Abstract
We report an unusual case of a woman in her 60s diagnosed with monomorphic epitheliotropic intestinal T-cell lymphoma who required a pancreatoduodenectomy (Whipple procedure) for duodenal obstruction. The patient was initially treated with several cycles of chemotherapy, with persistent disease of the duodenum at D3. She was symptomatic with obstructive symptoms and positron emission tomography (PET)-CT showed disease localised to the duodenum without evidence of active disease elsewhere. The patient underwent pancreatoduodenectomy for both palliation of obstructive symptoms and potential oncological benefit. The patient had mild symptoms of delayed gastric emptying requiring promotility agents postoperatively, but otherwise recovered well after surgery. Unfortunately, surgical pathology revealed diffuse disease through the resected portion of the duodenum and jejunum, with positive proximal and distal margins. We suspect she has diffuse small bowel disease which was occult by CT and PET-CT. Based on this case, we recommend consideration of bypass rather than resection when possible for surgical palliation due to likelihood for extensive bowel involvement.
Keywords: pancreas and biliary tract, gastrointestinal surgery, oncology, small intestine cancer, chemotherapy
Background
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is a rare and aggressive primary lymphoma of the gastrointestinal tract.1 Previously, MEITL was referred to as enteropathy-associated T-cell lymphoma type II, differentiated from type I which was associated with coeliac disease.1
While the most common site of involvement of MEITL is the jejunum,2 previous cases of duodenal involvement have been documented.3 4 The clinical presentation of MEITL includes non-specific gastrointestinal complaints such as abdominal pain and diarrhoea, with many patients ultimately diagnosed after acute complications such as intestinal perforation or gastrointestinal bleeding.2 Intestinal obstruction can occur in about 20% of cases of MEITL.5 Despite treatment with chemotherapy and/or surgery, the median survival of MEITL is less than 7 months.5
There is no standardised treatment for MEITL, resulting in heterogeneous use of surgery, chemotherapy, radiation and autologous stem cell transplantation.4 Previous studies have shown that a majority of patients undergo some surgical intervention for histological confirmation of diagnosis or management of an acute surgical complication.2 The role of palliative surgery and debulking for intestinal obstruction is less well defined.
This case report describes the surgical treatment of a patient with duodenal obstruction secondary to MEITL.
Case presentation
The patient was a woman in her 60s with a medical history of Lyme disease and MEITL diagnosed a year prior, after presenting with a jejunal perforation. The diagnosis of MEITL was made on surgical pathology which demonstrated CD30 negative and T-cell receptor beta gene rearrangement. T-cell receptor gamma gene rearrangement was negative.
Investigations
Staging positron emission tomography (PET)/CT at that time of diagnosis was unremarkable for any other sites of disease outside of the duodenum. She underwent six cycles of cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone. A repeat PET/CT demonstrated progression of disease with increased fluorodeoxyglucose (FDG) avidity in the third portion of the duodenum (figure 1). Maximum standardised uptake value (SUVmax) was 8.81, increased from 5.82 prior to treatment. Endoscopy demonstrated a stricture in the third portion of the duodenum with biopsy confirming MEITL. The patient then underwent two cycles of ifosfamide, carboplatin and etoposide therapy followed by PET/CT, which demonstrated partial response (figure 2) with a decrease in SUVmax to 3.2.
Figure 1.
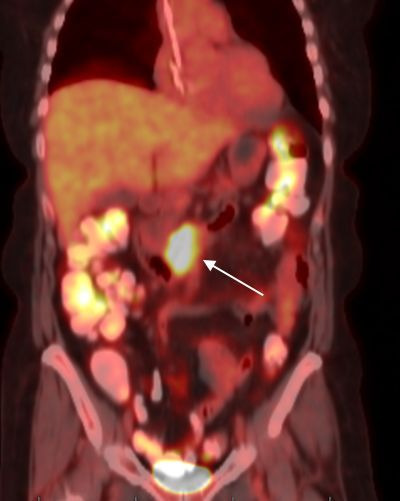
Positron emission tomography-CT demonstrating isolated fluorodeoxyglucose (FDG) avidity in the third portion of the duodenum (white arrow) after six cycles of cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone.
Figure 2.
