Abstract
Biopolymers are natural polymers sourced from plants and animals, which include a variety of polysaccharides and polypeptides. The inclusion of biopolymers into biomedical hydrogels is of great interest due to their inherent biochemical and biophysical properties, such as cellular adhesion, degradation, and viscoelasticity. The objective of this review is to provide a detailed overview of the design and development of biopolymer hydrogels for biomedical applications, with an emphasis on biopolymer chemical modifications and crosslinking methods. First, the fundamentals of biopolymers and chemical conjugation methods to introduce crosslinking groups are described. Crosslinking methods to form biopolymer networks are then discussed in detail, including i) covalent crosslinking (e.g., free radical chain polymerization, click crosslinking, crosslinking due to oxidation of phenolic groups), ii) dynamic covalent crosslinking (e.g., Schiff base formation, disulfide formation, reversible Diels-Alder reactions), and iii) physical crosslinking (e.g., guest-host interactions, hydrogen bonding, metal-ligand coordination, grafted biopolymers). Finally, recent advances in the use of chemically-modified biopolymer hydrogels for the biofabrication of tissue scaffolds, therapeutic delivery, tissue adhesives and sealants, as well as the formation of interpenetrating network biopolymer hydrogels, are highlighted.
Graphical Abstract
1. Introduction
Hydrogels are water-swollen polymer networks that have great utility for biomedical applications.1 Hydrogels can mimic features of many tissues, and there have been great advances in the tailoring of hydrogel properties (e.g., mechanics, degradation) for widespread biomedical applications. Many important advances have been made with the use of synthetic polymers to construct hydrogels due to precise control over chemical structures, low batch variability, and ease of sourcing.2 However, recent trends have included the fabrication of hydrogels from biological molecules, such as biopolymers, to introduce specific inherent biofunctionality to hydrogels.3
Biopolymers are natural polymers that are sourced from animals and plants, including a wide range of polysaccharides (e.g., sugars) and polypeptides (e.g., proteins). Representative examples of polysaccharides include hyaluronic acid, chondroitin sulfate, heparin, dextran, alginate, cellulose, chitin, and chitosan. Representative examples of polypeptides include gelatin, silk fibroin, albumin, elastin, keratin, and unique polypeptides engineered for specific functionality. The selection of specific polysaccharides or polypeptides introduces inherent properties to hydrogels, such as cell adhesion and degradability.
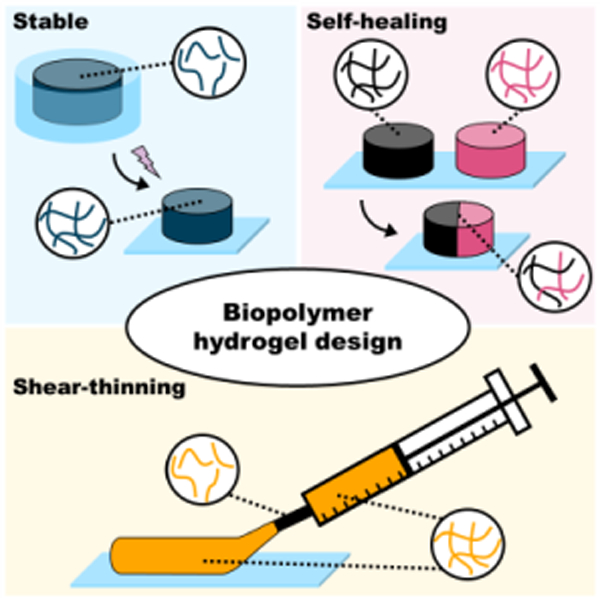
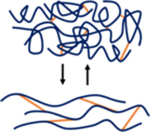
It is often necessary to chemically modify biopolymers to facilitate hydrogel formation. These modifications are performed on various chemical groups within the biopolymer repeat units (e.g., amines, hydroxyl groups, carboxylic acids) to allow for diverse methods of crosslinking (e.g., mixing, light, redox, thermal). The mechanical properties of formed hydrogels are generally driven by the extent of biopolymer modification, the degree of crosslinking, the biopolymer concentration, and the type of crosslinking chemistry used. If a very stable hydrogel is desired, chemical groups that permit covalent crosslinking (e.g., free radical chain polymerization, click reactions) are often used (Figure 1). However, dynamic covalent crosslinking (e.g., Schiff base, disulfides) can also be implemented to combine hydrogel stability with features such as self-healing behavior (Figure 1). If a less-stable hydrogel is desired, physical crosslinking (e.g., hydrogen bonding, metal-ligand coordination) is typically used, which exhibits properties such as shear-thinning and disassembly over time (Figure 1). Various biopolymer networks can also be combined (e.g., interpenetrating networks) to further vary hydrogel properties to match the needs of specific applications.
Figure 1. General hydrogel properties as a function of crosslink type.
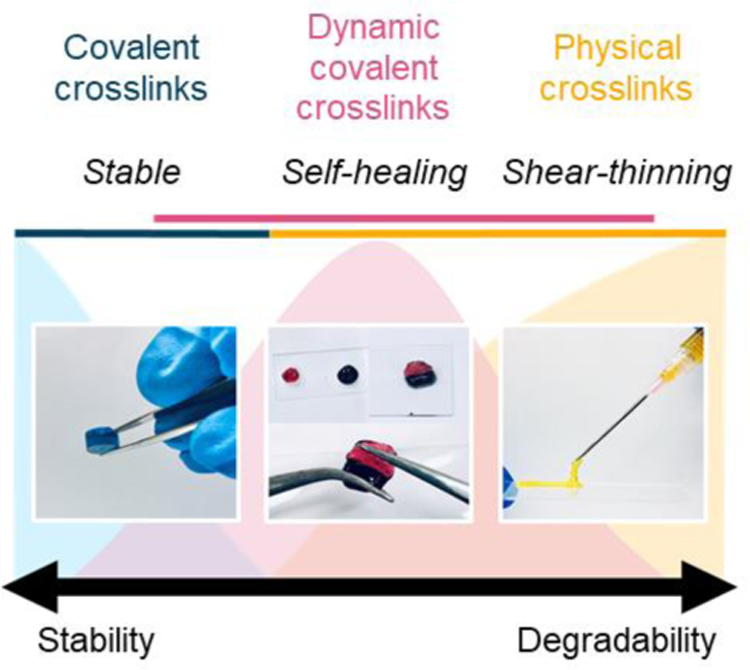
Schematic illustrating representative images of hydrogels formed from different crosslinking mechanisms (i.e., covalent [blue], dynamic covalent [pink], physical [yellow]).
The overall objective of this review is to provide the reader with an introduction to the use of biopolymers for the formation of biomedical hydrogels, with an emphasis on chemical modifications that facilitate hydrogel formation and control over hydrogel properties. There is great diversity in the modifications and resulting hydrogels properties, which is a strength to the use of biopolymers in hydrogel formation. Furthermore, specific examples of where biopolymer-based hydrogels are being used in biomedical applications of tissue engineering, biofabrication, and drug delivery are introduced, particularly where the use of a biopolymer and chemical modification was important to the hydrogel function.
2. Overview of biopolymers
Biopolymers are natural polymers that are derived from animals and plants. Biopolymers used for hydrogel formation generally fall into two classes of molecules: polysaccharides and polypeptides. Their repeat units consist of sugars or peptides, which guide the various biopolymer properties. Biopolymers inherently incorporate features that may be attractive in their use as biomaterials, including chemical compositions for cell interactions and degradation. Biopolymer hydrogels can be formed by polymer entanglement due to high molecular weight or high polymer concentration, by assembly (e.g., charge) due to the specific functionality of certain biopolymers, or by inter-polymer crosslinking due to chemical modifications of the biopolymer. In this section, we discuss the various biopolymers that are often chemically modified for hydrogel formation in the biomaterials field, including their general properties (e.g., molecular weight, adhesion to cells, degradability) and past use in commercial products.
2.1. Polysaccharides
Polysaccharides consist of monosaccharide or disaccharide repeat units and have important structural and biological functionality in living organisms (Table 1). For biomaterial applications, polysaccharides are often isolated from renewable sources such as plants and microorganisms. They may also be sourced from animal byproducts in the meat and fish industries. Many polysaccharides have been chemically modified to obtain natural hydrogels with a wide range of mechanical and biological properties.
Table 1. Polysaccharide-based biopolymers.
Representative chemical structures, function, and sources of various polysaccharide biopolymers that have been modified to form biomedical hydrogels.
| Polysaccharides | ||||
|---|---|---|---|---|
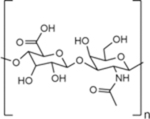
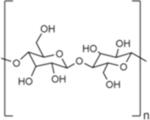
| Name | Hyaluronic Acid (HA) | Chondroitin Sulfate (CS) | Heparin Sulfate (HS) | Dextran |
| Chemical structure |
|
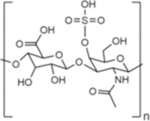
|
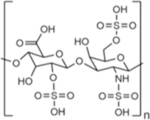
|
|
| Type | Linear GAG | Linear GAG | Linear GAG | Branched |
| Native function | Major component of ECM in human connective tissue | Major component of ECM in human connective tissue | Stored in mast cells in humans and secreted into vasculature upon injury | Component of some bacterial ECM |
| Source for biomedical applications | Bacterial culture; rooster comb19 | Animal sources (e.g., bovine trachea, porcine nasal septa)29 | Animal sources (e.g., porcine intestines)34 | Bacterial culture39 |
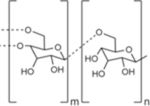
| Name | Alginate | Cellulose | Chitin | Chitosan |
| Chemical structure |
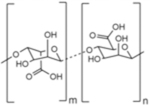
|
|
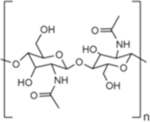
|
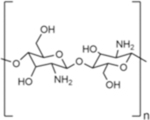
|
| Type | Linear | Linear | Linear | Linear |
| Native function | Structural support in seaweed | Structural support in plant cell walls; component in some bacterial ECM | Structural support in insect and crustacean exoskeletons | n/a |
| Source for biomedical applications | Brown algae42 | Bacterial culture49 | Shrimp and crab shells from food industry waste products56 | Deacetylated derivative of chitin57 |
2.1.1. Hyaluronic acid
Hyaluronic acid (HA) is a linear glycosaminoglycan (GAG) consisting of alternating D-glucuronic acid and N-acetyl-D-glucosamine repeat units that are linked together by alternating β-1,4 and β-1,3 glycosidic bonds.4,5 HA is a native component of the extracellular matrix (ECM) and is found throughout multiple tissues in the body, including cartilage, muscle, skin, and vocal folds.6,7 In its naturally occurring state, the size of HA can range from ~100 kDa in serum to ~8000 kDa in vitreous fluid.8 Through its structure and chemical properties, HA influences the mechanical and biological functionality of native tissues, as well as cellular responses in wound healing.9 HA is very hydrophilic, and one of its major roles is the maintenance of viscoelasticity and low-friction tissue interfaces, such as in synovial and vitreous fluids.5,10 Cells bind to HA through surface receptors such as the glycoprotein CD44.11 HA-CD44 binding interactions are very important in many cellular processes, such as chondrocyte proliferation and matrix production in cartilage tissue.12 HA can be degraded by oxidative species or enzymes such as hyaluronidase, glucuronidase, and hexosaminidase.5
Since the 1960s, HA has been utilized for many clinical applications including dermal fillers for soft-tissue augmentation,13–15 wound dressings,16 and intra-articular injections to manage symptoms of osteoarthritis.17 For research and clinical use, HA is either derived from streptococcal fermentation cultures or from animal sources, such as rooster combs.18,19 Unmodified HA can be crosslinked with 1,4-butanediol diglycidal ether (BDDE) or divinyl sulfone (DVS), either loosely to increase HA solution viscosity or more extensively to increase the mechanical integrity of HA hydrogels.13 To increase the diversity in properties of HA hydrogels for biomedical applications, a range of chemical modifications have been introduced to HA.4,20
2.1.2. Chondroitin sulfate
Chondroitin sulfate (CS) is a linear sulfated GAG consisting of ~40–100 repeat units of alternating β-1,3-linked-N-acetyl-galactosamine and β-1,4-linked-glucuronic acid sugar residues.21 CS is the main GAG found in aggrecan, a proteoglycan (PG) consisting of a protein core with GAG side chains.22 Aggrecan, and thus CS, plays an important role in cartilage mechanics by influencing tissue hydration, swelling, and lubrication.23 CS and PGs in general also play an important role in injury and disease recovery in the central nervous system.24 PGs formed with CS interact with ECM and cell adhesion molecules.25 CS can be degraded by enzymes such as chondroitinase ABC.26 Clinically, CS has been delivered orally for management of pain in knee and hip osteoarthritis, as CS stimulates PG synthesis in the joint space, as well as exhibits anti-inflammatory properties.27,28 For use in biomedical applications, CS is isolated from animal sources including bovine trachea, chicken keel, shark fins, and pig nasal septa.29
2.1.3. Heparin
Heparin is a linear GAG consisting of repeat units of uronic acid and D-glucosamine that are linked together by β-1,4 glycosidic bonds.30 Heparin is found on the cell surface and in the ECM, and is known to play essential roles in tissue development, angiogenesis, and anticoagulation.31 Heparin and heparan sulfate (HS), a sulfated derivative of heparin polysaccharides, interact with proteins to form PG coatings around cells,31 which enable cells to interact with many signaling molecules.31 Heparin binds to many biomolecules such as growth factors, cytokines, and adhesion proteins, including fibronectin.32 The molecular weight of naturally occurring heparin can range from 5 to 1000 kDa.30 Endothelial cells and macrophages preferentially bind to higher molecular weight heparin.33 Enzymes such as heparinase can degrade heparin and HS, which is important in ECM maintenance and remodeling.32
Clinically, heparin is used as a blood thinner to prevent the formation of blood clots. Heparin is one of only a few clinically approved polysaccharide drugs, and it is one of the oldest drugs still in clinical use.34 The World Health Organization (WHO) identifies heparin as one of the world’s Essential Medicines.35 For biomedical use, heparin is isolated from animal sources, most often porcine intestine.34 Heparin is often classified as either unfractionated heparin (UFH) or purified low molecular weight heparin (LMWH). As an anticoagulant, clinical use has shifted from UFH to LMWH for increased efficacy, as higher molecular weight heparin can adhere to endothelial cells and macrophages, and impede their anticoagulant ability.33 As a tissue engineering scaffold, higher molecular weight heparin may be of interest due to its increased affinity for endothelial cell adhesion.
2.1.4. Dextran
Dextran is a highly branched polysaccharide consisting of α-1,6-linked-glucose monomers and α-1,3 branching.36 Dextran is a major component of bacterial ECM, allowing for surface adhesion and biofilm formation.37 Dextran has been extensively researched in the dental field, as streptococci bacteria secrete dextran to form gelatinous plaques on teeth.38 Dextran can vary from molecular weights of ~10–150 kDa and ~5–30% degree of branching, depending on the bacteria and purification process used.36,39 Most dextran in commercial use is produced from Leuconostoc mesenteroides bacteria with ~5% degree of branching.39 Dextran can be degraded enzymatically by dextranase.40
Due to the ease of manufacturing and its biocompatibility, dextran has been widely used in many industries. Clinically, dextran is used as an antithrombotic agent to decrease vascular thrombosis by binding to erythrocytes, platelets, and vascular endothelium to reduce aggregation and make clots more easy to lyse.36 Dextran is also used as a lubricant in eye drops and as an additive in intravenous fluids to solubilize other factors.36 The clinical grades of dextran most often used include Dex-40 (40 kDa MW) and Dex-70 (70 kDa MW). The WHO includes Dex-70 on its List of Essential Medicines.35 In addition to its uses in medicine, dextran is extensively used in food and cosmetic products, as well as in waste water treatment processes.36 Due to the widespread availability and history of success in clinical use, dextran is a promising material for tissue engineering.
2.1.5. Alginate
Alginate is a linear polysaccharide consisting of repeat units of 1,4-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues.41 Alginate is found in the cell walls of brown algae (Phaeophysceae), providing a flexible mechanical structure to protect seaweed from damage due to strong water motion.42 Alginate rapidly crosslinks in the presence of divalent cations (e.g., Ca2+) due to ionic interactions with G residues. This ionic crosslinking mechanism has been used as a method to encapsulate biomolecules and cells for decades. For biomedical applications, alginate is frequently explored due to its biocompatible ionic gelation mechanisms, as well as its low cost and low toxicity.43
Purified alginate can be derived from brown algae cell walls as well as some bacterial strains, though commercially available alginate is derived exclusively from algal sources.44 Alginate is available across a range of molecular weights from ~30–400 kDa.43 Alginate has been used in many products for clinical applications, including reduction of gastrointestinal reflux, accelerated wound healing, and defect filling in musculoskeletal tissues.44 In addition to biomedical applications, alginate has been extensively used in the food industry as a thickening and gelation additive, colloid stabilizer, and sausage casing material.45
2.1.6. Cellulose
Cellulose is a linear polysaccharide consisting of repeating D-glucose units linked together by β-1,4 glycosidic bonds.46 Cellulose is considered to be the most abundant organic polymer on Earth.47 In plants, cellulose is a major component of the cell wall, where it forms strong microfibril crystal structures, leading to impressive mechanical properties.48 Cellulose derived from plant sources is used extensively for the production of paper, lumber, and cotton textiles.48 For biomedical applications, cellulose is typically derived from bacterial sources.49 Some bacteria produce cellulose to form flocs and create a mechanically robust microenvironment.48 Microbial-derived cellulose has been used in therapies for burns and ulcers, as well as in dental implants.49 Cellulose is also commonly utilized as an emulsion stabilizer in cosmetic and food products.49 Water-soluble derivatives of cellulose are manufactured by etherification reactions for use in food and medical industries.50 The most common cellulose derivatives used in hydrogel formation are carboxymethyl cellulose (CMC) and hydroxypropyl cellulose (HPC).50,51
2.1.7. Chitin and Chitosan
Chitin is a linear polysaccharide with similar structure to cellulose, except the hydroxyl group is replaced with an acetamide group, resulting in N-acetyl-D-glucosamine repeat units.52 Like cellulose, chitin monomers are linked by β-1,4 glycosidic bonds. The acetamide group allows for increased hydrogen bonding, resulting in increased strength in the chitin fibrillar matrix when compared to cellulose alone. Chitin is the primary component of exoskeletons of crustaceans and insects, and it is also found in fish scales, fungi cell walls, and cephalopod beaks.53 Behind cellulose, chitin is the second most abundant natural biopolymer on Earth.54 For biomedical applications, chitin is mostly sourced from shrimp and crab shells, which are waste products of the food industry.55,56 In its native form, chitin is hydrophobic, which can be utilized to form hard materials for tissue engineering applications.54 The electrical properties of chitin have also been explored for biomedical materials that benefit from electrical conductance.54 For hydrogel formation, water-soluble derivatives of chitin are often used.
Chitosan is produced by either chemical or enzymatic deacetylation of chitin isolated from crustaceans.57 It consists of glucosamine and N-acetyl-D-glucosamine repeat units.58 The degradation rate and hydrophilicity of chitosan is influenced by the degree of deacetylation, which may range from 30–95%.58 Lysozyme is the main enzyme that degrades chitosan in humans.58 Clinically, chitosan has been used in chitosan-based hemostatic dressings,59 as well as explored for use as a vaccine adjuvant.60
2.2. Polypeptides
Polypeptides are biopolymers consisting of amino acid repeat units, which are considered proteins when they consist of more than 50 amino acids (Table 2). Polypeptide- and protein-based hydrogels are of great interest in biomedical applications due to their potential to incorporate numerous cell interaction sites and to mimic native functions of the ECM. Polypeptides may be isolated from human, animal, or plant sources, or synthetically engineered using recombinant protein production or peptide synthesizers. The precision and diversity in polypeptide materials are attractive for many biomedical applications.
Table 2. Polypeptide-based biopolymers.
Representative structures, function, and sources of various polypeptide biopolymers that have been modified to form biomedical hydrogels.
| Polypeptides | |||||
|---|---|---|---|---|---|
| Name | Gelatin | Silk Fibroin | Albumin | Elastin | Keratin |
| Structure |
|

|
|
|
|
| Contains RGD motif for cell adhesion | Contains sequences that form crystalline β-sheets | Heart shaped, globular protein | Contains hydrophobic domains (i.e., VPGXG) for mechanical resilience | Contains high cysteine content for disulfide bond formation | |
| Native function | Denatured derivative of collagen (structural component of mammalian ECM) | Structural component of silk fibers produced by some arthropods | Modulates fluid distribution and provides oncotic pressure in blood plasma | Responsible for recoiling response when stress is applied in vertebrate connective tissue | Structural and protective component of hard tissues such as skin, hair, nails, horns, wool, feathers, and hooves |
| Source for biomedical applications | Animal sources (e.g., porcine skin)63 | Bombyx mori (silkworm)73 | Human (HSA) or bovine (BSA) blood plasma82 | Human donors or animal sources (e.g., bovine, murine)91 | Human hair,98 animal sources (e.g., feathers, wool)99,100 |
2.2.1. Gelatin
Gelatin is a hydrolyzed and denatured form of collagen, which is the main structural protein in mammalian connective tissue ECM.61 Collagen is the most abundant protein in mammals, making up 25–35% of the total protein content.61 To produce gelatin, collagen proteins are extracted from the skin and bones of animal sources (most often porcine skin) by acid or alkaline treatments, followed by a thermal-driven process of protein separation.62,63 Due to the heterogeneity in animal sources and gelatin isolation methods, the molecular weight of commercially available clinical-grade gelatin can range from ~103-106 Da.64 Much of the triple-helix structure of native collagen is denatured in gelatin production; however, the chemical structure of gelatin remains similar to collagen.63 Gelatin contains Gly-X-Y amino acid repeat sequences, where X is usually proline and Y is usually hydroxyproline.65 Gelatin also contains the RGD sequence (Arg-Gly-Asp), which is a cell adhesion site and binds to integrins.66 Adding gelatin (and thus RGD) to biomaterials has been shown to improve cell integration and tissue repair in many applications.66 In the body, gelatin can be degraded by proteases such as collagenase and metallo-proteases.67
Gelatin has been widely used in many industries, including those related to food, photography, and pharmaceuticals. For example, in food science, gelatin is used as a stabilizer, thickener, texturizer, and emulsifier,68 whereas in photography gelatin is used as a medium for making emulsions.68 In clinical use, gelatin is a major ingredient in hard and soft capsules, as well as tablet preparation,63,68 and gelatin sponges and particles have been widely used as hemostatic agents and to fill cartilage and bone defects.69 Towards tissue engineering, a major advantage to using gelatin is that it has biological functionality (RGD sequence) and thus mimics native ECM functions.70 Crosslinkers such as glutaraldehyde (GTA) and genipin can be used to directly form hydrogels with gelatin.71 To improve mechanical performance and increase the range of possible mechanical properties, gelatin can also be chemically modified with functional groups to undergo hydrogel formation.
2.2.2. Silk fibroin
Silk is produced through a series of proteins found in the glands of some arthropods including silkworms, spiders, scorpions, and bees.72 Silk is composed of two major proteins: silk fibroin, a semi-crystalline protein which provides structural stiffness and strength, and sericin, a glue-like protein that wraps around silk fibroin to hold fibers together.72 In biomedical applications, silk fibroin is of interest due to its excellent mechanical strength, biodegradability, and widespread availability. Silk fibroin consists of semi-crystalline polypeptides that have a heavy chain (MW ~390 kDa) and a light chain (MW ~26 kDa) linked together via a single disulfide bond.72 For clinical applications, silk fibroin is extracted from the Bombyx mori silkworm73 and consists mainly of Gly (43%), Ala (30%), and Ser (12%) amino acids.72 Silk fibroin contains hydrophobic domains that allow for the formation of stable anti-parallel β-sheet crystallites.72
Silk fibroin has been used in the textile industry for thousands of years.74 Towards clinical applications, silk has been used as a suture material for centuries.75,76 FDA-approved silk fibroin-based products include surgical meshes and ligament grafts.76 It has been shown that silk fibroin can be engineered for attachment and growth of human and animal cells.77 In vivo, silk fibroin scaffolds can be degraded by enzymes and hydrolysis,77 and the β-sheet crystalline content can be reduced in silk fibroin scaffolds to increase degradation rates.78 Chemically-modified silk fibroin scaffolds are being widely explored for applications in tissue repair and regeneration.
2.2.3. Albumin
Albumin is an endogenous protein produced mainly by the liver and secreted into the blood plasma.79 Human serum albumin (HSA) is the most abundant protein in blood plasma, accounting for 50–60% of total protein content.80 The primary role of HSA is the regulation of fluid distribution by providing ~80% of the total blood plasma oncotic pressure.81 HSA is a globular protein consisting of 585 amino acids with a molecular weight of 66 kDa.81 As determined by X-ray crystallography, the tertiary structure of HSA is a heart-shaped protein that is stabilized by 17 disulfide bridges formed between amino acids.82 Clinically, HSA has been used as a plasma expander for decades to restore and maintain circulating blood volume in response to trauma, surgery, and blood loss.81,83 HSA can be isolated from human blood plasma by many methods, including plasma fractionation followed by liquid chromatography.82 For biomedical research, albumin may be sourced from human blood plasma; however, bovine serum albumin (BSA) is also being widely explored as a cheaper and more abundant alternative.82
2.2.4. Elastin
Elastic fibers are an important ECM structural component and are responsible for the resilience and elasticity in many vertebrate connective tissues, including skin and cartilage.84,85 Elastic fibers are composed of ~90% elastin protein and a complex microfibrillar structure made of numerous other macromolecules.84 Elastin is composed of tropoelastin precursors that accumulate on the microfibrillar skeleton.86 The half-life of human elastin is around 70 years, making it an extremely durable biopolymer with low turn-over in healthy tissue.87 Elastic fibers can be degraded with disease or age due to proteolytic elastase enzymes.88 Many cell types interact with elastin, including through elastin receptors and integrins.84 Elastin is insoluble in water due to the presence of multiple hydrophobic domains; however, for hydrogel formation, water-soluble elastin-based materials have been explored.84
Various elastin formulations have been developed that include α-Elastin, a water-soluble elastin derivative that has been solubilized with oxalic acid,89 and tropoelastin, which is water soluble at low temperatures.90 Elastin-containing materials may be isolated from animal sources or human cadavers and processed into water-soluble derivatives for hydrogel formation.91 Using synthetic protein engineering, Elastin-like polypeptides (ELPs) have also been engineered for biomedical applications.92 ELPs contain the hydrophobic motif Val-Pro-Gly-X-Gly (VPGXG), where X is any amino acid except for Pro.93 VPGXG is one of the main hydrophobic motifs present in natural elastin that contributes to its unique mechanical properties. Elastin-based biopolymers can be crosslinked without chemical modification using crosslinkers such as GTA, disuccinimidyl suberate, and disuccinimidyl glutarate.89 Towards forming hydrogel scaffolds for tissue engineering applications, elastin-based materials are of particular interest due to their diverse biological and mechanical properties, which arise from the unique resilient behavior of elastin polypeptides.84
2.2.5. Keratin
Keratin is a fibrous protein rich in cysteine residues and is naturally found in hard integuments of animals, including in skin, hair, nails, wool, feathers, scales, and horns.94,95 Keratinous tissues serve structural and protective functions in a variety of animals.95 In humans, keratin is found in many epithelial tissues, including the epidermis and corneal epithelium, contributing to their role as a protective barrier.96 Keratin contains multiple cell adhesion sites, including RGD.97 The rich cysteine content allows for the formation of disulfide bonds, giving keratinous tissue strong and resilient mechanical properties.94 Keratins are often classified as either α-keratins (forming α-helices) or β-keratins (forming β-sheets).94 In the textile industry, keratin has been used as a raw material for centuries.94 For biomedical purposes, keratin may be extracted from numerous sources, including human hair,98 wool,99 and feathers.100 Keratin is of growing interest for use as a sustainable and cheap raw material in the biomedical field, as it can be easily sourced from the millions of tons of wool and feathers that are produced annually as by-products in livestock industries.94 In its native state, keratin is insoluble in most solvents, including water.101 Post-processing must be used to form water-soluble keratin for hydrogel formation, often involving the breaking of disulfide bonds with the addition of a reducing agent.98 Such a process results in free thiol groups on keratins that can be used for crosslinking or further functionalization.98,99
2.2.6. Engineered polypeptides
Advances in recombinant protein production and peptide synthesis have allowed for the design of engineered polypeptides that can be fabricated into hydrogels.102 Engineered polypeptides can be designed to mimic biological functions of naturally occurring peptides. For example, resilin-like polypeptides (RLPs) have been recombinantly engineered to fabricate hydrogel scaffolds that mimic the highly resilient mechanical properties of resilin protein found in arthropods.103 Using RLPs instead of native resilin allows for control over incorporation of other bioactive motifs into the polypeptide, such as MMP-sensitive and cell-binding sites.103 As another example, the engineered peptide poly(γ-propargyl-L-glutamate) (PPLG) has been used in combination with poly(ethylene glycol) (PEG) to form hydrogels.104 PPLG introduces cell-adhesion sites as well as nanoscale stiffness due to PPLG’s rod-like tertiary folding structure.104 Furthermore, self-assembling peptide hydrogels have been designed that result in nanofibrillar structures due to β-sheet formation.105 Other examples of hydrogels formed from engineered polypeptides include the use of novel pH-responsive engineered peptide amphiphiles for the formation of injectable nanofibrous scaffolds,106 and the use of engineered PEG-peptide copolymers for the formation of “Shear-thinning Hydrogels for Injectable Encapsulation and Long-term Delivery” (SHIELD).107 While engineered polypeptides may be designed for self-assembly, chemical modification of the engineered polypeptides may also be utilized for hydrogel formation, such as for crosslinking by azide-alkyne cycloaddition.108,109 Ultimately, the engineering of polypeptides expands potential hydrogel components well beyond those that are found in natural tissues and structures.
3. Conjugation reactions to modify biopolymers
As stated above, although many biopolymers have inherent inter-molecular interactions that can be used to form hydrogels, chemical modification is often needed for hydrogel formation or to improve upon formed hydrogel properties. Fortunately, biopolymers possess various chemical groups (e.g., hydroxyl, carboxyl, amine, thiol) available for modification through standard conjugation procedures. For example, all polysaccharides have hydroxyl groups (-OH), as do the amino acids serine and tyrosine, which often contribute to the hydrophilicity and hydrogen-bonding capabilities of biopolymers. The carboxyl group (-COOH) is found on two amino acids, aspartic acid and glutamic acid,110 and in numerous polysaccharides such as HA, alginate, and CS. Amines (-NH2 or -NR2) are common on biopolymers such as chitosan and within the amino acid lysine as a component of proteins and polypeptides.110 Lastly, thiols (-SH) are found in the amino acid cysteine, and the oxidation of thiols can lead to formation of a disulfide bond, which is commonly used in biopolymer hydrogel formation and fabrication.110
The most common conjugation reactions to chemically modify biopolymers include the formation of esters, amides, ethers, and carbamates, which involve hydroxyl, carboxyl, amine, and thiol groups on biopolymers (Figure 2). Ester formation is accomplished via the condensation of hydroxyl and carboxyl groups, usually in the presence of dehydrating reagents and appropriate catalysts. A common bioconjugation method is to use a carbodiimide such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) to activate a carboxylic acid group for esterification, in combination with a catalyst, such as 4-dimethylaminopyridine (DMAP). Esterification can also be achieved by combining a carboxylic acid with an epoxy, resulting in a hydroxyester. In bioconjugation, it is also common to combine acid anhydrides with functional hydroxyl groups on biopolymers to form esters. For example, methacrylic anhydride can be used to chemically modify biopolymers containing an aliphatic hydroxyl group with a methacrylate.111 Di-tert-butyl decarbonate (i.e., Boc anhydride, Boc2O) combined with DMAP can also be used to accomplish esterification.112
Figure 2. Common chemical reactions for modification of biopolymers.
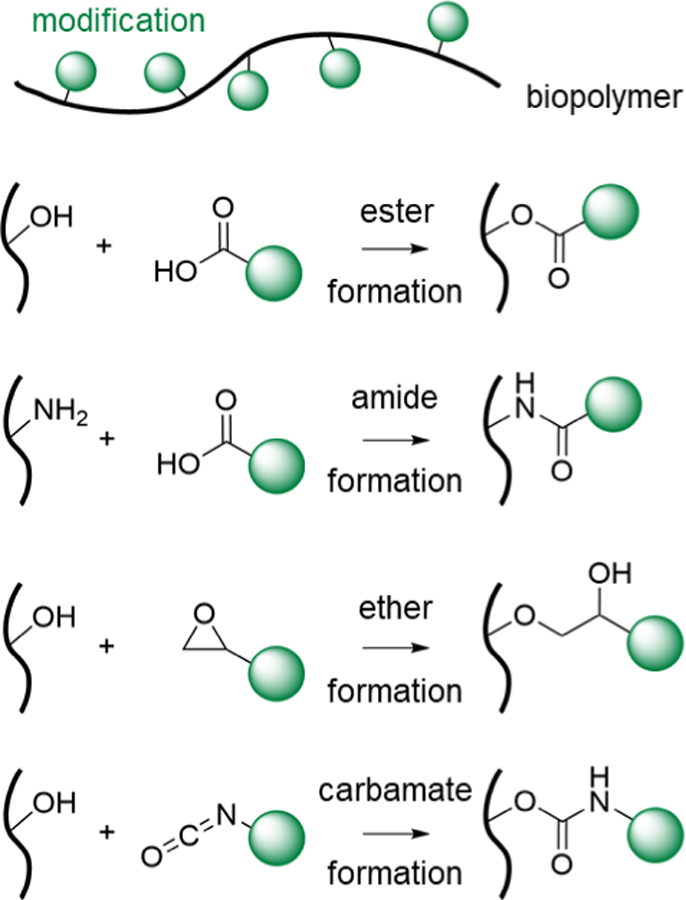
Schematic representation of chemically modifying biopolymers using common mechanisms. From top to bottom: esterification, amidation, etherification, and carbamate formation. The green circle denotes various chemical groups introduced onto biopolymers for potential use in hydrogel formation.
Amide formation can be achieved by condensing a carboxylic acid with an amine group. To accomplish this, the carboxylic acid group is usually first converted into an activated ester compound. Carbodiimides such as EDC activate the carboxylate group, and molecules like N-hydroxysuccinimide (NHS),113,114 hydroxybenzotriazole (HOBt),115,116 or benzotriazol-1-yloxytris (dimethylamino)phosphonium hexafluorophosphate (BOP)117 form activated ester compounds. The activated esters then readily form stable amide bonds with amine functional groups present in the reaction. Ethers can be formed by combining epoxide and hydroxyl functional groups under basic conditions. For example, this method is used to conjugate glycidyl methacrylate (which has an epoxide functional group) to biopolymers containing free hydroxyl groups,118–120 often in the presence of DMAP as a base. Lastly, compounds containing isocyanate functional groups can form carbamate bonds (also referred to as urethane bonds) with hydroxyl groups or can combine with thiols to form thiocarbamate bonds.121,122
There are numerous other examples of modifications directly to biopolymers for hydrogel formation. For example, Michael addition reactions can be used to chemically modify thiols on biopolymers, as well as to crosslink modified biopolymers for hydrogel formation (as described later in Section 4).123 Specifically, under basic conditions, thiolated molecules (Michael donors) can be combined with electron-deficient unsaturated compounds (Michael acceptors, such as maleimides, vinyl sulfones, acrylates, and acrylamides) via thiol-Michael addition, leading to the formation of a thioether bond.124 Polysaccharides can also be modified using ring-opening oxidation, resulting in free aldehyde groups on the biopolymer backbone, which can change degradation rates or be used for crosslinking (e.g., Schiff base formation).125,126 A common method to introduce aldehydes is to use sodium periodate as the oxidizing agent.127 Lastly, biopolymers such as keratin that have multiple disulfide bridges can be exposed to a reducing agent such as dithiothreitol (DTT) or mercaptoethanol to functionalize with free thiol groups,128 allowing for further biopolymer modification or crosslinking.
