonium bromide
- BPA
bisphenol A
- SF
silk fibroin
- EIS
electrochemical impedance spectroscopy
- Pβ-CD
poly-β-cyclodextrin
- ERGr
electro-reduced grapheme
- RhB
rhodamine B
- PME
poly(melamine)
- NBz
nitrobenzene
- PAA
poly(amic) acid
- 2-NP
2-nitrophenol
- 4-NA
4-nitroaniline
- DNBz
dinitrobenzene
- TNT
2,4,6-trinitrotoluene
- AdCSV
adsorptive cathodic stripping voltammetry
- PSA
poly(styrene-co-acrylic acid)
- L-MMS
lignin modified magnetic microspheres
- ErGO
electrochemically reduced graphene oxide
- P(O-PDA-CO-ANI)
poly(o-phenylenediamine-aniline
- DNT
2,4-dinitrotoluene
- PDDA
poly(diallyldimethylammoniumchloride)
- S/PPyA
sulfur-poly(pyrrole-co-aniline)
- PPyA
poly(pyrrole-co-aniline)
- PDA
polydopamine
- PTh
polythiophene
- g-C3N4
graphitic carbon nitride
Footnotes
Supporting Information
This information is available free of charge via the Internet at http://pubs.acs.org/. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/ac-s.iecr.0c04952.
Applications of polymer nanocomposites-based electrochemical sensors for the detection of various phenolic compounds in environmental samples (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.iecr.0c04952
The authors declare no competing financial interest.
Contributor Information
Somayeh Tajik, Research Center for Tropical and Infectious Diseases, Kerman University of Medical Sciences, Kerman 7616911319, Iran.
Hadi Beitollahi, Environment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman 7518934119, Iran.
Fariba Garkani Nejad, Environment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman 7518934119, Iran.
Zahra Dourandish, Environment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman 7518934119, Iran.
Mohammad A. Khalilzadeh, Department of Forest Biomaterials, College of Natural Resources, North Carolina State University, Raleigh, North Carolina 27695-8005, United States
Ho Won Jang, Department of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul 08826, Republic of Korea.
Richard A. Venditti, Department of Forest Biomaterials, College of Natural Resources, North Carolina State University, Raleigh, North Carolina 27695-8005, United States.
Rajender S. Varma, Chemical Methods and Treatment Branch, Water Infrastructure Division, Center for Environmental Solutions and Emergency Response, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268, United States; Regional Center of Advanced Technologies and Materials, Palacky University, Olomouc 783 71, Czech Republic.
Mohammadreza Shokouhimehr, Department of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul 08826, Republic of Korea.
REFERENCES
- (1).Wang Y; Hu S Applications of carbon nanotubes and graphene for electrochemical sensing of environmental pollutants. J. Nanosci. Nanotechnol 2016, 16, 7852–7872. [Google Scholar]
- (2).Zhang L; Fang M Nanomaterials in pollution trace detection and environmental improvement. Nano Today 2010, 5, 128–142. [Google Scholar]
- (3).Kuppusamy S; Palanisami T; Megharaj M; Venkateswarlu K; Naidu R Ex-situ remediation technologies for environmental pollutants: a critical perspective. Rev. Environ. Contam. Toxicol 2016, 236, 117–192. [DOI] [PubMed] [Google Scholar]
- (4).Tajik S; Beitollahi H; Garkani Nejad F; Kirlikovali KO; Le QV; Jang HW; Varma RS; Farha OK; Shokouhimehr M Recent Electrochemical Applications of Metal-Organic Framework-Based Materials. Cryst. Growth Des 2020, 20, 7034–7064. [Google Scholar]
- (5).Perera F; Herbstman J Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol 2011, 31, 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mates JM; Segura JA; Alonso FJ; Marquez J Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radical Biol. Med 2010, 49, 1328–1341. [DOI] [PubMed] [Google Scholar]
- (7).Escher BI; Baumgartner R; Koller M; Treyer K; Lienert J; McArdell CS Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res 2011, 45, 75–92. [DOI] [PubMed] [Google Scholar]
- (8).Huang YQ; Wong CKC; Zheng JS; Bouwman H; Barra R; Wahlström B; Neretin L; Wong MH Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ. Int 2012, 42, 91–99. [DOI] [PubMed] [Google Scholar]
- (9).Meng J; Shi C; Wei B; Yu W; Deng C; Zhang X Preparation of Fe3O4@ C@ PANI magnetic microspheres for the extraction and analysis of phenolic compounds in water samples by gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 2841–2847. [DOI] [PubMed] [Google Scholar]
- (10).Ghaedi M; Ahmadi F; Shokrollahi A Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J. Hazard. Mater 2007, 142, 272–278. [DOI] [PubMed] [Google Scholar]
- (11).Baig SA; Akhtera NA; Ashfaq M; Asi MR Determination of the organophosphorus pesticide in vegetables by high-performance liquid chromatography. Am. Eurasian J. Agric. Environ. Sci 2009, 6, 513–519. [Google Scholar]
- (12).Wang Q; Ma S; Huang H; Cao A; Li M; He L Highly sensitive and selective spectrofluorimetric determination of nitrite in food products with a novel fluorogenic probe. Food Control 2016, 63, 117–121. [Google Scholar]
