Abstract
Mice that lack the gene for expression of cytochrome P450 8B1 (P450 8B1) resist weight gain and improve glucose tolerance when fed a high-fat diet. Thus, the inhibition of P450 8B1 is a target to treat obesity-associated metabolic disorders. P450 8B1 is the enzyme that hydroxylates its substrate, 7α-hydroxy-cholest-4-en-3-one to 7α-,12α-dihydroxycholest-4-en-3-one, which ultimately results in the formation of cholic acid. Cholic acid is the 12α-hydroxylated bile acid implicated in enhanced absorption of cholesterol. The synthesis of a rationally designed inhibitor for P450 8B1 was achieved through the incorporation of a C12-pyridine in the C-ring of a steroid molecule. Seven days of new inhibitor treatment showed attenuation of glucose intolerance in mice that were fed a high fat and a high sucrose diet (HFHS) without affecting body weight. Taken together, these promising results will lead to a P450 8B1 inhibitor as a potential therapeutic strategy to treat obesity-associated insulin resistance.
Keywords: Bile acids, Cytochrome P450 8B1, Pyridine, Inhibitor, Organic synthesis
1. Introduction
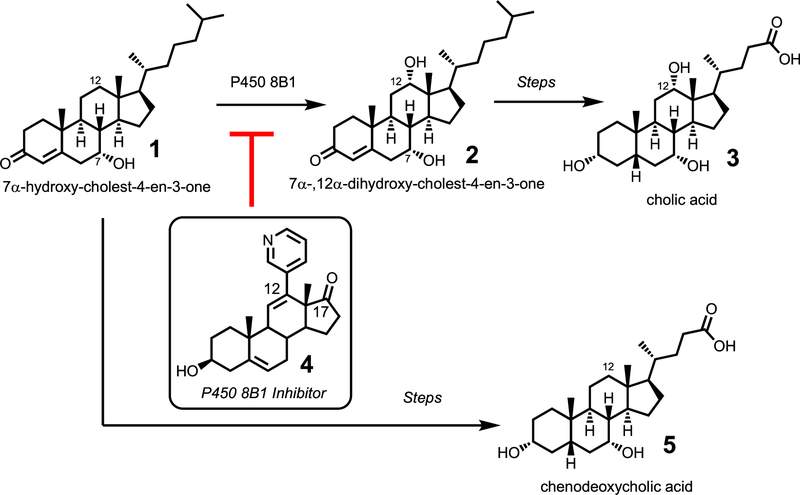
Cytochrome P450 8B1 (P450 8B1) is the enzyme that converts 7α-hydroxy-cholest-4-en-3-one to 7α-,12α-dihydroxycholest-4-en-3-one (Fig. 1). This enzyme was first characterized by Okuda and co-workers when the enzyme was isolated from rabbit liver microsomes. [1–2] The incorporation of the C12α-hydroxy group in the steroid backbone is important due to its ultimate formation of cholic acid, the bile acid implicated in enhanced absorption of cholesterol. [3] In fact, mice that lack the gene for expression of the P450 8B1 protein resist weight gain [4] and improved glucose homeostasis [5] when fed an obesogenic diet. Due to its potential in treating obesity-associated disorders, [6] the purpose of this study was to synthesize a selective inhibitor for cytochrome P450 8B1.
Fig. 1.
The enzymatic activity of cytochrome P450 8B1 involves the C12α-hydroxylation of its steroid-based substrate, 7α-hydroxy-cholest-4-en-3-one (1) to yield 7α-,12α-dihydroxy-cholest-4-en-3-one (2). This pathway ultimately forms cholic acid (3). Blocking the activity of P450 8B1 should lead to a decrease in cholic acid (3) and an increase in chenodeoxycholic acid (5).
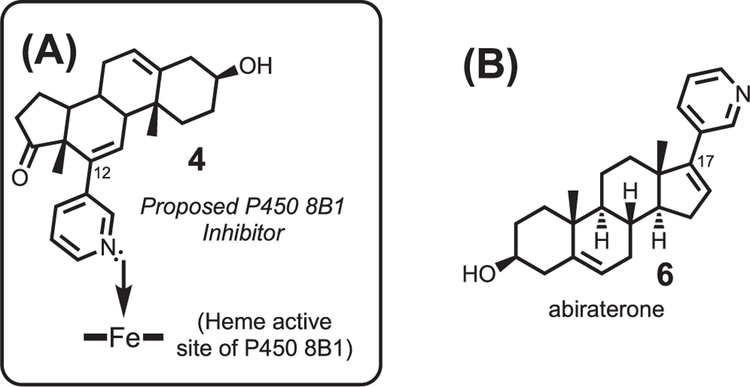
The steroid-based P450 8B1 inhibitor would bear a pyridine at the C12-position of the steroid, which would result in the coordination of the pyridine ring onto the iron active site of cytochrome P450 8B1 (Fig. 2A, 4). This rational approach to synthesizing an inhibitor for P450 8B1 was modeled after the strategy used to develop abiraterone, the steroid based inhibitor for P450 17A1 (Fig. 2B, 6). [7] P450 17A1 is the enzyme that hydroxylates the C17-position of its steroid substrates and subsequently cleaves the C17-C20 position to yield androgen products. [8] Abiraterone is a successful P450 17A1 inhibitor used to treat prostate cancer due to its ability to block androgen production (i.e. the activity of P450 17A1). [8] Abiraterone possesses a pyridine ring at the C17-position, which is the location of enzymatic activity of P450 17A1. Since P450 8B1 has enzymatic activity at the C12-position of its steroid substrates, we designed and synthesized a P450 8B1 inhibitor that possesses a pyridine at the C12-position of a steroid (Figs. 2 and 4). The compound (4) was tested in mice that were fed a a high fat and a high sucrose diet (HFHS) diet to show that the new P450 8B1 inhibitor has the anti-obesity effect in vivo.
Fig. 2.
(A) Rationally designed inhibitor for cytochrome P450 8B1. The incorporation of a pyridine ring at the C12-position should result in the coordination of the nitrogen lone pair from the inhibitor to the iron of the heme active site of cytochrome P450 8B1. (B) Structure of abiraterone for comparison.
Fig. 4.
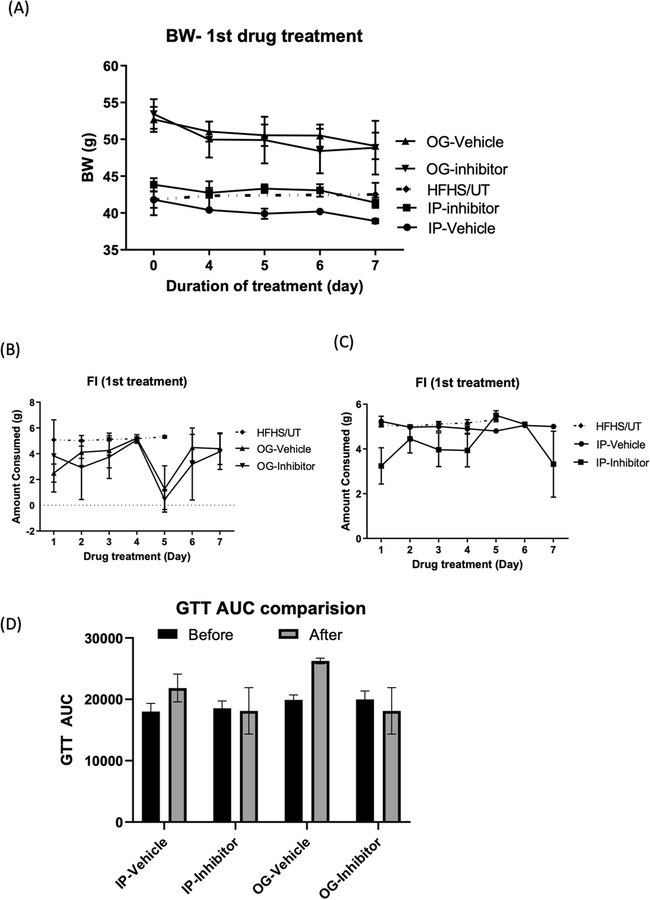
Effect of drug treatment on body weight, food intake, and glucose handling with 1st drug treatment. (A) Body weight (BW) decreased after 7 days of treatment. (B)-(C) Food intake was altered by the drug delivery method. (D) GTT AUC did not change in either the drug treatment or vehicle treatment. BW, body weight; GTT AUC, the area under the curve of glucose tolerance test. IP, intraperitoneal injection; OG, oral gavage. HFHS/UT (n = 8), mice fed with a high-fat and a high-sucrose diet (HFHS) only, indicated by closed diamond (◆); OG-vehicle (n = 2), HFHS/OB mice treated with vehicle, indicated by a closed triangle (▲); OG-inhibitor (n = 3), HFHS/OB mice treated with the inhibitor, indicated by a closed reversed triangle IP-inhibitor (n = 3), HFHS/IR mice treated with the inhibitor, indicated by a closed square (■); IP-vehicle (n = 2), HFHS/IR mice treated with the vehicle, indicated by a closed circle. Group differences were compared using one-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey’s multiple comparisons tests. A p-value of < 0.05 was considered a significant difference. OB: mice that developed insulin resistance with obesity. IR: mice that developed insulin resistance without obesity.