4. Covalent crosslinking
Hydrogels can be formed by the covalent crosslinking of functional groups attached to biopolymers. Covalent crosslinking mechanisms often require catalysts or initiators to induce covalent bond formation. Due to the general stability of covalent bonds, covalently crosslinked hydrogels have the potential to remain stable over long timescales both in vitro and in vivo, although this may be dependent on the ability of the network to undergo degradation. Both the mechanical and biological properties of the hydrogel formed are influenced by various components of the biopolymer and hydrogel design, such as the concentration of biopolymer, the type of crosslinking group introduced, and the degree of modification of the biopolymer. While there are many methods to form covalently crosslinked hydrogels, this review will focus on the most common approaches utilized in biopolymer hydrogel formation, including free radical chain polymerization, click chemistry, and oxidation of phenolic groups.
4.1. Crosslinking via free radical chain polymerization
Free radical chain polymerization consists of three steps: 1) initiation, 2) propagation, and 3) termination. During the initiation step, free radicals are generated from initiators, typically with changes in temperature, light, or redox conditions. During propagation, free radicals interact with unsaturated double bonds, and the free radical active center is transferred to propagate the kinetic chain, leading to crosslinking of the modified biopolymers (Figure 3a). The crosslinking reaction is terminated by either combination, disproportionation, or chain transfer events that stop the radical from propagating further. There are a wide range of functional groups that are used for the crosslinking of biopolymers in free radical chain polymerization (Figure 3a).
Figure 3. Crosslinking via free radical chain polymerization.
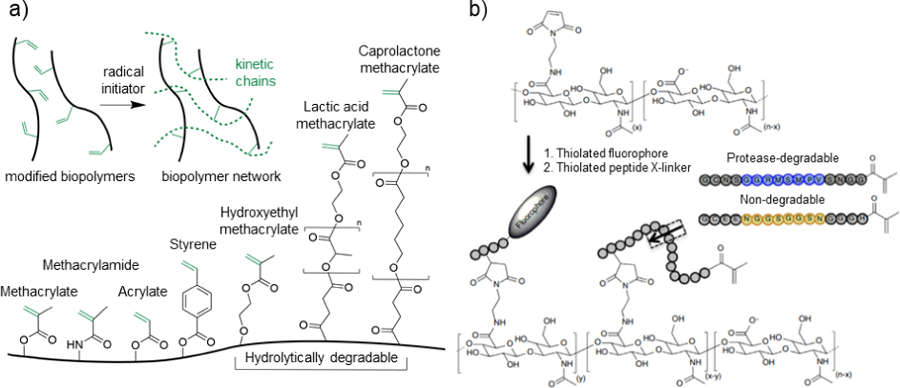
a) Schematic representation of the general crosslinking of modified biopolymers in the presence of an initiator to induce the formation of kinetic chains through the propagation of radical species (top), as well as common reactive groups used for biopolymer modification and hydrogel formation (bottom). b) Hyaluronic acid (HA) modified with maleimide groups to react with thiolated fluorophores and thiolated protease-degradable peptides capped with methacrylate groups for free radical chain polymerization. Peptide sequences are designed to be either protease degradable (blue) or non-degradable (yellow). Adapted with permission from Wade, et al.167 Copyright, 2015 Springer Nature.
Photoinitiation is a common method to generate free radicals in hydrogel formation.129 In this approach, a photoinitiator molecule cleaves in response to certain wavelengths of light, resulting in the generation of free radicals. Examples of water-soluble, biocompatible photoinitiators used in crosslinking include ultraviolet (UV) light-responsive molecules such as 2-hydroxy-4-(2-hydroxyethoxy)-2-methylpropiophenone (e.g., Irgacure, I2959)120,130 and visible light-responsive molecules such as lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP).131 Parameters such as light intensity and exposure time, as well as initiator and biopolymer concentrations, will affect the rates of polymerization and the resulting hydrogel properties. Oxidative-reductive (redox) mechanisms may also be used to generate free radicals. One of the most used redox initiators in biomedical applications is ammonium persulfate (APS) in combination with tetramethylethylenediamine (TEMED). Since radicals are generated upon mixing, injectable, in situ-forming hydrogels with tunable mechanical properties are possible with redox systems.132 Lastly, there are a number of thermal initiators in which a change in temperature is used to generate free radicals.133 Free radical chain polymerizations form stable hydrogels, as the kinetic chains formed are non-degradable, although degradable groups (e.g., hydrolytically degradable, enzymatically degradable) can be incorporated into the network to tailor erosion behaviors.
4.1.1. Meth(acrylates) and methacrylamides
To functionalize biopolymers for free radical chain polymerization, methacrylate groups are often conjugated to biopolymers. This can be accomplished through various reactions, including esterification with methacrylic anhydride134 and etherification with glycidyl methacrylate (GMA).135 Dextran was first modified with methacrylate groups by etherification between GMA and hydroxyl groups on the dextran backbone, forming GMA-Dex.118 GMA-Dex hydrogels were crosslinked in situ in the presence of APS/TEMED redox radical initiators.118 Kim et al. synthesized methacrylated dextran by esterification with methacrylic anhydride under basic conditions.136 It was demonstrated that model drugs such as fluorescently-labeled dextran and doxorubicin could be released in a sustained fashion from methacrylated dextran hydrogels crosslinked with UV light in vitro.137 To introduce micro- and macro-porous structures, PEG has been mixed into methacrylated dextran hydrogels, and liquid-liquid phase separation created different morphologies and porosities.119 In a similar approach, Ferreira et al. modified dextran with acrylate groups to form hydrogels containing tethered RGD and vascular endothelial growth factor (VEGF) encapsulated in poly(lactic-co-glycolic acid) (PLGA) microspheres for use as a scaffold for human embryonic stem cell vascular differentiation.138
HA modified with methacrylate moieties has been widely used in tissue engineering and drug delivery. Smeds et al. demonstrated that HA could be methacrylated (MeHA) using esterification with methacrylic anhydride and subsequently photocrosslinked to form HA hydrogels for sustained release of model drugs.134 Stiffness, swelling ratio, and degradation rates can be varied in HA hydrogels formed from MeHA as a function of the degree of substitution, polymer concentration, and photoinitiation conditions.111 In another chemical modification approach, Leach et al. showed that HA could be modified through etherification between hydroxyl groups on the polymer backbone and GMA (GMA-HA), and subsequently photocrosslinked using UV light.120 Furthermore, BSA was released as a model drug in a sustained fashion from GMA-HA for several weeks.139 Hydrogels from methacrylated HA have been used for a wide range of applications, including vocal fold tissue engineering,140 controlled human embryonic stem cell differentiation,141 and bioprinting.142
Numerous other polysaccharides have been modified with methacrylates for free radical chain polymerization, including cellulose,143 alginate,144 and CS.145 By altering reaction conditions, such as reagent concentrations and temperature, the degree of substitution of methacrylate groups on cellulose could be controlled, which later influenced hydrogel mechanical properties.143 HPC has been modified with methacrylates through reaction with methacrylic anhydride146 and processed with photolithography to create patterned hydrogel structures for diagnostics and tissue engineering applications.147 Towards cartilage tissue engineering, methacrylated alginate, methacrylated HA, and methacrylated CS have been used for chondrocyte encapsulation and proliferation.148–154
Chitin has been modified with methacrylate groups via esterification between methacrylic anhydride and free hydroxyl groups on water-soluble carboxymethyl chitin, resulting in a photocrosslinkable hydrogel.155 In another approach, chitin has been functionalized with methacrylate groups by carbamate bond formation between hydroxyl moieties on chitin and 2-isocyanatoethyl methacrylate.156 The modification resulted in a photocrosslinkable chitin hydrogel that could be micropatterned for controlled guidance of cells.
Beyond methacrylates, reactive methacrylamide groups have been used for free radical chain polymerization of modified biopolymers. For example, HA was modified with methacrylamides using amidation reactions in the presence of EDC.157 Park et al. showed that diacrylated PEG (PEGDA) could be incorporated into these methacrylamide-HA hydrogels to increase mechanical properties, and RGD could be tethered to the hydrogels to allow for cell adhesion and proliferation.157 Gelatin has also been used extensively after modification for free radical chain polymerization, mostly commonly through esterification with methacrylic anhydride,158 or by amidation with methacrylamide to form GelMA.159 GelMA has been shown to be a useful material for photopatterned, cell-laden microtissues and microfluidic devices.160 While GelMA has been a widely explored polypeptide for hydrogel formation, other polypeptides have also been explored. Kim et al. demonstrated that hydrolyzed silk fibroin could be methacrylated to form photocrosslinked hydrogels in the presence of UV light and LAP photoinitiator, where crosslinking is aided by β-sheet formation.161
4.1.2. Styrene
Although not used extensively, styrene moieties contain alkene groups that can be used for free radical chain polymerization. Styrene has been introduced to gelatin, HA, heparin, and albumin by either esterification with 4-vinylbenzoic acid or amidation with 4-vinylaniline.162 Styrenated gelatin has been explored for cartilage tissue engineering as a hydrogel for chondrocyte delivery.163 Furthermore, styrenated gelatin microspheres have been fabricated in a batch emulsion and subsequently explored for adipose tissue engineering.164
4.1.3. Degradable hydrogels from free radical chain polymerization
For some applications, hydrogels with high mechanical properties and low degradability may be preferred; however, more rapid hydrogel degradation may be desired for other applications. To introduce control over hydrogel degradation, hydrolytically degradable groups (e.g., esters) can be incorporated in between the biopolymer backbone and conjugated reactive groups. For example, hydroxyethyl methacrylate (HEMA) has been conjugated to many biopolymers, including dextran130 and HA,132 to modulate hydrogel degradation behavior. To increase hydrogel degradation rates, multiple lactic acid groups can be introduced.135 Sahoo et al. demonstrated that this could be achieved with HA, and that ECM distribution increases when mesenchymal stromal cells (MSCs) were cultured in these hydrolytically degradable hydrogels.165 The incorporation of lactic acid can result in hydrogels that degrade too quickly, resulting in cell clustering and altered cell morphology.166 To overcome this, caprolactone can be used instead as a hydrolytically degradable group with slower degradation rates. It has been shown that incorporating caprolactone groups between the HA backbone and methacrylate moieties allows for the tuning of hydrogel degradation rates to match the ECM deposition rates of MSC-laden hydrogels towards superior neocartilage formation in vitro.166 Beyond hydrolysis as a method of degradation, Wade et al. introduced protease-degradable peptides between the HA backbone and methacrylates, so that formed hydrogels respond to proteases (Figure 3b).167 These modified HA biopolymers were processed into degradable electrospun fibrous scaffolds, where degradation was dependent on the protease-sensitivity of the peptide sequence and the protease concentration.
4.2. Crosslinking via click chemistry
Click chemistry refers to a set of often biocompatible chemical reactions that result in the rapid formation of covalent bonds. Click chemistry reactions occur in a one-pot system, have a high thermodynamic driving force (greater than 20 kcal/mol), are not disturbed by water, have high specificity, and generate minimal byproducts.168 Due to the biocompatibility, reliability, and specificity of click chemistry reactions, they are often used in biomedical applications such as drug discovery and biomaterials engineering.169,170 Herein, we review some of the most common click chemistry reactions used for crosslinking of biopolymer hydrogels, including thiol-ene radical additions, thiol-ene Michael additions, azide-alkyne reactions, and tetrazine-norbornene cycloaddition.
4.2.1. Thiol-ene radical addition
Thiol-ene radical additions form a covalent thioether bond between an alkene and a thiol in the presence of a radical initiator. This click reaction is a powerful biomaterials tool due to its high yield, mild reaction conditions, regiospecificity and stereospecificity, and biorthogonality.171 Radical initiators convert thiols into thiyl radicals, which subsequently form thioether bonds with electron-deficient or strained enes (e.g., norbornene) and can be used to form hydrogels (Figure 4a). Although methacrylates, acrylates, styrenes, and maleimides can undergo both thiol-ene step growth and radical chain growth homopolymerization, norbornenes and vinyl ethers only undergo thiol-ene step growth, which permits better control over hydrogel formation.171 Thus, towards biopolymer hydrogel formation, the thiol-ene radical addition of thiols and norbornenes is most commonly used.
Figure 4. Crosslinking via a thiol-ene radical addition.
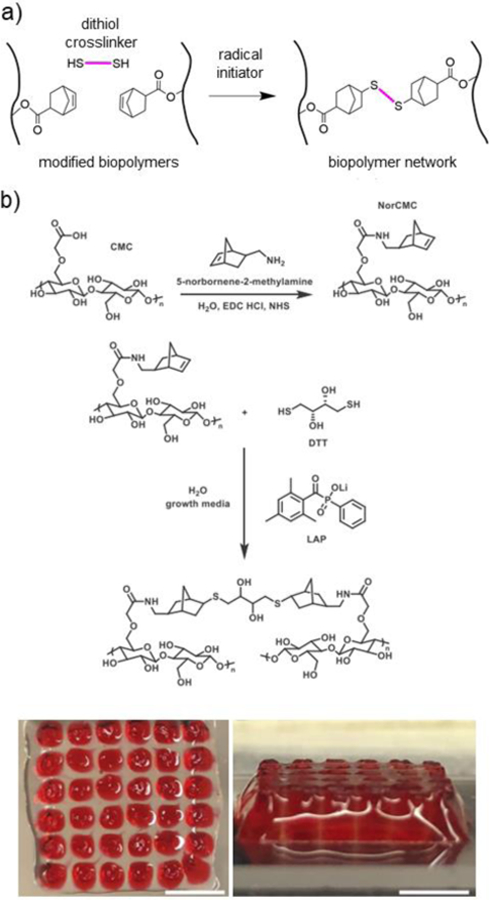
a) Schematic representation of norbornene-modified biopolymers (black) crosslinked with a dithiol crosslinker (pink) in the presence of a radical initiator. b) Thiol-norbornene crosslinked CMC hydrogels for bioprinting, showing (top) schematic representation of amidation reaction to synthesize norbornene-modified CMC (NorCMC), (middle) schematic representation of photocrosslinking reaction, and (bottom) bioprinted NorCMC scaffolds (clear) filled with Pluronic (red). Scale bars represent 5mm. Adapted with permission from Ji, et al.182 Copyright, 2020 Elsevier.
Norbornene is a bridged cyclic hydrocarbon with a strained carbon-carbon double bond. Many biopolymers have been functionalized with norbornene groups to undergo thiol-norbornene radical addition crosslinking, including HA,112,117,131,172 alginate,173,174 cellulose,114,175,176 gelatin,177–180 and silk fibroin.181 Biopolymers can be functionalized with norbornene derivatives using esterification112,177 or amidation117,174 reactions. DTT,112,181 multi-functional PEG dithiols,178 and enzymatically degradable dithiols114,173 have been explored as crosslinkers. While photoinitiator systems are commonly employed, redox-mediated radical initiators such as APS/TEMED can also be utilized for radical generation and thiol-norbornene crosslinking.176 Biopolymer, crosslinker, and initiator concentrations can be used to tune hydrogel mechanical properties across orders of magnitudes (~1–100kPa);112,114 however, the range of hydrogel stiffnesses that can be achieved may be hindered by limited chain mobility as the reaction progresses, resulting in “maximum possible” stiffnesses despite increased polymer or crosslinker concentrations.175
Gramlich, et al. demonstrated that HA could be functionalized with norbornene (NorHA) using esterification between 5-norbornene-2-carboxylic acid and the secondary alcohol group on HA.112 NorHA hydrogels could be spatiotemporally patterned by conjugating thiolated peptides to remaining free norbornenes using photomasks, demonstrating the ability to independently tune biochemical and mechanical properties.112 Vega, et al. further demonstrated the photopatterning capabilities of NorHA (synthesized through BOP coupling of 5-norbornene-2-methalamine to carboxylic acid via amidation) by encapsulating MSCs in a NorHA hydrogel with photopatterned gradients of thiolated- RGD and other peptides created using a sliding opaque photomask.117 In a single hydrogel, over 100 distinct biochemical formulations could be formed and screened for cartilage formation, demonstrating the promising application of thiol-norbornene radical addition in screening potential hydrogel formulations for tissue engineering.117 NorHA can also be utilized with other scaffold biofabrication techniques, including bioprinting131 and microgel formation.172
Other biopolymers have been modified with norbornenes for hydrogel formation. For example, norbornene-functionalized alginate hydrogels have been explored for many applications, including tissue engineered implantable constructs. Leuckgen et al. showed that norbornene-modified alginate hydrogels crosslinked with dithiolated enzymatically degradable crosslinkers allowed for cell and tissue infiltration in vivo after 8 weeks in a subcutaneous mouse study.173 Furthermore, Ooi, et al. demonstrated that norbornene-functionalized alginate could be used as a cell-laden bioink for bioprinting of tissue engineering scaffolds.174 CMC functionalized with norbornenes for thiol-ene radical addition crosslinking has also been explored for tissue engineering applications.114,175,176,182 Ji et al. showed that CMC can be modified with norbornene groups using either amidation or esterification reactions.182 Norbornene-modified CMC was combined with DTT to undergo thiol-ene photocrosslinking, and subsequently used as a cell-laden bioprinting ink (Figure 4b). Furthermore, Dadoo et al. showed that norbornene-modified CMC could be crosslinked with a thermally responsive dithiol-terminated poly(N-isopropyl acrylamide) crosslinker for spatiotemporal control over hydrogel swelling upon targeted temperature regulation.114 In another strategy, cellulose nanofibrils could be functionalized with norbornene to allow for conjugation with different thiolated molecules to create nanofibril hydrogel suspensions with a wide range mechanical properties.176 Lastly, Ryu, et al. demonstrated that silk fibroin modified with norbornenes could be combined with 4-arm PEG norbornene and DTT to create PEG hydrogels with embedded silk fibroin microgels, including with adenocarcinomic human alveolar basal epithelial cells.181
Gelatin can also be functionalized with norbornene groups using amidation between amines on the collagen backbone and carbic anhydride.177–179 Munoz, et al. demonstrated that norbornene-modified gelatin hydrogels supported more rapid and extensive cell spreading of encapsulated human MSCs (hMSCs) when compared to GelMA.177 This may be due to radical-mediated damage to proteins and cells due to kinetic chain growth in GelMA hydrogels, as well as the limited control over mesh size and molecular transport within GelMA hydrogels.177 Greene et al. demonstrated temporal control over crosslinking and thus mechanical properties of norbornene-modified gelatin hydrogels using intermittent light exposure, which was used to study hepatocellular carcinoma cell fate as a function of hydrogel matrix properties in vitro.178 While functionalizing biopolymers with norbornene groups is a common approach, biopolymers can also be functionalized with thiols and subsequently crosslinked with multi-arm norbornene crosslinkers. Holmes, et al. functionalized amine groups on collagen with thiols using 2-iminothiolane in the presence of DTT as a reducing agent.180 Thiolated collagen was then crosslinked with a multi-arm norbornene crosslinker for hydrogel formation. In another example, Yue et al. used thiol-modified keratin, along with a multi-arm PEG norbornene and Eosin Y photoinitiator, to create hydrogel constructs upon exposure to visible light.98 The keratin hydrogels could encapsulate cells with high viability and exhibited tunable compressive moduli up to 45 kPa.
In addition to norbornene modification, biopolymers including gelatin,183 chitosan,184 and starch185 have been modified with allyl groups through reaction with allyl glycidyl ether (AGE) or allyl chloride186 for thiol-ene radical addition crosslinking. AGE-modified gelatin (Gel-AGE) hydrogels crosslinked with DTT have been explored for bioprinting tissue engineered scaffolds for the encapsulation of chondrocytes.187 Kiliona et al. demonstrated that chitin nanocrystals and nanofibrils (nanochitin) could be functionalized with allyl groups by reaction with 10-undecenoyl chloride.188 When mixed with thiolated PEG, allyl-modified nanochitin was used to form organogels in the presence of UV light and photoinitiators.188 Hilderbrand et al. demonstrated that allyl-functionalized collagen mimetic peptides (CMPs) can be combined with thiolated PEG to fabricate a photocrosslinkable hydrogel for 3D cell culture.189 In another approach, biopolymers can be modified with pentenoate to functionalize with ene groups in order to undergo thiol-ene radical addition. For example, Mergy et al. modified both dextran and HA with pentenoate groups via esterification with pentenoic anhydride to undergo thiol-ene photocrosslinking in the presence of thiol crosslinkers.190 Further, pentenoate-modified gelatin and thiolated gelatin have been combined to form a photocrosslinkable hydrogel for cell encaulstion.191
4.2.2. Thiol-ene Michael addition
In Michael addition crosslinking, thiol-ene reactions can occur readily between thiols (Michael donors) and electron-deficient enes (Michael acceptors) without the need for radical initiators (Figure 5a).171 Thiol-ene Michael addition reactions can be either base-catalyzed or nucleophile-catalyzed.124 Common ene groups used for hydrogel crosslinking include maleimides, vinyl sulfones, acrylates, and methacrylates, in order of decreasing reactivity towards thiol-ene Michael addition (Figure 5b).124 Variations in the ene group, the pH, and the biopolymer and crosslinker concentrations allow for tuning of gelation times from a few seconds to several hours. Biopolymers may be functionalized with ene or thiol groups for crosslinking using esterification or amidation reactions. Biopolymer hydrogels crosslinked using Michael addition reactions are commonly utilized as injectable, in situ forming hydrogels due to the ability to tune gelation kinetics to clinically relevant timescales.
Figure 5. Crosslinking via thiol-ene Michael addition.
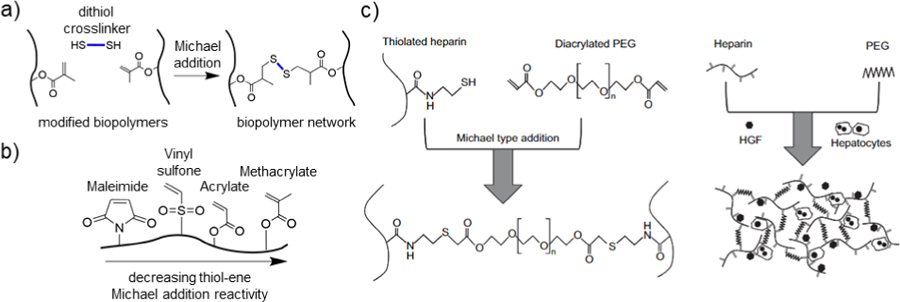
a) Schematic representation of methacrylate-modified biopolymers (black) crosslinked with a dithiol crosslinker (blue) under Michael addition conditions. b) Schematic representation of a biopolymer modified with multiple ene groups that can undergo thiol-ene Michael addition. From left to right, in decreasing order of Michael addition reactivity: maleimide, vinyl sulfone, acrylate, and methacrylate. c) Thiolated heparin is crosslinked with diacrylated PEG (PEGDA) via a thiol-ene Michael addition reaction, which was used for the culture of primary rat hepatocytes and hepatocyte growth factor (HGF). Adapted with permission from Kim, et al.205 Copyright, 2010 Elsevier.
Thiol-ene Michael addition reactions have been used for in situ crosslinking of injectable HA hydrogels for drug delivery and tissue engineering applications.192–194 Hahn et al. showed that MeHA crosslinked through Michael addition with DTT could be used as an injectable hydrogel for sustained release of erythropoietin.192 Gelation time varied from 30 minutes to 3 hours, and erythropoietin could be released over a 7 day period in a rat in vivo model.192 Vanderhooft et al. also demonstrated a wide range of gelation times (30 seconds – 2 hours) for hydrogels consisting of thiolated HA, thiolated gelatin, and various ene-functionalized PEG crosslinkers, including PEGDA and PEG-dimaleimide.193 Furthermore, the storage modulus of thiolated HA hydrogels crosslinked with PEGDA by Michael addition ranged from tens to thousands of Pa.195 Forgoing the need for small molecules or synthetic polymer crosslinkers, HA has been modified with methacrylates, acrylates, vinyl sulfones, and maleimides, and subsequently mixed with thiolated HA to crosslink via a Michael addition reaction, with gelation times ranging from instantaneous (maleimide) to ~45 minutes (methacrylate) as a function of the ene group present, pH, and polymer concentration.196 Towards cardiac tissue engineering, MeHA and thiolated HA were selected as an in situ crosslinking, injectable hydrogel formulation for mechanical stabilization of myocardial tissue after infarction.196 Michael addition crosslinking in HA hydrogels has been used for numerous applications, including cartilage tissue engineering197,198 and neural tissue engineering.199,200
A range of chemical modifications have been applied to dextran hydrogels for thiol-ene Michael addition hydrogel formation. Injectable, in situ crosslinking hydrogels consisting of thiolated dextran and either acrylated PEG or vinyl-sulfonated dextran have been developed with a wide range of mechanical and degradation properties.201 Degradation timescales can vary significantly (days to weeks) as a function of polymer concentration, Michael donors and acceptors selected, and spacing between conjugated functional groups and biopolymer backbone.201,202 For spatiotemporal control over degradation, Peng et al. chemically modified dextran with an acrylate functional group that contained a photolyzable o-nitro-benzyl moiety between the acrylate and dextran backbone.203 The hydrogel was crosslinked with a dithiolated PEG crosslinker using Michael addition, and subsequent exposure to UV light resulted in controlled degradation and release of model drugs.203
Many other biopolymers have been modified with thiols and/or ene functional groups for thiol-ene Michael addition crosslinking. For example, an injectable, thiolated chitosan hydrogel was developed by crosslinking with an acrylated PEG, resulting in tunable gelation times between ~10 seconds to 20 minutes.204 Cell attachment and spreading was demonstrated in vitro upon addition of an RGD peptide.204 Kim et al. showed that thiolated heparin could be mixed with PEGDA for Michael addition crosslinking to form a hydrogel useful for the encapsulation and in vitro culture of primary hepatocytes (Figure 5c).205 Furthermore, thiolated gelatin hydrogels mixed with PEGDA are promising for the rapid encapsulation of MSCs for wound repair applications.206 Xu et al. showed that, when applied to a full thickness wound rat model, these hydrogels supported accelerated wound closure, re-epithelialization, and vascularization.206 In another example, Zhang et al showed that thiolated keratin can be mixed with 4-arm PEG-vinyl sulfone (PEG-VS) to undergo Michael Addition crosslinking.99 The keratin hydrogel showed promise as a flexible strain sensor for future applications in wearable electronics.
4.2.3. Azide-alkyne [3+2] cycloaddition
Azide-alkyne [3+2] cycloaddition, also called Huisgen 1,3-dipolar cycloaddition, is a powerful click chemistry tool that is widely used in bioconjugation to form strong covalent bonds in a one-pot reaction.207 To perform the reaction under physiologically relevant conditions, the reaction is often catalyzed by Cu(I) or Cu(II).208 Towards hydrogel formation, biopolymers can be modified with azide or alkyne moieties to undergo [3+2] cycloaddition crosslinking, potentially in the presence of copper catalysts (Figure 6a). Li et al. fabricated a thermo-responsive albumin hydrogel by conjugating propargyl maleimide to thiol groups on BSA cysteine residues.209 The combination of alkyne-functionalized BSA with poly(N-isopropylacrylamide) (PNIPAAm) end-terminated with azide groups in the presence of Cu(II) catalysts yielded an azide-alkyne hydrogel.209 Gelatin hydrogels formed via azide-alkyne reactions have been engineered by conjugating propolic acid to lysine residues to add azide functionality and crosslinking with either 4,4′-diazido-2,2′stilbenedisulfonic acid or 1,8-diazidooctane, both of which are di-functionalized with alkyne groups.210 Upon exposure to Cu(II) catalysts, the gelatin hydrogels reached compressive moduli between 50 and 390 kPa.210 Other biopolymers that have been crosslinked by copper-catalyzed azide-alkyne cycloaddition reactions include HA,170,211,212 cellulose,213 and alginate.214
Figure 6. Crosslinking via azide-alkyne cycloaddition.
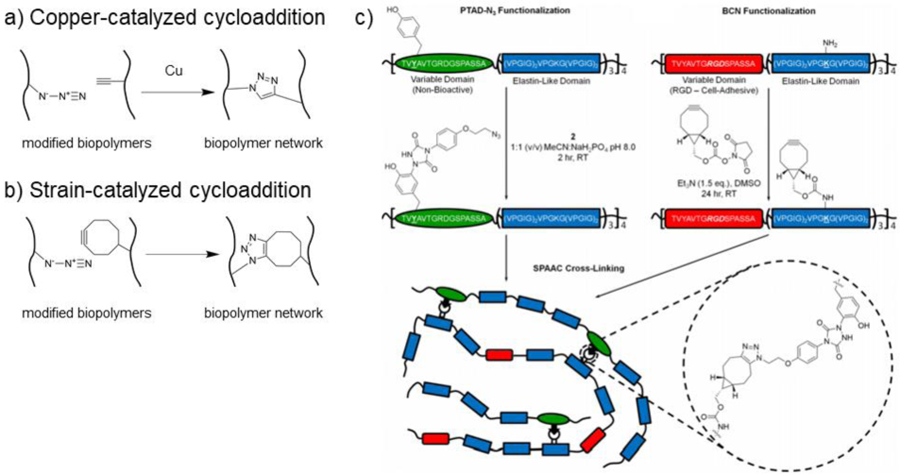
a) Schematic representation of copper-catalyzed azide-alkyne cycloaddition crosslinking of biopolymers. Biopolymers are modified with either azide or alkyne functional groups and upon combination in the presence of a copper catalyst, crosslinks form by azide-alkyne cycloaddition. b) Schematic representation of strain-catalyzed azide-alkyne cycloaddition crosslinking of biopolymers. Biopolymers are modified with either azide or strained alkyne (i.e., cyclooctyne) groups and upon combination, crosslinks form by azide-alkyne cycloaddition. c) Elastin-like polypeptides (ELPs) functionalized with either azide or bicyclononyne (BCN) groups for bio-orthogonal crosslinking due to the strain-promoted [3+2] azide-alkyne cycloaddition (SPAAC) reaction. Adapted with permission from Madl, et al.109 Copyright, 2017 American Chemical Society.
Copper-catalyzed azide-alkyne cycloadditions allows for rapid gelation; however, copper catalysts are often cytotoxic, limiting the ability for copper-catalyzed reactions to be used in cellular systems.215 To overcome this, strain-promoted [3+2] azide-alkyne cycloaddition (SPAAC) can be utilized by combining azides with strained cyclooctynes (Figure 6b).215 Wang et al. engineered metal-free, azide-alkyne crosslinked, injectable dextran hydrogels by modifying dextran with either azadibenzocyclooctyne (ADIBO-Dex) or azides (Dex-N3).216 Upon mixing the two components, gelation occurred within 1 to 10 minutes, resulting in hydrogels with storage moduli between 2 and 6 kPa.216 The hydrogels supported chondrocyte growth and cartilaginous tissue formation in vitro.216 In another approach to fabricate metal-free azide-alkyne hydrogels, chitosan was functionalized with azides by modification with azidopentanoic acid and subsequently mixed with 3-arm PEG-propiolate, a multifunctional alkyne crosslinker.217 The resulting hydrogel formed crosslinks within 5 to 60 minutes and reached compressive moduli between ~40 and 80 kPa.217 Lastly, ELPs have been functionalized with either azide or bicyclononyne moieties to undergo SPAAC crosslinking (Figure 6c).109 SPAAC-ELP hydrogels crosslinked within minutes.108,109 These hydrogels were used to rapidly encapsulate hMSCs and murine neural progenitor cells in vitro with high viability and phenotypic maintenance.
4.2.4. Tetrazine-norbornene
Tetrazine-norbornene reactions are useful to rapidly form irreversible covalent bonds. Hydrogels can be crosslinked by tetrazine-norbornene mechanisms for the encapsulation of cells and therapeutics (Figure 7a).218 Tetrazine-norbornene click chemistry offers similar advantages to metal-free azide-alkyne cycloaddition without the burden of the high cost of strained cyclooctyne groups.218 Biopolymers can be functionalized with tetrazine and norbornene groups to engineer injectable hydrogels that undergo in situ crosslinking.219,220 Desai et al. demonstrated that alginate could be functionalized with either benzylamino tetrazine or norbornene methylamine groups by EDC/NHS amidation.221 Cytocompatible alginate hydrogels could then be formed via tetrazine-norbornene click chemistry, with gelation times of approximately a few minutes and storage moduli between ~0.5 and 3 kPa.221,222 Furthermore, Lueckgen et al. showed that tetrazine-norbornene biopolymer networks containing oxidized alginate allowed for cell infiltration in an in vivo subcutaneous injection mouse model.222 HA has also been modified with tetrazine and norbornene moieties using EDC/NHS amidation mechanisms.219 The resulting HA hydrogels were engineered as an injectable delivery vehicle for the sustained release of protein therapeutics.219 Gelatin modified with tetrazine and norbornene groups has been explored for tissue engineered scaffold formation and as a delivery vehicle for contrast agents.220,223 For example, Koshy et al. developed a tetrazine-norbornene crosslinked gelatin hydrogel that supported cell growth and matrix remodeling in vitro, as well as cell infiltration in an in vivo subcutaneous mouse model (Figure 7b).223
Figure 7. Crosslinking via tetrazine-norbornene reactions.
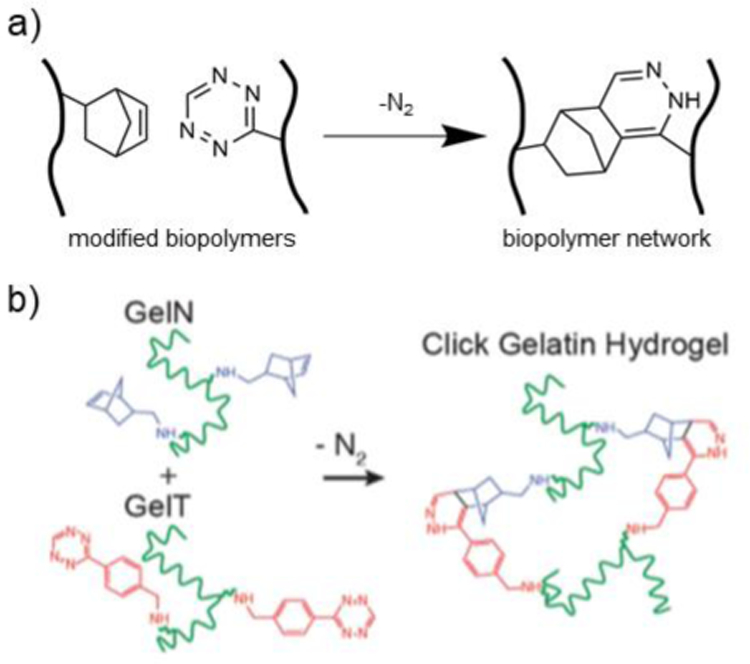
a) Schematic representation of biopolymers modified with either norbornene or tetrazine groups. Upon combination, crosslinks form by a tetrazine-norbornene reaction. b) Gelatin modified with either norbornene (GelN) or tetrazine (GelT) are mixed to form a tetrazine-norbornene click biopolymer network, which was cell-adhesive and degradable for use in cell encapsulation. Adapted with permission from Koshy, et al.223 Copyright 2016, Wiley.