- (13).Nie D; Li P; Zhang D; Zhou T; Liang Y; Shi G Simultaneous determination of nitroaromatic compounds in water using capillary electrophoresis with amperometric detection on an electrode modified with a mesoporous nano-structured carbon material. Electrophoresis 2010, 31, 2981–2988. [DOI] [PubMed] [Google Scholar]
- (14).Safavi A; Karimi MA Flow injection chemiluminescence determination of hydrazine by oxidation with chlorinated isocyanu-rates. Talanta 2002, 58, 785–792. [DOI] [PubMed] [Google Scholar]
- (15).Stradiotto NR; Yamanaka H; Zanoni MVB Electrochemical sensors: a powerful tool in analytical chemistry. J. Braz. Chem. Soc 2003, 14, 159–173. [Google Scholar]
- (16).He Q; Liu J; Liu X; Li G; Chen D; Deng P; Liang J A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar]
- (17).He Q; Liu J; Liu X; Li G; Chen D; Deng P; Liang J Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Tajik S; Beitollahi H; Garkani Nejad F; Safaei M; Zhang K; Le QV; Varma RS; Jang HW; Shokouhimehr M Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv 2020, 10, 21561–21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Magesa F; Wu Y; Tian Y; Vianney JM; Buza J; He Q; Tan Y Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem 2019, 23, No. e00064. [Google Scholar]
- (20).Tajik S; Beitollahi H; Mohammadi SZ; Azimzadeh M; Zhang K; Le QV; Yamauchi Y; Jang HW; Shokouhimehr M Recent developments in electrochemical sensors for detecting hydrazine with different modified electrodes. RSC Adv 2020, 10, 30481–30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Xing H; Xu J; Zhu X; Duan X; Lu L; Wang W; Zhang Y; Yang T Highly sensitive simultaneous determination of cadmium(II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J. Electroanal. Chem 2016, 760, 52–58. [Google Scholar]
- (22).Beitollahi H; Khalilzadeh MA; Tajik S; Safaei M; Zhang K; Jang HW; Shokouhimehr M Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 2020, 5, 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lakard B Electrochemical biosensors based on conducting polymers: a review. Appl. Sci 2020, 10, 6614. [Google Scholar]
- (24).He Q; Tian Y; Wu Y; Liu J; Li G; Deng P; Chen D Facile and ultrasensitive determination of 4-nitrophenol based on acetylene black paste and graphene hybrid electrode. Nanomaterials 2019, 9, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Tajik S; Dourandish Z; Zhang K; Beitollahi H; Le QV; Jang HW; Shokouhimehr M Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv 2020, 10, 15406–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Murugan E; Kumar K Fabrication of SnS/TiO2@GO composite coated glassy carbon electrode for concomitant determination of paracetamol, tryptophan, and caffeine in pharmaceutical formulations. Anal. Chem 2019, 91, 5667–5676. [DOI] [PubMed] [Google Scholar]
- (27).Tian Y; Deng P; Wu Y; Liu J; Li J; Li G; He Q High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode. Microchem. J 2020, 157, 104885. [Google Scholar]
- (28).Shankar SS; Shereema RM; Ramachandran V; Sruthi TV; Kumar VS; Rakhi RB Carbon Quantum Dot-Modified Carbon Paste Electrode-Based Sensor for Selective and Sensitive Determination of Adrenaline. ACS Omega 2019, 4, 7903–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).He Q; Wu Y; Tian Y; Li G; Liu J; Deng P; Chen D Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chekin F; Singh SK; Vasilescu A; Dhavale VM; Kurungot S; Boukherroub R; Szunerits S Reduced graphene oxide modified electrodes for sensitive sensing of gliadin in food samples. ACS Sens 2016, 1, 1462–1470. [Google Scholar]
- (31).He Q; Liu J; Xia Y; Tuo D; Deng P; Tian Y; Wu Y; Li G; Chen D Rapid and sensitive voltammetric detection of rhodamine B in chili-containing foodstuffs using MnO2 nanorods/electro-reduced graphene oxide composite. J. Electrochem. Soc 2019, 166, B805. [Google Scholar]
- (32).Beitollahi H; Dourandish Z; Tajik S; Ganjali MR; Norouzi P; Faridbod F Application of graphite screen printed electrode modified with dysprosium tungstate nanoparticles in voltammetric determination of epinephrine in the presence of acetylcholine. J. Rare Earths 2018, 36, 750–757. [Google Scholar]
- (33).Tian Y; Deng P; Wu Y; Li J; Liu J; Li G; He Q MnO2 Nanowires-Decorated Reduced Graphene Oxide Modified Glassy Carbon Electrode for Sensitive Determination of Bisphenol A. J. Electrochem. Soc 2020, 167, 046514. [Google Scholar]
- (34).Alshahrani LA; Liu L; Sathishkumar P; Nan J; Gu FL Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem 2018, 815, 68–75. [Google Scholar]
- (35).Alizadeh T; Ganjali MR; Akhoundian M; Norouzi P Voltammetric determination of ultratrace levels of cerium (III) using a carbon paste electrode modified with nano-sized cerium-imprinted polymer and multiwalled carbon nanotubes. Microchim. Acta 2016, 183, 1123–1130. [Google Scholar]