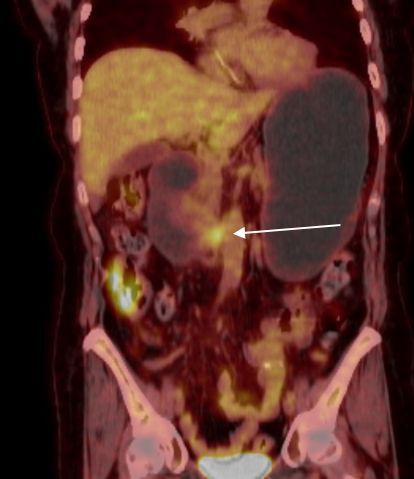
Repeat positron emission tomography-CT after two additional cycles of ifosfamide, carboplatin and etoposide therapy, demonstrating decreased fluorodeoxyglucose (FDG) avidity (white arrow).
Despite improvement demonstrated on PET, the patient developed progressive symptoms of nausea, emesis and intolerance of oral intake. Patient underwent repeat endoscopy revealing inflammation, oedema, erythema and ulceration of the entire duodenum, as well as a severe stenosis in the third portion of the duodenum with luminal diameter of 1 mm, which could not be traversed with a paediatric scope (figure 3).
Figure 3.
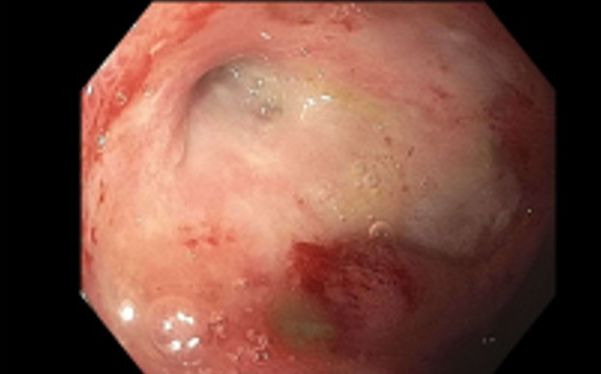
Endoscopic evaluation of the third portion of the duodenum demonstrating severe stricture with friable and edematous mucosa.
The patient was admitted to the hospital for nasogastric tube decompression. Extensive discussions were held in multidisciplinary fashion with involvement of medical oncology, surgical oncology, and the patient and her family. Given apparently isolated duodenal disease and partial response to chemotherapy, surgical resection with a duodenectomy versus Whipple was recommended for both palliation of symptoms and potential oncologic benefit.
Treatment
Intraoperatively, there was no evidence of metastatic disease visually or by palpation. The tumour was located approximately 1 cm distal to the ampulla. There were several areas of small bowel that were thickened. At the time, these areas were thought to be related to her prior surgery and were not biopsied as it would require a full thickness biopsy of bowel. Given the proximity of the disease to the ampulla, we proceeded with a Whipple procedure including resection of a portion of the proximal jejunum to encompass thickened bowel. The remainder of the procedure was performed in the standard fashion, and reconstruction was performed with a Blumgart duct-to-mucosa pancreaticojejunostomy, hepaticojejunostomy, and gastrojejunostomy. A nasojejunal (NJ) tube was left in place distal to the gastrojejunal anastomosis for feeding access.
Postoperatively, the patient had delayed gastric emptying treated with promotility agents, but otherwise recovered well. The patient was discharged on postoperative day 8 on a full liquid diet with supplemental NJ tube feeds due to inadequate oral intake. At her 1 month follow-up appointment, the patient was tolerating a regular diet and maintaining her weight, and the NJ tube was removed.
Outcome and follow-up
Surgical pathology revealed MEITL throughout the entirety of the resected duodenum and jejunum with positive margins both proximally and distally, indicating persistent refractory disease that was radiographically occult by PET/CT and not visualised endoscopically or intraoperatively. In retrospect, the intraoperatively noted diffuse small bowel thickening likely reflected disease involvement despite no mass-like component in these areas. Lymph nodes were all were negative for malignancy.
Although the patient recovered well initially, she experienced a decline in functional status and oral intake in the month after NJ tube removal. She declined evaluation or additional workup by the surgical team, and opted to pursue hospice care. She expired approximately 2 months after surgery.