2. Results and discussion
2.1. Chemical synthesis of C12-pyridine containing steroid
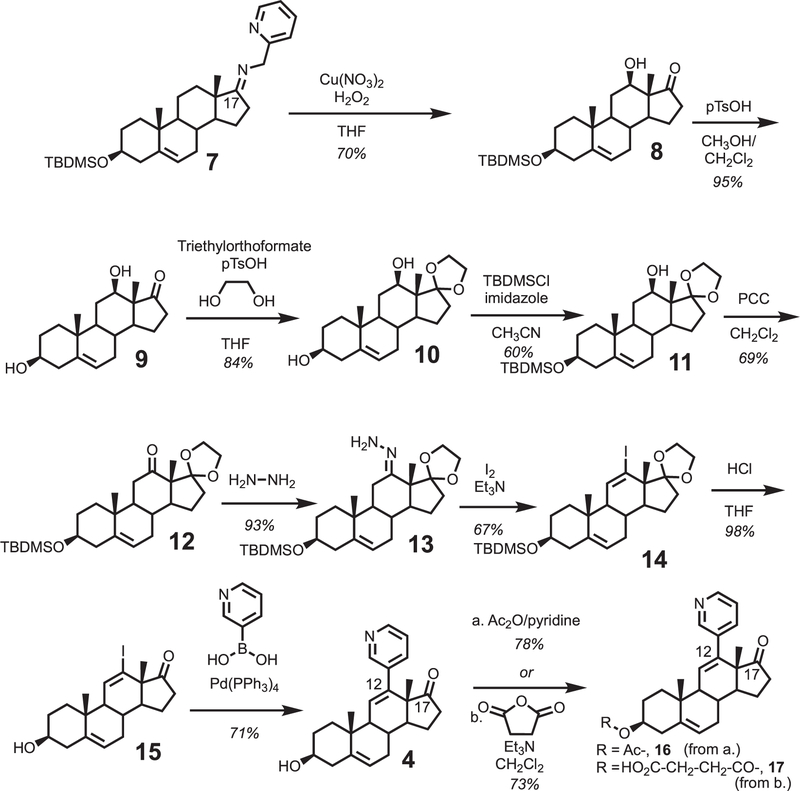
A C12-oxygenated dehydroepiandrosterone (DHEA) derivative containing a C12-ketone masked with a C17-ketal and a 3-tert-butyldimethylsilyl (TBDMS) ether (10) was synthesized through a similar route from our previous study (Scheme 1). [9] The key C12-hydroxylation step was performed using hydrogen peroxide as the oxidant (Scheme 1, 7 to 8), [10] which improved the yield from molecular oxygen (i.e. 70% vs. 20%). The use of the C3-TBDMS ether protecting group (compound 7) was necessary to improve extraction efficiency of the Schoenecker oxidation product. The C3-TBDMS group of 8 was subsequently removed with pTsOH in CH3OH/CH2Cl2 to yield diol 9. The C17-ketone of diol 9 was protected as the ketal with ethylene glycol and triethylorthoformate in the presence of pTsOH to afford ketal 10. Selective protection of the C3-alcohol with TBDMSCl gave TBDMS ether 11, which was subsequently oxidized with PCC to yield ketone 12. The resulting ketone (12) was refluxed with hydrazine and triethylamine in ethanol to yield hydrazone 13. Hydrazone 13 was treated with I2 and triethylamine to yield the vinyl iodide intermediate 14, which was obtained as an inseparable mixture with the C12-diiodide (not shown, see Supporting Information Part 2 for details). The C12-diiodide was eliminated to the vinyl iodide (14) using silver triflate in THF (Supporting Information, Part 2). The C17-ketal and C3-TBDMS groups of iodide 14 were deprotected with HCl in THF to yield vinyl iodide 15. Suzuki-cross coupling protocol with pyridine boronic acid introduced the pyridine moiety at the C12-position of the steroid to yield the inhibitor (compound 4).
Scheme 1.
Synthesis of a rationally designed inhibitor for P450 8B1. A pyridine moiety was incorporated at the C12-position of the steroid backbone.
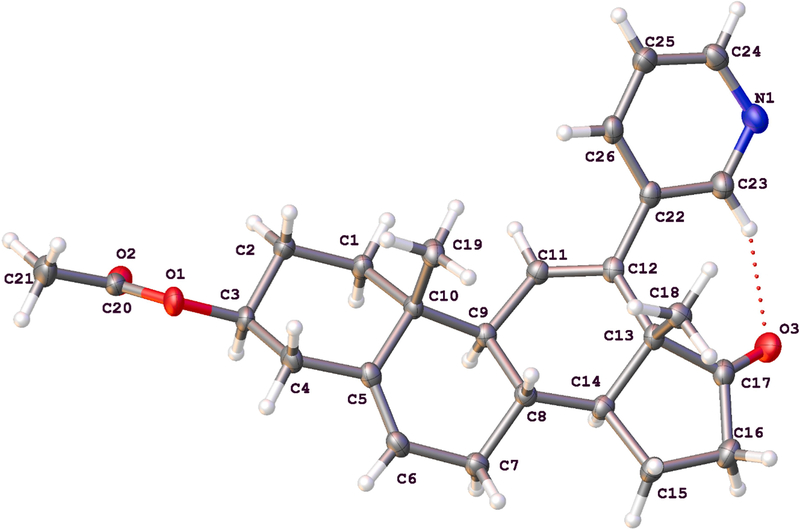
To enhance solubility of the designed inhibitor in a tween 80 buffer solution (see Second Drug Treatment section below, “TE”), the C3-hydroxy group was protected as the acetate (16) using acetic anhydride in pyridine. Abiraterone is used as the prodrug form, abiraterone acetate. Furthermore, a succinate version (17) was designed and synthesized to enhance solubility in water-based solvents (see Supporting Information, Part 3). Fig. 3 shows the crystal structure of compound 16.
Fig. 3.
Crystal structure of acetate 16, the rationally designed inhibitor of P450 8B1 reported in this study.
2.2. Treatment of mice with the C12-pyridine bearing steroid (Compound 4 or compound 16)
Two separate studies of treating mice with the designed P450 8B1 inhibitor were performed in this report. The first study used hydroxypropyl-β-cyclodextrin in buffer (HP-β-CD) as the solubilizing media for the drug and compound 4 was used (free 3-hydroxy group). The second study used a tween 80-based buffer (TE) as the solubilizing media for the drug and compound 16 was used (the C3-hydroxy was protected as the acetate).