- (36).Beitollahi H; Tajik S; Aflatoonian MR; Garkani Nejad F; Zhang K; Shahedi Asl M; Le QV; Cha JH; Shokouhimehr M; Peng W A Novel Screen-Printed Electrode Modified by graphene Nanocomposite for Detecting Clozapine. Int. J. Electrochem. Sci 2020, 15, 9271–9281. [Google Scholar]
- (37).Khan I; Saeed K; Khan I Nanoparticles: Properties, applications and toxicities. Arabian J. Chem 2019, 12, 908–931. [Google Scholar]
- (38).El Rhazi M; Majid S; Elbasri M; Salih FE; Oularbi L; Lafdi K Recent progress in nanocomposites based on conducting polymer: application as electrochemical sensors. Int. Nano Lett 2018, 8, 79–99. [Google Scholar]
- (39).Shirakawa H; Louis EJ; MacDiarmid AG; Chiang CK; Heeger AJ Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc., Chem. Commun 1977, 16, 578–580. [Google Scholar]
- (40).Shrivastava S; Jadon N; Jain R Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC, Trends Anal. Chem 2016, 82, 55–67. [Google Scholar]
- (41).Sengupta R; Chakraborty S; Bandyopadhyay SA; Dasgupta S; Mukhopadhyay R; Auddy K; Deuri AS A short review on rubber/clay nanocomposites with emphasis on mechanical properties. Polym. Eng. Sci 2007, 47, 1956–1974. [Google Scholar]
- (42).Fu Y; Yao J; Zhao H; Zhao G; Wan Z; Qiu Y Fabrication and magnetorheology of bidisperse magnetic microspheres coated with gelatin and multi-walled carbon nanotubes. Smart Mater. Struct 2018, 27, 125001. [Google Scholar]
- (43).Qi S; Yu M; Fu J; Zhu M; Xie Y; Li W An EPDM/MVQ polymer blend based magnetorheological elastomer with good thermostability and mechanical performance. Soft Matter 2018, 14, 8521–8528. [DOI] [PubMed] [Google Scholar]
- (44).Fu Y; Zhao G; Zhao H; Wan Z; Jia W Investigation into a Conductive Composite Matrix Based on Magnetically Sensitive Flexible Sponges. Ind. Eng. Chem. Res 2020, 59, 15967–15978. [Google Scholar]
- (45).Reynaud E; Gauthier C; Perez J Nanophases in polymers. Revue de Metallurgie 1999, 96, 169–176. [Google Scholar]
- (46).Young RJ; Kinloch IA; Gong L; Novoselov KS The mechanics of graphene nanocomposites: a review. Compos. Sci. Technol 2012, 72, 1459–1476. [Google Scholar]
- (47).Samanta S; Nandi AK Recyclable High Performance Palladium Heterogeneous Catalyst with Porous PVDF Binder from the Novel Gel Nanocomposite Route. J. Phys. Chem. C 2009, 113, 4721–4725. [Google Scholar]
- (48).Perez-Cappe E; Mosqueda Y; Martinez R; Milian CR; Sanchez O; Varela JA; Hortencia A; Souza E; Aranda P; Ruiz-Hitzky E Preparation and properties as positive electrodes of PANI-LiNi0.8Co0.2O2 nanocomposites. J. Mater. Chem 2008, 18, 3965–3971. [Google Scholar]
- (49).Macanas J; Parrondo J; Munoz M; Alegret S; Mijangos F; Muraviev DN Preparation and characterisation of metal-polymer nanocomposite membranes for electrochemical applications. Phys. Status Solidi A 2007, 204, 1699–1705. [Google Scholar]
- (50).Sender C; Dantras E; Dantras-Laffont L; Lacoste MH; Dandurand J; Mauzac M; Lacout JL; Lavergne C; Demont P; Bernes A; Lacabanne C Dynamic mechanical properties of a biomimetic hydroxyapatite/polyamide 6,9 nanocomposite. J. Biomed. Mater. Res., Part B 2007, 83B, 628–635. [DOI] [PubMed] [Google Scholar]
- (51).Zhou JP; Li R; Liu SL; Li Q; Zhang LZ; Zhang L; Guan JG Structure and Magnetic Properties of Regenerated Cellulose/Fe3O4 Nanocomposite Films. J. Appl. Polym. Sci 2009, 111, 2477–2484. [Google Scholar]
- (52).Deshmukh RD; Composto RJ Surface segregation and formation of silver nanoparticles created in situ in poly(methyl methacrylate) films. Chem. Mater 2007, 19, 745–754. [Google Scholar]
- (53).Pramanik N; Biswas SK; Pramanik P Synthesis and characterization of hydroxyapatite/poly(vinyl alcohol phosphate) nanocomposite biomaterials. Int. J. Appl. Ceram. Technol 2008, 5, 20–28. [Google Scholar]
- (54).Ramesan MT; Nidhisha V; Jayakrishnan P Synthesis, characterization and conducting properties of novel poly (vinyl cinnamate)/zinc oxide nanocomposites via in situ polymerization. Mater. Sci. Semicond. Process 2017, 63, 253–260. [Google Scholar]
- (55).Lee YR; Raghu AV; Jeong HM; Kim BK Properties of waterborne polyurethane/functionalized graphene sheet nanocomposites prepared by an in situ method. Macromol. Chem. Phys 2009, 210, 1247–1254. [Google Scholar]
- (56).Zeng C; Lee LJ Poly(methyl methacrylate) and polystyrene/clay nanocomposites prepared by in-situ polymerization. Macromolecules 2001, 34, 4098–4103. [Google Scholar]
- (57).Yang F; Ou Y; Yu Z Polyamide 6/silica nanocomposites prepared by in situ polymerization. J. Appl. Polym. Sci 1998, 69, 355–361. [Google Scholar]
- (58).Tamboli MS; Kulkarni MV; Patil RH; Gade WN; Navale SC; Kale BB Nanowires of silver-polyaniline nanocomposite synthesized via in situ polymerization and its novel functionality as an antibacterial agent. Colloids Surf., B 2012, 92, 35–41. [DOI] [PubMed] [Google Scholar]