4.3. Crosslinking via oxidation of phenolic groups
Phenols are aromatic hydrocarbons that contain one or more hydroxyl groups. Oxidative environments lead to the generation of phenolate radicals that form covalently bonded phenol dimers.224 This mechanism can be used to form crosslinks between biopolymers modified with phenolic moieties. Two of the most common approaches for hydrogel formation include the enzymatically driven crosslinking of tyramine and oxidation of catechol groups.
4.3.1. Tyramine
Tyramine is a naturally occurring amine derived from the tyrosine amino acid. In the presence of horse radish peroxidase (HRP) and hydrogen peroxide (H2O2), tyramine groups are converted into phenolate radicals that form either carbon-carbon bonds or di-tyramine adducts which can be used for hydrogel crosslinking. For example, radical photoinitiators can be combined with keratin, which contains tyrosine amino acids, to form a photocrosslinkable hydrogel upon exposure to UV light.225
Biopolymers can be modified with tyramine functional groups, usually by amidation between tyramine and carboxyl groups on biopolymer backbones, and subsequently covalently crosslinked by the addition of HRP and H2O2. (Figure 8a). Tyramine-based enzymatic crosslinking occurs rapidly within a few seconds to minutes, but can be tuned by varying concentrations of tyramine, HRP, and H2O2 in solution.116,226 While enzymatic crosslinking of tyramine moieties is most common, it has also been shown that tyramine can be crosslinked using visible light and a photoinitiator.227,228
Figure 8. Crosslinking via tyramine enzymatic reactions.
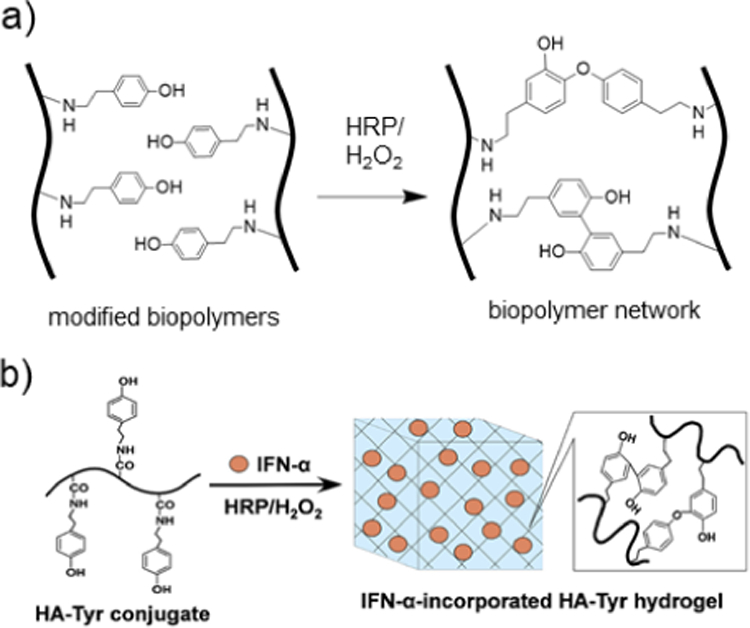
a) Schematic representation of biopolymers modified with tyramine that crosslink in the presence of HRP and H2O2 to form dityramine adducts. b) HA is modified with tyramine and subsequently exposed to horse radish peroxidase (HRP) and H2O2 to undergo enzymatic crosslinking by oxidation of tyramine groups, forming covalent dityramine adducts. Interferon-α (IFN-α) is encapsulated in the hydrogel for use as a prolonged-release delivery vehicle for renal carcinoma treatment. Adapted with permission from Ueda, et al.231 Copyright, 2016 Elsevier.
Using carbodiimide chemistry, tyramine groups can be conjugated to HA (HA-Tyr) by amidation with free carboxyl groups.116,229 Simultaneous injection of two solutions, one containing HA-Tyr and H2O2 and one containing HRP, can be used for rapid gelation in vivo upon mixing.116 It has been demonstrated that HA-Tyr hydrogels can be used for sustained delivery of proteins and anticancer therapeutics.230,231 For example, Ueda, et al. showed that HA-Tyr hydrogels could be used to rapidly encapsulate interferon-alpha (IFN-α) for sustained release as an immunotherapy treatment (Figure 8b).231 The HA-Tyr hydrogel was able to prolong the biological half-life of IFN-α and improve anticancer effects in vivo when evaluated in a human renal cell carcinoma xenograft mouse study.
Towards tissue engineering applications, Loebel et al. demonstrated the versatility of HA-Tyr hydrogels, comparing the influence of enzymatic crosslinking and visible light photocrosslinking on encapsulated MSCs.227 It was found that when HA-Tyr hydrogel stiffness was constant, enzymatically crosslinked biopolymer networks resulted in increased cell spreading and greater focal adhesion strength when compared to photocrosslinked hydrogels; however, photocrosslinked hydrogels resulted in increased cellular tractions.227 This highlights the importance of considering how crosslinking methods influence cell behavior. Furthermore, it has been shown that additional biopolymers, such as silk fibroin232 and tyramine-modified CS,233 can be added to enzymatically crosslinked HA-Tyr hydrogels to create multifunctional hydrogels for tissue engineering.
Using amidation, alginate can also be modified with tyramine groups (Alg-Tyr) for enzymatic crosslinking.234 It has been shown that enzymatically crosslinked Alg-Tyr hydrogels can retain their ability to undergo additional ionic crosslinking upon exposure to calcium (Ca2+).234,235 Furthermore, enzymatic crosslinking of Alg-Tyr hydrogels results in more stable hydrogels, overcoming the potential dissolution that occurs with ionic crosslinking during long-term cultures. In addition to Alg-Tyr, alginate modified with catechol moieties allow for HRP/H2O2 enzymatic crosslinking.236 Hou et al. showed that enzymatically crosslinked alginate hydrogels with catechol moieties results in improved tissue adhesiveness when compared to Alg-Tyr hydrogels.236
One method to functionalize dextran with tyramine groups is to first modify dextran with p-nitrophenyl chloroformate and to subsequently conjugate tyramines by urethane bond formation.121,226 In an alternative strategy, dextran can be modified with di-glycolic anhydride and subsequently functionalized with tyramine groups by amidation.121 Both synthesis methods result in tyramine-modified dextran hydrogels that can undergo enzymatic crosslinking; however, the latter method results in a hydrogel with increased hydrolytic degradability.121 Tyramine-modified dextran hydrogels have been promising towards cartilage repair, especially when combined with tyramine-modified heparin.226,237,238
Many other biopolymers have been modified with tyramine groups for enzymatic crosslinking, including cellulose derivatives,228,239 CS,240 pullulan,240 and chitin.241 Many polypeptides, such as silk fibroin, contain tyrosine residues for HRP/H2O2 enzymatic crosslinking.232,242 However, polypeptides can also be further modified with additional tyramine groups for increased enzymatic crosslinking in hydrogel formation.243,244
4.3.2. Catechol
Catechol, the ortho isomer of benzene diol, is a versatile functional group that can undergo crosslinking by the formation of covalent bonds, metal-ligand coordination, and hydrogen bonding.245 Catechol moieties occur widely in nature, with the famous example being mussel adhesion to dynamic wet surfaces due to the secretion of fluids rich in catechol groups.245,246 Inspired by mussels, tissue adhesive hydrogels containing catechol groups have been explored.246,247 Under oxidative conditions, catechol groups can form covalently crosslinked catechol dimers, which have been widely used in hydrogel formation (Figure 9a).245 Dopamine contains a catecholic moiety and has been conjugated to many biopolymers by EDC/NHS amidation reactions.248,249
Figure 9. Crosslinking via catechol reactions.
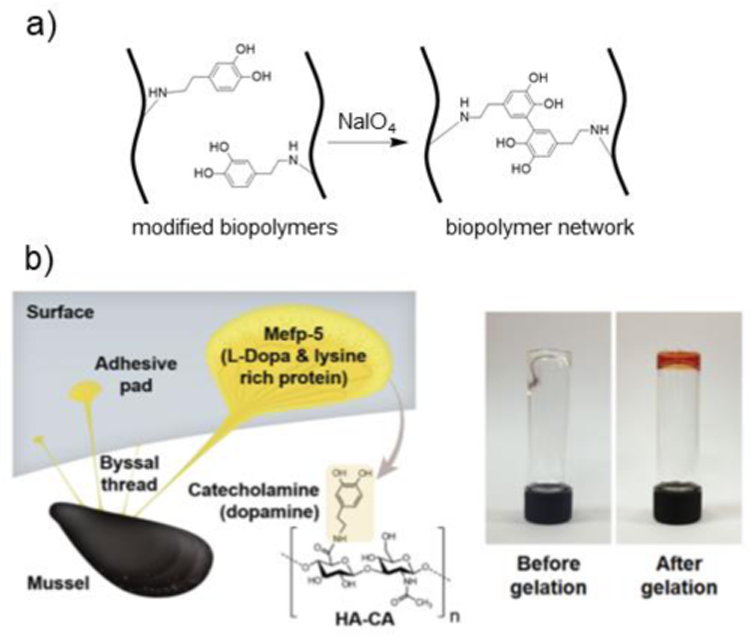
a) Schematic representation of biopolymers modified with catechol and crosslinking in the presence of NaIO4 to form dicatechol adducts. b) A mussel-inspired, HA hydrogel is formed by modifying HA with catechol moieties (HA-CA). HA-CA is covalently crosslinked in the presence of sodium periodate (NaIO4). Image shows HA-CA hydrogel before (clear) and after (red) gelation. Adapted with permission from Shin, et al.251 Copyright, 2015 Wiley.
Alginate,247 chitosan,250 and HA249 have all been modified with dopamine functional groups for hydrogel formation and tissue-adhesive applications. Lee et al. developed a catechol-modified alginate hydrogel that covalently crosslinks upon exposure to sodium periodate (NaIO4).248 The storage modulus could be tuned from 300 to 6000 Pa, depending on polymer concentration and degree of substitution.248 Furthermore, catechol-alginate hydrogels exhibited increased cytocompatibility when compared to ionically crosslinked alginate.248 Hong et al. demonstrated that catechol-modified HA (HA-CA) hydrogels exhibited strong adhesion to wet surfaces in acidic environments and increased mechanical stability due to the formation of covalent crosslinks in basic environments.249 Covalently crosslinked HA-CA hydrogels have been shown to adhere to both wet and beating tissues in vivo.251 Shin et al. engineered an HA-CA hydrogel for tissue adhesion applications (Figure 9b).251 The hydrogel adhered to liver and heart tissue for at least 1 month in an in vivo rat model. While exposing catechol-modified biopolymers to basic conditions yields rapid covalent crosslinking, Sato et al. showed that HA-CA hydrogels can undergo auto-oxidation and covalent crosslinking under physiological conditions (pH ~7.4) over a period of a few hours.252
5. Dynamic covalent crosslinking
Dynamic covalent crosslinking consists of a subset of reactions that allow for the formation of reversible covalent crosslinks between biopolymers. The dynamic nature of the covalent bonds may introduce shear-thinning and self-healing properties into the hydrogel while maintaining high mechanical moduli for structural integrity. Furthermore, dynamic covalent bonds can introduce stimuli-responsiveness in hydrogel assembly and disassembly. Gelation via dynamic covalent crosslinking may be achieved by one-pot mixing of reactive components. When compared to covalently crosslinked biopolymer networks, biopolymer networks crosslinked via dynamic covalent chemistries may experience faster degradation times and increased cell infiltration both in vitro and in vivo. Mechanical and biological properties of dynamic covalent biopolymer networks will be influenced by biopolymer and crosslinking group concentrations, as well as bond strength and bond formation kinetics. Dynamic covalent crosslinking in hydrogels has been explored for many applications, including tissue engineered scaffolds, drug delivery vehicles, and bioprinting inks. This review will focus on dynamic covalent crosslinking mechanisms commonly used towards biomedical applications, including Schiff base reactions, disulfide formation, and reversible Diels-Alder reactions.
5.1. Schiff base crosslinking
The Schiff base reaction was discovered by Hugo Schiff in 1864 and has been widely used as a click chemistry tool.253–255 Schiff bases are a type of imine, which has the structure R2C=NR’ and are either secondary aldimines or ketimines, where R’≠H. The condensation of carbonyl and primary amine groups results in Schiff base formation, with water as the only byproduct. The reversible reaction can proceed under mild conditions and is pH-responsive.253 For hydrogel formation, Schiff base crosslinks are formed by mixing aldehyde-functionalized and amine-functionalized biopolymers.254,255 Crosslink stability can be influenced by the neighboring atoms attached to the primary amine groups used in Schiff base formation. Imine crosslinks are formed when a carbonyl group condenses with a primary amine group attached to a hydrocarbyl group (Figure 10a). Hydrazone crosslinks, which are more stable than imine crosslinks, are formed when a carbonyl group condenses with a primary amine group attached to a nitrogen atom (Figure 10b).127,253 Oxime crosslinks are formed when a carbonyl condenses with a primary amine group attached to an oxygen atom and are more stable than both hydrazone and imine crosslinks.256,257 Hydrogel degradation can be tuned by selecting the type of Schiff base used in crosslinking (e.g., imine, hydrazone, oxime).127,256 For biopolymer hydrogel formation, imine and hydrazone functionalities are most commonly utilized to achieve dynamic crosslinking behavior.
Figure 10. Crosslinking via Schiff base formation.
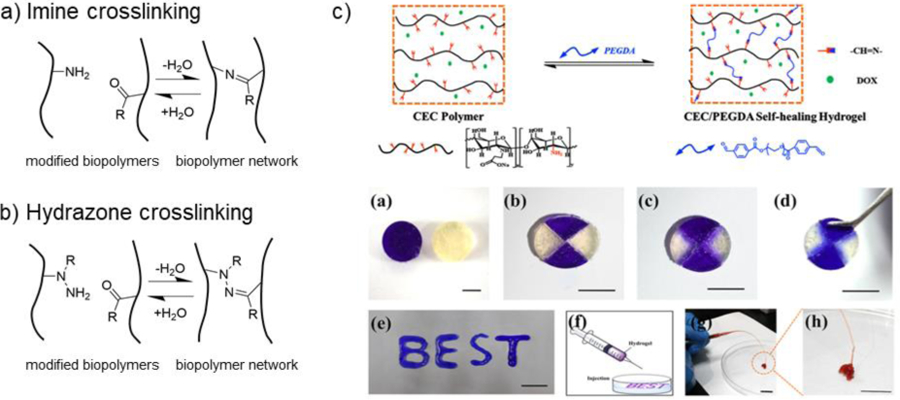
a) Schematic representation of imine dynamic covalent crosslinking by combining biopolymers modified with either primary amine or aldehyde groups. b) Schematic representation of hydrazone dynamic covalent crosslinking by combining biopolymers modified with either hydrazide or aldehyde groups. c) N-carboxyethyl chitosan (CEC) is combined with dialdehyde PEG (PEGDA, blue), where imine dynamic covalent crosslinks are formed between the amine groups on CEC and the aldehyde groups on PEGDA. Images show the self-healing (a-d) and shear-thinning (e-h) properties of the hydrogel. Adapted from Qu, et al.258 Copyright, 2017 Elsevier.
5.1.1. Imine crosslinks
Imine crosslinking is widely used in biopolymer hydrogel formation (Figure 10a).255 Sugar rings in polysaccharide backbones can be oxidized with sodium periodate to form dialdehyde-functionalized biopolymers. Furthermore, many biopolymers, such as chitosan and gelatin, have primary amine groups that can be used for imine crosslinking.258–260 Qu et al. developed an injectable N-carboxyethyl chitosan hydrogel crosslinked by imine formation between amino groups on chitosan and dialdehyde PEG crosslinkers (Figure 10c).258 The hydrogels were self-healing and showed promise as drug delivery vehicles for hepatocellular carcinoma therapy. In other examples, chitosan has been combined with many oxidized polysaccharides including cellulose,261,262 CS,263 and HA126,264 for hydrogel formation. Gelatin has been combined with many oxidized polysaccharides including alginate265,266 and pectin260 for hydrogel formation. The number of amine groups available for imine crosslinking can be increased by coupling gelatin with ethylenediamine using carbodiimide chemistry.265 Hydrolysis of the imine crosslinks results in hydrogel degradation.126 The dynamic nature of the imine crosslink formed can result in injectable hydrogels with shear-thinning and self-healing properties.126,259,263 To improve mechanical properties, imine-crosslinked hydrogels have also been reinforced with methacrylate-based covalent photo-crosslinking265 or with the incorporation of microgels into the hydrogel structure.263
5.1.2. Hydrazone crosslinks
Hydrazone crosslinks are more stable than imine crosslinks, and thus can be used to increase hydrogel stability.253 Hydrazone crosslinks can be formed by mixing oxidized polysaccharides with hydrazide-modified biopolymers (Figure 10b). Hydrazide modification is often accomplished using carbodiimide chemistry to conjugate adipic dihydrazide (ADH) to free carboxylic acid groups on biopolymer backbones.267,268 Due to the ease of chemical modification, HA has been extensively explored for hydrazone-based crosslinking.269–271 Wang et al. demonstrated that oxidized HA (HA-ALD) could be combined with adipic dihydrazide-modified HA (HA-ADH) to form a shear-thinning, self-healing hydrogel for bioprinting applications.271 Furthermore, combining HA-ALD/HA-ADH networks with a thiol-ene crosslinkable NorHA increased hydrogel storage moduli 3-fold, and allowed for thiol-norbornene photo-patterning of thiolated peptides within the bioprinted scaffolds.271 Domingues et al. demonstrated that injectable hydrazone-crosslinked HA networks could be strengthened by incorporating aldehyde-modified cellulose nanocrystals into the network.272 Hydrazone crosslinking has been explored for many other biopolymers in addition to HA, including alginate,270 cellulose,273 dextran,268 and xanthan gum.274
The oxidization of polysaccharide sugar rings using sodium periodate is a simple way to functionalize biopolymers with aldehydes; however, it can result in decreased biopolymer molecular weight. To improve hydrogel mechanical properties and stability, it may be desired to functionalize biopolymers with pendant aldehydes rather than oxidizing sugar rings in the polymer backbone. Biopolymers can be modified with pendent aldehydes using carbodiimide coupling of 3-amino-1,2-propanediol to carboxylic acid groups on the biopolymer backbone, followed by brief (~5 min) exposure to sodium periodate in order to oxidize the pendant diol for aldehyde formation.115 To further improve stability, biopolymers can be modified with carbohydrazide (CDH) instead of ADH for hydrazone-crosslinked hydrogel formation.127 Hozumi et al. showed that hydrogels formed by combining pendant aldehyde-modified HA and CDH-gelatin were stable for ~30 days, whereas hydrogels formed from combining ADH-gelatin and oxidized HA degraded within ~5 days.127
5.2. Disulfide crosslinking
Disulfide bonds (i.e., SS-bonds, disulfide bridges) are dynamic covalent interactions that can be cleaved and reformed in response to chemical or physical stimuli.275 Protein folding and structure rearrangement rely on the formation and shuffling of disulfide bonds, mostly involving cysteine residues containing thiol groups, which can undergo disulfide bond formation under oxidative conditions.276 Hydrogels crosslinked by disulfide bonds can exhibit shear-thinning, self-healing properties, while maintaining increased crosslink stability when compared to physical supramolecular interactions.275,277
Biopolymers have been functionalized with thiol groups for the formation of dynamic covalent disulfide crosslinks (Figure 11a). For example, HA has been modified with dithioiso(propanoic dihydrazide) (DTP) using carbodiimide amidation and subsequent reduction with DTT to result in HA-DTPH biopolymers for hydrogel formation.278 Shu et al. demonstrated that HA-DTPH hydrogels could be oxidized by exposure to ambient air for the formation of dynamic covalent disulfide crosslinks.278 In a later study, HA-DTPH and Gel-DTPH were combined to form a synthetic ECM hydrogel scaffold crosslinked by disulfide bonds for in vitro cell culture.279 Bermejo-Velasco et al. demonstrated that disulfide bond formation kinetics and stability could be increased by modifying HA with thiol moieties containing electron-withdrawing groups at the β-position (Figure 11b).280 HA modified with either cysteine or N-acetyl-cysteine groups formed disulfide biopolymer networks at neutral pH within minutes to hours, whereas thiolated HA was unable to form stable disulfide crosslinks at neutral pH within 24h.280 Alginate hydrogels crosslinked with disulfide bonds have been fabricated by modifying carboxyl groups on alginate with either cysteine or cysteamine via amidation.281 Zhao et al. demonstrated that these alginate hydrogels were pH responsive and underwent disassembly of disulfide crosslinks in the presence of DTT.281 In another strategy, self-healing cellulose hydrogels have been fabricated by chemically modifying cellulose nanocrystals to enable dynamic disulfide bond formation.282
Figure 11. Crosslinking via disulfide bond formation.
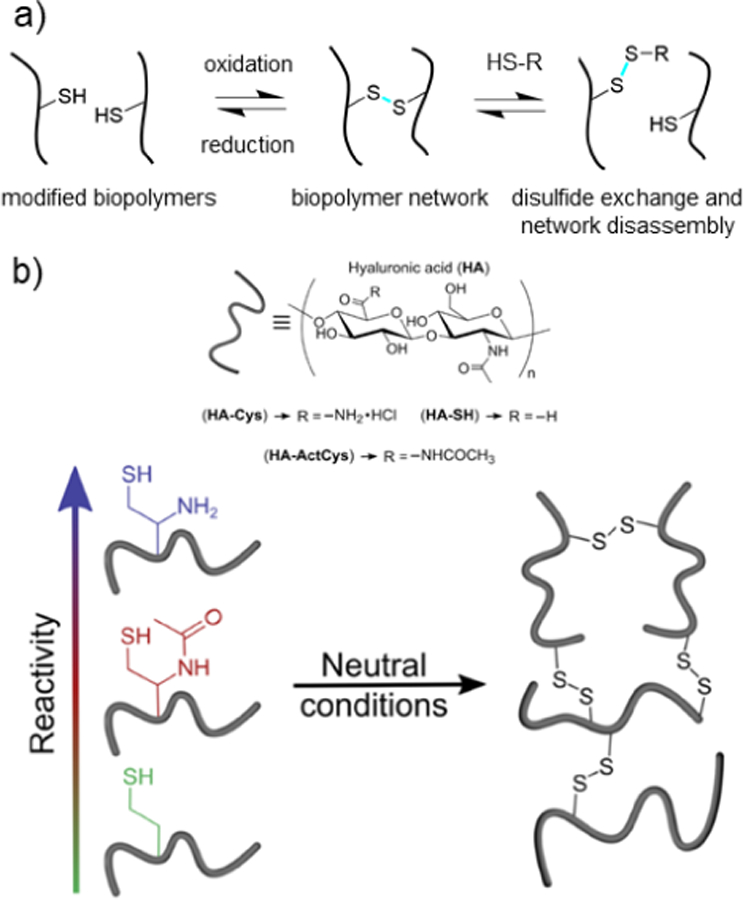
a) Schematic representation of thiolated biopolymers forming dynamic covalent crosslinks by disulfide bond formation (blue) under oxidative conditions. Upon the addition of a mono-thiolated component, disulfide exchange can result in disassembly of the hydrogel. b) Various thiolated HA biopolymers are synthesized for disulfide dynamic covalent crosslinking, including: HA-thiol (HA-SH, green), HA-acetyl cysteine (HA-ActCys, red), and HA-cysteine (HA-Cys, blue). Among the thiol groups, HA-Cys has the strongest electron-withdrawing group in the β-position, resulting in the most disulfide bond formation under neutral conditions. Adapted with permission from Bermejo-Velasco, et al.280 Copyright, 2019 American Chemical Society.
Polypeptide hydrogels can be crosslinked by forming disulfide bridges between cysteine amino acids. Sun et al. showed that injectable albumin hydrogels could be easily fabricated by combining BSA with H2O2.283 Wang et al. used a similar approach by combining keratin from chicken feathers with H2O2 to induce disulfide bond formation between free thiols on keratin.100 The resulting keratin hydrogel was explored for wound healing applications. To increase crosslinking, polypeptides can also be modified with excess thiol groups. Thi et al. fabricated a gelatin-based hydrogel with dual crosslinking functionality by mixing hydroxyphenyl propionic acid-modified gelatin (GH) with thiolated gelatin (GS).284 GH was crosslinked with HRP/H2O2, and the adhesive strength of the hydrogel increased 6-fold upon addition of GS due to the formation of disulfide crosslinks within the hydrogel and at the hydrogel-tissue interface.284 Engineered polypeptides have also been designed to form hydrogels via disulfide bonds. Shen et al. designed am artificial protein crosslinked by leucine zipper domains.285 It was shown that incorporating thiol groups within the leucine zipper structures allowed for the stabilization of crosslinks due to the formation of disulfide bonds.285
5.3. Reversible Diels-Alder crosslinking
Diels-Alder reactions, which are [4+2] cycloadditions between dienes and a dienophiles, are a widely used class of click chemistry tools (Figure 12a).286 For biomedical applications, the dynamic Diels-Alder reaction between furan and maleimide is of particular interest due to its reversibility at 100°C (i.e., retro-Diels-Alder).287 Hydrogels crosslinked with furan-maleimide Diels-Alder reactions degrade via hydrolysis of the maleimide groups as well as occurrence of the retro-Diels-Alder reaction.288 Synthetic polymers and crosslinkers containing furan and maleimide functionalities have been explored to engineer dynamic hydrogels with thermo-responsive behavior.289,290 To engineer hydrogels, many biopolymers have been modified with furan moieties and subsequently crosslinked with multifunctional maleimide crosslinkers.
Figure 12. Crosslinking via reversible Diels-Alder reactions.
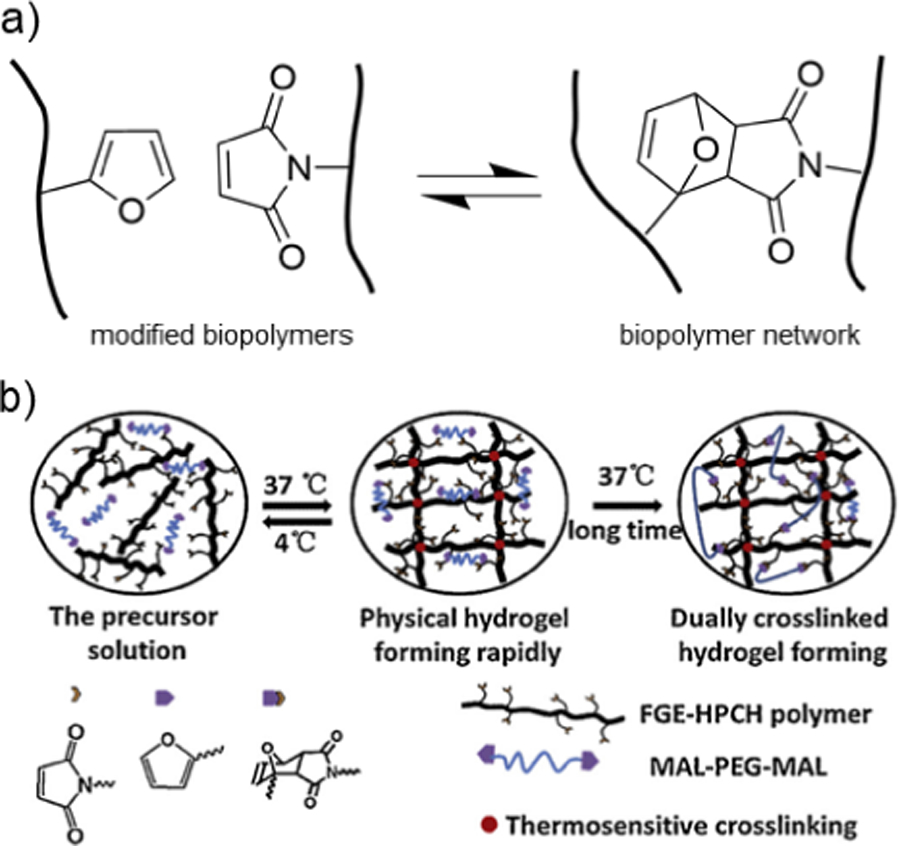
a) Schematic representation of dynamic covalent crosslinks formed by combining biopolymers modified with either furan or maleimide groups. b) Hydroxypropyl chitin (HPC, black) modified with furan groups and combined with PEG-bismaleimide crosslinks for hydrogel formation. Immediately, a thermo-responsive physical hydrogel forms due to interactions between HPC molecules, and over time, reversible Diels-Alder crosslinks form to stabilize the hydrogel structure. Adapted from Bi, et al.296 Copyright, 2019 Elsevier.
Amidation reactions have been used to conjugate furfurylamine groups onto many biopolymers including alginate,291 cellulose,292 chitosan,293 HA,294 and gelatin.295 Etherification and imine formation have also been explored to conjugate furan moieties to chitin296 and chitosan,297 respectively. Furan-modified biopolymers can then be crosslinked by synthetic bismaleimide crosslinkers to form hydrogels.291,296,298 These hydrogels can be thermo- and pH- responsive, and have been explored for tissue engineering scaffolds, drug delivery, and anti-microbial coatings.296,297,299–301 By tuning concentrations, furan-maleimide crosslinked biopolymer hydrogels can be injectable and undergo in situ crosslinking within minutes-to-hours.295,296 While synthetic bismaleimide crosslinkers are commonly used, maleimide groups can also be conjugated to the biopolymer backbone. For example, hydrogels consisting of HA modified with both furan and maleimide groups have been explored for sustained drug release.302
Bi et al. showed that chitin could be modified with furfuryl glycidyl ether to engineer a unique dual-gelation system (Figure 12b).296 At 37°C, physical interactions between chitin molecules result in immediate gelation. When combined with a PEG-based maleimide crosslinker, further gelation occurs over the period of hours-to-days due to long-term Diels-Alder formation. In another strategy, Yu et al. showed that HA could be modified with both furan and tyramine functional groups.303 Subsequent addition of HRP/H2O2 and a PEG-based maleimide crosslinker resulted in immediate gelatin due to enzymatic crosslinking, followed by further gelatin due to the Diels Alder reaction over 24 hours. Bai et al. created a dual-crosslinking system consisting of furan-modified CS, maleimide-modified Pluronic F127, and PEG-based maleimide.300 The formulation underwent rapid gelatin at 37°C due to Pluronic, followed by long-term gelation due to furan-maleimide Diels Alder reactions. The hydrogel showed promise in the field of bone tissue engineering.
6. Physical crosslinking
Physical crosslinking can be achieved by physical interactions between biopolymer chains modified with crosslinking groups. The reversible nature of physical interactions often leads to hydrogels with shear-thinning and self-healing properties, which may allow for injectable hydrogels. While mechanical moduli may be lower relative to hydrogels crosslinked with covalent bonds, utilizing physical crosslinks may allow for increased cell migration and diffusivity within a hydrogel system. Hydrogel assembly through physical interactions often occurs through one-pot mixing of two or more components, allowing for rapid gelation and facile encapsulation of cells or drugs. Such systems also typically do not require catalysts or initiators, which may improve cytocompatibility. Furthermore, some physical interactions can be designed to be stimuli-responsive in order to allow for controlled hydrogel assembly and disassembly or to bind to drugs to control their release from hydrogels. The mechanical and biological properties of physically crosslinked hydrogels depend on the concentrations of biopolymers and crosslinking groups, as well as the binding affinity between chemical groups. In this section, various types of physical crosslinking mechanisms that have been utilized with chemically-modified biopolymers are discussed, including the introduction of guest-host interactions, hydrogen bonding, metal-ligand coordination, and interactions between synthetic polymers grafted to biopolymers.
6.1. Guest-host interactions
Guest-host interactions involve the formation of inclusion complexes between a “host” macrocycle with a hydrophobic interior and one or more hydrophobic “guest” moieties (Figure 13).304 For biopolymer hydrogel formation, naturally-derived cyclodextrins and synthetic cucurbit[n]urils are the most commonly utilized host molecules due to their water-solubility, chemical diversity, low toxicity, cytocompatibility, and history of use.255,305,306 Numerous guest moieties have been explored for hydrogel assembly, some of which include stimuli-responsive properties to allow for controlled assembly and disassembly of the guest-host inclusion complex.305
Figure 13. Crosslinking via guest-host complexation.
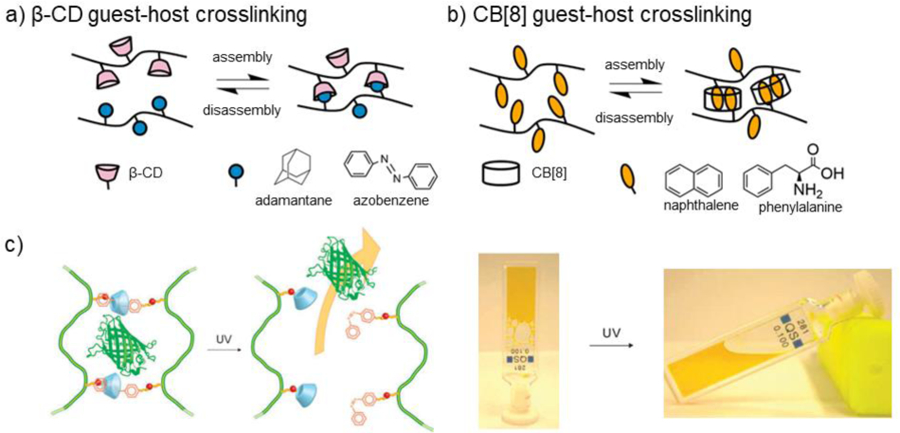
a) Schematic representation of biopolymers (black) modified with either host β-cyclodextrin (β-CD, pink) or guest (blue) functional groups to undergo reversible crosslinking due to guest-host complexation. Common guest groups for β-CD include adamantane (Ad) and azobenzene (Az). b) Schematic representation of biopolymers (black) modified with guest groups (yellow) and combined with host cucurbit[8]uril (CB[8]) to undergo reversible crosslinking due to guest-host complexation. CB[8] has a large host cavity to accommodate two guest groups, which commonly include naphthalene and phenylalanine. c) Dextran (green) is modified with either β-CD (blue) or trans Az (red). Upon mixing, hydrogel formation occurs due to guest-host complexation between β-CD and trans Az. Upon exposure to UV light, Az groups convert from trans to cis state, resulting in hydrogel disassembly and photoresponsive release of encapsulated proteins. Adapted from Peng, et al.123 Copyright, 2010, Royal Society of Chemistry.