The mice used in both studies (First Drug Treatment and Second Drug Treatment) were prepared as follows:
After 8 weeks of high-fat and a high-sucrose (HFHS) feeding, 5 out of 18 mice in the HFHS group developed obesity and insulin resistance (IR) as evidenced by impaired glucose tolerance tests (GTTs) (Fig. S1), which is common in diet-induced obesity models including mice [11] and primates [12] Between the first drug treatment and the second drug treatment, the mice underwent a 2-week “recovery phase” (described in the Experimental section). For further details regarding the mice in the two studies, see the descriptions below and the Supporting Information (Fig. S1, SI Part 1).
2.2.1. First drug treatment
In the first study (Drug Treatment 1): hydroxypropyl-β-cyclodextrin in 1x phosphate-buffered saline (PBS) (20% w/v) (HP-β-CD) was used as the vehicle. Compound 4 was dissolved in the vehicle (25 mg/ml). Initially, 150 mg/kg/day with compound 4 through oral gavage (OG) or intraperitoneal injection (IP) were planned, but because this high dose caused soft stool and ataxia, the dose was reduced to 100 mg/kg/day from the second day. The mice were treated at the same time each morning with 100 mg/kg/day for 7 days, and body weight was recorded. Intraperitoneal glucose tolerance test (GTT) was performed as described previously after 8 weeks of diet intervention and after drug treatment. The total area under the curve (AUC) was calculated by the trapezoidal method. Both the inhibitor and vehicle groups lost body weight regardless of delivery methods (OG and IP) compared to the untreated group with the same diet feeding (HFHS/UT) (Fig. 4A). Food Intake decreased in both vehicle and inhibitor of the OG group compared to HFHS/UT, while mice in the IP group did not have effects on food intake (Fig. 4B and 4C). After 7 days of the inhibitor treatment, we measured GTT and compared the results before and after treatment. As shown in Fig. 4D, the inhibitor (compound 4) attenuated impaired glucose tolerance although the results did not reach the statistical differences.
2.2.2. Second drug treatment
In the second drug treatment study in mice, only IP treated mice were used based on the findings that the OG-treatment from the first study resulted in less food consumption (Fig. 4B and 4C). After a 2-week recovery/wash-out phase in the mice from the first drug treatment, the vehicle was switched to 5% tween 80/5% ethanol in PBS (v/v) (TE). The vehicle was switched from HP-β-CD (see First Drug Treatment) to TE because a previous study demonstrated that oral administration of 100 mg/ml of HP-β-CD for 90 days in mice produced toxicity by increasing serum transaminase levels. [13] Furthermore, in order to enhance the solubility of the designed inhibitor (compound 4) in the new vehicle (TE), the C3-position of 4 was protected as the acetate to yield compound 16. The mice were treated with the same dose by IP injection (n = 2 for vehicle; n = 3 for compound 16).
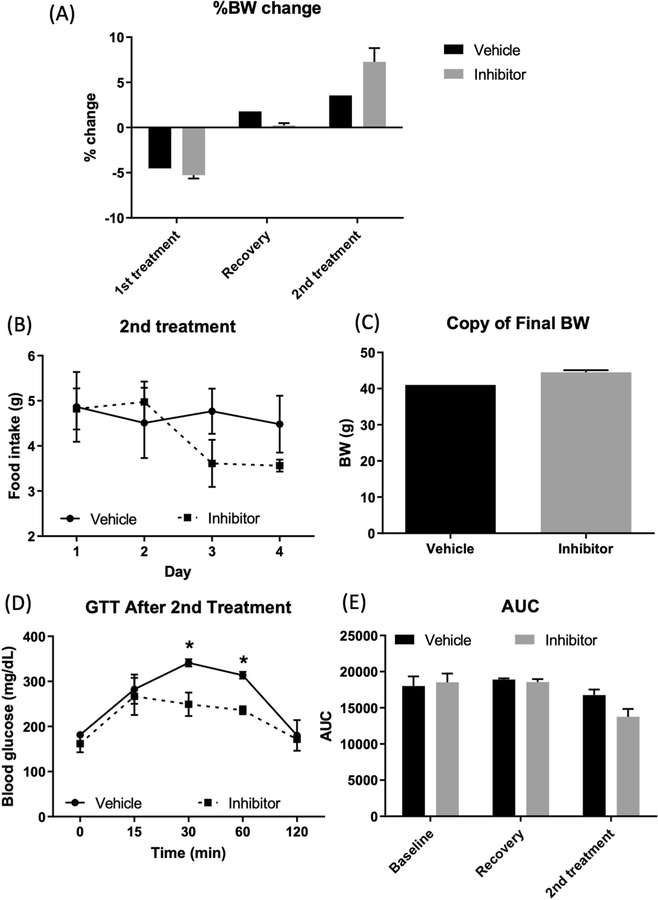
After treating the mice for 7 days with TE as the vehicle, the body weight did not decrease (Fig. 5A), suggesting that TE did not induce toxicity as in the case of HP-β-CD (Fig. 5A). The food intake was not significantly different between the two groups (vehicle and inhibitor) (Fig. 5B), which agrees with the previous study from cyp8b1 knock-out mice. [4] Unlike previous studies with cyp8b1 knock-out mice, [4,14–15] the final body weight was not different between the vehicle and inhibitor groups (Fig. 5C). Notably, mice treated with the inhibitor showed an improvement in glucose tolerance compared to the vehicle-treated group (Fig. 5D). GTT AUC throughout the study is shown in Fig. 5E.
Fig. 5.
Effect of drug treatment on body weight, food intake, and glucose handling with 2nd drug treatment. (A) % BW changes during the study period. 1st treatment of drug/vehicle with IP injection decreased body weight despite the mice were fed a HFHS, but during the 2 weeks of recovery, mice gained body weight. Drug treatment with TE vehicle increased body weight. (B) Food intake during 2nd drug treatment was not different. (C) Final body weight was not different between groups. (D) 2 days after 2nd drug treatment, glucose clearance was improved with drug treatment (IP-inhibitor) compared to the vehicle-treated group. (E) GTT AUC showed each treatment period. Baseline, before drug treatment; Recovery, 14 days of wash-out phase after 1st drug treatment; 2nd treatment, after 7 days of drug treatment IP-inhibitor (n = 3), HFHS/IR mice treated with the inhibitor, indicated by a closed square (■); IP-vehicle (n = 2), HFHS/IR mice treated with the vehicle, indicated by a closed circle. Group differences were compared using one-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey’s multiple comparisons tests. A p-value of <0.05 was considered a significant difference.
3. Conclusion
In summary, a rationally designed inhibitor for cytochrome P450 8B1 (the sterol 12α-hydroxylase enzyme) was achieved to treat obesity. The inhibitor was designed through the incorporation of a pyridine ring at the C12-position of the steroid ring (the location where P450 8B1 hydroxylates). In summary, our in vivo findings provide the proof-of-concept for the use of a cytochrome P450 8B1 inhibitor to improve insulin resistance associated with diet-induced obesity. Future studies are merited to extend our findings with a larger sample size and a longer duration of the treatment. Moreover, it is warranted to determine whether the positive effects of Cyp8b1 gene ablation shown in knock-out mice [16–17] can be recapitulated by the new inhibitor. Further modifications in the steroid ring will be made to test possible enhancements for selectivity and potency.
4. Experimental section
4.1. Materials and methods for mice experiments
4.1.1. Animals and diet
All experiments with mice were performed following the guidelines and regulations approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee (protocol # MU109). Five-week-old male CD1 mice were obtained from Charles River’s Laboratory (Wilmington, MA, USA) and were acclimated in the facility for 8 days on a standard laboratory chow diet (PicoLab Select Rodent 50 IF/6F, 5V5R, Lab Diet, St. Louis, MO, USA). Mice were weighed and randomly stratified by weight and group-housed and body weight was recorded weekly before the drug treatment. After 8-days of acclimatization, one cage (n = 5 mice/cage) was assigned to the control (CON) and fed with a refined control diet (Envigo, TD.200735, 62.4%, 19.4 %, and 18.2% of energy from carbohydrates, protein, and fat, respectively with 7% sucrose wt: wt). The remaining 4 cages (4–5 mice/cage) were assigned to a high-fat and a high sucrose diet (HFHS; Envigo, TD.08811, 40.7%, 14.7%, and 44.6 % of energy from carbohydrates, protein, and fat, respectively with 34 % sucrose wt:wt). The mice were fed on the same diets throughout the study.