- (59).Tanahashi M Development of Fabrication Methods of Filler/Polymer Nanocomposites: With Focus on Simple Melt-Compounding-Based Approach without Surface Modification of Nanofillers. Materials 2010, 3, 1593–1619. [Google Scholar]
- (60).Al-Saleh MH; Jawad SA Graphene nanoplatelet-polystyrene nanocomposite: dielectric and charge storage behaviors. J. Electron. Mater 2016, 45, 3532–3539. [Google Scholar]
- (61).Fukushima K; Abbate C; Tabuani D; Gennari M; Camino G Biodegradation of poly (lactic acid) and its nanocomposites. Polym. Degrad. Stab 2009, 94, 1646–1655. [Google Scholar]
- (62).Mensah B; Kim HG; Lee JH; Arepalli S; Nah C Carbon nanotube-reinforced elastomeric nanocomposites: a review. Int. J. Smart Nano Mater 2015, 6, 211–238. [Google Scholar]
- (63).Khan F; Kausar A; Siddiq M A review on properties and fabrication techniques of polymer/carbon nanotube composites and polymer intercalated buckypapers. Polym.-Plast. Technol. Eng 2015, 54, 1524–1539. [Google Scholar]
- (64).Marroquin JB; Rhee KY; Park SJ Chitosan nanocomposite films: enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym 2013, 92, 1783–1791. [DOI] [PubMed] [Google Scholar]
- (65).Zhang F; Zhang W; Yu Y; Deng B; Li J; Jin J Sol-gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J. Membr. Sci 2013, 432, 25–32. [Google Scholar]
- (66).Morselli D; Messori M; Bondioli F Poly (methyl methacrylate)-TiO2 nanocomposite obtained by non-hydrolytic sol-gel synthesis. J. Mater. Sci 2011, 46, 6609–6617. [Google Scholar]
- (67).Luan J; Ma K; Zhang L; Li M; Li Y; Pan B Research on different preparation methods of new photocatalysts. Curr. Org. Chem 2010, 14, 683–698. [Google Scholar]
- (68).Tripathi BP; Shahi VK 3-[[3-(Triethoxysilyl)propyl]-amino]propane-1-sulfonic Acid-Poly(vinyl alcohol) cross-linked zwit-terionic polymer electrolyte membranes for direct methanol fuel cell applications. ACS Appl. Mater. Interfaces 2009, 1, 1002–1012. [DOI] [PubMed] [Google Scholar]
- (69).Jaseela PK; Garvasis J; Joseph A Selective adsorption of methylene blue (MB) dye from aqueous mixture of MB and methyl orange (MO) using mesoporous titania (TiO2)-poly vinyl alcohol (PVA) nanocomposite. J. Mol. Liq 2019, 286, 110908. [Google Scholar]
- (70).Surudžić R; Janković A; Bibić N; Vukašinović-Sekulić M; Perić-Grujić A; Mišković-Stanković V; Park SJ; Rhee KY Physico-chemical and mechanical properties and antibacterial activity of silver/poly(vinylalcohol)/graphene nanocomposites obtained by electrochemical method. Composites, Part B 2016, 85, 102–112. [Google Scholar]
- (71).Bozlar M; Miomandre F; Bai JB Electrochemical synthesis and characterization of carbon nanotube/modified polypyrrole hybrids using a cavity microelectrode. Carbon 2009, 47, 80–84. [Google Scholar]
- (72).Janaky C; Visy C; Berkesi O; Tombacz E Conducting Polymer-Based Electrode with Magnetic Behavior: Electrochemical Synthesis of Poly(3-thiophene-acetic-acid)/Magnetite Nanocomposite Thin Layers. J. Phys. Chem. C 2009, 113, 1352–1358. [Google Scholar]
- (73).Gao Z; Yang W; Wang J; Yan H; Yao Y; Ma J; Wang B; Zhang M; Liu L Electrochemical synthesis of layer-by-layer reduced graphene oxide sheets/polyaniline nanofibers composite and its electrochemical performance. Electrochim. Acta 2013, 91, 185–194. [Google Scholar]
- (74).Tajik S; Beitollahi H; Aflatoonian MR; Mohtat B; Aflatoonian B; Sheikh Shoaie I; Khalilzadeh MA; Ziasistani M; Zhang K; Jang HW; Shokouhimehr M Fabrication of magnetic iron oxide-supported copper oxide nanoparticles (Fe3O4/CuO): modified screen-printed electrode for electrochemical studies and detection of desipramine. RSC Adv 2020, 10, 15171–15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Aragay G; Merkoçi A Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61. [Google Scholar]
- (76).Matlou GG; Nkosi D; Pillay K; Arotiba O electrochemical detection of Hg (II) in water using self-assembled single walled carbon nanotube-poly (m-amino benzene sulfonic acid) on gold electrode. Sens. Bio-Sens. Res 2016, 10, 27–33. [Google Scholar]
- (77).Zuo Y; Xu J; Zhu X; Duan X; Lu L; Gao Y; Xing H; Yanga T; Ye G; Yu Y Poly (3,4-ethylenedioxythiophene) nanorods/graphene oxide nanocomposite as a new electrode material for the selective electrochemical detection of mercury (II). Synth. Met 2016, 220, 14–19. [Google Scholar]
- (78).Khan A; Khan AAP; Rahman MM; Asiri AM; Alfaifi SY; Taib LA Toward Facile Preparation and Design of Mulberry-Shaped Poly (2-methylaniline)-Ce2(WO4) 3@CNT Nanocomposite and Its Application for Electrochemical Cd2+ Ion Detection for Environment Remediation. Polym.-Plast. Technol. Eng 2018, 57, 335–345. [Google Scholar]
- (79).Tarley CRT; Basaglia AM; Segatelli MG; Prete MC; Suquila FAC; de Oliveira LLG Preparation and application of nanocomposite based on imprinted poly (methacrylic acid)-PAN/MWCNT as a new electrochemical selective sensing platform of Pb2+ in water samples. J. Electroanal. Chem 2017, 801, 114–121. [Google Scholar]