Discussion
MEITL is a rare, but aggressive primary lymphoma of the gastrointestinal tract. MEITL of the small intestine is difficulty to diagnosis and is frequently associated with non-specific symptoms. Many cases are diagnosed after emergent surgery for perforation or obstruction, as occurred in this patient.6 Given the low incidence of the disease, available evidence is minimal and therefore no current guidelines exist regarding optimal diagnosis or treatment.1 Many case reports have demonstrated aggressive tumour biology resulting in poor survival despite treatment with chemotherapy and/or surgery,1 with mean overall survival of 7 months.5
In the largest case series to date, Mago et al examined 23 cases of MEITL. In the case series, 7 of 23 patients (30.4%) with small bowel MEITL underwent resection and only one patient who underwent resection survived more than a year. A majority of the resections were segmental small bowel resections, with no Whipple procedures included in this series. Outcomes from our case and others in the literature suggest that surgical resection may not be associated with improved survival in patients with MEITL.1 While MEITL is most frequently located in the jejunum, duodenal lesions have been previously reported and can result in obstructive jaundice, obstruction and gastrointestinal bleeding.4 7 A similar case to our patient was reported where the patient underwent gastrojejunal bypass rather than resection. This patient received several cycles of anthracycline chemotherapy, but had progression of disease, failed salvage chemotherapy treatment, and ultimately expired less than a year after diagnosis.4
The current case is unique in that our patient had both radiological and endoscopic evidence of a favourable response to chemotherapy treatment. Given the apparent favourable oncological behaviour, limited disease and overall good health and functional status of the patient, we opted to perform aggressive surgical resection for both symptom palliation and oncological benefit. Unfortunately, the extent of disease was not apparent by preoperative imaging, endoscopy or intraoperative examination, as surgical pathology revealed extensive, margin positive disease. While the surgical recovery was not complicated initially, we surmise that her extensive disease burden likely contributed to her decline in the months after her surgery. Due to her already protracted treatment course, the patient did not wish to undergo further evaluation or aggressive support, and expired approximately 14 months after her diagnosis.
While surgery for MEITL is feasible, this case demonstrates the aggressive and occult behaviour of this cancer. Surgical treatment of duodenal MEITL with an oncological intent is unlikely to provide a survival benefit and a palliative approach should be considered, as the average survival in this disease process approximates that of patients with metastatic pancreatic or gastric adenocarcinoma. Endoscopic decompression such as percutaneous venting gastrostomy tube, or palliative gastrojejunal bypass should be considered.
Learning points.
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is a rare and aggressive primary lymphoma of the gastrointestinal tract.
MEITL presents with non-specific gastrointestinal symptoms such as diarrhoea and abdominal pain, or acute surgical emergencies such as perforation and intestinal obstruction.
Attempted oncological resection in MEITL is unlikely to be successful due to diffuse nature of disease.
Based on aggressive biology of the disease, in the case of obstruction, palliative options should be considered.
Footnotes
Contributors: All authors (EJO, KR, JSP): substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Mago S, Mavilia M, Forouhar F, et al. Small bowel T-cell lymphoma: a MEITL-ing diagnosis. Clin J Gastroenterol 2021;14:1071–83. 10.1007/s12328-021-01417-3 [DOI] [PubMed] [Google Scholar]
- 2.Yi JH, Lee G-W, Do YR, et al. Multicenter retrospective analysis of the clinicopathologic features of monomorphic epitheliotropic intestinal T-cell lymphoma. Ann Hematol 2019;98:2541–50. 10.1007/s00277-019-03791-y [DOI] [PubMed] [Google Scholar]
- 3.Schmitt-Gräff A, Daum S, Hummel M, et al. Presence of clonal T-cell receptor gene rearrangements provides evidence of widespread intramucosal intestinal T-cell lymphoma. Z Gastroenterol 1996;34:680–5. [PubMed] [Google Scholar]
- 4.Kasinathan G. Monomorphic epitheliotropic intestinal T-cell lymphoma of the duodenum: an aggressive disease. Hematology, Transfusion and Cell Therapy 2021;43:518–20. 10.1016/j.htct.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse E, Kwong Y-L. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 2017;10:85. 10.1186/s13045-017-0452-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae JY, Ko BM, Min SK, et al. A case of enteropathy-type T-cell lymphoma diagnosed by small bowel enteroscopy: a perspective on imaging-enhanced endoscopy. Gut Liver 2012;6:516–9. 10.5009/gnl.2012.6.4.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishibashi H, Nimura S, Kayashima Y, et al. Multiple lesions of gastrointestinal tract invasion by monomorphic epitheliotropic intestinal T-cell lymphoma, accompanied by duodenal and intestinal enteropathy-like lesions and microscopic lymphocytic proctocolitis: a case series. Diagn Pathol 2016;11:66. 10.1186/s13000-016-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]