4.1.2. Drug treatment
After 8 weeks of diet intervention, 5 mice out of 18 mice developed obesity and insulin resistance (OB), while 5 mice out of 18 mice developed insulin resistance (IR) without altering body weight; thus, 10 mice were used initially. Previous studies [18–20] using abiraterone to treat mice delivered the drug (abiraterone) either through oral gavage (OG) or intraperitoneal injection (IP), so both delivery methods were tested in this current study. The mice induced with obesity with insulin resistance (OB mice) were treated with the drug through OG, while the mice induced with insulin resistance without obesity (IR mice) were treated with the drug through IP. The mode of delivery of the drug (OG or IP) for each type of mouse (OB or IR) was arbitrarily chosen. We tested several common vehicles for the drug study: 0.5% methylcellulose/0.2% tween 80 in water (MC-T), [21] hydroxypropyl-β-cyclodextrin (HP-β-CD), [13] or 5% tween 80/5% ethanol in PBS (TE). [18] The compound 4 did not dissolve in MC-T. Thus, in the first 7 days of treatment, the following vehicle was used: 100 mg/ml HP-β-CD. The dose was determined based on the previous abiraterone acetate or similar compounds, such as VT-464. [20] The 150 mg/kg/day caused soft stool and ataxia, so we reduced the dose to 100 mg/kg/day. The mice were treated at the same time each morning with 100 mg/kg/day for 7 days followed by 14 days without treatment (recovery phase).
After a 2-week recovery phase, the vehicle was switched to TE and the mice were treated with the same dose by IP injection (n = 2 for the vehicle; n = 3 for the compound) (Second Drug Treatment, see above). The mice were individually housed, administered for 7 days, and daily body weight changes and caloric intake were recorded. All conditions and handling of the animals were approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee. The study is reported following ARRIVE guidelines. [22]
4.1.3. Glucose tolerance test
Intraperitoneal glucose tolerance test (GTT) was performed as described previously [23] after 8 weeks of diet intervention, after 2-week recovery phase, and after 2nd treatment.
4.2. Chemical synthesis
A Laxco™ POL-300 Series Digital Automatic Polarimeter (Thermo Fisher Scientific, Waltham, MA) was used for optical rotation measurements. A 500 MHz Bruker (Billerica, MA) NMR spectrometer was used to obtain NMR spectra. LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA) connected to a Waters Acuity UPLC system (Milford, MA) was used to acquire high resolution mass spectrometry data.
4.2.1. . 12β-Hydroxy-dehydroepiandrosterone-3-tert-butyldimethyl silyl ether (Compound 8)
To 3 separate batches of the imine 7 (A, B and C, 150 g total, 50 g for each batch, 0.101 mol, 1.0 eq) in an oven dried 2 L round bottom flask (1 RBF for each batch) was added copper (II) nitrate hemi(pentahydrate) (26.1 g for each batch, 0.11 mol, 1.1 eq) and THF (3 × 500 ml). The solution was stirred for 15 min to completely dissolve the imine and copper nitrate salt resulting in an intense blue emulsion. Then H2O2 (30 % (w/w) in H2O, 31 ml for each batch, 0.304 mol, 3 eq) was added gradually to the reaction. The reaction became slightly exothermic. The color of the solution changed from a cloudy blue color to a clear dark green solution after H2O2 was added. The reaction was stirred for a further 15 min. Upon completion, the three reaction solutions (A, B, and C) were combined. The combined reaction solution was transferred to a stirring solution of saturated Na4EDTA (100 g of Na4EDTA in 500 ml of deionized H2O), and then ethyl acetate (500 ml) was added. The resulting solution was stirred for 1 h and then filtered using the fritted filter funnel to take out the Na4EDTA and copper salts. The filtrate was transferred into a separatory funnel and left to stand for at least 5 min to differentiate the organic layer from the aqueous layer. The organic layer was then separated from the aqueous layer. The aqueous layer was further extracted with ethyl acetate (3 × 500 ml). The organic solutions were combined and concentrated under reduced pressure to form a cream crude solid product. The crude cream solid was purified via column chromatography (20% ethyl acetate in hexanes → 70 % ethyl acetate in hexanes) to afford the 12β-hydroxylated product 8 as a white solid (89.6 g, 0.214 mol, 70.5 % from 150 g of imine, 0.304 mol). mp (170–173) °C; [α]D20 + 19° [0.002 in CHCl3]; Rf: 0.45 (4:1, hexanes: ethyl acetate, v/v); IR (ATR) 3545.09, 2950.11, 1732.84, 1470.74, 1253.78 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.34 (d, J = 6 Hz, 1H), 3.8 (dd, J = 11.3, 4.9 Hz, 1H), 3.47 (m, 1H), 3.06 (s, 1H), 2.47 (dd, J = 19, 10 Hz, 1H), 2.31–2.16 (m, 2H), 2.17–2.04 (m, 2H), 2.02–1.95 (m, 1H), 1.86–1.77 (dt, J = 13, 5 Hz, 2H), 1.76–1.70 (m, 1H), 1.69–1.57 (m, 3H), 1.57–1.50 (m, 1H), 1.49–1.37 (m, 1H), 1.29–1.19 (m, 1H), 1.16–1.06 (m, 2H), 1.06–0.99 (m, 1H), 1.03 (s, 3H), 0.95 (s, 3H), 0.88 (s, 9H), 0.07 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 223.20, 141.94, 120.35, 77.82, 77.51, 51.54, 49.72, 49.35, 42.83, 37.40, 37.00, 35.90, 32.10, 30.74, 30.55, 26.05, 21/84, 19.51, 18.38, 8.19, −4.45; HRMS (m/z, ESI +) calculated for: [M + H]+, C29H43O3Si+, m/z 419.2976; found m/z 419.2564 (△ 98 ppm).
4.2.2. 12β-Hydroxy-dehydroepiandrosterone (Compound 9)
In a 2 L round bottom flask containing compound 8 (78.4 g, 0.187 mol, 1 eq) was added methanol (250 ml) and dichloromethane (400 ml). The solution was stirred for 5 min. Then para-toluenesulfonic acid (p-TsOH) (8.05 g, 0.047 mol, 0.25 eq) was added to the stirring solution. The solution was stirred for a further 8 h and the progress was constantly monitored by TLC. The reaction was quenched with the addition saturated NaHCO3 (aqueous, 400 ml). The solution was transferred into a 2 L separatory funnel and extracted with dichloromethane (3 × 250 ml). The dichloromethane fractions were combined and concentrated under reduced pressure to form a crude white residue. The crude white solid was purified by column chromatography (50 % ethyl acetate in hexanes → 10% methanol in dichloromethane) to afford the diol 9 as white solid (54.4 g, 0.178 mol, 95 %); Rf: 0.61 (100% ethyl acetate); [α]D20 – 19.2° [c = 0.0013 in CHCl3]; mp (156 – 161) °C; IR (ATR) 3444.02, 2933.96, 1732.28, 1461.12 cm−1; 1H NMR (500 MHz CDCl3) δ 5.37 (apparent s, 1H), 3.80 (dd, J = 11.87, 4.80 Hz, 1H), 3.52 (m, 1H), 3.07 (s, 1H), 2.47 (dd, J = 18.91, 9.19 Hz, 1H), 2.33 (m, 1H), 2.27–2.19 (m, 1H), 2.16–2.07 (m, 1H), 2.02–1.95 (m, 1H), 1.88–1.78 (m, 3H), 1.72 – 1.58 (m, 4H), 1.55 – 1.40 (m, 2H), 1.29 – 1.21 (m, 1H), 1.15 – 1.05 (m, 2H), 1.04 (s, 3H), 0.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 222.95, 141.60, 120.89, 72.81, 71.68, 51.60, 51.53, 49.68, 49.26, 42.23, 37.38, 36.91, 35.89, 31.70, 30.72, 30.50, 28.33, 21.83, 19.48, 8.19; HRMS (m/z, ESI +) calculated for: [M + H]+, C19H29O3+, m/z 305.2111; found m/z 305.2077 (△ 11 ppm).