- (80).Zuo Y; Xu J; Jiang F; Duan X; Lu L; Xing H; Yang T; Zhang Y; Ye G; Yu Y Voltammetric sensing of Pb (II) using a glassy carbon electrode modified with composites consisting of Co3O4 nanoparticles, reduced graphene oxide and chitosan. J. Electroanal. Chem 2017, 801, 146–152. [Google Scholar]
- (81).Hussein MA; Ganash AA; Alqarni SA Electrochemical sensor-based gold nanoparticle/poly (aniline-co-o-toluidine)/graphene oxide nanocomposite modified electrode for hexavalent chromium detection: a real test sample. Polym-Plast. Technol 2019, 58, 1423–1436. [Google Scholar]
- (82).Dash S; Munichandraiah N Electroanalysis of As (III) at nanodendritic Pd on PEDOT. Analyst 2014, 139, 1789–1795. [DOI] [PubMed] [Google Scholar]
- (83).Chamjangali MA; Kouhestani H; Masdarolomoor F; Daneshinejad H A voltammetric sensor based on the glassy carbon electrode modified with multi-walled carbon nanotube/poly (pyrocatechol violet)/bismuth film for determination of cadmium and lead as environmental pollutants. Sens. Actuators, B 2015, 216, 384–393. [Google Scholar]
- (84).Promphet N; Rattanarat P; Rangkupan R; Chailapakul O; Rodthongkum N An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators, B 2015, 207, 526–534. [Google Scholar]
- (85).Dai H; Wang N; Wang D; Ma H; Lin M An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd (II) and Pb (II). Chem. Eng. J 2016, 299, 150–155. [Google Scholar]
- (86).Baghayeri M; Alinezhad H; Fayazi M; Tarahomi M; Ghanei-Motlagh R; Maleki B A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb(II) and Cd (II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar]
- (87).Maleki B; Baghayeri M; Ghanei-Motlagh M; Zonoz FM; Amiri A; Hajizadeh F; Hosseinifar A; Esmaeilnezhad E Polyamidoamine dendrimer functionalized iron oxide nanoparticles for simultaneous electrochemical detection of Pb2+ and Cd2+ ions in environmental waters. Measurement 2019, 140, 81–88. [Google Scholar]
- (88).Wei Y; Yang R; Liu JH; Huang XJ Selective detection toward Hg (II) and Pb (II) using polypyrrole/carbonaceous nanospheres modified screen-printed electrode. Electrochim. Acta 2013, 105, 218–223. [Google Scholar]
- (89).Wang N; Zhao W; Shen Z; Sun S; Dai H; Ma H; Lin M Sensitive and selective detection of Pb (II) and Cu (II) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuators, B 2020, 304, 127286. [Google Scholar]
- (90).Guo Z; Li DD; Luo XK; Li YH; Zhao QN; Li MM; Zhao YT; Sun TS; Ma C Simultaneous determination of trace Cd (II), Pb (II) and Cu (II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid Interface Sci 2017, 490, 11–22. [DOI] [PubMed] [Google Scholar]
- (91).Deshmukh MA; Celiesiute R; Ramanaviciene A; Shirsat MD; Ramanavicius A EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar]
- (92).Ruecha N; Rodthongkum N; Cate DM; Volckens J; Chailapakul O; Henry CS Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn(II), Cd (II), and Pb (II). Anal. Chim. Acta 2015, 874, 40–48. [DOI] [PubMed] [Google Scholar]
- (93).Kim KH; Kabir E; Jahan SA Exposure to pesticides and the associated human health effects. Sci. Total Environ 2017, 575, 525–535. [DOI] [PubMed] [Google Scholar]
- (94).Surucu O; Bolat G; Abaci S Electrochemical behavior and voltammetric detection of fenitrothion based on a pencil graphite electrode modified with reduced graphene oxide (RGO)/poly (E)-1-(4-((4-(phenylamino) phenyl) diazenyl) phenyl) ethanone (DPA) composite film. Talanta 2017, 168, 113–120. [DOI] [PubMed] [Google Scholar]
- (95).Bolat G; Abaci S Non-enzymatic electrochemical sensing of malathion pesticide in tomato and apple samples based on gold nanoparticles-chitosan-ionic liquid hybrid nanocomposite. Sensors 2018, 18, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Jeyapragasam T; Raju R; Chen SM; Saraswathi R; Hatamleh AA; Chen TW; Rwei SP Poly (o-phenylenediamine)-Multiwalled Carbon Nanotube Nanocomposite Based Electrochemical Sensing Platform for Paraquat Detection. Int. J. Electrochem. Sci 2019, 14, 8326–8339. [Google Scholar]
- (97).Zhang J; Wang C; Niu Y; Li S; Luo R Electrochemical sensor based on molecularly imprinted composite membrane of poly (o-aminothiophenol) with gold nanoparticles for sensitive determination of herbicide simazine in environmental samples. Sens. Actuators, B 2017, 249, 747–755. [Google Scholar]
- (98).Lei W; Wu Q; Si W; Gu Z; Zhang Y; Deng J; Hao Q Electrochemical determination of imidacloprid using poly (carbazole)/chemically reduced graphene oxide modified glassy carbon electrode. Sens. Actuators, B 2013, 183, 102–109. [Google Scholar]
- (99).Wu L; Lei W; Han Z; Zhang Y; Xia M; Hao Q A novel non-enzyme amperometric platform based on poly (3-methylthiophene)/nitrogen doped graphene modified electrode for determination of trace amounts of pesticide phoxim. Sens. Actuators, B 2015, 206, 495–501. [Google Scholar]