6.1.1. Cyclodextrins
Cyclodextrin (CD) is a cyclic macromolecule formed from either 6 (α-CD), 7 (β-CD), or 8 (γ-CD) glucopyranoside units, which has a long history of use for solubilization of drugs.307 The hydrophobic inner cavity of CD forms guest-host inclusion complexes with a variety of guest molecules including adamantane (Ad), cholesterol, azobenzene (Az), and ferrocene (Fc).255 CDs have been incorporated into hydrogels to act as host molecules for the sustained delivery of hydrophobic drugs.308–311 To form shear-thinning, self-healing hydrogels, many biopolymers have been modified with either CDs or guest moieties and subsequently mixed to allow for rapid guest-host mediated assembly (Figure 13a). Examples of biopolymers that have been chemically modified with CD groups include alginate,312,313 cellulose,314,315 chitosan,316 dextran,123 HA,317,318 and keratin.319 Furthermore, gelatin has been mixed with synthetic polymers containing β-CD to form hydrogels due to the guest-host interactions between β-CD and aromatic amino acid residues.320
Ad-CD guest-host interactions have been widely investigated and are known to have an association constant of about 105 M−1, which is relatively high for guest-host complexation.304 HA has been modified with either Ad (AdHA) or CD (CDHA) to form shear-thinning, self-healing guest-host hydrogels upon mixing for injectable tissue scaffolds, drug delivery, and bioprinting inks.321 Many methods have been utilized to conjugate Ad or CD to HA. For example, Rodell et al. demonstrated that carboxylic acid-derivatives of Ad can be conjugated to HA via esterification, and amine-derivatives of CD can be conjugated to HA via amidation.318 Once combined, AdHA and CDHA form injectable, self-healing guest-host hydrogels. Properties of AdHA-CDHA hydrogels depended on the concentration of modified HA, degree of modification, and the molar ratio of guest and host functional groups.318 Furthermore, Ad-CD guest-host networks can be combined with secondary covalent crosslinking to increase network mechanics and stability.196 There are many other guest-host pairings for CD that have been explored for hydrogel formation. In one example, alginate modified with β-CD has been combined with Pluronic F108, forming inclusion complexes between β-CD and the hydrophobic poly(propylene glycol) (PPG) block on the Pluronic.322 The resulting hydrogel had shear-thinning, self-healing properties in addition to increased thermo-responsive gelation due to the presence of the Pluronic.322
To increase the functionality of CD-containing guest-host hydrogels, stimuli-responsive guest molecules have also been explored. Az acts as a guest molecule for CD and undergoes trans-to-cis isomerization in response to UV light. While trans-Az has a high affinity for CD, cis-Az has a low affinity.323 Thus, hydrogels crosslinked with Az-CD inclusion complexes can undergo UV light-responsive disassembly, and subsequent re-assembly upon removal of the UV light.324 For biomedical applications, this system can be used to control scaffold degradability and drug release. UV-responsive Az-CD hydrogels have been formed with chemically-modified alginate,325 dextran,123 and HA.326 For example, Peng et al. showed that dextran can be modified with either β-CD or trans Az for guest-host hydrogel formation (Figure 13c).123 Upon exposure to UV light, Az undergoes a trans to cis isomerization, resulting in hydrogel disassembly and photoresponsive protein release. Fc can also be used as a stimuli-responsive guest molecule for CD.327 Tan et al. demonstrated that β-CD modified alginate could be mixed with Fc modified Pluronic F127 for hydrogel formation.328 NaOCl was used to oxidize Fc to form Fc+, which resulted in disassembly of guest-host complexes.328
6.1.2. Cucurbit[n]urils
Cucurbit[n]urils (CB[n]) are a class of cyclic macromolecules consisting of n glycoluril units that form a hydrophobic cavity with two openings.329 The strength of guest-host interactions between CB[n] and guest molecules depends on the binding affinity and CB[n] cavity size.329 For biomedical applications, CB[6], CB[7], and CB[8] are most often used,330 which have cavity sizes of 164, 279, and 479 Å, respectively.331 CB[8] host molecules provide a large enough cavity to bind two aromatic guest molecules simultaneously.331
Biopolymers have been modified with CB[n] derivatives and guest moieties for hydrogel formation. Park et al. developed an HA hydrogel crosslinked by guest-host complexation between CB[6] and polyamine chemical modifications for application as a tissue scaffold.332 Both diaminohexane (DAH) and spermine modifications were explored as guest molecules due to their high binding affinity with CB[6] (1010-1012 M−1).332 In a follow up study, CB[6]/DAH-HA hydrogels were used to encapsulate MSCs for potential use as an artificial ECM.154 CB[6]/DAH-HA guest-host hydrogels exhibited increased cell viability and retention when compared to Matrigel after 60 days in vivo in an subcutaneous mouse model.154 Furthermore, Sohail et al. engineered a tunable photoluminescent guest-host hydrogel consisting of CB[7] derivatives and alginate modified with dequalinium chloride hydrate guest moieties.333
CB[8] host molecules can accommodate two guest molecules due to the relatively large cavity size. Towards hydrogel formation, biopolymers can be chemically modified with guest moieties and subsequently mixed with free CB[8] compounds to form shear-thinning, self-healing hydrogels (Figure 13b).122,334 For example, cellulose derivatives have been modified with naphthalene and phenylalanine guest molecules for hydrogel formation with CB[8] hosts.122,335 HA modified with phenylalanine has also been shown to form injectable guest-host hydrogels with CB[8].334 Rowland et al. conjugated phenylalanine to HA using Michael addition between methacrylate-functionalized HA and free thiols on a cysteine-phenylalanine compound to form biopolymer hydrogels that showed promise for injectable drug delivery to the brain.336
6.2. Hydrogen bonding
Hydrogen bonding is a physical attractive interaction between hydrogen atoms and electronegative atoms such as oxygen and nitrogen. Many biological processes such as protein folding and DNA base-pairing are driven by hydrogen bonding interactions.337 For hydrogel formation driven by hydrogen bonding, biopolymers can be modified with functional groups such as ureidopyrimidone or gallol (Figure 14).
Figure 14. Crosslinking via hydrogen bonding.
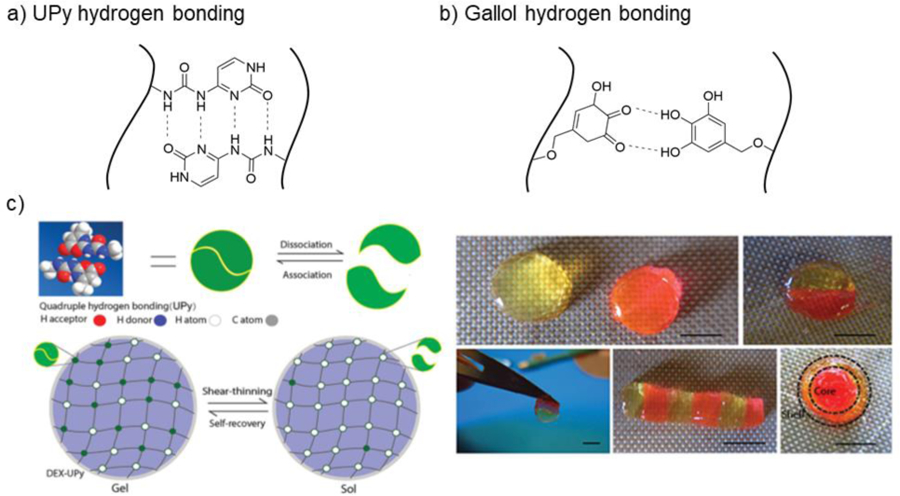
a) Schematic representation of biopolymers modified with ureidopyrimidone (UPy) and crosslinking due to hydrogen bonding. b) Schematic representation of biopolymers modified with gallol groups and crosslinking due to hydrogen bonding. c) Dextran is modified with UPy (Dex-UPy) to undergo hydrogel formation due to hydrogen bonding. The resulting hydrogel is shear-thinning and self-healing. Images show self-healing behavior of Dex-UPy hydrogels. Adapted with permission form Hou, et al.340 Copyright, 2015 Wiley.
6.2.1. Ureidopyrimidone
Ureidopyrimidone (UPy) is a quadruple hydrogen bonding motif that has been used as a dynamic crosslinker to prepare pH- and temperature-responsive injectable hydrogels (Figure 14a).338,339 The quadruple binding motif allows for transient network formation independent of stoichiometry.339 Injectable dextran hydrogels crosslinked by UPy hydrogen bonding have been developed for application in musculoskeletal tissue engineering, where isocyanate-functionalized UPy was conjugated to the hydroxyl groups on dextran (Figure 14c).340 The UPy-dextran hydrogel was able to sustain doxycycline drug release for a week and BSA release for more than a month.340 Gelatin has also been functionalized with UPy through urethane bond formation between isocyanate-modified UPy and amino groups on gelatin to create injectable hydrogels.341,342 Alavijeh et al. developed a UPy-modified gelatin hydrogel that demonstrated shape memory behavior for applications as a controlled drug delivery matrix.341 Furthermore, hydrogels have been formed using UPy-modified cellulose nanocrystals as well as UPy-modified heparin binding peptides.343,344
6.2.2. Gallol
Gallol is an aromatic ring structure with three hydroxyl groups that is associated with fruit browning.345 Gallol moieties can be conjugated to biopolymers for the formation of dynamic hydrogen bonds resulting in shear-thinning, self-healing injectable hydrogels (Figure 14b).346,347 Upon exposure to oxidative conditions (i.e., NaIO4), gallol moieties can convert to quinones for covalent stabilization of networks. Shin et al. developed an ECM-mimetic bioink by modifying both gelatin and HA with gallol moieties via EDC/NHS coupling between biopolymer carboxyl groups and hydroxydopamine.347 Hydrogen bonding between gallol functional groups resulted in a printable hydrogel ink, whereas slow spontaneous oxidation post-printing resulted in covalent stabilization of gallol crosslinks.347
6.3. Metal-ligand coordination
Metal-ligand coordination complexes consist of a central metallic atom surrounded by bounded molecules referred to as ligands. Many biological processes such as self-assembly and adhesion rely on the formation of metal-ligand complexes.348 For example, mussels make use of reversible metal-ligand complexes between catechol moieties and metal ions to form a self-healing protective fluid coating capable of protection against turbulent tidal motion.349 Hydrogel biomaterials inspired by reversible mussel adhesive chemistry have been explored for many applications, including engineered hydrogel actuators and bioprinting.350,351 The reversibility of metal-ligand complexation allows for the formation of shear-thinning, self-healing supramolecular hydrogels.352 Furthermore, hydrogels crosslinked via metal-ligand coordination can have pH-responsive behaviors and reach mechanical moduli near that of covalently bonded hydrogels.353
One of the most common approaches to introduce metal-ligand complexation into biopolymer hydrogels is to first conjugate catechol to the biopolymer backbone, which can then form metal-ligand complexes with many metallic atoms including Fe(III), Cu(II), and Al(III) (Figure 15a).352,354 Chitosan has been modified with catechol moieties and subsequently crosslinked via metal-ligand coordination to form shear-thinning, self-healing hydrogels.352 Injectable, self-healing catechol-chitosan hydrogels crosslinked by Fe(III) metal complexation have been developed as a promising drug delivery vehicle for breast cancer, showing capabilities of sustained release of anti-cancer drugs in vivo in a mouse model for more than 40 days (Figure 15b).355 Furthermore, catechol-modified chitosan hydrogels crosslinked with Fe(III) exhibit pH-responsiveness, adding another degree of tunability to hydrogel functionality.356,357
Figure 15. Crosslinking via metal-ligand complexation.
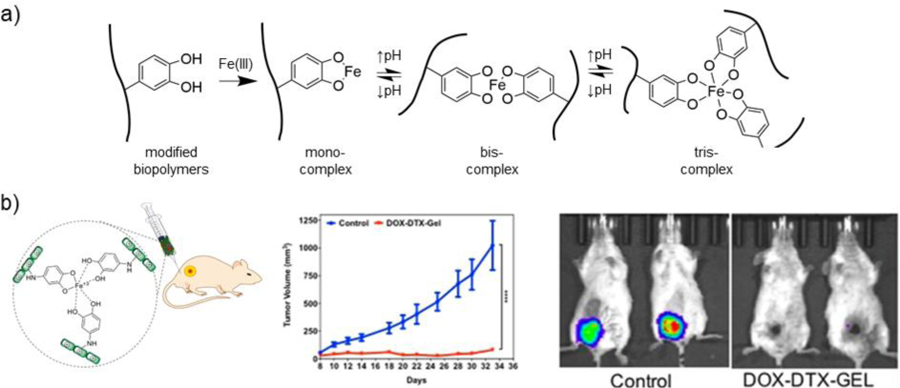
a) Schematic representation of biopolymers modified with catechol groups forming metal-ligand complexes with Fe(III). As pH increases, bis- and tris-complexation occurs, resulting in crosslink formation. b) Chitosan (green) is modified with catechol groups and forms a hydrogel metal-ligand complexation with Fe(III). The injectable hydrogel was used as a localized delivery vehicle for multiple chemotherapeutics. An increase in median survival rate was observed in murine lung and breast cancer models upon localized delivery of anticancer drugs from the hydrogel. Adapted with permission from Yavvari, et al.355 Copyright, 2017 American Chemical Society.
HA hydrogels crosslinked with metal-ligand coordination chemistry have also been developed.351,358 Lee et al. modified HA with catechol moieties using amidation between amino groups on dopamine and carboxyl groups on HA (HA-CA).358 HA-CA was crosslinked by the addition of Fe(III), as well as by oxidative conversion of catechol groups to covalently crosslinked quinones.358 Furthermore, HA-CA/Fe(III) crosslinks exhibited pH-responsiveness.358 Shin et al. developed granular hydrogels by jamming covalently crosslinked HA microgels containing gallol moieties.351 The microgels were mixed with silver nanoparticles (AgNPs) and the gallol groups formed metal-ligand coordination complexes between microgels with the AgNPs351. The resulting injectable hydrogel was conductive due to the presence of AgNPs, with potential applications in bioprinting of electroactive tissue constructs.351
6.4. Grafted biopolymers
To increase the range of functionalities in biopolymer hydrogels, synthetic polymers may be grafted to biopolymer backbones so they may interact with each other for assembly into hydrogels (Figure 16). For biomedical applications, two of the most common synthetic polymers grafted to biopolymers are poly(N-isopropylacrylamide) (PNIPAAm) and Pluronic.
Figure 16. Crosslinking via interactions between synthetic polymers grafted to biopolymers.
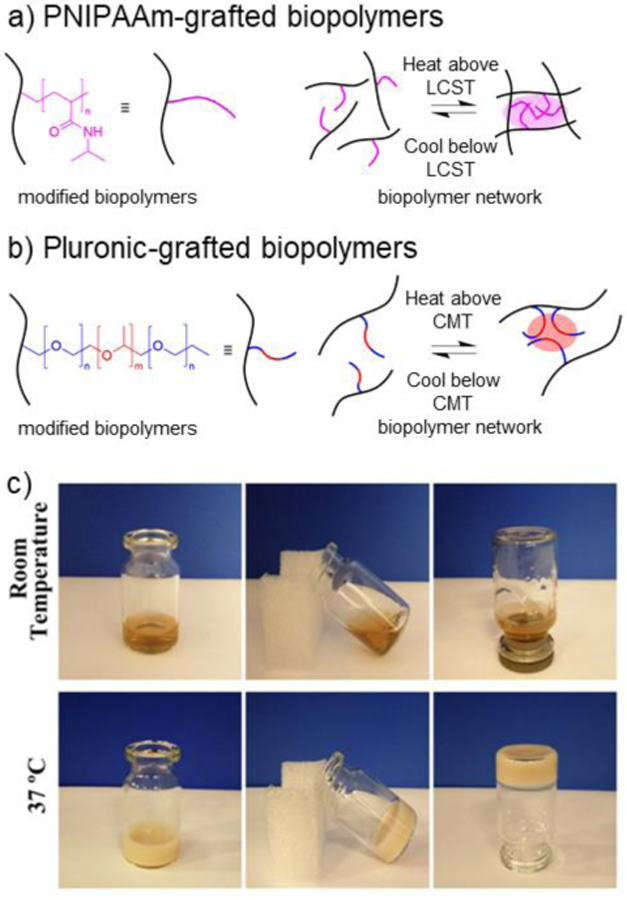
a) Schematic representation of poly(N-isopropylacrylamide) (PNIPAAm, pink) grafted to a biopolymer (black) (left). The grafted biopolymer can undergo reversible physical crosslinking above the lower critical solution temperature (LCST) (~30°C) of PNIPAAm due to hydrophobic interactions between PNIPAAm groups (right). b) Schematic representation of Pluronic grafted to a biopolymer (black). Pluronic is an A-B-A triblock copolymer consisting of poly(ethylene glycol) (PEG) blocks (blue) and poly(propylene glycol) (PPG) blocks (red) (left). The grafted biopolymer can undergo reversible physical crosslinking above the critical micelle temperature (CMT) (~25–40°C) of Pluronic due to hydrophobic interactions between PPG blocks. (right) c) A biopolymer of PNIPAAm grafted to keratin (keratin-g-PNIPAAm) exhibited an LCST around 28–32°C, resulting in thermo-responsive hydrogel formation due to hydrophobic interactions between PNIPAAm groups. Keratin-g-PNIPAAm was explored for applications in brain injury repair. Adapted with permission from Zhu, et al.366 Copyright 2019, Elsevier.
6.4.1. PNIPAAm
PNIPAAm is a synthetic polymer that is characterized by having both hydrophilic amide moieties and hydrophobic propyl moieties.359 PNIPAAm undergoes a reversible low critical solution temperature (LCST) around 34°C. At low temperatures, the amide groups are solvated by water, allowing for the existence of a PNIPAAm aqueous solution.359 Upon exposure to elevated temperature (e.g., >34°C), interactions between propyl hydrophobic groups strengthen, leading to the formation of a physically crosslinked network.359
PNIPAAm has been grafted to many biopolymers including alginate,360 HA,361 CS,362 chitosan,363 silk fibroin,364 and gelatin365 for the formation of thermo-responsive hydrogels (Figure 16a). For example, Zhu et al. grafted PNIPAAm onto keratin by first using thiol-ene radical addition to conjugate NIPAAm monomers onto free thiol groups on keratin, and subsequently polymerizing PNIPAAm off the keratin backbone using free radical kinetic chain polymerization.366 The resulting thermo-sensitive keratin-g-PNIPAAm hydrogel exhibited gelation around 28–32°C, depending on the grafting ratio (Figure 16c).366 The keratin-g-PNIPAAm hydrogel was explored for brain injury repair applications in vivo. In another approach, PNIPAAm has been grafted to HA by amidation between PNIPAAm end-terminated with carboxyl groups (PNIPAAm-COOH) and aminated HA.367 Tan et al. demonstrated that the injectable AHA-g-PNIPAAm hydrogels exhibited rapid gelation above 30°C.367 The hydrogel showed promise for adipose tissue engineering applications both in vitro and in vivo.367 Chitosan has also been functionalized with PNIPAAm using amidation between PNIPAAm-COOH and amine groups on chitosan using EDC/NHS carbodiimide chemistry.368 Yuan et al. combined chitosan-g-PNIPAAm with PEG to fabricate electrospun nanofibers for drug delivery and tissue engineering applications.363 In another example, gelatin-g-PNIPAAm hydrogels have been explored for use as an injectable delivery vehicle for intracameral delivery of the antiglaucoma drug, pilocarpine, in a rabbit glaucoma in vivo model.369
6.4.2. Pluronics
Pluronics are A-B-A triblock copolymers consisting of PEG-PPG-PEG that are manufactured by BASF.370 Pluronics have a critical micelle temperature (CMT) between 25–40°C, forming micelles with a hydrophobic core.370 Biopolymers grafted with Pluronics can form injectable hydrogels that undergo thermo-responsive gelation upon exposure to physiological temperatures (Figure 16b).371 The most common Pluronic used in hydrogel formation is Pluronic F127 (PEG100-PPG65-PEG100) due to its hydrophilicity at low temperatures.370,371 Many biopolymers have been modified with Pluronics for scaffold formation and drug delivery, including HA,372 chitosan,373 heparin,374 and gelatin.375 Lee et al. demonstrated that Pluronic F127 capped with amine groups could be grafted to carboxyl groups on HA using EDC/HOBt amidation.372 Bovine chondrocytes were encapsulated in the HA-g-F127 hydrogel for application as an injectable cell carrier for cartilage regenartion.372 Chitosan-g-F127 hydrogels have also been explored as an injectable hydrogel for cartilage repair.376 Park et al. showed that chitosan-g-F127 hydrogels reached gelation at a temperature of 25°C and could reach a storage modulus of 104 Pa.376
7. Recent applications of biopolymer hydrogels to biomedicine
Biopolymer hydrogels from chemically-modified biopolymers have been widely used across biomedical applications for several decades. In previous sections, we briefly mentioned some of these applications in the context of specific biopolymer modifications and crosslinking methods. In this section, we review representative recent advances (last 5 years) that have been made using chemically-modified biopolymer hydrogels, including for the biofabrication of tissue scaffolds, therapeutic molecule delivery, tissue adhesives and sealants, and the formation of interpenetrating network hydrogels. Although it is not possible to be comprehensive with all of the biomedical examples of biopolymer hydrogels, the studies discussed throughout this section were selected to highlight diverse biopolymers and chemical modifications and to emphasize examples where these biopolymer modifications were integral to the application.
7.1. Biofabrication of hydrogel scaffolds
7.1.1. Bioprinted scaffolds
Bioprinting has rapidly evolved as a widely adopted biofabrication technique to engineer tissue constructs with complex microarchitectures. Chemically-modified biopolymer hydrogels have been developed as bioinks to embed cells and bioprint tissue constructs, particularly with photocrosslinkable biopolymers to obtain stable tissue constructs.377
Traditional extrusion-based bioprinting utilizes viscous, shear-thinning photocrosslinkable bioinks to print constructs that are crosslinked by light exposure post-deposition.377 GelMA has been widely explored as a bioink for extrusion-based bioprinting. For example, Bejleri et al. bioprinted a cardiac patch composed of GelMA, decellularized cardiac ECM, and human cardiac progenitor cells.378 The addition of cardiac ECM to the GelMA bioink improved printability and the pro-angiogenic potential of embedded cells. The bioprinted patches were evaluated in an in vivo rat model and supported vascularization after 14 days. Towards musculoskeletal tissue engineering applications, Cidonio et al. engineered a bioink composed of GelMA, Laponite® nanoclay, and hMSCs for bone defect repair.379 The incorporation of nanoclay improved print fidelity when compared to GelMA alone, and the addition of VEGF in the scaffold resulted in increased vascularization in an ex vivo chick chorioallantoic model.
HA bioinks have also been widely adopted in bioprinting. For example, Petta et al. developed bioinks from HA-Tyr for extrusion-based bioprinting.380 Various cell types, including hMSCs and bovine chondrocytes, were encapsulated in HA-Tyr bioinks that were lightly enzymatically crosslinked with HRP/H2O2 before printing. After deposition, constructs were stabilized by secondary photocrosslinking and supported high cell viability with culture. In another approach, Kuss et al. combined MeHA, GelMA, polycaprolactone (PCL), and hydroxyapatite to engineer an extrusion bioprinted scaffold for craniofacial defect repair.381 Hybrid scaffolds were fabricated with layers of PCL/hydroxyapatite and then MeHA/GelMA containing embedded stromal vascular fraction of adipose tissue. After short-term culture in hypoxic conditions in vitro, the scaffolds were subcutaneously implanted into an in vivo mouse model and demonstrated microvasculature formation 4 weeks post-implantation. In another approach, Kesti et al. combined HA-g-PNIPAAm with MeHA to create a dually-crosslinked hydrogel.382 The rapid gelation of printed constructs occurred upon contact with a substrate at 37°C, providing immediate structural support for the constructs. Subsequent free radical chain polymerization of methacrylate groups provided long-term mechanical stability.382
Numerous other biopolymers have been chemically modified for use as extrusion-based 3D printing bioinks. For example, methacrylated alginate, methacrylated chondroitin sulfate, and GelMA have been combined with a graphene oxide nanofiller for use as a multicomponent photocrosslinkable bioink, which was explored for cartilage tissue engineering.383 Recently, norbornene-modified cellulose bioinks were engineered by functionalizing cellulose with a norbornene group using either amide or ester bond formation (Figure 4).182 The bioink was photocrosslinkable by thiol-ene radical addition and showed high cell viability for cell-laden bioprinted scaffolds.
One of the challenges with traditional extrusion-based bioprinting is the need for high bioink viscosity or rheological additives to ensure high print fidelity, which limits the use of many materials.377 To combat this, extrusion bioprinting with in situ crosslinking has emerged as a promising technique to permit extrusion-based printing of non-viscous bioinks.131,384 In this approach, bioinks are cured by photocrosslinking during the deposition process through the use of photopermeable capillary tubes. Ouyang et al. demonstrated that a wide variety of non-viscous bioinks, including GelMA, MeHA, and NorHA, could be printed using in situ crosslinking.384 Fibroblasts embedded in the non-viscous biopolymer inks exhibited high viability (>90%) post-printing. Galarraga et al. later showed that MSCs could be encapsulated in non-viscous NorHA bioinks to fabricate bioprinted constructs with in situ crosslinking for cartilage tissue engineering.131 Bioprinted constructs were cultured in chondrogenic media for 56 days, allowing for the formation of cartilaginous tissue. In a recent study, gelatin additives were used to process a wide variety of modified biopolymer bioinks, where the gelatin could be subsequently removed through heating after the printing was complete.385 This approach was used to bioprint soft hydrogels that were favorable for cell culture, but that would have been challenging to print otherwise. In another approach, Heo et al. developed a carbohydrazide-modified gelatin (Gel-CDH) bioink that was extruded into an oxidized alginate (OAlg) bath.386 Upon deposition of the bioink, hydrazide bonds formed in situ to stabilize the construct, allowing for good mechanical integrity of the scaffold as well as high cell viability when a cell-laden Gel-CDH bioink was used.
Beyond extrusion-based printing, lithography-based bioprinting has emerged as a promising biofabrication technique for printing constructs with non-viscous bioinks.387 Lithography-based bioprinting allows for the fabrication of constructs with high accuracy and precise spatial patterning.387 Dynamic light processing (DLP) is a technique that uses a digital light projector to selectively photocrosslink a bioresin on a computerized stage.377 The stage moves stepwise in the z direction to allow for layer-by-layer fabrication of constructs with significantly higher spatial resolution that extrusion-based techniques.
Biopolymers with photocrosslinkable groups have been explored as non-viscous bioinks for DLP-based biofabrication of tissue constructs. Zhu et al. engineered a bioink consisting of GelMA and GMA-HA with embedded human umbilical vein endothelial cells (HUVECs) and MSCs.388 DLP biofabrication was used to create prevascularized constructs by patterning complex microarchitectures into the HA/gelatin scaffold. Scaffolds exhibited high cell viability over 1 week in vitro. Constructs were subcutaneously implanted in vivo in a mouse model, and significantly improved vascular density was observed 2 weeks post-implantation when compared to a non-prevascularized control. In another example, Bertlein et al. developed a thiol-ene clickable Gel-AGE scaffold.187 The Gel-AGE bioink was used to bioprint constructs with high print fidelity using both extrusion-based and DLP bioprinting.
Kim et al. engineered a methacrylated silk fibroin bioink for scaffold formation using DLP biofabrication (Figure 17a).389 By tuning bioink composition, bioprinted scaffolds with a compressive modulus greater that 120 kPa were achieved. The use of DLP biofabrication allowed for the printing of precise, complex structures, including brain, ear, trachea, lung, and heart-shaped scaffolds. It was shown that fibroblasts could be encapsulated in the silk-based bioink and maintained with high viability throughout cultures up to 14 days. In another approach, Placone et al. engineered a photocrosslinkable keratin hydrogel scaffold using DLP biofabrication, which showed good print resolution and high biocompatibility.390
Figure 17: Representative examples of biofabricated hydrogel scaffolds made from chemically-modified biopolymers.
a) Silk modified with GMA (Sil-MA) is photocrosslinked using LAP as a photoinitiator (left). Scaffolds that mimic the shape of trachea, heart, lung, and vessel are printed using dynamic light processing (DLP) (right). Adapted with permission from Kim et al.389 Copyright, 2018 Springer Nature. b) GelMA (red) and ChitoMA (gray) microgels are fabricated using a microfluidic device and then mixed to form a self-healing granular hydrogel scaffold with ionic interparticle interactions. The scaffold is combined with human adipose-derived stem cells (hADSCs) to form a cell-laden network. Adapted with permission from Hsu, et al.401 Copyright 2019, Wiley. c) HA modified with norbornene (NorHA) and either hydrazides (NorHA-Hyd, red) or aldehydes (NorHA-Ald, green) are electrospun to create a multifiber network with dynamic covalent inter-fiber crosslinks (left). Luminal scaffolds are created by wrapping the multifiber network around a needle and visualized i) while removing the scaffold from the needle and ii) while extruding rhodamine-labeled dextran dye through the lumen. Adapted with permission from Davidson et al.415 Copyright, 2020 Wiley.
Stereolithography (SLA) is another lithographic printing approach that uses a focused laser light to crosslink specific areas in a resin vat in order to build a 3D structure.377 Smith et al. used methacrylated BSA (MA-BSA) to form an albumin-based scaffold via SLA biofabrication that demonstrated high fibroblast cell viability.391 Further, the albumin constructs were subject to thermal incubation in order to disrupt native albumin structure and introduce physical crosslinks between denatured albumin chains in addition to chemical crosslinks between methacrylate groups.
7.1.2. Granular hydrogel scaffolds
Granular hydrogels consist of hydrogel microparticles that are agglomerated into a jammed state.392 Hydrogel microparticles may be fabricated by various methods, including microfluidic devices, batch emulsions, lithography, and mechanical fragmentation.392 Granular hydrogels are injectable, microporous, and modular, making them a promising tool for engineering tissue scaffolds.392 Furthermore, interparticle crosslinks can be introduced between hydrogel microparticles to further stabilize granular hydrogel scaffolds.392 Granular hydrogel scaffolds fabricated from PEG have been explored for multiple applications, including wound closure.393,394 Recently, hydrogel microparticles fabricated from chemically-modified biopolymers have shown promise for the fabrication of granular hydrogel scaffolds.
HA microparticles have been widely explored for the fabrication of granular hydrogels. For example, acrylated HA microparticles have been formed by Michael addition in the presence of MMP-degradable dithiol crosslinkers using a microfluidic device.200,395 The HA microparticles could be annealed by enzymatic crosslinking, free radical chain polymerization, or amidation reactions between microparticles.395 The resulting granular hydrogel is termed a microporous annealed particle (MAP) scaffold: a granular hydrogel consisting of hydrogel microparticles annealed by interparticle crosslinking.393 HA MAP scaffolds annealed by enzymatic interparticle crosslinking have been shown to decrease scar formation and inflammation when injected into an in vivo mouse stroke model.200 MAP scaffolds have also been fabricated by NorHA microparticles with tetra-PEG-tetrazine, resulting in tetrazine-norbornene crosslinks between microparticles.396,397 The microparticles were fabricated in a water-in-oil batch emulsion and subsequently filtered to obtain a narrow size distribution. These tetrazine-norbornene annealed granular hydrogel scaffolds have been used to study polyplex-mediated gene delivery in vitro and have shown promise in stroke recovery applications.396,397
To fabricate injectable HA granular hydrogels, NorHA modified with Ad (AdNorHA) has been used to fabricate microparticles in a microfluidic device.398 AdNorHA microparticles were subsequently jammed by vacuum filtration in the presence of CDHA to create a shear-thinning, self-healing granular hydrogel with guest-host interparticle crosslinks.398 The hydrogel was used to study the disease-dependent behavior of hydrogel degradation in the myocardial infarction microenvironment using an in vivo rat model.398 Furthermore, it has been shown that granular hydrogels formed from jamming NorHA microparticles can be used as bioinks for bioprinting of microporous tissue structures.172
Many other biopolymers have been explored for granular hydrogel formation. Injectable granular hydrogels fabricated from cell-laden, norbornene-modified gelatin microparticles have been explored for hyaline cartilage tissue engineering.399 It was shown that the incorporation of 4-arm PEG-NHS between microparticles led to interparticle crosslinking for scaffold stabilization due to the formation of amide bonds.399 GelMA microparticles have been fabricated in a microfluidic device and subsequently annealed following additional UV light-mediated free radical crosslinking between microparticles.400 Within the granular hydrogels, stiffness and porosity could be tuned independently.400 It was shown that HUVEC cell migration into the GelMA granular hydrogel in vitro was significantly higher than for bulk GelMA controls.400 Furthermore, granular hydrogels have been fabricated by mixed microparticles formed from GelMA and methacrylated chitosan (ChitoMA), where ionic interactions between negatively-charged GelMA microparticles and positively-charged chitosan microparticles increased scaffold stability and mechanical properties (Figure 17b).401 The GelMA-ChitoMA granular hydrogels were used to support Schwann cell migration and axon growth in an in vivo sciatic nerve defect rat model.401
In another strategy, bulk hydrogels made from crosslinked cellulose nanofibrils (CNFs) were extruded through a microscale mesh to create granular gels with microscale porosity.402 The granular CNF gel exhibited increased fibroblast migration throughout the scaffold when compared to the control bulk hydrogel.402 In a recent study, granular hydrogels were formed by extruding bulk hydrogels through a mesh to create hydrogel microstrands, which were combined with cells for use as a cell-laden bioink.403 The microstrand fabrication process was explored using multiple biopolymers, including HA, gelatin, and carrageenan, a linear sulfated polysaccharide extracted from red seaweed.
7.1.3. Electrospun hydrogel scaffolds
Electrospinning is a method to fabricate fibrous materials by ejecting charged polymer solutions through a spinneret under a high-voltage electric field. The polymer solution solidifies through solvent evaporation, resulting in a stable nanofiber filament. Towards biomedical applications, electrospun biopolymers are of interest due to their ability to mimic the native fibrillar properties of the ECM.404 Hydrogels can be formed by crosslinking electrospun biopolymer fibers to form cellular scaffolds and drug delivery vehicles.404,405 There are many examples of hydrogels formed by crosslinking electrospun fibers made from non-modified biopolymers. For example, alginate fibers can be ionically crosslinked by immersion in Ca2+ solutions,406 fibrin fibers can be enzymatically crosslinked in the presence of thrombin,406 and silk fibroin fibers can be crosslinked by β-sheet formation.407
Towards the fabrication of chemically-modified biopolymer scaffolds made from electrospun fibers, GelMA and MeHA have been widely used to form fibrous scaffolds via free radical chain polymerization for crosslinking within and between fibers.152,408–412 For example, Sun et al. fabricated electrospun GelMA fibers that were subsequently photocrosslinked to form a fibrous hydrogel scaffold.410 The scaffold was evaluated in vivo, demonstrating increased microvasculature formation when compared to controls. In another example, Chen et al. fabricated an electrospun GelMA fibrous hydrogel scaffold for spinal cord repair and regeneration.411 Song et al. fabricated electrospun MeHA hydrogel scaffolds to investigate the influence of fibrous matrix stiffness on meniscal cell migration.412 Fibers were electrospun from MeHA with different degrees of modification to make soft (30% modification) and stiff (97% modification) MeHA fibrous hydrogel networks.412 The stiffer MeHA scaffolds supported enhanced cell invasion and collagen deposition when compared to the softer MeHA scaffolds. In another approach, Zhou et al. fabricated an electrospun scaffold from methacrylated chitosan that could be post-crosslinked using UV light to form a stable construct for potential use as a skin repair matrix.413
Other crosslinking chemistries have been explored to create biopolymer hydrogels from electrospun fibers. Zhang et al. fabricated electrospun fibers from solutions of alginate and thiolated-HA.406 The fibers were subsequently dually crosslinked by immersing in a solution of CaCl2 (ionic crosslinking) and PEGDA (for Michael addition crosslinking). Furthermore, NorHA has been electrospun and crosslinked with thiol-ene radical addition to form hydrogels.414 Davidson et al. developed a multifiber hydrogel network consisting of electrospun NorHA that was further modified with either hydrazide groups (NorHA-Hyd) or aldehyde groups (NorHA-Ald) (Figure 17c).415 NorHA-Hyd and NorHA-Ald were co-electrospun to create a multifiber network. Post-electrospinning, norbornene groups were crosslinked within and between fibers through thiol-ene radical addition in order to stabilize the fibrous scaffolds. Dynamic covalent interactions due to hydrazone crosslink formation between fibers resulted in increased scaffold stiffness and plastic deformation, permitting scaffold self-adhesion and the formation of layered and tubular constructs.