4.2.3. 12β-Hydroxy-dehydroepiandrosterone ethylene glycol 17-ketal (Compound 10)
To a solution of the diol 9 (10 g, 0.032 mol, 1 eq) in THF (50 ml) was added sequentially ethylene glycol (18 ml, 0.32 mol, 10 eq), p-TsOH (0.88 g, 0.005 mol, 0.16 eq) and triethylorthoformate (21 ml, 0.128 mol, 4 eq). The solution was stirred for 1 h with the diol ketal product crushing out as white solid in the reaction solution. The white solid was suction filtered from the solution using a fritted filter funnel. The solution was diluted with ethyl acetate (250 ml), transferred into a 1 L separatory funnel, and washed with saturated aqueous NaHCO3 (3 × 250 ml). The ethyl acetate fraction was then concentrated under reduced pressure to form a cream residue. Deionized water (50 ml) was added to the crude residue resulting in a suspension. The suspension was suction filtered using a fritted filter funnel to separate the white solids from the solution. The crude white solid gathered (crushed out solids from reaction and workup) was purified by column chromatography (80% ethyl acetate in hexanes → 10 % methanol in dichloromethane) to afford the diol ketal 10 as white solids (9.5 g, 0.027 mol, 84 %); mp (195 – 198) °C; [α]D20 – 126° [c = 0.001 in CHCl3]; Rf: 0.58 (100 % ethyl acetate); IR (ATR) 3375.03, 2942.42, 1408.06, 1301.85 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.34 (apparent s, 1H), 3.99 (m, 4H), 3.88 (m, 1H), 3.51 (m, 1H), 2.34 (m, 1H), 2.26 (m, 1H), 2.04 (m, 1H), 1.95 (m, 1H), 1.90–1.66 (m, 5H), 1.56 – 1.34 (m, 7H), 1.12 – 1.04 (m, 2H), 1.03 (s, 3H), 0.93 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 140.85, 121.42, 119.20, 71.75, 71.45, 64.72, 64.21, 49.36, 49.18, 48.86, 42.29, 37.36, 36.74, 33.90, 31.74, 31.36, 30.92, 29.52, 22.46, 19.51, 9.01; HRMS (m/z, ESI +) calculated for: [M + H]+, C21H33O4+, m/z 349.2373; found m/z 349.2331 (△ 12 ppm).
4.2.4. 12β-Hydroxy-dehydroepiandrosterone ethylene glycol 17-ketal 3-tert-butyldimethyl silyl ether (Compound 11)
In a 2 L round bottom flask containing the diol ketal 10 (84.3 g, 0.25 mol, 1 eq) in acetonitrile (750 ml) was added sequentially imidazole (83.7 g, 1.23 mol, 5 eq) and tert-butyldimethylsilyl chloride (111.6 g, 0.74 mol, 3 eq). The reaction was stirred for 2 h. After the completion of the reaction, the solution was diluted with ethyl acetate (500 ml) and transferred into a 2 L separatory funnel. The resulting solution was washed with deionized water (3 × 500 ml). The ethyl acetate fraction was concentrated under reduced pressure to afford a cream solid, which was purified by column chromatography to afford the silyl ether as a white solid 11 (69 g, 0.15 mol, 60 %); mp (107–110) °C; Rf: 0.74 (1:1, hexanes: ethyl acetate, v/v); [α]D20 + 4.6° [c = 0.00192 in CHCl3]; IR (ATR) 3573.26, 2929.83, 1469.58, 1381.05 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.31 (d, J = 5 Hz, 1H), 4.03–3.95 (m, 4H), 3.91–3.85 (m, 1H), 3.47 (m, 1H), 2.30–2.22 (m, 1H), 2.21–2.14 (m, 1H), 2.05–1.96 (m, 1H), 1.94–1.86 (m, 1H), 1.86–1.77 (m, 2H), 1.76–1.68 (m, 2H), 1.67–1.59 (m, 2H), 1.57–1.45 (m, 2H), 1.50–1.41 (m, 2H), 1.42–1.36 (m, 3H), 1.10–0.99 (m, 2H), 1.02 (s, 3H), 0.93 (s, 3H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 141.65, 120.89, 119.25, 72.59, 71.51, 49.41, 49.25, 48.87, 42.84, 37.48, 36.84, 32.10, 35.95, 32.15, 31.38, 30.97, 29.53, 26.07, 22.47, 19.55, 18.37, 9.01, −4.41; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H47O4Si+, 463.3238; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H47O4Si+, m/z 463.3238; found m/z 463.3241 (△ 0.6 ppm).
4.2.5. 12-Oxo-dehydroepiandrosterone ethylene glycol 17-ketal 3-tert-butyldimethyl silyl ether (Compound 12)
Pyridinium chlorochromate (78.3 g, 0.363 mol, 2 eq) was added to a solution of compound 11 (84.0 g, 0.23 mol, 1 eq) in dichloromethane (500 ml). The solution was stirred for 45 min. The solution was transferred into a 2 L separatory funnel and washed with 10% NaOH in water (w/v, 2 × 500 ml). The dichloromethane fraction was concentrated under reduced pressure to afford a crude brown slurry which was then purified by column chromatography (100% hexanes → 10 % ethyl acetate in hexanes) to afford the C12-ketone 12 as white solid; (73.9 g, 0.159 mol, 69 %); mp (115–119) °C; [α]D20 + 12° [c = 0.00153 in CHCl3]; Rf: 0.68 (4:1, hexanes: ethyl acetate, v/v); IR (ATR) 3541.16, 2929.25, 1732.87, 1470.60 cm−1; 1H NMR (500 MHz, CDCl3) δ 5.34 (d, J = 6 Hz, 1H), 4.28–4.15 (m, 1H), 4.15–4.08 (m, 1H), 4.01–3.90 (m, 1H), 3.90–3.75 (m, 1H), 3.47 (m, 1H), 2.42 (dd, J = 15, 11 Hz, 1H), 2.31–2.19 (m, 2H), 2.19–2.12 (m, 1H), 2.05–1.91 (m, 1H), 1.84–1.75 (m, 2H), 1.68–1.60 (m, 1H), 1.65–1.55 (m, 1H), 1.54–1.37 (m, 2H), 1.33–1.16 (m, 5H), 1.11 (s, 3H), 1.07–0.98 (m, 1H), 1.05 (s, 3H), 0.85 (s, 9H), 0.04 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 210.90, 141.29, 120.81, 116.92, 72.28, 65.59, 65.02, 57.73, 50.83, 49.96, 42.77, 38.64, 37.09, 37.04, 34.58, 31.92, 31.36, 30.79, 26.06, 20.92, 19.21, 18.37, −4.45; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H44O4Si+, 460.3009; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H45O4Si+, m/z 461.3082; found m/z 461.3025 (△ 12 ppm).