- (100).Xu M; Zhu J; Su H; Dong J; Ai S; Li R Electrochemical determination of methyl parathion using poly (malachite green)/graphene nanosheets-nafion composite film-modified glassy carbon electrode. J. Appl. Electrochem 2012, 42, 509–516. [Google Scholar]
- (101).Zeng Y; Yu D; Yu Y; Zhou T; Shi G Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes-poly (acrylamide) nanocomposite film modified electrode. J. Hazard. Mater 2012, 217, 315–322. [DOI] [PubMed] [Google Scholar]
- (102).Zhao L; Zhao F; Zeng B Electrochemical determination of methyl parathion using a molecularly imprinted polymer-ionic liquid-graphene composite film coated electrode. Sens. Actuators, B 2013, 176, 818–824. [Google Scholar]
- (103).ZHANG J; CHEN J; LI X Remove of phenolic compounds in water by low-temperature plasma: a review of current research. J. Water Resour. Prot 2009, 2, 99–109. [Google Scholar]
- (104).Nistor C; Emneus J; Gorton L; Ciucu A Improved Stability and Altered Selectivity of Tyrosinase Based Graphite Electrodes for Detection of Phenolic Compounds. Anal. Chim. Acta 1999, 387, 309–326. [Google Scholar]
- (105).Akyol H; Riciputi Y; Capanoglu E; Caboni MF; Verardo V Phenolic compounds in the potato and its byproducts: an overview. Int. J. Mol. Sci 2016, 17, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Yang CY; Chen SM; Tsai TH; Unnikrishnan B Poly (diphenylamine) with multi-walled carbon nanotube composite film modified electrode for the determination of phenol. Int. J. Electrochem. Sci 2012, 7, 12796–12807. [Google Scholar]
- (107).Yin H; Ma Q; Zhou Y; Ai S; Zhu L Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene-chitosan composite film modified glassy carbon electrode. Electrochim. Acta 2010, 55, 7102–7108. [Google Scholar]
- (108).Fan Y; Liu JH; Yang CP; Yu M; Liu P Graphene-polyaniline composite film modified electrode for voltammetric determination of 4-aminophenol. Sens. Actuators, B 2011, 157, 669–674. [Google Scholar]
- (109).Ezhil Vilian AT; Veeramani V; Chen SM; Madhu R; Huh YS; Han YK Preparation of a reduced graphene oxide/poly-Lglutathione nanocomposite for electrochemical detection of 4-aminophenol in orange juice samples. Anal. Methods 2015, 7, 5627–5634. [Google Scholar]
- (110).Yi Y; Zhu G; Wu X; Wang K Highly sensitive and simultaneous electrochemical determination of 2-aminophenol and 4-aminophenol based on poly (l-arginine)-β-cyclodextrin/carbon nano-tubes@graphene nanoribbons modified electrode. Biosens. Bioelectron 2016, 77, 353–358. [DOI] [PubMed] [Google Scholar]
- (111).Ndlovu T; Arotiba OA; Krause RW; Mamba BB Electrochemical detection of o-nitrophenol on a poly (propylenei-mine)-gold nanocomposite modified glassy carbon electrode. Int. J. Electrochem. Sci 2010, 5, 1179–1186. [Google Scholar]
- (112).Tang J; Zhang L; Han G; Liu Y; Tang W Graphene-chitosan composite modified electrode for simultaneous detection of nitrophenol isomers. J. Electrochem. Soc 2015, 162, B269–B274. [Google Scholar]
- (113).Arfin T; Bushra R; Mohammad F Electrochemical sensor for the sensitive detection of o-nitrophenol using graphene oxide-poly (ethyleneimine) dendrimer-modified glassy carbon electrode. Graphene Technol 2016, 1, 1–15. [Google Scholar]
- (114).Wei W; Yang S; Hu H; Li H; Jiang Z Hierarchically grown ZnFe2O4-decorated polyaniline-coupled-graphene nanosheets as a novel electrocatalyst for selective detecting p-nitrophenol. Microchem. J 2021, 160, 105777. [Google Scholar]
- (115).Wang B; Okoth OK; Yan K; Zhang J A highly selective electrochemical sensor for 4-chlorophenol determination based on molecularly imprinted polymer and PDDA-functionalized graphene. Sens. Actuators, B 2016, 236, 294–303. [Google Scholar]
- (116).Zhu X; Zhang K; Wang C; Guan J; Yuan X; Li B Quantitative determination and toxicity evaluation of 2,4-dichlorophenol using poly (eosin Y)/hydroxylated multi-walled carbon nanotubes modified electrode. Sci. Rep 2016, 6, 38657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Yin H; Zhang Q; Zhou Y; Ma Q; Zhu L; Ai S Electrochemical behavior of catechol, resorcinol and hydroquinone at graphene-chitosan composite film modified glassy carbon electrode and their simultaneous determination in water samples. Electrochim. Acta 2011, 56, 2748–2753. [Google Scholar]
- (118).Xu G; Li B; Luo X Carbon nanotube doped poly (3,4-ethylenedioxythiophene) for the electrocatalytic oxidation and detection of hydroquinone. Sens. Actuators, B 2013, 176, 69–74. [Google Scholar]
- (119).Nambiar SR; Aneesh PK; Rao TP Ultrasensitive voltammetric determination of catechol at a gold atomic cluster/poly (3,4-ethylenedioxythiophene) nanocomposite electrode. Analyst 2013, 138, 5031–5038. [DOI] [PubMed] [Google Scholar]
- (120).Tehrani RM; Ghadimi H; Ab Ghani S Electrochemical studies of two diphenols isomers at graphene nanosheet-poly (4-vinyl pyridine) composite modified electrode. Sens. Actuators, B 2013, 177, 612–619. [Google Scholar]