7.2. Therapeutic delivery
Injectable hydrogels have been widely used for localized and sustained delivery of therapeutics, including small molecules, nucleic acids, and proteins.416,417 To achieve sustained delivery by limiting passive diffusion, therapeutic molecules are often either physically bound to a hydrogel or encapsulated in nano- or microparticles within a hydrogel. However, larger therapeutic molecules, such as proteins, may be directly encapsulated within hydrogels for localized and sustained delivery.
CD guest-host complexation has been explored for sustained release of hydrophobic molecules.418 In addition, therapeutics such as nucleic acids can be modified with hydrophobic groups such as cholesterol to undergo guest-host complexation with CD.419 The reversible physical association between CD and hydrophobic therapeutic molecules allows for sustained release over days to weeks. Injectable HA hydrogels with β-CD moieties have been engineered for sustained release of cholesterol-modified RNAs.420,421 For example, shear-thinning, self-healing guest-host HA hydrogels were formed by mixing CDHA and AdHA, and cholesterol-modified miRNA formed guest-host inclusion complexes with unoccupied CD hosts.420 The injectable hydrogel sustained the delivery of miRNA to promote cardiomyocyte proliferation in an in vivo mouse myocardial infarction (MI) model. In another approach, dynamic covalent HA hydrogels were formed by mixing hydrazide-modified HA (HA-ADH) with aldehyde-modified HA (HA-ALD).421 The HA-ALD polymers also contained a CD (CD-ALD-HA) moiety for guest-host complexation with cholesterol-modified siRNA for sustained release. The hydrogel was used to deliver siRNA against MMP2 in order to prevent pathological remodeling in a rat MI model. Towards sustained release of small molecule drugs, Thi et al. engineered a tyramine-modified gelatin hydrogel that underwent enzymatic crosslinking in the presence of HRP/H2O2.422 Oxidized β-CD molecules were encapsulated within the hydrogel, as well as model hydrophobic drugs including dexamethasone and curcumin. The hydrophobic model drugs formed inclusion complexes with the oxidized β-CD, and the aldehyde groups on the oxidized β-CD formed dynamic imine crosslinks with free amino groups on gelatin. Furthermore, incorporation of aldehyde groups from oxidized β-CD resulted in significantly increased tissue adhesion.
In another approach to achieve sustained delivery, therapeutics may be entrapped within a nanoparticle, which is then encapsulated in a biopolymer hydrogel. Segovia et al. developed a dextran-based hydrogel for nanoparticle-mediated delivery of siRNA as an anticancer therapeutic.423 The siRNA formed nanoparticles by aggregation with poly(amidoamine) (PAMAM) dendrimers. The nanoparticles were mixed with oxidized dextran to form a dynamic covalent hydrogel with imine linkages between aldehyde groups on dextran and amine groups on the PAMAM nanoparticles. The hydrogel was used to deliver anti-luciferase siRNA in an in vivo xenograft mouse model of human breast cancer that contained luciferase-expressing tumor cells. Sustained release of anti-luciferase siRNA resulted in 70% luciferase silencing after 6 days, reaching improved transfection efficiencies when compared to commercially available siRNA-delivery agents. In a later study, the injectable aldehyde-dextran hydrogel was used to complex miRNA-PAMAM nanoparticles for sustained release of miRNA, leading to nearly 90% tumor shrinkage 2 weeks post-implant in a triple negative murine breast cancer model.424
To facilitate sustained delivery, therapeutic molecules may be encapsulated in a hydrogel microparticle. Chen et al. demonstrated that interleukin 10 (IL-10) could be encapsulated in NorHA microgels formed in a microfluidic device by photo-mediated thiol-ene radical addition.425 The NorHA microgels were incorporated into a CDHA-AdHA guest-host hydrogel to form a composite injectable delivery vehicle for sustained release of IL-10 in an in vivo rat MI model. Reduced macrophage density, as well as improved scar thickness and ejection fraction, were observed 4 weeks post-injection.
As an example of encapsulating and releasing molecules directly from hydrogels, Schirmer et al. engineered a heparin-based hydrogel for sustained delivery of interleukin 4 (IL-4) to facilitate improved wound healing.426 The hydrogel was formed via Michael addition by mixing maleimide-modified heparin with thiol-modified 4-arm star PEG. Heparin was selected due to the physical complexation that occurs between heparin and IL-4. Heparin acts as a stabilizer to protect IL-4 against thermal and proteolytic degradation, resulting in more sustained delivery of the therapeutic. In an in vitro study, primary murine macrophages adopted the pro-wound healing M2 phenotype upon exposure to the heparin hydrogels containing IL-4. In another example of hydrogel-based protein delivery, Turabee et al. developed a novel polypeptide triblock copolymer that undergoes thermal- and pH-responsive gelation.427 The polypeptide triblock copolymer contained a PEG block, a temperature-sensitive poly(γ-benzyl-L-glutamate) polypeptide block, and a pH-responsive oligo(sulfamethazine) block. The triblock copolymer formed a viscoelastic hydrogel upon subcutaneous injection into a rat model. Sustained release of lysozyme, a model protein, was demonstrated over a 1-week period in vivo.
7.3. Tissue adhesives and sealants
Hydrogel-based tissue adhesives and sealants have been researched for decades as alternatives to staples and sutures in order to promote improved wound closure and incision sealing.428 These hydrogels must be able to adhere to wet surfaces, withstand dynamic forces, and exhibit biocompatibility.428,429 Ideally, adhesives and sealants should be engineered to meet the needs of specific tissue and wound types.429 Many tissue adhesives fabricated from biopolymers have had clinical success, such as Tisseel (fibrin), Evicel (fibrin), PreveLeak (BSA), BioGlue (BSA), and LifeSeal (gelatin).429 Recent advances in hydrogels fabricated from chemically-modified biopolymers have furthered research into new tissue adhesives and sealants.
Adhesives and sealants fabricated from gelatin hydrogels show promise in research and clinical settings. It has been shown that photocrosslinked GelMA hydrogels can be used as tissue adhesives and sealants that exhibit a low inflammatory response, degrade quickly, and allow for rapid wound healing in vivo.430 In an in vivo rat lung incision model, GelMA sealant was able to recover healthy lung burst pressures 7 days after application.430 Tayafoghi et al. showed that combining GelMA with methacrylated alginate resulted in an injectable, photo-crosslinkable tissue adhesive hydrogel.431 The incorporation of an ionically crosslinked alginate network resulted in a 600% improvement in hydrogel toughness when compared to GelMA alone.431 Wei et al. engineered a mussel-inspired gelatin adhesive hydrogel, where gelatin was modified with isothiocyanate-functionalized dopamine by thiocarbamate bond formation.432 The resulting gelatin hydrogel contained both catechol and thiourea functional groups, where the thiourea groups crosslinked catechol moieties and also reduced oxidized quinone groups on catechol.432 The mussel-inspired gelatin hydrogel achieved the same adhesion strength as commercially available Tisseel adhesives in a T-peel porcine pericardium tissue assay.432
Chemically-modified HA has also been researched for applications in tissue adhesives and sealants. Kim et al. engineered an injectable and sprayable HA-Tyr hydrogel tissue adhesive.433 The hydrogel was biocompatible in an in vivo mouse subcutaneous model, and strong adhesion strengths were observed in an ex vivo mouse skin adhesion assay.433 In another approach, Bermejo-Velasco et al. modified HA with either enolizable or non-enolizable aldehyde groups to form a tissue adhesive hydrogel.434 Combining the two biopolymers resulted in rapid gelation due to the formation of aldol crosslinks.434 Residual aldehydes remaining after crosslinking resulted in tissue adhesive properties, as demonstrated by successful bonding of bone tissues ex vivo.434
Other biopolymers are being explored for fabrication of tissue adhesives and sealants. Hong et al. recently demonstrated that alginate could be modified with boronic acid derivatives to undergo pH-responsive curing and adhesion.435 The hydrogel showed strong adhesive strength to mouse intestinal tissue in an ex vivo study.435 Azuma et al. demonstrated that methacrylated chitin combined with chitin nanofibers could achieve faster tissue adhesion than some commercially available tissue adhesives in vivo.436 Annabi et al. engineered a methacrylated tropoelastin tissue adhesive from human recombinant tropoelastin.437 The hydrogel successfully sealed rat arteries and lungs in an in vivo model.437 Rat lungs sealed by the hydrogel exhibited higher burst pressure over lungs sealed with Evicel and Cosseal.437 In another approach, Wang et al. fabricated an adhesive, photocrosslinkable hydrogel by modifying chitosan with both methacryloyl and catechol groups.438 The hydrogel was explored for use as an antibacterial wound dressing.
7.4. Interpenetrating network biopolymer hydrogels
Most of the hydrogels discussed above consisted of single networks of biopolymers that are crosslinked together. Although different biopolymers may have been combined (e.g., CDHA and AdHA to form a guest-host network), the resulting hydrogel was formed from a single network. To build additional complexity into biopolymer hydrogel design, the development of interpenetrating polymer networks (IPNs) allows for further modulation of hydrogel behavior by combining properties of more than one network (Figure 18a). Utilizing IPNs increases the range of possible chemical, mechanical, and biological behaviors within a single hydrogel material. IPN hydrogels involve the combination of multiple independent polymer networks to form hydrogels, where the individual networks are inter-mixed, but not linked together. IPN hydrogels from biopolymers are reviewed in detail elsewhere.439
Figure 18. Interpenetrating network (IPN) biopolymer hydrogels.
a) IPN hydrogels are formed through various synthesis techniques, including the sequential (swelling of first network in a secondary monomer/macromer) or simultaneous (orthogonal crosslinking of both first and second networks) introduction of networks. Adapted with permission from Dhand, et al.439 Copyright 2020, Elsevier. b) An IPN is formed by combining bis-maleimide-PEG, furan-modified gelatin (Gel-Furan), and chitosan grafted with Pluronic F127 (Chitosan-g-Pluronic). Initially, Chitosan-g-Pluronic formed a physically crosslinked, thermosensitive hydrogel network. After 2 h, Diels-Alder crosslinks between bis-maleimide-PEG and Gel-Furan covalently stabilize the hydrogel. Adapted with permission from Abandansari, et al.442 Copyright 2018, Elsevier.
Biopolymers and synthetic polymers may be combined in an IPN hydrogel to take advantage of favorable properties from both networks. For example, Gan et al. engineered an IPN for nucleus pulposus replacement with both natural and synthetic polymer components.440 The primary network consisted of oxidized dextran mixed with amine-modified gelatin crosslinked by imine bond formation, which allowed for hydrogel bioactivity. The secondary network consisted of photocrosslinkable acrylated PEG, which allowed for increased mechanical stability. In another approach, Zhao et al. combined a synthetic catechol-modified polymer network that crosslinked by metal-ligand coordination with UPy-modified gelatin, which crosslinked by quadruple hydrogen bonding, to create an adhesive wound dressing with fast shape adaptability and self-healing properties.441 The catechol-modified synthetic polymer network allowed for strong adhesion, and the UPy-modified gelatin allowed for integration of biological signals and rapid self-healing behavior. As a last example, Abandansari et al. fabricated an IPN consisting of bis-maleimide-PEG, furan-modified gelatin (Gel-Furan), and Chitosan-g-Pluronic (CP) (Figure 18b).442 The physically-crosslinked CP network allowed for immediate stabilization of the injected hydrogel as well as thermo-responsive behavior, while the Mal-PEG-Mal and Gel-Furan network allowed for long-term covalent stabilization due to Diels-Alder crosslinking after multiple hours. The hydrogel showed promise for use as a thermosensitive, injectable cell delivery vehicle.
IPNs can also be designed such that both networks are formed from biopolymers. For example, Suo et al. combined photocrosslinkable GelMA and physically crosslinked chitosan into a single network.443 In another strategy, Chen et al. combined a modified collagen network crosslinked by azide-alkyne cycloaddition with a modified HA network crosslinked by Michael addition for use as an injectable, in situ-forming corneal defect filler.444
IPNs have been useful for the formation of complex stimuli-responsive hydrogels. In this approach, one network provides the responsiveness to stimuli, whereas the other network maintains hydrogel stability or controls hydrogel properties. For example, heparin was incorporated into IPN hydrogels with PNIPAAm to allow the formation of thermo-responsive hydrogels for the controlled release of growth factors.445 As another example, protease-sensitive crosslinks can be introduced into one network of an IPN hydrogel to allow for enzyme-responsive behavior.446,447
One specific class of IPN hydrogels are double network (DN) hydrogels, which consist of two networks that have asymmetric and contrasting properties and where the molar concentration of the secondary network is often >20 times that of the primary network. The first network is sacrificial and brittle, while the second network is ductile, allowing for the formation of hydrogels with high strength and toughness. This is due to the protection of the secondary network from fracture by the first network via energy dissipation. Although not with biopolymers, the first reports of DN hydrogels were from Gong and colleagues.448
A wide range of biopolymers have been processed into DN hydrogels, using the chemical modifications described above. For example, HA has been modified with either catechol or methacrylate groups and processed into DN hydrogels, through mussel-inspired and reversible Fe3+-catechol interactions and free-radical chain polymerization, respectively.449 In another strategy, Xiao et al. combined silk fibroin and methacrylated HA to fabricate a DN hydrogel for cell encapsulation.450 In another example, chitosan networks were combined with GelMA networks to form DN hydrogels that were explored for cartilage repair.443 There is significant potential to increase the diversity of properties in biopolymer hydrogels through the formation of IPNs.
8. Concluding remarks and future outlook
Our intent with this review was to motivate the use of biopolymers, their chemical modifications, and their crosslinking to form hydrogels for various biomedical applications. Numerous conjugation techniques have been utilized to introduce a range of chemical modifications onto biopolymers, where the type of modification, extent of modification, and concentration of components drive the resulting hydrogel properties. For example, we classify the types of crosslinking as covalent, dynamic covalent, and physical, with generally reduced mechanics and stability across these classifications.
Biopolymer hydrogels have been implemented in diverse biomedical applications, and we provide examples throughout on their use as cell culture substrates, scaffolds for tissue engineering, drug delivery vehicles, and tissue adhesives, where the selection of biopolymer and chemical modification drives the hydrogel utility. Evolution in biopolymer hydrogel complexity and control will advance their usefulness in these applications, as well as expand their utility into other applications. As one example, IPN biopolymer hydrogels are allowing for the combination of features of independent hydrogel networks. Further, the engineering of new polypeptides may introduce new properties that are not attainable with sourced biopolymers.
Despite these advances, we must pay attention to the balance between complexity and simplicity in biopolymer hydrogel design, particularly with regards to translational use. Bearing this in mind, there are a number of factors that should be considered when designing a chemically-modified biopolymer hydrogel for biomedical applications, particularly with translation in mind. For example, when selecting a biopolymer for biomedical hydrogel formation, one may consider whether cell adhesion sites are required for successful hydrogel function. If so, biopolymers such as HA, gelatin, and keratin that contain native cell adhesion sequences may be favorable. While cell adhesion peptides such as RGD may be easily conjugated to other biopolymers in a laboratory setting, such a step would require more elaborate processing on a larger scale, potentially hindering translation.
Batch variability is another important factor to consider when selecting a biopolymer for hydrogel formation. When extracting biopolymers from any natural source, either plant or animal, there is concern for batch-to-batch variability between sources. Biopolymers that can be mass-produced from microbial manufacturing, such as dextran, HA, and cellulose, may mitigate concerns over batch variability. In addition, one may consider the environmental sustainability of the biopolymer source. Biopolymers such as cellulose, chitin, keratin, and alginate are naturally abundant, providing plentiful green resources for hydrogel raw materials. In contrast, biopolymers isolated from animal sources are viewed as less sustainable. For example, commercial gelatin is mostly isolated from porcine and bovine sources and relies heavily on production in the meat industry. There is growing concern over the unsustainable nature of meat production.451 Thus, there is a need to develop alternate sustainable methods for manufacturing biopolymers that are traditionally obtained from animal sources.452,453
Another aspect to consider is the water solubility of the biopolymer. For example, some biopolymers such as cellulose, chitin, silk fibroin, and keratin are hydrophobic in their native states. Thus, extensive processing is required to create water-soluble biopolymer derivatives that can be processed into a hydrogel. While some of these processes have been successfully implemented on a large scale, there is growing concern over the environmental impact of these processes, motivating the need for the eco-friendly synthesis of water-soluble derivatives of such biopolymers.454,455
When selecting a chemical modification, it is important to consider the simplicity of the chemical modification process. For example, many reactions to functionalize hydrophilic biopolymers with methacrylates can be completed in a one-step aqueous reaction by addition of water-soluble molecules such as methacrylic anhydride or glycidyl methacrylate.145,456 In contrast, the functionalization of hydrophilic biopolymers with hydrophobic groups, such as norbornenes or β-CD, currently require additional processing steps, which may hinder production scale-up.322,457
As another consideration, the crosslinking mechanism used must be evaluated for feasibility in the application of interest. For instance, covalent crosslinking techniques that require a radical photoinitiator may work well for the fabrication of implantable, pre-formed scaffolds. However, injectable hydrogels requiring the application of light within the body for hydrogel formation may raise concerns over cytotoxicity, considering the generation of free radicals and potential damage to native cells. As such, hydrogels formed by mixing two components (e.g., guest-host interactions, hydrazone formation) may be more favorable for applications requiring injection. In another approach, advanced delivery systems can be developed for in situ application of light in order to photocrosslink hydrogels.458 However, translation of such an approach requires production scale-up of both the hydrogel and delivery device.
Storage and sterilization are important to consider in the design of biopolymer hydrogels for clinical translation. For example, temperature-sensitive biopolymers and crosslinking chemistries may require additional storage procedures. Further, the application of FDA-approved sterilization treatments, such as autoclaving and γ-irradiation, can have adverse effects on the biological and mechanical behavior of chemically modified hydrogel properties,459 and thus, must be considered and characterized.
Most biopolymer-based hydrogels that have been implemented in clinical applications involve either unmodified biopolymers or very simple modifications and at low levels.460 Chemical modifications introduce added scrutiny during translation. For successful clinical translation, it is important to consider that every component of a hydrogel system must be able to meet current good manufacturing practices (cGMP), including biopolymers, crosslinkers, initiators, and encapsulated therapeutics.460 Thus, when engineering a hydrogel for clinical translation, it is vital for a researcher to consider how each added complexity in a chemically-modified biopolymer hydrogel system is essential for successful hydrogel function.
With these considerations in mind, there is still great potential for the field of biomedical hydrogels to grow and succeed. In the past few decades, there have been nearly 30 injectable hydrogels that were FDA- and/or EMA-approved for clinical use.460 Of those injectable hydrogel products, 11 contain HA, 7 contain collagen, 4 contain CMC, and 1 contains alginate.460 Further, there are hundreds of hydrogels in clinical trial for biomedical applications ranging from injectable therapeutic delivery, wound dressings, regenerative medicine, and tissue sealants, many of which are formed from biopolymers.460 Ultimately, the next decades are likely to see increased translation of new biopolymer hydrogels to clinical use.
Acknowledgments
The authors would like to acknowledge support from the National Institutes of Health (R01AR077362, R01AR056624); the National Science Foundation through a graduate research fellowship (to VGM), the UPenn MRSEC (DMR-1720530), and the Center for Engineering MechanoBiology STC (CMMI: 15-48571); and the Tau Beta Pi Fellowship Program (to VGM).
Glossary
- Biopolymers
natural polymers that are sourced from animals and plants, including a wide range of polysaccharides (e.g., sugars) and polypeptides (e.g., proteins).
- Click chemistry
a set of biocompatible chemical reactions that result in the rapid formation of covalent bonds.
- Covalent crosslinking
the process of chemically linking polymer chains together via covalent bonds, primarily to form a polymer network.
- Dynamic covalent crosslinking
a subset of crosslinking reactions that allow for the formation of reversible covalent crosslinks between biopolymers.
- Free radical chain polymerization
a reaction in which either a polymer or crosslinks between polymers are formed via the propagation of radical species in the form of a growing kinetic chain.
- Guest-host assembly
the formation of physical inclusion complexes between a “host” macrocycle with a hydrophobic interior and one or more hydrophobic “guest” moieties
- Interpenetrating network (IPN)
the combination of multiple independent polymer networks, where the individual networks are inter-mixed, but not linked together.
- Metal-ligand coordination
the formation of complexes consisting of a central metallic atom surrounded by bounded molecules (e.g., ligands).
- Michael addition
an addition reaction that can occur readily between thiols (e.g., Michael donors) and electron-deficient enes (e.g., Michael acceptors) without the need for radical initiators.
- Photocrosslinking
the use of light to facilitate a crosslinking reaction between polymers, primarily to form a polymer network.
- Photoinitiator
a molecule that cleave in response to certain wavelengths of light, resulting in the generation of free radicals.
- Physical crosslinking
the process of forming a polymer network by physical (e.g., non-chemical) interactions between polymer chains.
Abbreviations
- Ad
adamantane
- APS
ammonium persulfate
- BSA
bovine serum albumin
- CB[n]
cucurbit[n]uril
- CD
cyclodextrin
- CMC
Carboxymethyl cellulose
- CS
chondroitin sulfate
- DMAP
4-dimethylaminopyridine
- DN
double network
- DTT
dithiothreitol
- ECM
extracellular matrix
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- ELP
elastin-like peptide
- GAG
glycosaminoglycan
- GelMA
methacrylated gelatin
- GMA
glycidyl methacrylate
- HA
hyaluronic acid
- HA-CA
catechol-modified hyaluronic acid
- HA-Tyr
tyramine-modified hyaluronic acid
- HOBt
hydroxybenzotriazole
- HRP
horse radish peroxidase
- I2959
Irgacure, 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone
- IPN
interpenetrating network
- LAP
lithium phenyl-2,4,6-trimethylbenzoylphosphinate
- MeHA
methacrylated hyaluronic acid
- MSCs
mesenchymal stromal cells
- NHS
N-hydroxysuccinimide
- NorHA
norbornene-modified hyaluronic acid
- PEG
poly(ethylene glycol)
- PEGDA
diacrylated poly(ethylene glycol)
- PG
proteoglycan
- PNIPAAm
poly(N-isopropylacrylamide)
- TEMED
tetramethylethylenediamine
- UPy
ureidopyrimidone
- UV
ultraviolet
- β-CD
β-cyclodextrin
Biographies
Victoria Grace Muir is currently a PhD candidate studying under the advisement of Dr. Jason A. Burdick in the Department of Bioengineering at the University of Pennsylvania. For her graduate studies, Victoria was awarded a National Science Foundation Graduate Research Fellowship and a Tau Beta Pi Fellowship. Victoria received her honors bachelor’s degree with distinction in the Department of Chemical and Biomolecular Engineering at the University of Delaware as a Eugene D. du Pont Scholar. As an undergraduate student, she conducted research on polymer nanoparticles for siRNA delivery under the advisement of Dr. Thomas H. Epps, III and Dr. Millicent O. Sullivan. For her undergraduate research efforts, Victoria received a Barry Goldwater Scholarship. Her current research focuses on the development of granular hydrogels for musculoskeletal tissue repair. She recently received the Penn Prize for Excellence in Graduate Teaching as well as the inaugural Poddar Award for Rising Chemical Engineers from the American Institute of Chemical Engineers.
Jason A. Burdick, PhD is the Robert D. Bent Professor of Bioengineering at the University of Pennsylvania. Dr. Burdick’s research involves the development of hydrogels through techniques such as photocrosslinking and self-assembly and their processing using approaches such as electrospinning and 3D printing. The applications of his research range from controlling stem cell differentiation through material cues to fabricating scaffolding for regenerative medicine and tissue repair. Jason currently has over 260 peer-reviewed publications and he is on the editorial boards of Journal of Biomedical Materials Research A, Biofabrication, and Advanced Healthcare Materials, and is an Associate Editor for ACS Biomaterials Science & Engineering. He has been recognized through numerous awards such as a Packard Fellowship in Science and Engineering, an American Heart Association Established Investigator Award, the Clemson Award for Basic Science through the Society for Biomaterials, and the Acta Biomaterialia Silver Medal Award. Lastly, Jason has been elected as a Fellow to the American Institute for Medical and Biological Engineering and the National Academy of Inventors and has founded several companies to translate technology developed in his laboratory.
9. References
- (1).Caló E; Khutoryanskiy VV Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J 2015, 65, 252–267. [Google Scholar]
- (2).Janouskova O Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res 2018, 67. [DOI] [PubMed] [Google Scholar]
- (3).Van Vlierberghe S; Dubruel P; Schacht E Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [DOI] [PubMed] [Google Scholar]
- (4).Burdick JA; Prestwich GD Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater 2011, 23, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Necas J; Bartosikova L; Brauner P; Kolar J Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. (Praha) 2008, 53, 397–411. [Google Scholar]
- (6).Fraser JRE; Laurent TC; Laurent UBG Hyaluronan: Its Nature, Distribution, Functions and Turnover. J. Intern. Med 1997, 242, 27–33. [DOI] [PubMed] [Google Scholar]
- (7).Ward PD; Thibeault SL; Gray SD Hyaluronic Acid: Its Role in Voice. J. Voice 2002, 16, 303–309. [DOI] [PubMed] [Google Scholar]
- (8).Prestwich GD Hyaluronic Acid-Based Clinical Biomaterials Derived for Cell and Molecule Delivery in Regenerative Medicine. J. Control. Release 2011, 155, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Toole BP Hyaluronan in Morphogenesis. Semin. Cell Dev. Biol 2001, 12, 79–87. [DOI] [PubMed] [Google Scholar]
- (10).Ogston BYAG; Stanier JE The Physiological Function of Hyaluronic Acid in Synovial Fluid; Viscous, Elastic, and Lubricant Properties. J. Physiol 1953, 119, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karjalainen JM; Tammi RH; Tammi MI; Eskelinen MJ; Ågren UM; Parkkinen JJ; Alhava EM; Kosma VM Reduced Level of CD44 and Hyaluronan Associated with Unfavorable Prognosis in Clinical Stage I Cutaneous Melanoma. Am. J. Pathol 2000, 157, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ishida O; Tanaka Y; Morimoto I; Takigawa M; Eto S Chondrocytes Are Regulated by Cellular Adhesion through CD44 and Hyaluronic Acid Pathway. J. Bone Miner. Res 1997, 12, 1657–1663. [DOI] [PubMed] [Google Scholar]
- (13).Gold M The Science and Art of Hyaluronic Acid Dermal Filler Use in Esthetic Applications. J. Cosmet. Dermatol 2009, 8, 301–307. [DOI] [PubMed] [Google Scholar]
- (14).Rohrich RJ; Ghavami A; Crosby MA The Role of Hyaluronic Acid Fillers (Restylane) in Facial Cosmetic Surgery: Review and Technical Considerations. Plast. Reconstr. Surg 2007, 120, 41–54. [DOI] [PubMed] [Google Scholar]
- (15).Carruthers J; Carruthers A A Prospective, Randomized, Parallel Group Study Analyzing the Effect of BTX-A (Botox) and Nonanimal Sourced Hyaluronic Acid (NASHA, Restylane) in Combination Compared with NASHA (Restylane) Alone in Severe Glabellar Rhytides in Adult Female Subjects: Trea. Dermatologic Surg 2003, 29, 802–809. [DOI] [PubMed] [Google Scholar]
- (16).Lee M; Han SH; Choi WJ; Chung KH; Lee JW Hyaluronic Acid Dressing (Healoderm) in the Treatment of Diabetic Foot Ulcer: A Prospective, Randomized, Placebo-Controlled, Single-Center Study. Wound Repair Regen 2016, 24, 581–588. [DOI] [PubMed] [Google Scholar]
- (17).Bannuru RR; Natov NS; Dasi UR; Schmid CH; McAlindon TE Therapeutic Trajectory Following Intra-Articular Hyaluronic Acid Injection in Knee Osteoarthritis - Meta-Analysis. Osteoarthr. Cartil 2011, 19, 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Brown MB; Jones SA Hyaluronic Acid: A Unique Topical Vehicle for the Localized Delivery of Drugs to the Skin. J. Eur. Acad. Dermatology Venereol 2005, 19, 308–318. [DOI] [PubMed] [Google Scholar]
- (19).Beer K A Randomized, Evaluator-Blinded Comparison of Efficacy of Hyaluronic Acid Gel and Avian-Sourced Hylan B plus Gel for Correction of Nasolabial Folds. Dermatologic Surg 2007, 33, 928–936. [DOI] [PubMed] [Google Scholar]
- (20).Highley CB; Prestwich GD; Burdick JA Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol 2016, 40, 35–40. [DOI] [PubMed] [Google Scholar]
- (21).Sugahara K; Mikami T; Uyama T; Mizuguchi S; Nomura K; Kitagawa H Recent Advances in the Structural Biology of Chondroitin Sulfate and Dermatan Sulfate. Curr. Opin. Struct. Biol 2003, 13, 612–620. [DOI] [PubMed] [Google Scholar]
- (22).Malavaki C; Mizumoto S; Karamanos N; Sugahara K Recent Advances in the Structural Study of Functional Chondroitin Sulfate and Dermatan Sulfate in Health and Disease. Connect. Tissue Res 2008, 49, 133–139. [DOI] [PubMed] [Google Scholar]
- (23).Baeurle SA; Kiselev MG; Makarova ES; Nogovitsin EA Effect of the Counterion Behavior on the Frictional-Compressive Properties of Chondroitin Sulfate Solutions. Polymer (Guildf) 2009, 50, 1805–1813. [Google Scholar]
- (24).Galtrey CM; Fawcett JW The Role of Chondroitin Sulfate Proteoglycans in Regeneration and Plasticity in the Central Nervous System. Brain Res. Rev 2007, 54, 1–18. [DOI] [PubMed] [Google Scholar]
- (25).Bukalo O; Schachner M; Dityatev A Modification of Extracellular Matrix by Enzymatic Removal of Chondroitin Sulfate and by Lack of Tenascin-R Differentially Affects Several Forms of Synaptic Plasticity in the Hippocampus. Neuroscience 2001, 104, 359–369. [DOI] [PubMed] [Google Scholar]
- (26).Zuo J; Neubauer D; Dyess K; Ferguson T; Muir D Degradation of Chondroitin Sulfate Proteoglycan Enhances the Neurite-Promoting Potential of Spinal Cord Tissue. Exp. Neurol 1998, 654–662. [DOI] [PubMed] [Google Scholar]
- (27).Vangsness CT; Spiker W; Erickson J A Review of Evidence-Based Medicine for Glucosamine and Chondroitin Sulfate Use in Knee Osteoarthritis. Arthrosc. - J. Arthrosc. Relat. Surg 2009, 25, 86–94. [DOI] [PubMed] [Google Scholar]
- (28).Muzzarelli RAA; Greco F; Busilacchi A; Sollazzo V; Gigante A Chitosan, Hyaluronan and Chondroitin Sulfate in Tissue Engineering for Cartilage Regeneration: A Review. Carbohydr. Polym 2012, 89, 723–739. [DOI] [PubMed] [Google Scholar]
- (29).Schiraldi C; Cimini D; De Rosa M Production of Chondroitin Sulfate and Chondroitin. Appl. Microbiol. Biotechnol 2010, 87, 1209–1220. [DOI] [PubMed] [Google Scholar]
- (30).Rabenstein DL Heparin and Heparan Sulfate: Structure and Function. Nat. Prod. Rep 2002, 19, 312–331. [DOI] [PubMed] [Google Scholar]
- (31).Sasisekharan R; Venkataraman G Heparin and Heparan Sulfate: Biosynthesis, Structure and Function. Curr. Opin. Chem. Biol 2000, 4, 626–631. [DOI] [PubMed] [Google Scholar]
- (32).Xu X; Dai Y Heparin: An Intervenor in Cell Communication. J. Cell. Mol. Med 2010, 14, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Weitz DS; Weitz JI Update on Heparin: What Do We Need to Know? J. Thromb. Thrombolysis 2010, 29, 199–207. [DOI] [PubMed] [Google Scholar]
- (34).Liu H; Zhang Z; Linhardt RJ Lessons Learned from the Contamination of Heparin. Nat. Prod. Rep 2009, 26, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).World Health Organization. WHO Model List of Essential Medicines - 19th List (April 2015); 2015.