4.2.6. Dehydroepiandrosterone 12-hydrazone 3-tert-butyldimethyl silyl ether ethylene glycol 17-ketal (Compound 13)
To the solution of the ketone 12 (50 g, 0.108 mol, 1 eq) in dry anhydrous ethanol (500 ml) was added triethylamine (20 ml, 0.14 mol, 1.3 eq) and hydrazine monohydrate (100 ml, 2.02 mol, 18.7 eq). The solution was reflux for 1 h. The progress of the reaction was monitored by NMR (appearance of the ‘dd’ at 2.7 ppm). The reaction was cooled to room temperature after completion and concentrated under reduced pressure to remove the ethanol solvent. The oily residue formed was diluted with dichloromethane (500 ml). The solution was transferred into a 2 L separatory funnel and washed with deionized water (3 × 500 ml) and dried over magnesium sulfate (1 g). The magnesium sulfate was filtered-off using a cotton plug funnel. The CH2Cl2 fraction was concentrated under reduced pressure to afford the C12-hydrazone 13 as white waxy solid (47.5 g, 0.100 mol, 93 %). No further purification was done at this point. ***The use of acetone was avoided throughout the reaction and workup procedure because acetone condenses with the terminal amino group of the hydrazone***; mp (72–76) °C; IR (ATR) 2928.37, 1470.68, 1140.99, 1000.66 cm−1; [α]D20 −83.3° [c = 0.0014 in CHCl3]; 1H NMR CDCl3 δ 5.32 (apparent s, 1H), 4.90 (broad s, 1H), 4.19 (m, 1H), 4.04 (m, 1H), 3.88 (m, 2H), 3.48 (m, 1H), 2.70 (dd, J = 16.44, 6.52 Hz, 1H), 2.29–2.14 (m, 3H), 2.09–1.92 (m, 4H), 1.89–1.47 (m, 7H), 1.33–1.22 (m, 2H), 1.12–1.02 (m, 1H), 1.05 (s, 3H), 1.03 (s, 3H), 0.87 (s, 9H), 0.04 (s, 6H); 13CNMR CDCl3 δ 154.85, 141.31, 121.02, 118.47, 72.27, 65.72, 65.25, 51.49, 49.79, 49.00, 42.78, 37.25, 37.23, 35.10, 31.96, 31.52, 31.01, 26.03, 22.55, 21.24, 20.08, 19.07, 18.33, 17.02, −4.47; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H47N2O3Si+, m/z 475.3350; found m/z 475.3288 (△ 13 ppm).
4.2.7. 12-Iodo-androsta-5,11-diene-3β-tert-butyldimethyl silyl ether ethylene glycol 17-ketal (Compound 14)
To the solution of the hydrazone 13 (47.5 g, 0.100 mol, 1 eq) in dry CH2Cl2 (300 ml) under nitrogen environment was added triethylamine (35 ml, 0.25 mol, 2.5 eq). Iodine solid (51 g, 0.2 mol, 2 eq) was dissolved in dry THF (50 ml) and gradually to the stirring solution with the aid of a syringe. The resulting dark solution was stirred for a further 30 min. The progress of the reaction was monitored by TLC. The reaction was concentrated under reduced pressure to form a black crude oil. The crude oil was purified by column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes) to afford the major C12-vinyl iodide product which co-eluted with the di-iodide as the minor product (38.7 g, 0.066 mol, 67 %) as an orange oil. ***A pure compound 14 was obtained for characterization after the S2/S3/S4/15 mixture was treated with silver triflate to convert the diiodide to the vinyl iodide (S2/15) - see SI Part 4 for details. The vinyl iodide (S2) was then protected at the C3-alcohol as the OTBDMS ether (14) ***Pure compound 14: cream solid (115–118) °C; Rf: 0.8 (4:1, hexanes: ethyl acetate, v/v); [α]D20–54.5° [c = 0.0004 in CHCl3]; IR (ATR) 2926.32, 1469.97, 1373.39, 1181.52 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.32 (d, J = 2.33 Hz, 1H), 5.36 (d, J = 5.64 Hz, 1H), 4.19 (m, 2H), 3.94 (m, 2H), 3.48 (m, 1H), 2.19 (m, 3H), 1.97 (m, 1H), 1.94 – 1.78 (m, 5H), 1.78 – 1.51 (m, 4H), 1.43 – 1.33 (m, 1H), 1.08 (m, 1H), 1.06 (s, 3H), 0.99 (s, 3H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 142.14, 139.80, 121.07, 117.97, 104.32, 72.55, 64.76, 63.93, 58.08, 51.25, 49.80, 42.78, 37.73, 36.74, 35.63, 31.82, 30.65, 30.22, 26.02, 21.17, 19.68, 18.34, 18.45, 17.66, −4.46; HRMS (m/z, ESI +) calculated for: [M + H]+, C27H44IO3Si+, m/z 571.2099; found m/z 571.2035 (△ 11 ppm).
4.2.8. 12-Iodo-androsta-5,11-diene-3β-ol 17-one (Compound 15)
To vinyl-iodide 14 (25 g, 0.044 mol, 1 eq) in THF (250 ml) was added HCl (100 ml, 2 M). The reaction was then refluxed for 12 h. The reaction was cooled to room temperature and quenched with the addition of saturated aq. NaHCO3 (250 ml). The resulting solution was extracted over ethyl acetate (3 × 250 ml). The ethyl acetate extract was concentrated under reduced pressure to form a crude colorless oil which was purified by column chromatography (100 % hexanes → 40 % ethyl acetate in hexanes) to afford the deprotected product as cream solid. The cream solid was subsequently treated with silver triflate to convert the 12,12-diiodide to the vinyl iodide 15 (See supporting information). (18.0 g, 0.043 mol, 98 %); mp (125–129) °C; [α]D20 – 245.4° [c = 0.00033 in CHCl3]; Rf: 0.55 (1:1, hexanes: ethyl acetate, v/v); IR (ATR) 3346.86, 2924.82, 1742.20, 1450.35 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.36 (d, J = 1.86 Hz, 1H), 5.44 (apparent s, 1H), 3.56 (m, 1H), 2.52 (dd, J = 19.25, 9.09 Hz, 1H), 2.35 (m, 1H), 2.26 – 2.06 (m, 3H), 2.03–1.73 (m, 7H), 1.63 – 1.43 (m, 2H), 1.16 (m, 1H), 1.07 (s, 3H), 1.02 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 212.15, 141.61, 139.86, 121.20, 100.84, 71.71, 58.12, 51.51, 50.39, 42.17, 37.78, 37.02, 36.66, 31.39, 30.36, 29.81, 19.77, 17.68, 1.15; HRMS (m/z, ESI +) calculated for: [M + H]+, C19H26IO2+, m/z 413.0972; found m/z 413.0916 (△ 14 ppm).
4.2.9. 12-(3-Pyridyl)-androsta-5,11-diene-3β-ol-17-one (Compound 4)
A 1 L round bottom flask was charged with the vinyl-iodide 15 (7.0 g, 0.017 mol, 1 eq), K2CO3 (11.70 g, 0.084 mol, 5 eq), 3-pyridine boronic acid (6.18 g, 0.051 mol, 3 eq) and bis(triphenylphosphine)palladium chloride (300 mg, 0.42 mmol, 2.5 %). THF (350 ml) and water (100 ml) were added to the reaction constituents. The resulting solution was refluxed for 12 h. After completion of the reaction as monitored by TLC and NMR, the reaction was cooled to room temperature and transferred into a 2 L separatory funnel. The solution was diluted with ethyl acetate (250 ml) and washed with deionized water (3 × 250 ml). The ethyl acetate extract was concentrated under reduced pressure to afford a crude brown oil which had traces of triphenyl phosphine oxide, which co-eluted with the pyridine product during column chromatography. Liquid-liquid extraction was performed on the reaction crude to remove the triphenyl phosphine oxide. Liquid-liquid extraction procedure to remove the triphenylphosphine oxide from the reaction: The brown oil was dissolved in CH2Cl2 (200 ml) and then transferred into a 2 L separatory funnel. The CH2Cl2 solution was washed repeatedly with aq. 1 M HCl (4 × 250 ml). The triphenylphosphine oxide was left in the CH2Cl2 layer while the pyridinium compound was protonated and extracted into the aq. HCl solution. The aq. HCl solutions gathered from the extraction were combined and basified with 20% NaOH (100 g NaOH/500 ml of deionized water). The pH of the solution was then determined using a universal pH indicator strip (pH was 14). The basified solution was transferred into another 2 L separatory funnel and then extracted with another portion of CH2Cl2 (4 × 250 ml). The CH2Cl2 fractions were combined, concentrated under reduced pressure to form a white foam. The white foam was purified by column chromatography (50 % ethyl acetate in hexanes → 100% ethyl acetate) to afford the pyridine product 4 as a white foamy solid (4.5 g, 0.012 mol, 71 %); mp (68–72) °C; [α]D20 −60.5° [c = 0.0014 in CHCl3]; Rf: 0.41 (100% ethyl acetate); IR (ATR) 3231.50, 2923.22, 1735.64, 1470.83 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.47 (m, 2H), 7.68 (dt, J = 7.71, 1.84 Hz, 1H), 7.24 (dd, J = 7.89, 1.14 Hz, 1H), 5.58 (d, J = 2.5 Hz, 1H), 5.48 (apparent s, 1H), 3.57 (m, 1H), 2.46 (dd, J = 9.08, 18.57 Hz, 1H), 2.38 (ddd, J = 12.80, 4.97, 2.27 Hz, 1H), 2.24 – 2.11 (m, 3H), 2.07 – 2.01 (m, 2H), 2.00–1.92 (m, 3H), 1.92 − 1.79 (m, 2H), 1.71 – 1.62 (m, 1H), 1.60 – 1.50 (m, 1H), 1.29 (s, 3H), 1.21 (td, J = 12.85, 3.75 Hz, 1H), 1.06 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 215.15, 148.97, 147.55, 142.07, 141.95, 136.95, 136.66, 130.18, 122.31, 121.46, 71.71, 54.15, 50.89, 49.26, 42.30, 37.85, 36.84, 36.70, 31.45, 30.52, 29.93, 20.45, 19.81, 19.45; HRMS (m/z, ESI +) calculated for: [M + H]+, C24H30NO2+, m/z 364.2271; found m/z 364.2226 (△ 12 ppm).