- (121).Li C; Liu W; Gu Y; Hao S; Yan X; Zhang Z; Yang M Simultaneous determination of catechol and hydroquinone based on poly (sulfosalicylic acid)/functionalized graphene modified electrode. J. Appl. Electrochem 2014, 44, 1059–1067. [Google Scholar]
- (122).Tian F; Li H; Li M; Li C; Lei Y; Yang B Synthesis of one-dimensional poly (3,4-ethylenedioxythiophene)-graphene composites for the simultaneous detection of hydroquinone, catechol, resorcinol, and nitrite. Synth. Met 2017, 226, 148–156. [Google Scholar]
- (123).Liu Y; Song N; Ma Z; Zhou K; Gan Z; Gao Y; Tang S; Chen C Synthesis of a poly (N-methylthionine)/reduced graphene oxide nanocomposite for the detection of hydroquinone. Mater. Chem. Phys 2019, 223, 548–556. [Google Scholar]
- (124).Cheng D; Kan X Simultaneous determination of dihydroxybenzene isomers based on gold dendritic/pEDOT electrochemical sensor. J. Electroanal. Chem 2020, 857, 113741. [Google Scholar]
- (125).Li L; Liu E; Wang X; Chen J; Zhang X Simultaneous determination of naphthol isomers at poly (3-methylthiophene)-nano-Au modified electrode with the enhancement of surfactant. Mater. Sci. Eng., C 2015, 53, 36–42. [DOI] [PubMed] [Google Scholar]
- (126).Yin H; Zhou Y; Ai S; Han R; Tang T; Zhu L Electrochemical behavior of bisphenol A at glassy carbon electrode modified with gold nanoparticles, silk fibroin, and PAMAM dendrimers. Microchim. Acta 2010, 170, 99–105. [Google Scholar]
- (127).Yin H; Cui L; Chen Q; Shi W; Ai S; Zhu L; Lu L Amperometric determination of bisphenol A in milk using PAMAM-Fe3O4 modified glassy carbon electrode. Food Chem 2011, 125, 1097–1103. [Google Scholar]
- (128).Zhang X; Wu L; Zhou J; Zhang X; Chen J A new ratiometric electrochemical sensor for sensitive detection of bisphenol A based on poly-β-cyclodextrin/electroreduced graphene modified glassy carbon electrode. J. Electroanal. Chem 2015, 742, 97–103. [Google Scholar]
- (129).Peng J; Feng Y; Han XX; Gao ZN Simultaneous determination of bisphenol A and hydroquinone using a poly (melamine) coated graphene doped carbon paste electrode. Microchim. Acta 2016, 183, 2289–2296. [Google Scholar]
- (130).Li Y; Wang H; Yan B; Zhang H An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-L-lysine nanocomposites. J. Electroanal. Chem 2017, 805, 39–46. [Google Scholar]
- (131).Bolat G; Yaman YT; Abaci S Highly sensitive electrochemical assay for Bisphenol A detection based on poly (CTAB)/MWCNTs modified pencil graphite electrodes. Sens. Actuators, B 2018, 255, 140–148. [Google Scholar]
- (132).Ramu AG; Telmenbayar L; Theerthagiri J; Yang D; Song M; Choi D Synthesis of a hierarchically structured Fe3O4-PEI nanocomposite for the highly sensitive electrochemical determination of bisphenol A in real samples. New J. Chem 2020, 44, 18633–18645. [Google Scholar]
- (133).Xu G; Li B; Wang X; Luo X Electrochemical sensor for nitrobenzene based on carbon paste electrode modified with a poly (3,4-ethylenedioxythiophene) and carbon nanotube nanocomposite. Microchim. Acta 2014, 181, 463–469. [Google Scholar]
- (134).Ren J; Li L; Cui M; Zhai M; Yu C; Ji X Nitrobenzene electrochemical sensor based on silver nanoparticle supported on poly-melamine functional multi-walled carbon nanotube. Ionics 2016, 22, 1937–1945. [Google Scholar]
- (135).Kariuki VM; Fasih-Ahmad SA; Osonga FJ; Sadik OA An electrochemical sensor for nitrobenzene using π-conjugated polymer-embedded nanosilver. Analyst 2016, 141, 2259–2269. [DOI] [PubMed] [Google Scholar]
- (136).Cotchim S; Thavarungkul P; Kanatharana P; Limbut W A new strategy for 2,4,6-Trinitrotoluene adsorption and electrochemical reduction on poly (melamine)/graphene oxide modified electrode. Electrochim. Acta 2015, 184, 102–110. [Google Scholar]
- (137).Mahmoud KA; Abdel-Wahab A; Zourob M Selective electrochemical detection of 2,4,6-trinitrotoluene (TNT) in water based on poly (styrene-co-acrylic acid) PSA/SiO2/Fe3O4/AuNPs/lignin-modified glassy carbon electrode. Water Sci. Technol 2015, 72, 1780–1788. [DOI] [PubMed] [Google Scholar]
- (138).Shamsipur M; Tabrizi MA; Mahkam M; Aboudi J A High Sensitive TNT Sensor Based on Electrochemically Reduced Graphene Oxide-Poly (amidoamine) Modified Electrode. Electro-analysis 2015, 27, 1466–1472. [Google Scholar]
- (139).Sağlam Ş;Üzer A; Tekdemir Y; Erçağ E; Apak R Electrochemical sensor for nitroaromatic type energetic materials using gold nanoparticles/poly (o-phenylenediamine-aniline) film modified glassy carbon electrode. Talanta 2015, 139, 181–188. [DOI] [PubMed] [Google Scholar]
- (140).Yang T; Yu R; Chen H; Yang R; Wang S; Luo X; Jiao K Electrochemical preparation of thin-layered molybdenum disulfide-poly (m-aminobenzenesulfonic acid) nanocomposite for TNT detection. J. Electroanal. Chem 2016, 781, 70–75. [Google Scholar]