- (36).Bhavani AL; Nisha J Dextran - The Polysaccharide with Versatile Uses. Int. J. Pharma Bio Sci 2010, 1, 569–573. [Google Scholar]
- (37).Meddens MJM; Thompson J; Leijh PCJ; Van Furth R Role of Granulocytes in the Induction of an Experimental Endocarditis with a Dextran-Producing Streptococcus Sanguis and Its Dextran-Negative Mutant. Br. J. Exp. Pathol 1984, 65, 257–265. [PMC free article] [PubMed] [Google Scholar]
- (38).Gibbons RJ; Fitzgerald RJ Dextran-Induced Agglutination of Streptococcus Mutans, and Its Potential Role in the Formation of Microbial Dental Plaques. J. Bacteriol 1969, 98, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ioan CE; Aberle T; Burchard W Structure Properties of Dextran. 2. Dilute Solution. Macromolecules 2000, 33, 5730–5739. [Google Scholar]
- (40).Iqbal S; Marchetti R; Aman A; Silipo A; Qader SAU; Molinaro A Enzymatic and Acidic Degradation of High Molecular Weight Dextran into Low Molecular Weight and Its Characterizations Using Novel Diffusion-Ordered NMR Spectroscopy. Int. J. Biol. Macromol 2017, 103, 744–750. [DOI] [PubMed] [Google Scholar]
- (41).Tønnesen HH; Karlsen J Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm 2002, 28, 621–630. [DOI] [PubMed] [Google Scholar]
- (42).Smidsrod O; Skjak-Braek G Alginate as Immobilization Matrix for Cells. Trend Biotechnol 1990. [DOI] [PubMed] [Google Scholar]
- (43).Lee KY; Mooney DJ Alginate: Properties and Biomedical Applications. Prog. Polym. Sci 2012, 37, 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Szekalska M; Puciłowska A; Szymańska E; Ciosek P; Winnicka K Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci 2016, 2016. [Google Scholar]
- (45).Wylie A Alginates as Food Additives. R.S.H 1973. [DOI] [PubMed]
- (46).O’Sullvian AC Cellulose: The Structure Slowly Unravels. Cellulose 1997, 4, 173–207. [Google Scholar]
- (47).Klemm D; Heublein B; Fink HP; Bohn A Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chemie - Int. Ed 2005, 44, 3358–3393. [DOI] [PubMed] [Google Scholar]
- (48).Ross P; Mayer R; Benziman M Cellulose Biosynthesis and Function in Bacteria. Microbiol. Rev 1991, 55, 35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Jonas R; Farah LF Production and Application of Microbial Cellulose. Polym. Degrad. Stab 1998, 59, 101–106. [Google Scholar]
- (50).Tosh B Esterification and Etherification of Cellulose: Synthesis and Application of Cellulose Derivatives. In Cellulose and Cellulose Derivatives; 2015. [Google Scholar]
- (51).Kabir SMF; Sikdar PP; Haque B; Bhuiyan MAR; Ali A; Islam MN Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater 2018, 7, 153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Aranaz I; Mengibar M; Harris R; Panos I; Miralles B; Acosta N; Galed G; Heras A Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol 2012, 3, 203–230. [Google Scholar]
- (53).Tang WJ; Fernandez JG; Sohn JJ; Amemiya CT Chitin Is Endogenously Produced in Vertebrates. Curr. Biol 2015, 25, 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Bedian L; Villalba-Rodríguez AM; Hernández-Vargas G; Parra-Saldivar R; Iqbal HMN Bio-Based Materials with Novel Characteristics for Tissue Engineering Applications – A Review. Int. J. Biol. Macromol 2017, 98, 837–846. [DOI] [PubMed] [Google Scholar]
- (55).Elieh Ali Komi D; Sharma L; Dela Cruz CS Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol 2018, 54, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ifuku S Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367–18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gómez-Ríos D; Barrera-Zapata R; Ríos-Estepa R Comparison of Process Technologies for Chitosan Production from Shrimp Shell Waste: A Techno-Economic Approach Using Aspen Plus®. Food Bioprod. Process 2017, 103, 49–57. [Google Scholar]
- (58).Di Martino A; Sittinger M; Risbud MV Chitosan: A Versatile Biopolymer for Orthopaedic Tissue-Engineering. Biomaterials 2005, 26, 5983–5990. [DOI] [PubMed] [Google Scholar]
- (59).Zhang YJ; Gao B; Liu XW Topical and Effective Hemostatic Medicines in the Battlefield. Int. J. Clin. Exp. Med 2015, 8, 10–19. [PMC free article] [PubMed] [Google Scholar]
- (60).Malik A; Gupta M; Gupta V; Gogoi H; Bhatnagar R Novel Application of Trimethyl Chitosan as an Adjuvant in Vaccine Delivery. Int. J. Nanomedicine 2018, 13, 7959–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Di Lullo GA; Sweeney SM; Körkkö J; Ala-Kokko L; San Antonio JD Mapping the Ligand-Binding Sites and Disease-Associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem 2002, 277, 4223–4231. [DOI] [PubMed] [Google Scholar]
- (62).Schrieber R; Gareis H Gelatin Handbook – Theory and Industrial Practice; 2007.
- (63).Duconseille A; Astruc T; Quintana N; Meersman F; Sante-Lhoutellier V Gelatin Structure and Composition Linked to Hard Capsule Dissolution: A Review. Food Hydrocoll 2015, 43, 360–376. [Google Scholar]
- (64).Ward AG; Courts A The Science and Technology of Gelatin; 1977.
- (65).Bailey AJ; Light ND Connective Tissue in Meat and Meat Products; 1989.
- (66).Sarker B; Singh R; Silva R; Roether JA; Kaschta J; Detsch R; Schubert DW; Cicha I; Boccaccini AR Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS One 2014, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Sellers A; Reynolds JJ; Meikle MC Neutral Metallo-Proteinases of Rabbit Bone. Biochem. J 1978, 171, 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Mariod AA; Adam HF Review: Gelatin, Source, Extraction and Industrial Applications. Acta Sci. Pol. Technol. Aliment 2013, 12, 135–147. [Google Scholar]
- (69).Bello AB; Kim D; Kim D; Park H; Lee SH Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. - Part B Rev 2020, 26, 164–180. [DOI] [PubMed] [Google Scholar]
- (70).Davidenko N; Schuster CF; Bax DV; Farndale RW; Hamaia S; Best SM; Cameron RE Evaluation of Cell Binding to Collagen and Gelatin: A Study of the Effect of 2D and 3D Architecture and Surface Chemistry. J. Mater. Sci. Mater. Med 2016, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Bigi A; Cojazzi G; Panzavolta S; Roveri N; Rubini K Stabilization of Gelatin Films by Crosslinking with Genipin. Biomaterials 2002, 23, 4827–4832. [DOI] [PubMed] [Google Scholar]
- (72).Qi Y; Wang H; Wei K; Yang Y; Zheng RY; Kim IS; Zhang KQ A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Kundu B; Rajkhowa R; Kundu SC; Wang X Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev 2013, 65, 457–470. [DOI] [PubMed] [Google Scholar]
- (74).Vepari Charu; Kaplan David L. Silk as Biomaterial. Prog. Polym. Sci 2007, 100, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Omenetto FG; Kaplan DL New Opportunities for an Ancient Material. Science (80-. ) 2010, 329, 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Melke J; Midha S; Ghosh S; Ito K; Hofmann S Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater 2016, 31, 1–16. [DOI] [PubMed] [Google Scholar]
- (77).Wang Y; Rudym DD; Walsh A; Abrahamsen L; Kim HJ; Kim HS; Kirker-Head C; Kaplan DL In Vivo Degradation of Three-Dimensional Silk Fibroin Scaffolds. Biomaterials 2008, 29, 3415–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Jin HJ; Park J; Karageorgiou V; Kim UJ; Valluzzi R; Cebe P; Kaplan DL Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater 2005, 15, 1241–1247. [Google Scholar]
- (79).Ong J; Zhao J; Justin AW; Markaki AE Albumin-Based Hydrogels for Regenerative Engineering and Cell Transplantation. Biotechnol. Bioeng 2019, 116, 3457–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Simard JR; Zunszain PA; Hamilton JA; Curry S Location of High and Low Affinity Fatty Acid Binding Sites on Human Serum Albumin Revealed by NMR Drug-Competition Analysis. J. Mol. Biol 2006, 361, 336–351. [DOI] [PubMed] [Google Scholar]
- (81).Caraceni P; Tufoni M; Bonavita ME Clinical Use of Albumin. Blood Transfus 2013, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Raoufinia R; Mota A; Keyhanvar N; Safari F; Shamekhi S; Abdolalizadeh J Overview of Albumin and Its Purification Methods. Adv. Pharm. Bull 2016, 6, 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Matejtschuk P; Dash CH; Gascoigne EW Production of Human Albumin Solution: A Continually Developing Colloid. Br. J. Anaesth 2000, 85, 887–895. [DOI] [PubMed] [Google Scholar]
- (84).Mithieux BSM; Weiss AS Elastin Is a Key Extracellular Matrix Protein That Is Critical to the Elasticity I. Elastic Fiber The Extracellular Matrix Imparts Structural Integrity on the Tissues And. Advances 2006, 70, 437–461. [Google Scholar]
- (85).Oxlund H; Manschot J; Viidik A The Role of Elastin in the Mechanical Properties of Skin. J. Biomech 1988, 21, 213–218. [DOI] [PubMed] [Google Scholar]
- (86).Baldock C; Oberhauser AF; Ma L; Lammie D; Siegler V; Mithieux SM; Tu Y; Chow JYH; Suleman F; Malfois M; et al. Shape of Tropoelastin, the Highly Extensible Protein That Controls Human Tissue Elasticity. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 4322–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Debelle L; Tamburro AM Elastin: Molecular Description and Function. Int. J. Biochem. Cell Biol 1999, 31, 261–272. [DOI] [PubMed] [Google Scholar]
- (88).Faury G Function-Structure Relationship of Elastic Arteries in Evolution: From Microfibrils to Elastin and Elastic Fibres. Pathol. Biol 2001, 49, 310–325. [DOI] [PubMed] [Google Scholar]
- (89).Annabi N; Mithieux SM; Weiss AS; Dehghani F The Fabrication of Elastin-Based Hydrogels Using High Pressure CO2. Biomaterials 2009, 30, 1–7. [DOI] [PubMed] [Google Scholar]
- (90).Mithieux SM; Rasko JEJ; Weiss AS Synthetic Elastin Hydrogels Derived from Massive Elastic Assemblies of Self-Organized Human Protein Monomers. Biomaterials 2004, 25, 4921–4927. [DOI] [PubMed] [Google Scholar]
- (91).Yeo GC; Aghaei-Ghareh-Bolagh B; Brackenreg EP; Hiob MA; Lee P; Weiss AS Fabricated Elastin. Adv. Healthc. Mater 2016, 4, 2530–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Lim DW; Nettles DL; Setton LA; Chilkoti A In Situ Cross-Linking of Elastin-like Polypeptide Block Copolymers for Tissue Repair. Biomacromolecules 2008, 9, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Trabbic-Carlson K; Setton LA; Chilkoti A Swelling and Mechanical Behaviors of Chemically Cross-Linked Hydrogels of Elastin-like Polypeptides. Biomacromolecules 2003, 4, 572–580. [DOI] [PubMed] [Google Scholar]
- (94).Feroz S; Muhammad N; Ranayake J; Dias G Keratin - Based Materials for Biomedical Applications. Bioact. Mater 2020, 5, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Wang B; Yang W; McKittrick J; Meyers MA Keratin: Structure, Mechanical Properties, Occurrence in Biological Organisms, and Efforts at Bioinspiration. Prog. Mater. Sci 2016, 76, 229–318. [Google Scholar]
- (96).Irwin McLean WH; Moore CB Keratin Disorders: From Gene to Therapy. Hum. Mol. Genet 2011, 20, 189–197. [DOI] [PubMed] [Google Scholar]
- (97).Wang Y; Zhang W; Yuan J; Shen J Differences in Cytocompatibility between Collagen, Gelatin and Keratin. Mater. Sci. Eng. C 2016, 59, 30–34. [DOI] [PubMed] [Google Scholar]
- (98).Yue K; Liu Y; Byambaa B; Singh V; Liu W; Li X; Sun Y; Zhang YS; Tamayol A; Zhang P; et al. Visible Light Crosslinkable Human Hair Keratin Hydrogels. Bioeng. Transl. Med 2018, 3, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Zhang L; Hu F; Zhu S; Lin Y; Meng Z; Yu R; Liu XY Meso-Reconstruction of Wool Keratin 3D “Molecular Springs” for Tunable Ultra-Sensitive and Highly Recovery Strain Sensors. Small 2020, 16, 1–9. [DOI] [PubMed] [Google Scholar]
- (100).Wang J; Hao S; Luo T; Cheng Z; Li W; Gao F; Guo T; Gong Y; Wang B Feather Keratin Hydrogel for Wound Repair: Preparation, Healing Effect and Biocompatibility Evaluation. Colloids Surfaces B Biointerfaces 2017, 149, 341–350. [DOI] [PubMed] [Google Scholar]
- (101).Idris A; Vijayaraghavan R; Rana UA; Fredericks D; Patti AF; MacFarlane DR Dissolution of Feather Keratin in Ionic Liquids. Green Chem 2013, 15, 525–534. [Google Scholar]
- (102).Kumar D; Workman VL; O’Brien M; McLaren J; White L; Ragunath K; Rose F; Saiani A; Gough JE Peptide Hydrogels—A Tissue Engineering Strategy for the Prevention of Oesophageal Strictures. Adv. Funct. Mater 2017, 27. [Google Scholar]
- (103).Li L; Tong Z; Jia X; Kiick K Resilin-Like Polypeptide Hydrogels Engineered for Versatile Biological Functions. Soft M 2013, 9, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).He H; Sofman M; Wang AJS; Ahrens CC; Wang W; Griffith LG; Hammond PT Engineering Helical Modular Polypeptide-Based Hydrogels as Synthetic Extracellular Matrices for Cell Culture. Biomacromolecules 2020, 21, 566–580. [DOI] [PubMed] [Google Scholar]
- (105).Koch F; Müller M; König F; Meyer N; Gattlen J; Pieles U; Peters K; Kreikemeyer B; Mathes S; Saxer S Mechanical Characteristics of Beta Sheet-Forming Peptide Hydrogels Are Dependent on Peptide Sequence, Concentration and Buffer Composition. R. Soc. Open Sci 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Lin BF; Megley KA; Viswanathan N; Krogstad DV; Drews LB; Kade MJ; Qian Y; Tirrell MV PH-Responsive Branched Peptide Amphiphile Hydrogel Designed for Applications in Regenerative Medicine with Potential as Injectable Tissue Scaffolds. J. Mater. Chem 2012, 22, 19447–19454. [Google Scholar]
- (107).Steele AN; Cai L; Truong VN; Edwards BB; Goldstone AB; Eskandari A; Mitchell AC; Marquardt LM; Foster AA; Cochran JR; et al. A Novel Protein-Engineered Hepatocyte Growth Factor Analog Released via a Shear-Thinning Injectable Hydrogel Enhances Post-Infarction Ventricular Function. Biotechnol. Bioeng 2017, 114, 2379–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Madl CM; Katz LM; Heilshorn SC Bio-Orthogonally Crosslinked, Engineered Protein Hydrogels with Tunable Mechanics and Biochemistry for Cell Encapsulation. Adv. Funct. Mater 2016, 26, 3612–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Madl CM; Heilshorn SC Tyrosine-Selective Functionalization for Bio-Orthogonal Cross-Linking of Engineered Protein Hydrogels. Bioconjug. Chem 2017, 28, 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Baslé E; Joubert N; Pucheault M Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol 2010, 17, 213–227. [DOI] [PubMed] [Google Scholar]
- (111).Burdick JA; Chung C; Jia X; Randolph MA; Langer R Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Biomacromolecules 2005, 6, 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Gramlich WM; Kim IL; Burdick JA Synthesis and Orthogonal Photopatterning of Hyaluronic Acid Hydrogels with Thiol-Norbornene Chemistry. Biomaterials 2013, 34, 9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Jeon O; Bouhadir KH; Mansour JM; Alsberg E Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [DOI] [PubMed] [Google Scholar]
- (114).Dadoo N; Landry SB; Bomar JD; Gramlich WM Synthesis and Spatiotemporal Modification of Biocompatible and Stimuli-Responsive Carboxymethyl Cellulose Hydrogels Using Thiol-Norbornene Chemistry. Macromol. Biosci 2017, 17, 1–12. [DOI] [PubMed] [Google Scholar]
- (115).Martínez-Sanz E; Ossipov DA; Hilborn J; Larsson S; Jonsson KB; Varghese OP Bone Reservoir: Injectable Hyaluronic Acid Hydrogel for Minimal Invasive Bone Augmentation. J. Control. Release 2011, 152, 232–240. [DOI] [PubMed] [Google Scholar]
- (116).Kurisawa M; Chung JE; Yang YY; Gao SJ; Uyama H Injectable Biodegradable Hydrogels Composed of Hyaluronic Acid-Tyramine Conjugates for Drug Delivery and Tissue Engineering. Chem. Commun 2005, No. 34, 4312–4314. [DOI] [PubMed] [Google Scholar]
- (117).Vega SL; Kwon MY; Song KH; Wang C; Mauck RL; Han L; Burdick JA Combinatorial Hydrogels with Biochemical Gradients for Screening 3D Cellular Microenvironments. Nat. Commun 2018, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).van Dijk-Wotthuis WNE; Franssen O; Talsma H; van Steenbergen MJ; Kettenes-van den Bosch JJ; Hennink WE Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar]
- (119).Lévesque SG; Lim RM; Shoichet MS Macroporous Interconnected Dextran Scaffolds of Controlled Porosity for Tissue-Engineering Applications. Biomaterials 2005, 26, 7436–7446. [DOI] [PubMed] [Google Scholar]
- (120).Leach JB; Bivens KA; Patrick CW; Schmidt CE Photocrosslinked Hyaluronic Acid Hydrogels: Natural, Biodegradable Tissue Engineering Scaffolds. Biotechnol. Bioeng 2003, 82, 578–589. [DOI] [PubMed] [Google Scholar]
- (121).Jin R; Hiemstra C; Zhong Z; Feijen J Enzyme-Mediated Fast in Situ Formation of Hydrogels from Dextran-Tyramine Conjugates. Biomaterials 2007, 28, 2791–2800. [DOI] [PubMed] [Google Scholar]
- (122).Appel EA; Loh XJ; Jones ST; Biedermann F; Dreiss CA; Scherman OA Ultrahigh-Water-Content Supramolecular Hydrogels Exhibiting Multistimuli Responsiveness. J. Am. Chem. Soc 2012, 134, 11767–11773. [DOI] [PubMed] [Google Scholar]
- (123).Peng K; Tomatsu I; Kros A Light Controlled Protein Release from a Supramolecular Hydrogel. Chem. Commun 2010, 46, 4094–4096. [DOI] [PubMed] [Google Scholar]
- (124).Nair DP; Podgórski M; Chatani S; Gong T; Xi W; Fenoli CR; Bowman CN The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater 2014, 26, 724–744. [Google Scholar]
- (125).Su H; Jia Q; Shan S Synthesis and Characterization of Schiff Base Contained Dextran Microgels in Water-in-Oil Inverse Microemulsion. Carbohydr. Polym 2016, 152, 156–162. [DOI] [PubMed] [Google Scholar]
- (126).Kim DY; Park H; Kim SW; Lee JW; Lee KY Injectable Hydrogels Prepared from Partially Oxidized Hyaluronate and Glycol Chitosan for Chondrocyte Encapsulation. Carbohydr. Polym 2017, 157, 1281–1287. [DOI] [PubMed] [Google Scholar]
- (127).Hozumi T; Kageyama T; Ohta S; Fukuda J; Ito T Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [DOI] [PubMed] [Google Scholar]
- (128).Sinkiewicz I; Śliwińska A; Staroszczyk H; Kołodziejska I Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste and Biomass Valorization 2017, 8, 1043–1048. [Google Scholar]
- (129).Ifkovits JL; Burdick JA Review: Photopolymerizable and Degradable Biomaterials for Tissue Engineering Applications. Tissue Eng 2007, 13, 2369–2385. [DOI] [PubMed] [Google Scholar]
- (130).De Geest BG; Urbanski JP; Thorsen T; Demeester J; De Smedt SC Synthesis of Monodisperse Biodegradable Microgels in Microfluidic Devices. Langmuir 2005, 21, 10275–10279. [DOI] [PubMed] [Google Scholar]
- (131).Galarraga JH; Kwon MY; Burdick JA 3D Bioprinting via an in Situ Crosslinking Technique towards Engineering Cartilage Tissue. Sci. Rep 2019, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Tous E; Ifkovits JL; Koomalsingh KJ; Shuto T; Soeda T; Kondo N; Gorman JH; Gorman RC; Burdick JA Influence of Injectable Hyaluronic Acid Hydrogel Degradation Behavior on Infarction-Induced Ventricular Remodeling. Biomacromolecules 2011, 12, 4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Studer K; Nesvadba P; Jung T; Benkhoff J; Powell K; Lordelot C Novel Curing Agents: Thermal Radical Initiators as Viable Alternatives to Peroxides. Prog. Org. Coatings 2008, 61, 119–125. [Google Scholar]
- (134).Smeds KA; Pfister-Serres A; Hatchell DL; Grinstaff MW Synthesis of a Novel Polysaccharide Hydrogel. J.M.S.-PURE APPL. CHEM 1999. [Google Scholar]
- (135).Van Dijk-Wolthuis WNE; Tsang SKY; Kettenes-Van Den Bosch JJ; Hennink WE A New Class of Polymerizable Dextrans with Hydrolyzable Groups: Hydroxyethyl Methacrylated Dextran with and without Oligolactate Spacer. Polymer (Guildf) 1997, 38, 6235–6242. [Google Scholar]
- (136).Kim SH; Chu CC Synthesis and Characterization of Dextran-Methacrylate Hydrogels and Structural Study by SEM. J. Biomed. Mater. Res 2000, 49, 517–527. [DOI] [PubMed] [Google Scholar]
- (137).Kim SH; Chu CC In Vitro Release Behavior of Dextran-Methacrylate Hydrogels Using Doxorubicin and Other Model Compounds. J. Biomater. Appl 2000, 15. [DOI] [PubMed] [Google Scholar]
- (138).Ferreira LS; Gerecht S; Fuller J; Shieh HF; Vunjak-Novakovic G; Langer R Bioactive Hydrogel Scaffolds for Controllable Vascular Differentiation of Human Embryonic Stem Cells. Biomaterials 2007, 28, 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Leach JB; Schmidt CE Characterization of Protein Release from Photocrosslinkable Hyaluronic Acid-Polyethylene Glycol Hydrogel Tissue Engineering Scaffolds. Biomaterials 2005, 26, 125–135. [DOI] [PubMed] [Google Scholar]
- (140).Jia X; Burdick JA; Kobler J; Clifton RJ; Rosowski JJ; Zeitels SM; Langer R Synthesis and Characterization of in Situ Cross-Linkable Hyaluronic Acid-Based Hydrogels with Potential Application for Vocal Fold Regeneration. Macromolecules 2004, 37, 3239–3248. [Google Scholar]
- (141).Gerecht S; Burdick JA; Ferreira LS; Townsend SA; Langer R; Vunjak-Novakovic G Hyaluronic Acid Hydrogel for Controlled Self-Renewal and Differentiation of Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Ouyang L; Highley CB; Rodell CB; Sun W; Burdick JA 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng 2016, 2, 1743–1751. [DOI] [PubMed] [Google Scholar]
- (143).Marsano E; De Paz L; Tambuscio E; Bianchi E Cellulose Methacrylate: Synthesis and Liquid Crystalline Behaviour of Solutions and Gels. Polymer (Guildf) 1998, 39, 4289–4294. [Google Scholar]
- (144).Smeds KA; Pfister-Serres A; Miki D; Dastgheib K; Inoue M; Hatchell DL; Grinstaff MW Photocrosslinkable Polysaccharides Forin Situ Hydrogel Formation. J. Biomed. Mater. Res 2001, 55, 254–255. [DOI] [PubMed] [Google Scholar]
- (145).Wang LF; Shen SS; Lu SC Synthesis and Characterization of Chondroitin Sulfate-Methacrylate Hydrogels. Carbohydr. Polym 2003, 52, 389–396. [Google Scholar]
- (146).Marsano E; Gagliardi S; Ghioni F; Bianchi E Behaviour of Gels Based on (Hydroxypropyl) Cellulose Methacrylate. Polymer (Guildf) 2000, 41, 7691–7698. [Google Scholar]
- (147).Qi A; Hoo SP; Friend J; Yeo L; Yue Z; Chan PPY Hydroxypropyl Cellulose Methacrylate as a Photo-Patternable and Biodegradable Hybrid Paper Substrate for Cell Culture and Other Bioapplications. Adv. Healthc. Mater 2014, 3, 543–554. [DOI] [PubMed] [Google Scholar]
- (148).Jeon O; Powell C; Ahmed SM; Alsberg E Biodegradable, Photocrosslinked Alginate Hydrogels with Independently Tailorable Physical Properties and Cell Adhesivity. Tissue Eng. - Part A 2010, 16, 2915–2925. [DOI] [PubMed] [Google Scholar]
- (149).Rouillard AD; Berglund CM; Lee JY; Polacheck WJ; Tsui Y; Bonassar LJ; Kirby BJ Methods for Photocrosslinking Alginate Hydrogel Scaffolds with High Cell Viability. Tissue Eng. - Part C Methods 2011, 17, 173–179. [DOI] [PubMed] [Google Scholar]
- (150).Levett PA; Melchels FPW; Schrobback K; Hutmacher DW; Malda J; Klein TJ A Biomimetic Extracellular Matrix for Cartilage Tissue Engineering Centered on Photocurable Gelatin, Hyaluronic Acid and Chondroitin Sulfate. Acta Biomater 2014, 10, 214–223. [DOI] [PubMed] [Google Scholar]
- (151).Bian L; Hou C; Tous E; Rai R; Mauck RL; Burdick JA The Influence of Hyaluronic Acid Hydrogel Crosslinking Density and Macromolecular Diffusivity on Human MSC Chondrogenesis and Hypertrophy. Biomaterials 2013, 34, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Kim IL; Khetan S; Baker BM; Chen CS; Burdick JA Fibrous Hyaluronic Acid Hydrogels That Direct MSC Chondrogenesis through Mechanical and Adhesive Cues. Biomaterials 2013, 34, 5571–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Kwon MY; Vega SL; Gramlich WM; Kim M; Mauck RL; Burdick JA Dose and Timing of N-Cadherin Mimetic Peptides Regulate MSC Chondrogenesis within Hydrogels. Adv. Healthc. Mater 2018, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Yeom J; Kim SJ; Jung H; Namkoong H; Yang J; Hwang BW; Oh K; Kim K; Sung YC; Hahn SK Supramolecular Hydrogels for Long-Term Bioengineered Stem Cell Therapy. Adv. Healthc. Mater 2015, 4, 237–244. [DOI] [PubMed] [Google Scholar]
- (155).Kang W; Bi B; Zhuo R; Jiang X Photocrosslinked Methacrylated Carboxymethyl Chitin Hydrogels with Tunable Degradation and Mechanical Behavior. Carbohydr. Polym 2017, 160, 18–25. [DOI] [PubMed] [Google Scholar]
- (156).Pradhan S; Moore KM; Ainslie KM; Yadavalli VK Flexible, Microstructured Surfaces Using Chitin-Derived Biopolymers. J. Mater. Chem. B 2019, 7, 5328–5335. [DOI] [PubMed] [Google Scholar]
- (157).Park YD; Tirelli N; Hubbell JA Photopolymerized Hyaluronic Acid-Based Hydrogels and Interpenetrating Networks. Biomater. Silver Jubil. Compend 2002, 24, 203–210. [DOI] [PubMed] [Google Scholar]
- (158).Van Den Bulcke AI; Bogdanov B; De Rooze N; Schacht EH; Cornelissen M; Berghmans H Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [DOI] [PubMed] [Google Scholar]
- (159).Brinkman WT; Nagapudi K; Thomas BS; Chaikof EL Photo-Cross-Linking of Type I Collagen Gels in the Presence of Smooth Muscle Cells: Mechanical Properties, Cell Viability, and Function. Biomacromolecules 2003, 4, 890–895. [DOI] [PubMed] [Google Scholar]
- (160).Nichol JW; Koshy ST; Bae H; Hwang CM; Yamanlar S; Khademhosseini A Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Kim HH; Kim JW; Choi J; Park YH; Ki CS Characterization of Silk Hydrogel Formed with Hydrolyzed Silk Fibroin-Methacrylate via Photopolymerization. Polymer (Guildf) 2018, 153, 232–240. [Google Scholar]
- (162).Matsuda T; Magoshi T Preparation of Vinylated Polysaccharides and Photofabrication of Tubular Scaffolds as Potential Use in Tissue Engineering. Biomacromolecules 2002, 3, 942–950. [DOI] [PubMed] [Google Scholar]
- (163).Hoshikawa A; Nakayama Y; Matsuda T; Oda H; Nakamura K; Mabuchi K Encapsulation of Chondrocytes in Photopolymerizable Styrenated Gelatin for Cartilage Tissue Engineering. Tissue Eng 2006, 12, 2333–2341. [DOI] [PubMed] [Google Scholar]
- (164).Masuda T; Furue M; Ph D Photocured, Styrenated Gelatin-Based Microspheres for de Novo Adipogenesis through Corelease of Basic Fibroblast Growth Factor, Insulin, and Insulin-Like Growth Factor I. Tissue Eng 2004, 10. [DOI] [PubMed] [Google Scholar]
- (165).Sahoo S; Chung C; Khetan S; Burdick JA Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9, 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Chung C; Beecham M; Mauck RL; Burdick JA The Influence of Degradation Characteristics of Hyaluronic Acid Hydrogels on in Vitro Neocartilage Formation by Mesenchymal Stem Cells. Biomaterials 2009, 30, 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Wade RJ; Bassin EJ; Rodell CB; Burdick JA Protease-Degradable Electrospun Fibrous Hydrogels. Nat. Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Kolb HC; Finn MG; Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie - Int. Ed 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- (169).Moses JE; Moorhouse AD The Growing Applications of Click Chemistry. Chem. Soc. Rev 2007, 36, 1249–1262. [DOI] [PubMed] [Google Scholar]
- (170).Crescenzi V; Cornelio L; Di Meo C; Nardecchia S; Lamanna R Novel Hydrogels via Click Chemistry: Synthesis and Potential Biomedical Applications. Biomacromolecules 2007, 8, 1844–1850. [DOI] [PubMed] [Google Scholar]
- (171).Hoyle CE; Bowman CN Thiol-Ene Click Chemistry. Angew. Chemie - Int. Ed 2010, 49, 1540–1573. [DOI] [PubMed] [Google Scholar]
- (172).Highley CB; Song KH; Daly AC; Burdick JA Jammed Microgel Inks for 3D Printing Applications. Adv. Sci 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Lueckgen A; Garske DS; Ellinghaus A; Mooney DJ; Duda GN; Cipitria A Enzymatically-Degradable Alginate Hydrogels Promote Cell Spreading and in Vivo Tissue Infiltration. Biomaterials 2019, 217, 119294. [DOI] [PubMed] [Google Scholar]
- (174).Ooi HW; Mota C; Tessa Ten Cate A; Calore A; Moroni L; Baker MB Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).McOscar TVC; Gramlich WM Hydrogels from Norbornene-Functionalized Carboxymethyl Cellulose Using a UV-Initiated Thiol-Ene Click Reaction. Cellulose 2018, 25, 6531–6545. [Google Scholar]
- (176).Fein K; Bousfield DW; Gramlich WM The Influence of Versatile Thiol-Norbornene Modifications to Cellulose Nanofibers on Rheology and Film Properties. Carbohydr. Polym 2020, 230. [DOI] [PubMed] [Google Scholar]
- (177).Mũnoz Z; Shih H; Lin CC Gelatin Hydrogels Formed by Orthogonal Thiol-Norbornene Photochemistry for Cell Encapsulation. Biomater. Sci 2014, 2, 1063–1072. [DOI] [PubMed] [Google Scholar]
- (178).Greene T; Lin CC Modular Cross-Linking of Gelatin-Based Thiol-Norbornene Hydrogels for in Vitro 3D Culture of Hepatocellular Carcinoma Cells. ACS Biomater. Sci. Eng 2015, 1, 1314–1323. [DOI] [PubMed] [Google Scholar]
- (179).Perera MM; Ayres N Gelatin Based Dynamic Hydrogels: Via Thiol-Norbornene Reactions. Polym. Chem 2017, 8, 6741–6749. [Google Scholar]
- (180).Holmes R; Yang X. Bin; Dunne A; Florea L; Wood D; Tronci G Thiol-Ene Photo-Click Collagen-PEG Hydrogels: Impact of Water-Soluble Photoinitiators on Cell Viability, Gelation Kinetics and Rheological Properties. Polymers (Basel) 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Ryu S; Kim HH; Park YH; Lin CC; Um IC; Ki CS Dual Mode Gelation Behavior of Silk Fibroin Microgel Embedded Poly(Ethylene Glycol) Hydrogels. J. Mater. Chem. B 2016, 4, 4574–4584. [DOI] [PubMed] [Google Scholar]
- (182).Ji S; Abaci A; Morrison T; Gramlich WM; Guvendiren M Novel Bioinks from UV-Responsive Norbornene-Functionalized Carboxymethyl Cellulose Macromers. Bioprinting 2020, 18, e00083. [Google Scholar]
- (183).Li TD; Tang XL; Yang XD; Guo H; Cui YZ; Xu J Studies on the Reaction of Allyl Glycidyl Ether with Gelatin by Van Slyke Method. Asian J. Chem 2013, 25, 858–860. [Google Scholar]
- (184).Illy N; Robitzer M; Auvergne R; Caillol S; David G; Boutevin B Synthesis of Water-Soluble Allyl-Functionalized Oligochitosan and Its Modification by Thiol-Ene Addition in Water. J. Polym. Sci. Part A Polym. Chem 2014, 52, 39–48. [Google Scholar]
- (185).Duanmu J; Gamstedt EK; Rosling A Hygromechanical Properties of Composites of Crosslinked Allylglycidyl-Ether Modified Starch Reinforced by Wood Fibres. Compos. Sci. Technol 2007, 67, 3090–3097. [Google Scholar]
- (186).Hu H; You J; Gan W; Zhou J; Zhang L Synthesis of Allyl Cellulose in NaOH/Urea Aqueous Solutions and Its Thiol-Ene Click Reactions. Polym. Chem 2015, 6, 3543–3548. [Google Scholar]
- (187).Bertlein S; Brown G; Lim KS; Jungst T; Boeck T; Blunk T; Tessmar J; Hooper GJ; Woodfield TBF; Groll J Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater 2017, 29, 1–6. [DOI] [PubMed] [Google Scholar]