4.2.10. 12-(3-Pyridyl)-androsta-5,11-diene-3β-acetoxy-17-one (Compound 16)
To the pyridine coupled steroid 4 (6.7 g, 0.018 mol, 1 eq) dissolved in pyridine (50 ml, 0.98 mol, 54 eq) was added acetic anhydride (10 ml, 0.105 mol, 5.7 eq). The reaction was stirred for 12 h with the progress monitored by TLC and NMR. After completion of the reaction, the solution was concentrated under reduced pressure to remove solvents (pyridine and acetic anhydride). The crude yellow oil was dissolved with ethyl acetate (100 ml) and transferred into a 1 l separatory funnel. The resulting solution was washed with several portions of deionized water (3 × 250 ml). Then the organic solution was washed with saturated aq. NaHCO3 (3 × 250 ml). The organic layer was concentrated under reduced pressure to form the crude colorless oil which was purified by column chromatography (50 % ethyl acetate in hexanes → 100 % ethyl acetate) to afford the C3 acetylated product 16 (5.8 g, 0.014 mol, 78 %) as white solid; mp (115–119) °C; [α]D20 – 50.8° [c = 0.0019in CHCl3]; Rf: 0.70 (100% ethyl acetate); IR (ATR) 2896.83, 1720.44, 1479.99, 1244.51 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.49 (dd, J = 4.83, 1.76 Hz, 1H), 8.45 (apparent d, J = 2.48 Hz, 1H), 7.66 (dt, J = 7.94, 1.95 Hz, 1H), 7.23 (dd, J = 7.49, 4.90 Hz, 1H), 5.57 (d, J = 2.25 Hz, 1H), 5.52 (apparent s, 1H), 4.64 (m, 1H), 2.49 – 2.38 (m, 2H), 2.31 – 2.11 (m, 3H), 2.04 (s, 3H), 2.07 – 1.81 (m, 7H), 1.71 – 1.60 (m, 2H), 1.27 (m, 1H), 1.25 (s, 3H), 1.08 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.65, 149.24, 147.87, 142.31, 140.83, 136.75, 129.85, 122.52, 122.26, 73.80, 54.04, 50.92, 49.23, 38.22, 37.93, 36.72, 36.61, 30.50, 29.95, 27.57, 21.53, 20.47, 19.70, 19.45; HRMS (m/z, ESI +) calculated for: [M + H]+, C26H32NO3+ m/z 406.2377; found 406.2355 (△ 5 ppm).
4.2.11. 12-(3-Pyridyl)-androsta-5,11-diene-3β-succinyl-17-one (Compound 17)
To a 250 ml RBF charged with the pyridine compound 4 (1.6 g, 4.4 mmol, 1 eq) was added succinic anhydride (0.66 g, 6.6 mmol, 1.5 eq) and CH2Cl2 (50 ml). Triethylamine (1.2 ml, 8.8 mmol, 2 eq) was added to the reaction solution. The reaction was stirred for 6 h. A second portion of the succinic anhydride (0.66 g, 6.6 mmol, 1.5 eq) was added. The reaction was left to stir until completion, which was for another 3 h. The solution was concentrated under reduce pressure to yield a crude brown oil which was purified by column chromatography (50 % ethyl acetate in hexanes → 10 % methanol in CH2Cl2) to afford the succinic acid product 17 as cream solids (1.5 g, 3.2 mmol, 73 %); mp (174 – 178) °C; Rf: 0.57 (10% MeOH inCH2Cl2);[α]D20 + 75° [c = 0.0014 in CHCl3]; IR (ATR) 2925.50, 1724.48, 1410.81, 1215.88 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.48 (d, J = 8.06 Hz, 2H), 7.76 (d, J = 8.33 Hz, 1H), 7.31 (m, 1H), 5.59 (d, J = 2 Hz, 1H), 5.51 (d, J = 5 Hz, 1H), 4.67 (m, 1H), 2.71 – 2.58 (m, 4H), 2.49 – 2.38 (m, 2H), 2.32 – 2.24 (m, 1H), 2.22 − 2.11 (m, 2H), 2.07 – 1.90 (m, 6H), 1.88 – 1.81 (m, 1H), 1.71 – 1.60 (m, 2H), 1.30 – 1.20 (m, 1H), 1.25 (s, 3H), 1.06 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 215.15, 175.75, 171.99, 147.56, 146.19, 141.74, 140.79, 138.08, 137.24, 130.50, 122.71, 122.49, 74.13, 54.09, 50.84, 49.19, 38.11, 37.92, 36.68, 36.57, 30.47, 29.93, 29.64, 29.20, 27.47, 20.45, 19.77, 19.53, HRMS (m/z, ESI +) calculated for: [M + H]+, C28H34NO5+, 464.2431; found 464.2371 (△ 13 ppm).
4.3. X-ray crystallography of compound 16
Single crystals of C26H31NO3 (16) were prepared by slow evaporation of an acetone solution (500 mg of the steroid was dissolved in 20 ml of acetone in an 11-dram vial and left to sit open to air for 4 days). A suitable colorless block-like crystal, with dimensions of 0.200 mm × 0.155 mm × 0.095 mm, was mounted in paratone oil onto a nylon loop. All data were collected at 100.0(1) K, using a XtaLAB Synergy/Dualflex, HyPix fitted with CuKα radiation (λ = 1.54184 Å). Data collection and unit cell refinement were performed using CrysAlisPro software. [24] The total number of data were measured in the 9.10° < 2θ < 152.8°, using ω scans. Data processing and absorption correction, giving minimum and maximum transmission factors (0.378, 1.000) were accomplished with CrysAlisProv [24] and SCALE3 ABSPACK, [25] respectively. The structure, using Olex2, [26] was solved with the ShelXT [27] structure solution program using direct methods and refined (on F2) with the ShelXL [28] refinement package using full-matrix, least-squares techniques. All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atom positions were determined by geometry and refined by a riding model.
Supplementary Material
Acknowledgments
Francis K. Yoshimoto, Ph.D. holds a Voelcker Fund Young Investigator Award from the MAX AND MINNIE TOMERLIN VOELCKER FUND. Eunhee Chung, Ph.D. was supported by the National Institute of Health [NIH, NIGMS, grant GM125603]. NSF MRI grant for X-ray diffraction (Award No. 1920057) and NSF MRI grant for NMR spectrometer (Award No. 1625963) are also acknowledged.