- (141).Coleman DH; White RE; Hobbs DT A parallel-plate electrochemical reactor model for the destruction of nitrate and nitrite in alkaline waste solutions. J. Electrochem. Soc 1995, 142, 1152–1161. [Google Scholar]
- (142).Pandikumar A; Manonmani S; Ramaraj R TiO2-Au nanocomposite materials embedded in polymer matrices and their application in the photocatalytic reduction of nitrite to ammonia. Catal. Sci. Technol 2012, 2, 345–353. [Google Scholar]
- (143).Ye D; Luo L; Ding Y; Chen Q; Liu X A novel nitrite sensor based on graphene/polypyrrole/chitosan nanocomposite modified glassy carbon electrode. Analyst 2011, 136, 4563–4569. [DOI] [PubMed] [Google Scholar]
- (144).Wang Q; Yun Y A nanomaterial composed of cobalt nanoparticles, poly (3,4-ethylenedioxythiophene) and graphene with high electrocatalytic activity for nitrite oxidation. Microchim. Acta 2012, 177, 411–418. [Google Scholar]
- (145).Ning D; Zhang H; Zheng J Electrochemical sensor for sensitive determination of nitrite based on the PAMAM dendrimer-stabilized silver nanoparticles. J. Electroanal. Chem 2014, 717, 29–33. [Google Scholar]
- (146).Xu F; Deng M; Liu Y; Ling X; Deng X; Wang L Facile preparation of poly (diallyldimethylammonium chloride) modified reduced graphene oxide for sensitive detection of nitrite. Electrochem. Commun 2014, 47, 33–36. [Google Scholar]
- (147).Lin P; Chai F; Zhang R; Xu G; Fan X; Luo X Electrochemical synthesis of poly (3, 4-ethylenedioxythiophene) doped with gold nanoparticles, and its application to nitrite sensing. Microchim. Acta 2016, 183, 1235–1241. [Google Scholar]
- (148).Liu M; Zhang S; Gao J; Qian Y; Song H; Wang S; Xie K; Jiang W; Li A Enhanced electrocatalytic nitrite determination using poly (diallyldimethylammonium chloride)-coated Fe1.833(OH)0.5O2.5-decorated N-doped graphene ternary hierarchical nanocomposite. Sens. Actuators, B 2017, 243, 184–194. [Google Scholar]
- (149).Madhuvilakku R; Alagar S; Mariappan R; Piraman S Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3,4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk. Anal. Chim. Acta 2020, 1093, 93–105. [DOI] [PubMed] [Google Scholar]
- (150).Sahoo S; Sahoo PK; Sharma A; Satpati AK Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens. Actuators, B 2020, 309, 127763. [Google Scholar]
- (151).Amlathe S; Gupta VK Spectrophotometric determination of trace amounts of hydrazine in polluted water. Analyst 1988, 113, 1481–1483. [DOI] [PubMed] [Google Scholar]
- (152).Umar A; Rahman MM; Shim YB ZnO nanorods based hydrazine sensors. J. Nanosci. Nanotechnol 2009, 9, 4686–4691. [DOI] [PubMed] [Google Scholar]
- (153).Umar A; Rahman MM; Kim SH; Hahn YB Zinc oxide nanonail based chemical sensor for hydrazine detection. Chem. Commun 2008, 2, 166–168. [DOI] [PubMed] [Google Scholar]
- (154).Vernot E; Mac Ewen JD; Bruner RH; Haus CC; Kinkead ER Long-term inhalation toxicity of hydrazine. Fundam. Appl. Toxicol 1985, 5, 1050–1064. [DOI] [PubMed] [Google Scholar]
- (155).Yamada K; Yasuda K; Fujiwara N; Siroma Z; Tanaka H; Miyazaki Y; Kobayashi T Potential application of anion-exchange membrane for hydrazine fuel cell electrolyte. Electrochem. Commun 2003, 5, 892–898. [Google Scholar]
- (156).Peng H; Liang C Electrochemical Detection of Hydrazine Based on Facial Synthesized Sulfur-Poly (Pyrrole-Co-Aniline) Nano-Fiber. Int. J. Electrochem. Sci 2016, 11, 8145–8154. [Google Scholar]
- (157).Rahman MM; Khan A; Marwani HM; Asiri AM Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim. Acta 2016, 183, 1787–1796. [Google Scholar]
- (158).Kaur B; Srivastava R; Satpati B Copper nanoparticles decorated polyaniline-zeolite nanocomposite for the nanomolar simultaneous detection of hydrazine and phenylhydrazine. Catal. Sci. Technol 2016, 6, 1134–1145. [Google Scholar]
- (159).Peng H; Liang C Electrochemical determination of hydrazine based on polydopamine-reduced graphene oxide nanocomposite. Fullerenes, Nanotubes, Carbon Nanostruct 2017, 25, 29–33. [Google Scholar]
- (160).Faisal M; Harraz FA; Al-Salami AE; Al-Sayari SA; Al-Hajry A; Al-Assiri MS Polythiophene/ZnO nanocomposite-modified glassy carbon electrode as efficient electrochemical hydrazine sensor. Mater. Chem. Phys 2018, 214, 126–134. [Google Scholar]
- (161).Afshari M; Dinari M; Momeni MM The graphitic carbon nitride/polyaniline/silver nanocomposites as a potential electrocatalyst for hydrazine detection. J. Electroanal. Chem 2019, 833, 9–16. [Google Scholar]
- (162).Kaladevi G; Wilson P; Pandian K Silver nanoparticle-decorated PANI/reduced graphene oxide for sensing of hydrazine in water and inhibition studies on microorganism. Ionics 2020, 26, 3123–3133. [Google Scholar]
- (163).Saeb E; Asadpour-Zeynali K Facile synthesis of TiO2@PANI@Au nanocomposite as an electrochemical sensor for determination of hydrazine. Microchem. J 2021, 160, 105603. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