- (188).Kiliona KPS; Zhou M; Zhu Y; Lan P; Lin N Preparation and Surface Modification of Crab Nanochitin for Organogels Based on Thiol-Ene Click Cross-Linking. Int. J. Biol. Macromol 2020, 150, 756–764. [DOI] [PubMed] [Google Scholar]
- (189).Hilderbrand AM; Ford EM; Guo C; Sloppy JD; Kloxin AM Hierarchically Structured Hydrogels Utilizing Multifunctional Assembling Peptides for 3D Cell Culture. Biomater. Sci 2020, 8, 1256–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Mergy J; Fournier A; Hachet E; Auzély-Velty R Modification of Polysaccharides via Thiol-Ene Chemistry: A Versatile Route to Functional Biomaterials. J. Polym. Sci. Part A Polym. Chem 2012, 50, 4019–4028. [Google Scholar]
- (191).Russo L; Sgambato A; Visone R; Occhetta P; Moretti M; Rasponi M; Nicotra F; Cipolla L Gelatin Hydrogels via Thiol-Ene Chemistry. Monatshefte fur Chemie 2016, 147, 587–592. [Google Scholar]
- (192).Hahn SK; Oh EJ; Miyamoto H; Shimobouji T Sustained Release Formulation of Erythropoietin Using Hyaluronic Acid Hydrogels Crosslinked by Michael Addition. Int. J. Pharm 2006, 322, 44–51. [DOI] [PubMed] [Google Scholar]
- (193).Vanderhooft JL; Mann BK; Prestwich GD Synthesis and Characterization of Novel Thiol-Reactive Poly(Ethylene Glycol) Cross-Linkers for Extracellular-Matrix-Mimetic Biomaterials. Biomacromolecules 2007, 8, 2883–2889. [DOI] [PubMed] [Google Scholar]
- (194).Serban MA; Prestwich GD Synthesis of Hyaluronan Haloacetates and Biology of Novel Cross-Linker-Free Synthetic Extracellular Matrix Hydrogels. Biomacromolecules 2007, 8, 2821–2828. [DOI] [PubMed] [Google Scholar]
- (195).Vanderhooft JL; Alcoutlabi M; Magda JJ; Prestwich GD Rheological Properties of Cross-Linked Hyaluronan-Gelatin Hydrogels for Tissue Engineering. Macromol. Biosci 2009, 9, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Rodell CB; MacArthur JW; Dorsey SM; Wade RJ; Wang LL; Woo YJ; Burdick JA Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties in Vivo. Adv. Funct. Mater 2015, 25, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Liu Y; Shu XZ; Prestwich GD Osteochondral Defect Repair with Autologous Bone. Tissue Eng 2006, 12. [DOI] [PubMed] [Google Scholar]
- (198).Jin R; Teixeira LSM; Krouwels A; Dijkstra PJ; Van Blitterswijk CA; Karperien M; Feijen J Synthesis and Characterization of Hyaluronic Acid-Poly(Ethylene Glycol) Hydrogels via Michael Addition: An Injectable Biomaterial for Cartilage Repair. Acta Biomater 2010, 6, 1968–1977. [DOI] [PubMed] [Google Scholar]
- (199).Tay A; Sohrabi A; Poole K; Seidlits S; Di Carlo DA 3D Magnetic Hyaluronic Acid Hydrogel for Magnetomechanical Neuromodulation of Primary Dorsal Root Ganglion Neurons. Adv. Mater 2018, 30, 1–8. [DOI] [PubMed] [Google Scholar]
- (200).Nih LR; Sideris E; Carmichael ST; Segura T Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater 2017, 29, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Hiemstra C; Van Der Aa LJ; Zhong Z; Dijkstra PJ; Feijen J Novel in Situ Forming, Degradable Dextran Hydrogels by Michael Addition Chemistry: Synthesis, Rheology, and Degradation. Macromolecules 2007, 40, 1165–1173. [Google Scholar]
- (202).Hiemstra C; van der Aa LJ; Zhong Z; Dijkstra PJ; Feijen J Rapidly in Situ-Forming Degradable Hydrogels from Dextram Triols through Michael Addition. Biomacromolecules 2007, 8, 1548–1556. [DOI] [PubMed] [Google Scholar]
- (203).Peng K; Tomatsu I; Van Den Broek B; Cui C; Korobko AV; Van Noort J; Meijer AH; Spaink HP; Kros A Dextran Based Photodegradable Hydrogels Formed via a Michael Addition. Soft Matter 2011, 7, 4881–4887. [Google Scholar]
- (204).Yu Y; Deng C; Meng F; Shi Q; Feijen J; Zhong Z Novel Injectable Biodegradable Glycol Chitosan-Based Hydrogels Crosslinked by Michael-Type Addition Reaction with Oligo(Acryloyl Carbonate)-b-Poly(Ethylene Glycol)-b-Oligo(Acryloyl Carbonate) Copolymers. J. Biomed. Mater. Res. - Part A 2011, 99 A, 316–326. [DOI] [PubMed] [Google Scholar]
- (205).Kim M; Lee JY; Jones CN; Revzin A; Tae G Heparin-Based Hydrogel as a Matrix for Encapsulation and Cultivation of Primary Hepatocytes. Biomaterials 2010, 31, 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Xu K; Cantu DA; Fu Y; Kim J; Zheng X; Hematti P; Kao WJ Thiol-Ene Michael-Type Formation of Gelatin/Poly(Ethylene Glycol) Biomatrices for Three-Dimensional Mesenchymal Stromal/Stem Cell Administration to Cutaneous Wounds. Acta Biomater 2013, 9, 8802–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Wang Q; Chan TR; Hilgraf R; Fokin VV; Sharpless KB; Finn MG Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc 2003, 125, 3192–3193. [DOI] [PubMed] [Google Scholar]
- (208).Meldal M; Tornøe CW Cu-Catalyzed Azide - Alkyne Cycloaddition 2008, 2952–3015. [DOI] [PubMed] [Google Scholar]
- (209).Li M; De Priyadarsi; Gondi SR; Sumerlin BS Responsive Polymer-Protein Bioconjugates Prepared by RAFT Polymerization and Copper-Catalyzed Azide-Alkyne Click Chemistry. Macromol. Rapid Commun 2008, 29, 1172–1176. [Google Scholar]
- (210).Piluso S; Vuki R; Nöchel U; Lendlein A Sequential Alkyne-Azide Cycloadditions for Functionalized Gelatin Hydrogel Formation 2018, 100, 77–85. [Google Scholar]
- (211).Engkagul V; Sereemaspun A; Chirachanchai S One Pot Preparation of Chitosan/Hyaluronic Acid-Based Triple Network Hydrogel via in Situ Click Reaction, Metal Coordination and Polyion Complexation in Water. Carbohydr. Polym 2018, 200, 616–623. [DOI] [PubMed] [Google Scholar]
- (212).Piluso S; Hiebl B; Gorb SN; Kovalev A; Lendlein A; Axel T Hyaluronic Acid-Based Hydrogels Crosslinked by Copper-Catalyzed Azide-Alkyne Cycloaddition with Tailorable Mechanical Properties 2011, 34, 192–197. [DOI] [PubMed] [Google Scholar]
- (213).Koschella A; Hartlieb M; Heinze TA “Click-Chemistry” Approach to Cellulose-Based Hydrogels. Carbohydr. Polym 2011, 86, 154–161. [Google Scholar]
- (214).Reddy KR; Rajgopal K; Kantam ML Copper-Alginates : A Biopolymer Supported Cu ( II ) Catalyst for 1, 3-Dipolar Cycloaddition of Alkynes with Azides and Oxidative Coupling of 2-Naphthols and Phenols in Water 2007, 114, 36–40. [Google Scholar]
- (215).Agard NJ; Prescher JA; Bertozzi CR A Strain-Promoted [3 + 2] Azide-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc 2004, 126, 15046–15047. [DOI] [PubMed] [Google Scholar]
- (216).Wang X; Li Z; Shi T; Zhao P; An K; Lin C; Liu H Injectable Dextran Hydrogels Fabricated by Metal-Free Click Chemistry for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2017, 73, 21–30. [DOI] [PubMed] [Google Scholar]
- (217).Truong VX; Ablett MP; Gilbert HTJ; Bowen J; Richardson SM; Hoyland JA; Dove AP In Situ-Forming Robust Chitosan-Poly(Ethylene Glycol) Hydrogels Prepared by Copper-Free Azide– Alkyne Click Reaction for Tissue Engineering. Biomater. Sci 2014, 2, 167–175. [DOI] [PubMed] [Google Scholar]
- (218).Alge DL; Azagarsamy MA; Donohue DF; Anseth KS Synthetically Tractable Click Hydrogels for Three-Dimensional Cell Culture Formed Using Tetrazine-Norbornene Chemistry. Biomacromolecules 2013, 14, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Famili A; Rajagopal K Bio-Orthogonal Cross-Linking Chemistry Enables in Situ Protein Encapsulation and Provides Sustained Release from Hyaluronic Acid Based Hydrogels. Mol. Pharm 2017, 14, 1961–1968. [DOI] [PubMed] [Google Scholar]
- (220).Hong S; Carlson J; Lee H; Weissleder R Bioorthogonal Radiopaque Hydrogel for Endoscopic Delivery and Universal Tissue Marking. Adv. Healthc. Mater 2016, 5, 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Desai RM; Koshy ST; Hilderbrand SA; Mooney DJ; Joshi NS Versatile Click Alginate Hydrogels Crosslinked via Tetrazine-Norbornene Chemistry. Biomaterials 2015, 50, 30–37. [DOI] [PubMed] [Google Scholar]
- (222).Lueckgen A; Garske DS; Ellinghaus A; Desai RM; Stafford AG; Mooney DJ; Duda GN; Cipitria A Hydrolytically-Degradable Click-Crosslinked Alginate Hydrogels. Biomaterials 2018, 181, 189–198. [DOI] [PubMed] [Google Scholar]
- (223).Koshy ST; Desai RM; Joly P; Li J; Bagrodia RK; Lewin SA; Joshi NS; Mooney DJ Click-Crosslinked Injectable Gelatin Hydrogels. Adv. Healthc. Mater 2016, 5, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Magario I; García Einschlag FS; Rueda EH; Zygadlo J; Ferreira ML Mechanisms of Radical Generation in the Removal of Phenol Derivatives and Pigments Using Different Fe-Based Catalytic Systems. J. Mol. Catal. A Chem 2012, 352, 1–20. [Google Scholar]
- (225).Navarro J; Swayambunathan J; Lerman M; Santoro M; Fisher JP Development of Keratin-Based Membranes for Potential Use in Skin Repair. Acta Biomater 2019, 83, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Jin R; Moreira Teixeira LS; Dijkstra PJ; Zhong Z; Van Blitterswijk CA; Karperien M; Feijen J Enzymatically Crosslinked Dextran-Tyramine Hydrogels as Injectable Scaffolds for Cartilage Tissue Engineering. Tissue Eng. - Part A 2010, 16, 2429–2440. [DOI] [PubMed] [Google Scholar]
- (227).Loebel C; Szczesny SE; Cosgrove BD; Alini M; Zenobi-Wong M; Mauck RL; Eglin D Cross-Linking Chemistry of Tyramine-Modified Hyaluronan Hydrogels Alters Mesenchymal Stem Cell Early Attachment and Behavior. Biomacromolecules 2017, 18, 855–864. [DOI] [PubMed] [Google Scholar]
- (228).Shin JY; Yeo YH; Jeong JE; Park SA; Park WH Dual-Crosslinked Methylcellulose Hydrogels for 3D Bioprinting Applications. Carbohydr. Polym 2020, 238, 116192. [DOI] [PubMed] [Google Scholar]
- (229).Darr A; Calabro A Synthesis and Characterization of Tyramine-Based Hyaluronan Hydrogels. J. Mater. Sci. Mater. Med 2009, 20, 33–44. [DOI] [PubMed] [Google Scholar]
- (230).Lee F; Chung JE; Kurisawa M An Injectable Hyaluronic Acid-Tyramine Hydrogel System for Protein Delivery. J. Control. Release 2009, 134, 186–193. [DOI] [PubMed] [Google Scholar]
- (231).Ueda K; Akiba J; Ogasawara S; Todoroki K; Nakayama M; Sumi A; Kusano H; Sanada S; Suekane S; Xu K; et al. Growth Inhibitory Effect of an Injectable Hyaluronic Acid-Tyramine Hydrogels Incorporating Human Natural Interferon-α and Sorafenib on Renal Cell Carcinoma Cells. Acta Biomater 2016, 29, 103–111. [DOI] [PubMed] [Google Scholar]
- (232).Raia NR; Partlow BP; McGill M; Kimmerling EP; Ghezzi CE; Kaplan DL Enzymatically Crosslinked Silk-Hyaluronic Acid Hydrogels. Biomaterials 2017, 131, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Zhang Y; Chen H; Zhang T; Zan Y; Ni T; Cao Y; Wang J; Liu M; Pei R Injectable Hydrogels from Enzyme-Catalyzed Crosslinking as BMSCs-Laden Scaffold for Bone Repair and Regeneration. Mater. Sci. Eng. C 2019, 96, 841–849. [DOI] [PubMed] [Google Scholar]
- (234).Sakai S; Kawakami K Synthesis and Characterization of Both Ionically and Enzymatically Cross-Linkable Alginate. Acta Biomater 2007, 3, 495–501. [DOI] [PubMed] [Google Scholar]
- (235).Prodanovic O; Spasojevic D; Prokopijevic M; Radotic K; Markovic N; Blazic M; Prodanovic R Tyramine Modified Alginates via Periodate Oxidation for Peroxidase Induced Hydrogel Formation and Immobilization. React. Funct. Polym 2015, 93, 77–83. [Google Scholar]
- (236).Hou J; Li C; Guan Y; Zhang Y; Zhu XX Enzymatically Crosslinked Alginate Hydrogels with Improved Adhesion Properties. Polym. Chem 2015, 6, 2204–2213. [Google Scholar]
- (237).Jin R; Moreira Teixeira LS; Dijkstra PJ; Van Blitterswijk CA; Karperien M; Feijen J Chondrogenesis in Injectable Enzymatically Crosslinked Heparin/Dextran Hydrogels. J. Control. Release 2011, 152, 186–195. [DOI] [PubMed] [Google Scholar]
- (238).Moreira Teixeira LS; Bijl S; Pully VV; Otto C; Jin R; Feijen J; Van Blitterswijk CA; Dijkstra PJ; Karperien M Self-Attaching and Cell-Attracting in-Situ Forming Dextran-Tyramine Conjugates Hydrogels for Arthroscopic Cartilage Repair. Biomaterials 2012, 33, 3164–3174. [DOI] [PubMed] [Google Scholar]
- (239).Ogushi Y; Sakai S; Kawakami K Synthesis of Enzymatically-Gellable Carboxymethylcellulose for Biomedical Applications. J. Biosci. Bioeng 2007, 104, 30–33. [DOI] [PubMed] [Google Scholar]
- (240).Chen F; Yu S; Liu B; Ni Y; Yu C; Su Y; Zhu X; Yu X; Zhou Y; Yan D An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep 2016, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (241).Bi B; Liu H; Kang W; Zhuo R; Jiang X An Injectable Enzymatically Crosslinked Tyramine-Modified Carboxymethyl Chitin Hydrogel for Biomedical Applications. Colloids Surfaces B Biointerfaces 2019, 175, 614–624. [DOI] [PubMed] [Google Scholar]
- (242).Partlow BP; Hanna CW; Rnjak-kovacina J; Moreau JE; Applegate MB; Burke KA; Marelli B; Mitropoulos AN; Omenetto FG; Kaplan DL Highly Tunable Elastomeric Silk Biomaterials 2014, 4615–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Kripotou S; Stefanopoulou E; Culebras-Martínez M; Morales-Román RM; Gallego Ferrer G; Kyritsis A Water Dynamics and Thermal Properties of Tyramine-Modified Hyaluronic Acid - Gelatin Hydrogels. Polymer (Guildf) 2019, 178, 121598. [Google Scholar]
- (244).Hasturk O; Jordan KE; Choi J; Kaplan DL Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (245).Yang J; Cohen Stuart MA; Kamperman M Jack of All Trades: Versatile Catechol Crosslinking Mechanisms. Chem. Soc. Rev 2014, 43, 8271–8298. [DOI] [PubMed] [Google Scholar]
- (246).Filippidi E; Cristiani TR; Eisenbach CD; Herbert Waite J; Israelachvili JN; Kollbe Ahn B; Valentine MT Toughening Elastomers Using Mussel-Inspired Iron-Catechol Complexes. Science (80-. ) 2017, 358, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Cholewinski A; Yang FK; Zhao B Underwater Contact Behavior of Alginate and Catechol-Conjugated Alginate Hydrogel Beads. Langmuir 2017, 33, 8353–8361. [DOI] [PubMed] [Google Scholar]
- (248).Lee C; Shin J; Lee JS; Byun E; Ryu JH; Um SH; Kim DI; Lee H; Cho SW Bioinspired, Calcium-Free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [DOI] [PubMed] [Google Scholar]
- (249).Hong S; Yang K; Kang B; Lee C; Song IT; Byun E; Park KI; Cho SW; Lee H Hyaluronic Acid Catechol: A Biopolymer Exhibiting a PH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater 2013, 23, 1774–1780. [Google Scholar]
- (250).Xu J; Strandman S; Zhu JXX; Barralet J; Cerruti M Genipin-Crosslinked Catechol-Chitosan Mucoadhesive Hydrogels for Buccal Drug Delivery. Biomaterials 2015, 37, 395–404. [DOI] [PubMed] [Google Scholar]
- (251).Shin J; Lee JS; Lee C; Park HJ; Yang K; Jin Y; Ryu JH; Hong KS; Moon SH; Chung HM; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater 2015, 25, 3814–3824. [Google Scholar]
- (252).Sato T; Aoyagi T; Ebara M; Auzély-Velty R Catechol-Modified Hyaluronic Acid: In Situ-Forming Hydrogels by Auto-Oxidation of Catechol or Photo-Oxidation Using Visible Light. Polym. Bull 2017, 74, 4069–4085. [Google Scholar]
- (253).Zhang Z; He C; Chen X Hydrogels Based on PH-Responsive Reversible Carbon-Nitrogen Double-Bond Linkages for Biomedical Applications. Mater. Chem. Front 2018, 2, 1765–1778. [Google Scholar]
- (254).Jiang Y; Chen J; Deng C; Suuronen EJ; Zhong Z Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [DOI] [PubMed] [Google Scholar]
- (255).Uman S; Dhand A; Burdick JA Recent Advances in Shear-Thinning and Self-Healing Hydrogels for Biomedical Applications. J. Appl. Polym. Sci 2019, 48668, 1–20. [Google Scholar]
- (256).Boehnke N; Cam C; Bat E; Segura T; Maynard HD Imine Hydrogels with Tunable Degradability for Tissue Engineering. Biomacromolecules 2015, 16, 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Kalia J; Raines RT Hydrolytic Stability of Hydrazones and Oximes. Angew. Chemie - Int. Ed 2008, 47, 7523–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Qu J; Zhao X; Ma PX; Guo B PH-Responsive Self-Healing Injectable Hydrogel Based on N-Carboxyethyl Chitosan for Hepatocellular Carcinoma Therapy. Acta Biomater 2017, 58, 168–180. [DOI] [PubMed] [Google Scholar]
- (259).Xu C; Zhan W; Tang X; Mo F; Fu L; Lin B Self-Healing Chitosan / Vanillin Hydrogels Based on Schi Ff -Base Bond / Hydrogen Bond Hybrid Linkages. Polym. Test 2018, 66, 155–163. [Google Scholar]
- (260).Gupta B; Tummalapalli M; Deopura BL; Alam MS Preparation and Characterization of In-Situ Crosslinked Pectin – Gelatin Hydrogels. Carbohydr. Polym 2014, 106, 312–318. [DOI] [PubMed] [Google Scholar]
- (261).Lü S; Liu M; Ni B An Injectable Oxidized Carboxymethylcellulose/N-Succinyl-Chitosan Hydrogel System for Protein Delivery. Chem. Eng. J 2010, 160, 779–787. [Google Scholar]
- (262).Kimura S; Isobe N; Wada M; Kuga S; Ko JH; Kim UJ Enzymatic Hydrolysis of Chitosan-Dialdehyde Cellulose Hydrogels. Carbohydr. Polym 2011, 83, 1850–1853. [Google Scholar]
- (263).Fan M; Ma Y; Tan H; Jia Y; Zou S; Guo S; Zhao M; Huang H; Ling Z; Chen Y; et al. Covalent and Injectable Chitosan-Chondroitin Sulfate Hydrogels Embedded with Chitosan Microspheres for Drug Delivery and Tissue Engineering 2017, 71, 67–74. [DOI] [PubMed] [Google Scholar]
- (264).Tan H; Chu CR; Payne KA; Marra KG Biomaterials Injectable in Situ Forming Biodegradable Chitosan – Hyaluronic Acid Based Hydrogels for Cartilage Tissue Engineering. Biomaterials 2009, 30, 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Yuan L; Wu Y; Gu Q. sheng; El-Hamshary H; El-Newehy M; Mo X Injectable Photo Crosslinked Enhanced Double-Network Hydrogels from Modified Sodium Alginate and Gelatin. Int. J. Biol. Macromol 2017, 96, 569–577. [DOI] [PubMed] [Google Scholar]
- (266).Sarker B; Papageorgiou DG; Silva R; Zehnder T; Gul-E-Noor F; Bertmer M; Kaschta J; Chrissafis K; Detsch R; Boccaccini AR Fabrication of Alginate-Gelatin Crosslinked Hydrogel Microcapsules and Evaluation of the Microstructure and Physico-Chemical Properties. J. Mater. Chem. B 2014, 2, 1470–1482. [DOI] [PubMed] [Google Scholar]
- (267).Luo Y; Kirker KR; Prestwich GD Cross-Linked Hyaluronic Acid Hydrogel FIlms New Biomaterials. J. Control. Release 2000, 69, 169–184. [DOI] [PubMed] [Google Scholar]
- (268).Tan H; Hu X Injectable In Situ Forming Glucose-Responsive Dextran-Based Hydrogels to Deliver Adipogenic Factor for Adipose Tissue Engineering. J. Appl. Polym. Sci 2012, 126. [Google Scholar]
- (269).Jha AK; Hule RA; Jiao T; Teller SS; Clifton RJ; Duncan RL; Pochan DJ; Jia X Structural Analysis and Mechanical Characterization of Hyaluronic Acid-Based Doubly Cross-Linked Networks. Macromolecules 2009, 42, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (270).Dahlmann J; Krause A; Möller L; Kensah G; Möwes M; Diekmann A; Martin U; Kirschning A; Gruh I; Dräger G Biomaterials Fully de Fi Ned in Situ Cross-Linkable Alginate and Hyaluronic Acid Hydrogels for Myocardial Tissue Engineering. Biomaterials 2013, 34, 940–951. [DOI] [PubMed] [Google Scholar]
- (271).Wang LL; Highley CB; Yeh YC; Galarraga JH; Uman S; Burdick JA Three-Dimensional Extrusion Bioprinting of Single- and Double-Network Hydrogels Containing Dynamic Covalent Crosslinks. J. Biomed. Mater. Res. - Part A 2018, 106, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Domingues RMA; Silva M; Gershovich P; Betta S; Babo P; Caridade SG; Mano JF; Motta A; Reis RL; Gomes ME Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem 2015, 26, 1571–1581. [DOI] [PubMed] [Google Scholar]
- (273).Luo P; Liu L; Xu W; Fan L; Nie M Preparation and Characterization of Aminated Hyaluronic Acid/Oxidized Hydroxyethyl Cellulose Hydrogel. Carbohydr. Polym 2018, 199, 170–177. [DOI] [PubMed] [Google Scholar]
- (274).Sharma PK; Taneja S; Singh Y Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan-Poly(Ethylene Glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [DOI] [PubMed] [Google Scholar]
- (275).Bang EK; Lista M; Sforazzini G; Sakai N; Matile S Poly(Disulfide)S. Chem. Sci 2012, 3, 1752–1763. [Google Scholar]
- (276).Wedemeyer WJ; Welker E; Narayan M; Scheraga HA Disulfide Bonds and Protein Folding. Biochemistry 2000, 39, 4207–4216. [DOI] [PubMed] [Google Scholar]
- (277).Barcan GA; Zhang X; Waymouth RM Structurally Dynamic Hydrogels Derived from 1,2-Dithiolanes. J. Am. Chem. Soc 2015, 137, 5650–5653. [DOI] [PubMed] [Google Scholar]
- (278).Shu XZ; Liu Y; Luo Y; Roberts MC; Prestwich GD Disulfide Cross-Linked Hyaluronan Hydrogels. Biomacromolecules 2002, 3, 1304–1311. [DOI] [PubMed] [Google Scholar]
- (279).Shu XZ; Liu Y; Palumbo F; Prestwich GD Disulfide-Crosslinked Hyaluronan-Gelatin Hydrogel Films: A Covalent Mimic of the Extracellular Matrix for in Vitro Cell Growth. Biomaterials 2003, 24, 3825–3834. [DOI] [PubMed] [Google Scholar]
- (280).Bermejo-Velasco D; Azémar A; Oommen OP; Hilborn J; Varghese OP Modulating Thiol PKa Promotes Disulfide Formation at Physiological PH: An Elegant Strategy to Design Disulfide Cross-Linked Hyaluronic Acid Hydrogels. Biomacromolecules 2019, 20, 1412–1420. [DOI] [PubMed] [Google Scholar]
- (281).Zhao Y; Gao S; Zhao S; Li Y; Cheng L; Li J; Yin Y Synthesis and Characterization of Disulfide-Crosslinked Alginate Hydrogel Scaffolds. Mater. Sci. Eng. C 2012, 32, 2153–2162. [Google Scholar]
- (282).Li W; Lu S; Zhao M; Lin X; Zhang M; Xiao H; Liu K; Huang L; Chen L; Ouyang X; et al. Self-Healing Cellulose Nanocrystals-Containing Gels via Reshuffling of Thiuram Disulfide Bonds. Polymers (Basel) 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Sun Y; Huang Y Disulfide-Crosslinked Albumin Hydrogels. J. Mater. Chem. B 2016, 4, 2768–2775. [DOI] [PubMed] [Google Scholar]
- (284).Hoang Thi TT; Lee Y; Ryu SB; Nguyen DH; Park KD Enhanced Tissue Adhesiveness of Injectable Gelatin Hydrogels through Dual Catalytic Activity of Horseradish Peroxidase. Biopolymers 2018, 109, 1–11. [DOI] [PubMed] [Google Scholar]
- (285).Shen W; Lammertink RGH; Sakata JK; Kornfield JA; Tirrell DA Assembly of an Artificial Protein Hydrogel through Leucine Zipper Aggregation and Bisulfide Bond Formation. Macromolecules 2005, 38, 3909–3916. [Google Scholar]
- (286).Jin Y; Yu C; Denman RJ; Zhang W Recent Advances in Dynamic Covalent Chemistry. Chem. Soc. Rev 2013, 42, 6634–6654. [DOI] [PubMed] [Google Scholar]
- (287).Gandini A The Furan/Maleimide Diels-Alder Reaction: A Versatile Click-Unclick Tool in Macromolecular Synthesis. Prog. Polym. Sci 2013, 38, 1–29. [Google Scholar]
- (288).Kirchhof S; Strasser A; Wittmann HJ; Messmann V; Hammer N; Goepferich AM; Brandl FP New Insights into the Cross-Linking and Degradation Mechanism of Diels-Alder Hydrogels. J. Mater. Chem. B 2015, 3, 449–457. [DOI] [PubMed] [Google Scholar]
- (289).Wei HL; Yang Z; Zheng LM; Shen YM Thermosensitive Hydrogels Synthesized by Fast Diels-Alder Reaction in Water. Polymer (Guildf) 2009, 50, 2836–2840. [Google Scholar]
- (290).Koehler KC; Anseth KS; Bowman CN Diels-Alder Mediated Controlled Release from a Poly(Ethylene Glycol) Based Hydrogel. Biomacromolecules 2013, 14, 538–547. [DOI] [PubMed] [Google Scholar]
- (291).García-Astrain C; Avérous L Synthesis and Evaluation of Functional Alginate Hydrogels Based on Click Chemistry for Drug Delivery Applications. Carbohydr. Polym 2018, 190, 271–280. [DOI] [PubMed] [Google Scholar]
- (292).Kramer RK; Belgacem MN; Carvalho AJF; Gandini A Thermally Reversible Nanocellulose Hydrogels Synthesized via the Furan/Maleimide Diels-Alder Click Reaction in Water. Int. J. Biol. Macromol 2019, 141, 493–498. [DOI] [PubMed] [Google Scholar]
- (293).Montiel-Herrera M; Gandini A; Goycoolea FM; Jacobsen NE; Lizardi-Mendoza J; Recillas-Mota M; Argüelles-Monal WM N-(Furfural) Chitosan Hydrogels Based on Diels-Alder Cycloadditions and Application as Microspheres for Controlled Drug Release. Carbohydr. Polym 2015, 128, 6–12. [DOI] [PubMed] [Google Scholar]
- (294).Nimmo CM; Owen SC; Shoichet MS Diels-Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. Biomacromolecules 2011, 12, 824–830. [DOI] [PubMed] [Google Scholar]
- (295).García-Astrain C; Algar I; Gandini A; Eceiza A; Corcuera MÁ; Gabilondo N Hydrogel Synthesis by Aqueous Diels-Alder Reaction between Furan Modified Methacrylate and Polyetheramine-Based Bismaleimides. J. Polym. Sci. Part A Polym. Chem 2015, 53, 699–708. [Google Scholar]
- (296).Bi B; Ma M; Lv S; Zhuo R; Jiang X In-Situ Forming Thermosensitive Hydroxypropyl Chitin-Based Hydrogel Crosslinked by Diels-Alder Reaction for Three Dimensional Cell Culture. Carbohydr. Polym 2019, 212, 368–377. [DOI] [PubMed] [Google Scholar]
- (297).Guaresti O; García–Astrain C; Palomares T; Alonso–Varona A; Eceiza A; Gabilondo N Synthesis and Characterization of a Biocompatible Chitosan–Based Hydrogel Cross–Linked via ‘Click’ Chemistry for Controlled Drug Release. Int. J. Biol. Macromol 2017, 102, 1–9. [DOI] [PubMed] [Google Scholar]
- (298).Owen SC; Fisher SA; Tam RY; Nimmo CM; Shoichet MS Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir 2013, 29, 7393–7400. [DOI] [PubMed] [Google Scholar]
- (299).Wang G; Zhu J; Chen X; Dong H; Li Q; Zeng L; Cao X Alginate Based Antimicrobial Hydrogels Formed by Integrating Diels-Alder “Click Chemistry” and the Thiol-Ene Reaction. RSC Adv 2018, 8, 11036–11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Bai X; Lü S; Cao Z; Ni B; Wang X; Ning P; Ma D; Wei H; Liu M Dual Crosslinked Chondroitin Sulfate Injectable Hydrogel Formed via Continuous Diels-Alder (DA) Click Chemistry for Bone Repair. Carbohydr. Polym 2017, 166, 123–130. [DOI] [PubMed] [Google Scholar]
- (301).García-Astrain C; Guaresti O; González K; Santamaria-Echart A; Eceiza A; Corcuera MA; Gabilondo N Click Gelatin Hydrogels: Characterization and Drug Release Behaviour. Mater. Lett 2016, 182, 134–137. [Google Scholar]
- (302).Tan H; Rubin JP; Marra KG Direct Synthesis of Biodegradable Polysaccharide Derivative Hydrogels through Aqueous Diels-Alder Chemistry. Macromol. Rapid Commun 2011, 32, 905–911. [DOI] [PubMed] [Google Scholar]
- (303).Yu F; Cao X; Li Y; Zeng L; Yuan B; Chen X An Injectable Hyaluronic Acid/PEG Hydrogel for Cartilage Tissue Engineering Formed by Integrating Enzymatic Crosslinking and Diels-Alder “Click Chemistry.” Polym. Chem 2014, 5, 1082–1090. [Google Scholar]
- (304).Chen G; Jiang M Cyclodextrin-Based Inclusion Complexation Bridging Supramolecular Chemistry and Macromolecular Self-Assembly. Chem. Soc. Rev 2011, 40, 2254–2266. [DOI] [PubMed] [Google Scholar]
- (305).Teng L; Chen Y; Jia YG; Ren L Supramolecular and Dynamic Covalent Hydrogel Scaffolds: From Gelation Chemistry to Enhanced Cell Retention and Cartilage Regeneration. J. Mater. Chem. B 2019, 7, 6705–6736. [DOI] [PubMed] [Google Scholar]
- (306).Wang H; Heilshorn SC Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater 2015, 27, 3717–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Del Valle EMM Cyclodextrins and Their Uses: A Review. Process Biochem 2004, 39, 1033–1046. [Google Scholar]
- (308).Kono H; Teshirogi T Cyclodextrin-Grafted Chitosan Hydrogels for Controlled Drug Delivery. Int. J. Biol. Macromol 2015, 72, 299–308. [DOI] [PubMed] [Google Scholar]
- (309).Pluemsab W; Sakairi N; Furuike T Synthesis and Inclusion Property of α-Cyclodextrin-Linked Alginate. Polymer (Guildf) 2005, 46, 9778–9783. [Google Scholar]
- (310).Mateen R; Hoare T Injectable, in Situ Gelling, Cyclodextrin-Dextran Hydrogels for the Partitioning-Driven Release of Hydrophobic Drugs. J. Mater. Chem. B 2014, 2, 5157–5167. [DOI] [PubMed] [Google Scholar]
- (311).Heyraud A; Guenot P; Rinaudo M Controlled Synthesis and Inclusion Ability of a Hyaluronic Acid Derivative Bearing -Cyclodextrin Molecules 2006, 907–913. [DOI] [PubMed] [Google Scholar]
- (312).Gomez CG; Chambat G; Heyraud A; Villar M; Auzély-Velty R Synthesis and Characterization of a β-CD-Alginate Conjugate. Polymer (Guildf) 2006, 47, 8509–8516. [Google Scholar]
- (313).Izawa H; Kawakami K; Sumita M; Tateyama Y; Hill JP; Ariga K β-Cyclodextrin-Crosslinked Alginate Gel for Patient-Controlled Drug Delivery Systems: Regulation of Host-Guest Interactions with Mechanical Stimuli. J. Mater. Chem. B 2013, 1, 2155–2161. [DOI] [PubMed] [Google Scholar]
- (314).Zhang L; Zhou J; Zhang L Structure and Properties of β-Cyclodextrin/Cellulose Hydrogels Prepared in NaOH/Urea Aqueous Solution. Carbohydr. Polym 2013, 94, 386–393. [DOI] [PubMed] [Google Scholar]
- (315).Medronho B; Andrade R; Vivod V; Ostlund A; Miguel MG; Lindman B; Voncina B; Valente AJM Cyclodextrin-Grafted Cellulose: Physico-Chemical Characterization. Carbohydr. Polym 2013, 93, 324–330. [DOI] [PubMed] [Google Scholar]
- (316).Tojima T; Katsura H; Sang-mun H; Tanida F; Nishi N; Tokura S; Sakairi N Preparation of an a -Cyclodextrin – Linked Chitosan Derivative Via. J. Polym. Sci. Part A 1998, 36, 1965–1968. [Google Scholar]
- (317).Zawko SA; Truong Q; Schmidt CE Drug-Binding Hydrogels of Hyaluronic Acid Functionalized with b -Cyclodextrin. 2008. [DOI] [PubMed] [Google Scholar]
- (318).Rodell CB; Kaminski AL; Burdick JA Rational Design of Network Properties in Guest-Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14, 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Kongprathet T; Wanichwecharungruang S Sustaining Guest Molecules on Bio-Surfaces by Grafting the Surfaces with Cyclodextrins. Carbohydr. Polym 2015, 119, 110–117. [DOI] [PubMed] [Google Scholar]
- (320).Feng Q; Wei K; Lin S; Xu Z; Sun Y; Shi P; Li G; Bian L Mechanically Resilient, Injectable, and Bioadhesive Supramolecular Gelatin Hydrogels Crosslinked by Weak Host-Guest Interactions Assist Cell Infiltration and in Situ Tissue Regeneration. Biomaterials 2016, 101, 217–228. [DOI] [PubMed] [Google Scholar]
- (321).Charlot A; Auzely-Velty R Synthesis of Novel Supramolecular Assemblies Based on Hyaluronic Acid Derivatives Bearing Bivalent - Cyclodextrin and Adamantane Moieties. Macromolecules 2007, 40, 1147–1158. [Google Scholar]
- (322).Miao T; Fenn SL; Charron PN; Oldinski RA Self-Healing and Thermoresponsive Dual-Cross-Linked Alginate Hydrogels Based on Supramolecular Inclusion Complexes. Biomacromolecules 2015, 16, 3740–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Tomatsu I; Hashidzume A; Harada A Contrast Viscosity Changes upon Photoirradiation for Mixtures of Poly(Acrylic Acid)-Based α-Cyclodextrin and Azobenzene Polymers. J. Am. Chem. Soc 2006, 128, 2226–2227. [DOI] [PubMed] [Google Scholar]
- (324).Tamesue S; Takashima Y; Yamaguchi H; Shinkai S; Harada A Photoswitchable Supramolecular Hydrogels Formed by Cyclodextrins and Azobenzene Polymers. Angew. Chemie - Int. Ed 2010, 49, 7461–7464. [DOI] [PubMed] [Google Scholar]
- (325).Chiang CY; Chu CC Synthesis of Photoresponsive Hybrid Alginate Hydrogel with Photo-Controlled Release Behavior. Carbohydr. Polym 2015, 119, 18–25. [DOI] [PubMed] [Google Scholar]