Abbreviations:
- CON
Control mice
- CYP8B1
Cytochrome P450 8B1
- HFHS
High fat high sucrose diet
- HP-β-CD
Hydroxypropyl-β-cyclodextrin
- LC-HRMS
Liquid chromatography high-resolution mass spectrometry
- IP
Intraperitoneal
- IR
Insulin resistant mice without obesity
- MC-T
0.5% Methylcellulose/0.2% tween 80 in water
- OB
Insulin resistant mice with obesity
- OG
Oral gavage
- TBDMS
tert-Butyldimethylsilyl
- TE
5% Tween 80/5% ethanol in PBS
- THF
Tetrahydrofuran
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.steroids.2021.108952.
References
- [1].Murakami K, Okada Y, Okuda K, Purification and characterization of 7alpha-hydroxy-4-cholesten-3-one 12alpha-monooxygenase, J. Biol. Chem. 257 (14) (1982) 8030–8035. [PubMed] [Google Scholar]
- [2].Ishida H, Noshiro M, Okuda K, Coon MJ, Purification and characterization of 7alpha-hydroxy-4-cholesten-3-one 12alpha-hydroxylase, J. Biol. Chem. 267 (30) (1992) 21319–21323. [PubMed] [Google Scholar]
- [3].Woollett LA, Buckley DD, Yao L, Jones PJH, Granholm NA, Tolley EA, Tso P, Heubi JE, Cholic acid supplementation enhances cholesterol absorption in humans, Gastroenterology 126 (3) (2004) 724–731. [DOI] [PubMed] [Google Scholar]
- [4].Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, Chan RB, Wang L, Haeusler RA, Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption, Am J Physiol Endocrinol Metab. 313 (2) (2017) E121–E133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, Groen AK, Verchere CB, Singaraja RR, Hayden MR, Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1, Diabetes 64 (2015) 1168–1179. [DOI] [PubMed] [Google Scholar]
- [6].Higuchi S, Ahmad TR, Argueta DA, Perez PA, Zhao C, Schwartz GJ, DiPatrizio NV, Haeusler RA, Bile acid composition regulates GPR119- dependent intestinal lipid sensing and food intake regulation in mice, Gut 69 (9) (2020) 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Potter GA, Barrie SE, Jarman M, Rowlands MG, Novel Steroidal Inhibitors of Human Cytochrome P45017alpha (17alpha-Hydroxylase-C17,20-lyase): Potential Agents for the Treatment of Prostatic Cancer, J. Med. Chem. 38 (1995) 2463–2471. [DOI] [PubMed] [Google Scholar]
- [8].Yoshimoto FK, Auchus RJ, The diverse chemistry of cytochrome P450 17A1 (P450C17, CYP17A1), J. Steroid Biochem. Mol. Biol. 151 (2015) 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Offei SD, Arman HD, Baig MO, Chavez LS, Paladini CA, Yoshimoto FK, Chemical synthesis of 7-oxygenated 12α-hydroxy steroid derivatives to enable the biochemical characterization of cytochrome P450 8B1, the oxysterol 12a-hydroxylase enzyme implicated in cardiovascular health and obesity, Steroids 140 (2018) 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Trammell R, See YY, Herrmann AT, Xie N, Díaz DE, Siegler MA, Baran PS, Garcia-Bosch I, Decoding the Mechanism of Intramolecular Cu-Directed Hydroxylation of sp3 C-H Bonds, J. Org. Chem. 82 (15) (2017) 7887–7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choi J-Y, McGregor RA, Kwon E-Y, Kim YJ, Han Y, Park JHY, Lee KW, Kim S-J, Kim J, Yun JW, Choi M-S, The metabolic response to a high-fat diet reveals obesity-prone and -resistant phenotypes in mice with distinct mRNA-seq transcriptome profiles, Int J Obes (Lond). 40 (9) (2016) 1452–1460. [DOI] [PubMed] [Google Scholar]
- [12].McCurdy CE, Schenk S, Hetrick B, Houck J, Drew BG, Kaye S, Lashbrook M, Bergman BC, Takahashi DL, Dean TA, Nemkov T, Gertsman I, Hansen KC, Philp A, Hevener AL, Chicco AJ, Aagaard KM, Grove KL and Friedman JE. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight. 2016;1:e86612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thackaberry EA, Kopytek S, Sherratt P, Trouba K and McIntyre B. Comprehensive investigation of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl-beta-cyclodextrin for use in general toxicology studies. Toxicol. Sci. 2010;117:485–492. [DOI] [PubMed] [Google Scholar]
- [14].Axling U, Cavalera M, Degerman E, Gåfvels M, Eggertsen G, Holm C, Increased whole body energy expenditure and protection against diet-induced obesity in Cyp8b1-deficient mice is accompanied by altered adipose tissue features, Adipocyte. 9 (1) (2020) 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bonde Y, Eggertsen G, Rudling M, Peterson J, Mice abundant in muricholic bile acids show resistance to dietary induced steatosis, weight gain, and to impaired glucose metabolism, PLoS ONE 11 (1) (2016) e0147772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Slätis K, Gåfvels M, Kannisto K, Ovchinnikova O, Paulsson-Berne G, Parini P, Jiang Z-Y, Eggertsen G, Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice, J. Lipid Res. 51 (11) (2010) 3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chevre R, Trigueros-Motos L, Castaño D, Chua T, Corlianó M, Patankar JV, Sng L, Sim L, Juin TL, Carissimo G, Ng LFP, Yi CNJ, Eliathamby CC, Groen AK, Hayden MR, Singaraja RR, Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice, FASEB J. 32 (7) (2018) 3792–3802. [DOI] [PubMed] [Google Scholar]
- [18].Liu C, Armstrong CM, Lou W, Lombard A, Evans CP, Gao AC, Inhibition of AKR1C3 Activation Overcomes Resistance to Abiraterone in Advanced Prostate Cancer, Mol. Cancer Ther. 16 (1) (2017) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maity SN, Titus MA, Gyftaki R, Wu G, Lu JF, Ramachandran S, Li-Ning-Tapia EM, Logothetis CJ, Araujo JC, Efstathiou E, Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer, Sci. Rep. 6 (2016) 35354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toren PJ, Kim S, Pham S, Mangalji A, Adomat H, Guns EST, Zoubeidi A, Moore W, Gleave ME, Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer, Mol. Cancer Ther. 14 (1) (2015) 59–69. [DOI] [PubMed] [Google Scholar]
- [21].Sellers RS, Antman M, Phillips J, Khan KN, Furst SM, Effects of miglyol 812 on rats after 4 weeks of gavage as compared with methylcellulose/tween 80, Drug Chem. Toxicol. 28 (4) (2005) 423–432. [DOI] [PubMed] [Google Scholar]
- [22].Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T and Wurbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chung E, Campise SN, Joiner HE, Tomison MD, Kaur G, Dufour JM, Cole L, Ramalingam L, Moustaid-Moussa N, Shen C-L, Effect of annatto-extracted tocotrienols and green tea polyphenols on glucose homeostasis and skeletal muscle metabolism in obese male mice, J. Nutr. Biochem. 67 (2019) 36–43. [DOI] [PubMed] [Google Scholar]
- [24].CrysAlisPro 11714063a (Rigaku Oxford Diffraction, 2019). [Google Scholar]
- [25].SCALE3 ABSPACK - An Oxford Diffraction program (1.0.4, gui: 1.0.3) Oxford Diffraction Ltd. 2005. [Google Scholar]
- [26].Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H, OLEX2: a Complete Structure Solution, Refinement and Analysis Program, J. Appl. Crystallogr. 42 (2) (2009) 339–341. [Google Scholar]
- [27].Sheldrick GM, SHELXT - integrated space-group and crystal-structure determination, Acta Cryst. 71 (1) (2015) 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sheldrick GM, A short history of SHELX, Acta Cryst. 64 (1) (2008) 112–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.