- (326).Rosales AM; Rodell CB; Chen MH; Morrow MG; Anseth KS; Burdick JA Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjug. Chem 2018, 29, 905–913. [DOI] [PubMed] [Google Scholar]
- (327).Saleem M; Yu H; Wang L; Zain-ul-Abdin; Khalid H; Akram M; Abbasi NM; Huang J Review on Synthesis of Ferrocene-Based Redox Polymers and Derivatives and Their Application in Glucose Sensing. Anal. Chim. Acta 2015, 876, 9–25. [DOI] [PubMed] [Google Scholar]
- (328).Tan L; Liu Y; Ha W; Ding LS; Peng SL; Zhang S; Li BJ Stimuli-Induced Gel-Sol Transition of Multi-Sensitive Supramolecular β-Cyclodextrin Grafted Alginate/Ferrocene Modified Pluronic Hydrogel. Soft Matter 2012, 8, 5746–5749. [Google Scholar]
- (329).Murray J; Kim K; Ogoshi T; Yao W; Gibb BC The Aqueous Supramolecular Chemistry of Cucurbit[: N] Urils, Pillar [n] Arenes and Deep-Cavity Cavitands. Chem. Soc. Rev 2017, 46, 2479–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Walker S; Oun R; McInnes FJ; Wheate NJ The Potential of Cucurbit[n]Urils in Drug Delivery. Isr. J. Chem 2011, 51, 616–624. [Google Scholar]
- (331).Isaacs L Cucurbit[n]Urils: From Mechanism to Structure and Function. Chem. Commun 2009, No. 6, 619–629. [DOI] [PubMed] [Google Scholar]
- (332).Park KM; Yang JA; Jung H; Yeom J; Park JS; Park KH; Hoffman AS; Hahn SK; Kim K In Situ Supramolecular Assembly and Modular Modification of Hyaluronic Acid Hydrogels for 3D Cellular Engineering. ACS Nano 2012, 6, 2960–2968. [DOI] [PubMed] [Google Scholar]
- (333).Sohail A; Alnaqbi MA; Saleh N Alginate/Cucurbit[7]Uril/Dequalinium-Based Supramolecular Carbohydrates: Modulation of FRET Signals by Temperature Control. Macromolecules 2019, 52, 9023–9031. [Google Scholar]
- (334).Rowland MJ; Atgie M; Hoogland D; Scherman OA Preparation and Supramolecular Recognition of Multivalent Peptide-Polysaccharide Conjugates by Cucurbit[8]Uril in Hydrogel Formation. Biomacromolecules 2015, 16, 2436–2443. [DOI] [PubMed] [Google Scholar]
- (335).Li C; Rowland MJ; Shao Y; Cao T; Chen C; Jia H; Zhou X; Yang Z; Scherman OA; Liu D Responsive Double Network Hydrogels of Interpenetrating DNA and CB[8] Host-Guest Supramolecular Systems. Adv. Mater 2015, 27, 3298–3304. [DOI] [PubMed] [Google Scholar]
- (336).Rowland MJ; Parkins CC; McAbee JH; Kolb AK; Hein R; Loh XJ; Watts C; Scherman OA An Adherent Tissue-Inspired Hydrogel Delivery Vehicle Utilised in Primary Human Glioma Models. Biomaterials 2018, 179, 199–208. [DOI] [PubMed] [Google Scholar]
- (337).Wahl MC; Sundaralingam M C-H...O Hydrogen Bonding in Biology. Trends Biochem. Sci 1997, 22, 97–102. [DOI] [PubMed] [Google Scholar]
- (338).Mol EA; Lei Z; Roefs MT; Bakker MH; Goumans MJ; Doevendans PA; Dankers PYW; Vader P; Sluijter JPG Injectable Supramolecular Ureidopyrimidinone Hydrogels Provide Sustained Release of Extracellular Vesicle Therapeutics. Adv. Healthc. Mater 2019, 8. [DOI] [PubMed] [Google Scholar]
- (339).Thompson CB; Korley LTJ Harnessing Supramolecular and Peptidic Self-Assembly for the Construction of Reinforced Polymeric Tissue Scaffolds. Bioconjug. Chem 2017, 28, 1325–1339. [DOI] [PubMed] [Google Scholar]
- (340).Hou S; Wang X; Park S; Jin X; Ma PX Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv. Healthc. Mater 2015, 4, 1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (341).Zamani Alavijeh R; Shokrollahi P; Barzin J A Thermally and Water Activated Shape Memory Gelatin Physical Hydrogel, with a Gel Point above the Physiological Temperature, for Biomedical Applications. J. Mater. Chem. B 2017, 5, 2302–2314. [DOI] [PubMed] [Google Scholar]
- (342).Zhang G; Lv L; Deng Y; Wang C Self-Healing Gelatin Hydrogels Cross-Linked by Combining Multiple Hydrogen Bonding and Ionic Coordination. Macromol. Rapid Commun 2017, 38, 1–9. [DOI] [PubMed] [Google Scholar]
- (343).Liu S; Jin M; Chen Y; Gao H; Shi X; Cheng W; Ren L; Wang Y High Internal Phase Emulsions Stabilised by Supramolecular Cellulose Nanocrystals and Their Application as Cell-Adhesive Macroporous Hydrogel Monoliths. J. Mater. Chem. B 2017, 5, 2671–2678. [DOI] [PubMed] [Google Scholar]
- (344).Bonito V; Smits AIPM; Goor OJGM; Ippel BD; Driessen-Mol A; Münker TJAG; Bosman AW; Mes T; Dankers PYW; Bouten CVC Modulation of Macrophage Phenotype and Protein Secretion via Heparin-IL-4 Functionalized Supramolecular Elastomers. Acta Biomater 2018, 71, 247–260. [DOI] [PubMed] [Google Scholar]
- (345).Quideau S; Deffieux D; Douat-Casassus C; Pouységu L Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chemie - Int. Ed 2011, 50, 586–621. [DOI] [PubMed] [Google Scholar]
- (346).Shin M; Lee H Gallol-Rich Hyaluronic Acid Hydrogels: Shear-Thinning, Protein Accumulation against Concentration Gradients, and Degradation-Resistant Properties. Chem. Mater 2017, 29, 8211–8220. [Google Scholar]
- (347).Shin M; Galarraga JH; Kwon MY; Lee H; Burdick JA Gallol-Derived ECM-Mimetic Adhesive Bioinks Exhibiting Temporal Shear-Thinning and Stabilization Behavior. Acta Biomater 2019, 95, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Holm RH; Kennepohl P; Solomon EI Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev 1996, 96, 2239–2314. [DOI] [PubMed] [Google Scholar]
- (349).Harrington MJ; Masic A; Holten-andersen N Iron-Clad Fibers : A Metal-Based Biological Strategy for Hard Flexible Coatings. Science (80-. ) 2010, 328, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Lee BP; Konst S Novel Hydrogel Actuator Inspired by Reversible Mussel Adhesive Protein Chemistry. Adv. Mater 2014, 26, 3415–3419. [DOI] [PubMed] [Google Scholar]
- (351).Shin M; Song KH; Burrell JC; Cullen K; Burdick JA Injectable and Conductive Granular Hydrogels for 3D Printing and Electroactive Tissue Support. Advanced Science 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Yavvari PS; Srivastava A Robust, Self-Healing Hydrogels Synthesised from Catechol Rich Polymers. J. Mater. Chem. B 2015, 3, 899–910. [DOI] [PubMed] [Google Scholar]
- (353).Holten-Andersen N; Harrington MJ; Birkedal H; Lee BP; Messersmith PB; Lee KYC; Waite JH PH-Induced Metal-Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 2651–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (354).Menyo MS; Hawker CJ; Waite JH Versatile Tuning of Supramolecular Hydrogels through Metal Complexation of Oxidation-Resistant Catechol-Inspired Ligands. Soft Matter 2013, 9, 10314–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (355).Yavvari PS; Pal S; Kumar S; Kar A; Awasthi AK; Naaz A; Srivastava A; Bajaj A Injectable, Self-Healing Chimeric Catechol-Fe(III) Hydrogel for Localized Combination Cancer Therapy. ACS Biomater. Sci. Eng 2017, 3, 3404–3413. [DOI] [PubMed] [Google Scholar]
- (356).Guo Z; Ni K; Wei D; Ren Y Fe3+-Induced Oxidation and Coordination Cross-Linking in Catechol-Chitosan Hydrogels under Acidic PH Conditions. RSC Adv 2015, 5, 37377–37384. [Google Scholar]
- (357).Ghadban A; Ahmed AS; Ping Y; Ramos R; Arfin N; Cantaert B; Ramanujan RV; Miserez A Bioinspired PH and Magnetic Responsive Catechol-Functionalized Chitosan Hydrogels with Tunable Elastic Properties. Chem. Commun 2016, 52, 697–700. [DOI] [PubMed] [Google Scholar]
- (358).Lee J; Chang K; Kim S; Gite V; Chung H; Sohn D Phase Controllable Hyaluronic Acid Hydrogel with Iron(III) Ion-Catechol Induced Dual Cross-Linking by Utilizing the Gap of Gelation Kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar]
- (359).Haq MA; Su Y; Wang D Mechanical Properties of PNIPAM Based Hydrogels: A Review. Mater. Sci. Eng. C 2017, 70, 842–855. [DOI] [PubMed] [Google Scholar]
- (360).Lencina MMS; Rizzo C; Demitri C; Andreucetti N; Maffezzoli A Rheological Analysis of Thermo-Responsive Alginate/PNIPAAm Graft Copolymers Synthesized by Gamma Radiation. Radiat. Phys. Chem 2019, 156, 38–43. [Google Scholar]
- (361).Ekerdt BL; Fuentes CM; Lei Y; Adil MM; Ramasubramanian A; Segalman RA; Schaffer DV Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater 2018, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Wiltsey C; Kubinski P; Christiani T; Toomer K; Sheehan J; Branda A; Kadlowec J; Iftode C; Vernengo J Characterization of Injectable Hydrogels Based on Poly(N- Isopropylacrylamide)-g-Chondroitin Sulfate with Adhesive Properties for Nucleus Pulposus Tissue Engineering. J. Mater. Sci. Mater. Med 2013, 24, 837–847. [DOI] [PubMed] [Google Scholar]
- (363).Yuan H; Li B; Liang K; Lou X; Zhang Y Regulating Drug Release from PH- and Temperature-Responsive Electrospun CTS-g-PNIPAAm/Poly(Ethylene Oxide) Hydrogel Nanofibers. Biomed. Mater 2014, 9. [DOI] [PubMed] [Google Scholar]
- (364).Radu IC; Biru IE; Damian CM; Ion AC; Iovu H; Tanasa E; Zaharia C; Galateanu B Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Ren Z; Wang Y; Ma S; Duan S; Yang X; Gao P; Zhang X; Cai Q Effective Bone Regeneration Using Thermosensitive Poly(N-Isopropylacrylamide) Grafted Gelatin as Injectable Carrier for Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 19006–19015. [DOI] [PubMed] [Google Scholar]
- (366).Zhu Q; Gong Y; Guo T; Deng J; Ji J; Wang B; Hao S Thermo-Sensitive Keratin Hydrogel against Iron-Induced Brain Injury after Experimental Intracerebral Hemorrhage. Int. J. Pharm 2019, 566, 342–351. [DOI] [PubMed] [Google Scholar]
- (367).Tan H; Ramirez CM; Miljkovic N; Li H; Rubin JP; Marra KG Thermosensitive Injectable Hyaluronic Acid Hydrogel for Adipose Tissue Engineering. Biomaterials 2009, 30, 6844–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (368).Chen JP; Cheng TH Preparation and Evaluation of Thermo-Reversible Copolymer Hydrogels Containing Chitosan and Hyaluronic Acid as Injectable Cell Carriers. Polymer (Guildf) 2009, 50, 107–116. [Google Scholar]
- (369).Lai JY; Hsieh AC A Gelatin-g-Poly(N-Isopropylacrylamide) Biodegradable in Situ Gelling Delivery System for the Intracameral Administration of Pilocarpine. Biomaterials 2012, 33, 2372–2387. [DOI] [PubMed] [Google Scholar]
- (370).Kabanov AV; Batrakova EV; Alakhov VY Pluronic® Block Copolymers as Novel Polymer Therapeutics for Drug and Gene Delivery. J. Control. Release 2002, 189–212. [DOI] [PubMed] [Google Scholar]
- (371).Le PN; Huynh CK; Tran NQ Advances in Thermosensitive Polymer-Grafted Platforms for Biomedical Applications. Mater. Sci. Eng. C 2018, 92, 1016–1030. [DOI] [PubMed] [Google Scholar]
- (372).Lee H; Park TG Photo-Crosslinkable, Biomimetic, and Thermo-Sensitive Pluronic Grafted Hyaluronic Acid Copolymers for Injectable Delivery of Chondrocytes. J. Biomed. Mater. Res. - Part A 2009, 88, 797–806. [DOI] [PubMed] [Google Scholar]
- (373).Chung HJ; Go DH; Bae JW; Jung IK; Lee JW; Park KD Synthesis and Characterization of Pluronic® Grafted Chitosan Copolymer as a Novel Injectable Biomaterial. Curr. Appl. Phys 2005, 5, 485–488. [Google Scholar]
- (374).Choi JH; Joung YK; Bae JW; Choi JW; Quyen TN; Park KD Self-Assembled Nanogel of Pluronic-Conjugated Heparin as a Versatile Drug Nanocarrier. Macromol. Res 2011, 19, 180–188. [Google Scholar]
- (375).Kim DH; Heo S; Shin J; Mun CW; Park KM; Park KD Preparation of Thermosensitive Gelatin-Pluronic Copolymer for Cartilage Tissue Engineering 2010, 18, 387–391. [Google Scholar]
- (376).Park KM; Lee SY; Joung YK; Na JS; Lee MC; Park KD Thermosensitive Chitosan – Pluronic Hydrogel as an Injectable Cell Delivery Carrier for Cartilage Regeneration. Acta Biomater 2009, 5, 1956–1965. [DOI] [PubMed] [Google Scholar]
- (377).Lim KS; Galarraga JH; Cui X; Lindberg GCJ; Burdick JA; Wood TBF Fundamentals and Applications of Photo-Cross-Linking in Bioprinting [DOI] [PubMed] [Google Scholar]
- (378).Bejleri D; Streeter BW; Nachlas ALY; Brown ME; Gaetani R; Christman KL; Davis ME A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater 2018, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Cidonio G; Alcala-Orozco CR; Lim KS; Glinka M; Mutreja I; Kim YH; Dawson JI; Woodfield TBF; Oreffo ROC Osteogenic and Angiogenic Tissue Formation in High Fidelity Nanocomposite Laponite-Gelatin Bioinks. Biofabrication 2019, 11. [DOI] [PubMed] [Google Scholar]
- (380).Petta D; Armiento AR; Grijpma D; Alini M; Eglin D; D’Este M 3D Bioprinting of a Hyaluronan Bioink through Enzymatic-and Visible Light-Crosslinking. Biofabrication 2018, 10. [DOI] [PubMed] [Google Scholar]
- (381).Kuss MA; Harms R; Wu S; Wang Y; Untrauer JB; Carlson MA; Duan B Short-Term Hypoxic Preconditioning Promotes Prevascularization in 3D Bioprinted Bone Constructs with Stromal Vascular Fraction Derived Cells. RSC Adv 2017, 7, 29312–29320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Kesti M; Müller M; Becher J; Schnabelrauch M; Este MD; Eglin D; Zenobi-wong M Acta Biomaterialia A Versatile Bioink for Three-Dimensional Printing of Cellular Scaffolds Based on Thermally and Photo-Triggered Tandem Gelation. Acta Biomater 2015, 11, 162–172. [DOI] [PubMed] [Google Scholar]
- (383).Olate-Moya F; Arens L; Wilhelm M; Mateos-Timoneda MA; Engel E; Palza H Chondroinductive Alginate-Based Hydrogels Having Graphene Oxide for 3D Printed Scaffold Fabrication. ACS Appl. Mater. Interfaces 2020, 12, 4343–4357. [DOI] [PubMed] [Google Scholar]
- (384).Ouyang L; Highley CB; Sun W; Burdick JA A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-Crosslinkable Inks. Adv. Mater 2017, 29. [DOI] [PubMed] [Google Scholar]
- (385).Ouyang L; Armstrong JPK; Lin Y; Wojciechowski JP; Lee-Reeves C; Hachin D; Zhou K; Burdick JA; Stevens MM Expanding and Optimizing 3D Bioprinting Capabilities Using Complementary Network Bioinks. Sci. Adv 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (386).Heo DN; Alioglu MA; Wu Y; Ozbolat V; Ayan B; Dey M; Kang Y; Ozbolat IT 3D Bioprinting of Carbohydrazide-Modified Gelatin into Microparticle-Suspended Oxidized Alginate for the Fabrication of Complex-Shaped Tissue Constructs. ACS Appl. Mater. Interfaces 2020, 12, 20295–20306. [DOI] [PubMed] [Google Scholar]
- (387).Melchels FPW; Feijen J; Grijpma DW A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [DOI] [PubMed] [Google Scholar]
- (388).Zhu W; Qu X; Zhu J; Ma X; Patel S; Liu J; Wang P; Lai CSE; Gou M; Xu Y; et al. Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials 2017, 124, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (389).Kim SH; Yeon YK; Lee JM; Chao JR; Lee YJ; Seo YB; Sultan MT; Lee OJ; Lee JS; Yoon S Il; et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat. Commun 2018, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (390).Placone JK; Navarro J; Laslo GW; Lerman MJ; Gabard AR; Herendeen GJ; Falco EE; Tomblyn S; Burnett L; Fisher JP Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng 2017, 45, 237–248. [DOI] [PubMed] [Google Scholar]
- (391).Smith PT; Narupai B; Tsui JH; Millik SC; Shafranek RT; Kim DH; Nelson A Additive Manufacturing of Bovine Serum Albumin-Based Hydrogels and Bioplastics. Biomacromolecules 2020, 21, 484–492. [DOI] [PubMed] [Google Scholar]
- (392).Daly AC; Riley L; Segura T; Burdick JA Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Griffin DR; Weaver WM; Scumpia PO; Di Carlo D; Segura T Accelerated Wound Healing by Injectable Microporous Gel Scaffolds Assembled from Annealed Building Blocks. Nat. Mater 2015, 14, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).de Rutte JM; Koh J; Di Carlo D Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater 2019, 29, 1–10. [Google Scholar]
- (395).Sideris E; Griffin DR; Ding Y; Li S; Weaver WM; Di Carlo D; Hsiai T; Segura T Particle Hydrogels Based on Hyaluronic Acid Building Blocks. ACS Biomater. Sci. Eng 2016, 2, 2034–2041. [DOI] [PubMed] [Google Scholar]
- (396).Truong NF; Kurt E; Tahmizyan N; Lesher-Pérez SC; Chen M; Darling NJ; Xi W; Segura T Microporous Annealed Particle Hydrogel Stiffness, Void Space Size, and Adhesion Properties Impact Cell Proliferation, Cell Spreading, and Gene Transfer. Acta Biomater 2019, 94, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Darling NJ; Xi W; Sideris E; Anderson AR; Pong C; Carmichael ST; Segura T Click by Click Microporous Annealed Particle (MAP) Scaffolds. Adv. Healthc. Mater 2020, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Mealy JE; Chung JJ; Jeong HH; Issadore D; Lee D; Atluri P; Burdick JA Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater 2018, 30, 1–7. [DOI] [PubMed] [Google Scholar]
- (399).Li F; Truong VX; Fisch P; Levinson C; Glattauer V; Zenobi-Wong M; Thissen H; Forsythe JS; Frith JE Cartilage Tissue Formation through Assembly of Microgels Containing Mesenchymal Stem Cells. Acta Biomater 2018, 77, 48–62. [DOI] [PubMed] [Google Scholar]
- (400).Sheikhi A; de Rutte J; Haghniaz R; Akouissi O; Sohrabi A; Di Carlo D; Khademhosseini A Microfluidic-Enabled Bottom-up Hydrogels from Annealable Naturally-Derived Protein Microbeads. Biomaterials 2019, 192, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Hsu RS; Chen PY; Fang JH; Chen YY; Chang CW; Lu YJ; Hu SH Adaptable Microporous Hydrogels of Propagating NGF-Gradient by Injectable Building Blocks for Accelerated Axonal Outgrowth. Adv. Sci 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (402).Gehlen DB; Jürgens N; Omidinia-Anarkoli A; Haraszti T; George J; Walther A; Ye H; De Laporte L Granular Cellulose Nanofibril Hydrogel Scaffolds for 3D Cell Cultivation. Macromol. Rapid Commun 2020, 41, 1–10. [DOI] [PubMed] [Google Scholar]
- (403).Kessel B; Lee M; Bonato A; Tinguely Y; Tosoratti E; Zenobi-Wong M 3D Bioprinting of Macroporous Materials Based on Entangled Hydrogel Microstrands. Adv. Sci 2020, 2001419, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Lim SH; Mao HQ Electrospun Scaffolds for Stem Cell Engineering. Adv. Drug Deliv. Rev 2009, 61, 1084–1096. [DOI] [PubMed] [Google Scholar]
- (405).Li J; Pan K; Tian H; Yin L The Potential of Electrospinning / Electrospraying Technology in the Rational Design of Hydrogel Structures 2020, 2000285, 1–26. [Google Scholar]
- (406).Zhang S; Liu X; Barreto-ortiz SF; Yu Y; Ginn BP; Desantis NA; Hutton DL; Grayson WL; Cui F; Korgel BA; et al. Creating Polymer Hydrogel Micro Fibres with Internal Alignment via Electrical and Mechanical Stretching. Biomaterials 2014, 35, 3243–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Ghalei S; Nourmohammadi J; Solouk A; Mirzadeh H Enhanced Cellular Response Elicited by Addition of Amniotic Fluid to Alginate Hydrogel-Electrospun Silk Fibroin Fibers for Potential Wound Dressing Application. Colloids Surfaces B Biointerfaces 2018, 172, 82–89. [DOI] [PubMed] [Google Scholar]
- (408).Sundararaghavan HG; Burdick JA Gradients with Depth in Electrospun Fibrous Scaffolds for Directed Cell Behavior 2011, 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (409).Davidson MD; Song KH; Lee M; Llewellyn J; Du Y; Baker BM; Wells RG; Burdick JA Engineered Fibrous Networks To Investigate the Influence of Fiber Mechanics on Myofibroblast Differentiation 2019. [DOI] [PubMed] [Google Scholar]
- (410).Sun X; Lang Q; Zhang H; Cheng L; Zhang Y; Pan G; Zhao X; Yang H; Zhang Y; Santos HA; et al. Electrospun Photocrosslinkable Hydrogel Fibrous Scaffolds for Rapid In Vivo Vascularized Skin Flap Regeneration. Adv. Funct. Mater 2017, 27, 1–12. [Google Scholar]
- (411).Chen C; Tang J; Gu Y; Liu L; Liu X; Deng L; Martins C; Sarmento B; Cui W; Chen L Bioinspired Hydrogel Electrospun Fibers for Spinal Cord Regeneration. Adv. Funct. Mater 2019, 29, 1–11. [Google Scholar]
- (412).Song KH; Heo S; Peredo AP; Davidson MD; Mauck RL; Burdick JA Influence of Fiber Stiffness on Meniscal Cell Migration into Dense Fibrous Networks 2020, 1901228, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (413).Zhou Y; Liang K; Zhang C; Li J; Yang H; Liu X; Yin X; Chen D; Xu W; Xiao P Photocrosslinked Methacrylated Chitosan-Based Nanofibrous Scaffolds as Potential Skin Substitute. Cellulose 2017, 24, 4253–4262. [Google Scholar]
- (414).Wade RJ; Bassin EJ; Gramlich WM; Burdick JA Nanofibrous Hydrogels with Spatially Patterned Biochemical Signals to Control Cell Behavior 2015, 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Davidson MD; Ban E; Schoonen ACM; Lee MH; D’Este M; Shenoy VB; Burdick JA Mechanochemical Adhesion and Plasticity in Multifiber Hydrogel Networks. Adv. Mater 2020, 32, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Wang LL; Burdick JA Engineered Hydrogels for Local and Sustained Delivery of RNA-Interference Therapies. Adv. Healthc. Mater 2017, 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (417).Dimatteo R; Darling NJ; Segura T In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev 2018, 127, 167–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Zhang J; Ma PX Cyclodextrin-Based Supramolecular Systems for Drug Delivery: Recent Progress and Future Perspective. Adv. Drug Deliv. Rev 2013, 65, 1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (419).Ercole F; Whittaker MR; Quinn JF; Davis TP Cholesterol Modified Self-Assemblies and Their Application to Nanomedicine. Biomacromolecules 2015, 16, 1886–1914. [DOI] [PubMed] [Google Scholar]
- (420).Wang LL; Liu Y; Chung JJ; Wang T; Gaffey AC; Lu M; Cavanaugh CA; Zhou S; Kanade R; Atluri P; et al. Sustained MiRNA Delivery from an Injectable Hydrogel Promotes Cardiomyocyte Proliferation and Functional Regeneration after Ischaemic Injury. Nat. Biomed. Eng 2018, 1, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Wang LL; Chung JJ; Li EC; Uman S; Atluri P; Burdick JA Injectable and Protease-Degradable Hydrogel for SiRNA Sequestration and Triggered Delivery to the Heart. J. Control. Release 2018, 285, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Hoang Thi TT; Lee Y; Ryu SB; Sung HJ; Park KD Oxidized Cyclodextrin-Functionalized Injectable Gelatin Hydrogels as a New Platform for Tissue-Adhesive Hydrophobic Drug Delivery. RSC Adv 2017, 7, 34053–34062. [Google Scholar]
- (423).Segovia N; Pont M; Oliva N; Ramos V; Borrós S; Artzi N Hydrogel Doped with Nanoparticles for Local Sustained Release of SiRNA in Breast Cancer. Adv. Healthc. Mater 2015, 4, 271–280. [DOI] [PubMed] [Google Scholar]
- (424).Conde J; Oliva N; Atilano M; Song HS; Artzi N Self-Assembled RNA-Triple-Helix Hydrogel Scaffold for MicroRNA Modulation in the Tumour Microenvironment. Nat. Mater 2016, 15, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (425).Chen MH; Chung JJ; Mealy JE; Zaman S; Li EC; Arisi MF; Atluri P; Burdick JA Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci 2019, 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (426).Schirmer L; Atallah P; Werner C; Freudenberg U StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv. Healthc. Mater 2016, 5, 3157–3164. [DOI] [PubMed] [Google Scholar]
- (427).Turabee MH; Thambi T; Duong HTT; Jeong JH; Lee DS A PH- and Temperature-Responsive Bioresorbable Injectable Hydrogel Based on Polypeptide Block Copolymers for the Sustained Delivery of Proteins: In Vivo. Biomater. Sci 2018, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- (428).Bré LP; Zheng Y; Pêgo AP; Wang W Taking Tissue Adhesives to the Future: From Traditional Synthetic to New Biomimetic Approaches. Biomater. Sci 2013, 1, 239–253. [DOI] [PubMed] [Google Scholar]
- (429).Taboada GM; Yang K; Pereira MJN; Liu SS; Hu Y; Karp JM; Artzi N; Lee Y Overcoming the Translational Barriers of Tissue Adhesives. Nat. Rev. Mater 2020, 5, 310–329. [Google Scholar]
- (430).Assmann A; Vegh A; Ghasemi-Rad M; Bagherifard S; Cheng G; Sani ES; Ruiz-Esparza GU; Noshadi I; Lassaletta AD; Gangadharan S; et al. A Highly Adhesive and Naturally Derived Sealant. Biomaterials 2017, 140, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Tavafoghi M; Sheikhi A; Tutar R; Jahangiry J; Baidya A; Haghniaz R; Khademhosseini A Engineering Tough, Injectable, Naturally Derived, Bioadhesive Composite Hydrogels. Adv. Healthc. Mater 2020, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Wei K; Senturk B; Matter MT; Wu X; Herrmann IK; Rottmar M; Toncelli C Mussel-Inspired Injectable Hydrogel Adhesive Formed under Mild Conditions Features Near-Native Tissue Properties. ACS Appl. Mater. Interfaces 2019, 11, 47707–47719. [DOI] [PubMed] [Google Scholar]
- (433).Kim SH; Lee SH; Lee JE; Park SJ; Kim K; Kim IS; Lee YS; Hwang NS; Kim BG Tissue Adhesive, Rapid Forming, and Sprayable ECM Hydrogel via Recombinant Tyrosinase Crosslinking. Biomaterials 2018, 178, 401–412. [DOI] [PubMed] [Google Scholar]
- (434).Bermejo-Velasco D; Kadekar S; Tavares Da Costa MV; Oommen OP; Gamstedt K; Hilborn J; Varghese OP First Aldol Cross-Linked Hyaluronic Acid Hydrogel: Fast and Hydrolytically Stable Hydrogel with Tissue Adhesive Properties. ACS Appl. Mater. Interfaces 2019, 11, 38232–38239. [DOI] [PubMed] [Google Scholar]
- (435).Hong SH; Shin M; Park E; Ryu JH; Burdick JA; Lee H Alginate-Boronic Acid: PH-Triggered Bioinspired Glue for Hydrogel Assembly. Adv. Funct. Mater 2020, 30, 1–8. [Google Scholar]
- (436).Azuma K; Nishihara M; Shimizu H; Itoh Y; Takashima O; Osaki T; Itoh N; Imagawa T; Murahata Y; Tsuka T; et al. Biological Adhesive Based on Carboxymethyl Chitin Derivatives and Chitin Nanofibers. Biomaterials 2015, 42, 20–29. [DOI] [PubMed] [Google Scholar]
- (437).Annabi N; Zhang YN; Assmann A; Sani ES; Cheng G; Lassaletta AD; Vegh A; Dehghani B; Ruiz-Esparza GU; Wang X; et al. Engineering a Highly Elastic Human Protein-Based Sealant for Surgical Applications. Sci. Transl. Med 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (438).Wang L; Zhang X; Yang K; Fu YV; Xu T; Li S; Zhang D; Wang LN; Lee CS A Novel Double-Crosslinking-Double-Network Design for Injectable Hydrogels with Enhanced Tissue Adhesion and Antibacterial Capability for Wound Treatment. Adv. Funct. Mater 2020, 30. [Google Scholar]
- (439).Dhand A; Galarraga JH; Burdick JA Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol 2020, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (440).Gan Y; Li P; Wang L; Mo X; Song L; Xu Y; Zhao C; Ouyang B; Tu B; Luo L; et al. An Interpenetrating Network-Strengthened and Toughened Hydrogel That Supports Cell-Based Nucleus Pulposus Regeneration. Biomaterials 2017, 136, 12–28. [DOI] [PubMed] [Google Scholar]
- (441).Zhao X; Liang Y; Huang Y; He J; Han Y; Guo B Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/PH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater 2020, 30. [Google Scholar]
- (442).Abandansari HS; Ghanian MH; Varzideh F; Mahmoudi E; Rajabi S; Taheri P; Nabid MR; Baharvand H In Situ Formation of Interpenetrating Polymer Network Using Sequential Thermal and Click Crosslinking for Enhanced Retention of Transplanted Cells. Biomaterials 2018, 170, 12–25. [DOI] [PubMed] [Google Scholar]
- (443).Suo H; Zhang D; Yin J; Qian J; Wu ZL; Fu J Interpenetrating Polymer Network Hydrogels Composed of Chitosan and Photocrosslinkable Gelatin with Enhanced Mechanical Properties for Tissue Engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [DOI] [PubMed] [Google Scholar]
- (444).Chen F; Le P; Lai K; Fernandes-Cunha GM; Myung D Simultaneous Interpenetrating Polymer Network of Collagen and Hyaluronic Acid as an in Situ-Forming Corneal Defect Filler. Chem. Mater 2020, 32, 5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (445).Sievers J; Zschoche S; Dockhorn R; Friedrichs J; Werner C; Freudenberg U Temperature-Induced Mechanomodulation of Interpenetrating Networks of Star Poly(Ethylene Glycol)-Heparin and Poly(N-Isopropylacrylamide). ACS Appl. Mater. Interfaces 2019, 11, 41862–41874. [DOI] [PubMed] [Google Scholar]
- (446).Wen C; Lu L; Li X Mechanically Robust Gelatin-Alginate IPN Hydrogels by a Combination of Enzymatic and Ionic Crosslinking Approaches. Macromol. Mater. Eng 2014, 299, 504–513. [Google Scholar]
- (447).Park S; Edwards S; Hou S; Boudreau R; Yee R; Jeong KJ A Multi-Interpenetrating Network (IPN) Hydrogel with Gelatin and Silk Fibroin. Biomater. Sci 2019, 7, 1276–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Gong JP Why Are Double Network Hydrogels so Tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar]
- (449).Guo Z; Mi S; Sun W A Facile Strategy for Preparing Tough, Self-Healing Double-Network Hyaluronic Acid Hydrogels Inspired by Mussel Cuticles. Macromol. Mater. Eng 2019, 304, 1–7. [Google Scholar]
- (450).Xiao W; Qu X; Li J; Chen L; Tan Y; Li K; Li B; Liao X Synthesis and Characterization of Cell-Laden Double-Network Hydrogels Based on Silk Fibroin and Methacrylated Hyaluronic Acid. Eur. Polym. J 2019, 118, 382–392. [Google Scholar]
- (451).Ong S; Choudhury D; Naing MW Cell-Based Meat: Current Ambiguities with Nomenclature. Trends Food Sci. Technol 2020, 102, 223–231. [Google Scholar]
- (452).Zhang X; Ye T; Meng X; Tian Z; Pang L; Han Y; Li H; Lu G; Xiu F; Yu HD; et al. Sustainable and Transparent Fish Gelatin Films for Flexible Electroluminescent Devices. ACS Nano 2020. [DOI] [PubMed] [Google Scholar]
- (453).Ma Y; Zeng X; Ma X; Yang R; Zhao W A Simple and Eco-Friendly Method of Gelatin Production from Bone: One-Step Biocatalysis. J. Clean. Prod 2019, 209, 916–926. [Google Scholar]
- (454).Pang J; Liu X; Yang J; Lu F; Wang B; Xu F; Ma M; Zhang X Synthesis of Highly Polymerized Water-Soluble Cellulose Acetate by the Side Reaction in Carboxylate Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate. Sci. Rep 2016, 6, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Schmidt S; Liebert T; Heinze T Synthesis of Soluble Cellulose Tosylates in an Eco-Friendly Medium. Green Chem 2014, 16, 1941–1946. [Google Scholar]
- (456).Bae S. Bin; Kim MH; Park WH Electrospinning and Dual Crosslinking of Water-Soluble Silk Fibroin Modified with Glycidyl Methacrylate. Polym. Degrad. Stab 2020, 179, 109304. [Google Scholar]
- (457).Loebel C; Rodell CB; Chen MH; Burdick JA Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3D-Printing. Nat. Protoc 2017, 12, 1521–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Schmocker A; Khoushabi A; Frauchiger DA; Gantenbein B; Schizas C; Moser C; Bourban PE; Pioletti DP A Photopolymerized Composite Hydrogel and Surgical Implanting Tool for a Nucleus Pulposus Replacement. Biomaterials 2016, 88, 110–119. [DOI] [PubMed] [Google Scholar]
- (459).Rizwan M; Chan SW; Comeau PA; Willett TL; Yim EKF Effect of Sterilization Treatment on Mechanical Properties, Biodegradation, Bioactivity and Printability of GelMA Hydrogels. Biomed. Mater 2020, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (460).Mandal A; Clegg JR; Anselmo AC; Mitragotri S Hydrogels in the Clinic. Bioeng. Transl. Med 2020, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


